Abstract
The β-cyclodextrin shell of synthesized silver nanoparticles (βCD-AgNPs) are found to enhance the detection of hydrogen peroxide in urine when compared to the Horse Radish Peroxidase assay kit. Nanoparticles are confirmed by the UV-Vis absorbance of their localized surface plasmonic resonance (LSPR) at 384 nm. The mean size of the βCD-AgNPs is 53 nm/diameter; XRD analysis shows a face-centered cubic structure. The crystalline structure of type 4H hexagonal nature of the AgNPs with 2.4 nm β-CD coating onto is confirmed using aberration corrected high-resolution transmission electron microscopy (HRTEM). A silver atomic lattice at 2.50 Å and 2.41 Å corresponding to (100) and (101) Miller indices is confirmed using the HRTEM. The scope of βCD-AgNPs to detect hydrogen peroxide (H2O2) in aqueous media and human urine is investigated. The test is optimized by examining the effect of volumes of nanoparticles, the pH of the medium, and the kinetic and temperature effect on H2O2 detection. The βCD-AgNPs test is used as a refined protocol, which demonstrated improved sensitivity towards H2O2 in urine compared to the values obtained by the Horse Radish Assay kit. Direct assessment of H2O2 by the βCD-AgNPs test presented always with a linear response in the nM, μM, and mM ranges with a limit of detection of 1.47 nM and a quantitation limit of 3.76 nM. While a linear response obtained from 1.3 to 37.3 nmoles of H2O2/mole creatinine with a slope of 0.0075 and regression coefficient of 0.9955 when the βCD-AgNPs is used as refined test of creatinine. Values ranging from 34.62 ± 0.23 nmoles of H2O2/mole of creatinine and 54.61 ± 1.04 nmoles of H2O2/mole of creatinine when the matrix is not diluted and between 32.16 ± 0.42 nmoles of H2O2/mole of creatinine and 49.66 ± 0.80 nmoles of H2O2/mole of creatinine when the matrix is twice diluted are found in freshly voided urine of seven apparent healthy men aged between 20 and 40 years old.
Keywords: β-cyclodextrin, silver nanoparticles, reactive oxygen species, hydrogen peroxide, creatinine
1. Introduction
Reactive oxygen species (ROS) are very reactive unstable products of oxygen metabolism in all organisms, formed through different mechanisms. They are generated in mitochondria as result of energy production, or also as part of an antimicrobial or antiviral responses [1]. The process of generating ROS can be favored under exogenous stimuli such as environmental pollutants’ exposures, UV radiation, air pollution, and cigarette smoking [2]. ROS are used as biomarkers to gain information about the status of biological redox system, progression, and state of diseases and the status of health enhancement by enzymatic and non-enzymatic antioxidants in humans [3]. ROS are found to have a direct role in cell signaling namely: gene expression, apoptosis, and overall activation of cell signaling cataracts, and could serve as intra and intercellular messengers [4,5]. Mitochondrial electron transport process is found to be responsible for generating superoxide () which is converted to hydrogen peroxide (H2O2) under the effect of superoxide dismutase or even under spontaneous dismutation [3]. In metal catalyzed reactions ROS are necessary intermediates that host electrons released by the oxidation reactions. Superoxide, for instance, is produced by electron transfer to O2 at Qo site of ubiquinol-cytochrome c oxidoreductase, a complex formed from large number of polypeptides, three heme groups, and a Fe-S center [6]. Atomic oxygen has two lone pairs of electrons that occupy two different orbitals, which makes it more vulnerable to changes especially radicals’ formation, the consecutive acceptance of electrons by oxygen lead to the formation of many ROS species, namely: superoxide (); hydrogen peroxide (); hydroxyl radical (); and hydroxyl ion (). Other oxygen-containing ROS includes: alkoxy radicals (), nitric oxide (), peroxyl radical ), sulfate radical (), and lipids hydroperoxides (LOOH), all these ROS share the property of being able to cause oxidation of essential components of the cell [7]. The imbalance between the produced and removed ROS is referred to as “oxidative stress”. All survival organisms detoxify ROS through defense mechanisms that provide a balance between produced and removed ROS [8]. Oxidoreductase enzymes, such as superoxide dismutase catalyze the reaction of conversion of two superoxide molecules to one hydrogen peroxide molecule and one molecular oxygen [9]. Hydrogen peroxide is considered as important biomarker between other ROS due to superoxide’s dismutation by the superoxide dismutase (SOD) which acts as first line defense against oxidative stress [10]. In addition of being a major product of oxidase catalyzed reactions, H2O2 level is responsible for many cellular damages [11,12,13]. Sensing of H2O2 is significant in many fields, mainly medical and environmental, measuring the concentration of H2O2 in micro and nano levels is essential to control quantities of many important biomolecules in our diet such as carbohydrate, lipids, and proteins, and their monomeric molecules [14]. Many analytical assays have been used to quantify the concentration of H2O2 such as electrochemical [15,16,17,18], enzymatic [19,20,21], and colorimetric assays are still the most attractive due to their simplicity and cost effective [22,23,24,25,26]. The disadvantage of the analytical methods based on colorimetric detection of H2O2, is mainly due to their low sensitivity. A satisfactory reproducibility and high sensitivity for H2O2 detection is therefore difficult to obtain.
Recently there has been a lot of focus on metals nanoparticles characteristics for ROS detection methods [27,28]. This is due to their small size and extraordinary optical, magnetic, catalytic, and powered properties of nanoparticles that are not found in bulk materials [29,30]. Based on the hypothetical attributes of the nanomaterials such as nanoparticles, nanotubes and nanofibers these are used in the form of quantum dots (QDs) or noble metal nanoparticles (NPs); for instance, gold and silver for the development of chemical sensors and biosensors [31,32].
On the other hand, cyclodextrins are macromolecule oligosaccharides that occur naturally, and consist of six, seven, and eight glucose cycles that are joined by glycosidic bonds. They are created from enzymatic starch conversion [33]. Cyclodextrins are arranged like cones, and they can host small molecules, such as aromatic molecules and steroids inside their cavities. The interior cavity has a lipophilic character. However, the external surface of cyclodextrins is very soluble in water. This is what explain their highest solubility in water [34].
The encapsulation character of cyclodextrins lead to their wide use in drug delivery. β-cyclodextrin is occupying a middle position because of its moderated size cavity of 0.6 nm compared to α and γ-cyclodextrins [35]. Herein, a novel strategy based on mediated creatinine encapsulation by the β-cyclodextrin organic shell of the nanoparticles is developed. Due to the hosting ability of the β-cyclodextrin, the creatinine could easily be hosted by the shell and improves the stability and reproducibility of H2O2 concentration in urine. The test results are validated through comparison with previous assays and a commercially available assay kit.
