Abstract
Prevalence of allergy to fungi is around 3–10%. The most prevalent species involved in sensitizations are Alternaria alternata, Aspergillus fumigatus, Cladosporium herbarum, and Penicillium notatum. Our main objective was to estimate the prevalence of fungal sensitization and its variation across Spain. Following the ICH-GCP, we recruited 1156 patients from 15 allergy departments in Spain. Hospitals were selected by bioclimatic areas. Patients underwent a skin prick test (SPT) with fungi, pollens, house dust mites, and animal dander. Specific IgE to Alternaria alternata and Alt a 1 was assessed in patients with positive SPT to fungi. Of the 233 patients (20.2%) sensitized to at least one of the five fungi tested, 162 (69.5%) were sensitized to Alternaria alternata and Alt a 1, of whom 113 (69.8%) were children; 181 (77.7%) were also polysensitized to other allergens. Alternaria alternata and Alt a 1 sensitization was present in 25.4% of patients in the Continental area, 12.0% in the Mediterranean area, 7.0% in the Semidesertic area, and 2.3% in the Oceanic area. Prevalence of sensitization to the other tested sources was 63.8% to pollens, 60.5% to house dust mite, and 38.1% to animal dander. We concluded that the prevalence of fungal allergy is increasing. Fungi are still the fourth source of allergen sensitization. Alternaria alternata sensitization is the most prevalent in allergic patients to fungi. Alt a 1 is present in almost 90% of the patients sensitized to Alternaria alternata.
Keywords: Alternaria alternata, fungus, fungal allergy, rhinitis, prevalence, Alt a 1, asthma, bioclimatic areas
1. Introduction
Allergy to fungi has an estimated prevalence around 3–10% [1,2]. The most prevalent species involved in allergic disease or sensitizations are Alternaria alternata, Aspergillus fumigatus, Cladosporium herbarum, and Penicillium notatum. Alternaria alternata represents 60% of the positive skin prick tests (SPT) among fungi sensitized patients [3].
The European Community Respiratory Health Survey showed that 4.4% of the adult population studied (n = 11,355) were sensitized to Alternaria alternata [4]. GA2LEN initiative performed a study in 14 countries of the European Community (n = 3034) and showed a prevalence of sensitization to Alternaria alternata of 9%, with ratios between 2% (Finland) and 23.8% (Greece) [5]. In the United States, the prevalence of positive SPT to Alternaria alternata was estimated at around 12.9% in subjects aged between 6 to 74 years old (n = 20,322) [6]. In Spain, this prevalence was estimated at around 20% [7].
Fungi are the fourth source of sensitization, after pollens and dust mites, involved in allergic respiratory diseases, Alternaria alternata and its major allergen Alt a 1 being the most relevant and most studied in allergic diseases [8,9,10,11,12,13]. Although there are several epidemiologic studies about its prevalence, it varies according to the diagnosis [14,15].
Monosensitization to fungi is rare. This sensitization is usually associated to other allergens [3], and some studies have described the capacity of fungi for activating the immune system and increasing the inflammatory response induced by other allergens such as grass pollen [16]. In addition, fungi sensitization has a strong genetic influence [17] and it is related to an increase of asthma severity and admission to hospitals [18,19,20].
Alternaria alternata is considered an outdoor fungus, saprophyte in plants, food, and soil. Its optimal temperature is around 21 °C, but it can survive between 2–32 °C, with humidity conditions greater than 84%. Sporulation takes place between May and November, presenting its higher incidence in summer and autumn [21,22,23].
Outdoor spores counting can reach 7500 spores/m3 of air; indoor counting is about 280 spores/m3 [24]. Indoor presence is associated with high humidity, poor air circulation, and the presence of cockroaches or cat dander, linked to the “sick building syndrome” [25]. A Polish study in adults (n = 500) correlated a concentration of 80 spores/m3 with a flare-up of allergic symptoms and levels higher than 300 spores/m3 are associated with dyspnea [26].
We have estimated the prevalence of Alternaria alternata sensitization in Spain and have studied the characteristics of sensitized patients to provide the best diagnosis and treatment.
2. Materials and Methods
This is an observational, cross-sectional, and multicentric study aimed to draw a new map of fungal sensitization in Spain, considering bioclimatic areas (areas where the climate, fauna, and flora are similar) [27].
