Abstract
In this paper, we discuss the role of particle therapy—a novel radiation therapy (RT) that has shown rapid progress and widespread use in recent years—in multidisciplinary treatment. Three types of particle therapies are currently used for cancer treatment: proton beam therapy (PBT), carbon-ion beam therapy (CIBT), and boron neutron capture therapy (BNCT). PBT and CIBT have been reported to have excellent therapeutic results owing to the physical characteristics of their Bragg peaks. Variable drug therapies, such as chemotherapy, hormone therapy, and immunotherapy, are combined in various treatment strategies, and treatment effects have been improved. BNCT has a high dose concentration for cancer in terms of nuclear reactions with boron. BNCT is a next-generation RT that can achieve cancer cell-selective therapeutic effects, and its effectiveness strongly depends on the selective 10B accumulation in cancer cells by concomitant boron preparation. Therefore, drug delivery research, including nanoparticles, is highly desirable. In this review, we introduce both clinical and basic aspects of particle beam therapy from the perspective of multidisciplinary treatment, which is expected to expand further in the future.
Keywords: particle beam therapy, proton beam therapy, carbon-ion beam therapy, boron neutron capture therapy, combination therapy, drug delivery
1. Background: Particle Beam Therapy as a Novel Radiotherapy
1.1. Promotion and Expansion of Particle Therapy Facilities
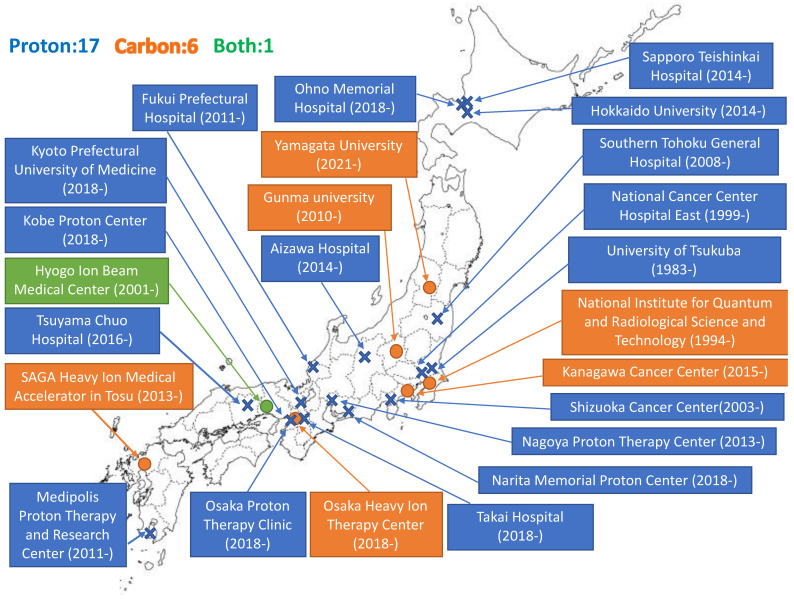
Particle beam therapy is a type of radiotherapy (RT). Particle beam therapy delivers a high radiation dose to tumors and as technology has improved, enables antitumor effects. The application of drug therapy is an integral part of development. We review the present status and future aspects of particle beam therapy in terms of drug therapy. Proton beam therapy (PBT) and carbon-ion beam therapy (CIBT) are common particle beam therapies. In 1946, Wilson suggested for the first time the use of accelerated protons in radiation therapy (RT) [1]. He investigated the depth-dose profile of protons accelerated at the cyclotron in Berkeley (CA, USA) and observed a steep increase in energy deposition at the end of the particle range, which is known as the Bragg peak. In 1958, the first clinical use of accelerated protons in the pituitary gland of 26 patients with advanced breast cancer was reported by the Lawrence Radiation Laboratory in Berkeley [2]. Several clinical studies have been conducted in the following decades. However, they were performed in a physics laboratory; thus, they had little beam time, and beam lines were not necessarily designed for medical use. The first medical treatment facilities to use PBT were constructed at the Clatterbridge Oncology Center (UK) in 1989, which uses a cyclotron and at Loma Linda University (CA, USA) in 1990, which uses a synchrotron. The first medical treatment facility for CIBT was initiated in HIMAC in Chiba (Japan), in 1994. As of 2019, over 250,000 patients have been treated with particle therapy (proton and carbon-ion [C-ion] beams). The number of treatment facilities has rapidly increased in recent years. Currently, treatment is provided at 100 facilities worldwide. The United States has the highest number with 40 facilities, followed by Japan with 24 facilities, Germany with seven facilities, and Russia with five facilities. There are 95 PBT facilities and 12 CIBT facilities [3]. Japan has more than 20 particle therapy facilities on its small land area and is one of the countries where particle therapy is becoming increasingly popular (Figure 1).
Figure 1.
Expansion of particle beam therapy facility in Japan.
1.2. Physical Aspects of Particle Therapy
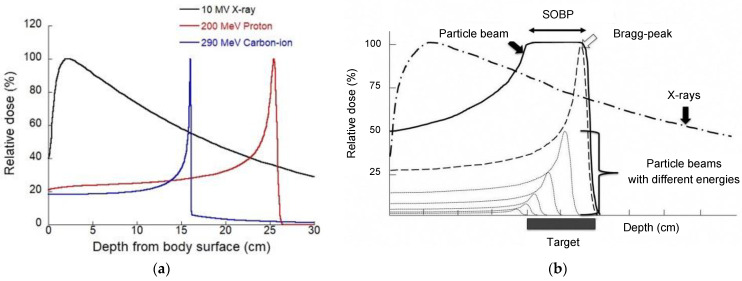
The dose distributions of protons and C-ions largely differ from those of photons, which are used in conventional RT. The biggest difference in physical characteristics is the existence of depth-dose distribution, the so-called Bragg peak, and by having this characteristic, particle beam therapy can give the maximum energy to the surroundings near the stop (cancer part) (Figure 2a). Although the dose distributions of protons and C-ions are very similar, the difference is that C-ion beams have narrower penumbra and longer fragmentation tails than proton beams [4].
Figure 2.
(a) Depth-dose distributions of clinical X-ray, proton, and carbon-ion beams and (b) the spread-out Bragg peak (SOBP).
In particle therapy, an extended Bragg peak (spread-out Bragg peak; SOBP) is formed to fit this Bragg peak to the size of the cancer (Figure 2b). Beam formation technology has evolved dramatically in recent years, and an increasing number of facilities are introducing an active scanning system (ASS) in addition to the conventional passive scattering system (PSS). PSS uses a rotating scatterer to expand the beam vertically and horizontally, and a collimator and bolus are used to optimize the beam to the cancer shape in front of the patient [5]. In contrast, the ASS uses a thin pencil beam, which is deflected vertically and horizontally by electromagnets, and the depth can be adjusted by changing the energy of the accelerator [6]. The advantage of the active scanning technique is its application in complexly shaped target volumes and reduction in the cost of manufacturing patient-specific devices, such as compensators, and the disadvantage is its larger lateral penumbra [7,8,9,10,11].
