Abstract
This study investigated peptide fractions from fish skin collagen for antibacterial activity against Escherichia coli and Salmonella strains. The collagen was hydrolyzed with six commercial proteases, including trypsin, Alcalase, Neutrase, Flavourzyme, pepsin and papain. Hydrolyzed samples obtained with trypsin and Alcalase had the largest number of small peptides (molecular weight <10 kDa), while the hydrolysate produced with papain showed the lowest degree of hydrolysis and highest number of large peptides. Four hydrolysates were found to inhibit the growth of the Gram-negative bacteria, with papain hydrolysate showing the best activity against E. coli, and Neutrase and papain hydrolysates showing the best activity against S. abony; hydrolysates produced with trypsin and pepsin did not show detectable antibacterial activity. After acetone fractionation of the latter hydrolysates, the peptide fractions demonstrated enhanced dose-dependent inhibition of the growth (colony-forming units) of four Salmonella strains, including S. abony (NCTC 6017), S. typhimurium (ATCC 13311), S. typhimurium (ATCC 14028) and S. chol (ATCC 10708). Shotgun peptidomics analysis of the acetone fractions of Neutrase and papain hydrolysates resulted in the identification of 71 and 103 peptides, respectively, with chain lengths of 6–22 and 6–24, respectively. This work provided an array of peptide sequences from fish skin collagen for pharmacophore identification, structure–activity relationship studies, and further investigation as food-based antibacterial agents against pathogenic microorganisms.
Keywords: sturgeon fish (Huso huso), collagen, bioactive peptides, antimicrobial peptides, peptidomics, Salmonella
1. Introduction
The increasing prevalence of bacterial resistance to commercial antibiotics leading to failed treatments and increased expenses has become a public health concern [1]. Moreover, the increasing constraint on the use of chemical preservatives and the growing demand for natural antimicrobial agents in foods or food supplements has spurred emerging research towards the discovery of novel natural antimicrobial agents with a broad spectrum of antibiotic activity [2]. Consequently, the development of sustainable alternatives to synthetic antibiotics has become a research priority. Antimicrobial peptides (AMPs) are essential components of the innate immune response and a diverse group of individually unique small peptides (less than 50 amino acid residues) that can participate in different organisms as immune modulators and exhibit broad-spectrum antimicrobial activity towards pathogens (Gram-positive and Gram-negative bacteria, viruses, parasites, protozoa, yeast, and fungi) [3]. Natural AMPs can be isolated from practically every living organism, from prokaryotes to humans. The food source of proteins or peptides with antibacterial activities against Gram-positive and Gram-negative strains is diverse, including whole milk [4], lactoferrin [5], ovotransferrin [6], casein [7] and β-lactoglobulin [8]. Moreover, proteins from fish sources have shown promise as functional food ingredients as they are a valuable source of bioactive peptides. These peptides are encrypted within the primary protein sequence and can be released upon enzymatic hydrolysis [9]. Bioactive peptides isolated from various fish protein hydrolysates have short chain lengths, with only 3–20 amino acid residues, and have shown many bioactivities, such as antithrombotic, immunomodulatory, antihypertensive, anticoagulant, antioxidative, and antimicrobial properties [10,11].
Several studies have reported that treatment of marine organisms by enzymatic hydrolysis yielded AMPs and hydrolysates with activity against pathogenic bacteria. For instance, antibacterial peptide fractions isolated from Atlantic mackerel (Scomber scombrus) by-products were reported to inhibit the growth of Gram-positive and Gram-negative bacteria [12]. Sila et al. [13,14] also demonstrated potent inhibitory activity against pathogenic bacteria using antibacterial peptides from barbel muscle protein hydrolysates produced with Alcalase. In addition, antibacterial peptides and fractions have been isolated from pepsin hydrolysate of half-fin anchovy [2] and rainbow trout by-products [15], and from several crab species, including Atlantic rock crab Cancer irroratus [16], snow crab Chionoecetes opilio [17], blue crab Callinectes sapidus [18,19], Chinese mitten crab Eriocheir sinensis [20], mud crab Scylla paramamosain [21], shore crab Carcinus meanas [22], and green crab Carcinus meanas [23]. The structural diversity of the marine-derived proteins provides a unique platform for the discovery of a wide range of AMPs for combating several pathogenic bacteria and overcoming antimicrobial resistance.
Fish collagens and their hydrolysates have been used as sources of biologically active peptides with beneficial effects and potential application in the nutraceutical, cosmeceutical, biomedical, and pharmaceutical industries [24]. According to Ennaas et al. [25], collagencin, an antibacterial peptide isolated from fish collagen hydrolysate produced with Protamex, potently inhibited the growth of Gram-positive and Gram-negative bacteria. Considering the need for fish by-product upcycling and the structural diversity of collagen-derived peptides, the objectives of this study were to investigate the antibacterial activities of sturgeon fish skin collagen hydrolysates produced with six different commercial enzymes and to fractionate and identify the collagen peptides with anti-Salmonella activity from the most active hydrolysates.
2. Materials and Methods
2.1. Chemicals
A high-molecular-weight marker was obtained from Bio-Rad Laboratories (Hercules, CA, USA) and N,N,N,N-tetramethyl ethylene diamine (TEMED) was purchased from Sigma Aldrich (St. Louis, MO, USA). Trypsin (from bovine pancreas; ≥7500 U/g) and pepsin (from porcine gastric mucosa; ≥500 U/g) were purchased from Sigma Chemical Co. (St, Louis, MO, USA). Alcalase (protease from Bacillus licheniformis; ≥2.4 U/g), Neutrase (protease from Bacillus amyloliquefaciens; ≥0.8 U/g), papain (from papaya latex), and Flavourzyme (protease from Aspergillus oryzae; ≥500 U/g) were purchased from Sigma Chemical Co. (Bagsveard, Denmark). O-Phthaldialdehyde, 8-anilo-1-naphthalenesulfonic acid (ANS), Coomassie Brilliant Blue R-250, sodium dodecyl sulfate (SDS), and β-mercaptoethanol (βME) were purchased from Merck (Darmstadt, Germany). All other chemicals and reagents used were of analytical grade.
2.2. Enzymatic Hydrolysis of Fish Skin Collagen
Collagen was isolated from sturgeon fish skin as previously reported [26]. Lyophilized pepsin-solubilized collagen (1 g) was suspended in 200 mL of deionized water and hydrolyzed separately with six commercial enzymes at their optimal conditions (Table 1) with continuous stirring. During enzymatic hydrolysis, the pH of the mixtures was adjusted by adding 1 M NaOH or 1 M HCl. After 3 h of hydrolysis, the enzymatic reaction was terminated by changing pH as shown in Table 1 to inactivate each of the enzymes. The reaction mixtures were then centrifuged at 10,000× g for 30 min at 4 °C and the supernatants lyophilized using a freeze-drier to yield the collagen hydrolysates and stored at −20 °C until use.
Table 1.
Hydrolysis conditions and degree of hydrolysis of Sturgeon fish skin collagen with various commercial proteases.
| Enzyme | Optimum Conditions | DH (%) |
||||
|---|---|---|---|---|---|---|
| E/S | Time (h) |
Temp. (°C) |
pH | pH (Inactivation) |
||
| Trypsin | 1:100 | 3 | 37 | 8 | 3 | 40.35 ± 0.07 a |
| Alcalase | 1:100 | 3 | 50 | 8 | 4 | 24.37 ± 0.01 b |
| Neutrase | 1:100 | 3 | 50 | 8 | 4 | 19.63 ± 0.01 c |
| Flavourzyme | 1:100 | 3 | 50 | 7 | 4 | 14.43 ± 0.03 d |
| Pepsin | 1:100 | 3 | 37 | 2 | 6.5–8 | 12.35 ± 0.02 d |
| Papain | 1:100 | 3 | 37 | 6.5 | 3 | 7.38 ± 0.01 e |
E/S, enzyme–substrate ratio; DH, degree of hydrolysis. Mean values in the DH column with different letters are significantly different with p < 0.05.
2.3. Characterization of the Protein Hydrolysates
2.3.1. Degree of Hydrolysis (DH)
DH of the collagen hydrolysates was determined in a 96-well plate using the O-phthaldialdehyde (OPA) method described by Nielsen et al. [27], with some modifications. Briefly, 30 µL sample solution, standard (serine) or blank control (deionized water) was mixed with 225 µL OPA reagent, and after 2 min shaking at room temperature, absorbance the mixtures were measured at 340 nm using a microplate reader. Thereafter, DH was calculated as previously reported [28].
2.3.2. SDS Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The molecular weight profile of the collagen and collagen hydrolysates was determined by SDS-PAGE according to the method of Laemmli [29], with a slight modification, using gradient resolving gel (6%, 9%, 12%, 15%, and 18%) with 3% (w/v) stacking gel. To prepare the samples, collagen and collagen hydrolysate powders (1 mg/mL) were dissolved in 0.02 M sodium phosphate buffer (pH 7.2) containing 1% (w/v) SDS and 3.5 M urea, and the solutions were gently stirred at 4 °C for 12 h. The mixtures were centrifuged at 5000× g for 5 min to remove undissolved debris. Thereafter, the samples were mixed with the sample buffer (2% SDS, 20% glycerol, and 0.5% bromophenol blue in 62.5 mM Tris-HCl buffer, pH 6.8, containing 1 M β-mercaptoethanol), followed by heat denaturation at boiling temperature for 5 min. Electrophoresis was conducted at a constant current at 120 V for 2 h, and the gel was stained overnight with Coomassie Brilliant Blue R-250 solution and distained with Milli-Q water by shaking for 4 h. A standard molecular weight protein marker ranging from 10 to 250 kDa was used to estimate the molecular weight of the collagen peptides. Image scanning of gels was done using the ChemiDoc Imaging System (BioRad Inc., Mississauga, ON, Canada).