The study was conducted in the allergy department of 15 hospitals located in 6 Spanish regions (Andalusia, Catalonia, Extremadura, Valencian Community, Galicia, and Basque Country). Each department should enroll 100 consecutive patients diagnosed with allergic rhinitis or rhinoconjunctivitis with or without asthma. Patients included were between 3–70 years of age; older than 16 years were considered adults and younger than 16 were considered children.
Participating patients gave informed consent. Demographic variables of the patients were collected (age, sex, previous allergy pathologies, and smoking habits). To classify rhinitis and asthma, the Allergic Rhinitis and its Impact on Asthma (ARIA) guideline [28], the Spanish guidelines for the management of asthma (GEMA 5.0) [29], and the asthma control test (ACT) [30,31] were used. A SPT to fungi, pollens, animal dander, and house dust mites was performed to all patients. SPT was considered positive if the papule diameter was bigger than 3 mm; this SPT were performed with Diater laboratories extracts. Specific IgE to Alternaria alternata and to Alt a 1 was assessed in patients who were positive to any of the tested fungi. They were asked to fill a Mold questionnaire (Appendix A and Appendix B).
Primary objective was to describe the sensitization to Alternaria alternata and its major allergen Alt a 1 in Spain. To achieve this objective, we calculated the percentage of patients sensitized and its confidence interval at 95% for the whole sample and for each center separately, comparing them with chi-square test.
Secondary objectives were to determine the percentage of patients with positive SPT to the tested fungi, house dust mite, pollens, and animal dander; the percentage of patients with positive IgE to Alternaria alternata and Alt a 1 among the patients sensitized to fungi by SPT; and correlate the demographic characteristics of these patients.
After estimating the prevalence of Alternaria alternata and Alt a 1 sensitization in each center, patients were grouped by bioclimatic area. The selected areas were Mediterranean, Semidesert, Continental, and Oceanic.
The statistical analysis was carried out by SAS®® v9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Recruitment
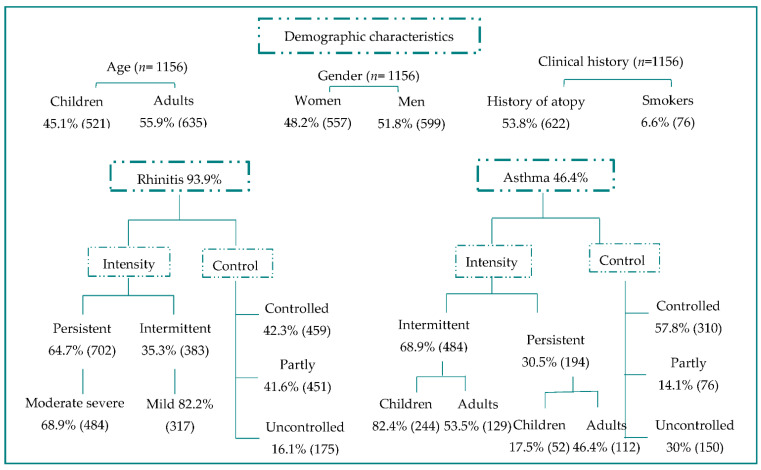
We recruited a total of 1191 patients, 35 of them were excluded from the analysis per protocol, so we obtained 1156 patients. Patients were excluded because 34 did not have any sensitization and one patient did not want to do the tests. Table 1 shows the distribution of patients by center and Figure 1 shows the characteristics of patients included in the study.
Table 1.
Distribution of patients included in the study by center.
| Centers | Children | Adults | Total (%) |
|---|---|---|---|
| Andalusia | 126 | 269 | 395 (34.1) |
| Catalonia | 181 | 106 | 287 (24.8) |
| Extremadura | 96 | 99 | 195 (16.9) |
| Valencian Community | 98 | 50 | 148 (12.8) |
| Galicia | 18 | 82 | 100 (8.7) |
| Basque Country | 2 | 29 | 31 (2.7) |
| Total | 521 | 635 | 1156 (100) |
Figure 1.
Characteristics of patients included in the study (n = 1156).
The selected centers were located as follows: Mediterranean region (Barcelona, Manresa, Valencia, Seville, and Huelva); Semidesert region (Almeria); Continental region (Badajoz and Lleida); and Oceanic region (Lugo and Vitoria).
3.2. Primary Objective
The main objective of the study was to determine the percentage of patients sensitized to Alternaria alternata/Alt a 1 in each region.