1.3. Biological Aspects of Particle Therapy
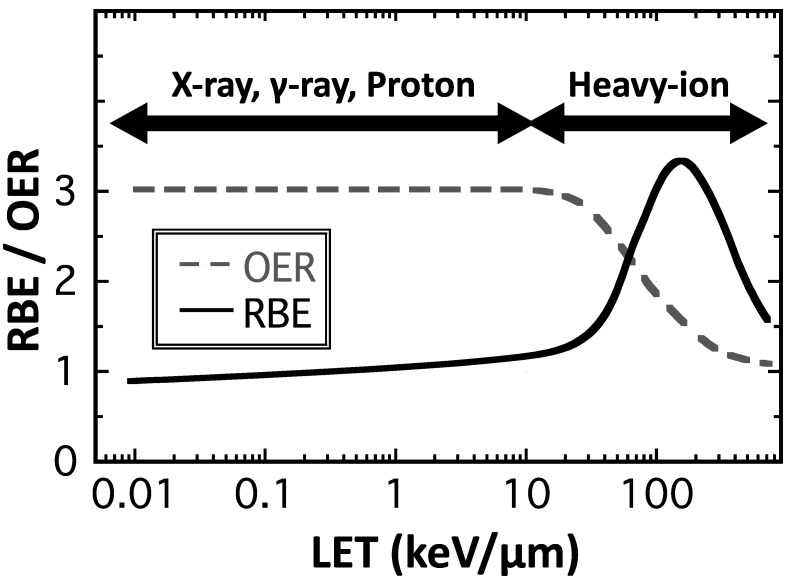
The concept of quality and quantity is important to understand the biological effects of PBT. The quality of PBT is defined as the amount of energy transferred per track (linear energy transfer; LET). Radiation with high LET can cause more severe damage to cells, represented by complex DNA damage that is difficult to repair. A parameter that quantitatively expresses the difference in biological effects due to this difference in LET is the relative biological effectiveness (RBE), which is defined as the dose ratio between the reference photon radiation and the particle radiation that produces the same biological effect. The international standard for proton RBE is 1.1; however, recent studies have shown that the distal end of a proton-SOBP shows a slightly higher RBE value depending on the increase in LET [12]. In contrast, carbon lines with high LET show high RBE values, with approximately 1.5 as the biological RBE and 3.0 as the clinical RBE [4,13,14]. Furthermore, it is important to recognize that although RBE is a useful parameter for expressing relative effects, its value may vary depending on the biological endpoint and the level of biological effect [15]. In addition, it is known that C-ions with higher LET radiation have a killing effect on hypoxic and generally radioresistant tumors [16]. The biological effect of radiation is enhanced in the presence of oxygen (oxygen effect) [17]. The oxygen enhancement ratio (OER) is a parameter that indicates the difference in biological effects depending on the presence or absence of oxygen. The OER is expressed as the ratio of the absorbed doses required to induce the same biological effect with and without oxygen. RBE and OER are LET-dependent variables (Figure 3), and the higher the LET, the higher the RBE, which reaches its maximum at approximately 100–200 keV/µm and then decreases (overkill effect). The OER shows a value of approximately 2.5–3.0 at low LET and asymptotically approaches 1 at high LET. High RBE [15,18,19,20,21] and low OER [16,22,23,24] values are characteristic biological properties of heavy-ion beams with high LETs.
Figure 3.
Relationship between RBE, OER, and LETs.
2. The Role of Particle Beam Therapy in Multidisciplinary Treatments in Clinics
This chapter outlines particle beam therapy, which is often used in combination with drug therapy.
2.1. Esophageal Cancer
In 2020, it was estimated that 18,440 new patients developed esophageal cancer and 16,170 died in the United States [25]. Hence, the case fatality risk of esophageal cancer is still high, and the disease is currently the sixth leading cause of cancer-related deaths [26]. The gold standard treatment for localized esophageal cancer is surgery. However, some patients are unfit for surgery because of their advanced tumors (unresectable), poor general conditions (medically inoperable), refusal to undergo surgery, and concurrent chemoradiotherapy (CCRT) is considered for them as an alternative curative treatment to surgery [27]. However, cardiopulmonary toxicities after CCRT using photon beams are still associated with late adverse effects and would disturb the administration of high-dose chest irradiation in the treatments for esophageal and lung cancers [28,29]. Especially, a standard total irradiation dose in the treatment for esophageal cancer (50.4 Gy in 28 fractions) is less than that for lung cancer (60 Gy in 30 fractions) because larger irradiation fields are necessary to entirely cover the primary tumor and regional lymph nodes of thoracic esophageal cancers [30]. It is known that local and/or regional recurrences are the most frequent failure patterns after CCRT for esophageal cancer [31] and there are significant relationships between cardiopulmonary toxicity and irradiated volumes and doses in the lung and heart [32,33]. Therefore, dose escalation to the target while avoiding unnecessary doses to the lung and heart is a reasonable approach to improve treatment outcomes for esophageal cancer.
PBT offers advantageous physical properties to RT for the treatment of various cancers and can reduce the radiation dose and irradiated volume in organs at risk, such as the lung and heart, in RT for esophageal cancer compared with photon therapy [34,35]. We initially reported the usefulness of PBT alone or a PBT boost following photon RT without concurrent chemotherapy for patients with unresectable esophageal cancer [36]. In the case of PBT alone, the median total dose was 80 Gy RBE (Gy [RBE]), but patients were given a median dose of 46 Gy using photons with a boost to 80 Gy (RBE) using protons. Thereafter, CCRT using cisplatin/5 fluorouracil added to a median dose of 60 Gy (RBE) using PBT alone was performed in 40 patients, and no grade 3 cardiopulmonary toxicities were observed in our recent study [37]. In a Japanese multicenter retrospective study, clinical outcomes obtained from 202 patients (195 with squamous cell carcinoma and seven with adenocarcinoma) treated between 2009 and 2013 at four institutes were investigated. The 5-year rates of overall survival (OS) and local control of patients with stages I and II were 75.4% and 74.0%, respectively, and the corresponding rates of stages III and IV were 36.8% and 53.8%, respectively. Furthermore, grade 3 cardiopulmonary toxicities were observed in only three (1.5%) patients [38]. Table 1 shows that the incidence of cardiopulmonary toxicities in CCRT for esophageal cancer after PBT was less than that after photon RT [39,40,41,42,43]. A recent randomized study compared the total toxicity burden (TTB) and progression-free survival (PFS) between intensity-modulated radiotherapy (IMRT) and PBT and revealed that there was no significant difference in PFS between the two arms. However, the mean TTB, which was the other primary endpoint of the study, was 2.3 times higher for IMRT (39.9; 95% highest posterior density interval, 26.2–54.9) than for PBT (17.4; 10.5–25.0) and the PBT arm experienced numerically fewer cardiopulmonary toxicities and postoperative complications [43].
Table 1.
Cardiopulmonary toxicities of photon RT and PBT for esophageal cancer.
| Author | N | RT Modality | Treatment | Endpoint | Late Toxicity Rate | |
|---|---|---|---|---|---|---|
| Heat | Lung | |||||
| DeCesaris [15] | 36 | Photon RT | Preope/definitive | Perioperative death | 13.9% | |
| 18 | Proton | 0% | ||||
| Wang [6] | 320 | IMRT | Preope/definitive | Grade 3 (2y/5y) | 18%/21% | NA |
| 159 | Proton | 11%/13% | NA | |||
| Wang [17] | 208 | 3DCRT | Preoperative | Perioperative complication | 15.9% | 30.3% |
| 164 | IMRT | 17.1% | 23.8% | |||
| 72 | Proton | 9.7% | 13.9% | |||
| Makishima [10] | 19 | 3DCRT | Definitive | Grade 3 | 0% | 10.3% |
| 25 | Proton | 0% | 0% | |||
| Xi [18] | 211 | IMRT | Preope/definitive | Grade 3 | 2.4% | 4.7% |
| 132 | Proton | 0.8% | 2.3% | |||
| Lin [19] | 61 | IMRT | Preope/definitive | Grade 3 | 5 * | 11 * |
| 46 | Proton | 3 * | 5 * | |||
RT, radiotherapy; IMRT, intensity-modulated radiotherapy; 3DCRT, three-dimensional conformal radiotherapy; NA, not assessed; *, number of events.