2. Materials and Methods
2.1. Chemicals
All chemicals are purchased from Sigma Aldrich through a local company (Labcoltd-Dubai, UAE), they are used without any further purification as received unless otherwise stated. Soluble β-cyclodextrin (C42H70O35), sodium borohydride (NaBH4), hydrogen peroxide (H2O2, 35% w/w, d = 1.13 g/mL), buffer pH = 4.0 (0.1 M citric acid/sodium hydroxide), buffer pH = 7.0 (0.1 M potassium dihydrogen phosphate, disodium hydrogen phosphate, 12 hydrates), buffer pH = 10.0 (0.1 M borax/sodium hydrate), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, 99.95%), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, analytical grade), copper(II) sulfate pentahydrate (CuSO4·5H2O, analytical grade), Manganese(II) nitrate tetrahydrate (Mn(NO3)2·4H2O, analytical grade), nickel(II) nitrate (Ni(NO3)2, analytical grade), lead(II) nitrate (Pb(NO3)2, ACS reagent), zinc chloride (ZnCl2, ACS reagent) and sodium fluoride (NaF, ACS reagent, 99.99%) urea (CO(NH2)2, ACS reagent), D(+)-glucose (C6H12O6, anhydrous), ascorbic acid (C6H8O6, ≥99%) are purchased from Sigma Aldrich. Silver nitrate (AgNO3, 99.9+%) is purchased from Alfa Aesar, and ultrapure deionized water (from Millipore) is employed to formulate aqueous solutions. Hydrogen peroxide assay kits are acquired from Biomedical Scientific Services LLC, Al-Ain, UAE.
2.2. Preparation of β-CDAgNPs
β-cyclodextrin capped nanoparticles are prepared subsequent the modified procedure described by Yingju et al. [36]. In a 500 mL one neck round bottom flask, 200 mL of saturated solution of β-cyclodextrin (0.2% w/v) is stirred for a period of 30 min, then 3.0 mL of a 0.1 M solution of AgNO3 as source of Ag+ and 6.0 mL of 0.1 M of newly prepared NaBH4 are added to the mixture. A color change occurs instantly after the addition of NaBH4 from uncolored to greenish. This is a strong signal of the creation of silver nanoparticles. The solution is stirred for an additional time of 30 min. The produced nanoparticles are stable for a period of more than 6 months; nanoparticles are centrifuged to ×4000 rpm for 10 min preceding any utilization. The β-CDAgNPs are brought to analysis and afterward are used for the detecting of H2O2. Because the absorbance of the bare nanoparticles is very high, nanoparticles are diluted 3 times in all experiments.
2.3. Optimization of the H2O2 Detection by β-CDAgNPs
The detection of H2O2 by β-CDAgNPs is optimized using batch equilibrium experimentations at room temperature. Preliminary and final concentrations of H2O2 are assessed from Δabs = Aref − Asample (measured between the reference and the sample) using a UV-vis spectrophotometry. The effect of the volume of nanoparticles used is assessed as follow; in 9 test tubes, volumes of 0.75 mL of fixed concentration of H2O2 (40 mM), are reacted with volumes ranging from 0.30 to 2.70 mL of the nanoparticles’ solution. Experiments are completed at the pH of the suspension without modification (pHi). After achieving equilibrium, the solutions are analyzed using UV-vis spectrophotometry. While the effect of pH on the detection of H2O2 is assessed by adding H2O2 concentration spanning in the range of 5.0 to 50.0 mM, solutions are prepared from a 50 mM stock solution. To 10 test tubes containing each 0.75 mL of the nanoparticles’ solutions, 0.3 mL of H2O2 with concentration varying between 5.0 and 50.0 mM are added. Tubes are left to equilibrate for 3.0 min and then absorbances of samples and the reference (βCD-AgNPs) are recorded between 200 and 800 nm using the UV-vis spectrophotometry. Similar experiments are repeated in pH = 4.0 and pH = 10.0 using corresponding 0.1 M buffers to fix the pH. To study the effect of time (kinetic of the reaction), 0.3 mL of 40.0 mM H2O2 is prepared then added to 0.75 mL of β-CDAgNPs, absorbances are recorded at different timing from 0.0 to 10.0 min by UV-Vis spectrometry. The effect of temperature is studied to gain information about the spontaneity of the process. For this purpose, the kinetic of 0.3 mL of 40.0 mM H2O2 mixed with 0.75 mL of β-CDAgNPs is studied at three different temperatures of 25 °C, 37 °C, and 45 °C.
2.4. H2O2 Detection in Urine and Limit of Detection
The βCD-AgNPs test is compared with a peroxidase assay test as reference. The peroxidase assay allows the development of a pink color when Horse Radish peroxidase (HRP) is mixed with the OxiRed probe and H2O2 sample. The working standards of peroxidase assay are made ranging from 1.0 to 10.0 nmoles of H2O2.
Working standards of the H2O2 detection using βCD-AgNPs are made in three different ranges 4.7 to 32.0 nM, 4.7 to 32.0 μM and 4.7 to 32.0 mM by mixing varying volumes from 0.1 to 2.8 mL of their parent stock solution of 40.0 nM, 40.0 μM, and 40.0 mM with 0.75 mL of βCD-AgNPs. A corrected creatinine H2O2 test is made in the 1.33 to 37.3 nmoles of H2O2/mole of creatinine by mixing varied volumes of 0.1 to 2.8 mL of 40.0 nM H2O2 with 0.75 mL of βCD-AgNPs in the presence of 300 µL of 1.0 mM creatinine.
Midstream urine samples were collected from seven healthy-looking men volunteers between 20 and 40 years in age. The collected samples were stored at 4 °C for a period of 12 h followed by centrifugation for 15 min at ×1000 rpm before use. H2O2 concentrations are measured in triplicates in the samples’ supernatants. To reduce the effect of the matrix, analysis of H2O2 was studied in three sets: the pure urine samples, twice and ten times diluted urine samples. Stock solutions and working standards of H2O2 are prepared from the 30% concentrated H2O2, from which 0.88 mM and 0.2 mM are gradually diluted using MilliQ ultra-pure water with a resistivity of 17.5 MΩ·cm (Fisherbrand Accu20 Ultrapure Water System, Loughborough, UK). Then, working standards ranging from 1.0 to 10.0 nmoles/microplate are freshly prepared from 0.2 mM using 0.1 M buffer solution supplied with the assay kit. The standards as well as urine samples were treated similarly as mentioned in Abcam Assay Kit’s procedure: 46 µL of 0.1 M buffer, 2 µL of OxiRed probe, and 2 µL of HRP are blended, forming a reaction mixture to which 50 µL of the standard or sample is added. The working standards and urine samples are incubated for 3 h, prior to absorbances recording versus the reaction mixture blank for the peroxidase assay kit at 570 nm. However, for the βCD-AgNPs assay, 150 µL of the working standard or sample are mixed with 150 µL of the nanoparticles. Samples and working standards are incubated for 40 min, and absorbances are recorded at 384 nm against the 0.1 M buffer as a blank. The limit of detection (LOD) of βCD-AgNPs assay test is verified using the standard deviation of the absorbance and slope of the calibration curve after the equation LOD = 3.3 σ/S [29], where σ is the standard deviation of three reading of the lowest possible concentration, made by mixing 0.75 mL of βCD-AgNPs with 100 µL of H2O2 to give a total volume of 0.95 mL. The limit of quantitation (LOQ) is defined using the equation LOQ = 10 Lv/S, where Lv, is the lowest possible concentration identified by the test. S signifies the slope of the calibration curve in the nM range. Conversion between molar concentration and moles of H2O2 is made by multiplying the concentration by 300 μL, representing the volume of the microplate’s well. Measurements are made in triplicates and presented as Mean ± SD.
