Abstract
We previously reported that the role of reactive oxygen intermediates (ROIs) in NF-κB activation by proinflammatory cytokines was cell specific. However, the sources for ROIs in various cell types are yet to be determined and might include 5-lipoxygenase (5-LOX) and NADPH oxidase. 5-LOX and 5-LOX activating protein (FLAP) are coexpressed in lymphoid cells but not in monocytic or epithelial cells. Stimulation of lymphoid cells with interleukin-1β (IL-1β) led to ROI production and NF-κB activation, which could both be blocked by antioxidants or FLAP inhibitors, confirming that 5-LOX was the source of ROIs and was required for NF-κB activation in these cells. IL-1β stimulation of epithelial cells did not generate any ROIs and NF-κB induction was not influenced by 5-LOX inhibitors. However, reintroduction of a functional 5-LOX system in these cells allowed ROI production and 5-LOX-dependent NF-κB activation. In monocytic cells, IL-1β treatment led to a production of ROIs which is independent of the 5-LOX enzyme but requires the NADPH oxidase activity. This pathway involves the Rac1 and Cdc42 GTPases, two enzymes which are not required for NF-κB activation by IL-1β in epithelial cells. In conclusion, three different cell-specific pathways lead to NF-κB activation by IL-1β: a pathway dependent on ROI production by 5-LOX in lymphoid cells, an ROI- and 5-LOX-independent pathway in epithelial cells, and a pathway requiring ROI production by NADPH oxidase in monocytic cells.
The interaction of interleukin-1β (IL-1β) with its type 1 cell surface receptor initiates a cascade of intracellular reactions leading to the activation of transcription factors and the expression of target genes. One of the major transcription factors mediating IL-1β biological activities is NF-κB (for reviews, see references 2, 3, and 22). This factor is sequestered in the cytoplasm by an inhibitor from the IκB family. IL-1β cellular stimulation leads to a rapid phosphorylation and degradation of IκBα, the most common NF-κB inhibitor. This reaction allows NF-κB to translocate to the nucleus, to bind DNA, and to activate the transcription of specific genes (2, 55).
Following its interaction with IL-1, the type 1 IL-1 receptor recruits the IL-1 receptor-associated kinase (IRAK) protein, which subsequently interacts with the TRAF6 adapter protein (15, 16, 30, 61, 62, 65). TRAF6 is required for IL-1β-induced NF-κB activation, as demonstrated in 293 cells (16).
However, the signaling pathways leading to NF-κB activation from the IL-1β receptors are still controversial. It has been demonstrated that TRAF6 interacts with a MAP kinase kinase kinase (MAPKKK) known as NIK and that NIK is required for IL-1β- or tumor necrosis factor alpha (TNF-α)-dependent NF-κB activation (39, 56). Large (500 to 900 kDa) multimeric protein kinase complexes have been purified from HeLa cells and transmit the signal from the TNF receptor type 1 (TNFR-1) and type 1 IL-1 receptors to the NF-κB/IκB cytoplasmic complex (17, 20, 33, 41). From these complexes three IκB kinases, IKK-α, IKK-β, and IKK-γ, have been purified, and their genes were cloned (20, 42, 49, 66). Other investigators have cloned IKK kinases by virtue of their association with the NIK protein kinase (47, 64). Moreover, inactivation of these kinases by dominant negative mutants suppresses IL-1β and TNF-α induction of NF-κB. These studies indicate that the activated NIK kinase phosphorylates and activates the IKK protein kinases. IKK protein kinases can in turn phosphorylate the IκBα protein on serines located at positions 32 and 36, a reaction which targets IκBα for ubiquitination and rapid degradation by the proteasome (12, 58, 59). These reactions are extremely rapid, and the cellular IκBα protein is completely degraded within minutes following TNF-α or IL-1β cell stimulation (4, 13).
Despite this simplified linear receptor-TRAF-NIK-IKK axis for IκBα phosphorylation and degradation, other intermediates might be involved in NF-κB activation by TNF-α or IL-1β. First, several components of the large signaling complex remain to be identified, as the three IKK protein kinases do not account for the molecular weight of the whole complex. Second, a large number of studies, some of them being a matter of controversy, have identified other intermediates which seem to be required for TNF-α- or IL-1β-mediated NF-κB activation. These intermediates are Raf-1, MAP kinases, the PKC ζ and λ/ι isoforms, Rho and Rac proteins, and ceramide or reactive oxygen intermediates (ROIs) (19, 24, 25, 32, 33, 38, 46, 50–53, 57). Such a large number of controversial studies might be explained by cell type specificities. Indeed, most of these studies were performed with a single cell line, although as we reported the roles of sphingomyelinases, PKC λ/ι, and ROIs in NF-κB activation by IL-1β were cell specific (6–8).
We reported that an oxidative stress favored replication of the human immunodeficiency virus type 1 (HIV-1) containing a tandem κB site in its long terminal repeat (LTR) (35). Later on, the authors of several studies proposed that ROIs were produced by all NF-κB-activating agents and were required for NF-κB activation (50–52). So far, however, the IκB kinases activated by ROIs have not yet been identified. More recently, however, it was reported that the effects of oxidants and antioxidants on NF-κB activation were dependent on the cell line and on the applied stimulus (10, 11). We also reported that IL-1β induced NF-κB through the production of ROIs in lymphoid cells but independently of any ROI production in epithelial cells (6). These data indicate that the role of ROIs in NF-κB activation is probably cell specific.
The sources of ROIs following TNF-α or IL-1β treatment are still largely unknown. CD28 stimulation of T lymphocytes induces ROI production through an increase of the activity of 5-lipoxygenase (5-LOX) (37), an enzyme which is also required for NF-κB-dependent transcription induced by IL-1β in endothelial cells (34). The 5-LOX enzyme catalyzes the production of leukotrienes and ROIs from arachidonic acid (29, 36). Its activity requires FLAP (5-LOX activating protein), which transports 5-LOX to the nuclear membrane (23, 43, 48, 63). Recently, we reported that the inhibition of 5-LOX in lymphoid cells blocked NF-κB activation after IL-1β or arachidonic acid stimulation (7). Similarly, according to our previous data, the absence of ROI induction in IL-1β-treated epithelial cells might be due to low 5-LOX and high catalase activities (7). However, there are other sources of cellular ROIs, including the NADPH oxidase which is expressed not only in monocytes/macrophages and polymorphonuclear neutrophils but also in lymphocytes (54). In the present study, we investigated the roles of 5-LOX, FLAP, and NADPH oxidase in the IL-1β-induced ROI production and NF-κB activation in different cell types.
MATERIALS AND METHODS
Cell culture and biological reagents.
The cell lines HL-60, U937, Jurkat, EL-4, Raji, OVCAR-3, SKOV-3, MCF7 A/Z, HCT-116, HT-29, and HTM-29 were grown in RPMI 1640 medium (Life Technologies) supplemented with 1% antibiotics, 1% glutamine, and 10% fetal bovine serum. The mouse pre-B-cell line 70Z/3 was grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% antibiotics, 1% glutamine, and 10 mM β-mercaptoethanol. Before stimulation, 70Z/3 cells were grown for one day in medium without β-mercaptoethanol. The breast cancer cell line MCF7 A/Z was a generous gift from M. Mareel (University of Ghent, Belgium).