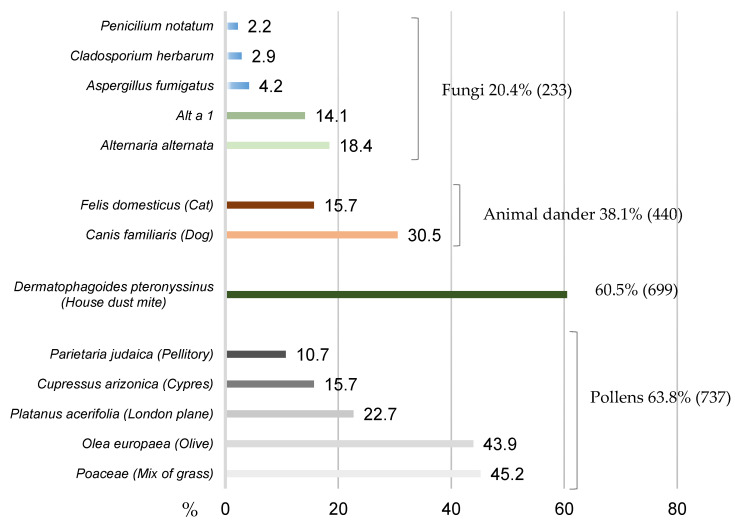
Figure 2 shows the SPT results by allergen source. Table 2 shows the distribution of patients sensitized to any fungi and Alternaria alternata and Alt a 1 by region and Table 3 shows patients sensitized to any fungi and Alternaria alternata or Alt a 1 by bioclimatic region.
Figure 2.
SPT results by allergen source (%).
Table 2.
Patients sensitized to fungi and Alternaria alternata and Alt a 1 by center.
| Centers | Patients Included N |
Positive SPT to Any Fungi N (%) |
Positive IgE to A. alternata and Alt a 1 N (%) |
Positive SPT to A. alternata and Alt a 1 |
|
|---|---|---|---|---|---|
| Children N (%) |
Adults N (%) |
||||
| Andalusia | 395 | 78 (19.7) | 55 (13.9) | 20 (5.1) | 28 (7.1%) |
| Catalonia | 287 | 81 (28.2) | 51 (17.8) | 34 (11.8) | 22 (7.7%) |
| Extremadura | 195 | 53 (27.2) | 41 (21.0) | 30 (15.4) | 12 (6.2%) |
| Valencian Community | 148 | 15 (10.1) | 12 (8.1) | 8 (5.4) | 2 (1.4%) |
| Galicia | 100 | 1 (1.0) | 1 (1.0) | 1 (1.0) | 0 (0%) |
| Basque Country | 31 | 5 (16.1) | 2 (6.5) | 0 (0) | 2 (6.5%) |
| Total | 1156 | 233 (20.2) | 162 (14.0) | 93 (8.0) | 64 (5.5%) |
Table 3.
Patients sensitized to fungi and Alternaria alternata or Alt a 1 by bioclimatic regions.
| Bioclimatic Areas | Patients Included N |
Positive SPT to Any Fungi N (%) |
Positive IgE to A. alternata or Alt a 1 N (%) |
|---|---|---|---|
| Mediterranean | 592 | 124 (20.9) | 71 (12.0) |
| Semidesert | 201 | 27 (13.4) | 14 (7.0) |
| Continental | 232 | 76 (32.8) | 59 (25.4) |
| Oceanic | 131 | 6 (4.6) | 3 (2.3) |
| Total | 1156 | 233 (20.2) | 147 (12.7) |
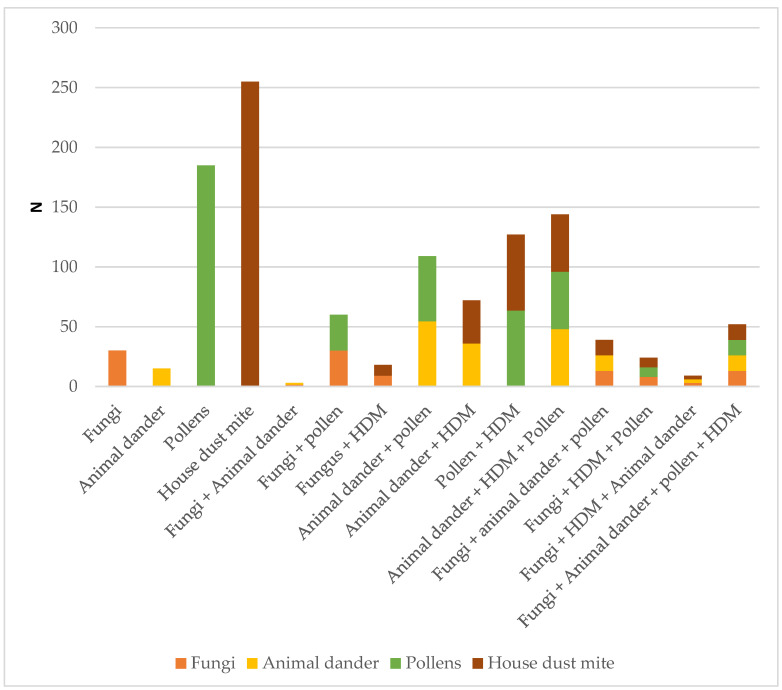
Polysensitization was seen in 56.5% of the study population. Of patients sensitized to any fungi, 77.7% were also polysensitized to other allergens. Figure 3 shows the distribution of patients by allergen groups.