2.5. Instrumentation
The XRD analysis are made on a Bruker D8 Analytical diffractometer operated by the software DIFFRAC.SUITE at 40 kV and 40 mA, equipped with a copper anode and a graphite monochromator to select CuKα1 radiation (λ = 1.540 Å). Diffractions are recorded from 2θ = 5.0° to 80° with step increase of 0.03° and time of 0.5 s/step. To determine the abundance of elements in βCD-AgNPs, EDS is made concomitantly with SEM using a Tescan VEGA XM electron microscope machine provided with an X-Max 50 EDS detector’s Oxford Instruments (Tescan, Brno, Czech Republic), at 125 eV resolution and managed by the AZtecEnergy analysis software. The software VEGA TC is used to record SEM images of the βCD-AgNPs using an accelerating voltage of 30 kV. TEM images are recorded by transferring the βCD-AgNPs onto the TEM grid (Electron Microscopy Sciences 300 mesh grid made of a carbon layer deposited on copper). The aberration corrected TEM (Hillsboro, Oregon, USA, Thermo Fisher Scientific formerly FEI, Titan G2) is used for the structural characterization of the βCD-AgNPs, run at 300 kV and using spherical aberration)-correction. The hydrodynamic sizes of the nanoparticles are measured using the Nanotrac Wave II DLS analyzer (Microtrac, Pennsylvania, USA) working between 0.8 nm and 6.5 and is run for 15 cycles at a 90° angle and 26 °C.
3. Results and Discussion
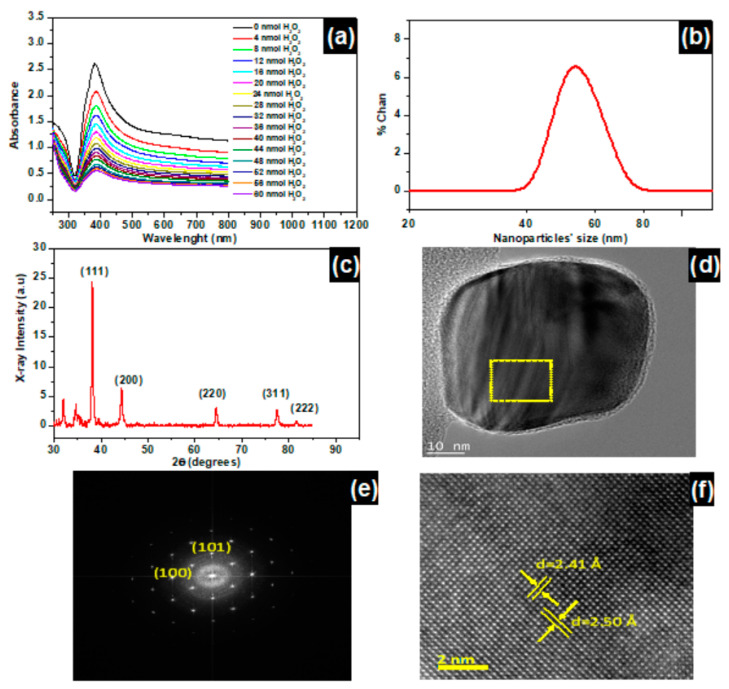
Figure 1a represents the UV-Vis absorbance of the surface plasmon peaks of βCD-AgNPs with a characteristic peak at 384 nm. This peak is found to decrease as the concentration of H2O2 increases. The color of the nanoparticles faded from a green olive color to uncolored with the increase of the concentration of H2O2 (Figure S1, Supplementary Materials), suggesting a proportionality between the color change of the βCD-AgNPs and concentration change of H2O2. Consequently, color change is exploited as an optical test for the hydrogen peroxide detection.
Figure 1.
(a) βCD-AgNPs LSPR absorbances recorded at λmax = 384 nm as function of H2O2 concentration increase, and nanoparticles diluted 3 times. (b) Size of βCD-AgNPs using dynamic Light scattering (DLS). (c) X-ray diffraction of βCD-AgNPs. (d) HRTEM micrograph of a typical 50 nm βCD-AgNPs with 2.4 nm of βCD macromolecule coating (marked between two yellow lines in the image). (e) Fast Fourier Transform HRTEM image with Miller indices of the electron diffraction, indicating the crystalline structure of type 4H hexagonal nature of the nanoparticle. (f) HRTEM micrograph of chosen area marked with square in (d), showing that the interplanar distances are 2.50 Å and 2.41 Å corresponding to (100), (101) Miller indices.
The color change of the βCD-AgNPs, from green olive to uncolored, is accompanied by a gradual decrease in the absorption intensity of the characteristic plasmonic peak of the βCD-AgNPs at 384 nm due to atomic dissolution of βCD-AgNPs in the presence of H2O2, and this explanation is in accordance with the proposed mechanism by which the βCD-AgNPs are oxidized in the presence of H2O2 to release Ag+ to the medium. H2O2 may induce aggregation of the AgNPs, as is indicated by Wang et al. [37] during the regeneration of AuNPs from an aromatic thiol (ArSH) dissolved AuNPs solution. However, aggregation cannot be an overall mechanism of βCD-AgNPs interaction with H2O2 because the resulting solution does not have any absorbance.
The first parameter to check when optimizing a test is its selectivity, the βCD-AgNPs are mixed volume by volume with Ca2+, Co2+, Cu2+, Mn2+, Ni2+, Pb2+, Zn2+, F−, urea, glucose, and ascorbic acid as possible interfering species with H2O2. Little changes are assessed in the case of all interfering species, with the most pronounced change found for H2O2, results of this analysis are presented in Figure S2 (Supplementary Materials). Energy Disperse Spectroscopy (EDS) presented in Figure S3 (Supplementary Materials) demonstrates the presence of silver, carbon, and oxygen, and proves that β-cyclodextrin macromolecules successfully capped the silver metal. The weight percent of the silver is found to be the highest at 44.1 wt% followed by oxygen and carbon.
The βCD-AgNPs size distribution is determined using the dynamic light scattering (DLS) and the aberration-corrected high-resolution transmission electron microscopy (HRTEM) represented in Figure 1b,d (and Figure S4 in Supplementary Materials), respectively. The DLS size is found to be 52.0 nm/dimeter, however the HRTEM shows regular spherical shaped nanoparticles with average size varying between 19.4 and 53 nm/dimeter, and the high diameter presented by DLS has been attributed to the DLS measuring the hydrodynamic size of the particle which is the size of the βCD macromolecules shell and the liquid layer all around the particle. Shells may be involved in various forms of intermolecular forces (van der Waals forces and hydrogen bonding), while the HRTEM gives the actual size of the nanoparticle [38]. Zheng et al. demonstrated, however, that the hydrodynamic diameters of the nanoparticles can significantly vary with nanoparticles’ concentration and incident beam used in the instrument [39].
Figure 1c represents the XRD pattern of an oven dried βCD-AgNPs at 100 °C for a period of 24 h. The peaks at 38.12°, 44.35°, 64.60°, 77.33°, and 81.45° are correlated to the (111), (200), (220), (311), and (222) reticular planes of the crystalline Ag of the βCD-AgNPs [40]. An enlargement of the βCD-AgNPs peaks in comparison to metallic silver (PDF no. 04–0783), is assigned to a nanosized Ag in the βCD-AgNPs material [41]. The Ag atomic lattice of the nanoparticles is also confirmed using HRTEM (Figure S5 in supplementary materials), Figure 1d presents a selected 50 nm βCD-AgNPs with 2.4 nm of βCD coating. Fast Fourier Transform HRTEM image with Miller indices of the electron diffraction is shown in Figure 1e. The image clearly shows the crystalline structure of type 4H hexagonal nature of the nanoparticle. The interplanar distances in HRTEM are 2.50 Å and 2.41 Å correspond to (100), (101) Miller indices [42].