IL-1β treatment was carried out at a 50-U/ml concentration (specific activity, >5 × 107 U/mg; Boehringer, Mannheim, Germany). N-Acetyl-cysteine (NAC) or pyrrolidine-9-dithiocarbamate (PDTC) was added to the medium 150 min before IL-1β stimulation. Cells were incubated with the FLAP inhibitor MK-886, a generous gift from J. Evans (Merck Frosst Centre for Therapeutic Research, Quebec, Canada) (40), for 10 min before IL-1β stimulation, with phenylarsine oxide (PAO; Sigma) for 1 h prior to IL-1β treatment, or with diphenyleneiodonium chloride (DPI; Sigma) or eicosatetraynoic acid (ETYA; Sigma) for 40 min before IL-1β stimulation.
Immunoblots.
Protein extracts (30 μg) obtained by sodium dodecyl sulfate (SDS) lysis were separated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel. After transfer to a nylon membrane (Immobilon-P; Millipore, Bedford, Mass.) and overnight blocking at 4°C with Tris-buffered saline–Tween (20 mM Tris [pH 7.5], 500 mM NaCl, 0.2% Tween 20 plus 5% dry milk), the membranes were incubated for 1 h with specific antibodies directed against human 5-LOX and FLAP (Merck Frosst Centre for Therapeutic Research), washed, and then incubated with the second peroxidase-conjugated antibody. The reaction was revealed by the enhanced chemiluminescence detection method (ECL kit; Amersham, Little Chalfont, Buckinghamshire, United Kingdom).
Fluorescence measurement of intracellular ROIs.
Formation of ROIs was measured using dichlorofluorescein diacetate (DFCH) (6). The cells were incubated in 175-cm2 flasks with 20 μM DCFH in phenol red-free medium 199. After 15 min, the medium was removed and the cells were washed and incubated with IL-1β or H2O2 for 15 min. Fluorescence was measured by spectrofluorometry with excitation at 505 nm and detection of light emission at 525 nm. ROI concentrations in cytoplasmic extracts were measured as previously described (6) and expressed as a function of the protein content in nuclear extracts from the same cells. The measurements were performed on 3 × 106 cells for all cell types with the exception of selected CD20-positive cells, for which measurements were made on 1.2 × 106 cells.
Nuclear protein extraction and EMSA.
Nuclear protein extracts were prepared as described (7). Briefly, the pelleted nuclei were resuspended in nuclear buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 0.2 mM EDTA, 0.63 M NaCl, 25% glycerol, protease inhibitors [protease inhibitor kit; Boehringer]), incubated for 20 min at 4°C, and centrifuged for 30 min at 14,000 rpm in an Eppendorf 5415C centrifuge. Protein amounts were quantified with the Micro BCA Protein Assay Reagent kit (Pierce, Rockford, Ill.). Electrophoretic mobility shift assays (EMSA) were performed as described (26). The palindromic (PD)-κB probe was 5′-TTGGCAACGGCAGGGGAATTCCCCTCTCCTTA-3′.
Plasmids.
The reporter plasmid HIV-κB-CAT contained the two κB sites from the HIV LTR cloned upstream of a chloramphenicol acetyltransferase (CAT) reporter gene (9). Expression vectors for wild-type and mutant RhoA, Rac1, and Cdc42 were generous gifts from L. Cross (National Institutes of Health, Bethesda, Md.) (18, 46). The 5-LOX and FLAP expression vectors were received from J. Evans (Merck Frosst Centre for Therapeutic Research). The pCMV-CD20 (cluster of differentiation 20 antigen) plasmid was kindly provided by Jim Koh (Laboratory of Molecular Oncology, Massachusetts General Hospital and Harvard Medical School, Cambridge, Mass.).
Transfections.
Expression vectors (1 μg) and the HIV-κB-CAT reporter plasmid (4 μg) were transfected into HCT-116 and MCF7 A/Z cells by using the DOTAP liposomal transfection reagent (Boehringer Mannheim). After 6 h, the DNA-containing medium was removed and replaced by fresh medium. Cells were then left untreated or were stimulated with 50 U of IL-1β/ml for 6 h. Cellular extracts were prepared and CAT activity was determined as described previously (9).
U937 and THP-1 cells were transfected with DEAE-dextran (Amersham). Cells (8 × 105 cells) were incubated for 90 min at 37°C in 1 ml of Tris-buffered saline (pH 7.6) containing 5 μg of total DNA and 400 μg of DEAE-dextran. After 10% dimethyl sulfoxide was added for 2 min, 15 ml of Tris-buffered saline was added. Cells were centrifuged and resuspended in 1 ml of culture medium for 24 h. The IL-1β stimulation was then performed after the addition of 2 ml of fresh medium.
For stable transfections, MCF7 A/Z cells were transfected with the linearized pcDNA3 (10 μg) empty vector or with the 5-LOX expression vector (10 μg) (MCF7 A/Z LOX) by using the DOTAP system (Boehringer Mannheim). Forty-eight hours later, transfected cells were selected in a medium containing G418 at 0.5 mg/ml. After 15 days of selection, 10 G418-resistant colonies were isolated, grown separately, and selected for 5-LOX expression by immunoblotting.
Positive selection of CD20-positive cells.
Cells transfected with the CD20 expression vectors were washed, incubated in the presence of a fluorescein isothiocyanate (FITC)-conjugated anti-CD20 monoclonal antibody (Sanver Tech, Boechout, Belgium) for 30 min, washed again, and then incubated with magnetic beads coated with a monoclonal anti-FITC antibody (Miltenyi Biotec, Germany). The beads were loaded on columns in a magnetic field, and positive cells were finally eluted in culture medium.
Magnetic cell sorting (MACS)-selected cells were further purified by flow cytometry. Briefly, cells were incubated with the same FITC-conjugated anti-CD20 monoclonal antibody and selected by using a flow cell sorter (FACStar Plus; Becton Dickinson, San Jose, Calif.) with a 100 mW air-cooled argon laser (Spinnaker 1161; Spectra Physics, Mountain View, Calif.) and the CellQuest software (Becton Dickinson) for Macintosh Facstation. CD20-positive cells (1.2 × 106 cells) (purity, >90%) were stimulated with IL-1β, and the ROIs were measured, as described.
Statistical analysis.
Data are presented as means ± standard deviations (SDs). Differences between measures were assessed by Student’s t tests for unpaired data. A P value of less than 0.05 was considered significant.
RESULTS
Expression of 5-LOX and FLAP in cell lines.
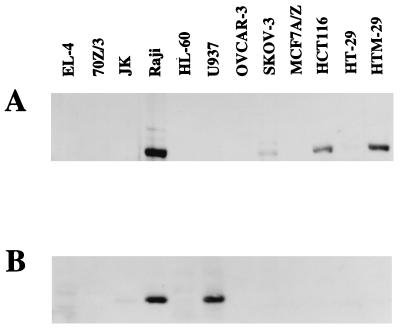
The expression of 5-LOX and FLAP proteins was analyzed by immunoblotting in various cell lines from different lineages, as follows (Fig. 1): monocytic cells (HL-60 and U937), lymphoid B and T cells (Jurkat, EL-4, 70Z/3, and Raji), ovarian carcinoma cells (OVCAR-3 and SKOV-3), breast carcinoma cells (MCF7 A/Z), and colon carcinoma cells (HCT-116, HT-29, and HTM-29) (Fig. 1A). Using an antibody directed against the human 5-LOX, the protein was detected in Raji, SKOV-3, HCT-116, HT-29 (faint band), and HTM-29 cells but not in the murine cell lines. Moreover, we could not detect any 5-LOX expression in the THP-1 monocytic cells (data not shown). 5-LOX mRNA expression was also detected by reverse transcription (RT)-PCR in the murine lymphoid cell lines 70Z/3 and EL-4 (reference 7 and data not shown). In other words, we could not observe any 5-LOX expression, either by immunoblotting or by RT-PCR, in monocytic cells or in breast or ovarian carcinoma cells.