Recently, the impact of maintaining host immunity on patient survival has brought focus on CCRT for esophageal cancer, and lymphocyte count during CCRT may be a representative landmark for predicting survival [44,45]. Since PBT avoids unnecessary doses to the body of patients compared with photon RT, the studies showed that lymphocyte counts during PBT were well maintained and OS after PBT tended to be better than that after photon RT [45,46]. A pencil beam scanning method that provides a better dose distribution than passive scattering, which reduces the doses to the normal tissues close to the target, is currently available at most institutes, and future prospective studies may further confirm the true efficacy including survival outcomes of PBT for esophageal cancer.
2.2. Pancreatic Cancer
Pancreatic cancer is well known as one of the cancers with a poor prognosis and remains challenging to treat. According to a survey, there are an estimated 57,600 new patients and 47,050 deaths worldwide annually [26]. Surgery is the gold standard for the curative treatment of pancreatic cancer, and chemotherapy for pancreatic cancer has made great strides in the last few decades. The role of RT is to control local lesions, such as original tumors and locoregional lymph node metastases. Since the pancreas is surrounded by organs that are at risk, such as the stomach and duodenum, it is difficult to deliver a higher radiation dose for conventional photon RT. Therefore, particle beam therapy, which has an excellent dose concentration, is highly recommended for the treatment of pancreatic cancer and is mainly used for curative therapy of unresectable diseases and preoperative therapy for resectable diseases.
There have been few reports of particle beam therapy for pancreatic cancer before 2010, but it has been increasing rapidly since then. This is largely due to advances in chemotherapy and technological advances in particle beam therapy. Table 2 shows a review of concurrent PBT combined with chemotherapy for pancreatic cancer. Hong et al. conducted preoperative CCRT using proton beams and capecitabine in 25 patients with resectable pancreatic cancer and reported a 75% 1-year OS rate in 2011 [47]. After that, some clinical studies using proton beams commonly combined with capecitabine, gemcitabine, 5-FU, and S-1, have been reported, and Hong et al. reported a 2-year OS rate of 42% in their subsequent report in 2014 [48]. In the curative treatment for the unresectable or borderline resectable disease, the 1- and 2-year OS rates after PBT were 62–77.5% and 40–50.8%, respectively [49,50,51,52]. Maemura et al. performed induction chemotherapy followed by CCRT using proton beams. In their study, gemcitabine was used for induction therapy, and S-1 was concurrently combined. The irradiation dose was either a standard dose of 50 Gy or escalated dose of 67.5 Gy (RBE) with 25 fractions and achieved a 1-year OS rate of 80% [53]. Regarding toxicity, gastrointestinal ulcers have been reported as a late adverse event in some studies [48,49,53]. Pancreatic cancer using CIBT has been reported at several institutions in Japan, and Kawashiro et al. summarized the data as a retrospective multi-institutional study [54]. They investigated 72 patients whose irradiation dose was 52.8 and 55.2 Gy (RBE) with 12 fractions and concurrent chemotherapy was performed in 56 patients using gemcitabine or S-1. The OS rates were 73% and 46% at 1 and 2 years, respectively, and late adverse events (ulcers) were found in one patient. Using in vitro and in vivo studies, Sai et al. demonstrated that C-ion beam combined with gemcitabine had a superior potential to kill pancreatic cancer stem-like cells [55]. Chemotherapy for pancreatic cancer has improved prognosis with the advent of gemcitabine and has since been further improved with the combination of other drugs, such as nab-paclitaxel [56,57]. In addition, FOLFIRINOX has been demonstrated to have a higher antitumor effect than gemcitabine [58]. To date, the use of FOLFIRINOX in combination with particle beam therapy has been uncertain due to its strong side effects, however, in Europe, a prospective study on the combination of FOLFIRINOX as induction therapy for preoperative CIBT was conducted [59]. With regard to the technical progress of particle beam therapy, the concomitant boost [49,50,52,60], active scanning [61], and layer-stacking boost techniques [62] have been attempted.
Table 2.
Concurrent particle beam therapy combined with chemotherapy for pancreatic cancer.
| Author | N | RT | Dose | Chemotherapy | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Hong [46] | 25 | proton | 30GyRBE/10fr 25GyRBE/5fr |
capecitabine | preoperative | OS: 75%/1Y |
| Terashima [48] | 50 | proton | 50GyRBE/25fr 70.2GyRBE/26fr 67.5GyRBE/25fr |
gemcitabine | curative | OS: 76.8%/1Y, PFS: 64.3%/1Y |
| Hong [47] | 50 | proton | 25GyRBE/5fr | capecitabine | preoperative | OS: 42%/2Y |
| Maemura [52] | 10 | proton | 50GyRBE/25fr 67.5GyRBE/25fr |
gemcitabine, S-1 | curative | OS: 80, 45, 22.5%/1, 2, 3Y |
| Kim [49] | 37 | proton | 45GyRBE/10fr | capecitabine, 5-FU | curative | OS: 75.7%/1Y, PFS: 64.8%/1Y, 19.3M |
| Jethea [50] | 13 | proton | 50GyRBE/25fr | capecitabine, 5-FU | curative | OS: 62, 40%/1, 2Y, 16M |
| Hiroshima [51] | 42 | proton | 50–67.5GyRBE/25-33fr | gemcitabine, S-1 | curative | OS: 77.5, 50.8%/1, 2Y, 25.6M |
| Kawashiro [53] | 72 | carbon | 52.8GyRBE/12fr 55.2GyRBE/12fr |
gemcitabine, S-1 (n = 56) | curative | OS: 73, 46%/1, 2Y, 21.5M |
| Vitolo [58] | carbon | 38.4GyRBE/4fr | FOLFIRINOX, gemcitabine |
preoperative |
Value in outcome represents overall survival rate, progression-free survival rate, and median survival time. OS: overall survival, PFS: progression-free survival.
2.3. Prostate Cancer
Prostate cancer (PC) screening and early diagnosis using prostate-specific antigen have indicated that there are approximately 192,000 new patients per year in the US [26]. In Japan, the annual number of newly diagnosed patients also increases every year, and over 12,500 PC-related deaths were estimated in 2019 [63]. Furthermore, almost half of Japanese patients with PC are over 75 years old, and RT plays an important role as a representative nonsurgical and curative treatment, especially for elderly patients. Since PC control after RT is dose-dependent [64], recent advances in modern RT techniques, including IMRT and brachytherapy, under image guidance can provide successful treatment outcomes by allowing delivery of higher doses to the prostate with less toxicity to the organs at risk, such as the rectum and bladder, compared with conventional three-dimensional conformal RT [65,66].