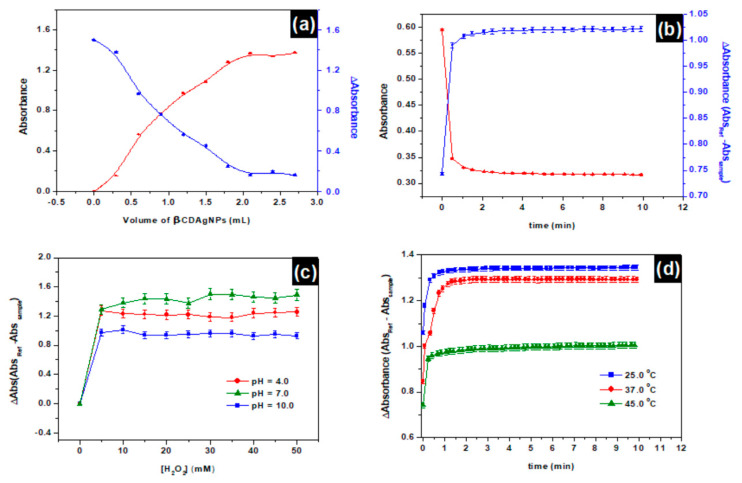
Figure 2a represents the evolution of absorbances of different samples at different volumes of βCD-AgNPs. An increase in the concentration of βCD-AgNPs (volume increase) is faced with a decrease in the sample’s absorbance, ΔAbs represents the difference between the reference unreacted βCD-AgNPs minus the absorbance of the sample, which is found to increase with concentration increase of H2O2. Even though the volume increase of βCD-AgNPs nanoparticles is expected to increase the number of active sites of interaction with H2O2, however, the number of H2O2 molecules circumvent the sites of interaction’s number provided by the material. At 2.0 mL of βCD-AgNPs, enough active sites of interactions have been provided by the material to interact in a mole-to-mole ratio with the H2O2 in the medium. After this point, the number of active sites provided exceed the required number and a saturation is observed on words. The kinetic of H2O2 interaction with βCD-AgNPs is presented in Figure 2b, it can be observed that increasing the time of the reaction lead to direct increase in the ΔAbs until it reaches a plateau. In the beginning, the material has many sites of interaction, with time increase these sites are occupied by H2O2, while the time proceeding these sites are fully occupied by H2O2 and therefore saturation is achieved. The kinetic of the reaction is found to be very fast, equilibrium is attained at time less than 0.5 min, the sites of interaction of βCD-AgNPs almost consumed after 0.5 min to attain equilibrium.
Figure 2.
(a) Effect of βCD-AgNPs volume on the detection of H2O2, Experiment condition [H2O2] = 40 mM, equilibrium time (3 min), pHi = 9.28, T = 22.5 °C. (b) Impact of time (kinetic) of H2O2 recognition by βCD-AgNPs as a function of equilibration time at 25 °C; [H2O2]i = 40 mM; V (AgNPs)= 0.75 mL; pHi (βCD-AgNPs) ~ 9.28; (c) Effect of different pH on H2O2 by βCD-AgNPs. Experiment condition [H2O2] = from 1.0 to 50.0 mM increasing with a step of 5.0 mM, equilibration time (3 min), T = 25 °C. (d) Kinetic of the effect of temperature on H2O2 detection by βCD-AgNPs; V(βCD-AgNPs) = 0.75 mL; pH = 9.28; [H2O2]i = 40.0 mM; Absorbances in all experiments are recorded at LSPR λmax = 384 nm and nanoparticles were diluted 3 times.
Figure 2c shows an increase in ∆Abs by increasing the concentration of H2O2 until ∆Abs reaches a plateau for the three pH (pH = 4.0, pH = 7.0, and pH = 10.0). The ΔAbs is lower in alkaline pH compared to neutral pH = 7.0 and acidic pH = 4.0, lower ∆Abs corresponds to a higher Abs, which is indicative that the nanoparticles are more stable in alkaline mediums, this behavior could be explained based on hydroxide anions could enhance aggregation of the nanoparticles. The acidic pH = 4 is still presenting a ΔAbs lower (high Abs) than the neutral pH = 7.0 which may be explained by competition reaction between the hydronium ion and H2O2. From Figure 2d, it can be concluded that βCD-AgNPs operate very well at high temperature, since 45 °C is presented with lowest ∆Abs (highest Abs). Thirty-seven degrees, the physiological temperature where most of the biological samples are extracted, presented with the second lowest ∆Abs when adopted for biological analysis. The temperature increases the speed of collision between the H2O2 and sites of interactions on the βCD-AgNPs. Increasing the temperature slows down the process. The reaction is therefore classified as exothermic.
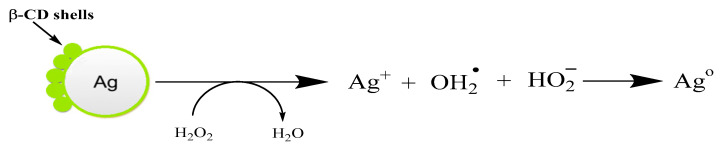
The mechanism of interaction of βCD-AgNPs, with H2O2 represented in Figure 3, is based on the oxidative dissolution of the nanoparticles to release Ag+ and other reactive oxygen species such as OH• which will further dissolute the nanoparticles. Molleman and Hiemstra [43] demonstrated that the shape of the AgNPs plays a big role in their reactivity, and they found that nanorods and nanoprisms are more reactive than nanospheres because they exhibit exposed facets and therefore react faster.
Figure 3.
Schematic representation of the mechanism of βCD-AgNPs reaction with hydrogen peroxide.
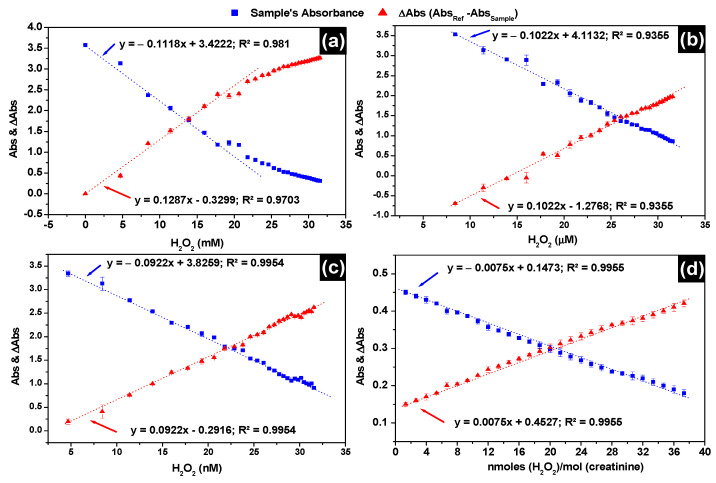
To measure the level of H2O2 in patients’ urine, calibration curves are established in mM, μM and nM concentration ranges, which are presented in Figure 4a–c, respectively. Markedly, the βCD-AgNPs test presented with a large linear range from 5.0 nM through the whole μM range up to 17.7 mM concentration (Figure 4a) where the deviation from linearity start showing. In Figure 4d, this is represented by a refined protocol of H2O2 response in the presence of 10 mM of creatinine. The sensitivity is almost 12-folds lower, using the refined protocol with linearity maintained from 1.3 up to 37.3 nmoles of H2O2/mole of creatinine. This decrease in sensitivity is due to the encapsulation creatinine with β-CD shell of the nanoparticles. β-CD is well known to form 1:1 complexes with neutral guest species, in solution, the nonpolar cavity of β-CD is occupied with water molecules which is not favorable energetically. Water molecules are substituted with appropriate guest molecules such as creatinine that are less polar than water molecule. Limit of H2O2 detection using βCD-AgNPs is determined in the nM calibration curve applying the equation LOD = 3σ/S, where σ is the standard deviation of the response of three urine samples replicates at wavelength of 384 nm. The limit of detection is found to be 1.47 nM when Absorbance of βCD-AgNPs is used directly to assess H2O2, while 0.041 nM is found when ΔAbs is used instead. The detection limit is very low compared to other H2O2 sensors reported in Table 1. The limit of quantitation (LOQ) is found from the formula: LOQ = 10(Sy/S), where Sy and S are the slope and standard deviation of the response obtained from the calibration of the test. A LOQ of 3.76 nM is found for the βCD-AgNPs test when Abs is used and 0.14 nM when ΔAbs is used, indicating that ΔAbs always yield lower LOD and LOQ compared to Abs.