FIG. 1.
5-LOX and FLAP expression in cell lines. Protein extracts from various cell lines were prepared and analyzed for 5-LOX (A) and FLAP (B) expression by immunoblots revealed with specific antibodies. The 5-LOX antibody was directed against the human protein and could not detect the murine enzyme in EL-4 and 70Z/3 cells. The FLAP antibody could recognize both the human and the murine proteins, and the murine protein gave a faint and more slowly migrating band in EL-4 and 70Z/3 cells, which is visible after longer exposures of the gel.
Similarly, the FLAP protein was detected by Western blotting in the four analyzed lymphoid cell lines (Fig. 1). Its expression was high in Raji cells and considerably lower in Jurkat cells. A faint and more slowly migrating specific band was also detected with the human antibody in the murine cell lines EL-4 and 70Z/3. The expression of FLAP was not observed in any of the adenocarcinoma cell lines. Among monocytic cell lines, FLAP was expressed in U937 and THP-1 cells but not in HL-60 cells (Fig. 1 and data not shown). We could thus conclude that only the lymphoid cells expressed simultaneously 5-LOX and FLAP proteins and could therefore demonstrate 5-LOX enzymatic activity.
ROI production following IL-1β stimulation.
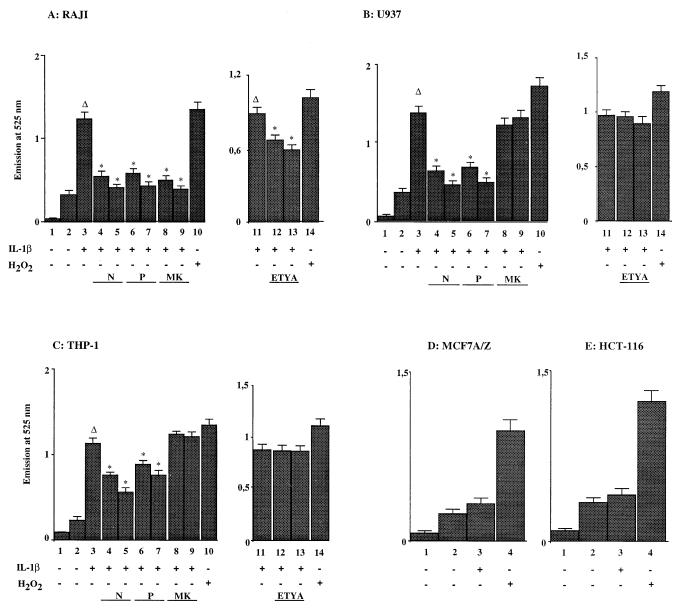
We previously reported that IL-1β stimulation led to the generation of ROIs in lymphoid cells but not in epithelial transformed cells (6). In order to determine whether ROI production was due to 5-LOX activity, U937, Raji, MCF7 A/Z, and HCT-116 cells were stimulated with IL-1β (50 U/ml) for 15 min and analyzed with a DFCH probe for ROI detection (Fig. 2). Under these conditions, IL-1β treatment of Raji cells led to the production of ROIs (Fig. 2A; compare columns 2 and 3). As expected, preincubation of Raji cells with the antioxidant NAC or PDTC abolished ROI accumulation (columns 4 to 7). Interestingly, preincubation of Raji cells prior to IL-1β stimulation with MK886, a specific inhibitor of FLAP (40) (columns 8 and 9), or with the 5-LOX inhibitor ETYA (columns 12 and 13) completely abolished or markedly decreased ROI production, confirming that 5-LOX activity was required for IL-1β-induced intracellular oxidative stress in lymphoid cells. As a positive control, we measured ROI production in H2O2-treated cells (250 μM) (columns 10 and 14); MK886 did not influence the ROI production after H2O2 treatment, indicating that this inhibitor exerts a specific action (data not shown).
FIG. 2.
Production of ROIs following IL-1β stimulation. Formation of ROIs was measured using a DFCH probe in unstimulated and IL-1β-stimulated cells. Panels A, B, and C show the relative fluorescence emission at 525 nm of the DFCH probe in Raji, U937, and THP-1 cells. Columns 1, background (no DFCH probe); columns 2, unstimulated cells; columns 3, stimulation with IL-1β (50 U/ml); columns 4, as in columns 3 plus NAC (10 mM); columns 5, as in columns 3 plus NAC (20 mM); columns 6, as in columns 3 plus PDTC (60 μM); columns 7, as in columns 3 plus PDTC (100 μM); columns 8, as in columns 3 plus MK886 (0.5 μM); columns 9, as in columns 3 plus MK886 (1 μM); columns 11, stimulation with IL-1β (50 U/ml); columns 12, as in columns 11 plus ETYA (35 μM); columns 13, as in columns 11 plus ETYA (65 μM); columns 10 and 14, stimulation with H2O2 (250 μM). Panels D and E show the relative fluorescence emission at 525 nm of the DFCH probe in MCF7 A/Z and HCT-116 cells. Columns 1, background (no DFCH probe); columns 2, unstimulated cells; columns 3, stimulation with IL-1β (50 U/ml); columns 4, stimulation with H2O2 (250 μM). Asterisks indicate that the values were statistically different from the reference values (Δ).
Stimulation of U937 or THP-1 monocytic cells with IL-1β also generated ROIs (Fig. 2B and C; compare columns 2 and 3). Again, the production of ROIs was completely or partially abolished by preincubation of these cells with the antioxidant NAC or PDTC (columns 4 to 7). Similar data were obtained with HL60 cells (data not shown). However, contrasting with the observation made with Raji cells, MK886 and ETYA failed to block ROI induction in monocytic cells (columns 8, 9, 12, and 13), demonstrating that 5-LOX activity was not responsible for this production.
Stimulation of HCT116 or MCF7 A/Z cells with IL-1β did not generate any significant ROI production (Fig. 2D and E; compare columns 2, without IL-1β, and columns 3, in the presence of IL-1β). The last observation confirmed our previously reported data obtained with ovarian carcinoma OVCAR-3 cells (6), indicating that IL-1β did not generate any oxidative stress in epithelial transformed cells.
Induction of NF-κB following IL-1β stimulation.