Figure 3.
Polysensitization of the study population by allergen groups.
Among the patients sensitized to any of the fungi tested (233); 214 patients had allergic rhinitis; description of these patients is below in Table 4.
Table 4.
Presence (%) of allergic rhinitis in sensitized patients to each fungal allergen.
| Fungus | Sensitized (n = 233) | With Allergic Rhinitis (n = 214)/% |
|---|---|---|
| Alternaria alternata and Alt a 1 | 162 | 149/92% |
| Cladosporium herbarum | 31 | 31/100% |
| Aspergillus fumigatus | 45 | 43/95.5% |
| Penicilium notatum | 24 | 23/95.8% |
3.3. Secondary Objectives
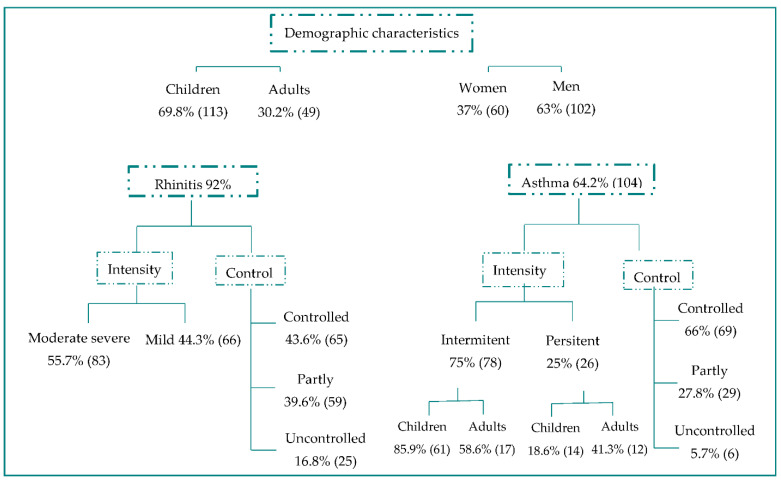
We analyzed different variables among the sensitized subjects to Alternaria alternata and Alt a 1. Figure 4 shows the characteristics of patients sensitized to Alternaria alternata and Alt a 1.
Figure 4.
Characteristics of patients sensitized to Alternaria alternata and Alt a 1.
In total, 113 (69.8%) of the children were sensitized to Alternaria alternata and Alt a 1 and 49 (30.2%) of adults. There was a significant (p = 0.0387) difference between the percentage of children sensitized to Alternaria alternata and that of those non-sensitized.
In total, 102 (63%) of patients sensitized to Alternaria alternata and Alt a 1 were men; a significant difference (p = 0.0292) (2:1 male: female) was found between those sensitized to Alternaria alternata and those who were not.
There have been no significant differences found between patients sensitized to Alternaria alternata and Alt a 1 and those who were not sensitized regarding asthma and rhinitis or its control.
Positive SPT to Alternaria alternata was associated to the patients who had specific IgE to Alternaria alternata and Alt a 1: 167 (88.8%) of the patients with positive SPT to Alternaria alternata had specific IgE to Alternaria alternata and Alt a 1, and 134 (91.2%) with positive SPT to Alt a1 had specific IgE to Alternaria alternata and Alt a 1.
4. Discussion
This study estimates the prevalence of sensitization to fungi in Spain, focusing on Alternaria alternata.
