Abstract
Jun N-terminal kinases (JNKs) are serine-threonine kinases that play a critical role in the regulation of cell growth and differentiation. We previously observed that JNK activity is suppressed by all-trans-retinoic acid (t-RA), a ligand for retinoic acid nuclear receptors (RARs), in normal human bronchial epithelial cells, which are growth inhibited by t-RA. In this study, we investigated the mechanism by which t-RA inhibits JNK and the possibility that this signaling event is blocked in non-small cell lung cancer (NSCLC) cells. Virtually all NSCLC cell lines are resistant to the growth-inhibitory effects of t-RA, and a subset of them have a transcriptional defect specific to retinoid nuclear receptors. We found that in NSCLC cells expressing functional retinoid receptors, serum-induced JNK phosphorylation and activity were inhibited by t-RA in a bimodal pattern, transiently within 30 min and in a sustained fashion beginning at 12 h. Retinoid receptor transcriptional activation was required for the late, but not the early, suppression of JNK activity. t-RA inhibited serum-induced JNK activity by blocking mitogen-activated protein (MAP) kinase kinase 4-induced signaling events. This effect of t-RA was phosphatase dependent and involved an increase in the expression of the dual-specificity MAP kinase phosphatase 1 (MKP-1). t-RA did not activate MKP-1 expression or inhibit JNK activity in a NSCLC cell line with retinoid receptors that are refractory to ligand-induced transcriptional activation. These findings provide the first evidence that t-RA suppresses JNK activity by inhibiting JNK phosphorylation. Retinoid receptor transcriptional activation was necessary for the sustained inhibition of JNK activity by t-RA, and this signaling event was disrupted in NSCLC cells with retinoid receptors that are refractory to ligand-induced transcriptional activation.
Jun N-terminal kinases (JNKs) are serine-threonine kinases that mediate cellular responses to growth factors, cytokines, and environmental stress (reviewed in reference 20). JNKs are activated by phosphorylation on threonine 183 and tyrosine 185 by mitogen-activated protein (MAP) kinase kinase 4 (MKK-4) and JNK kinase 2 (9, 25, 28, 43). Downstream effectors are ATF-2, c-Jun, and Elk-1, which are directly phosphorylated by JNKs (reviewed in reference 20). Biologic responses attributed to JNK activation include apoptosis, apoptosis inhibition, DNA repair, and neoplastic transformation (6, 12, 33, 35, 44). Activated JNKs are inhibited through dephosphorylation by MAP kinase phosphatases (MKPs) (8, 14, 31, 40). MKPs are a family of dual-specificity phosphatases that recognize the homologous tripeptide phosphorylation sites required for activation of extracellular signal-regulated protein kinases (ERKs) and JNKs, TEY and TPY, respectively. The MKPs shown to target JNKs include MKP-1, MKP-2, and M3/6.
We previously observed that JNK activity was inhibited by all-trans-retinoic acid (t-RA) (23). t-RA is a ligand for retinoic acid nuclear receptors (RARs), and 9-cis-retinoic acid binds to both RARs and retinoid X receptors (RXRs) (reviewed in reference 27). These receptors form RXR-RAR heterodimers and RXR homodimers. Retinoid receptor function is modulated by binding to transcriptional coactivators and corepressors (4, 15, 19, 34, 41). RXR-RAR heterodimers and RXR homodimers bind to distinct retinoic acid response elements (RAREs). Through these mechanisms, retinoid receptors activate gene expression directly by binding to RAREs within gene promoters and indirectly by interacting with other transcription factors. Several studies have demonstrated the importance of retinoid receptors in mediating the biologic effects of t-RA (5, 29). We have shown that t-RA activates RXR-RAR heterodimers in normal human bronchial epithelial (HBE) cells, which are growth suppressed by t-RA treatment (23). In contrast, non-small cell lung cancer (NSCLC) cells, which are derived from normal HBE cells, are refractory to the growth-inhibitory effects of t-RA (30). Potentially contributing to this retinoid refractoriness, a proportion of NSCLC cell lines have a transcriptional defect specific to retinoid nuclear receptors (30). In these cells, RARs and RXRs are expressed but are not transcriptionally activated by t-RA, and t-RA does not increase the expression of genes typically activated by RXR-RAR heterodimers such as RAR-β, which contains in its promoter an RARE that positively regulates RAR-β expression (30).
We have sought to identify the retinoid signaling events that mediate growth inhibition in normal HBE cells and the mechanism by which NSCLC cells become retinoid resistant. Normal HBE cell growth is regulated through autocrine and paracrine pathways involving a number of receptor tyrosine kinases and their ligands (17). Receptor tyrosine kinases mediate their effects through activation of MAP kinases. We have observed that t-RA inhibits epidermal growth factor-induced JNK activity in normal HBE cells, which could contribute to the growth-inhibitory effects of t-RA (24). Here, we investigated the mechanism by which t-RA inhibits JNK activity and the possibility that this signaling event is blocked in NSCLC cells. In NSCLC cells expressing functional retinoid receptors, t-RA inhibited JNK activity by decreasing JNK phosphorylation. This effect of t-RA was phosphatase dependent and involved increased expression of MKP-1. Suppression of JNK activity and activation of MKP-1 expression were mediated through RAR-dependent pathways and did not occur in an NSCLC cell line expressing retinoid receptors that are refractory to ligand-induced transcriptional activation. These findings reveal a novel mechanism of retinoid signaling that may be a crucial regulator of cell growth.
MATERIALS AND METHODS
Cell lines and culture conditions.
The NSCLC cell line H661 was obtained from the American Type Culture Collection, and H226Br (46) was a gift from Jack Roth, M. D. Anderson Cancer Center. These cells were maintained in RPMI 1640 with 10% fetal calf serum. Cycloheximide, orthovanadate, arsenite, and okadaic acid were purchased from Sigma Chemical Co. (St. Louis, Mo.). The effects of t-RA on cell growth were examined by performing MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue) assays as previously described (23).
Retinoids.
t-RA was purchased from Sigma. The RAR-selective antagonist LG100815 (1) was a generous gift from Richard Heyman (Ligand Pharmaceuticals, San Diego, Calif.).
Northern analysis.