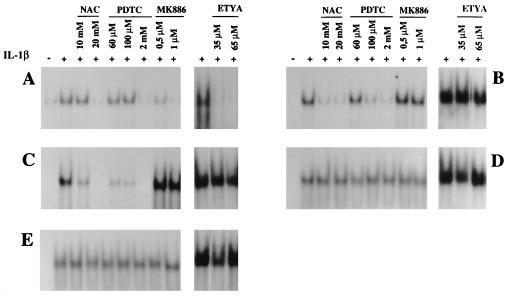
Several reports stated that NF-κB induction following a number of stimuli depended on ROI production (50–52). However, we reported that IL-1β induced NF-κB in adenocarcinoma cells independently of any cellular oxidative stress (6), an observation confirmed by the experiments described in Fig. 2. We therefore further evaluated the role of 5-LOX activity in NF-κB activation after IL-1β stimulation of lymphoid or monocytic cells. Stimulation of Raji, U937, or THP-1 cells with IL-1β induced nuclear NF-κB DNA-binding activity as demonstrated by EMSA (Fig. 3A, B, and C). Pretreatment with antioxidant NAC or PDTC completely abolished NF-κB activation in the three cell lines. However, pretreatment with the FLAP inhibitor MK-886 or the 5-LOX inhibitor ETYA inhibited IL-1β-dependent NF-κB activation in Raji cells (Fig. 3A) while it did not affect this induction in U937 or THP-1 cells (Fig. 3B and C). Similarly, antioxidants, MK-866, or ETYA failed to abolish NF-κB activation by IL-1β in MCF7 A/Z and HCT-116 cells (Fig. 3D and E), confirming our previous observation in OVCAR-3 and SKOV-3 epithelial cells (6, 7). These data thus indicate that 5-LOX activity plays an important role in ROI production and NF-κB activation in lymphoid cells but not in monocytic or epithelial cells.
FIG. 3.
ROI or 5-LOX inhibitors blocked NF-κB activation by IL-1β in a cell-specific manner. Nuclear extracts were prepared from Raji (A), U937 (B), THP-1 (C), HCT116 (D), or MCF7 A/Z (E) cells, either untreated or stimulated with IL-1β (50 U/ml). The same cells were preincubated prior to IL-1β stimulation with increasing concentrations of NAC, PDTC, MK886, or ETYA, as indicated in the figure. These extracts were analyzed by EMSA for binding to a specific κB probe.
Reconstitution of the 5-LOX/FLAP pathway in adenocarcinoma cells.
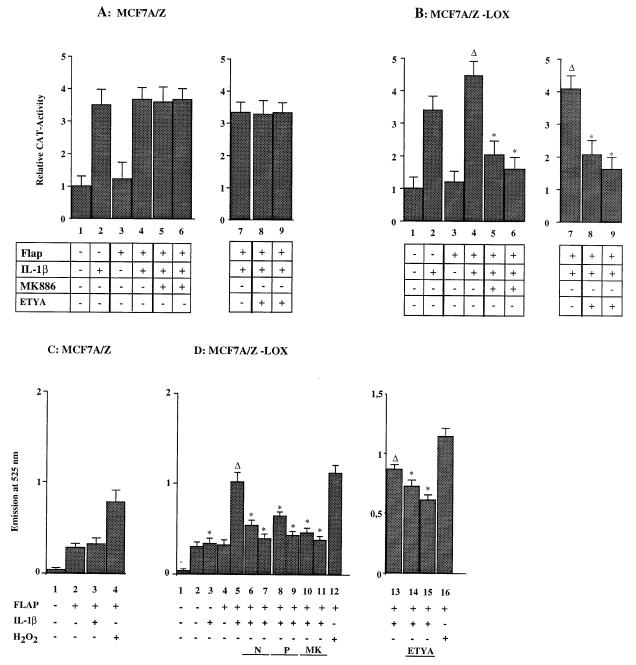
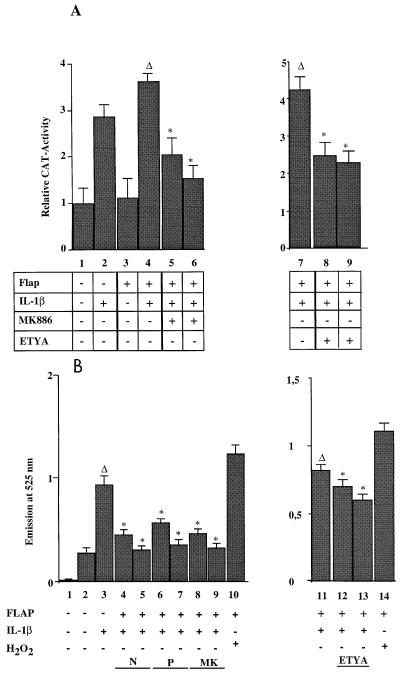
In order to confirm the data obtained with EMSAs, MCF7 A/Z cells were transfected with a reporter plasmid containing a CAT gene driven by the two κB sites from the HIV LTR (HIV-κB-CAT) and then stimulated with IL-1β. Such a treatment induced CAT activity three- to fourfold over the basal activity observed in untreated control cells (Fig. 4A). Cotransfection of the FLAP expression vector did not allow any further induction of CAT activity following IL-1β stimulation (columns 3 and 4). Moreover, the 5-LOX inhibitors MK-886 and ETYA failed to block IL-1β induction of NF-κB-dependent transcription in either FLAP-expressing (Fig. 4A, columns 5, 6, 8, and 9) or nonexpressing cells (data not shown).
FIG. 4.
Transfection of 5-LOX and FLAP expression vectors restored ROI production and ROI-dependent NF-κB activity in MCF7 A/Z cells. (A) MCF A/Z cells were transfected with the HIV-κB-CAT reporter plasmid alone or together with a FLAP expression vector, and CAT activities were measured in unstimulated cells or in cells stimulated with IL-1β for 6 h (50 U/ml), as indicated in the figure. The IL-1β treatment was performed in the absence or in the presence of either the FLAP inhibitor MK886 at 0.5 μM (column 5) and 1 μM (column 6) or the 5-LOX inhibitor ETYA at 35 μM (column 8) and 65 μM (column 9). Each column represents the mean of three independent experiments (± SD). We did not observe any statistically significant differences between cells stimulated by IL-1β in the presence or absence of the inhibitors. The total amount of transfected DNA was kept constant throughout the experiment by addition of appropriate amounts of the expression vector without insert. (B) MCF A/Z cells stably transfected with the 5-LOX expression vector (MCF A/Z-LOX) were transfected with the HIV-κB-CAT reporter plasmid alone or together with a FLAP expression vector, and CAT activities were measured in unstimulated cells or in cells stimulated with IL-1β as described for panel A. Asterisks indicate that values were statistically different from the reference values (Δ). (C) Formation of ROIs was measured by using DFCH in MCF7 A/Z cells transiently transfected with the FLAP expression vector. Column 1, background (no DFCH probe); column 2, unstimulated cells; column 3, stimulation with IL-1β (50 U/ml); column 4, stimulation with H2O2 (250 μM). (D) ROI production in MCF7 A/Z-LOX cells either unmodified or transiently transfected with the FLAP expression vector. Column 1, background (no DFCH probe); column 2, unstimulated cells; column 3, stimulation with IL-1β (50 U/ml); column 4, FLAP-transfected unstimulated cells; column 5, FLAP-transfected cells stimulated with IL-1β (50 U/ml); column 6, as in column 5 plus NAC (10 mM); column 7, as in column 5 plus NAC (20 mM); column 8, as in column 5 plus PDTC (60 μM); column 9, as in column 5 plus PDTC (100 μM); column 10, as in column 5 plus MK886 (0.5 μM); column 11, as in column 5 plus MK886 (1 μM); column 13, stimulation with IL-1β (50 U/ml); column 14, as in column 13 plus ETYA (35 μM); column 15, as in column 13 plus ETYA (65 μM); columns 12 and 16, stimulation with H2O2 (250 μM). Asterisks indicate that values were statistically different from the reference values (Δ).