2.3.3. Surface Hydrophobicity (Ho)
Surface hydrophobicity (Ho) of the collagen hydrolysates was determined by the fluorescence method using ANS as the hydrophobic probe. Samples at concentrations ranging from 0.005 to 0.025% were mixed with 20 µL ANS in a Grenier UV-Star (96-well) microplate. Fluorescence of the mixture was then measured at excitation and emission wavelengths of 390 and 470 nm, respectively, using a Spark multimode microplate reader (Tecan, Switzerland). The slope of the fluorescence intensity vs. concentration plot was used to represent the surface hydrophobicity (Ho), as previously reported [30].
2.3.4. Dynamic Light Scattering (DLS) Analysis
Zeta (ζ)-potential and mean particle size of the collagen hydrolysates were evaluated by DLS using the Zetasizer Nano Series Nano-ZS (Malvern Instruments Ltd., Malvern, UK) at 25 °C. The hydrolyzed samples (0.05 mg/mL) were mixed in water and used to determine the zeta potential and particle size of the samples. All measurements were taken in triplicates at 25 °C with the Smoluchowski model at F (ka) 1.50 and backscattered angle of 173°.
2.4. Solvent Fractionation of the Hydrolysates
Selected collagen hydrolysates were further subjected to solvent fractionation to separate the bioactive peptides as previously reported [12]. High-molecular-weight peptides in the samples were precipitated using ice-cold acetone (50%, v/v) followed by centrifugation at 8000× g for 20 min at 4 °C. Thereafter, acetone in the supernatants containing low-molecular-weight peptides was evaporated using a centrifugal vacuum evaporator, and the samples were freeze-dried to obtain the peptide fraction powders.
2.5. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) and Peptidomics Data Analysis
Shotgun proteomics was used to identify peptides in the collagen peptide fractions. Lyophilized samples were separated by a 60-min gradient elution at a flow rate of 250 nL/min using an EASY-nLC integrated nano-HPLC system (Thermo Fisher, San Jose, CA, USA), which was directly interfaced with a quadrupole Orbitrap (Q-Exactive) mass spectrometer (Thermo Fisher, San Jose, CA, USA). The analytical column used was a PepMap RSLC EASY-Spray column (75 μm × 50 cm) packed with C18 resin (2 μm). Eluted peptides were then introduced into the Orbitrap Q-Exactive mass spectrometer, operated in the data-dependent acquisition mode using the MaxQuant software with a single full-scan spectrum (400–1500 m/z, 70,000 resolution) followed by 10 data-dependent MS/MS scans in the Orbitrap mass analyzer. For peptide identification, the spectral data were processed with MaxQuant version 1.6.10.43 software (Max Planck Institute of Biochemistry, Planegg, Germany) using the Andromeda peptide search engine, as reported by Tyanova et al. [31].
2.6. Antibacterial Activity of Collagen Peptides
Antibacterial activity of the collagen hydrolysates and the acetone fractions was performed using a microtest polystyrene 96-well microplate (VWR Tissue Culture Plates, Randor, PA, USA) as previously described [32]. Briefly, the microplate wells were filled by distributing 100 µL of BHI medium. Medium alone and medium with each strain inoculum were used as blank and control, respectively, on the same microplate. Then, 100 µL of each collagen hydrolysates and acetone fractions prepared in the medium were added to each well (Column 3 to Column 12) and 2-fold diluted with the medium starting from 100 mg/mL for collagen peptides and from 10 mg/mL for acetone fractions. The wells, containing 100 µL of media or sample solution, were inoculated with 100 µL overnight culture of the target strains diluted to a final concentration of 105 CFU/mL. The microplates were subsequently incubated at 37 °C, and absorbance at 650 nm was measured at 20 min intervals for 24 h, using the Spark multimode microplate. Viable bacterial strains were quantified after 6 h incubation with the acetone fractions or BHI medium as control using the standard plate counting method on BHI agar and expressed as CFU/mL.
2.7. Statistical Analysis
Data were collected in triplicate and analyzed using SPSS (Chicago, IL, USA). Results were expressed as mean ± standard deviation, and the differences between mean values of different samples were determined by the Least Significant Difference (LSD) test (p < 0.05).
3. Results
3.1. Degree of Hydrolysis and Surface Hydrophobicity of the Hydrolysates
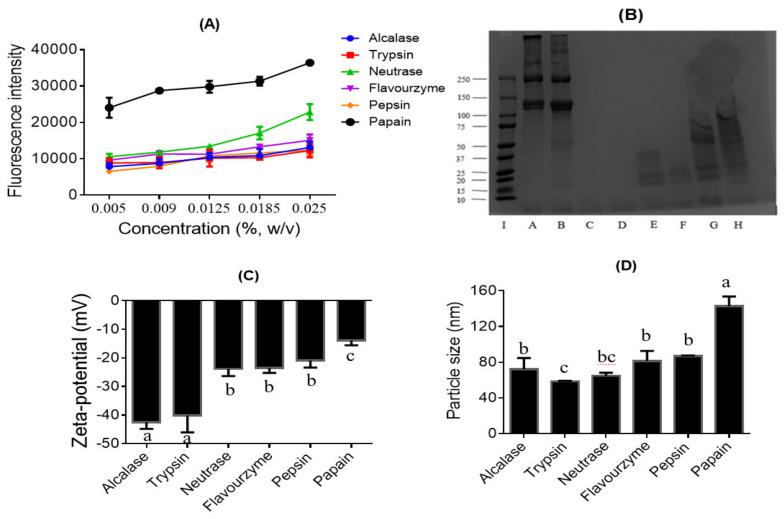
DH of the collagen hydrolysates produced with different enzymes (trypsin, Alcalase, Neutrase, Flavourzyme, pepsin, and papain) at their optimal temperature and pH conditions are shown in Table 1. Enzymes were used at the same enzyme–substrate ratio to compare their hydrolytic efficiencies. As shown in Table 1, trypsin and papain hydrolysate demonstrated the highest and lowest DH (p < 0.05), respectively, in producing the collagen hydrolysates. Interestingly, there was no significant difference between the DH of hydrolysates obtained with the single enzyme, pepsin and the multi-enzyme, Flavourzme. In contrast, the highest surface hydrophobicity was observed for collagen hydrolysate produced with papain, whereas hydrolysates obtained with Alcalase and trypsin had similar and the lowest H0 values (Figure 1A). There was no defined pattern in the DH or H0 with respect to the origin of the enzymes (plant, animal or microbe) used in collagen hydrolysis.
Figure 1.
Properties of the collagen protein hydrolysates. (A) Surface hydrophobicity (H0), (B) SDS-PAGE protein profile, (C) zeta potential, and (D) mean particle size of the collagen hydrolysates produced by Alcalase, trypsin, Neutrase, Flavourzyme, pepsin and papain. Mean values with different letters in (C) and (D) are significantly different with p < 0.05. SDS-PAGE label: Lanes I: High molecular-weight protein marker, A = Acid-solubilized collagen (ASC), B = Pepsin-solubilized collagen (PSC), and collagen hydrolysates obtained using C = trypsin, D = Alcalase, E = Flavourzyme, F = Neutrase, G = pepsin, and H = papain.
3.2. Molecular Weight Distribution of the Collagen Hydrolysates
The molecular weight profiles of the fish skin collagen and its hydrolysates, determined by SDS-PAGE, are shown in Figure 1B. After treating collagen with the six commercial enzymes, the samples obtained with trypsin (lane C) and Alcalase (lane D) displayed peptide bands with the lowest molecular weights (<10 kDa). In contrast, hydrolysates obtained with the pepsin (lane G) and papain (lane H) showed bands with high molecular weights ranging from ~100 to <10 kDa. The molecular weight profile follows a similar pattern as the DH (Table 1); hydrolysates with higher DH showed lower molecular weight bands on the SDS-PAGE gels, indicating extensive protein hydrolysis by the enzymes, and vice versa. Furthermore, SDS-PAGE showed the disappearance of the two major bands present in the original collagen samples (lanes A and B) in all the hydrolysates (lanes C-H), indicating that all six enzymes hydrolyzed the parent protein, although to different extents.
3.3. Surface Charge and Particle Size of the Collagen Hydrolysates
As shown in Figure 1C,D, the collagen hydrolysate particles had a net negative surface charge when dispersed in water. The magnitude of zeta potential for the hydrolysates produced with Alcalase and trypsin was significantly higher than the rest, and papain had the lowest surface charge. Conversely, similar particle sizes were observed for the hydrolysates produced with Alcalase, Flavourzyme, and pepsin, while that produce with papain had the highest particle size, and those produced with trypsin and Neutrase showed the lowest values. The particle size results are inversely correlated to the DH (Table 1) and surface charge results (Figure 2C).