Total cellular RNA was prepared as previously described (24). RNA was subjected to electrophoresis (30 μg per lane) on a 1% agarose gel containing 2% formaldehyde, transferred to a nylon membrane (Duralon UV; Stratagene, La Jolla, Calif.), hybridized to an [α-32P]dCTP-labeled cDNA probe, washed in high-stringency conditions, and autoradiographed for 24 to 48 h. The human MKP-1 cDNA probe used in this study was a gift from Kun-Liang Guan (University of Michigan Medical School, Ann Arbor), and the RAR-β cDNA was a gift from Bill Lamph (Ligand Pharmaceuticals).
Western analysis.
Whole-cell lysates were prepared in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 0.2 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM dithiothreitol, (1 mM phenylmethylsulfonyl fluoride, 20 mM sodium fluoride, 5 mM sodium orthovanadate, aprotinin [10 mg/ml], leupeptin [10 mg/ml], pepstatin [2 mg/ml], 1 mM benzamidine). Cells were incubated for 20 min on ice and clarified by centrifugation at 13,000 × g for 15 min. Supernatants were transferred to new tubes. Protein lysate was separated by electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide gel, transferred to a BA-S-83-reinforced nitrocellulose membrane (Schleicher & Schuell, Inc., Burlington, Vt.), and western blotted overnight at 4°C with primary monoclonal antibodies purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.) that recognize JNK-1, total c-Jun, c-Jun phosphorylated at serine 63, MKP-1, MKP-2, and hemagglutinin (HA). Additional antibodies that recognize JNK-1 and -2 dually phosphorylated at threonine 183 and tyrosine 185 were purchased from Promega, Inc. (Madison, Wis.). Binding was detected by using an enhanced chemiluminescence kit (Amersham, Inc., Arlington Heights, Ill.) according to the manufacturer’s directions. Autoradiographs were densitometrically analyzed by using Image IC software (Scion Corp.) on a Hewlett-Packard Scanjet 4C.
Reporter plasmids and expression vectors.
The luciferase reporter plasmids used contained (i) RAREs (AGTTCA) in direct repeat separated by five nucleotides (DR5) in the context of a thymidine kinase (TK) heterologous promoter (RARE-LUC) or (ii) a control plasmid containing the TK promoter but no RARE (TK-LUC) (11). The constitutively active mutant human MKK-4 (SEK-1) cDNA (E-D mutant), under the control of the elongation factor (EBG) promoter, was a gift from John M. Kyriakis (Harvard Medical School, Boston, Mass.) (36). The dominant negative mutant human MAP kinase/ERK kinase kinase (MEKK-1) cDNA (K432M mutant) under the control of the simian virus 40 (SV40) promoter was a gift from F. X. Claret (M. D. Anderson Cancer Center) (28). An expression vector containing the full-length human JNK-1 cDNA fused with HA was a gift from Bing Su (M. D. Anderson Cancer Center). SV40-driven expression vectors were purchased; these contain the DNA-binding domain (DBD) of GAL4 alone (GAL-DBD) or fused to the transcription activation domain of c-Jun (Jun-GAL-DBD) (Stratagene). GAL-UAS-LUC (Stratagene) is a luciferase reporter plasmid containing five repeats of the GAL4 binding element. The C-terminally truncated RAR-α cDNA lacking the ligand-binding domain (LBD) (403* [7]) under the control of a cytomegalovirus modified (CMX) promoter was a gift from Richard Heyman.
Transient transfection assays.
The day after seeding 105 cells into six-well plates, we transfected the cells with the indicated plasmids for 6 h, using Lipofectamine (GIBCO-BRL). The transfection solution was removed, and the cells were cultured overnight in serum-free medium. Cells were then untreated or treated with retinoids for 24 h. In experiments that involved reporters which contain GAL4 response elements, the cells were then treated for 6 h with 10% serum. Cells were subjected to luciferase assays as previously described (22). Luciferase activities were expressed as the means and standard deviations of five identical wells and were normalized to cell number (105 cells per well). Variations in luciferase activities attributable to differences in transfection efficiencies between H226Br and H661 cells were corrected by comparing β-galactosidase activities of cells transfected with a β-galactosidase expression vector under the control of the β-actin promoter (22). β-Galactosidase assays were performed with the Galactolight Plus reporter system (Tropix, Inc., Bedford, Mass.) according to the manufacturer’s instructions.
JNK assays.
Immune complex kinase assays were performed as previously described (24) to examine JNK activity. Briefly, H661 cells were grown for 24 h in serum-free medium, treated for the indicated time periods with 10−6 M t-RA alone or in combination with LG100815, and then treated for 20 min with 10% serum to activate JNK. Cells were removed from plates with a rubber policeman and lysed in lysis buffer, and JNK-1 and -2 were immunoprecipitated from 100 μg of cell extracts with antibodies (1 μg) that recognize JNK-1 (Santa Cruz Biotechnology) by rotation at 4°C for at least 2 h. The total volume was adjusted to 0.5 ml with lysis buffer. Protein A-G–agarose beads (20 μl; Santa Cruz Biotechnology) were added and incubated at 4°C for 1 h. The beads were washed three times with lysis buffer and once with kinase buffer (20 mM HEPES [pH 7.5], 20 mM β-glycerol phosphate, 10 mM p-nitrophenol phosphate, 10 mM MgCl2, 1 mM dithiothreitol, 50 mM sodium vanadate). Kinase assays were performed by incubating the beads with 30 μl of kinase buffer to which 20 μM cold ATP, 5 μCi of [γ32P]ATP (2,000 cpm/pmol), and 2 μg of glutathione S-transferase (GST)–c-Jun(1-79) (Santa Cruz Biotechnology), were added. The kinase reaction was performed at 30°C for 20 min. The samples were suspended in Laemmli buffer and boiled for 5 min, and the samples were analyzed by SDS-polyacrylamide gel electrophoresis. The gel was dried and autoradiographed.
Immune complex assays were also performed on H661 cells transiently transfected with expression vectors. In these experiments, H661 cells were transfected with the indicated vectors by using Lipofectamine for 6 h, incubated overnight in serum-free medium, treated for 24 h with 10−6 M t-RA, and then treated for 20 min with 10% serum to activate JNK. Cell lysates were prepared and immune complex assays were performed to measure JNK activity as described above.
In vitro phosphatase assays.