Protons and C-ions are charged particles and have been used for PC treatment through particle therapy for several decades. PBT was initially used as a tool for dose escalation after photon RT. The first randomized study compared the results between photon RT and PBT in patients with advanced PC. In the study, patients in the standard-dose and high-dose groups were treated with pelvic RT at 50.4 Gy/28 fr, followed by local photon RT at 16.8 Gy/8 fr (total dose: 67.2 Gy/36 fr) and local PBT at 25.2 Gy equivalents (GyRBE)/12 fr (total dose: 75.6 GyRBE/40 fr), respectively, and a better local control was achieved in the high-dose PBT group than in the standard-dose photon RT group (8-year local control rate: 73% vs. 59%) [67]. In addition, the incidence of grade 3 rectal bleeding was recorded in only 2.9% of the patients in the high-dose PBT group, although the corresponding rate of RTOG9413 using pelvic RT followed by local photon boost was 4.3% despite using a 70.2 Gy dose (5.4 Gy lower than the abovementioned 75.6 Gy PBT dose in the randomized study) [68].
The PROG95-09 trial tested a prostate PBT boost of either 19.8 GyRBE/11 fr (standard-dose group) or 28.8 GyRBE/16 fr (high-dose group) following 50.4 Gy/28 fr by local photon RT without regional irradiation for patients with stage T1–2 PC; the 10-year biochemical failures were observed in 32.4% and 16.7% of patients in the standard-dose and high-dose groups, respectively (p < 0.0001) [69]. Regarding toxicity, there were no significant differences between the standard-dose and high-dose groups; grade 3 gastrointestinal (GI) and genitourinary (GU) events were only noted in 1% and 2% of patients in the high-dose group, respectively, and 0% and 2% in the standard-dose group, respectively. At almost the same time, a randomized trial was conducted to determine the effectiveness of dose escalation using photon RT. The 10-year recurrence-free rates in the standard-dose group (70 Gy/35 fr) and in the high-dose group (78 Gy/39 fr) were 50% and 73%, respectively (p = 0.004); however, grade 3 GI and GU adverse events in the high-dose group were observed in 3.3% and 6.6% of patients, respectively, suggesting higher event rates compared with those in the 79.2 GyRBE dose of the high-dose group in the PROG95-09 trial described above [70] (Table 3).
Table 3.
Clinical outcomes of photon and PBT trials for prostate cancer.
| Author | N | RT Modality |
Total Dose (Gy) |
Photon (Gy) | Proton (GyRBE) | Efficacy (%) | Late toxicity (Grade 3) (%) | |
|---|---|---|---|---|---|---|---|---|
| GI | GU | |||||||
| Shipley [67] | 202 | Photon +Proton | 75.6 67.2 |
50.4 (pelvis) 50.4 (pelvis) + 16.8 (local) |
25.2 (local) | 8y-LC:73 | 2.9 | NA |
| Photon | - | 59 | 0 | NA | ||||
| Roach [68] | 440 | Photon | 70.2 70.2 |
50.4 (pelvis) + 19.8 (local) 70.2 (local) | 7y-PFS:40 | 4.3 | 3 | |
| Photon | 27 | 0 | 0 | |||||
| Local prostate irradiation | ||||||||
| Zeitman [69] | 393 | Photon +Proton | 79.2 70.2 |
50.4 (local) 50.4 (local) |
28.8 (local) | 10y-bRF:83 | 1 | 2 |
| Photon +Proton | 19.8 (local) | 67 | 0 | 2 | ||||
| Kuban [70] | 301 | Photon | 78.0 70.0 |
78.0 (local) 78.0 (local) |
10y-FFF:73 | 7 | 3 | |
| Photon | 50 | 1 | 5 | |||||
GI, gastrointestinal; GU, genitourinary; LC, local control; PFS, progression-free survival; bRF, biochemical relapse-free; FFF, freedom from any failures.
In the same period, the efficacy of a combination of androgen deprivation therapy (ADT) with RT for intermediate- and high-risk patients with PC on not only disease control but also overall survival was shown in a randomized trial [71] and high-dose local PBT without photon RT yielded favorable outcomes regarding both PC control and adverse effects [72]. The clinical outcomes of local high-dose PBT combined with ADT were equal to or superior to those of local high-dose photon RT or PBT combined with pelvic or prostate RT. Furthermore, C-ions have been used for patients with PC as a local RT since 1995 at the National Institute of Quantum and Radiological Science and Technology Hospital, and the optimal RT dose and appropriate use of ADT in CIBT for PC have been investigated through several clinical trials [73]. At present, ADT is combined with CIBT for 2–6 months in intermediate-risk PC patients and for 2 years in high-risk PC patients based on previous phase I/II and II clinical trials; however, the appropriate indication criteria and duration of ADT use for PC in combination with high-dose RT, including PBT and CIBT, remains unknown.
Multi-institutional cohort studies involving four Japanese institutes were conducted to determine the appropriate use of ADT combined with PBT, and 520 intermediate-risk and 555 high-risk PC patients treated with PBT between 2008 and 2011 were analyzed [74]. Overall, the use of short-term ADT for ≤6 months improved the biochemical recurrence-free survival (bRFS), but no benefit of ADT for> 6 months was observed. The effectiveness of short-term ADT on bRFS was more evident in patients with two or three intermediate-risk factors than in those with a single factor. In contrast, short-term ADT for ≤6 months did not improve bRFS in the high-risk group. The study revealed that ADT for 12 and 21 months is preferable for patients with single and multiple risk factors, respectively, with high-dose PBT. Since ADT may cause dysfunction in lipid, glucose, and mineral metabolisms, especially in the elderly, prospective studies are necessary to determine the optimal ADT use and avoid unnecessary ADT administration in combination with high-dose RT for patients with PC.
2.4. Pediatric Cancer
The large difference between radiation therapy for aged patients and pediatric patients is the possibility of several complications. The following are typical complications and various complications that have been studied using photon RT: (1) Reproductive dysfunction: Sex cells are highly radiosensitive, and it is said that sperm cell depletion and morphological abnormalities can occur even at 10 cGy or less. Sanders et al. reported that 463 boys received total body irradiation of 10, 12, 14, and 15.5 Gy, and only five (1%) became fathers [75]. The Childhood Cancer Survivor Study (CCSS) examined 1915 patients after treatment for pediatric cancers, and the rate of miscarriage increased 3.6 times in cases with whole-brain and whole-spinal irradiation and 1.7 times in cases with pelvic irradiation [76]. (2) Cardiac complications: According to a CCSS, the relative risk of death from cardiac complications was as high as 11.9 (95% confidence interval (CI): 9.1–15. (3) in 2717 five-year-old or older survivors after chemotherapy for pediatric Hodgkin lymphoma. In cases where the mediastinum was irradiated with 42 Gy or more, it was reported to be 41.5 (95% CI: 18.1–82.1) [77]. PBT has a superior dose concentration with cytotoxicity equivalent to photons; thus, it is expected to be utilized for the treatment of patients with pediatric cancer. Moreover, although there are few clinical reports because secondary cancers develop years–decades later, PBT is expected to reduce secondary cancers. In Japan, PBT for patients with pediatric cancer became the first medical insurance coverage for all cancer diseases in 2016, which indicates high expectations for pediatric cancer treatment.