Our results showed that fungi are the fourth cause of allergic sensitization, after pollens and dust mites; in line with Alergológica [32] and D’Amato et al. [7]. We have observed that 233 (20.2%) of patients were sensitized to at least one of the five fungi tested. Among these 233 patients, 219 (94.0%) were sensitized to Alternaria alternata and/or Alt a 1, and 162 (69.5%) were sensitized to both Alternaria alternata and Alt a 1, which represents 14% of the sample accordingly with the 10–13% reported in 2015 [32].
Prevalence of sensitization to Alternaria alternata was higher in Extremadura and Catalonia followed by Andalusia and Basque Country; lower sensitization was found in Valencian Community and Galicia. Compared to the prevalence of sensitization in patients with rhinitis studied in 2015 in Spain, we have seen that the prevalence in general has increased and we perceived differences in some regions.
In five of the six regions studied, the sensitization to fungi doubled its prevalence. On the other hand, Galicia has shown a decrease from 6.5% to 1%. This decrease might be due to the population recruited. Most of patients were adults and the consecutive recruitment could have influenced.
In general, we have detected a higher prevalence of sensitization to Alternaria alternata in the inner regions of Spain, such as Lleida with a prevalence of 45% and Badajoz with 21.5%.
The good correlation between SPT and serologic test (specific IgE) for Alternaria alternata and Alt a 1 is noteworthy. Thus, Alt a 1 should be one of the allergens tested in the SPT regular allergen battery, because this protein gives us precise information about patient sensitization and helps us in prescribing the best treatment option for each patient.
Most patients sensitized to any fungi (203, 87.1%) were polysensitized to other allergen sources, mainly pollens, such as olive and grass [33]. At this point we would like to underline the perception of Alternaria alternata being a trigger allergen to the atopic march, due to its high prevalence in children and the high rate of polysensitization when they grow up, and the importance of an early treatment in order to prevent further sensitizations. Does this sensitization decrease as we get older and become sensitized to other sources?
Cross reactivity among fungi is not well studied but there are some statements about it. From a phylogenetical point of view, there are two classes of cross reactivity: species-specific and cross-reactive allergens. Among them, Asp f 1, Alt a 1, Cop c 1, and Mala s 1 represent specie-specific proteins that cannot be found in any other genera. However, except these and perhaps some other few exceptions, most of the fungal allergens represent cross-reactive structures covering different protein families [34]. In this study we saw that most of our patients were sensitized to Alternaria alternata or Alt a 1, which could explain that the most prevalent fungal genera among allergic patients in Spain is Alternaria alternata. As we see (Table 4) this sensitization to fungus relays on allergic pathology and we can consider all of them, most of all due to its prevalence Alternaria alternata, a relevant cause of sensitization and allergens to take into account when diagnosing patients.
Regarding our studied population, almost 70% of patients sensitized to Alternaria alternata were younger than 16 years old, even though there were more adults included in our studied population and 63% were male. Children sensitized to both allergens (Alternaria alternata and Alt a 1) had a worse control of allergic pathology.
Patients sensitized to Alternaria alternata have moderate to severe partly controlled allergic rhinitis according to ARIA, and episodic asthma partly controlled according to GEMA 5.0. [29] This sensitization is statically related to asthma. Alternaria alternata sensitization is supposed to be a risk factor for developing asthma.
Chew’s questionnaire [35], which is based on identifying a relation among house and environment [36,37] characteristics and presence of fungi, shows that patients’ sensitization worsens at the end of summer and autumn. They do not seem to be affected by other items such as rain, humidity, or fungi at their home.
5. Conclusions
We concluded that fungi are an important source of sensitization in Spain and its incidence is increasing; patients have mild control of their symptoms in general and they might have benefits from an early diagnosis and treatment in order to prevent them from developing asthma and other sensitizations.
Our unmet needs and proposal of new studies are to further study the relationship between sensitization and atopic march, and to relate polysensitization to environment conditions and exposures.
We have updated the Spanish fungal map relating it to regions.
Acknowledgments
Luis Alonso; José Batllés; Jose Antonio Bejarano; María Capataz; Francisco Carballada; Eduardo Fernandez; Laia Ferré; Alba Gairi; Mª Angeles Gonzalo; Silvia Lara; Celia Morales; Mª Carmen Moya; Antonio Nieto; Ana Mª Plaza; Macarena Piñero; Joaquín Quiralte; Juan José Zapata for the investigation and data recorded.