H661 cells were treated first for 24 h with 10−6 M t-RA or medium alone and then for 20 min with 10% serum. Cells were washed three times with ice-cold phosphate-buffered saline (PBS) and then lysed in protein tyrosine phosphatase (PTPase) buffer (50 mM HEPES [pH 7.6], 10 mM EDTA, 10 mM EGTA, protease inhibitors phenylmethylsulfonyl fluoride, pepstatin, aprotinin, and leupeptin) alone or with phosphatase inhibitor (pervanadate, arsenite, or okadaic acid). Pervanadate was prepared from orthovanadate by treatment with a 30% solution of H2O2 as described elsewhere (49). Cells were lysed by sonication followed by freeze-thawing in liquid nitrogen. Cell lysates were centrifuged (15,000 × g) for 10 min to remove cell debris. Activated JNK was isolated by immunoprecipitation from serum-treated H661 cells by using antibodies that recognize JNK-1. JNK immunoprecipitates were washed twice in 50 mM HEPES (pH 7.6) to remove any divalent ions. Activated JNK was incubated at 30°C for 30 min with 100 μg of cell lysate. The JNK immune complex was reisolated and washed three times with PTPase buffer containing 0.1% SDS and twice with kinase buffer. The samples were then subjected to kinase assay using GST–c-Jun(1-79) as a substrate as described above.
Metabolic labeling.
H661 cells were grown to confluency and then serum starved for 24 h. Cells were then treated with t-RA alone or with LG100815 at the indicated concentrations for 24 h, washed with PBS, and incubated in RPMI 1640 without methionine or cysteine (Sigma) for 1 h. Cells were pulse-labeled for 30 min by treatment with 35S-labeled methionine and cysteine (0.5 mCi; ICN Pharmaceuticals, Costa Mesa, Calif.). Serum (10%) was added to the medium for the last 20 min of the pulse. The medium was then changed to RPMI 1640 containing cold methionine and cysteine to final concentrations of 150 mg/liter. At different time points, cells were washed in ice-cold PBS and lysed in radioimmunoprecipitation assay buffer. Cell extracts were subjected to immunoprecipitation at 4°C overnight with an antibody to MKP-1 (Santa Cruz Biotechnology). Immunoprecipitates were washed with lysis buffer, electrophoresed on an SDS–12% polyacrylamide gel, and autoradiographed.
RESULTS
t-RA inhibits JNK in a biphasic pattern.
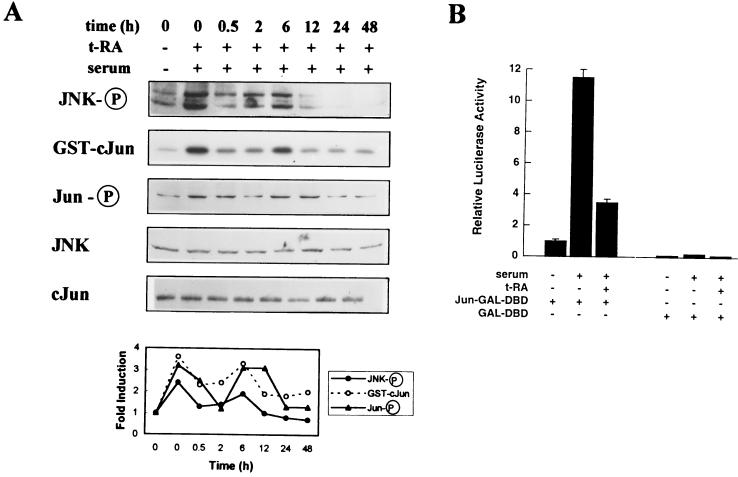
We investigated whether JNK is a component of retinoid signaling in H661, an NSCLC cell line previously shown to express retinoid receptors that are transcriptionally activated by t-RA (30). Western analysis was performed on H661 cells treated first with 10−6 M t-RA for different time periods and then for 20 min with 10% serum to activate JNK, using antisera specific for JNK-1 and -2 dually phosphorylated on Thr-183 and Tyr-185. t-RA inhibited JNK phosphorylation by serum in a biphasic pattern (Fig. 1A). A transient reduction was observed at 30 min, and a sustained reduction appeared at 12 h. Using extracts from the same experiment, we examined JNK activity by immune complex assays using GST–c-Jun(1-79) fusion protein as the substrate. t-RA inhibited JNK activation by serum in a pattern that correlated with JNK phosphorylation (Fig. 1A).
FIG. 1.
t-RA inhibits serum-induced JNK activation in H661 cells. (A) H661 cells were serum starved and then treated with medium alone or 10−6 M t-RA for the indicated time periods, followed by 10% serum for 20 min to activate JNK. Western analysis was performed with antibodies specific for JNK-1 and -2 dually phosphorylated on Thr-183 and Tyr-185 (JNK-P), c-Jun phosphorylated at serine 63 (Jun-P), JNK-1 (JNK), or total c-Jun (cJun). Using the same extracts, we performed immune complex assays with GST–c-Jun(1-79) as a substrate. Laser densitometry was performed to measure intensities of the bands relative to that of serum-starved cells. (B) Transient cotransfection assays were performed to measure JNK activity in H661 cells, using the GAL-UAS-LUC reporter and 1 μg of the GAL-DBD or Jun-GAL-DBD expression vector as a substrate for JNK. Following transfection, the cells were treated for 24 h with (+) or without (−) 10−6 M t-RA, followed by 10% serum for 6 h, and then subjected to luciferase assays. Luciferase activities represent the means and standard deviations of values from five identical wells.
We examined the effect of t-RA on the phosphorylation and transcriptional activity of c-Jun, a JNK target that is a component of the AP-1 complex. Western analysis using antisera specific for c-Jun phosphorylated on serine 63 revealed that t-RA inhibited c-Jun phosphorylation by serum in a pattern that correlated with JNK activity (Fig. 1A). JNK and c-Jun protein levels did not appreciably change with t-RA treatment (Fig. 1A). Transient cotransfection experiments were performed with a reporter containing GAL4 response elements (GAL-UAS-LUC) and an expression vector containing the GAL4 DBD alone (GAL-DBD) or fused with the transcriptional activating domain of c-Jun (Jun-GAL-DBD). Serum increased c-Jun transcriptional activity, and t-RA inhibited c-Jun transcriptional activation by serum (Fig. 1B).
Distinct pathways mediate early and late suppression of JNK activity by t-RA.
