Abstract
The microbiota is the set of commensal microorganisms, residing in the organism, helping proper functioning of organs and systems. The role that the microbiota plays in maintaining the health of vertebrates is widely accepted, particularly in the gastrointestinal system, where it is fundamental for immunity, development, and conversion of nutrients. Dysbiosis is an alteration of the microbiota which refers to a disturbed balance, which can cause a number of pathologies. Probiotics have proven to be effective in modulating the microbiota of the gastrointestinal system and, therefore, in promoting the health of the individual. In particular, Lactobacilli are a group of Gram-positive bacteria, which are able to produce lactic acid through glucose metabolism. They are present in different microenvironments, ranging from the vagina, to the mouth, to different tracts of the small intestine. In the present review, we will discuss the use of Limosilactobacillus in human health in general and more specifically in diverticulitis. In particular we analyze the role of Limosilactobacillus reuteri and its anti-inflammatory action. For this review, articles were identified using the electronic PubMed database through a comprehensive search, conducted by combining key terms such as “diverticulitis”, “Limosilactobacillus reuteri”, “human health and disease”, “probiotics”. We selected all the articles published in the last 10 years and screened 1017 papers. Articles referenced in the screened papers were evaluated if considered interesting for our topic. Probiotics have proven to be effective in modulating the microbiota of the gastrointestinal system and, therefore, in promoting the health of the individual. The importance of probiotics in treating diverticular disease and acute diverticulitis can be further understood if taking into consideration some pathophysiological aspects, associated to the microbiota. L. reuteri plays an important role in human health and disease. The effectiveness of L. reuteri in stimulating a correct bowl motility partly explains its effectiveness in treating diverticulitis. The most important action of L. reuteri is probably its immunomodulating activity. Levels of IL-6, IL-8, and Tumor necrosis factor (TNF-alpha) are reduced after supplementation with different strands of Lactobacilli, while T-regulatory cells increase in number and activity. Anyway, new mechanisms of action of probiotics come to light from the many investigations currently taking place in numerous centres around the world and to improve how exactly probiotic administration could make the difference in the management of diverticular disease and acute diverticulitis.
Keywords: diverticulitis, Lactobacillus, acute diverticolitis, Lactobacillus reuteri, immune system, inflammatory diseases, microbiota, probiotic
1. Introduction
The microbiota is the set of commensal microorganisms, residing in the organism, helping proper functioning of organs and systems. The role that the microbiota plays in maintaining the health of vertebrates is widely accepted, particularly in gastrointestinal system, where it is fundamental for immunity, development, and conversion of nutrients. Dybiosis is an alteration of the microbiota which refers to a disturbed balance, which can cause a number of pathologies. Probiotics have proven to be effective in modulating the microbiota of the gastrointestinal system and, therefore, in promoting the health of the individual.
In particular, Lactobacilli are a group of Gram-positive bacteria, which are able to lactic acid through glucose metabolism. They are present in different microenvironments, ranging from the vagina, to the mouth, to different tracts of the small intestine.
In the present review, we will discuss the use of Limosilactobacillus in human health in general and more specifically in diverticulitis. In particular, we analyse the role of Limosilactobacillus reuteri and its anti-inflammatory action.
2. Lactobacilli as Probiotics: Which Ones and Where?
The microbiota is the set of commensal microorganisms, residing in the organism, helping proper functioning of organs and systems [1]. The role that the microbiota plays in maintaining the health of vertebrates is widely accepted, particularly in gastrointestinal system, where it is fundamental for immunity, development, and conversion of nutrients [2].
Dybiosis is an alteration of the microbiota which refers to a disturbed balance, which can cause a number of pathologies [2].
Probiotics have proven to be effective in modulating the microbiota of the gastrointestinal system and, therefore, in promoting the health of the individual [3].
Probiotics are defined by the World Health Organization as “live microorganisms which, if administered in the right amount, benefit the host”; to be considered effective, a probiotic must have specific requirements and characteristics: it must have the ability to survive in the gastrointestinal tract, adhere to the epithelium of the mucosa, be resistant to gastric acids, and must be free of transferable genes of antibiotic resistance [4]. Indeed, there is evidence that some species of Lactobacilli, if not used correctly, can determine severe diseases [5]. Lactobacilli are a group of Gram-positive bacteria, which are able to produce lactic acid through glucose metabolism. They are present in different microenvironments, ranging from the vagina [6], to the mouth [7], to different tracts of the small intestine [8].
There are about 170 different species of Lactobacilli, which play an important role in different contexts [9] but are often difficult to differentiate from one another [5]. For instance, in the vaginal microbiota, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jensenii and Lactobacillus iners are the most common found species, while they are not as common in other microenvironments [10]. Additionally, in the case of probiotics, some species are more effective than others.
Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus brevis, Lactobacillus fermentum, Lactobacillus parabuchneri, Lactobacillus bulgaricus, Lactobacillus rhamnosus GG are the most commonly used species, particularly for commercial purposes, both in producing probiotics and in fermentation of different types of food [5]. All these species have proven effective in treating different types of diseases. Lactobacillus acidophilus can be used in traveller’s diarrhoea, Lactobacillus casei in constipation and rheumatoid arthritis, Lactobacillus brevis in biliary disorders, Lactobacillus fermentum in vaginosis and in Staphylococcus infections, Lactobacillus bulgaricus improves immune status, Lactobacillus rhamnosus GG also improves immune status, particularly in viral infections [11].
While not as commonly used as other species, Limosilactobacillus is also effective in different contexts, such as bacteraemia, gastroenteritis, colitis, hospitalizations, particularly in infants [11].
In Table 1, we present a short summary of the different species of Lactobacilli, with implications on human health.
Table 1.