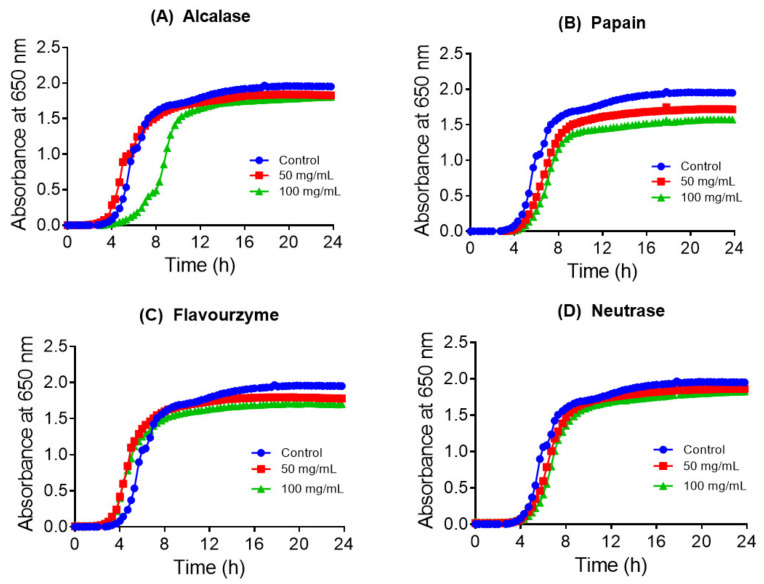
Figure 2.
Growth curves of E. coli in the absence (control) and presence of collagen hydrolysates produced with four enzymes (A) Alcalase, (B) papain, (C) Flavourzyme, and (D) Neutrase. Experiments were conducted three times, and each data point represents the mean value.
3.4. Antibacterial Activity of the Collagen Hydrolysates and Peptide Fractions
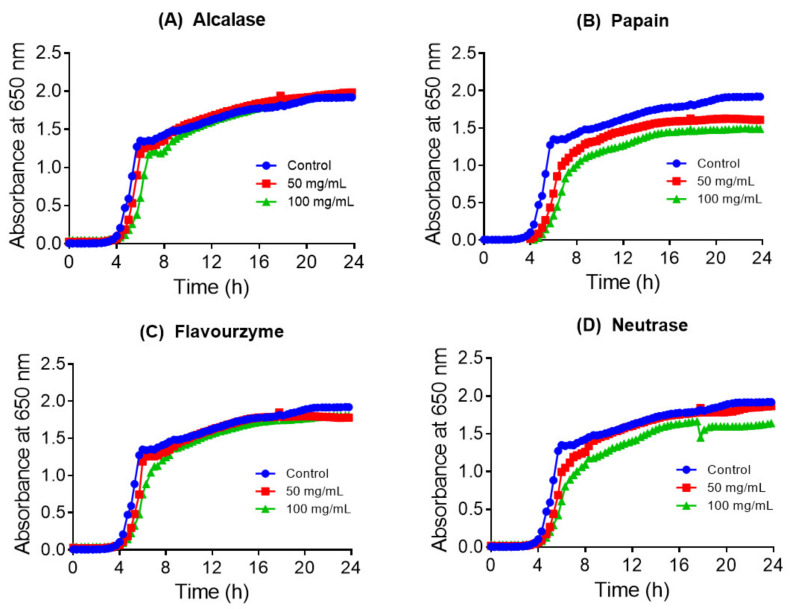
The collagen hydrolysates and their acetone fractions were evaluated for inhibitory activity against E. coli and four Salmonella strains using microdilution assay. As shown in Figure 2 and Figure 3, some collagen hydrolysate at a concentration of 100 mg/mL moderately inhibited the growth of E. coli and S. abony strains, with crude hydrolysates obtained with papain, Alcalase, and Neutrase being the most potent. Specifically, hydrolysate produced with papain inhibited the growth of both strains, while Neutrase and Alcalase produced hydrolysates with selective antibacterial activity against E. coli and S. abony, respectively. To a lesser extent, collagen hydrolysate obtained with Flavourzyme exhibited a weak growth inhibitory effect against S. abony. Collagen hydrolysates produced with pepsin and trypsin did not show detectable antibacterial activity in both strains under our experimental conditions.
Figure 3.
Growth curves of S. abony in the absence (control) and presence of collagen hydrolysates produced with four enzymes (A) Alcalase, (B) papain, (C) Flavourzyme, and (D) Neutrase. Experiments were conducted three times, and each data point represents the mean value.
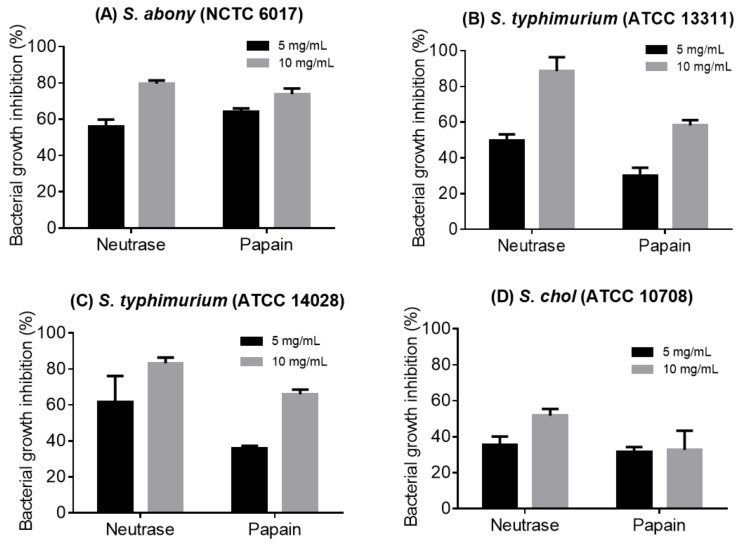
Fractionation using acetone increased the specific activity of inhibitory substances in most samples. After 6-h incubation, the acetone fractions of hydrolysates produced with Neutrase and papain exhibited the highest and dose-dependent anti-Salmonella growth inhibitory activities (Figure 4). In contrast, fractions from hydrolysates produced with Alcalase and Flavourzyme showed less potency against the four Salmonella strains (data not shown). Neutrase produced the hydrolysate with the most potent activity against the four Salmonella strains, with the highest growth inhibition of 90% observed at 10 mg/mL against S. typhimurium ATCC 13311.
Figure 4.
Growth inhibition of Salmonella strains in the presence of collagen acetone fractions (A): S. abony (NCTC 6017); (B): S. typhimurium (ATCC 13311); (C): S. typhimurium (ATCC 14028) and (D): S. chol (ATCC 10708).
The anti-Salmonella activity results were confirmed using the viable bacterial cell counts (CFU) in the presence of acetone peptide fractions of collagen hydrolysates produced with Neutrase and papain (10 mg/mL) after 6 h incubation (Table 2). When treated with Neutrase peptide fraction at 10 mg/mL, the CFU counts of the Salmonella strains were significantly (p < 0.05) reduced by more than two logs, with S. typhimurium (ATCC 13311) being the most sensitive strain (2.28 log reduction). The Salmonella strains were less, but significantly, affected by the papain peptide fractions with a log reduction range of 0.32–1.26.
Table 2.
Cell viable counts (log CFU/mL) of Salmonella strains in the presence of collagen acetone peptide fractions derived from two enzymatic hydrolysates.
| Strains | Control | Neutrase | Papain |
|---|---|---|---|
| S. abony (NCTC 6017) | 9.30 ± 0.22 a | 7.83 ± 0.12 b | 8.67 ± 0.10 a |
| S. chol (ATCC 10708) | 9.10 ± 0.17 a | 8.54 ± 0.10 a | 8.78 ± 0.21 a |
| S. typhimurium (ATCC 13311) | 9.15 ± 0.11 a | 6.87 ± 0.21 b | 7.89 ± 0.12 b |
| S. typhimurium (ATCC 14028) | 9.21 ± 0.12 a | 7.56 ± 0.12 b | 7.81 ± 0.12 b |
Control, bacteria in BHI medium without the peptide treatment; Neutrase and Papain, bacterial treated with 10 mg/mL of acetone fraction of collagen hydrolysates produced with Neutrase and papain, respectively. Different letters in each row represent significantly different mean values (p < 0.05).
3.5. Prole of Peptides in the Acetone Fractions
Peptide profiles of the collagen hydrolysate fractions determined by shogun peptidomics are shown in Table 3 and Table 4 for samples produced with Neutrase and papain, respectively. A total of 71 and 103 peptides were identified in the fractions of collagen hydrolysates produced using Neutrase and papain, respectively, which displayed the most potent antibacterial activities against the Gram-negative bacteria. The length of identified peptides ranged from 6 to 22 and 6 to 24 amino acid residues, and the molecular mass ranged from 612.3 to 2266 Da and 540.2 to 2586 Da for the Neutrase and papain fractions, respectively. The vast majority of peptides in both samples were derived from Type 1 collagen alpha 1 chain, the main collagen protein component. In general, lower molecular weight peptides with 2–5 amino acid residues are less accurately identified by the shotgun peptidomics approach and, if present in the fractions, were not detected in this study.
Table 3.