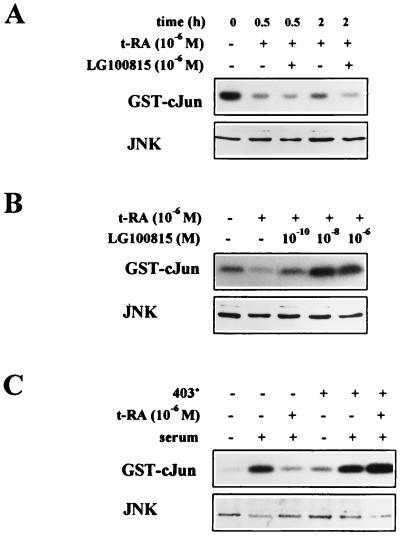
The bimodal regulation of JNK phosphorylation and activity raised the possibility that t-RA inhibits JNK activity through more than one pathway. We investigated whether the early suppression and late suppression of JNK activity differ in their dependence on retinoid receptor transcriptional activation. H661 cells were treated with the RAR antagonist LG100815, a retinoid that binds but does not transcriptionally activate RARs (1). The suppression of JNK activity by t-RA LG100815 was not detectably altered by LG100815 within the first 2 h (Fig. 2A) but was blocked by LG100815 in a concentration-dependent manner at 24 h (Fig. 2B). The role of RARs was further investigated by transfection of an RAR-α cDNA that has a C-terminal truncation in the LBD (403*) and functions as a dominant negative mutant. Transfection of H661 cells with 403* blocked the suppression of JNK activity by t-RA at 24 h (Fig. 2C). These findings suggest that retinoid receptor transcriptional activation was necessary for the late, sustained suppression of JNK activity but not for the earlier, transient effects of t-RA on JNK.
FIG. 2.
RAR transcriptional activation is required for the late, but not the early, inhibition of JNK activity. Immune complex assays were performed, using GST–c-Jun(1-79) as a substrate, on H661 cells treated for the indicated time periods with t-RA alone or in combination with the RAR antagonist LG100815 (A), or for 24 h with t-RA and the indicated doses of LG100815 (B), followed by 10% serum for 20 min to activate JNK. Western analysis of JNK levels (JNK) was performed on the same extracts. (C) Immune complex assays were performed on H661 cells transiently transfected with expression vectors (5 μg) containing 403* or, in the absence of 403*, an empty CMX-driven control vector. The transfectants were treated for 24 h with (+) or without (−) 10−6 M t-RA, followed by 10% serum for 20 min, and then subjected to immune complex assays using GST–c-Jun(1-79) as a substrate or Western analysis using an antibody that recognizes JNK-1 (JNK).
t-RA blocks JNK activation by MKK-4 through a phosphatase-dependent pathway.
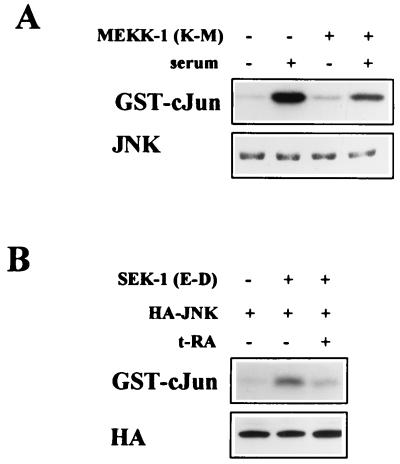
We investigated the mechanism by which t-RA inhibited serum-induced JNK phosphorylation. We examined whether t-RA inhibited JNK activation by upstream kinases. JNK is a substrate of MKK-4, which is a substrate of MEKK-1 (20). Transient transfection of a dominant negative mutant MEKK-1 cDNA partially suppressed JNK activation by serum (Fig. 3A), providing evidence that serum activated JNK through MEKK-1. This result underestimated the effect of the dominant negative mutant MEKK-1 because of the low efficiency of transient transfection. Transient transfection of a constitutively active mutant MKK-4 cDNA stimulated JNK, and t-RA inhibited JNK activation in MKK-4-transfected cells (Fig. 3B). Thus, t-RA inhibited serum-induced JNK activation by blocking MKK-4-induced signaling events.
FIG. 3.
t-RA inhibits serum-induced JNK activity by blocking MKK-4-induced signaling events. (A) Immune complex assays were performed on H661 cells transiently transfected with an expression vector containing a dominant negative MEKK-1 mutant cDNA (K432M) under the control of the SV40 promoter or, in its absence, an empty SV40-driven control vector. After being treated for 24 h with or without 10% serum, the transfectants were subjected to immune complex assays using GST–c-Jun(1-79) as a substrate or Western analysis using an antibody that recognizes JNK-1 (JNK). (B) Immune complex assays were performed on H661 cells transiently cotransfected with an expression vector containing HA-tagged JNK-1 (HA-JNK) and a constitutively active SEK-1 mutant cDNA (E-D) under the control of the EBG promoter or, in its absence, an empty EBG-driven vector. After being treated for 24 h with or without 10−6 M t-RA, the transfectants were subjected to immune complex assays using GST–c-Jun as a substrate or Western analysis using an antibody that recognizes HA.
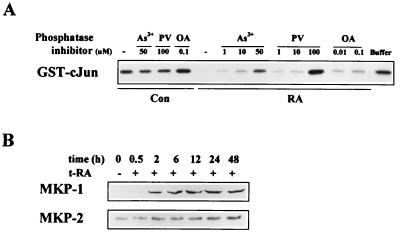
Because the MKK-4 mutant cDNA was constitutively active, we hypothesized that t-RA blocked JNK activation by inhibiting MKK-4-induced JNK phosphorylation. MKK-4 phosphorylates JNK at Thr-183 and Tyr-185, and JNK phosphorylation at these sites is inhibited by dual-specificity phosphatases (8, 14, 31, 40). To investigate the role of phosphatases in retinoid actions, we examined whether the inhibition of JNK activity by t-RA could be blocked by phosphatase inhibitors. Activated JNK was isolated from serum-treated H661 cells by immunopurification and incubated with extracts of untreated or t-RA-treated H661 cells that were lysed in the presence or absence of phosphatase inhibitors. The immunopurified JNK was then washed, reisolated, and subjected to immune complex kinase assays using GST–c-Jun as a substrate. JNK activity was inhibited by extracts of t-RA-treated cells but not untreated cells (Fig. 4A). PTPase inhibitors (pervanadate and arsenite) reversed, in a concentration-dependent manner, the inhibition of JNK activity by extracts of t-RA treated cells, but the serine-threonine phosphatase inhibitor okadaic acid did not (Fig. 4A). These findings suggest that t-RA-treated H661 cells contain a JNK phosphatase(s) that is sensitive to arsenite and pervanadate but not okadaic acid. Pervanadate and arsenite did not measurably alter JNK activity when added to extracts of untreated cells (Fig. 4A), suggesting that the phosphatase(s) was not active in untreated cells.