Lactobacilli and their effect on human health.
| Species | Application | Reference |
|---|---|---|
| Lactobacillus acidophilus | Reduction of cholesterol; reduced in type 2 diabetes; improvement of Irritable bowel syndrome (IBS) symptoms; reduced inflammation. | [12,13,14,15,16] |
| Lactobacillus casei | Immune modulation; improvement of Chron’s disease and rheumatoid arthritis symptoms; alleviation of constipation. | [17,18,19] |
| Lactobacillus brevis | Improved gut barrier function; oxidative stress reduction. | [20,21] |
| Lactobacillus fermentum | Symptomatic colitis improvement; protective against Staphylococcus infection; improved response to viral infections. | [22,23,24] |
| Lactobacillus bulgaricus | Immune modulation; improved humoral response: promotion of wound healing | [25,26,27] |
| Lactobacillus rhamnosus GG | Improves symptoms of gastroenteritis; reduction of antibiotic resistance. | [28,29] |
| Limosilactobacillus reuteri | Improves diarrhoea; reduced symptoms of uncomplicated diverticulitis; metabolic modulation | [30,31,32] |
3. Limosilactobacillus Reuteri in Human Health and Disease
Lactobacilli spp. are the most used probiotics and one of the species most frequently found in food products [33]. As discussed above, numerous species belong to this genus including L. acidophilus, L. rhamnosus, L. bulgaricus, L. casei, and L. reuteri. L. reuteri possesses all the requisites to be considered an effective probiotic [2]. It has proven to be effective in influencing the diversity, composition, and metabolic function of the microbiota, not only in the intestine but also in the oral and vaginal area [34,35].
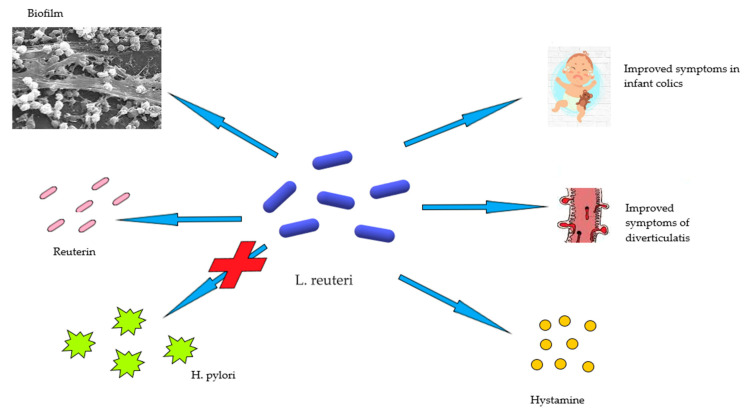
The antimicrobial and immunomodulating power of L. reuteri lies in the metabolites it can produce [2]. Among these, one of the most important is reuterin, a molecule consisting of a mixture of three hydroxypropionaldehyde 3 hpa, which in turn breaks down spontaneously into acrolein or a cytotoxic electrophile, capable of inhibiting a large amount of gram-negative bacteria [2,36].
Other metabolites that make L. reuteri effective against many types of gastrointestinal infections are acetic acid, ethanol and lactic acid [37,38] 1. Another metabolite that confers immunomodulating properties in the gastrointestinal tract to L. reuteri is histamine [2].
Some strains of L. reuteri are also able to convert the amino acid L-histidine, of dietary origin, into biogenic histamine. Some studies have shown that histamine produced by L. reuteri can suppress the production of tumour necrosis factor (TNF), produced by stimulated human monocytes [39]. The clusters of chromosomal genes responsible for these properties are histidine decarboxylase A-B and C. The research has also found that oral administration of hdc + L. reuteri could effectively suppress intestinal inflammation in a mouse colitis model [39].
L. reuteri is also able to produce lipopolysaccharide (LPS), which is extremely important for the adhesion of L. reuteri to intestinal cells and the formation of a biofilm [40]. The formation of biofilm is an important feature for L. reuteri, because it allows it to survive in the intestinal environment and to protect the epithelium from the adhesion of other pathogenic microorganisms through both steric hindrance and competitive inhibition and by triggering the immune response of the host [41]. It has also been shown that LPS produced by L. reuteri can avoid the adhesion of E. coli to intestinal epithelial cells in pigs in vitro and also suppresses the expression of some E. coli-induced pro-inflammatory cytokines such as interleukin (IL)-6 and IL-1b [42].
The tryptophan catabolites have been recognized as ligands for the aryl hydrocarbon receptor, whose activation induces the expression of IL-22 and other cytokines by innate lymphoid cells. In addition, tryptophan derivatives can induce the development of double-positive regulatory CD4+ and CD8+ intraepithelial lymphocytes in anaryl hydrocarbon receptor (AhR)-dependent manner [43].
One of the infections in which L. reuteri has proved effective in eradicating and reducing symptoms is chronic H. pylori infection [2], one of the most frequent chronic infections and one of the main causes of peptic ulcers, as well as a risk factor for developing some types of gastric cancer. L. reuteri acts by competing with H. pylori thus, inhibiting its adhesion to glycosidic receptors [44]. This mechanism of action is very effective in eradicating the infection and in reducing symptoms, while avoiding the side effects associated with antibiotic therapies [45].
The integrity of the intestinal barrier is essential to prevent the entry of pathogens and external toxins. When it is disturbed, the intestine becomes permeable allowing the passage of antigens and toxins into the intestinal lumen thus, triggering a pathological process. Probiotics, in particular L. reuteri, are very effective in ensuring the integrity of the barrier itself [46].
A study carried out on mice has shown that the administration of a mixture of Lactobacilli, including L. reuteri, can increase the expression of tight junction proteins in intestinal epithelial cells. This finding has been further confirmed by studies carried out on pigs [38,47].
The ability to maintain the intestinal barrier permeability of L. reuteri has also been demonstrated in humans. In children with atopic dermatitis, for instance, in which the increase in intestinal permeability was related to the pathogenesis of the disease [48], the administration of L. reuteri reduced intestinal permeability, as demonstrated by the reduction of the lactulose/mannitol ratio, and associated symptoms [49].
L. Reuteri has also been proven effective in disorders of the early stages of life such as colic or disorders characterized by excessive crying, which affects 10–30% of newborns. In a study conducted by Guolin et al., breastfed infants younger than 4 months of age were recruited and divided into two groups, a placebo and a group to which L. reuteri was administered. At the end of the 4 weeks supplementation, it was found that 100% of the children treated with L. reuteri had a reduction in mean crying time and a decrease in maternal depression as a secondary effect. The same effects were found in only the 15, 7% of children treated with placebo, suggesting that L. reuteri may be a viable option for the treatment of infantile colic [49] (Figure 1).