Figure 4.
Calibration curves of H2O2 detection using LSPR absorbances of the βCD-AgNPs and LSPR ΔAbsorbance between the reference and the sample (a) 4.7 to 32.0 mM H2O2 range; (b) 4.7 to 32.0 µM H2O2 range; (c) 4.7 to 32.0 nM H2O2 range; (d) 1.3 to 37.3 nmoles of H2O2/mole of creatinine range in the presence of 10 mM creatinine. Absorbances in all experiments are recorded at LSPR λmax = 384 nm and nanoparticles were diluted 3 times.
Table 1.
Analytical performance of the βCD-AgNPs during H2O2 detection in comparison with other H2O2 non-enzymatic tests.
| Method | Limit of Detection (LOD) | Linear Range | Reference |
|---|---|---|---|
| Cu/Electrochemically reduced graphene oxide | 1.87 nM | 0.01–1 mM | [44] |
| CoHCF Nanoparticles | 9.31 μM | 8–7500 μM | [45] |
| Co-NC/NF | 10 μM | 0–5.0 mM | [28] |
| GC/ILC/(CNT-Fe-Ni) | 0.971 nM | 0.007–1000 μM | [46] |
| N, S co-doped G/CNT-Fe3C | 272 nM | 0.002–10.37 mM | [47] |
| Ti/ML-TNT/HRP/Nafion | 12 nM | 41.2 nM–6.0 μM | [48] |
| CuCo2O4/rGO | 80 nM | 30–5010 μM | [49] |
| Brown Algae AgNPs | 8.6 nM | 1–120 μM | [50] |
| Algae-AgNPs | 1.77 nM | 4.70–32.0 nM | [51] |
| Abs of βCD-AgNPs | 1.47 nM | 5.0–31.0 nM | This work |
| ΔAbs of βCD-AgNPs | 0.041 nM |
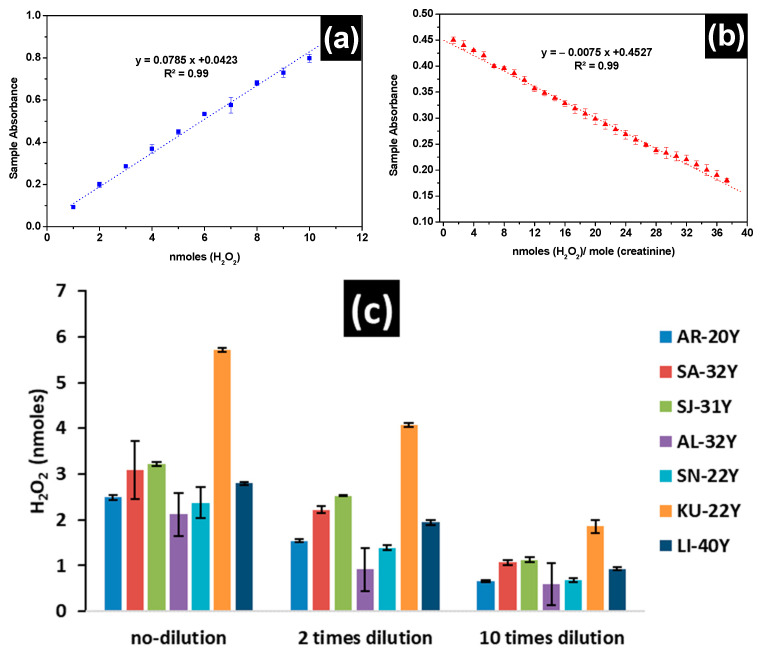
In Figure 5a,b the calibration curves used to assess the levels of H2O2 in freshly voided midstream urine of the 7 healthy men aged between 20 and 40 years are presented, using the assay kit and the LSPR of βCD-AgNPs, respectively. Linear responses are obtained for both tests. For the assay kit a linear response was in the range of 1.0 nmoles to 10.0 nmoles (10–100 μM) with a slope of 0.0785 and a regression coefficient of 0.995, while a linear response 1.33 to 37.3 nmoles of H2O2/moles of creatinine with a slope of 0.0075 and regression coefficient of 0.9955 when the βCD-AgNPs test is used in the presence of 10.0 mM of creatinine. The concentration of H2O2 is dependent on the dilution for all participants, except KU-22, all participants showed lower concentration of H2O2 when samples are diluted 10 times and the assay kit is used (Figure 5c). The average concentration of H2O2 in urine using the assay kit is found to vary between 2.11 ± 0.048 nmoles (21.1 ± 0.48 μM) and 3.22 ± 0.046 nmoles (32.2 ± 0.46 μM) when the matrix is not diluted, between 1.38 ± 0.042 nmoles (13.8 ± 0.42 μM) and 2.52 ± 0.019 nmoles (25.2 ± 0.19 μM) when the matrix is diluted twice and between 0.60 ± 0.051 nmoles (6.0 ± 0.51 μM) and 1.12 ± 0.050 nmoles (12.1 ± 0.50 μM) when the matrix is diluted 10 times. In this current study, the H2O2 total concentration in freshly voided urine in the 7 healthy subjects using the assay kit are comparable to values reported by Long et al. [52] who found values between 11 and 173 μM when analyzing freshly secreted urine of subjects aged from 19 to 38 years old using oxoglutarate decarboxylation assay. H2O2 levels of 17.28 μM are also reported in normal participants not suffering from any disease [53]. However, very high concentrations of H2O2 were noticed in ill subjects, 89.10 8.73, 85.37 15.45, 91.35 17.79, and 68.02 7.23 μM are reported in subjects suffering from helminth + protozoa, protozoa only, helminth and intestinal parasitic, respectively. Lower levels of H2O2 in the order of 0.84 to 5.71 µM are also reported in fasting subjects [54].
Figure 5.