Collagen peptides identified in the acetone fraction of the hydrolysate produced with Neutrase.
| No | Sequence | Chain Length | Mass (Da) | Net Charge | Fragment Position | Protein Name |
|---|---|---|---|---|---|---|
| 1 | AAGDAGKPGERG | 12 | 1084.5261 | −2;3 | 581–592 | Type1 collagen alpha1 chain |
| 2 | AAGPPGATGFPG | 12 | 998.48214 | −2 | 860–871 | Type1 collagen alpha1 chain |
| 3 | AAGPPGATGFPGAAGR | 16 | 1353.6789 | −2 | 860–875 | Type1 collagen alpha1 chain |
| 4 | AEIKAQY | 7 | 821.42832 | −2 | 274–280 | |
| 5 | AGEELWSLLAD | 11 | 1202.5819 | +2 | 562–572 | Afamin |
| 6 | AGPPGADGQAGAK | 13 | 1095.5309 | −2 | 807–819 | Type1 collagen alpha1 chain |
| 7 | ASGPAGPRGPA | 11 | 936.47773 | −2 | 1127–1137 | Type1 collagen alpha1 chain |
| 8 | ASGPAGPRGPAGPA | 14 | 1161.5891 | −2 | 1127–1140 | Type1 collagen alpha1 chain |
| 9 | ATGPAGARGSPGSPGND | 17 | 1467.6702 | −2 | 683–699 | Type1 collagen alpha1 chain |
| 10 | CHRWVSL | 7 | 956.46506 | +2 | 428–434 | |
| 11 | DAFLGSFLYEY | 11 | 1323.6023 | +2 | 347–357 | Serum albumin |
| 12 | DFGFVAQ | 7 | 782.3599 | −1 | 1190–1196 | Type1 collagen alpha1 chain |
| 13 | DGAKGDSGPAGPK | 13 | 1155.552 | −3 | 267–279 | Type1 collagen alpha1 chain |
| 14 | DGAKGDSGPAGPKGEPGSSGE | 21 | 1855.8184 | −2;3 | 267–287 | Type1 collagen alpha1 chain |
| 15 | DQLEGALQQ | 9 | 1000.4825 | +2 | 399–407 | Keratin, type II cytoskeletal 72 |
| 16 | DVNRDDACDLLV | 12 | 1403.635 | +2 | 256–267 | Inter-alpha-trypsin inhibitor heavy… |
| 17 | ENEVALRQSVE | 11 | 1272.631 | −2 | 239–249 | Keratin, type I cytoskeletal 10 |
| 18 | FSGLDGAKGDSGPAGPK | 17 | 1559.758 | +3 | 263–279 | Type1 collagen alpha1 chain |
| 19 | FSGLPGPTGEPGKQGPGGPSGE | 22 | 2008.949 | −3 | 965–986 | Type1 collagen alpha1 chain |
| 20 | FYAPELLYYANK | 12 | 1490.7446 | +2 | 172–183 | Serum albumin |
| 21 | GAAGDAGKPGE | 11 | 928.42502 | +2 | 580–590 | Type1 collagen alpha1 chain |
| 22 | GAAGDAGKPGERG | 13 | 1141.5476 | +2;3 | 580–592 | Type1 collagen alpha1 chain |
| 23 | GERGFPGERGGPG | 13 | 1271.6007 | +3 | 670–682 | Type1 collagen alpha1 chain |
| 24 | GFPGERGGPGA | 11 | 1000.4726 | −2 | 673–683 | Type1 collagen alpha1 chain |
| 25 | GGDGAPGKDGIRG | 13 | 1155.5632 | −3 | 745–757 | Type1 collagen alpha1 chain |
| 26 | GGDGAPGKDGIRGM | 14 | 1286.6037 | +3 | 745–758 | Type1 collagen alpha1 chain |
| 27 | GGPGAKGEVGPAGGRGSDGPQGARG | 25 | 2148.042 | −3 | 340–364 | Type1 collagen alpha1 chain |
| 28 | GGPGATGPAGAR | 12 | 967.48354 | +2 | 679–690 | Type1 collagen alpha1 chain |
| 29 | GKNGDRGESGPAGPA | 15 | 1368.6382 | −2 | 1054–1068 | Type1 collagen alpha1 chain |
| 30 | GKNGDRGESGPAGPAGPA | 18 | 1593.7495 | −2 | 1054–1071 | Type1 collagen alpha1 chain |
| 31 | GKNGDRGESGPAGPAGPAGPA | 21 | 1818.8609 | −2 | 1054–1074 | Type1 collagen alpha1 chain |
| 32 | GKNGDRGESGPAGPAGPAGPAGA | 23 | 1946.9195 | −2 | 1054–1076 | Type1 collagen alpha1 chain |
| 33 | GKNGDRGESGPAGPAGPAGPAGAR | 24 | 2103.0206 | −3 | 1054–1077 | Type1 collagen alpha1 chain |
| 34 | GKNGDRGESGPAGPAGPAGPAGARG | 25 | 2160.042 | −2;3 | 1054–1078 | Type1 collagen alpha1 chain |
| 35 | GPAGPAGARG | 10 | 809.4144 | +2 | 1069–1078 | Type1 collagen alpha1 chain |
| 36 | GPAGPRGPA | 9 | 778.40859 | +2 | 1129–1137 | Type1 collagen alpha1 chain |
| 37 | GPAGPRGPAGPA | 12 | 1003.5199 | −2 | 1129–1140 | Type1 collagen alpha1 chain |
| 38 | GPSGPQGAR | 9 | 825.40931 | −2 | 238–246 | Type1 collagen alpha1 chain |
| 39 | GSRGSPGERGESGPPGPAG | 19 | 1707.7925 | −2 | 787–805 | Type1 collagen alpha1 chain |
| 40 | IGPAGPPGTPGPPGPPGPPGGGFD | 24 | 2048.9956 | +2 | 1167–1190 | Type1 collagen alpha1 chain |
| 41 | IVGLPGQRGERG | 12 | 1237.6891 | +2 | 953–964 | Type1 collagen alpha1 chain |
| 42 | KPKYGLVTY | 9 | 1067.6015 | +2 | 305–313 | |
| 43 | LDGAKGDSGPAGPK | 14 | 1268.6361 | −2;3 | 266–279 | Type1 collagen alpha1 chain |
| 44 | LGRVVDP | 7 | 754.43374 | +2 | 443–449 | |
| 45 | LQAETEGL | 8 | 859.42871 | −2 | 333–340 | |
| 46 | LQMDYSK | 7 | 883.41095 | +2 | 255–261 | Thyroxine-binding globulin |
| 47 | LSGAPGEAGREG | 12 | 1099.5258 | +2 | 998–1009 | Type1 collagen alpha1 chain |
| 48 | LTGSPGSPGPDGKTGPAGPAGQ | 22 | 1904.9228 | +2 | 533–554 | Type1 collagen alpha1 chain |
| 49 | LTYTSNDSALFILPDKGKM | 19 | 2113.0765 | +2 | 259–277 | Serpin A3–4 |
| 50 | MESTEVFTKKT | 11 | 1299.6381 | +2 | 141–151 | |
| 51 | MNRDSNKNTLI | 11 | 1304.6507 | +2 | 1434–1444 | |
| 52 | NGDRGESGPAGPAGPAGPAGAR | 22 | 1917.9041 | −3 | 1056–1077 | Type1 collagen alpha1 chain |
| 53 | PGAAGPA | 7 | 539.27036 | −1 | 839–845 | |
| 54 | QDPVTGLTVN | 10 | 1042.5295 | +2 | 681–690 | Inter-alpha-trypsin inhibitor heavy… |
| 55 | QLQISVDQHGDNLKNTKSEI | 20 | 2266.1553 | +2 | 410–429 | Cytokeratin-4 |
| 56 | RADLERQ | 7 | 886.46208 | +2 | 377–383 | Keratin, type I cuticular Ha8 |
| 57 | RGDKGEAGEAGERG | 14 | 1387.644 | −2;3 | 1086–1099 | Type1 collagen alpha1 chain |
| 58 | RGESGPAGAPGAPGAPGA | 18 | 1475.7117 | −2 | 1029–1046 | Type1 collagen alpha1 chain |
| 59 | RGESGPPGPAGF | 12 | 1127.536 | −2 | 795–806 | Type1 collagen alpha1 chain |
| 60 | RGPPGPMGPPG | 11 | 1018.5018 | −2 | 987–997 | Type1 collagen alpha1 chain |
| 61 | RGPPGPMGPPGL | 12 | 1131.5859 | +2 | 987–998 | Type1 collagen alpha1 chain |
| 62 | SAGAQGARGDKGEAGE | 16 | 1459.6651 | +2 | 1079–1094 | Type1 collagen alpha1 chain |
| 63 | SAGAQGARGDKGEAGEAGER | 20 | 1872.8674 | +3 | 1079–1098 | Type1 collagen alpha1 chain |
| 64 | SGAPGEAGREG | 11 | 986.44174 | +2 | 999–1009 | Type1 collagen alpha1 chain |
| 65 | SGAPGEAGREGAAG | 14 | 1185.5374 | +2 | 999–1012 | Type1 collagen alpha1 chain |
| 66 | SRTSFSSVSRS | 11 | 1199.5895 | +2 | 28–38 | |
| 67 | TSGLLGAHASAITA | 14 | 1268.6725 | +2 | 1182–1195 | |
| 68 | VAGAPGALG | 9 | 711.39154 | −2 | 593–601 | Type1 collagen alpha1 chain |
| 69 | VGATGPKGSRG | 11 | 985.53049 | +2 | 849–859 | Type1 collagen alpha1 chain |
| 70 | VPGQRG | 6 | 612.33436 | −1 | 424–429 | |
| 71 | VRLCPG | 6 | 700.36903 | −2 | 347–352 | Alpha-2 HS-glycoprotein |
Table 4.