PBT is used for the treatment of cancers, such as brain tumors, neuroblastoma, and soft tissue tumors. PBT is not often administered alone and is often multidisciplinary with surgery or chemotherapy. Glioma, medulloblastoma, ependymoma, germinoma, and craniopharyngioma make up a lot of brain tumors. Eaton et al. compared the treatment results of photon RT and PBT for pediatric medulloblastoma. Both treatment protocols were the same as craniospinal and focal irradiation, with vincristine, cisplatin, cyclophosphamide, and lomustine. Second cancers were found in three of 43 patients in the photon RT group and none of the 45 in the PBT group [78]. They also investigated the endocrine result of photon RT and PBT in 77 patients and reported hypothyroidism as 69% and 23%, sex hormone deficiency as 19% and 3%, and any endocrine replacement therapy as 78% and 55%, respectively, and concluded that PBT may reduce the risk of some endocrine abnormalities [79]. Rhabdomyosarcoma (RMS) is a high-grade tumor characterized by local invasiveness and is the most common soft tissue sarcoma in pediatric patients. RMS can occur all over the body; however, the most common sites are the head and neck, followed by the pelvis. RMS is treated using a multidisciplinary approach. Clinical experiences in the treatment of pediatric RMS with PBT indicated safety and effectiveness with low acute toxicities and disease control comparable to photon irradiation [80,81,82]. Mizumoto et al. retrospectively summarized data from a multicenter study in Japan and reported the clinical outcome of 55 children aged 0–19 years (median, 5 years) who received PBT for RMS with doses ranging from 36–60 Gy (RBE). Surgical resection before PBT was performed in 41 patients (75%), and 53 patients (96%) received chemotherapy. The number of patients that enrolled during pre-PBT, pre- PBT and during PBT, and only during PBT was 17, 34, and two patients, respectively. The median follow-up time was 24.5 months. Acute toxicity of more than grade 3 was found in 16% of the patients, but they recovered well after PBT. A total of 87% of the patients experienced hematologic toxicities more than grade 3, which were very likely to be related to PBT [83].
3. Current Basic Research on Combination with Particle Therapy
3.1. Combination Therapy
In recent years, the content and quality of cancer treatment have been greatly developed along with advancements in science and technology. In other words, the introduction of new therapeutic concepts and methods into clinical practice through the invention and improvement in medical devices that enable high-precision RT, such as particle beam therapy, has brought progress in the cancer treatment. Early detection of early-stage cancers using modern diagnostic imaging technologies contributes to the improvement in survival rates of cancer patients; however, the clinical outcomes of many advanced-stage cancers using a single treatment method remain disappointing. Therefore, a multidisciplinary treatment combining RT with surgery, chemotherapy, molecular targeted therapy, or immunotherapy has been tested, especially for advanced-stage cancers, for a long period. How can a combined protocol be adapted for particle beam therapy? Recent basic biological studies have reported the possibility that higher sensitization effects can be demonstrated with proton beams, which have similar biological effects to photon beams. However, it is expected that combination experiments with C-ions, rather than protons, will show unexpected results. In this section, we summarize the latest findings on the combined effects of particle RT and various cancer treatment modalities.
3.2. Chemotherapy
Chemotherapy is often combined with RT for advanced-stage cancers, given before (neoadjuvant), at the same time (concomitant), and after (adjuvant) RT, and the efficacy and feasibility of the combination therapy have been confirmed in most of them. Many of the goals of RT and chemotherapy combinations are to treat locally and systemically distributed cancers, respectively.
However, it is well known that a combination of drugs and radiation can have local synergistic effects. As a typical example, concurrent chemoradiotherapy consisting of photon RT combined with platinum compounds for head and neck squamous cell carcinoma had a significant benefit of OS with a 10% reduction, but neoadjuvant or adjuvant chemotherapy did not [84]. In contrast, heavy particle beams with a high LET have a smaller sensitizing effect by various chemotherapeutic agents than photon beams. The sensitizing effect of camptothecin, cisplatin, gemcitabine, and paclitaxel, which are frequently used in the field of RT, is remarkable for X-rays, but extremely small for C-ions of 103 keV/µm [85]. Similarly, when glioblastoma (GBM) cells were treated with carbon wire and temozolomide (TMZ), only additive effects were observed [86]. In contrast, a remarkable synergistic effect with a decrease in the shoulder of the cell survival curve was observed in S-phase human colon cancer-derived cells when antimetabolite gemcitabine (2′,2′-difluoro-2′-deoxycytidine, dFdCyd) was combined with C-ions [87]. Proton beams, which exhibit biological effects similar to those of photon beams, can be expected to be more effective in combination with chemotherapeutic agents than C-ions, and concurrent chemoradiotherapy using protons is widespread in clinical practice. However, in recent years, differences between X-rays and proton beams have been reported, such as in gene expression and protein levels due to various double-helix DNA cleavages and DNA responses and disorders in the process of cell death [88,89]. It has been reported that overall DNA damage differs to some extent after proton and X-ray irradiation and that proton-irradiated cells preferentially undergo homologous recombination [90]. This result suggests that the distribution of DNA repair types differs between protons and X-rays. Our research also confirmed that the recovery phenomenon is less likely to occur after proton irradiation than after X-ray irradiation [91]. Furthermore, it has been reported that the sensitizing effect of CDDP may differ between X-rays and protons [92]. The combination index for CDDP in the three types of cells was 0.82–1.00 by X-ray, whereas it was 0.73–0.89 by proton beam, indicating that the proton beam has a stronger sensitizing effect. Analysis using the Cell Cycle Indicator System (fluorescent ubiquitination-based cell cycle indicator or FUCCI) also showed that CDDP and proton beam increased apoptotic cells and G2/M arrest induction more significantly than X-rays. This difference was particularly pronounced in radiation-resistant cells, suggesting that chemoradiotherapy with X-rays and protons may have different effects on radiation-resistant cancers.
3.3. Molecular Targeted Therapy
The toxicity of normal tissues is often increased by chemoradiotherapy, limiting the amount of drug or radiation that can be administered [93]. This fact is far from ideal, and more effective and less toxic combination therapies are needed for the systemic therapy combined with radiation. Against this background, research and development of molecular-targeted drugs are underway. It specifically inhibits cell survival, information exchange with surrounding tissues, and signal transduction associated with responses to internal and external stresses, including radiation. Recent developments in the fields of biotechnology and cell biology have revealed and rapidly elucidated signal transduction and intracellular crosstalk.
A suitable target molecule for a drug used for RT should be overexpressed in many tumors to be treated and not in the normal tissue surrounding the tumor [94,95]. Molecules involved in the mechanism of radioresistance are also ideal. Given these conditions, good examples of reasonable targets related to RT include epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway, Ras pathway, and histone deacetylation. The efficacy of these molecular-targeted drugs in combination with photon therapy has been demonstrated in both basic research and clinically, for example, with EGFR inhibitors.