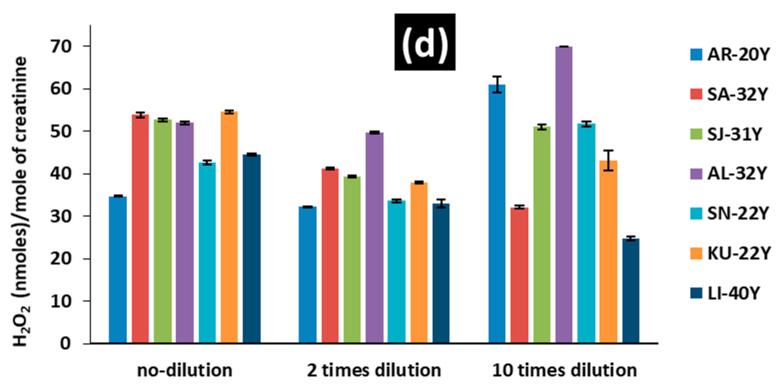
Calibration curves used for the measurement of H2O2 levels in 7 patients’ urine samples (a) using horse radish commercial assay kit, (b) using LSPR sample absorbance when mixed with βCD-AgNPs in the presence of 10 mM creatinine, and (c,d) H2O2 levels of freshly voided midstream urine of the 7 healthy participants (all men) aged between 20 and 40 years old, using the commercial assay kit and βCD-AgNPs, respectively.
The detection and reproducibility of H2O2 in urine is enhanced when the creatinine corrected test is used instead of direct test for H2O2, because of the probable interaction between creatinine and H2O2. Even though no clear reaction has been documented in the literature, Hanif et al. [55] proposed a mechanism for the interaction of creatinine with H2O2 in the presence of a catalyst such as Co2+ which generates a tetrahedral spirodioxetane intermediates. The unstable spirodioxetane intermediates decompose and results in the formation of an excited-state species with concomitant elimination of CO2.
In terms of creatinine corrected H2O2 levels in urine, βCD-AgNPs test shows values ranging from 34.62 ± 0.23 nmoles of H2O2/mole of creatinine and 54.61 ± 1.04 nmoles of H2O2/mole of creatinine when the matrix is not diluted and between 32.16 ± 0.42 nmoles of H2O2/mole of creatinine and 49.66 ± 0.80 nmoles of H2O2/mole of creatinine when the matrix is twice diluted. When the matrix is diluted 10 times, the level of H2O2 is not consistent, yet it increases for AR-20, AL-32, and SN-22, while decreasing for SA-32, SJ-31, KU-22, and Li-40. This lack of consistency in the test results at 10-times dilution could be attributed to the looseness of the nanoparticles LSPR at high dilutions. Minimal data are available in the literature about creatinine corrected H2O2 concentration. Yuen et al. [56], for instance, reported values between 90–1164 μmoles H2O2/mole creatinine using a refined protocol taking into account the presence of creatinine in the H2O2 matrix that increases the concentration of H2O2. These values are significantly higher than values found for βCD-AgNPs test and the variations are mainly due to the stability and biological variations of the sample which limit the use of hydrogen peroxide in urine as a potential biomarker of whole-body oxidative stress.
4. Conclusions
βCD-AgNPs have been successfully synthesized, and the synthesized NPs are characterized by UV−visible, DLS, EDX, XRD, and HRTEM analysis. The assay using βCD acted as a shell to cap and stabilize silver nanoparticles. Furthermore, a simple and instant colorimetric assay for H2O2 detection in the commercial oxidant solution is established via synthesized βCD-AgNPs. The H2O2 detection optimum conditions were studied in detail. An increase in the volume of nanoparticles is faced with a decrease in the LSPR absorbance of the suspended nanoparticles mixed with 40.0 mM H2O2 concentration. The optimum pH is found to be equal to 10.0. The kinetic of the reaction is found to be fast, and equilibrium is established between the nanoparticles and H2O2 in the first 0.5 min. The thermodynamics of the equilibrium show that the process is exothermic, and the reaction is favored at 37 °C compared to 25 °C. The β-cyclodextrin organic shell of the synthesized silver nanoparticles (βCD-AgNPs) are found to enhance the detection of hydrogen peroxide in urine through encapsulation mediated process of creatinine when compared to the commercial assay kit. Surely the assay kit gives reproducible results of total H2O2 in urine, values varying between 0.60 ± 0.051 nmoles (6.0 ± 0.51 μM) and 3.22 ± 0.046 nmoles (32.2 ± 0.46 μM) are found at different dilution rates, suggesting that the test is affected by the matrix complexity. βCD-AgNPs test provided an optical creatinine corrected test with high selectivity and sensitivity for H2O2, with values ranging from 24.82 ± 1.50 nmoles of H2O2/mole of creatinine and 70.0 ± 1.57 nmoles of H2O2/mole of creatinine. The test is also affected by the complexity of the matrix. These values are lower than creatinine corrected H2O2 reported in the literature (90–1164 μmoles H2O2/mole creatinine), and the variations may be due to the stability of the samples and limit the use of hydrogen peroxide in urine as a potential biomarker of whole-body oxidative stress.
Acknowledgments
Authors would like to thank Taif University for the support through the Researchers Supporting Project number (TURSP-2020/90), Taif University, Taif, Saudi Arabia, and the Advanced Materials Research Center, University of Sharjah, Sharjah, United Arab Emirates for X-ray diffraction and EDS analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11081897/s1, Figure S1: Photographs of βCD-AgNPs exposed to increasing concentrations of H2O2 from the right to the left, Figure S2: LSPR absorbances of βCD-AgNPs reacted with potential interfering species equilibrium time = 3.0 min; pH0 = 7.0, T = 25 °C, Figure S3: (a) Scanning Electron microscopy (SEM) and (b) Energy Disperse Spectroscopy (EDS) of βCD-AgNPs, Figure S4: (a,b) TEM micrograph of a typical βCD-AgNPs. The dotted rectangle indicates the region of interest (ROI) used for the analysis. (c) A 2.4 nm βCD macromolecule coating is indicated by the lines and arrows in the figure. The intensity profile along the dotted line in (c) is shown in (d) indicating 2.4 nm thickness. The ROI and profile are realized using the Gatan digital micrograph platform, Figure S5: TEM micrograph of βCD-AgNPs, with (a) 1.0 μm, (b) 50 nm, (c) 100 nm, and (d) 200 nm size scale bars taken at different magnification.