Collagen peptides identified in the acetone fraction of the hydrolysate produced with papain.
| No | Sequence | Chain Length | Mass (Da) | Net Charge | Fragment Position | Protein Name |
|---|---|---|---|---|---|---|
| 1 | AAGDAGKPGERG | 12 | 1084.5261 | −2;3 | 581–592 | Type1 collagen alpha1 chain |
| 2 | AGPAGPAGAR | 10 | 823.43005 | −2 | 1068–1077 | Type1 collagen alpha1 chain |
| 3 | AGPPGADGQAGA | 12 | 967.43592 | −2 | 807–818 | Type1 collagen alpha1 chain |
| 4 | AGPPGADGQAGAKGEPGDS | 19 | 1637.7281 | −2 | 807–825 | Type1 collagen alpha1 chain |
| 5 | AGRPGEPGPAGPPGPTGE | 18 | 1599.7641 | −2;3 | 909–926 | Type1 collagen alpha1 chain |
| 6 | AKGEPGDSGAKGDAG | 15 | 1315.6004 | −2;3 | 818–832 | Type1 collagen alpha1 chain |
| 7 | AKGETGPAGAPG | 12 | 1011.4985 | +2 | 701–712 | Type1 collagen alpha1 chain |
| 8 | AKIQLCPPPPQVPNACDMTTTV | 22 | 2437.1804 | +2 | 806–827 | Complement factor H |
| 9 | APDPFRHY | 8 | 1001.4719 | −2 | 1202–1209 | Type1 collagen alpha1 chain |
| 10 | APGEAGREGAAG | 12 | 1041.4839 | +2 | 1001–1012 | Type1 collagen alpha1 chain |
| 11 | APGEKGESGPAGPGGPTG | 18 | 1521.7059 | −2 | 770–787 | Type1 collagen alpha1 chain |
| 12 | APGEKGESGPAGPGGPTGS | 19 | 1608.738 | −2 | 770–788 | Type1 collagen alpha1 chain |
| 13 | APGFPGGPGA | 10 | 826.39735 | +2 | 335–344 | Type1 collagen alpha1 chain |
| 14 | ARGSPGSPGNDGAKGETGPAG | 21 | 1838.8507 | −2 | 689–709 | Type1 collagen alpha1 chain |
| 15 | ASGPAGPRGPAGPAGSSGKD | 20 | 1692.818 | −2;3 | 1127–1146 | Type1 collagen alpha1 chain |
| 16 | ASGPAGPRGPAGPAGSSGKDGVSG | 24 | 1992.9613 | −2;3 | 1127–1150 | Type1 collagen alpha1 chain |
| 17 | ATEAGHSAAAWLLTAQGSGTHSPL | 24 | 2333.14 | +3 | 53–76 | Peptidoglycan recognition protein 2 |
| 18 | DEGQDDRPKVGLG | 13 | 1384.6583 | +2 | 34–46 | Fibrinogen beta chain |
| 19 | DGAKGDSGPAGPKGEPGSSGE | 21 | 1855.8184 | −2;3 | 267–287 | Type1 collagen alpha1 chain |
| 20 | DGHARGDSVSQGTGLAPGSP | 20 | 1864.8664 | +3 | 270–289 | Fibrinogen alpha chain |
| 21 | DKGRLQSELKTMQD | 14 | 1647.825 | +3 | 279–292 | Cytokeratin-4 |
| 22 | DSALQLQDFYQEVANPLMTSVAF | 23 | 2586.2312 | +3 | 446–468 | Inter-alpha-trypsin inhibitor heavy... |
| 23 | DSGGPLACEKNG | 12 | 1203.519 | +2 | 576–587 | |
| 24 | EKGESGPAGPGGPT | 14 | 1239.5731 | +2 | 773–786 | Type1 collagen alpha1 chain |
| 25 | EKGEYFAFLETYGT | 14 | 1653.7563 | +2 | 336–349 | Complement component C9 |
| 26 | EKIGCSQPPQIDHG | 14 | 1564.7304 | +2 | 866–879 | Complement factor H |
| 27 | ENGLQQLTFPLSSE | 14 | 1561.7624 | +2 | 184–197 | Alpha-2-macroglobulin |
| 28 | ERGFPGE | 7 | 790.36097 | −2 | 671–677 | Type1 collagen alpha1 chain |
| 29 | EVVSLTVTCCAE | 12 | 1366.6109 | +2 | 66–77 | Vitamin D binding protein |
| 30 | EWNASQVLANLTW | 13 | 1530.7467 | +3 | 308–320 | Alpha-2-antiplasmin |
| 31 | FMQSVTGWNMGRAL | 14 | 1596.7541 | +2 | 245–258 | Angiotensinogen |
| 32 | GAAGDAGKPGERGVA | 15 | 1311.6531 | +3 | 580–594 | Type1 collagen alpha1 chain |
| 33 | GAAGPKGGPGE | 11 | 896.43519 | +2 | 493–503 | Type1 collagen alpha1 chain |
| 34 | GADGQAGAKGEPG | 13 | 1113.5051 | +2 | 811–823 | Type1 collagen alpha1 chain |
| 35 | GAKGDAGSPGPAGPTG | 16 | 1295.6106 | +2 | 826–841 | Type1 collagen alpha1 chain |
| 36 | GARGDKGEAGEAGE | 14 | 1302.58 | −2;3 | 1084–1097 | Type1 collagen alpha1 chain |
| 37 | GDRGESGPAG | 10 | 901.38897 | +2 | 1027–1036 | Type1 collagen alpha1 chain |
| 38 | GEPGDSGAKGDAGSPGPAGPTG | 22 | 1837.8078 | −2 | 820–841 | Type1 collagen alpha1 chain |
| 39 | GEPGPGGVQ | 9 | 796.37153 | −2 | 442–450 | Type1 collagen alpha1 chain |
| 40 | GEVGPAGGRGSDGPQGA | 17 | 1467.6702 | +2 | 346–362 | Type1 collagen alpha1 chain |
| 41 | GFPGADGAAGPKG | 13 | 1100.5251 | +2 | 487–499 | Type1 collagen alpha1 chain |
| 42 | GFPGPKGAAGDAGKP | 15 | 1325.6728 | +2 | 574–588 | Type1 collagen alpha1 chain |
| 43 | GGDGAPGKDGIR | 12 | 1098.5418 | +2 | 745–756 | Type1 collagen alpha1 chain |
| 44 | GGDGAPGKDGIRGM | 14 | 1286.6037 | +3 | 745–758 | Type1 collagen alpha1 chain |
| 45 | GGPGATGPAGA | 11 | 811.38243 | +2 | 679–689 | Type1 collagen alpha1 chain |
| 46 | GHRGFTGL | 8 | 843.43514 | +2 | 1102–1109 | Type1 collagen alpha1 chain |
| 47 | GIAGQRGIVG | 10 | 926.52976 | −2 | 946–955 | Type1 collagen alpha1 chain |
| 48 | GLVGPKGDTGE | 11 | 1028.5138 | +2 | 67–77 | Adiponectin |
| 49 | GMKGCPAVMPIDHVYGTLGI | 20 | 2115.0315 | +2 | 88–107 | periostin isoform X7 |
| 50 | GPAGPAGPAG | 10 | 750.36605 | −2 | 1063–1072 | Type1 collagen alpha1 chain |
| 51 | GPAGPAGSSGK | 11 | 884.43519 | +2 | 1135–1145 | Type1 collagen alpha1 chain |
| 52 | GPAGPRGPA | 9 | 778.40859 | −2 | 1129–1137 | Type1 collagen alpha1 chain |
| 53 | GPAGPRGPAGPAG | 13 | 1060.5414 | +2 | 1129–1141 | Type1 collagen alpha1 chain |
| 54 | GPMGPRGPPGPA | 12 | 1089.5389 | −2 | 175–186 | Type1 collagen alpha1 chain |
| 55 | GPMGPRGPPGPAG | 13 | 1146.5604 | −2 | 175–187 | Type1 collagen alpha1 chain |
| 56 | GPRGPPGPAG | 10 | 861.4457 | +1 | 178–187 | Type1 collagen alpha1 chain |
| 57 | GRSGRSGSFLYQ | 12 | 1313.6476 | +2 | 2406–2417 | Truncated profilaggrin |
| 58 | GRSRSFLYQVSSHE | 14 | 1651.8067 | +3 | 1436–1449 | Truncated profilaggrin |
| 59 | GSAGAQGARGDKGEAGE | 17 | 1516.6866 | −2 | 1078–1094 | Type1 collagen alpha1 chain |
| 60 | GSIQIENGYFVHYF | 14 | 1672.7886 | +3 | 251–264 | Inter-alpha-trypsin inhibitor heavy... |
| 61 | GSPGERGESGPPGPAG | 16 | 1407.6379 | +2 | 790–805 | Type1 collagen alpha1 chain |
| 62 | GSPGSPGNDGAKGETGPAG | 19 | 1611.7125 | −2 | 691–709 | Type1 collagen alpha1 chain |
| 63 | GVCISSLSCSRVGS | 14 | 1467.681 | +2 | 46–59 | |
| 64 | HRGFSGL | 7 | 772.39802 | +2 | 260–266 | Type1 collagen alpha1 chain |
| 65 | HRGFTGL | 7 | 786.41367 | −2 | 1103–1109 | Type1 collagen alpha1 chain |
| 66 | IRDVWGIEGPID | 12 | 1368.7038 | +2 | 192–203 | vitronectin |
| 67 | KGDAGSPGPAGPTG | 14 | 1167.552 | +2 | 828–841 | Type1 collagen alpha1 chain |
| 68 | KNGDRGESGPAGPAGPAGPA | 20 | 1761.8394 | −2 | 1055–1074 | Type1 collagen alpha1 chain |
| 69 | KNGDRGESGPAGPAGPAGPAG | 21 | 1818.8609 | −2 | 1055–1075 | Type1 collagen alpha1 chain |
| 70 | KNGDRGESGPAGPAGPAGPAGA | 22 | 1889.898 | −2 | 1055–1076 | Type1 collagen alpha1 chain |
| 71 | KNGDRGESGPAGPAGPAGPAGAR | 23 | 2045.9991 | −3 | 1055–1077 | Type1 collagen alpha1 chain |
| 72 | KSENARLVLQI | 11 | 1269.7405 | +3 | 158–168 | Keratin, type I cuticular Ha6 |
| 73 | LDGAKGDSGPAGPK | 14 | 1268.6361 | +3 | 266–279 | Type1 collagen alpha1 chain |
| 74 | LGIANPATDF | 10 | 1017.5131 | −2 | 728–737 | Inter-alpha-trypsin inhibitor heavy... |
| 75 | LMGEVARHSVQDGK | 14 | 1525.7671 | +3 | 103–116 | Peptidoglycan recognition protein 2 |
| 76 | LPGPTG | 6 | 540.29076 | −1 | 968–973 | |
| 77 | LPGPTGEPGKQGPGGPSGE | 19 | 1717.8271 | +2 | 968–986 | Type1 collagen alpha1 chain |
| 78 | LVDTELNCTVLQMD | 14 | 1649.7641 | +2 | 245–258 | Thyroxine-binding globulin |