The same experiment was then performed in MCF7 A/Z cells stably transfected with a 5-LOX expression vector (MCF7 A/Z-LOX). In these cells, IL-1β induced NF-κB transcriptional activity and this activity was not modified in the presence of MK886 (Fig. 4B and data not shown). Transient transfection of MCF7 A/Z-LOX cells with the FLAP expression vector led to a small increase of the IL-1β-induced CAT activity (Fig. 4B, column 4). In the presence of FLAP, treatment of MCF7 A/Z-LOX cells with MK-886 or ETYA completely abolished IL-1β-dependent transcriptional activity (columns 5, 6, 8, and 9).
Consistently with these CAT assays, transient transfections of MCF7 A/Z cells with the FLAP expression vector did not allow any generation of ROIs, even after IL-1β stimulation (Fig. 4C; compare columns 2 and 3). However, transient transfection of the FLAP expression vector in MCF7 A/Z-LOX cells led to a marked increase in ROI production after IL-1β treatment (Fig. 4D; compare columns 4 and 5) while stable expression of 5-LOX alone was not sufficient for such an effect (columns 2 and 3). As expected, NAC, PDTC, MK886, and ETYA reduced ROI production in MCF7 A/Z cells transfected stably with the 5-LOX expression vector and transiently with the FLAP expression vector (columns 6 to 11, 14, and 15).
In HCT-116 cells, which express the 5-LOX enzyme but not the FLAP protein, IL-1β stimulated threefold the NF-κB-dependent CAT activity (Fig. 5A) and this stimulation was not blocked by MK886 (data not shown). Again, after cotransfection of the FLAP expression vector, we observed a small increase in the transcription induced by IL-1β (column 4), an effect which was inhibited by preincubation of the cells with MK886 (columns 5 and 6) or ETYA (columns 8 and 9). Similarly, transient transfection of the FLAP expression vector in HCT-116 cells was sufficient to allow the production of ROIs after IL-1β stimulation (Fig. 5B), a production which was inhibited by preincubation with NAC, PDTC, MK886, or ETYA.
FIG. 5.
Transfection of the FLAP expression vector restored ROI production and ROI-dependent NF-κB activity in HCT-116 cells. (A) HCT-116 cells were transfected with the HIV-κB-CAT reporter plasmid alone or together with a FLAP expression vector, and CAT activities were measured in unstimulated cells or in cells stimulated with IL-1β for 6 h (50 U/ml), as indicated in the figure. The IL-1β treatment was performed in the absence or in the presence of either the FLAP inhibitor MK886 at 0.5 μM (column 5) and 1 μM (column 6) or the 5-LOX inhibitor ETYA at 35 μM (column 8) and 65 μM (column 9). Each column represents the mean of three independent experiments (± SD). Asterisks indicate that values were statistically different from the reference values (Δ). (B) Formation of ROIs in HCT-116 cells transiently transfected with the FLAP expression vector. Column 1, background (no DFCH probe); column 2, unstimulated, FLAP transfected cells; column 3, stimulation with IL-1β (50 U/ml); column 4, as in column 3 plus NAC (10 mM); column 5, as in column 3 plus NAC (20 mM); column 6, as in column 3 plus PDTC (60 μM); column 7, as in column 3 plus PDTC (100 μM); column 8, as in column 3 plus MK886 (0.5 μM); column 9, as in column 3 plus MK886 (1 μM); column 11, stimulation with IL-1β (50 U/ml); column 12, as in column 11 plus ETYA (35 μM); column 13, as in column 11 plus ETYA (65 μM); columns 10 and 14, stimulation with H2O2 (250 μM). Asterisks indicate that values are statistically different from the reference values (Δ).
We can thus conclude that coexpression of 5-LOX and FLAP is required to restore the 5-LOX-dependent pathway leading to production of ROIs and consequent NF-κB activation.
NADPH oxidase is required for ROI production in IL-1β-treated monocytic cells.
Monocytic cells such as U937 or THP-1 cells produce ROIs in response to IL-1β stimulation but do not express 5-LOX. They must therefore rely on another source for ROI production, and this source was deemed most likely to be NADPH oxidase.
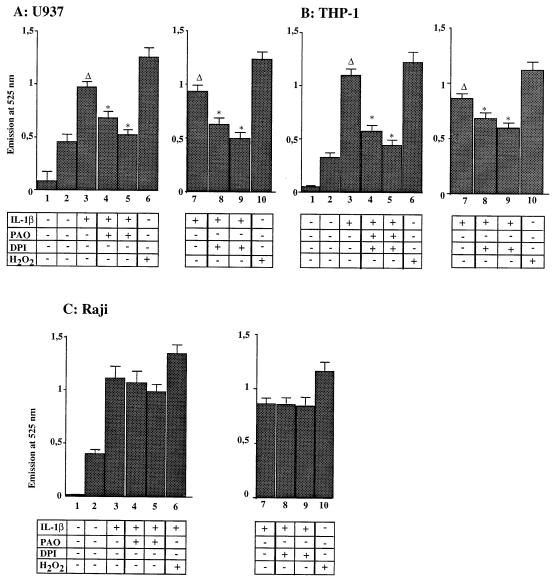
We first investigated whether NADPH oxidase inhibitors could block ROI production in IL-1β-stimulated monocytic cells. As already shown in Fig. 2, IL-1β treatment induced ROIs in THP-1 and U937 cells (Fig. 6A and B; compare columns 2 and 3). Preincubation of these cells with DPI or PAO inhibited in a dose-dependent manner IL-1β-induced ROI production (Fig. 6A and B, columns 4, 5, 8, and 9). Interestingly, preincubation of Raji cells with the same NADPH oxidase inhibitors did not block ROI production (Fig. 6C).
FIG. 6.
NADPH oxidase inhibitors blocked ROI production induced by IL-1β stimulation in monocytic cells. Formation of ROIs was measured by using a DFCH probe in U937 (A), THP-1 (B), and Raji (C) cells after IL-1β stimulation, with and without preincubation in the presence of the NADPH oxidase inhibitors DPI and PAO. Columns 1, background (no DFCH probe); columns 2, unstimulated cells; columns 3, stimulation with IL-1β (50 U/ml); columns 4, as in columns 3 plus PAO (30 μg/ml); columns 5, as in columns 3 plus PAO (60 μg/ml); columns 7, stimulation with IL-1β (50 U/ml); columns 8, as in columns 7 plus DPI (10 μM); columns 9, as in columns 7 plus DPI (30 μM); columns 6 and 10, stimulation with H2O2 (250 μM). Asterisks indicate that values are statistically different from the reference values (Δ).
To investigate whether NADPH oxidase was involved in NF-κB activation by IL-1β, nuclear extracts were prepared from cells preincubated with the same inhibitor, DPI or PAO, prior to IL-1β stimulation. These inhibitors did not modify NF-κB activation induced by IL-1β in Raji, MCF7 A/Z, or HCT-116 cells, as demonstrated on EMSAs (Fig. 7A, D, and E). However, they abolished in a dose-dependent manner the induction of NF-κB in U937 and THP-1 monocytic cells (Fig. 7B and C), indicating that NADPH oxidase activity was required for the IL-1β-dependent signaling pathway leading to IκBα degradation in these cells.