Appendix A. Mold Questionnaire Part 1
| Sensitization | Alternaria alternata /Alt a 1 | |
| Part 1 | N | % |
|
161 | 100.0 |
|
76 | 47.2 |
|
85 | 52.8 |
|
162 | 100.0 |
|
76 | 46.9 |
|
86 | 53.1 |
|
160 | 100.0 |
|
52 | 32.5 |
|
108 | 67.5 |
|
160 | 100.0 |
|
71 | 44.4 |
|
89 | 55.6 |
|
161 | 100.0 |
|
50 | 31.1 |
|
111 | 68.9 |
|
161 | 100.0 |
|
81 | 50.3 |
|
80 | 49.7 |
|
161 | 100.0 |
|
97 | 60.2 |
|
64 | 39.8 |
Appendix B. Mold Questionnaire Part 2
| Sensitization | Alternaria alternata /Alt a 1 | |
| Part 2 | N | % |
|
162 | 100.0 |
|
37 | 22.8 |
|
125 | 77.2 |
|
162 | 100.0 |
|
9 | 5.6 |
|
4 | 2.5 |
|
4 | 2.5 |
|
37 | 22.8 |
|
108 | 66.7 |
|
162 | 100.0 |
|
42 | 25.9 |
|
120 | 74.1 |
|
162 | 100.0 |
|
55 | 34.0 |
|
107 | 66.0 |
|
162 | 100.0 |
|
7 | 4.3 |
|
154 | 95.7 |
Author Contributions
Conceptualization, R.P.P. and D.R.G.; Methodology, R.P.P., D.R.G. and J.M.Q.; Project administration, R.P.P. and D.R.G.; Supervision, R.P.P. and D.R.G.; Validation, J.M.Q.; Writing—original draft, V.P.L.C., R.P.P. and D.R.G.; Writing—review & editing, V.P.L.C., R.P.P., M.T.-G. and D.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Diater laboratorios S.A.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Ethics Committee of CEIm-E (Comité de Etica de la Investigación con Medicamentos de Euskadi) (protocol code DLA-ALT-2018-01 approved on Euskadi 24 April 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Authors ensure that data shared are in accordance with consent provided by participants on the use of confidential data, following Reglamento (UE) 2016/679 del Parlamento europeo y del Consejo de 27 de abril de 2016 de Protección de Datos (RGPD) and the good clinical practice and declaration of Helsinki. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to GDPR.
Conflicts of Interest
V.P.L.C., D.R.G. and R.P.P. work for Diater Laboratorios. M.T.-G. and J.M.Q. has no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hawksworth D.L. The fungal dimension of biodiversity: Magnitude, significance and conservation. Micol. Res. 2006;95:641–645. doi: 10.1016/S0953-7562(09)80810-1. [DOI] [Google Scholar]
- 2.Twaroch T.E., Curin M., Valenta R., Swoboda I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015;7:205–220. doi: 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mari A., Scheneider P., Wally V., Breitenbach M., Simon-Nobbe B. Sensitization to fungi: Epidemiology, comparative skin test and IgE reactivity of fungal extracts. Clin. Exp. Allergy. 2003;33:1429–1438. doi: 10.1046/j.1365-2222.2003.01783.x. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet P.J., Hooper R., Kogevinas M., Jarvis D., Burney P. Number of allergens to be tested to asses allergenic sensitization in epidemiologicstudies: Results of the European Community Respiratory Health Survey I. Clin. Exp. Allergy. 2007;37:780–787. doi: 10.1111/j.1365-2222.2007.02714.x. [DOI] [PubMed] [Google Scholar]
- 5.Heinzeding L.I., Burbaeh G.J., Edenharter G. GA2LEN skin test study: GA2LEN harmonization of skin prick testing: Novel sensitization patterns for inhalant allergens in Europe. Allergy. 2009;64:1498–1506. doi: 10.1111/j.1398-9995.2009.02093.x. [DOI] [PubMed] [Google Scholar]
- 6.Arbes S.J., Jr., Gergen P.J., Elliott L., Zeldin D.C. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2005;116:377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 7.D’amato G., Chatzigeorgiou G., Corsico R., Gioulekas D., Jäger L., Jäger S., Kontou-Fili K., Kouridakis S., Liccardi G., Meriggi A., et al. Evaluation of the prevalence of skin prick test positivity to Alternaria and Cladosporium in patients with suspected respiratory allergy. A European multicenter study promoted by the Subcommittee on Aerobiology and Environmental aspects of inhalant allergens of the European Academy of Allergology and Clinical Immunology. Allergy. 1997;52:711–716. doi: 10.1111/j.1398-9995.1997.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 8.Deards M.J., Montagne A.E. Purification and characterization of a major allergen of Alternaria alternata. Mol. Immunol. 1991;28:408–415. doi: 10.1016/0161-5890(91)90154-C. [DOI] [PubMed] [Google Scholar]
- 9.Chruszcz M., Chapman M.D., Osinski T., Solberg R., Demas M., Porebski P., Majorek K.A., Pomés A., Minor W. Alternaria alternata allergen Alt a 1: A unique β-barrel protein dimer found exclusively in fungi. J. Allergy Clin. Immunol. 2012;130:241–247. doi: 10.1016/j.jaci.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twaroch T.E., Arcalís E., Sterflinger K., Stöger E., Swoboda I., Valenta R. Predominant localization of the major Alternaria alternata allergen Alt a 1 in the cell wall of airborne spores. J. Allergy Clin. Immunol. 2012;129:1148–1149. doi: 10.1016/j.jaci.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez-Rodríguez A., Postigo I., Guisantes J.A., Suñén E., Martínez J. Identification of allergen homologous to Alt a 1 from Stemphylium botryosum and Ulocladium botrytis. Med. Mycol. 2011;49:892–896. doi: 10.3109/13693786.2011.576350. [DOI] [PubMed] [Google Scholar]