| 79 | MHGLISDAEERGER | 14 | 1598.7471 | +2 | 409–422 | Keratin, type II cytoskeletal 1b |
| 80 | MSAPGPMGPMGPRGPPGPAG | 20 | 1817.8375 | +3 | 168–187 | Type1 collagen alpha1 chain |
| 81 | MSAPGPMGPMGPRGPPGPAGSN | 22 | 2018.9125 | +2 | 168–189 | Type1 collagen alpha1 chain |
| 82 | NGDRGESGPAGPAGPAGPAGA | 21 | 1761.803 | −2 | 1056–1076 | Type1 collagen alpha1 chain |
| 83 | NGDRGESGPAGPAGPAGPAGAR | 22 | 1917.9041 | −2;3 | 1056–1077 | Type1 collagen alpha1 chain |
| 84 | PAGPAGQDGRAGPPGPSGARG | 21 | 1828.8929 | +3 | 548–568 | Type1 collagen alpha1 chain |
| 85 | PGPTGEPGKQGPGGPSGE | 18 | 1604.7431 | −2 | 969–986 | Type1 collagen alpha1 chain |
| 86 | PYRVYCDMKTEKG | 13 | 1645.7592 | +2 | 269–281 | Fibrinogen beta chain |
| 87 | QLEPEE | 6 | 743.33375 | +1 | 234–239 | Complement C3 |
| 88 | RGDKGEAGEAGE | 12 | 1174.5214 | −2 | 1086–1097 | Type1 collagen alpha1 chain |
| 89 | RGEGGPAGAPGF | 12 | 1071.5098 | +2 | 624–635 | Type1 collagen alpha1 chain |
| 90 | RGESGPAGPAGPAGPAGA | 18 | 1475.7117 | +2 | 1059–1076 | Type1 collagen alpha1 chain |
| 91 | RGPPGPMGPPG | 11 | 1018.5018 | +2 | 987–997 | Type1 collagen alpha1 chain |
| 92 | RGSAGAQGARGDKGEAGE | 18 | 1672.7877 | +3 | 1077–1094 | Type1 collagen alpha1 chain |
| 93 | RGSAGAQGARGDKGEAGEA | 19 | 1743.8248 | −3 | 1077–1095 | Type1 collagen alpha1 chain |
| 94 | RGSPGSPGNDGAKGETGPAG | 20 | 1767.8136 | −2 | 690–709 | Type1 collagen alpha1 chain |
| 95 | SGAPGEAGREGAAGN | 15 | 1299.5804 | −2 | 999–1013 | Type1 collagen alpha1 chain |
| 96 | SGAPGEAGREGAAGNEGAPGRD | 22 | 1981.8838 | +2 | 999–1020 | Type1 collagen alpha1 chain |
| 97 | SGPAGPRGPAGPA | 13 | 1090.552 | +2 | 1128–1140 | Type1 collagen alpha1 chain |
| 98 | SGPPGPAG | 8 | 638.30239 | +1 | 798–805 | Type1 collagen alpha1 chain |
| 99 | SRGERGFPGERGGPGATGPAG | 21 | 1968.9514 | +3 | 668–688 | Type1 collagen alpha1 chain |
| 100 | SVMADATSVPVTE | 13 | 1305.6122 | +2 | 25–37 | Protein HP-25 homolog 2 |
| 101 | VAQPSQE | 7 | 757.36063 | −2 | 1194–1200 | Type1 collagen alpha1 chain |
| 102 | VKGGDGAPGKDGIRG | 15 | 1382.7266 | −2 | 743–757 | Type1 collagen alpha1 chain |
| 103 | VKGGDGAPGKDGIRGM | 16 | 1513.7671 | +3 | 743–758 | Type1 collagen alpha1 chain |
4. Discussion
Biological activities of protein hydrolysates are dependent on the protein substrate, enzyme used for proteolysis, and hydrolysis conditions, such as the enzyme–substrate ratio (E/S), incubation time, temperature, and pH [33]. The DH of the proteins influences the size and structure of the released peptides [34]. Moreover, functional properties of food protein hydrolysates, such as protein solubility, surface hydrophobicity, emulsification, and foaming capacity, depend on the degree of hydrolysis. For instance, the solubility of protein hydrolysate increases with increased DH as observed in samples from yellow stripe trevally [35], Alaska pollack [36], barbel [13,14], anchovy [37], and Atlantic mackerel [12]. High DH would ensure that the peptides become soluble and accessible to interact with their targets in aqueous physiological environments. However, extensive hydrolysis may lead to the loss of the bioactive motif in a peptide. As reported previously, the fish skin collagens in this study consist of two different subunits with an average molecular weight of 110–150 kDa (α1 and α2 at band intensity ratio of 2:1), β (dimers) and small amounts of γ (trimers) [38]. These protein bands were hydrolyzed differently depending on the enzyme used. Interestingly, trypsin gave the highest DH, even higher than multi-enzyme Alcalase and Flavourzyme, resulting in peptides with the lowest MW profiles. Conversely, papain and pepsin gave the lowest DH resulting in several high MW bands. Notably, the original collagen protein bands were not detected in all the samples, signifying their hydrolysis by the enzymes. Similar results have been reported by Suárez-Jiménez et al. [39], Chi et al. [40] and Felician et al. [10], who observed the presence of low molecular weight polypeptide bands for collagens isolated from squid by-products, fish cartilage fish, and jellyfish, respectively.
Surface hydrophobicity of the protein hydrolysates in this study was inversely related to DH. Whereas the smaller sized peptides would be highly soluble, it is expected that the high molecular weight peptides from limited hydrolysis would interact more easily to form hydrophobic pockets or aggregates that bind the fluorescent molecular probe [41,42]. Furthermore, surface charge distribution is an important parameter for the determination of biomolecular structure and interactions in aqueous environments [30]. Results from this study indicated weak electrostatic stabilization (except for hydrolysates produced with Alcalase and trypsin) and inclination of the hydrolysate particles to aggregate in aqueous solution. The net surface charges of the hydrolysates in aqueous solution also decreased in magnitude with an increase in surface hydrophobicity, which is an outcome of peptide aggregation. The molecular profile, surface properties and intermolecular interactions of peptides are important determinants of their bioaccessibility and binding to molecular targets, which would influence their bioactivities.
Antimicrobial peptides have been derived from various food sources by enzymatic hydrolysis, fermentation or gastrointestinal digestion for combating food-borne pathogens [43]. Results in this study revealed that the extent of the antibacterial activity of collagen hydrolysates against the S. abony and E. coli varies with different enzymes used for hydrolysis. Sila et al. [14] demonstrated that hydrolysates with higher DH (16.2% and 14.53%) or lower DH (2.8%) did not inhibit the growth of Gram-positive and Gram-negative bacteria, compared to hydrolysates with DH 6.6%, which showed antibacterial effect against a broad spectrum of Gram-positive (Listeria monocytogenes, Staphylococcus aureus, Enterococcus faecalis, Micrococcus luteus and Bacillus cereus) and Gram-negative bacteria (Escherichia coli, Salmonella enterica, Pseudomonas aeruginosa, Klebsiella pneumonia, and Enterobacter sp.). In our study, the DH had no significant effect (p > 0.05) on antibacterial activity. [15] and [44] observed similar results in their studies on trout protein hydrolysate and yak kappa-casein hydrolysate, respectively. The biological attributes of peptides are largely influenced by their molecular structural properties, including amino acid composition, sequence, net charge, and chain length [43]. These properties may have influenced the antibacterial activities of the collagen hydrolysates. Notably, hydrophobicity is thought to be important because it facilitates the interaction of the peptides with bacterial cytoplasmic membranes [44]. This feature relates more to the molecular hydrophobicity of individual peptides instead of surface hydrophobicity.