FIG. 7.
NADPH oxidase inhibitors blocked NF-κB activation by IL-1β in monocytic cells. Nuclear extracts were prepared from Raji (A), U937 (B), THP-1 (C), HCT116 (D), or MCF7 A/Z (E) cells, either untreated or stimulated with IL-1β (50 U/ml). The same cells were preincubated for 1 h prior to IL-1β stimulation with increasing concentrations of DPI or PAO, as indicated in the figure. These extracts were analyzed by EMSA for binding to a specific κB probe.
Rac and Cdc42 are required for ROI production and NF-κB activation in IL-1β-treated monocytic cells.
Rho, Rac1, and Cdc42, all members of the Ras superfamily of GTPases, have been noted to play a role in several transduction processes, including the JNK/SAPK and MAPK pathways (60). More recently, it has been reported that Rho, Rac1, and Cdc42 are involved in NF-κB induction by TNF-α or bradykinin (45, 46). The NADPH oxidase component p67phox associates with Cdc42/Rac, and Rac1 is required for the activation of NADPH oxidase (1, 5, 21).
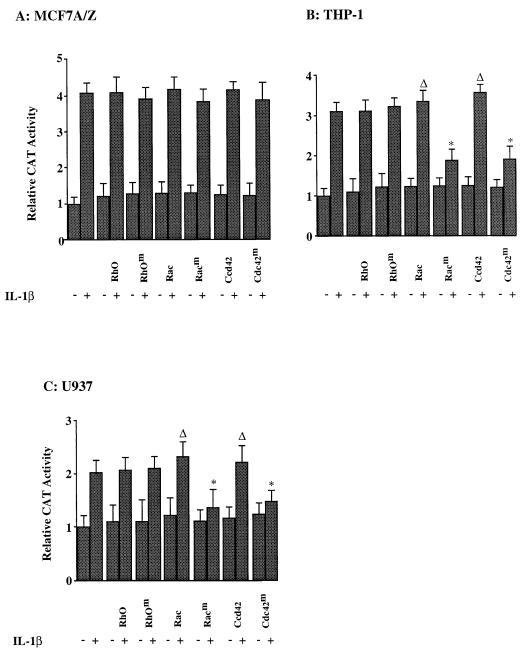
Therefore, we investigated whether these GTPases are involved in IL-1β-induced NF-κB activation in adenocarcinoma or monocytic cells. MCF7 A/Z cells were cotransfected with the HIV-κB-CAT reporter plasmid together with expression vectors coding for wild-type or dominant-negative RhoA, Rac1, or Cdc42 (Fig. 8A). Again, IL-1β stimulation induced three- to four-fold the basal CAT activity. This transcriptional activation was not modified in the presence of wild-type or mutant Rho, Rac1, or Cdc42 proteins. Similarly, treatment of MCF7 A/Z cells with TNF-α or arachidonic acid also activated NF-κB-dependent transcription of the CAT reporter gene. Again, the expression of wild-type or mutant GTPases did not modify this induction (data not shown). We thus concluded that RhoA, Rac1, and Cdc42 are not required for NF-κB activation by IL-1β, TNF-α, or arachidonic acid in MCF7 A/Z cells.
FIG. 8.
GTPases are required for NF-κB transcriptional activity in monocytic cells. MCF7 A/Z (A), THP-1 (B), or U937 (C) cells were transfected with the HIV-κB-CAT reporter plasmid alone or together with expression vectors for wild-type or dominant-negative RhoA, Rac1, and Cdc42. Cells were either untreated (−) or stimulated with IL-1β (+) for 6 h, and CAT activities were measured. Each column represents the mean of three independent experiments (± SD). Asterisks indicate that values are statistically different from the reference values (Δ).
In monocytic cells (THP-1 and U937), however, while transfection of expression vectors for wild-type RhoA, Rac1, or Cdc42 did not modify transcription of an NF-κB-dependent reporter plasmid, the expression of the Rac1 and Cdc42 dominant-negative mutants almost completely inhibited IL-1β-induced NF-κB-dependent gene transcription (Fig. 8B and C).
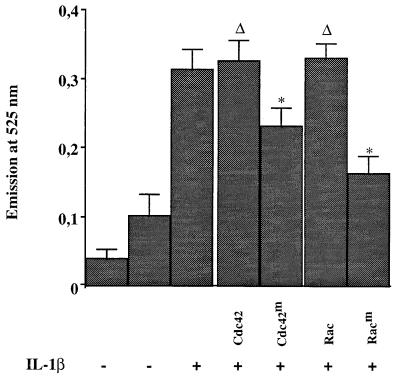
In order to demonstrate that Cdc42 and Rac1 dominant-negative mutants could block ROI production in monocytic cells, U937 cells were transiently transfected with expression vectors coding for these mutants or the wild-type enzymes. Since the transfection efficiency was very low and did not allow the detection of any difference in ROI production, these cells were then cotransfected with the mentioned plasmids together with an expression vector coding for the CD20 antigen. Transfected cells were purified through two rounds of positive selection with an anti-CD20 monoclonal antibody, first by MACS and then by flow cytometry. Purified transfected cells (1.2 × 106 cells) were then treated with IL-1β, and ROI production was measured as described above. Under these conditions, IL-1β stimulation generated ROIs (Fig. 9). This production of ROIs was not modified by the expression of wild-type Cdc42 or Rac1 enzyme, while it was significantly reduced by the two dominant-negative mutants, confirming their implied roles in induced ROI production in monocytic cells.
FIG. 9.
GTPases are required for ROI production in response to IL-1β in monocytic cells. U937 cells were transfected with the CD20 expression vector and with plasmids coding for wild-type (Cdc42 and Rac) or dominant-negative (Cdc42m and Racm) Rac1 and Cdc42. CD20-expressing transfected cells were positively selected with an anti-CD20 monoclonal antibody through MACS and flow cytometry cell sorting. Selected cells were either untreated (−) or stimulated with IL-1β (+) for 15 min, and formation of ROIs was measured by using a DFCH probe. Asterisks indicate that values are statistically different from the reference values (Δ).
DISCUSSION
The signaling pathways leading to NF-κB activation following cellular stimulation with proinflammatory cytokines have been the subject of many investigations but remain a matter of discussion. The recent discovery of IκBα kinases (IKK-α, IKK-β, and IKK-γ) and their activation by the NIK kinase led to the hypothesis that TNF-α or IL-1β receptors are connected to IκBα through TRAF adapter proteins, NIK and IKKs (20, 33, 39, 42, 56, 66). However, such a model did not incorporate the results of previous studies indicating the role of other signaling intermediates such as ROIs, ceramide, or Rho proteins and left unidentified other members of the large signaling complex. These discrepancies could be explained by cell type specificities. Indeed, most of the studies reported so far investigated a single cell line, and the models derived from such experiments might not be confirmed in different settings.
We previously reported that ROIs and the PKC λ/ι isoform play a cell-specific role in NF-κB activation by IL-1β or TNF-α (6–8). In this study, we explored the sources of ROIs in different cell types and their roles in NF-κB activation by IL-1β. At least three different pathways that lead to induction of nuclear NF-κB DNA-binding activity and transactivation of a reporter gene after cellular treatment with IL-1β can be identified (Fig. 10). Lymphoid cells, such as Raji or 70Z/3 cells, express 5-LOX and its activating protein FLAP, and 5-LOX enzymatic activity is required for ROI production and NF-κB activation following stimulation by IL-1β. In these cells, the ROIs are essential for IκBα degradation and NF-κB activation (6) but it is not known whether ROIs can directly or indirectly activate the IKK kinases or whether other IκBα kinases are involved.