- 12.Moreno A., Pineda F., Alcover J., Rodríguez D., Palacios R., Martínez-Naves E. Orthologous allergens and diagnostic utility of major allergen Alt a 1. Allergy Asthma Immunol. Res. 2016;8:428–435. doi: 10.4168/aair.2016.8.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon-Nobbe B., Probst G., Kajava A.V., Oberkofler H., Susani M., Crameri R., Ferreira F., Ebner C., Breitenbach M. IgE-binding epitopes of enolases, a class of highly conserved fungal allergens. J. Allergy Clin. Immunol. 2000;106:887–895. doi: 10.1067/mai.2000.110799. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel M.F., Postigo I., Gutierrez-Rodriguez A., Sunen E., Guisantes J., Tomaz C.T., Martinez J. Characterization of Alternaria alternata manganese-dependent superoxide dismutase, a cross-reactive allergen homologue to Asp f 6. Immunology. 2015;220:851–858. doi: 10.1016/j.imbio.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel M.F., Postigo I., Tomaz C.T., Martínez J. Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016;89–90:71–80. doi: 10.1016/j.envint.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.-K., Lund S., Baum R., Rosenthal P., Khorram N., Doherty T.A. Innate Type 2 Response to Alternaria Extract Enhances Ryegrass-Induced Lung Inflammation. Int. Arch. Allergy Immunol. 2014;163:92–105. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantani A., Clachi V. Epidemiology of Alternaria alternata allergy: A prospective study in 6840 Italian asthmatic children. Eur. Rev. Med. Pharmacol. Sci. 2004;8:289–294. [PubMed] [Google Scholar]
- 18.Burbach G.J., Heinzerling L.M., Edenharter G., Bachert C., Bindslev-Jensen C., Bonini S., Bousquet J., Bousquet-Rouanet L., Bousquet P.J., Bresciani M., et al. GA2LEN skin test study II: Clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64:1507–1515. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 19.Zureik M., Neukirch C., Leynaert B., Liard R., Bousquet J., Neukirch F. European Community Respuratory Health Survey. Sensitization to airborne molds and severity of asthma: Cross sectional study from European Community Respiratory Health Survey. BMJ. 2002;125:411–414. doi: 10.1136/bmj.325.7361.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black P.N., Udy A.A., Brodie S.M. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–504. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 21.Pose G., Patriarca A., Kyanko V., Pardo A., Pinto V.F. Effect of water activity and temperature on growth of Alternaria alternata on a syntetic tomato medium. Int. J. Food Microbiol. 2009;135:60–63. doi: 10.1016/j.ijfoodmicro.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Pose G., Patriarca A., Kyanko V., Pardo A., Pinto V.F. Water activity and temperature effects on mycotoxin production by Alternaria alternata on a syntetic tomato médium. Int. J. Food Microbiol. 2010;142:348–353. doi: 10.1016/j.ijfoodmicro.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Weber R.W. Alternaria alternata. Ann. Allergy Asthma Immunol. 2001;87:A-4. doi: 10.1016/s1081-1206(10)62913-4. [DOI] [PubMed] [Google Scholar]
- 24.Bush R.K., Prochman J.J. Case study: Alternaria induced asthma. J. Allergy Clin. Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi M., Tonori H., Miki T., Miyajima E., Kudo Y., Tsunoda M., Sakabe K., Aizawa Y. Classification of patients complaining of skin house syndrome and/or multiple chemical sensitivity. Tohoku J. Exp. Med. 2007;211:223–233. doi: 10.1620/tjem.211.223. [DOI] [PubMed] [Google Scholar]
- 26.Rapiejko P., Lipiec A., Wojdas A., Jurkiewicz D. Treshold pollen concentration necessary to evoke allergic symptoms. Int. Rev. Allergol. Clin. 2004;10:91–94. [Google Scholar]
- 27.Javier Martín Vide y Jorge Olcina . Climas y Tiempos de España. Alianza Editorial; Madrid, Spain: 2001. p. 258. [Google Scholar]
- 28.Brożek J.L., Bousquet J., Agache I., Agarwal A., Bachert C., Bosnic-Anticevich S., Brignardello-Petersen R., Canonica G.W., Casale T., Chavannes N.H., et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J. Allergy Clin. Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 29.GEMA 5.0 Guía Española Para el Manejo del Asma. [(accessed on 2 August 2021)]; Available online: www.gemasma.com.