Figure 1.
The role of L. reuteri in human health and disease.
4. Acute Diverticulitis
Colon diverticula are blind-ended extroversions involving the mucosal and submucosal layer of the intestinal wall [50]. They represent one of the most common pathologies in inpatient and outpatient patients [51] and the most frequently encountered finding in colonoscopies [52]. They can occur anywhere in the large intestine and their distribution depends heavily on the country of origin. In the industrialized Western world, diverticula strains are much more frequent at the sigmoid level. They can be single but are frequently fund as multiple [53].
The prevalence of diverticula is very similar in men and women [51] and it is often directly proportional to the patient’s age [54]. Studies have estimated an increase in diverticula occurring in about half of people between 60 and 80 years old, up to 70% in people aged 80 [55,56].
To date, the process driving the pathogenesis is still not fully understood; however, it is clear that it lies within the interaction of various risk factors determining an increase in intraluminal pressure and causing herniation of the intestinal wall, where the rectus vessels penetrate the colon wall [57].
The risk factors are represented by age and genetics, as demonstrated by the incidence of the disease among homozygote twins, when compared to heterozygotes [58]. Poor colon wall motility, low-fibre diet, obesity, smoking, consumption of red meat, constipation, alterations of the intestinal microbiota, use of some drugs in particular non-steroidal anti-inflammatory drugs, aspirin, opioids, and comorbidities are also important factors in the development of the disease. In the literature, it is possible to find various associations between the development of diverticula and other diseases, in particular diabetes and hypertension [55,59].
Diverticula can be asymptomatic for life or can become inflamed and symptomatic [57]. However, recent studies have estimated that less than 5% of patients with diverticulosis will become symptomatic [60].
Diverticulitis can be divided into two categories: simple and complicated [59]. Simple diverticulitis is characterized by the inflammation of one or more adjacent diverticula and the pericolic tissue [61]. The complicated form, which occur in 12% of cases, is represented primarily by an abscess followed by perforation, obstruction, and fistulisation [59].
Simple diverticulitis is also known to cause pain, generally localized to the left lower quadrant and may be accompanied by nausea, vomiting, alterations of the alvo [59]. Occult blood in the stool is particularly rare, while hemodynamic instability and abdominal rigidity are findings compatible with the complications, such as free perforation and/or generalized peritonitis [62].
Since diverticula are asymptomatic in most cases, they are often an occasional finding during a colonoscopy performed for other reasons [55].
Abdominal computed tomography scan (CT) scan is used in case of acute symptoms, when colonoscopy is not indicated [55]. An alternative to CT can be abdominal ultrasound, with a sensitivity and specificity of about 90%. Magnetic MRI can also be used in suspected diverticulitis in patients for whom CT or ultrasound are not indicated. X-ray can be used in suspicion of abdominal perforation or to exclude other causes of abdominal pain such as intestinal obstruction. However, this methodology cannot be used to diagnose diverticulitis and/or abscess [59].
Uncomplicated acute diverticulitis can be treated directly in the hospital facilities and includes a treatment involving a liquid diet and use of antibiotics, in particular a combination of metronidazole and ciprofloxacin.
Hospitalization is indicated in all those patients that are unable to take oral therapy, are suffering from severe comorbidities, or do not present any improvement despite oral therapy or face complications. Clinical improvement usually occurs within 34 days of onset of symptoms [57].
The role of surgery has changed through time: abscesses are treated with antibiotic therapy or percutaneous drainage while the use of surgery is reserved for cases of peritonitis. Besides, the associated comorbidities and the recurrence of episodes of inflammation are the factors to be considered when evaluating the surgical approach [62].
There is no consensus on the optimal therapy to be taken as secondary prevention. Although, a diet with a high fibre content is recommended, but also the use of rifaximin to be taken in cycles [57].
Studies have also shown that the use of mesalazine is not superior to placebo in reducing the likelihood of relapse but reduces abdominal symptoms [63].
5. Microbiota and Acute Diverticulitis
As discussed above, diverticulitis is defined as an inflammation of a herniation of colonic mucosa and submucosa through the muscle layer. Inflammation is caused in particular by local factors, including the microbiota, but it is not completely clear whether or not the microbiota could play a role in determining the herniation in the first place [64].
Dietary factors are key in determining the onset of diverticular disorder and they can also change the composition of one’s microbiota. It is not possible to say without doubt whether the changes in the composition of the microbiota may act as an enhancing factor in the development of diverticulitis, but it is worth nothing that the microbial species associated to diverticular disease are Enterobacteriaceae, Streptococcus and Bacteroides, while so called “good bacteria” (e.g., Bifidobacteria and Lactobacilli) are reduced [65].
The role of microbiota in determining diverticular inflammation is instead more straightforward. Recurring diverticulitis not susceptible to surgery has been treated in a successful and lasting way with faecal transplant, [66]. Further confirmation of the role microbiota plays in the development and progression of diverticular disease, is that patients receiving faecal transplant for C. difficile infection and who also presented mild forms of diverticular disease, developed after the procedure their first episode of diverticulitis [67].
A reduction in taxa with anti-inflammatory activity, such as Clostridium cluster IV, Lactobacilli and Bacteroides are all changes that have been observed in patients who were about to manifest acute diverticulitis. At the same time, also an overgrowth of Bifidobacteria, Enterobacteriaceae and Akkermansia have been reported [64]. Additionally, an increase in Proteobacteria is quite typical, and these changes could be used to diagnose the disease in its earlier stages [68].
Finally, the importance of the microbiota in diverticular disease has been demonstrated indirectly by the therapies used to treat it. Rifaximin and probiotics have proven to be effective, at least in clinical trials.
Rifaximin has been used to treat Symptomatic Uncomplicated Diverticular Disease in a clinical trial and results were encouraging, in terms of symptom control. Interestingly, the changes in clinical presentation, also corresponded to changes in faecal microbiota composition, with a reduction of Roseburia, Veillonella, Streptococcus and Haemophilus [69]. In another study, also Akkermansia resulted reduced [70].