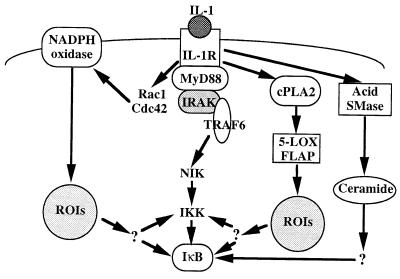
FIG. 10.
Signaling pathways for NF-κB activation by IL-1β. Following the interaction of IL-1 with the type 1 IL-1 receptor, the NIK-IKK pathway can be activated through the MyD88, IRAK, and TRAF6 signaling proteins. Alternatively, Rac1 and Cdc42 can activate the NADPH oxidase complex and induce NF-κB activity through the production of ROIs. Other pathways involve, in lymphoid cells, the cytoplasmic phospholipase A2, the 5-LOX, and FLAP complex and the production of ROIs or, in epithelial cells, the acid sphingomyelinase (SMase) and the production of ceramide. CPLA2, cytosolic phospholipase A2.
Epithelial cells do not express the 5-LOX/FLAP enzymatic complex and do not produce ROIs in response to IL-1β or TNF-α. Reconstitution of the 5-LOX/FLAP system in these cells showed that, despite high catalase activity (7), this enzymatic activity was sufficient to restore ROI production and ROI-dependent NF-κB activation. In other words, once the 5-LOX pathway is functional, it becomes predominant. These data, as well as those obtained with monocytic and lymphoid cells, suggest that the ROIs might activate the signalsome. In the absence of 5-LOX, as we had previously reported, an alternative pathway which involves the activation of acid sphingomyelinase and the production of ceramide could be essential for NF-κB activation by IL-1β in epithelial cells (7), but such an hypothesis remains to be confirmed by genetic studies.
Finally, monocytic cells (THP-1 or U937 cells) do not express the 5-LOX enzyme but produce ROIs in response to IL-1β or TNF-α. In these cells, ROIs are generated by the NADPH oxidase and are required for NF-κB activation by proinflammatory cytokines. Moreover, the induction of NF-κB by IL-1β, TNF-α, or arachidonic acid in monocytic cells also requires the activity of small GTPases. We concluded that Rho and Rac proteins stimulated ROI production through NADPH oxidase activation in macrophages/monocytes. As for lymphoid cells, we do not know how ROIs are connected to IκBα kinases.
These data thus disclosed two distinct, cell-specific sources of ROIs, which are each required for NF-κB activation by proinflammatory cytokines, and a third pathway leading to NF-κB activation independently of ROI production (Fig. 10). Such cell specificity might be explained by the association of distinct proteins with the IL-1 receptor. Indeed, IL-1R associates with TRAF6, MyD88, IRAK, and IRAK-2 proteins, which are all required for NF-κB activation (14–16, 31, 44, 61). Moreover, other proteins might also associate with this receptor. It is possible that the expression of each of these receptor-associated adapter proteins or kinases is cell specific and therefore initiates distinct transduction pathways. Indeed, it has been shown that, although TRAF6, MyD88, IRAK, and IRAK-2 mRNAs could be detected in most cell types and organs, their levels of expression vary considerably (15, 16, 28, 44). It would be most interesting to determine whether different proteins are associated with the IL-1 type I receptor in lymphoid, monocytic, or epithelial cells and play specific, cell type-related, signaling roles.
These signaling pathways leading to NF-κB activation might be related to distinct IL-1β biological activities in different cell types. The nature of the activated NF-κB complexes might be different for each pathway and lead to the transcription of distinct target genes. Each pathway is also controlled differently. For instance, antioxidant cellular defenses could regulate pathways involving ROI production while distinct phosphatases could dephosphorylate kinase substrates and specifically regulate signal transduction. Finally, the activation of distinct pathways leads to the concomitant activation of NF-κB and other specific transcription factors. In a given cellular context, the activation of a specific set of transcription factors, including NF-κB, certainly regulates the expression of distinct target genes and thus allows an appropriate cellular response to the applied stimulus. Further experiments are required in order to identify NF-κB target genes specifically induced by IL-1β in lymphoid, monocytic, or epithelial cells and to determine cell-specific mechanisms of regulation for each of them.
It has been shown that IL-1β can exert pro- or anti-apoptotic activities in HeLa and L929 cells (27). Moreover, high IL-1β levels have been measured in the sera of patients with ovarian adenocarcinomas (67). It is therefore crucial to better understand the role of IL-1β in normal and transformed epithelial cells and to identify the biochemical pathways mediating its biological activities.
ACKNOWLEDGMENTS
We thank J. Evans (Merck Frosst Centre for Therapeutic Research, Quebec, Canada) for the MK-886 inhibitor, for the 5-LOX and FLAP expression vectors, and for the FLAP and 5-LOX antibodies, L. Cross (National Institutes of Health, Bethesda, Md.) for the RhoA, Rac1, and Cdc42 constructs, and Jim Koh (Laboratory of Molecular Oncology, Massachusetts General Hospital and Harvard Medical School) for the CD20 expression plasmid. We are most thankful to J. Gielen for his support and critical comments about the manuscript, to O. Giet for his help with the MACS selection, and to F. Bureau for statistical analysis.
M.-P.M. and V.B. are research associates and J.P. is a research director at the National Fund for Scientific Research (Belgium). G.B. is a fellow from the Biotechnology Programme, European Commission. This work has been supported by grants from the National Fund for Scientific Research (FNRS, Belgium), FNRS-Télévie (Belgium), the Centre Anti Cancéreux (University of Liège, Liège, Belgium), and the EEC Biomed II program (grant BMH4-CT97-2387).