Although there is insufficient clinical data on the use of particle beams in combination with molecularly targeted drugs and the efficacy of these drugs is not yet clear, many interesting data have been reported from basic biological experiments using cells and mice. The number of relevant studies has increased from approximately 30 in 2010 to over 140 in 2020. The combination of the proton beam and a VEGF inhibitor, bevacizumab, decreased cell survival, increased apoptosis levels, and cell cycle arrest in melanoma cells [96]. The EGFR inhibitor gefitinib preferentially sensitized non-small cell lung cancer cells (H460 and H1299) to proton radiation compared to gamma radiation [97]. Cetuximab (Cmab) also preferentially sensitizes cancer stem cells (SQ-20B/CSCs) isolated from head and neck squamous cell carcinoma (SQ-20B) to carbon beam irradiation compared to photon irradiation [98]. Furthermore, C-ions + Cmab strongly inhibits the enhanced invasion ability of SQ-20B/CSCs, and it has been suggested that the combination therapy is a very promising treatment that suppresses the migration and infiltration process of all cancers as well as CSCs. A PARP inhibitor (PARPi), AZD2281, enhanced the effect of protons on human lung cancer cells (A549) and pancreatic cancer cells (MIAPaCa-2), inducing an increase in γ-H2AX counts and an increase in G2/M arrest [99]. Another PARPi, olaparib and niraparib, sensitized the cytotoxic effects of protons on lung, pancreatic, esophageal, and head and neck cancer cells as well as on X-rays [100,101,102]. These inhibitors have sustained DNA damage from irradiation, delayed apoptosis, prolonged cell cycle arrest, promoted aging, and had more synergistic effects on cells with high proliferation rates. In addition, PARP inhibitors have been effective against C-ions, and the PARPi ADZ2281 enhances the cell-killing effect of carbon rays on pancreatic cancer cells [103]. The combination of talazoparib and carbon beams dramatically reduced the number of GBM stem cells (GSCs), induced prominent and long-term G2/M block, and reduced GSC proliferation [104]. Owing to its multifunctional role and low expression in normal cells, Hsp90 inhibitors are considered a good target for cancer therapy; however, there are few reports on its effects in combination with particle therapy, and we found two reports on its combination with carbon beams [105,106]. 17AAG enhanced the cytotoxic effect on lung cancer cells and the antitumor effect on lung cancer transplanted tumors after C-ions to the same extent as X-rays [105]. This sensitizing effect was due to the inhibition of homologous recombination repair by 17AAG, and as a result, the strong induction of G2 arrest was confirmed. The combination of C-ions with different LET (14 and 50 keV/µm) and another Hsp90 inhibitor, PU-H71, showed higher or similar enhancement ratios compared to X-rays [106]. This result suggests a dependence of the enhancement ratio of molecular-targeted drugs on LET. Valproic acid, a histone deacetylase inhibitor (HDACi), sensitizes hepatocellular carcinoma cells to proton beams more strongly than photon beams by prolonging DNA damage and enhancing apoptosis [107]. In addition, Tsuboi et al. reported that another HDACi, suberoylanilide hydroxamic acid, enhanced the cell-killing effect of C-ions [108]. The combination of novel molecularly targeted drugs and particle radiotherapy has great potential to improve patient outcomes. However, the basic biological knowledge of this combination therapy is scarce, and further research is needed both in vitro and in vivo.
3.4. Nanoparticles
Metal nanoparticles (MNPs), such as gold and platinum, have been reported to exhibit radiosensitizing effects in photon-irradiated cells and animal models [109,110,111]. Experiments have shown that the mechanism is largely due to the interaction of photons with high-Z metal atoms to produce low-energy electrons (especially Auger electrons) [112,113], which in turn promotes the production of reactive oxygen species [114]. Currently, several attempts are being made to introduce nanoparticles into clinical practice.
However, because the interaction of charged particles is largely independent of the atomic number Z of the target, the sensitization of particle beams by MNPs is expected to be even smaller than that of photon beams. However, several experiments have confirmed the large sensitizing effect of the combination of proton beams with MNPs [114,115]. C-ions with high LET, in combination with magnetic nanoparticles, reduce the fluence per unit dose, making the probability of direct interaction at therapeutic doses unrealistic.
Furthermore, in recent years, the application of biomaterials, such as polymer nanofibers, for cancer treatment and drug delivery systems has increased [116,117]. Polymeric nanofibers are an exciting new class of materials and have attracted great attention because of their remarkable properties, such as high specific surface area, high porosity, high molecular alignment, and nano-size effects [118,119,120]. Additionally, many different types of molecules can be easily incorporated into nanofibers to greatly expand their drug-loading capacity or to provide a sustained release of the embedded drug molecules [121,122]. Nanofibers with continuous drug release properties that can enhance the efficacy of anticancer drugs, molecularly targeted drugs, and hyperthermia are being developed [116,123,124,125], and we believe that developments in this field will greatly benefit the sensitization of radiation.
3.5. Immunotherapy
The idea of using immunotherapy in combination with particle beam therapy is completely different from the combination of anticancer drugs and molecular-targeted drugs. It has been shown that local irradiation of tumors acts as an immune adjuvant and can elicit a systemic tumor immune response by killing tumor cells in situ [126,127,128]. Its basic mechanism is the induction of immunogenic cell death in the tumor microenvironment and the sequential activation of systemic cell-mediated immunity [129,130]. Danger signals and the release of tumor-specific antigens after ionizing radiation can turn the irradiated tumor into an in-situ vaccine. Recently, important angiogenic and immunosuppressive factors, such as vascular endothelial growth factor, interleukin 6 and 8, and hypoxia-inducing factor 1α, have been significantly downregulated after high-energy proton irradiation [131]. It has also been reported that proton irradiation mediates cell surface expression of calreticulin on tumor cells and increases susceptibility to cytotoxic T lymphocyte killing [132]. These findings suggest that PBT can inhibit angiogenesis and has an immunosuppressive mechanism, and thus, its therapeutic use can be expanded [133]. The C-ions correlated more with immune activation and prevention of metastasis in mouse models when used in combination with dendritic cell infusion [134,135,136]. In addition, in clinical cases, abscopal reactions have been reported in patients who received topical C-ion treatment [137,138].
In clinical studies using PBT, a phase I study in which an intratumoral injection of hydroxyapatite was used as an immune adjuvant after PBT was locally advanced or recurrent to prevent local or distant recurrence due to the activation of the immune system. It was performed in patients with hepatocellular carcinoma. The conditions of four of the nine patients were reported to have exacerbated [139]. Regarding C-ions, abscopal reactions have been reported in patients receiving topical C-ion treatment [137,138]. A promising approach to using this strategy to remove cancer cells that have spread to unirradiated areas is to combine particle therapy with immune system regulators, such as immune checkpoint inhibitors and cytokines. In addition, tumor-specific immune responses can be obtained by converting the tumor into an effective in situ vaccine using particle therapy.