Lactobacilli have demonstrated to reduce Symptomatic Uncomplicated Diverticular Disease, with a reduction of bloating and abdominal pain [71], while Lactobacillus salivarius, Lactobacillus acidophilus and Bifidobacterium lactis have proven effective in the treatment of acute diverticulitis [72].
6. Limosilactobacillus and Diverticulitis
As discussed above L. reuteri plays an important role in human health and disease. Its effects on gut health are quite interesting, studied in particular in terms of fighting chronic constipation. Given its relatively safe profile, supplementation has been used to treat chronic constipation in children, with encouraging results [73].
The effectiveness of L. reuteri in stimulating a correct bowl motility partly explains its effectiveness in treating diverticulitis.
While data on this particular topic are not abundant, one study in particular, by Petruziello et al. [32], has highlighted that supplementation with this particular strand significantly reduced symptoms in acute uncomplicated diverticulitis. Interestingly, also inflammatory markers were reduced, further reinforcing the results of the study.
The mechanisms through which these positive responses take place are different: while on the one hand an action on intestinal motility does take place and is important, it is worth noting that the most important action of L. reuteri is probably its immunomodulating activity. Levels of IL-6, IL-8, and TNF-alpha are reduced after supplementation with different strands of Lactobacilli, while T-regulatory cells increase in number and activity [74].
Additionally, there appears to be a higher expression of tight junction proteins claudin-1, occludin, and zonulin-1.
The described actions are likely to be at least in part modulated by the increased production of butyrate, induced by this strand of bacteria, including L. reuteri.
While they do not directly produce butyrate, they are capable of activating it [75].
The effects of these supplements strongly depend on the host’s immunity, particularly on T-regulatory cells, even though it has been suggested by recent studies that L. reuteri might be capable of modulating the activity of this compartment of immunity. Liu et al. have observed in murine models that, while other strains of Lactobacilli are not as effective in reducing inflammation if T-regulatory cells are deficient, L. reuteri might be still capable of modulating inflammation and microbiota, which suggests there are other mechanisms underlying its action [76].
7. Probiotics in Diverticulitis: Mechanisms of Action
The importance of probiotics in treating diverticular disease and acute diverticulitis can be further understood if taking into consideration some pathophysiological aspects, associated to the microbiota. Changes in microbiota composition have been associated to altered nervous response in the gut, which leads to neuronal and muscular dysfunction, and eventually to abdominal symptoms [77].
Additionally, a decreased presence of anti-inflammatory bacterial species might be linked to mucosal inflammation, resulting in a vitious circle, in which dysbiosis and inflammation promote each other [78].
Dysbiosis and mucosal inflammation can also lead to dysmotility, further promoting bacterial translocation from the lumen of the diverticulum to perivisceral area. In such way, it is possible for Toll-like receptors of innate immunity to be stimulated and activated, with a subsequent inflammatory reaction at the level of perivisceral tissues [79].
This evidence has led researchers to consider changes in peri-diverticular bacterial flora as a critical element in acute diverticulitis pathogenesis, in a similar way to acute appendicitis. Overall, stasis of faecal material within diverticula can be favoured by a prolonged colonic transit, which promotes an altered microflora and bacterial overgrowth. Mucosal barrier function can be then impaired, determining an inflammatory reaction, through of cytokine release; a low-grade, localized microscopic colitis can take place, evolving towards microperforation and acute diverticulitis [80].
It appears clear that microbial colonization plays an important role in at least promoting diverticular disease, thus changing its composition through probiotic becomes an interesting therapeutic strategy. Interestingly, use of probiotics also results in direct changes in inflammatory patterns, as reported by Quigley. Indeed, probiotics have the ability to modify localized and persistent inflammation which is constantly present in some patients suffering from acute diverticulitis, even between bouts of the disease, which may also sustain symptoms’ development in individuals affected by uncomplicated diverticular disease [81]. Foligne and colleagues [82] have studied, in the context of IBD, thirteen strains of probiotics in terms of anti-inflammatory properties and, among these, L. acidophilus and L. salivarius Ls33 seemed to be the best-performing, in terms of increased induction of IL-10 and decreased induction of IL-12.
Data from in vitro and in vivo studies concerning L. salivarius Ls33 suggest that it is linked to an improved recovery of tissue inflammation in a rat colitis model [83].
The intestinal bacterial flora also produces outer membrane vesicles, which play an important role in microbiota–host communication, through the action of adhesins, sulfatases and proteases and pathways such as micropinocytosis, clathrin- and lipid raft-dependent endocytosis [84].
These outer membrane vesicles positively impact mucosal immunity and its signaling pathways. While this could be an interesting further explanation of the mechanisms underlying the effectiveness in treating diverticulitis and abdominal disorders with probiotics, it is still not completely understood and data are still lacking [85,86].
Brandimarte et al. [87] have discussed the pros and cons, regarding evidence on probiotic action in diverticular disease. Overgrowth and alteration of gut microbiota play an important role in the development of inflammation, which is key to diverticular disease development, thus there is a clear rationale for the use of probiotics, aiming to restore a healthy microenvironment in the colon. The involved mechanisms, range from bacterial translocation, inflammation, competitive inhibition of pathogenic bacteria, immune modulation and are all potentially a target of probiotic therapy [88,89].
8. Methods
For this review, articles were identified using the electronic PubMed database through a comprehensive search, conducted by combining key terms such as “diverticulitis”, Limosilactobacillus Reuteri”, “human health and disease”, “probiotics”.
English-language articles were screened for relevance. Full review of publications for the relevant studies was conducted, including additional publications that were identified from individual article reference lists. At first, the literature search was individually conducted by the single authors, who subsequently confronted each other in order to include in the review only the most recent and most relevant articles.
We selected all the articles published in the last 10 years and screened 1017 papers between March and June 2021. Articles referenced in the screened papers were evaluated if considered interesting for our topic.
9. Conclusions and Discussion
Probiotics have proven to be effective in modulating the microbiota of the gastrointestinal system and, therefore, in promoting the health of the individual.