FIG. 4.
Inhibition of JNK activity by t-RA is phosphatase dependent, and t-RA activates the expression of MKP-1 and -2. (A) An in vitro phosphatase assay was performed on H661 cells treated for 24 h with 10−6 M t-RA (RA) or medium alone (Con), followed by 10% serum for 20 min. Whole-cell extracts were prepared in the presence or absence (−) of arsenite (As3+), pervanadate (PV), or okadaic acid (OA) at the indicated concentrations, and the extracts were incubated for 30 min with activated JNK immunopurified from serum-treated H661 cells. As a control, immunopurified JNK was incubated with buffer alone (buffer). The immunopurified JNK was then washed and subjected to immune complex assays using GST–c-Jun as a substrate. (B) Western analysis of MKP-1 and -2. H661 cells were treated with medium alone or 10−6 M t-RA for the indicated time points, followed by 10% serum for 20 min, and then subjected to Western analysis.
t-RA increases MKP expression.
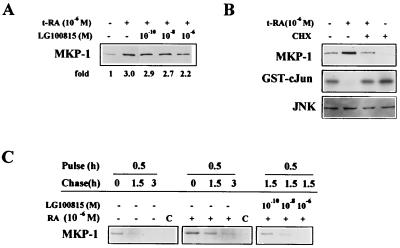
We investigated whether t-RA alters the expression of dual-specificity phosphatases known to inhibit JNK activity. We found that t-RA increased MKP-1 and, to a lesser extent, MKP-2 protein levels in a time-dependent manner (Fig. 4B). The mechanism by which t-RA activated MKP-1 expression was investigated. LG100815 partially blocked the increase in MKP-1 levels induced by t-RA (Fig. 5A), demonstrating that retinoid receptor transcriptional activation was required for the increase in MKP-1 expression. However, retinoid receptors did not directly activate MKP-1 gene transcription, as Northern analysis of H661 cells revealed that t-RA did not measurably alter MKP-1 mRNA levels (data not shown). Cycloheximide abrogated the increase in MKP-1 protein and the suppression of JNK activity by t-RA (Fig. 5B), supporting a role for protein synthesis in the activation of MKP-1 expression and the inhibition of JNK activity by t-RA. Without evidence for transcriptional regulation of MKP-1, new protein synthesis could be required for translational or posttranslational regulation of MKP-1. We investigated whether MKP-1 expression was regulated posttranslationally by performing pulse-chase experiments. t-RA increased the stability of MKP-1 protein, and this effect was blocked in a concentration-dependent manner by LG100815 (Fig. 5C). These findings suggest that t-RA increased MKP-1 protein levels posttranslationally through a retinoid receptor-dependent mechanism.
FIG. 5.
t-RA activates MKP-1 expression posttranslationally through a retinoid receptor-dependent mechanism. (A) Western analysis of MKP-1 and -2 was performed on H661 cells treated for 24 h with t-RA alone or in combination with the indicated doses of LG100815, followed by 10% serum for 20 min. Laser densitometry was performed to quantitate the density of the MKP-1 bands relative to that of cells treated in the absence of t-RA and LG100815. (B) Immune complex assays, using GST–c-Jun as a substrate, and Western analysis of MKP-1 and JNK-1 and -2 (JNK) were performed on H661 cells treated for 24 h with or without 10−6 M t-RA and 10 μg of cycloheximide (CHX) per ml and then with 10% serum for 20 min. (C) MKP-1 protein stability was examined by pulse-chase analysis. H661 cells were treated for 24 h with t-RA (RA), t-RA combined with the indicated doses of LG100815, or medium alone. Cells were then cultured for 1 h in methionine- and cysteine- poor medium, pulsed for 30 min with 35S-labeled methionine and cysteine, chased with cold methionine and cysteine for the indicated time periods, and subjected to immunoprecipitation using an antibody specific for MKP-1 or rabbit preimmune serum (lanes C). The immunoprecipitant was subjected to electrophoresis and autoradiography.
JNK suppression does not occur in an NSCLC cell with defective retinoid receptors.
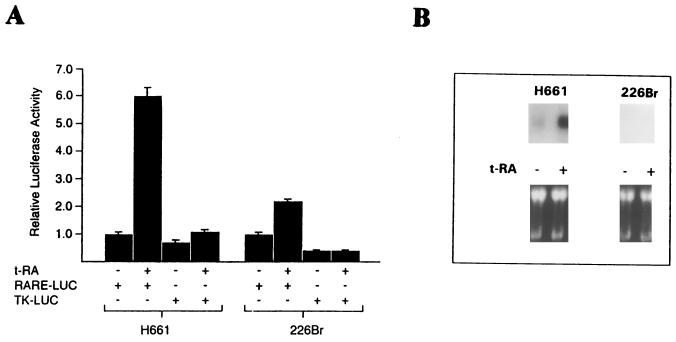
Our findings paint a complex picture in which retinoid receptor transcriptional activation is necessary for some, but not all, of the effects of t-RA on the regulation of JNK activity. To further investigate the importance of ligand-induced retinoid receptor transcriptional activation in the regulation of JNK activity, we examined the effect of t-RA on the H226Br cell line, which is representative of a subset of NSCLC cells that has a transcriptional defect specific to retinoid receptors (30). In comparison to H661 cells, H226Br cells transiently transfected with a reporter containing an RARE (RARE-LUC) only minimally increased RARE activity in response to t-RA treatment (Fig. 6A). Further, in contrast to H661 cells, t-RA treatment of H226Br cells did not detectably increase the mRNA levels of RAR-β (Fig. 6B), demonstrating that a gene which is transcriptionally activated by retinoid receptors is not detectable in these cells.
FIG. 6.