4. Relationship between Boron Neutron Capture Therapy (BNCT) and Boron Compounds
4.1. The Principle of BNCT
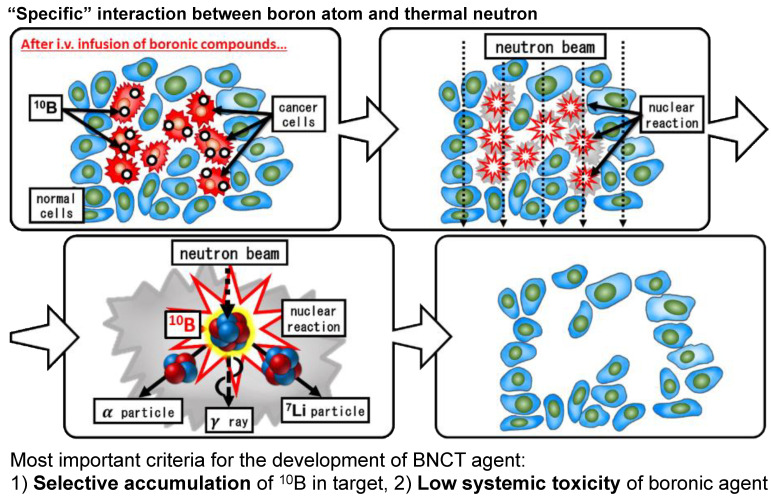
The principle of boron neutron capture therapy was proposed four years after the discovery of neutrons by Chadwick in 1932 [140,141]. It uses 10B, which has a large absorbable cross section for thermal neutrons. It is one of the RT classified as particle beam RT and is characterized by the external irradiation of thermal neutrons, which have no electric charge, and the use of high-LET particle beams (alpha rays and lithium nuclei) produced by the reaction of neutrons with 10B in the body for cellular damage (treatment of malignant tumors). In principle, if boron can be directed to the target area, the reaction will occur only in that area and shows a cell-killing effect.
Briefly, it is common to use 10B as a compound that reacts easily with thermal neutrons, that is, a compound with a large absorbable cross section (3850 barns, 1 barn = 10−24 cm2). A 10B compound with tumor-accumulating properties is administered intravenously to the organism beforehand. When 10B has accumulated in the tumor, thermal neutrons are injected into the affected area by external irradiation. The neutrons react with 10B to produce alpha rays (helium nuclei) and lithium nuclei (Figure 4). Both of these have a short range of approximately 10 microns (about the size of a cell) and can provide high energy without exceeding the diameter of the cell. The principle is that the more selective the distribution of 10B to tumor cells, the less the damage to normal tissues and the higher the antitumor effect.
Figure 4.
Selective cell destruction using boron neutron capture therapy (BNCT).
In RT, the accuracy of spatial positioning has been improved by increasing the accuracy of the treatment device and body sides. These techniques include respiratory synchronization, image-guided RT, and stereotactic RT. However, BNCT is a bimodal therapy, which is different from conventional RT in that it utilizes the difference in drug distribution, especially drug uptake between normal and tumor tissues, to achieve an effect. Thus, BNCT consists of two steps: the drug is responsible for tumor selectivity and the external irradiation of thermal neutrons is used to produce the effect. Therefore, it was necessary to develop a boron-containing compound with tumor-accumulating properties and a high-intensity thermal neutron source capable of delivering a sufficient dose (flux) for the treatment.
4.2. The History of BNCT
In the early days of research and development, the necessary thermal neutrons could only be obtained from experimental reactors. Clinical studies using experimental reactors were initiated in the United States in the 1960s, but the inadequate performance of 10B compounds prevented the discovery of promising results [142,143,144].
Since then, basic and clinical research has been conducted in Japan and Europe [145,146,147,148]. Neutron sources have generally been used through beam shaping, in which the beam from the core of a research reactor is attenuated to increase the proportion of low-energy thermal neutrons [149,150,151,152]. However, in recent years, as the use of nuclear reactors has become increasingly difficult for both commercial and research purposes, high-power neutron sources, such as the Japan Proton Accelerator Research Complex (J-PARK, Tokai, Japan), have been developed in the field of physics, and research on thermal neutron sources for therapeutic use without using nuclear reactors has become popular as a spin-out of these sources [153,154,155,156]. Accelerator neutron sources can be installed in hospitals for medical use, and they have a higher potential for medical applications than the use of nuclear reactors because they are not subject to the strict regulations of nuclear reactors.
Japan was the first country to begin its clinical application. An accelerator-based BNCT clinical study in Japan was developed based on the previous BNCT treatment in nuclear reactors, that is, malignant brain tumors and head and neck cancers were the first targets [157,158,159]. In June 2020, Stella Pharma’s L-p-boronophenylalanine (BPA) agents and Sumitomo Heavy Industries’ NeuCure were the first drugs and devices for BNCT to be covered by health insurance in Japan. As a result, BNCT has become a medical treatment option that utilizes drugs and medical devices, and its use as a full-fledged treatment for a limited number of indications has started. In addition, several research institutes and companies in the United States or China have succeeded in producing thermal neutron beams that can be installed in hospitals [160,161].
4.3. Reactor and Accelerator-Based Neutron Source
In terms of the medical use of nuclear reactors, the research reactor (JRR-4) at the Japan Atomic Energy Research Institute (the Japan Atomic Energy Agency) was modified for medical use in collaboration with the Department of Neurosurgery at the University of Tsukuba, with an operating room, simulation room, irradiation room, remote anesthesia equipment, and even experimental equipment [162,163]. With the development of a simulation calculation code, noncircumferential irradiation using epithermal neutrons can now be handled [164,165,166,167]. Epithermal neutrons are slightly higher in energy than thermal neutrons, and after entering the body, they become thermal neutrons and cause a neutron capture reaction, which has the advantage of allowing treatment of brain tumors with external irradiation [168,169]. While thermal neutrons themselves can only treat superficial lesions with a depth of approximately 2 cm, epithermal neutrons can treat lesions with a depth of approximately 6 cm, and the clinical application of epithermal neutrons will shift from intraoperative irradiation by craniotomy to external irradiation without anesthesia. The clinical application of thermal external neutrons has shifted from intraoperative irradiation by craniotomy to external irradiation without anesthesia.
In addition, unlike charged particles, neutron beams cannot be directly accelerated because they do not have an electric charge, and their handling is more difficult than charged particles [170].
4.4. Head and Neck Cancers
In head and neck cancer, the involvement of the human papilloma virus and the introduction of IMRT have reduced complications, and nivolumab has been introduced to treat recurrence. Head and neck cancer BNCT was used as a salvage treatment for patients who had already been irradiated and were at the time of recurrence or inoperable. It has attracted attention as a new treatment option for patients who have no other choice but palliative treatment, and because of the large number of cases compared to malignant brain tumors, it is thought to have been approved ahead of other treatments. The clinical study cases of head and neck cancer treated with BNCT are summarized in Table 4.
Table 4.
The summary of the clinical study cases of head and neck cancer treated using BNCT.
| Facility | Neutron Source | Year | Tumor | Patients No. | Boron Agents | Clinical Course |
|---|---|---|---|---|---|---|
| Osaka University [171,172] | KUR JRR4 | 2001–2014 | Rec H&N | 45 | BSH, BPA | 5y 32% 10y 21% PR 29% CR 51% |
| Kawasaki Medical College [173] | KUR JRR4 | 2003–2011 | Rec H&N | 20 | BPA | PR 35% CR 55% |
| Kawasaki Medical College | KUR JRR4 | 2006–2012 | H&N preop. | 7 | BPA | 5y 42% PR 1/7 CR 5/7 |
| Helsinki University Central Hospital [174] | FiR-1 | 2003–2008 | Rec H&N | 30 | BPA | MST 13mo PR31% CR 45% |
| Taipei Veterans General Hospital [175] | THOR | 2010–2011 | Rec H&N | 10 | BPA | PR 40% CR 30% |
KUR: Kyoto Research Reactor, Kumatori, Osaka, Japan; JRR4: Japan Research Reactor No.4; Tokai, Ibaraki, Japan; FiR-1: Finland Reactor 1, Otaniemi, Finland, THOR: Tsing Hua open-pool Reactor, Hsinchu Taiwan, Rec H&N: recurrent head and neck cancer, H&N preoperative: head and neck cancer patients of preoperative state, BSH: disodium mercaptoundecahydrododecaborate, BPA: L-p boronophenylalanine, 5y: 5 year survival rate, 10y: 10 year survival rate, PR: Partial response rate, CR: Complete response rate, MST: Median overall survival time.