The importance of probiotics in treating diverticular disease and acute diverticulitis can be further understood if taking into consideration some pathophysiological aspects, associated to the microbiota.
Changes in microbiota composition have been associated to altered nervous response in the gut, which lead to neuronal and muscular dysfunction, and eventually to abdominal symptoms.
Additionally, a decreased presence of anti-inflammatory bacterial species might be linked to mucosal inflammation, resulting in a vitious circle, in which dysbiosis and inflammation promote each other.
In the present review, we will discuss the use of Limosilactobacillus in human health in general and more specifically in diverticulitis.
In particular, the effectiveness of L. reuteri in stimulating a correct bowl motility partly explains its effectiveness in treating diverticulitis.
L. Reutery significantly reduced symptoms in acute uncomplicated diverticulitis and it is shown by the reduced levels of inflammatory markers.
The mechanisms is probably its immunomodulating activity. Levels of IL-6, IL-8, and TNF-alpha are reduced after supplementation with different strands of Lactobacilli, while T-regulatory cells increase in number and activity.
Yet, the data present on this matter are not enough to find robust conclusions on the efficacy of probiotics in diverticular disease, as confirmed by a recent expert consensus with a submaximal level of agreement [90].
Overall, there still is no standard protocol in terms of probiotic use in diverticular disease, given that the data we have do not come from large studies and there still is a lack of robust information [87].
New mechanisms of action for probiotics have come to light from the many investigations currently taking place in numerous centres around the world.
New protocols should be established in order to study how exactly probiotic administration could make the difference in the management of diverticular disease and acute diverticulitis.
Author Contributions
Conceptualization, A.P. and V.O.; methodology, L.F.; software, C.Z.; validation, A.P., F.F. and V.O.; formal analysis, A.S.; investigation, V.V.; resources, M.C. (Marcello Candelli); data curation, M.C. (Marcello Covino); writing—original draft preparation, L.F.; writing—review and editing, A.P.; visualization, F.F.; supervision, V.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Belkaid Y., Harrison O.J. Homeostatic Immunity and the Microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mu Q., Tavella V.J., Luo X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018;9:757. doi: 10.3389/fmicb.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S.K., Guevarra R.B., Kim Y.T., Kwon J., Kim H., Cho J.H., Kim H.B., Lee J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019;29:1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 4.Montalban-Arques A., De Schryver P., Bossier P., Gorkiewicz G., Mulero V., Gatlin D.M., 3rd, Galindo-Villegas J. Selective Manipulation of the Gut Microbiota Improves Immune Status in Vertebrates. Front. Immunol. 2015;6:512. doi: 10.3389/fimmu.2015.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein E.J.C., Tyrrell K.L., Citron D.M. Lactobacillus Species: Taxonomic Complexity and Controversial Susceptibilities. Clin. Infect. Dis. 2015;60(Suppl. 2):S98–S107. doi: 10.1093/cid/civ072. [DOI] [PubMed] [Google Scholar]
- 6.Amabebe E., Anumba D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caufield P.W., Schön C.N., Saraithong P., Li Y., Argimón S. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res. 2015;94(Suppl. 9):110s–118s. doi: 10.1177/0022034515576052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitetta L., Vitetta G., Hall S. Immunological Tolerance and Function: Associations Between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018;9:2240. doi: 10.3389/fimmu.2018.02240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shokryazdan P., Sieo C.C., Kalavathy R., Liang J.B., Alitheen N.B., Faseleh Jahromi M., Ho Y.W. Probiotic Potential of Lactobacillus Strains with Antimicrobial Activity against Some Human Pathogenic Strains. BioMed Res. Int. 2014;2014:927268. doi: 10.1155/2014/927268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrova M.I., Lievens E., Malik S., Imholz N., Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fijan S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health. 2014;11:4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Zhou B., Zhou X., Wang Y., Wang H., Jia S., Zhang Z., Chu C., Mu J. Combined Lowering Effects of Rosuvastatin and L. acidophilus on Cholesterol Levels in Rat. J. Microbiol. Biotechnol. 2019;29:473–481. doi: 10.4014/jmb.1806.06004. [DOI] [PubMed] [Google Scholar]
- 13.Halawa M.R., El-Salam M.A., Mostafa B.M., Sallout S.S. The Gut Microbiome, Lactobacillus acidophilus; Relation with Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2019;15:480–485. doi: 10.2174/1573399815666190206162143. [DOI] [PubMed] [Google Scholar]
- 14.Sadrin S., Sennoune S.R., Gout B., Marque S., Moreau J., Grillasca J., Pons O., Maixent J.M. Lactobacillus acidophilus versus placebo in the symptomatic treatment of irritable bowel syndrome: The LAPIBSS randomized trial. Cell. Mol. Biol. 2017;63:122–131. doi: 10.14715/cmb/2017.63.9.21. [DOI] [PubMed] [Google Scholar]
- 15.Hoseinifar S.H., Roosta Z., Hajimoradloo A., Vakili F. The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri) Fish Shellfish Immunol. 2015;42:533–538. doi: 10.1016/j.fsi.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Guandalini S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011;45:S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.Y., Lee S.Y., Jung S.H., Kim M.R., Choi I.D., Lee J.L., Sim J.H., Pan C.H., Kang K. Protective effect of Lactobacillus casei HY2782 against particulate matter toxicity in human intestinal CCD-18Co cells and Caenorhabditis elegans. Biotechnol. Lett. 2020;42:519–528. doi: 10.1007/s10529-020-02814-3. [DOI] [PubMed] [Google Scholar]