Author Contributions
A.E.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing—Original draft preparation, Writing Review & Editing, Visualization, Supervision, Project administration, Funding acquisition, C.N.: Investigation, Data curation, Validation, A.B.: Investigation, Data curation, Validation, S.A.I.M.: Investigation, Data curation, A.H.S.A.A.: Investigation, Data curation, S.S.A.: Conceptualization, Methodology, Writing Review & Editing, S.A.E.A.: Conceptualization, Methodology, Writing Review & Editing, S.P.P.: Conceptualization, Methodology, Writing Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Institute of Science and Engineering (RISE), University of Sharjah, Sharjah, United Arab Emirates, competitive grant number 1702142052-P, Undergraduate students support grant number C.C.R.G/R1243, 2017. Researchers Supporting Project number (TURSP-2020/90), Taif University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukhenouna S., Wilson M.A., Bahmed K., Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid. Med. Cell. Longev. 2018;2018:5730395. doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrocco I., Altieri F., Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Longev. 2017;2017:6501046. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J., Zhao D., Lu H., Huang W., Yu D. Apoptosis Signal-Regulating Kinase 1 (ASK1) Activation is Involved in Silver Nanoparticles Induced Apoptosis of A549 Lung Cancer Cell Line. J. Biomed. Nanotechnol. 2017;13:349–354. doi: 10.1166/jbn.2017.2359. [DOI] [PubMed] [Google Scholar]
- 5.Endo T., Yanagida Y., Hatsuzawa T. Quantitative determination of hydrogen peroxide using polymer coated Ag nanoparticles. Measurement. 2008;41:1045–1053. doi: 10.1016/j.measurement.2008.03.004. [DOI] [Google Scholar]
- 6.Bae Y.S., Oh H., Rhee S.G., Do Yoo Y. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezayian M., Niknam V., Ebrahimzadeh H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019;6:1309–1313. doi: 10.1016/j.toxrep.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Puertas M.C., Corpas F.J., Sandalio L.M., Leterrier M., Rodríguez-Serrano M., Del Río L.A., Palma J.M. Glutathione reductase from pea leaves: Response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol. 2006;170:43–52. doi: 10.1111/j.1469-8137.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 10.Mallick N., Mohn F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000;157:183–193. doi: 10.1016/S0176-1617(00)80189-3. [DOI] [Google Scholar]
- 11.Zorov D.B., Plotnikov E.Y., Jankauskas S.S., Isaev N.K., Silachev D.N., Zorova L.D., Pevzner I.B., Pulkova N.V., Zorov S.D., Morosanova M.A. The phenoptosis problem: What is causing the death of an organism? Lessons from acute kidney injury. Biochemistry. 2012;77:742–753. doi: 10.1134/S0006297912070073. [DOI] [PubMed] [Google Scholar]
- 12.Kozlov A.V., Bahrami S., Calzia E., Dungel P., Gille L., Kuznetsov A.V., Troppmair J. Mitochondrial dysfunction and biogenesis: Do ICU patients die from mitochondrial failure? Ann. Intensive Care. 2011;1:41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bie M.K., Buiten M.S., Rabelink T.J., Jukema J.W. How to reduce sudden cardiac death in patients with renal failure. Heart. 2012;98:335–341. doi: 10.1136/heartjnl-2011-300693. [DOI] [PubMed] [Google Scholar]
- 14.Gaikwad R., Thangaraj P.R., Sen A.K. Direct and rapid measurement of hydrogen peroxide in human blood using a microfluidic device. Sci. Rep. 2021;11:2960. doi: 10.1038/s41598-021-82623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zong L., Ruan L., Li J., Marks R.S., Wang J., Cosnier S., Zhang X., Shan D. Fe-MOGs-based enzyme mimetic and its mediated electrochemiluminescence for in situ detection of H2O2 released from Hela cells. Biosens. Bioelectron. 2021;184:113216. doi: 10.1016/j.bios.2021.113216. [DOI] [PubMed] [Google Scholar]
- 16.Yin T., Wang H., Li J., Yuan B., Qin W. Translating potentiometric detection into non-enzymatic amperometric measurement of H2O2. Talanta. 2021;232:122489. doi: 10.1016/j.talanta.2021.122489. [DOI] [PubMed] [Google Scholar]
- 17.Fiore L., Mazzaracchio V., Galloni P., Sabuzi F., Pezzola S., Matteucci G., Moscone D., Arduini F. A paper-based electrochemical sensor for H2O2 detection in aerosol phase: Measure of H2O2 nebulized by a reconverted ultrasonic aroma diffuser as a case of study. Microchem. J. 2021;166:106249. doi: 10.1016/j.microc.2021.106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao N., Xu Y., Wang L., Yang W., Liu Y. Hollow porous N-doped carbon-based Co4N with peroxidase-like activity for detection of H2O2 under non-physiologic conditions. Microchem. J. 2021;166:106206. doi: 10.1016/j.microc.2021.106206. [DOI] [Google Scholar]
- 19.Atlante A., Passarella S. Detection of reactive oxygen species in primary cultures of cerebellar granule cells. Brain Res. Protoc. 1999;4:266–270. doi: 10.1016/S1385-299X(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 20.Gomes A., Fernandes E., Lima J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Poulsen A.K., Scharff-Poulsen A.M., Olsen L.F. Horseradish peroxidase embedded in polyacrylamide nanoparticles enables optical detection of reactive oxygen species. Anal. Biochem. 2007;366:29–36. doi: 10.1016/j.ab.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Liu X., Wang M., Wang X., Ma W., Li J. Facile synthesis of CDs@ZIF-8 nanocomposites as excellent peroxidase mimics for colorimetric detection of H2O2 and glutathione. Sens. Actuators B Chem. 2021;329:129115. doi: 10.1016/j.snb.2020.129115. [DOI] [Google Scholar]
- 23.Guo T., Xu T., Xia W., Carrier A.J., Wang L., Zhang X. Graphene oxide and CuO double quantum dot composites (GOQD-q-CuO) with enhanced haloperoxidase-like activity and its application in colorimetric detection of H2O2 and glucose. Mater. Chem. Phys. 2021;260:124126. doi: 10.1016/j.matchemphys.2020.124126. [DOI] [Google Scholar]
- 24.Chen P., Zhong H., Li X., Li M., Zhou S. Palygorskite@Co3O4 nanocomposites as efficient peroxidase mimics for colorimetric detection of H2O2 and ascorbic acid. Appl. Clay Sci. 2021;209:106109. doi: 10.1016/j.clay.2021.106109. [DOI] [Google Scholar]
- 25.Remani K.C., Binitha N.N. Cobalt doped ceria catalysts for the oxidative abatement of gaseous pollutants and colorimetric detection of H2O2. Mater. Res. Bull. 2021;139:111253. doi: 10.1016/j.materresbull.2021.111253. [DOI] [Google Scholar]
- 26.Chi K., Guan Y., Zhang X., Yang T., Meng S., Hu R., Yang Y. Iodide/metal-organic frameworks (MOF) -mediated signal amplification strategy for the colorimetric detection of H2O2, Cr2O72− and H2S. Anal. Chim. Acta. 2021;1159:338378. doi: 10.1016/j.aca.2021.338378. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Luo P., Yang H., Pan S., Liu H., Hu X. Regulating the enzymatic activities of metal-ATP nanoparticles by metal doping and their application for H2O2 detection. Sens. Actuators B Chem. 2021;335:129671. doi: 10.1016/j.snb.2021.129671. [DOI] [Google Scholar]
- 28.Riaz M.A., Yuan Z., Mahmood A., Liu F., Sui X., Chen J., Huang Q., Liao X., Wei L., Chen Y. Hierarchically porous carbon nanofibers embedded with cobalt nanoparticles for efficient H2O2 detection on multiple sensor platforms. Sens. Actuators B Chem. 2020;319:128243. doi: 10.1016/j.snb.2020.128243. [DOI] [Google Scholar]