The bioactivity mechanisms of antibacterial peptides can be by interaction with negatively charged cell surface components, such as lipoteichoic acid in the outer membranes of Gram-positive bacteria and lipopolysaccharides in the cell wall of Gram-negative bacteria. Antibacterial peptides produce pores, channels or peptide–lipid complexes in the outer membrane or the cell wall of bacteria, and disruption of cytoplasmic membranes occurs followed by cell lysis, which results in inhibition of cell function or death [43,45]. Because of the increasing bacterial resistance against many antibiotics and advances in peptide design and synthesis, there has been a heightened interest in the discovery of new effective antibiotics [46]. Moreover, food-based sources provide potentially cheaper and safer alternatives to chemical antibacterial agents [43]. In our study, the collagen hydrolysates contained several peptides, some of which may be inactive. Acetone fractionation of the most potent hydrolysates (produced with Neutrase and papain) resulted in significantly increased antibacterial activity against four S. species, even at lower concentrations. This indicates that the downstream processing led to the concentration of active peptides in the resulting fraction. Ennaas et al. [12] reported a similar pattern with acetone fractionation of mackerel by-product hydrolysates with antibacterial activity against Gram-positive (L. innocua) and Gram-negative bacteria (E. coli). The increase in antibacterial activity after fractionation with acetone was attributed to the hydrophobic nature of the fractionated peptides. Similarly, Ruangsri et al. [47] reported that acetonitrile fractions extracted from different Atlantic cod (Gadus morhua) tissues had higher antibacterial activity than the aqueous fractions.
Peptides present in the acetone fractions are important candidates for further evaluation as antibacterial agents or for identification of important bioactive structural motifs. Several of the fish collagen peptides obtained with Neutrase (71 peptides) and papain (103 peptides) had arginine (R) and/or histidine (H), and/or lysine (K) residues in their sequences. These cationic amino acid residues, in addition to the presence of hydrophobic domains in sequences of antibacterial peptides (AMPs), contribute to the formation of amphiphilic topology for adherence of the peptides to bacterial membrane [43,48]. Furthermore, identified peptides from both samples revealed the high occurrence of glycine (G) and proline (P) residues, which are common structural characteristics of several AMPs [11,12,49,50]. Previously, a 12-mer collagencin (GLPGPLGPAGPK; f291–302 of Petromyzon marinus collagen, S4R4C5_PETMA) identified in a fraction from Scomber scombrus (Atlantic mackerel) by-product hydrolysate was reported to have antimicrobial activity against six Gram-positive and Gram-negative bacteria, including L. innocua, Lactococcus lactis, Carnobacterium divergens, Staphylococcus aureus, Streptococcus pyogenes, and E. coli [25]. Notably, the C-terminal hexamer fragment of collagencin (GPAGPK) was found in seven peptides in our study, including peptides 13, 14, 18 and 19 released by Neutrase (Table 3) and peptides 19 and 73 released by papain (Table 4). It is likely that these motifs played a role in the antibacterial activity of their respective fractions.
5. Conclusions
In this study, fish skin collagen was hydrolyzed with different commercial enzymes to release peptides possessing potent antibacterial activity. The results demonstrated that the degree of hydrolysis had no significant effect on antibacterial activity. Collagen hydrolysate produced with Neutrase and papain showed the most potent inhibitory activity against Gram-negative bacteria (Salmonella strains), and viable count confirmed the decrease of the cell population. Acetone fractionation significantly enhanced the growth inhibitory activity against four Salmonella strains, providing products with stronger potential for use against food-borne pathogens. Antibacterial peptides not only alter the cytoplasmic membrane but also inhibit intracellular targets such as nucleic acid synthesis, protein synthesis or enzymatic activity. Therefore, the large number of peptide sequences identified in the fractions by shotgun peptidomics suggest further investigation of a potentially multi-targeted approach to the antimicrobial effects. Taken together, this study provided an array of peptide sequences from the fish skin by-products for elucidating molecular mechanisms and further exploration as value-added antimicrobial nutraceutical products against food-borne pathogens.
Acknowledgments
We acknowledge Nico Hüttmann of the Department of Chemistry and Biomolecular Science, University of Ottawa, for technical assistance with LC-MS/MS analysis.
Author Contributions
Conceptualization, M.A., S.M.O., R.H. and C.C.U.; methodology, M.A., Y.A.C., R.H., C.C.U.; validation, M.A., Y.A.C., R.H. and C.C.U.; formal analysis, M.A. and Y.A.C.; investigation, M.A. and Y.A.C.; resources, C.C.U., R.H. and S.M.O.; writing—original draft preparation, M.A.; writing—review and editing, M.A., Y.A.C., R.H., S.M.O., A.M.L., M.E. and C.C.U.; visualization, M.A., Y.A.C. and C.C.U.; supervision, C.C.U., R.H. and S.M.O.; funding acquisition, R.H. and C.C.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), reference numbers RGPIN-2018-06839 (C.C.U.) and RGPIN-2018-06059 (R.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mor A. Peptide-based antibiotics: A potential answer to raging antimicrobial resistance. Drug. Dev. Res. 2000;50:440–447. doi: 10.1002/1098-2299(200007/08)50:3/4<440::AID-DDR27>3.0.CO;2-4. [DOI] [Google Scholar]
- 2.Song R., Wei R.-B., Luo H.-Y., Wang D.-F. Isolation and Characterization of an Antibacterial Peptide Fraction from the Pepsin Hydrolysate of Half-Fin Anchovy (Setipinna taty) Moleclues. 2012;17:2980–2991. doi: 10.3390/molecules17032980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atef M., Ojagh S.M. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods. 2017;35:673–681. doi: 10.1016/j.jff.2017.06.034. [DOI] [Google Scholar]
- 4.Pellegrini A. Antimicrobial Peptides from Food Proteins. Curr. Pharm. Des. 2003;9:1225–1238. doi: 10.2174/1381612033454865. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-F. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim H.R., Iwamori E., Sugimoto Y., Aoki T. Identification of a distinct antibacterial domain within the N-lobe of ovotransferrin. Biochim. Biophys. Acta Bioenerg. 1998;1401:289–303. doi: 10.1016/S0167-4889(97)00132-8. [DOI] [PubMed] [Google Scholar]
- 7.Zucht H.-D., Raida M., Adermann K., Mägert H.-J., Forssmann W.-G. Casocidin-I: A casein-αs2derived peptide exhibits antibacterial activity. FEBS Lett. 1995;372:185–188. doi: 10.1016/0014-5793(95)00974-E. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrini A., Dettling C., Thomas U., Hunziker P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochim. Biophys. Acta Gen. Subj. 2001;1526:131–140. doi: 10.1016/S0304-4165(01)00116-7. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen R.S., Morrissey M.T. Marine Biotechnology for Production of Food Ingredients. Adv. Food Nutr. Res. 2007;52:237–292. doi: 10.1016/S1043-4526(06)52005-4. [DOI] [PubMed] [Google Scholar]
- 10.Felician F.F., Yu R.-H., Li M.-Z., Li C.-J., Chen H.-Q., Jiang Y., Tang T., Qi W.-Y., Xu H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019;22:12–20. doi: 10.1016/j.cjtee.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemil I., Abdelhedi O., Nasri R., Mora L., Jridi M., Aristoy M.-C., Toldrá F., Nasri M. Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res. Int. 2017;100:121–133. doi: 10.1016/j.foodres.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Ennaas N., Hammami R., Beaulieu L., Fliss I. Production of antibacterial fraction from Atlantic mackerel (Scomber scombrus) and its processing by-products using commercial enzymes. Food Bioprod. Process. 2015;96:145–153. doi: 10.1016/j.fbp.2015.07.014. [DOI] [Google Scholar]
- 13.Sila A., Hedhili K., Przybylski R., Ellouz-Chaabouni S., Dhulster P., Bougatef A., Nedjar-Arroume N. Antibacterial activity of new peptides from barbel protein hydrolysates and mode of action via a membrane damage mechanism against Listeria monocytogenes. J. Funct. Foods. 2014;11:322–329. doi: 10.1016/j.jff.2014.10.006. [DOI] [Google Scholar]
- 14.Sila A., Nedjar-Arroume N., Hedhili K., Chataigné G., Balti R., Nasri M., Dhulster P., Bougatef A. Antibacterial peptides from barbel muscle protein hydrolysates: Activity against some pathogenic bacteria. LWT. 2014;55:183–188. doi: 10.1016/j.lwt.2013.07.021. [DOI] [Google Scholar]