- 18.Llopis M., Antolin M., Carol M., Borruel N., Casellas F., Martinez C., Espín-Basany E., Guarner F., Malagelada J.R. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm. Bowel Dis. 2009;15:275–283. doi: 10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 19.Ou Y., Chen S., Ren F., Zhang M., Ge S., Guo H., Zhang H., Zhao L. Lactobacillus casei Strain Shirota Alleviates Constipation in Adults by Increasing the Pipecolinic Acid Level in the Gut. Front. Microbiol. 2019;10:324. doi: 10.3389/fmicb.2019.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin M.Y., Yong C.C., Oh S. Regulatory Effect of Lactobacillus brevis Bmb6 on Gut Barrier Functions in Experimental Colitis. Foods. 2020;9:864. doi: 10.3390/foods9070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X., Gu S., Liu D., Zhao L., Xia S., He X., Chen H., Ge J. Lactobacillus brevis 23017 Relieves Mercury Toxicity in the Colon by Modulation of Oxidative Stress and Inflammation Through the Interplay of MAPK and NF-κB Signaling Cascades. Front. Microbiol. 2018;9:2425. doi: 10.3389/fmicb.2018.02425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang Y.J., Kim W.K., Han D.H., Lee K., Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10:696–711. doi: 10.1080/19490976.2019.1589281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastor-Villaescusa B., Hurtado J.A., Gil-Campos M., Uberos J., Maldonado-Lobón J.A., Díaz-Ropero M.P., Bañuelos O., Fonollá J., Olivares M. Effects of Lactobacillus fermentum CECT5716 Lc40 on infant growth and health: A randomised clinical trial in nursing women. Benef. Microbes. 2020;11:235–244. doi: 10.3920/BM2019.0180. [DOI] [PubMed] [Google Scholar]
- 24.West N.P., Pyne D.B., Cripps A.W., Hopkins W.G., Eskesen D.C., Jairath A., Christophersen C.T., Conlon M.A., Fricker P.A. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011;10:30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evivie S.E., Abdelazez A., Li B., Lu S., Liu F., Huo G. Lactobacillus delbrueckii subsp. bulgaricus KLDS 1.0207 Exerts Antimicrobial and Cytotoxic Effects in vitro and Improves Blood Biochemical Parameters In Vivo against Notable Foodborne Pathogens. Front. Microbiol. 2020;11:583070. doi: 10.3389/fmicb.2020.583070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto Y., Saruta J., Takahashi T., To M., Shimizu T., Hayashi T., Morozumi T., Kubota N., Kamata Y., Makino S., et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: A randomized controlled trial. Acta Odontol. Scand. 2019;77:517–524. doi: 10.1080/00016357.2019.1609697. [DOI] [PubMed] [Google Scholar]
- 27.Mohtashami M., Mohamadi M., Azimi-Nezhad M., Saeidi J., Nia F.F., Ghasemi A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2020 doi: 10.1002/bab.2064. [DOI] [PubMed] [Google Scholar]
- 28.Mantegazza C., Molinari P., D’Auria E., Sonnino M., Morelli L., Zuccotti G.V. Probiotics and antibiotic-associated diarrhea in children: A review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol. Res. 2018;128:63–72. doi: 10.1016/j.phrs.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Szajewska H., Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 2015;42:1149–1157. doi: 10.1111/apt.13404. [DOI] [PubMed] [Google Scholar]
- 30.Ojetti V., Ianiro G., Tortora A., D’Angelo G., Di Rienzo T.A., Bibbò S., Migneco A., Gasbarrini A. The effect of Lactobacillus reuteri supplementation in adults with chronic functional constipation: A randomized, double-blind, placebo-controlled trial. J. Gastrointest. Liver Dis. JGLD. 2014;23:387–391. doi: 10.15403/jgld.2014.1121.234.elr. [DOI] [PubMed] [Google Scholar]
- 31.Mobini R., Tremaroli V., Ståhlman M., Karlsson F., Levin M., Ljungberg M., Sohlin M., Bertéus Forslund H., Perkins R., Bäckhed F., et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017;19:579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 32.Petruzziello C., Migneco A., Cardone S., Covino M., Saviano A., Franceschi F., Ojetti V. Supplementation with Lactobacillus reuteri ATCC PTA 4659 in patients affected by acute uncomplicated diverticulitis: A randomized double-blind placebo controlled trial. Int. J. Colorectal Dis. 2019;34:1087–1094. doi: 10.1007/s00384-019-03295-1. [DOI] [PubMed] [Google Scholar]
- 33.Giraffa G., Chanishvili N., Widyastuti Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010;161:480–487. doi: 10.1016/j.resmic.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Macklaim J.M., Clemente J.C., Knight R., Gloor G.B., Reid G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 2015;26:27799. doi: 10.3402/mehd.v26.27799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romani Vestman N., Chen T., Lif Holgerson P., Öhman C., Johansson I. Oral Microbiota Shift after 12-Week Supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289; A Randomized Control Trial. PLoS ONE. 2015;10:e0125812. doi: 10.1371/journal.pone.0125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talarico T.L., Dobrogosz W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1989;33:674–679. doi: 10.1128/AAC.33.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gänzle M.G., Vogel R.F. Studies on the mode of action of reutericyclin. Appl. Environ. Microbiol. 2003;69:1305–1307. doi: 10.1128/AEM.69.2.1305-1307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang F., Wang A., Zeng X., Hou C., Liu H., Qiao S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015;15:32. doi: 10.1186/s12866-015-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas C.M., Hong T., van Pijkeren J.P., Hemarajata P., Trinh D.V., Hu W., Britton R.A., Kalkum M., Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Candelli M., Franza L., Pignataro G., Ojetti V., Covino M., Piccioni A., Gasbarrini A., Franceschi F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021;22:6242. doi: 10.3390/ijms22126242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salas-Jara M.J., Ilabaca A., Vega M., García A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms. 2016;4:35. doi: 10.3390/microorganisms4030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kšonžeková P., Bystrický P., Vlčková S., Pätoprstý V., Pulzová L., Mudroňová D., Kubašková T., Csank T., Tkáčiková Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016;141:10–19. doi: 10.1016/j.carbpol.