- 29.Elgamouz A., Bajou K., Hafez B., Nassab C., Behi A., Haija M.A., Patole S.P. Optical Sensing of Hydrogen Peroxide Using Starch Capped Silver Nanoparticles, Synthesis, Optimization and Detection in Urine. Sens. Actuators Rep. 2020;2:100014. doi: 10.1016/j.snr.2020.100014. [DOI] [Google Scholar]
- 30.Ju-Nam Y., Lead J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008;400:396–414. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 31.Angioletti-Uberti S. Theory, simulations and the design of functionalized nanoparticles for biomedical applications: A Soft Matter Perspective. NPJ Comput. Mater. 2017;3:48. doi: 10.1038/s41524-017-0050-y. [DOI] [Google Scholar]
- 32.Yang D., Chen Y., Peng H., Chen G., Lin Z. An integrated experimental and theoretical study on the optical properties of uniform hairy noble metal nanoparticles. Nanoscale. 2018;10:22750–22757. doi: 10.1039/C8NR07115B. [DOI] [PubMed] [Google Scholar]
- 33.Jansook P., Ogawa N., Loftsson T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018;535:272–284. doi: 10.1016/j.ijpharm.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998;98:1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 35.Dodero A., Schlatter G., Hébraud A., Vicini S., Castellano M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021;264:118042. doi: 10.1016/j.carbpol.2021.118042. [DOI] [PubMed] [Google Scholar]
- 36.Fan Y., Liu Z., Zhan J. Synthesis of starch-stabilized Ag nanoparticles and Hg 2 recognition in aqueous media. Nanoscale Res. Lett. 2009;4:1230. doi: 10.1007/s11671-009-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F., He C., Han M., Wu J.H., Xu G.Q. Chemical controlled reversible gold nanoparticles dissolution and reconstruction at room-temperature. Chem. Commun. 2012;48:6136–6138. doi: 10.1039/c2cc31319g. [DOI] [PubMed] [Google Scholar]
- 38.Patil R.B., Chougale A.D. Analytical methods for the identification and characterization of silver nanoparticles: A brief review. Mater. Today Proc. 2021 doi: 10.1016/j.matpr.2021.03.384. [DOI] [Google Scholar]
- 39.Zheng T., Bott S., Huo Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl. Mater. Interfaces. 2016;8:21585–21594. doi: 10.1021/acsami.6b06903. [DOI] [PubMed] [Google Scholar]
- 40.Shameli K., Ahmad M.B., Jazayeri S.D., Shabanzadeh P., Sangpour P., Jahangirian H., Gharayebi Y. Investigation of antibacterial properties silver nanoparticles prepared via green method. Chem. Cent. J. 2012;6:73–83. doi: 10.1186/1752-153X-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zargar M., Hamid A.A., Bakar F.A., Shamsudin M.N., Shameli K., Jahanshiri F., Farahani F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules. 2011;16:6667–6676. doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virgen-Ortiz A., Limón-Miranda S., Soto-Covarrubias M.A., Apolinar-Iribe A., Rodríguez-León E., Iñiguez-Palomares R. Biocompatible silver nanoparticles synthesized using rumex hymenosepalus extract decreases fasting glucose levels in diabetic rats. Dig. J. Nanomater. Biostruct. 2015;10:927–933. [Google Scholar]
- 43.Molleman B., Hiemstra T. Surface Structure of Silver Nanoparticles as a Model for Understanding the Oxidative Dissolution of Silver Ions. Langmuir. 2015;31:13361–13372. doi: 10.1021/acs.langmuir.5b03686. [DOI] [PubMed] [Google Scholar]
- 44.Temur E., Eryiğit M., Kurt Urhan B., Demir Ü., Öznülüer Özer T. Cu/Electrochemically reduced graphene oxide layered nanocomposite for non-enzymatic H2O2 sensor. Mater. Today Proc. 2021 doi: 10.1016/j.matpr.2021.03.273. [DOI] [Google Scholar]
- 45.Banavath R., Srivastava R., Bhargava P. Improved non-enzymatic H2O2 sensors using highly electroactive cobalt hexacyanoferrate nanostructures prepared through EDTA chelation route. Mater. Chem. Phys. 2021;267:124593. doi: 10.1016/j.matchemphys.2021.124593. [DOI] [Google Scholar]
- 46.Atta N.F., Abdel Gawad S.A., Galal A., Razik A.A., El-Gohary A.R.M. Efficient electrochemical sensor for determination of H2O2 in human serum based on nano iron-nickel alloy/carbon nanotubes/ionic liquid crystal composite. J. Electroanal. Chem. 2021;881:114953. doi: 10.1016/j.jelechem.2020.114953. [DOI] [Google Scholar]
- 47.Karuppiah C., Venkatesh K., Arunachalam P., Ramaraj S.K., Al-Mayouf A.M., Yang C. Optimization of S-dopant on N, S co-doped graphene/CNT-Fe3C nanocomposite electrode for non-enzymatic H2O2 sensor. Mater. Lett. 2021;285:129001. doi: 10.1016/j.matlet.2020.129001. [DOI] [Google Scholar]
- 48.Chithra Lekha P., Ram Babu Y., Fidal Kumar V.T., Chandra T.S., Roy S.C. Investigation of Photo-induced Enhancement of Sensitivity and Electrochemical Surface Phenomenon of Multileg TiO2 Sensor Device towards H2O2. J. Electroanal. Chem. 2021;895:115399. doi: 10.1016/j.jelechem.2021.115399. [DOI] [Google Scholar]
- 49.Jiang L., Zhao Y., Zhao P., Zhou S., Ji Z., Huo D., Zhong D., Hou C. Electrochemical sensor based on reduced graphene oxide supported dumbbell-shaped CuCo2O4 for real-time monitoring of H2O2 released from cells. Microchem. J. 2021;160:105521. doi: 10.1016/j.microc.2020.105521. [DOI] [Google Scholar]
- 50.Farrokhnia M., Karimi S., Momeni S., Khalililaghab S. Colorimetric sensor assay for detection of hydrogen peroxide using green synthesis of silver chloride nanoparticles: Experimental and theoretical evidence. Sens. Actuators B Chem. 2017;246:979–987. doi: 10.1016/j.snb.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 51.Elgamouz A., Idriss H., Nassab C., Bihi A., Bajou K., Hasan K., Abu Haija M., Patole S.P. Green Synthesis, Characterization, Antimicrobial, Anti-Cancer, and Optimization of Colorimetric Sensing of Hydrogen Peroxide of Algae Extract Capped Silver Nanoparticles. Nanomaterials. 2020;10:1861. doi: 10.3390/nano10091861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long L.H., Evans P.J., Halliwell B. Hydrogen peroxide in human urine: Implications for antioxidant defense and redox regulation. Biochem. Biophys. Res. Commun. 1999;262:605–609. doi: 10.1006/bbrc.1999.1263. [DOI] [PubMed] [Google Scholar]
- 53.Chandramathi S., Suresh K., Anita Z.B., Kuppusamy U.R. Elevated levels of urinary hydrogen peroxide, advanced oxidative protein product (AOPP) and malondialdehyde in humans infected with intestinal parasites. Parasitology. 2009;136:359–363. doi: 10.1017/S0031182008005465. [DOI] [PubMed] [Google Scholar]
- 54.Varma S.D. Radio-isotopic determination of subnanomolar amounts of peroxide. Free Radic. Res. Commun. 1989;5:359–368. doi: 10.3109/10715768909073419. [DOI] [PubMed] [Google Scholar]
- 55.Hanif S., John P., Gao W., Saqib M., Qi L., Xu G. Chemiluminescence of creatinine/H2O2/Co2+ and its application for selective creatinine detection. Biosens. Bioelectron. 2016;75:347–351. doi: 10.1016/j.bios.2015.08.053. [DOI] [PubMed] [Google Scholar]
- 56.Yuen J., Benzie I. Hydrogen peroxide in urine as a potential biomarker of whole body oxidative stress. Free Radic. Res. 2003;37:1209–1213. doi: 10.1080/10715760310001616032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.