Relative to their function in H661 cells, retinoid receptors in H226Br cells are refractory to ligand-induced transcriptional activation. H661 and H226Br cells were transiently transfected with reporters (2 μg) containing a DR5 RARE under the control of a TK heterologous promoter (RARE-LUC) or a control vector (TK-LUC), treated for 24 h with 10−6 M t-RA, and subjected to luciferase assays. Results represent the means and standard deviations of luciferase values from five identical wells. (B) Northern analysis of RAR-β was performed on total cellular RNA (30 μg per lane) prepared from H661 and H226Br cells treated for 24 h with 10−6 M t-RA or medium alone. A photograph of an ethidium bromide-stained gel indicates relative amounts of RNA loaded per well.
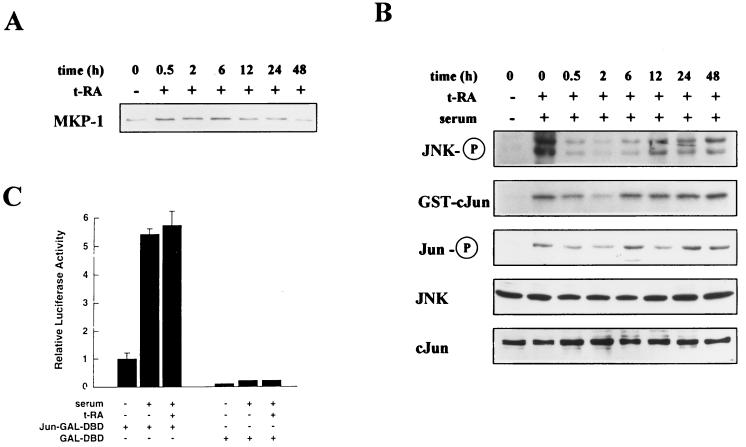
We hypothesized that H226Br cells would be deficient in the ability to activate MKP-1 expression and suppress JNK activity in response to t-RA treatment. Western analysis of MKP-1 expression by H226Br cells treated first with 10−6 M t-RA for different time periods and then for 20 min with 10% serum revealed a transient increase in MKP-1 detectable at 30 min that was, by 12 h, reduced to the level of untreated cells (Fig. 7A). In the same cell extracts, JNK phosphorylation was transiently decreased by t-RA, but a sustained reduction was not observed (Fig. 7B). t-RA transiently inhibited JNK activation and c-Jun phosphorylation in a pattern that correlated with JNK phosphorylation (Fig. 7B). JNK and c-Jun protein levels did not measurably change with t-RA treatment (Fig. 7B). Transient cotransfection assays using GAL4-based vectors demonstrated no detectable effect of t-RA on serum-induced c-Jun transcriptional activity (Fig. 7C). These results provide evidence that t-RA had transient, but no sustained, effects on MKP-1 expression and JNK activity in cells with retinoid receptors that are refractory to ligand-induced transcriptional activation, supporting the notion that t-RA inhibited JNK activity through two distinct pathways that are distinguishable on the basis of their requirement for retinoid receptor transcriptional activation. Further, these findings demonstrate that NSCLC cells can have a signaling defect involving JNK, an important modulator of cell growth, that is linked to retinoid receptor function.
FIG. 7.
t-RA does not activate MKP-1 expression or induce a sustained inhibition of JNK in H226Br cells. (A) Western analysis of MKP-1 expression was performed on H226Br cells treated with 10−6 M t-RA for the indicated time periods, followed by 10% serum for 20 min. (B) H661 cells were serum starved and then treated with medium alone or 10−6 M t-RA for the indicated time periods, followed by 10% serum for 20 min to activate JNK. Western analysis was performed with antibodies specific for JNK-1 and -2 dually phosphorylated on Thr-183 and Tyr-185 (JNK-P), c-Jun phosphorylated at serine 63 (Jun-P), JNK-1 (JNK), or total c-Jun (cJun). Using the same extracts, we performed immune complex assays with GST–c-Jun as a substrate. (C) Transient cotransfection assays were performed to measure JNK activity in H661 cells, using the GAL-UAS-LUC reporter and 1 μg of GAL-DBD or Jun-GAL-DBD expression vector as a substrate for JNK. Following transfection, the cells were treated for 24 h with (+) or without (−) 10−6 M t-RA, followed by 10% serum for 6 h, and then subjected to luciferase assays. Luciferase activities represent the means and standard deviations of values from five identical wells.
DISCUSSION
In this study, we investigated the mechanism by which t-RA inhibits JNK activity. We provide the first evidence that t-RA inhibits serum-induced JNK phosphorylation, suppressing JNK activity in a phosphatase-dependent manner. This has not been described previously and represents a novel mechanism by which retinoids regulate AP-1 activity. Interestingly, JNK suppression was biphasic, raising the possibility that t-RA inhibited JNK through more than one mechanism. Supporting this possibility, retinoid receptor transcriptional activation was required for the late, sustained JNK suppression but not for the early, transient effect of t-RA on JNK. Potentially contributing to the early suppression of JNK activity by t-RA, RARs interact with some proteins in a ligand-dependent manner, altering the activity of signal transduction pathways through mechanisms that do not require transcriptional activation of downstream genes (19). We also found that serum activated JNK through MKK-4-dependent pathways. This is consistent with prior observations that serum activates JNK through stimulation of phosphoinositol 3-kinase, which activates MKK-4 through Rac-dependent pathways (26, 32).
Evidence presented here suggests that t-RA inhibits AP-1 through its effects on JNK substrates. t-RA inhibited c-Jun phosphorylation at serine 63. Dephosphorylation at this site negatively regulates c-Jun transcriptional activity (38). We previously showed that in addition to inhibiting c-Jun transcriptional activity, t-RA decreased c-fos gene transcription through JNK inhibition (24). JNK activates the c-fos promoter by phosphorylating Elk-1 (42), a component of the ternary complex factor. Further, t-RA inhibits AP-1 through sequestration of the transcriptional coactivator CREB binding protein (CBP) (19). These findings suggest that t-RA inhibits AP-1 through multiple mechanisms and point to AP-1 as a central component of retinoid signaling. Our findings differ in some respects from previous observations. Caelles et al. (2) reported that suppression of UV light-induced JNK activity by dexamethasone required the presence of the glucocorticoid receptor (GR), but a transcriptionally inactive GR mutant was as efficient as wild-type receptor in suppressing JNK activity, and actinomycin D treatment did not block JNK suppression by dexamethasone, suggesting that GR inhibited JNK through a transcription-independent mechanism. In addition, dexamethasone did not change MKP-1 protein levels. One possible explanation for this disparity is that the mechanism by which steroid receptors inhibit JNK is specific to the JNK-activating stimulus (UV light or serum) and the cell type examined (lung cancer or HeLa cells). An alternative explanation is that GR and retinoid receptors differ in the mechanism by which they inhibit JNK. Previous studies support the notion that GR and retinoid receptors inhibit AP-1 through different mechanisms. GR directly associates with AP-1, converting AP-1 into a transcriptional suppressor (18, 21), while retinoid receptors interact with AP-1 indirectly through competition for CBP (19).