- 15.Wald M., Schwarz K., Rehbein H., Bußmann B., Beermann C. Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by-products with trout pepsin. Food Chem. 2016;205:221–228. doi: 10.1016/j.foodchem.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Beaulieu L., Thibodeau J., Bonnet C., Bryl P., Carbonneau M.-É. Detection of antibacterial activity in an enzymatic hydrolysate fraction obtained from processing of Atlantic rock crab (Cancer irroratus) by-products. PharmaNutrition. 2013;1:149–157. doi: 10.1016/j.phanu.2013.05.004. [DOI] [Google Scholar]
- 17.Beaulieu L., Thibodeau J., Desbiens M., Saint-Louis R., Zatylny-Gaudin C., Thibault S. Evidence of Antibacterial Activities in Peptide Fractions Originating from Snow Crab (Chionoecetes opilio) By-Products. Probiotics Antimicrob. Proteins. 2010;2:197–209. doi: 10.1007/s12602-010-9043-6. [DOI] [PubMed] [Google Scholar]
- 18.Noga E.J., Stone K.L., Wood A., Gordon W.L., Robinette D. Primary structure and cellular localization of callinectin, an antimicrobial peptide from the blue crab. Dev. Comp. Immunol. 2011;35:409–415. doi: 10.1016/j.dci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo L., Robinette D.W., Noga E.J. Callinectin, an Antibacterial Peptide from Blue Crab, Callinectes sapidus, Hemocytes. Mar. Biotechnol. 1999;1:44–51. doi: 10.1007/PL00011750. [DOI] [PubMed] [Google Scholar]
- 20.Mu C., Zheng P., Zhao J., Wang L., Zhang H., Qiu L., Gai Y., Song L. Molecular characterization and expression of a crustin-like gene from Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 2010;34:734–740. doi: 10.1016/j.dci.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Imjongjirak C., Amparyup P., Tassanakajon A., Sittipraneed S. Molecular cloning and characterization of crustin from mud crab Scylla paramamosain. Mol. Biol. Rep. 2008;36:841–850. doi: 10.1007/s11033-008-9253-0. [DOI] [PubMed] [Google Scholar]
- 22.Relf J.M., Chisholm J.R.S., Kemp G.D., Smith V.J. Purification and characterization of a cysteine-rich 11.5-kDa antibacterial protein from the granular haemocytes of the shore crab, Carcinus maenas. JBIC J. Biol. Inorg. Chem. 1999;264:350–357. doi: 10.1046/j.1432-1327.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 23.Schnapp D., Kemp G.D., Smith V.J. Purification and Characterization of a Proline-Rich Antibacterial Peptide, with Sequence Similarity to Bactenecin-7, from the Haemocytes of the Shore Crab, Carcinus Maenas. JBIC J. Biol. Inorg. Chem. 1996;240:532–539. doi: 10.1111/j.1432-1033.1996.0532h.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Olsen K., Grossi A., Otte J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013;141:2343–2354. doi: 10.1016/j.foodchem.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 25.Ennaas N., Hammami R., Gomaa A., Bédard F., Biron Éric, Subirade M., Beaulieu L., Fliss I. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochem. Biophys. Res. Commun. 2016;473:642–647. doi: 10.1016/j.bbrc.2016.03.121. [DOI] [PubMed] [Google Scholar]
- 26.Kittiphattanabawon P., Benjakul S., Visessanguan W., Kishimura H., Shahidi F. Isolation and Characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum) Food Chem. 2010;119:1519–1526. doi: 10.1016/j.foodchem.2009.09.037. [DOI] [Google Scholar]
- 27.Nielsen P.M., Petersen D., Dambmann C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001;66:642–646. doi: 10.1111/j.1365-2621.2001.tb04614.x. [DOI] [Google Scholar]
- 28.Sun X., Ohanenye I.C., Ahmed T., Udenigwe C.C. Microwave treatment increased protein digestibility of pigeon pea (Cajanus cajan) flour: Elucidation of underlying mechanisms. Food Chem. 2020;329:127196. doi: 10.1016/j.foodchem.2020.127196. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Mohan A., Udenigwe C.C. Towards the design of hypolipidaemic peptides: Deoxycholate binding affinity of hydrophobic peptide aggregates of casein plastein. J. Funct. Foods. 2015;18:129–136. doi: 10.1016/j.jff.2015.06.064. [DOI] [Google Scholar]
- 31.Tyanova S., Temu T., Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016;11:2301–2319. doi: 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- 32.Hammami R., Zouhir A., Ben Hamida J., Neffati M., Vergoten G., Naghmouchi K., Fliss I. Antimicrobial properties of aqueous extracts from three medicinal plants growing wild in arid regions of Tunisia. Pharm. Biol. 2009;47:452–457. doi: 10.1080/13880200902822604. [DOI] [Google Scholar]
- 33.Kristinsson H.G., Rasco B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- 34.Jang H.L., Liceaga A.M., Yoon K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods. 2016;20:433–442. doi: 10.1016/j.jff.2015.11.020. [DOI] [Google Scholar]
- 35.Klompong V., Benjakul S., Kantachote D., Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- 36.Jia J., Zhou Y., Lu J., Chen A., Li Y., Zheng G. Enzymatic hydrolysis of Alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010;90:635–640. doi: 10.1002/jsfa.3861. [DOI] [PubMed] [Google Scholar]
- 37.Wu H., Liu Z., Zhao Y., Zeng M. Enzymatic preparation and characterization of iron-chelating peptides from anchovy (Engraulis japonicus) muscle protein. Food Res. Int. 2012;48:435–441. doi: 10.1016/j.foodres.2012.04.013. [DOI] [Google Scholar]
- 38.Atef M., Ojagh S.M., Latifi A.M., Esmaeili M., Udenigwe C.C. Biochemical and structural characterization of sturgeon fish skin collagen (Huso huso) J. Food Biochem. 2020;44:e13256. doi: 10.1111/jfbc.13256. [DOI] [PubMed] [Google Scholar]
- 39.Suárez-Jiménez G.M., Robles-Sánches R.M., Yépiz-Plascencia G., Burgos-Hernández A., Ezquerra-Brauer J.M. In vitro antioxidant, antimutagenic and antiproliferative activities of collagen hydrolysates of jumbo squid (Dosidicus gigas) byproducts. Food Sci. Technol. 2015;35:421–427. doi: 10.1590/1678-457X.6658. [DOI] [Google Scholar]
- 40.Chi C., Hu F., Li Z., Wang B., Luo H., Hu F. Influence of different hydrolysis processes by trypsin on the physicochemical, antioxidant, and functional properties of collagen hydrolysates from Sphyrna lewini, Dasyatis akjei, and Raja porosa. J. Aquat. Food Prod. Technol. 2015;25:616–632. doi: 10.1080/10498850.2014.898004. [DOI] [Google Scholar]
- 41.Wu W.U., Hettiarachchy N.S., Qi M. Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J. Am. Oil Chem. Soc. 1998;75:845–850. doi: 10.1007/s11746-998-0235-0. [DOI] [Google Scholar]
- 42.Boachie R.T., Okoro F.L., Imai K., Sun L., Elom S.O., Nwankwo J.O., Ejike C.E.C.C., Udenigwe C.C. Enzymatic release of dipeptidyl peptidase-4 inhibitors (gliptins) from pigeon pea (Cajanus cajan) nutrient reservoir proteins: In silico and in vitro assessments. J. Food Biochem. 2019;43:e13071. doi: 10.1111/jfbc.13071. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed T., Hammami R. Recent insights into structure-function relationships of antimicrobial peptides. J. Food Biochem. 2018;43:e12546. doi: 10.1111/jfbc.12546. [DOI] [PubMed] [Google Scholar]
- 44.Cheng X., Tang X., Wang Q., Mao X. Antibacterial effect and hydrophobicity of yak κ-casein hydrolysate and its fractions. Int. Dairy J. 2013;31:111–116. doi: 10.1016/j.idairyj.2012.12.004. [DOI] [Google Scholar]
- 45.Yasir M., Willcox M.D.P., Dutta D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials. 2018;11:2468. doi: 10.3390/ma11122468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013;31:379–382. doi: 10.1038/nbt.2572. [DOI] [PubMed] [Google Scholar]
- 47.Ruangsri J., Fernandes J.M., Brinchmann M.F., Kiron V. Antimicrobial activity in the tissues of Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2010;28:879–886. doi: 10.1016/j.fsi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Dashper S.G., Liu S.W., Reynolds E.C. Antimicrobial Peptides and their Potential as Oral Therapeutic Agents. Int. J. Pept. Res. Ther. 2007;13:505–516. doi: 10.1007/s10989-007-9094-z. [DOI] [Google Scholar]
- 49.Ilić N., Novkovic M., Guida F., Xhindoli D., Benincasa M., Tossi A., Juretić D. Selective antimicrobial activity and mode of action of adepantins, glycine-rich peptide antibiotics based on anuran antimicrobial peptide sequences. Biochim. Biophys. Acta Biomembr. 2013;1828:1004–1012. doi: 10.1016/j.bbamem.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Dong N., Ma Q., Shan A., Lv Y., Hu W., Gu Y., Li Y. Strand Length-Dependent Antimicrobial Activity and Membrane-Active Mechanism of Arginine- and Valine-Rich β-Hairpin-Like Antimicrobial Peptides. Antimicrob. Agents Chemother. 2012;56:2994–3003. doi: 10.1128/AAC.06327-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings are available within the article.