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Cervantes-Barragan L., Chai J.N. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franceschi F., Cazzato A., Nista E.C., Scarpellini E., Roccarina D., Gigante G., Gasbarrini G., Gasbarrini A. Role of probiotics in patients with Helicobacter pylori infection. Helicobacter. 2007;12(Suppl. 2):59–63. doi: 10.1111/j.1523-5378.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 45.Francavilla R., Polimeno L., Demichina A., Maurogiovanni G., Principi B., Scaccianoce G., Ierardi E., Russo F., Riezzo G., Di Leo A., et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: A randomized, double-blind, placebo-controlled study. J. Clin. Gastroenterol. 2014;48:407–413. doi: 10.1097/MCG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 46.Mu Q., Kirby J., Reilly C.M., Luo X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mu Q., Zhang H., Liao X., Lin K., Liu H., Edwards M.R., Ahmed S.A., Yuan R., Li L., Cecere T.E., et al. Control of lupus nephritis by changes of gut microbiota. Microbiome. 2017;5:73. doi: 10.1186/s40168-017-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Benedetto A., Rafaels N.M., McGirt L.Y., Ivanov A.I., Georas S.N., Cheadle C., Berger A.E., Zhang K., Vidyasagar S., Yoshida T., et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011;127:773–786.e7. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mi G.L., Zhao L., Qiao D.D., Kang W.Q., Tang M.Q., Xu J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Van Leeuwenhoek. 2015;107:1547–1553. doi: 10.1007/s10482-015-0448-9. [DOI] [PubMed] [Google Scholar]
- 50.Violi A., Cambiè G., Miraglia C., Barchi A., Nouvenne A., Capasso M., Leandro G., Meschi T., De’ Angelis G.L., Di Mario F. Epidemiology and risk factors for diverticular disease. Acta Bio-Med. Atenei Parm. 2018;89(Suppl. 9):107–112. doi: 10.23750/abm.v89i9-S.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munie S.T., Nalamati S.P.M. Epidemiology and Pathophysiology of Diverticular Disease. Clin. Colon Rectal Surg. 2018;31:209–213. doi: 10.1055/s-0037-1607464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schieffer K.M., Kline B.P., Yochum G.S., Koltun W.A. Pathophysiology of diverticular disease. Expert Rev. Gastroenterol. Hepatol. 2018;12:683–692. doi: 10.1080/17474124.2018.1481746. [DOI] [PubMed] [Google Scholar]
- 53.McSweeney W., Srinath H. Diverticular disease practice points. Aust. Fam. Physician. 2017;46:829–832. [PubMed] [Google Scholar]
- 54.Bernades P. Natural history of diverticular disease of the colon. Ann. Gastroenterol. D’Hepatol. 1986;22:209–211. [PubMed] [Google Scholar]
- 55.Thompson A.E. Diverticulosis and Diverticulitis. JAMA. 2016;316:1124. doi: 10.1001/jama.2016.3592. [DOI] [PubMed] [Google Scholar]
- 56.Feuerstein J.D., Falchuk K.R. Diverticulosis and Diverticulitis. Mayo Clin. Proc. 2016;91:1094–1104. doi: 10.1016/j.mayocp.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Tursi A., Papa A., Danese S. Review article: The pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment. Pharmacol. Ther. 2015;42:664–684. doi: 10.1111/apt.13322. [DOI] [PubMed] [Google Scholar]
- 58.Strate L.L., Morris A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology. 2019;156:1282–1298.e1. doi: 10.1053/j.gastro.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson S.M., Strate L.L. Acute Colonic Diverticulitis. Ann. Intern. Med. 2018;168:Itc65–itc80. doi: 10.7326/AITC201805010. [DOI] [PubMed] [Google Scholar]
- 60.Germer C.T. Diverticular disease of the colon. Der Chir. Z. Fur Alle Geb. Der Oper. Medizen. 2014;85:280. doi: 10.1007/s00104-013-2616-7. [DOI] [PubMed] [Google Scholar]
- 61.Bharucha A.E., Parthasarathy G., Ditah I., Fletcher J.G., Ewelukwa O., Pendlimari R., Yawn B.P., Melton L.J., Schleck C., Zinsmeister A.R. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am. J. Gastroenterol. 2015;110:1589–1596. doi: 10.1038/ajg.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gargallo Puyuelo C.J., Sopeña F., Lanas Arbeloa A. Colonic diverticular disease. Treatment and prevention. Gastroenterol. Y Hepatol. 2015;38:590–599. doi: 10.1016/j.gastrohep.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Stollman N., Magowan S., Shanahan F., Quigley E.M. A randomized controlled study of mesalamine after acute diverticulitis: Results of the DIVA trial. J. Clin. Gastroenterol. 2013;47:621–629. doi: 10.1097/MCG.0b013e31828003f6. [DOI] [PubMed] [Google Scholar]
- 64.Ticinesi A., Nouvenne A., Corrente V., Tana C., Di Mario F., Meschi T. Diverticular Disease: A Gut Microbiota Perspective. J. Gastrointest. Liver Dis. JGLD. 2019;28:327–337. doi: 10.15403/jgld-277. [DOI] [PubMed] [Google Scholar]
- 65.Piccioni A., Franza L., Brigida M., Zanza C., Torelli E., Petrucci M., Nicolò R., Covino M., Candelli M. Gut Microbiota and Acute Diverticulitis: Role of Probiotics in Management of This Delicate Pathophysiological Balance. J. Pers. Med. 2021;11:298. doi: 10.3390/jpm11040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer D.C., Hill S.S., Bebinger D.M., McDade J.A., Davids J.S., Alavi K., Maykel J.A. Resolution of multiply recurrent and multifocal diverticulitis after fecal microbiota transplantation. Tech. Coloproctol. 2020;24:971–975. doi: 10.1007/s10151-020-02275-w. [DOI] [PubMed] [Google Scholar]
- 67.Mandalia A., Kraft C.S., Dhere T. Diverticulitis after fecal microbiota transplant for C. difficile infection. Am. J. Gastroenterol. 2014;109:1956–1957. doi: 10.1038/ajg.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daniels L., Budding A.E., de Korte N., Eck A., Bogaards J.A., Stockmann H.B., Consten E.C., Savelkoul P.H., Boermeester M.A. Fecal microbiome analysis as a diagnostic test for diverticulitis. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2014;33:1927–1936. doi: 10.1007/s10096-014-2162-3. [DOI] [PubMed] [Google Scholar]