In this study, JNK phosphatase activity was detected in H661 NSCLC cells treated with t-RA. Several findings support the possibility that this activity reflects the presence of a dual-specificity phosphatase. First, the phosphatase activity was induced by t-RA, and most members of the MKP group are inducible (31, 40). Second, the phosphatase activity was inhibited by pervanadate and arsenite but not okadaic acid, which reflects the sensitivity of dual-specificity phosphatases to phosphatase inhibitors. Pervanadate inhibits all PTPases at concentrations of between 10 and 100 μM (49). Arsenite has been shown to inhibit the activity of low-Mr PTPases (3, 48), which include the dual-specificity phosphatases. Okadaic acid is a potent inhibitor of the serine-threonine protein phosphatases 1 and 2A (39) but not dual-specificity phosphatases (3). Third, we previously observed that t-RA inhibited the activity of ERKs (24), which are additional targets of dual-specificity phosphatases (31, 40). These findings point to a role for dual-specificity phosphatases in the suppression of JNK activity by t-RA. One candidate is MKP-1, which increased in abundance with t-RA treatment in this study. Although MKP-1 induction did not correlate with the late suppression of JNK activity, it clearly correlated with the early suppression, which is consistent with a role for MKP-1 in at least some of the effects of t-RA. Other dual-specificity phosphatases could also have contributed to the suppression of JNK activity by t-RA, and t-RA could have increased the activity of MKPs through mechanisms other than increasing their expression, such as posttranslational modification of MKPs by protein phosphorylation or farnesylation, which are important regulators of phosphatase activity (10, 37). Supporting a role for posttranslational regulation, MKP-1 protein stability was enhanced by t-RA in this study.
Retinoids potently inhibit the growth of normal HBE cells but have either no effect or minimally inhibit the growth of virtually all NSCLC cell lines (22, 30), including H661 and H226Br (data not shown). Growth inhibition by t-RA requires transcriptional activation of retinoid receptors, but retinoid receptor transcriptional activation does not occur in a subset of NSCLC cells lines (5, 29, 30). We found evidence that retinoid receptors were transcriptionally activated by t-RA in H661 cells but not in H226Br cells. A previous report on H661 cells differs from ours in that t-RA did not increase RAR-β mRNA but was similar in the activation of an RARE (47). These findings suggest that the basis of retinoid resistance in NSCLC cells is complex, involving disruption of retinoid signaling at the level of retinoid receptor function in some cells (H226Br) and events downstream of retinoid receptor activation in others (H661). The growth-inhibitory signals downstream of retinoid receptor activation that are dysregulated in NSCLC cells have not been defined. We propose JNK as a potentially important target of retinoid receptor activation. JNK has been implicated in the regulation of apoptosis, both positively and negatively (6, 12, 33, 44). However, findings presented here suggest that JNK inhibition is not sufficient to inhibit growth, as H661 cells were resistant to the growth-inhibitory effects of t-RA. Retinoids activate a number of other growth-inhibitory signaling pathways in normal HBE cells, including those activated by insulin-like growth factor binding proteins, transglutaminases, and transforming growth factor β (13, 45). Combined with JNK suppression, these pathways may inhibit cell growth in an additive or synergistic manner, and retinoid resistance may be conferred by the dysregulation of one or more of these pathways. Dysregulation of growth-inhibitory pathways occurs in cancer cells as a consequence of inactivating mutations in genes encoding adenomatous polyposis coli, transforming growth factor β receptor, SMAD-4, and pRB (reviewed in reference 16). It is postulated that these genes encode tumor suppressors, and inactivation of these gene products is central to the development of many types of cancer (16). Similarly, the transcriptional defect specific to retinoid receptors and the signaling abnormalities downstream of retinoid receptor activation may inactivate a tumor suppressor pathway, potentially contributing to lung cancer development.
ACKNOWLEDGMENTS
We thank Melanie Cobb (University of Texas at Southwestern Medical School) for helpful discussion.
This work was supported in part by NIH grants R29 CA67353 and P50 CA70907 (Lung Cancer SPORE).