REFERENCES
- 1.Abo A, Boyhan A, West I, Thrasher A J, Segal A W. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1 and cytochrome b-245. J Biol Chem. 1992;267:16767–16770. [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P J, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Finco T S, Nantermet P V, Baldwin A S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokoch G M. Regulation of the phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 1995;5:109–113. doi: 10.1016/s0962-8924(00)88960-6. [DOI] [PubMed] [Google Scholar]
- 6.Bonizzi G, Dejardin E, Piret B, Piette J, Merville M-P, Bours V. IL-1β induces NF-κB in epithelial cells independently of the production of reactive oxygen intermediates. Eur J Biochem. 1996;242:544–549. doi: 10.1111/j.1432-1033.1996.0544r.x. [DOI] [PubMed] [Google Scholar]
- 7.Bonizzi G, Piette J, Merville M-P, Bours V. Distinct signal transduction pathways mediate nuclear factor-κB induction by IL-1β in epithelial and lymphoid cells. J Immunol. 1997;159:5264–5272. [PubMed] [Google Scholar]
- 8.Bonizzi, G., S. Schoonbroodt, J. Piette, M.-P. Merville, and V. Bours. Implication of the protein kinase C λ/ι isoform in NF-κB activation by IL-1β or TNF-α: cell type specificities. Biochem. Pharmacol., in press. [DOI] [PubMed]
- 9.Bours V, Burd P R, Brown K, Villalobos J, Park S, Ryseck R-P, Bravo R, Kelly K, Siebenlist U. A novel mitogen-inducible gene product related to p50/p105-NF-κB participates in transactivation through a κB site. Mol Cell Biol. 1992;12:685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowie A, Moynagh P N, O’Neill L A J. Lipid peroxidation is involved in the activation of NF-κB by tumor necrosis factor but not interleukin-1 in the human endothelial cell line ECV304. J Biol Chem. 1997;272:25941–25950. doi: 10.1074/jbc.272.41.25941. [DOI] [PubMed] [Google Scholar]
- 11.Brennan P, O’Neill L A J. Effects of oxidants and antioxidants on nuclear factor κB activation in three different cell lines: evidence against a universal hypothesis involving oxygen radicals. Biochim Biophys Acta. 1995;1260:167–175. doi: 10.1016/0167-4781(94)00186-7. [DOI] [PubMed] [Google Scholar]
- 12.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 13.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-κB and its inhibitor, IκB-α. Proc Natl Acad Sci USA. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer J-L, Di Marco F, French L, Tschopp J. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 15.Cao Z, Henzel W J, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 16.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 18.Coso O A, Chiarello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1148. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Meco M T, Berra E, Municio M M, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera M T, Alcami J, Payà C V, Arenzana-Seisdedos F, Virelizier J-L, Moscat J. A dominant negative protein kinase C ζ subspecies blocks NF-κB activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcriptional factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 21.Diekmann D, Abo A, Johnston C, Segal A W, Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 22.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 23.Dixon R A F, Diehl R E, Opas E, Rands E, Vickers P J, Evans J F, Gillard J W, Miller D K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez I, Sanz L, Arenzana-Seisdedos F, Diaz-Meco M T, Virelizier J-L, Moscat J. Inhibition of protein kinase C ζ subspecies blocks the activation of an NF-κB-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993;13:1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finco T S, Baldwin A S J. κB site-dependent induction of gene expression by diverse inducers of nuclear factor κB requires Raf-1. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 26.Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 27.Friedlander R M, Gagliardini V, Rotello R J, Yuan J. Functional role of interleukin 1β (IL-1β) in IL-1β-converting enzyme-mediated apoptosis. J Exp Med. 1996;184:717–724. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardiman G, Rock F L, Balasubramanian S, Kastelein R A, Bazan J F. Molecular characterization and modular analysis of human MyD88. Oncogene. 1996;13:2467–2475. [PubMed] [Google Scholar]
- 29.Harrison K A, Murphy R C. Isoleukotrienes are biologically active free radical products of lipid peroxidation. J Biol Chem. 1995;270:17273–17278. doi: 10.1074/jbc.270.29.17273. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Gao X, Cao Z. Recruitment of IRAK to the interleukin 1 receptor complex requires interleukin 1 receptor accessory protein. Proc Natl Acad Sci USA. 1997;94:12829–12832. doi: 10.1073/pnas.94.24.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanakaraj P, Schafer P H, Cavender D E, Wu Y, Ngo K, Grealish P F, Wadsworth S A, Peterson P A, Siekierka J J, Harris C A, Fung-Leung W-P. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J Exp Med. 1998;187:2073–2079. doi: 10.1084/jem.187.12.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolesnick R, Golde D W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 33.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Felts K A, Parry G C N, Armacost L M, Cobb R R. Inhibition of 5-lipoxygenase blocks IL-1β-induced vascular adhesion molecule-1 gene expression in human endothelial cells. J Immunol. 1997;158:3401–3407. [PubMed] [Google Scholar]
- 35.Legrand-Poels S, Vaira D, Pincemail J, Van de Vorst A, Piette J. Activation of human immunodeficiency virus type 1 by an oxidative stress. AIDS Res Hum Retroviruses. 1990;6:1389–1397. doi: 10.1089/aid.1990.6.1389. [DOI] [PubMed] [Google Scholar]
- 36.Lewis R A, Austen K F, Soberman R J. Leukotrienes and other products of the 5-lipoxygenase pathway. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 37.Los M, Schenk H, Hexel K, Baeuerle P A, Dröge W, Schulze-Osthoff K. IL-2 gene expression and NF-κB activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14:3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machleidt T, Wiegmann K, Henkel T, Schütze S, Baeuerle P, Krönke M. Sphingomyelinase activates proteolytic IκB-α degradation in a cell-free system. J Biol Chem. 1994;269:13760–13765. [PubMed] [Google Scholar]
- 39.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 40.Mancini J A, Prasit P, Coppolino M G, Charleson P, Leger S, Evans J F, Gillard J W, Vickers P J. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Mol Pharmacol. 1991;41:267–272. [PubMed] [Google Scholar]
- 41.Maniatis T. Catalysis by a multiprotein IκB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- 42.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennet B L, Li J W, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–865. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 43.Miller D K, Gillard J W, Vickers P J, Sadowski S, Léveillé C, Mancini J A, Charleson P, Dixon R A F, Ford-Hutchinson H A W, Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- 44.Muzio M, Ni J, Feng P, Dixit V M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 45.Pan Z K, Ye R D, Christiansen S C, Jagels M A, Bokoch G M, Zuraw B L. Role of the Rho GTPase in bradykinin-stimulated nuclear-κB activation and IL-1β gene expression in cultured epithelial cells. J Immunol. 1998;160:3038–3045. [PubMed] [Google Scholar]
- 46.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal J C. Activation of the nuclear factor-κB by Rho, CDC42 and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 47.Régnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 48.Reid G K, Kargman S, Vickers P J, Mancini J A, Léveillé C, Ethier D, Miller D K, Gillard J W, Dixon R A F, Evans J F. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase and cellular leukotriene synthesis. J Biol Chem. 1990;265:19818–19823. [PubMed] [Google Scholar]
- 49.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 50.Schreck R, Albermann K, Baeuerle P A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–227. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 51.Schreck R, Meier B, Mannel D N, Droge W, Baeuerle P A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreck R, Rieber P, Baeuerle P A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 54.Segal A W, Abo A. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem Sci. 1993;18:43–47. doi: 10.1016/0968-0004(93)90051-n. [DOI] [PubMed] [Google Scholar]
- 55.Siebenlist U, Franzoso G, Brown K. Structure, regulation, and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 56.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sulciner D J, Irani K, Yu Z-X, Ferrans V J, Goldschmidt-Clermont P, Finkel T. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traenckner E B-M, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Traenckner E B-M, Wilk S, Baeuerle P A. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of IκB-α that is still bound to NF-κB. EMBO J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Elst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 61.Wesche H, Henzel W J, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 62.Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin M U. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor associated kinase (IRAK) and stress-activated protein kinases (SAP kinases) J Biol Chem. 1997;272:7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- 63.Woods J W, Evans J F, Ethier D, Scott S, Vickers P J, Hearn L, Heiben J A, Charleson S, Singer I I. 5-Lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 65.Yamin T T, Miller D K. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- 66.Zandi E, Rothwarf D M, Delhasse M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 67.Zeisler H, Tempfer C, Joura E A, Sliutz G, Koebl H, Wagner O, Kainz C. Serum interleukin 1 in ovarian cancer patients. Eur J Cancer. 1998;34:931–933. doi: 10.1016/s0959-8049(97)10107-1. [DOI] [PubMed] [Google Scholar]