- 69.Tursi A., Franceschi M., Elisei W., Picchio M., Mario F.D., Brandimarte G. The natural history of symptomatic uncomplicated diverticular disease: A long-term follow-up study. Ann. Gastroenterol. 2021;34:208–213. doi: 10.20524/aog.2020.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tursi A., Mastromarino P., Capobianco D., Elisei W., Miccheli A., Capuani G., Tomassini A., Campagna G., Picchio M., Giorgetti G., et al. Assessment of Fecal Microbiota and Fecal Metabolome in Symptomatic Uncomplicated Diverticular Disease of the Colon. J. Clin. Gastroenterol. 2016;50(Suppl. 1):S9–S12. doi: 10.1097/MCG.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 71.Lahner E., Bellisario C., Hassan C., Zullo A., Esposito G., Annibale B. Probiotics in the Treatment of Diverticular Disease. A Systematic Review. J. Gastrointest. Liver Dis. JGLD. 2016;25:79–86. doi: 10.15403/jgld.2014.1121.251.srw. [DOI] [PubMed] [Google Scholar]
- 72.Ojetti V., Petruzziello C., Cardone S., Saviano L., Migneco A., Santarelli L., Gabrielli M., Zaccaria R., Lopetuso L., Covino M., et al. The Use of Probiotics in Different Phases of Diverticular Disease. Rev. Recent Clin. Trials. 2018;13:89–96. doi: 10.2174/1574887113666180402143140. [DOI] [PubMed] [Google Scholar]
- 73.García Contreras A.A., Vásquez Garibay E.M., Sánchez Ramírez C.A., Fafutis Morris M. Lactobacillus reuteri DSM 17938 and Agave Inulin in Children with Cerebral Palsy and Chronic Constipation: A Double-Blind Randomized Placebo Controlled Clinical Trial. Nutrients. 2020;12:2971. doi: 10.3390/nu12102971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J., Vitetta L. Inflammation-Modulating Effect of Butyrate in the Prevention of Colon Cancer by Dietary Fiber. Clin. Colorectal Cancer. 2018;17:e541–e544. doi: 10.1016/j.clcc.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y., Hoang T.K., Taylor C.M., Park E.S., Freeborn J., Luo M., Roos S., Rhoads J.M. Limosilactobacillus reuteri and Lacticaseibacillus rhamnosus GG differentially affect gut microbes and metabolites in mice with Treg deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2021;320:G969–G981. doi: 10.1152/ajpgi.00072.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wedel T., Böttner M. Anatomy and pathogenesis of diverticular disease. Der Chir. Z. Fur Alle Geb. Der Oper. Medizen. 2014;85:281–288. doi: 10.1007/s00104-013-2617-6. [DOI] [PubMed] [Google Scholar]
- 78.Petruzziello C., Marannino M., Migneco A., Brigida M., Saviano A., Piccioni A., Franceschi F., Ojetti V. The efficacy of a mix of three probiotic strains in reducing abdominal pain and inflammatory biomarkers in acute uncomplicated diverticulitis. Eur. Rev. Med. Pharmacol. Sci. 2019;23:9126–9133. doi: 10.1016/S1590-8658(20)30782-9. [DOI] [PubMed] [Google Scholar]
- 79.Severi C., Carabotti M., Cicenia A., Pallotta L., Annibale B. Recent advances in understanding and managing diverticulitis. F1000Research. 2018;7:971–983. doi: 10.12688/f1000research.14299.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Narula N., Marshall J.K. Role of probiotics in management of diverticular disease. J. Gastroenterol. Hepatol. 2010;25:1827–1830. doi: 10.1111/j.1440-1746.2010.06444.x. [DOI] [PubMed] [Google Scholar]
- 81.Quigley E.M. Gut microbiota, inflammation and symptomatic diverticular disease. New insights into an old and neglected disorder. J. Gastrointest. Liver Dis. JGLD. 2010;19:127–129. [PubMed] [Google Scholar]
- 82.Foligné B., Dewulf J., Breton J., Claisse O., Lonvaud-Funel A., Pot B. Probiotic properties of non-conventional lactic acid bacteria: Immunomodulation by Oenococcus oeni. Int. J. Food Microbiol. 2010;140:136–145. doi: 10.1016/j.ijfoodmicro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 83.Peran L., Camuesco D., Comalada M., Nieto A., Concha A., Diaz-Ropero M.P., Olivares M., Xaus J., Zarzuelo A., Galvez J. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J. Gastroenterol. 2005;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Macia L., Nanan R., Hosseini-Beheshti E., Grau G.E. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int. J. Mol. Sci. 2019;21:107. doi: 10.3390/ijms21010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kameli N., Borman R., Lpez-Iglesias C., Savelkoul P., Stassen F.R.M. Characterization of Feces-Derived Bacterial Membrane Vesicles and the Impact of Their Origin on the Inflammatory Response. Front. Cell. Infect. Microbiol. 2021;11:667987. doi: 10.3389/fcimb.2021.667987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gilmore W.J., Johnston E.L., Zavan L., Bitto N.J., Kaparakis-Liaskos M. Immunomodulatory roles and novel applications of bacterial membrane vesicles. Mol. Immunol. 2021;134:72–85. doi: 10.1016/j.molimm.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 87.Brandimarte G., Bafutto M., Kruis W., Scarpignato C., Mearin F., Barbara G., Štimac D., Vranić L., Cassieri C., Lecca P.G., et al. Hot Topics in Medical Treatment of Diverticular Disease: Evidence Pro and Cons. J. Gastrointest. Liver Dis. JGLD. 2019;28:23–29. doi: 10.15403/jgld-554. [DOI] [PubMed] [Google Scholar]
- 88.Piscopo N., Ellul P. Diverticular Disease: A Review on Pathophysiology and Recent Evidence. Ulst. Med. J. 2020;89:83–88. [PMC free article] [PubMed] [Google Scholar]
- 89.Guslandi M. Medical treatment of uncomplicated diverticular disease of the colon: Any progress? Minerva Gastroenterol. E Dietol. 2010;56:367–370. [PubMed] [Google Scholar]
- 90.Binda G.A., Cuomo R., Laghi A., Nascimbeni R., Serventi A., Bellini D., Gervaz P., Annibale B. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Tech. Coloproctol. 2015;19:615–626. doi: 10.1007/s10151-015-1370-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.