REFERENCES
- 1.Apfel C, Bauer F, Crettaz M, Forni L, Kamber M, Kaufmann F, LeMotte P, Pirson W, Klaus M. A retinoic acid receptor α antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci USA. 1992;89:7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caelles C, González-Sancho J M, Muñoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J D, Evans R M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 5.Collins S J, Robertson K A, Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-α) Mol Cell Biol. 1990;10:2154–2163. doi: 10.1128/mcb.10.5.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosulich S, Clarke P. Apoptosis: does stress kill? Curr Biol. 1996;6:1586–1588. doi: 10.1016/s0960-9822(02)70779-3. [DOI] [PubMed] [Google Scholar]
- 7.Damm K, Heyman R A, Umesono K, Evans R M. Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc Natl Acad Sci USA. 1993;90:2898–2993. doi: 10.1073/pnas.90.7.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denu J M, Zhou G, Wu L, Zhao R, Yuvaniyama J, Saper M A, Dixon J E. The purification and characterization of a human dual-specific protein tyrosine phosphatase. J Biol Chem. 1995;270:3796–3803. doi: 10.1074/jbc.270.8.3796. [DOI] [PubMed] [Google Scholar]
- 9.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 10.DeSmedt F, Boom A, Pesesse X, Schiffmann S N, Erneux C. Post-translational modifications of human brain type I inositol-1,4,5-triphosphate 5-phosphatase by farnesylation. J Biol Chem. 1996;271:10419–10424. doi: 10.1074/jbc.271.17.10419. [DOI] [PubMed] [Google Scholar]
- 11.Glass C K, Lipkin S M, Devary O V, Rosenfeld M G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989;59:697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- 12.Goillot E, Raingeaud J, Ranger A, Tepper R I, Davis R J, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han G-R, Dohi D F, Lee H-Y, Rajah R, Walsh G L, Hong W-K, Cohen P, Kurie J M. All-trans retinoic acid increases transforming growth factor -β2 and insulin-like growth factor binding protein-3 expression through a retinoic acid receptor-α-dependent signaling pathway. J Biol Chem. 1997;272:13711–13716. doi: 10.1074/jbc.272.21.13711. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch D D, Stork P J S. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. J Biol Chem. 1997;272:4568–4575. doi: 10.1074/jbc.272.7.4568. [DOI] [PubMed] [Google Scholar]
- 15.Horlein A J, Näär A M, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass C K, Rosenfeld M G. Ligand-independent repression by the thyroid hormone receptor mediated by nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 16.Hussain S P, Harris C C. Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 1998;58:4023–4037. [PubMed] [Google Scholar]
- 17.Jetten A M, Vollberg T M, Nervi C, Grorge M D. Positive and negative regulation of proliferation and differentiation in tracheobronchial epithelial cells. Am Rev Respir Dis. 1990;142:S36–S39. doi: 10.1164/ajrccm/142.6_Pt_2.S36. [DOI] [PubMed] [Google Scholar]
- 18.Jonat C, Rahmsdorf H J, Park K-K, Cato A C B, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 19.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CPB integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 21.Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y-H, Dohi D F, Han G R, Zou C-P, Oridate N, Walsh G L, Nesbitt J C, Xu X-C, Hong W K, Lotan R, Kurie J M. Retinoid refractoriness occurs during lung carcinogenesis despite functional retinoid receptors. Cancer Res. 1995;55:5603–5610. [PubMed] [Google Scholar]
- 23.Lee H Y, Dohi D F, Kim Y H, Walsh G L, Consoli U, Andreeff M, Dawson M I, Hong W K, Kurie J M. All-trans retinoic acid converts E2F into a transcriptional suppressor and inhibits the growth of normal human bronchial epithelial cells through a retinoic acid receptor-dependent signaling pathway. J Clin Investig. 1998;101:1012–1019. doi: 10.1172/JCI1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H Y, Walsh G I, Dawson M I, Hong W K, Kurie J M. All-trans-retinoic acid inhibits jun N-terminal kinase-dependent signaling pathways. J Biol Chem. 1998;273:7066–7071. doi: 10.1074/jbc.273.12.7066. [DOI] [PubMed] [Google Scholar]
- 25.Lin A, Minden A, Martinetto H, Claret F-X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 26.Logan S K, Falaska M, Hu P, Schlessinger J. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangelsdorf D J, Umesono K, Evans R M. The retinoid receptors. In: Sporn M B, Roberts A B, Goodman D S, editors. The retinoids: biology, chemistry, and medicine. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1994. pp. 319–348. [Google Scholar]
- 28.Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases rac and cdc42. Cell. 1995;84:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 29.Moasser M M, DeBlasio A, Dmitrovsky E. Response and resistance to retinoic acid are mediated through the retinoic acid nuclear receptor gamma in human teratocarcinomas. Oncogene. 1994;9:833–840. [PubMed] [Google Scholar]
- 30.Moghal N, Neel B G. Evidence for impaired retinoic acid receptor-thyroid hormone receptor AF-2 cofactor activity in human lung cancer. Mol Cell Biol. 1995;15:3945–3959. doi: 10.1128/mcb.15.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muda M, Boscher U, Dickinson R, Martinou J C, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 32.Nimnual A S, Yatsula B A, Bar-Sagi D. Coupling of ras and rac guanosine triphosphates through the ras exchanger sos. Science. 1998;279:560–562. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 33.Nishina H, Fischer L D, Radvanyi L, Shahinian A, Hakem R, Rubie E A, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Stress-signalling kinase SEK1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 34.Oòate S A, Tsai S Y, Tsai M-J, O’Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 35.Potapova O, Haghigl A, Bost F, Liu C, Birrer M J, Gjerset R, Mercola D. The jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatinum. J Biol Chem. 1997;272:14041–14044. doi: 10.1074/jbc.272.22.14041. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez I R, Hughes T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:22–29. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 37.Shifrin V I, Davis R J, Neel B G. Phosphorylation of protein-tyrosine phosphatase PTP-1B on identical sites suggests activation of a common signaling pathway during mitosis and stress response in mammalian cells. J Biol Chem. 1997;272:2957–2962. doi: 10.1074/jbc.272.5.2957. [DOI] [PubMed] [Google Scholar]
- 38.Smeal T, Binetruy B, Mercola D A, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 39.Sugunama M, Fujiki H, Suguri H, Yoshizawa S, Hirota M, Nakayasu M, Ojika M, Wakamatsu K, Yamada K, Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci USA. 1988;85:1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H, Charles C H, Lau L F, Tonks N. MKP-1 (3CH134), an immediate early gene product, is a dual-specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 41.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 42.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 43.Wu Z, Wu J, Jacinto E, Karin M. Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol Cell Biol. 1997;17:7407–7416. doi: 10.1128/mcb.17.12.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L-X, Mills K J, Dawson M I, Collins S J, Jetten A M. Evidence for the involvement of retinoic acid receptor RAR-α-dependent signaling pathway in the induction of tissue transglutaminase and apoptosis by retinoids. J Biol Chem. 1995;270:6022–6029. doi: 10.1074/jbc.270.11.6022. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W W, Fang X, Mazur W, French B A, Georges R N, Roth J A. High-efficiency gene transfer and high-level expression of wild-type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther. 1994;1:5–13. [PubMed] [Google Scholar]
- 47.Zhang X-K, Liu Y, Lee M-O, Pfahl M. A specific defect in the retinoic acid response with human lung cancer cell lines. Cancer Res. 1994;54:5063–5069. [PubMed] [Google Scholar]
- 48.Zhang Z Y, Zhou G, Denu J M, Wu L, Tang X, Mondesert O, Russell P, Butch E, Guan K L. Purification and characterization of the low molecular weight protein tyrosine phosphatase, Stp 1, from the fission yeast Schizosaccharomyces pombe. Biochemistry. 1995;34:10560–10568. doi: 10.1021/bi00033a031. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Z, Tan Z, Diltz C D, You M, Fischer E H. Activation of mitogen-activated protein kinase pathway by pervanadate, a potent inhibitor of tyrosine phosphatases. J Biol Chem. 1996;271:22251–22255. doi: 10.1074/jbc.271.36.22251. [DOI] [PubMed] [Google Scholar]