- 30.Vega J.M., Badia X., Badiola C., López-Viña A., Olaguíbel J.M., Picado C., Sastre J., Dal-Ré R. Validation of the Spanish version of the asthma control test (ACT) J. Asthma. 2007;44:867–872. doi: 10.1080/02770900701752615. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Yarza E.G., Castro-Rodriguez J.A., Villa Asensi J.R., Garde Garde J., Hidalgo Bermejo F.J., on behalf of the VESCASI Group Validación de la versión en español de la prueba de control del asma infantil (ACT) para su uso en España [Validation of a Spanish version of the Childhood Asthma Control Test (Sc-ACT) for use in Spain] An. Pediatr. 2015;83:94–103. doi: 10.1016/j.anpedi.2014.10.031. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 32.Spanish Society of Allergology and Clinical Immunology Allergológica 2015. In Spanish Society of Allergology and Clinical Immunology. [(accessed on 2 August 2021)]; Available online: https://www.seaic.org/inicio/noticias-general/alergologica-2015.html.
- 33.Warm K., Hedman L., Lindberg A., Lötvall J., Lundbäck B., Rönmark E. Allergic sensitization isa ge-dependently associated with rhinitis, but les so with asthma. J. Allergy Clin. Immunol. 2015;136:1559–1565. doi: 10.1016/j.jaci.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Crameri R., Zeller S., Glaser A.G., Vilhelmsson M., Rhyner C. Cross-reactivity among fungal allergens: A clinically relevant phenomenon? Mycoses. 2009;52:99–106. doi: 10.1111/j.1439-0507.2008.01644.x. [DOI] [PubMed] [Google Scholar]
- 35.Chew G.L., Horner W.E., Kennedy K., Grimes C., Barnes C.S., Phipatanakul W., Larenas-Linnemann D., Miller J.D., Portnoy J., Levetin E., et al. Environmental Allergens Workgroup. Procedures to Assist Health Care Providers to Determine When Home Assessments for Potential Molds Exposure Are Warranted. J. Allergy Clin. Immunol. Pract. 2016;4:417–422.e2. doi: 10.1016/j.jaip.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karvonen A.M., Hyvarinen A., Korppi M., Haverinen-Shaughnessy U., Renz H., Pfefferle P.I., Remes S., Genuneit J., Pekkanen J. Moisture damage and asthma: A birth cohort study. Pediatrics. 2015;135:e598–e606. doi: 10.1542/peds.2014-1239. [DOI] [PubMed] [Google Scholar]
- 37.Larenas-Linnemann D., Baxi S., Phipatanakul W., Portnoy J.M., Barnes C., Grimes C., Horner W.E., Kennedy K., Levetin E., Miller J.D., et al. Environmental Allergens Workgroup. Clinical Evaluation and Management of Patients with Suspected Fungus Sensitivity. J. Allergy Clin. Immunol. Pract. 2016;4:405–414. doi: 10.1016/j.jaip.2015.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Authors ensure that data shared are in accordance with consent provided by participants on the use of confidential data, following Reglamento (UE) 2016/679 del Parlamento europeo y del Consejo de 27 de abril de 2016 de Protección de Datos (RGPD) and the good clinical practice and declaration of Helsinki. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to GDPR.