4.5. Malignant Brain Tumor
For malignant brain tumors, especially GBM, the combination of TMZ and radiation after maximum possible resection has been the standard of care since the breakthrough of TMZ [176], however, there has been no major technological innovation since then.
Malignant brain tumor BNCT has been used as the postoperative RT of choice in newly diagnosed cases or as salvage therapy in cases of recurrence [177]. The University of Tsukuba conducted a clinical study on newly diagnosed malignant glioma and showed that several protocols, including intraoperative irradiation of the craniotomy, prolonged survival by about two times compared to the standard treatment at that time using photon RT [178]. Chemotherapy at that time was in transition from nitrosoureas to TMZ. The median survival of 27 months was an epoch-making figure at that time, although the total number of patients was only a few dozen and there was selection bias, such as a lack of deep-seated disease. Similar to the head and neck region, clinical trials have been conducted for previously irradiated malignant gliomas that have recurred and are awaiting approval. Recent clinical studies on GBM treated using BNCT are summarized in Table 5. Malignant meningioma is another candidate for BNCT [179].
Table 5.
The summary of the recent clinical study cases of glioblastoma treated using BNCT.
| Facility | Neutron Source | Year | Tumor | Patients No. | Boron Agents | Clinical Course (Month) |
|---|---|---|---|---|---|---|
| University of Tsukuba [178] | JRR4 | 1998–2007 | GBM | 15 | BPA, BSH | MST 23.3 27.1 |
| Tokusima University [180,181] | KUR JRR4 | 1998–2008 | GBM | 23 | BSH | MST 15.5 19.5 26.2 |
| Osaka Medical College [182,183,184] | KUR | 2002–2006 | GBM | 21 | BPA, BSH | MST 14.5 23.5 |
| 2002–2007 | rGBM | 19 | BSH, BPA | MST 10.8 |
KUR: Kyoto Research Reactor, Kumatori, Osaka, Japan; JRR4: Japan Research Reactor No.4, Tokai, Ibaraki, Japan; GBM: glioblastoma multiforme; rGBM: recurrent glioblastoma; BSH: disodium mercaptoundecahydrododecaborate; BPA L-p boronophenylalanine; MST, median overall survival time.
4.6. Requirements for Drugs for BNCT and Current Boron Compounds
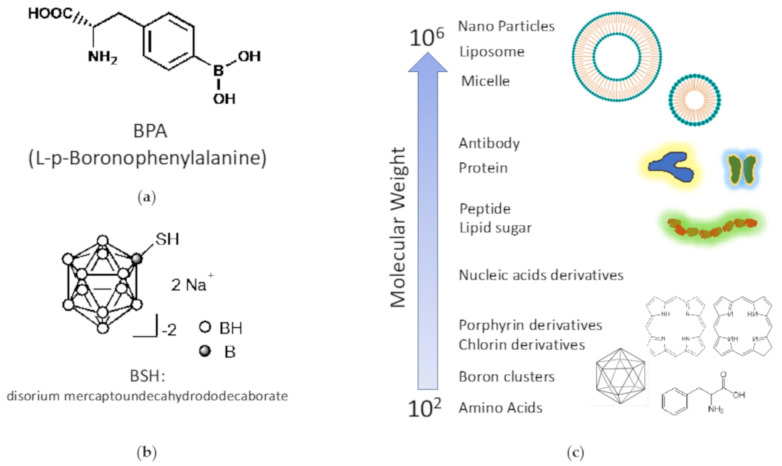
Research and development of boron compounds for BNCT continues; however, the number of clinically available agents is very limited, with only two compounds, BPA and mercaptoundecahydro-closo-dodecaborate (BSH). The ideal BNCT drug would be able to accumulate 10B only in tumors and would not have any toxicity or effect on its own. The concentration required for the treatment depends on the intensity of the neutron beam.
BPA is the main component of borofalan, a newly approved drug for BNCT in Japan (Figure 5a). BPA is composed of the amino acid phenylalanine bound to a single atom of boron. The boron content is thus not high (approximately 5% by weight). It is believed to be absorbed into the cytoplasm via amino acid transporters in tumor cells with an active metabolism. Since tyrosine is a substrate of melanin, it was first used in BNCT for malignant melanoma [146], and its accumulation effect was later found in many histological types of tumors; it was also used for head and neck cancer and malignant brain tumors [185,186]. BSH is a low molecular weight compound with a molecular weight of approximately 200 and has been used for malignant brain tumors (Figure 5b) [187,188]. They do not accumulate in tumors and penetrate tissues by diffusion, and they do not cross the blood-brain barrier in normal brain tissues. This point was reversed in a BNCT clinical study using BSH for malignant brain tumors, which utilized the fact that malignant brain tumors normally diffuse into the interstitium only around disrupted tumor blood vessels. Inevitably, there are also studies using protocols that expect additive effects by combining multiple drugs, that is, BPA and BSH in a two-drug combination, but at present, BSH alone is not used because of the complexity of the procedure and the low tumor/blood ratio.
Figure 5.
Chemical structures of (a) BPA and (b) BSH, and (c) molecular weight of various compounds.
In addition, as a technological development for diagnosis, fluorine-labeled BPA (18FBPA) has been developed for positron emission tomography (PET) examination [189,190,191]. The advantage of being able to determine the accumulation of boron drugs before BNCT treatment has been found to reliably predict the therapeutic effect of BNCT.
BNCT is a single-irRT, and the thermal neutron fluence (n/cm2: the number of particles passing through a unit area) required for a single treatment is approximately 1 × 1013 (within one hour of treatment), which can be generated by current accelerators. To achieve tumor control with this neutron fluence dose, a tissue or intracellular 10B concentration of 20–40 μg/mL is required. To maintain this concentration for the duration of irradiation, the dose of drug administered to the body must be extremely high and must be administered for a short period, and for BPA, a dose of 500 mg/kg is required. According to the dosage and administration approved in Japan, 30 g of the compound was administered in 3 h to a human weighing 60 kg. Compared to anticancer drugs and antibiotics, where the dosage ranges from a few hundred milligrams to a few grams at most, 30 g is a huge amount. Of course, the boron drug alone does not have an antitumor effect, but it does need to accumulate in the tumor.
The ideal conditions for a boron compound for BNCT are:
The concentration of 10B in the tumor tissue or cells must be at least 20 μg/mL during neutron irradiation.
It must be safely administered and excreted.
No toxicity is observed in bolus doses of several tens of grams.
It must be water-soluble
The tumor/normal tissue (T/N) ratio or tumor/blood (T/B) ratio should be as high as possible. The result is a drug with high therapeutic efficacy and reduced damage to normal tissues.
Attempts to develop new boron compounds that are more effective and safer have been ongoing for a long time, and applications of nanoparticles using drug delivery systems, antibody drugs, low-molecular-weight boron compounds have been studied and attempts to improve the tumor/normal tissue ratio as a standard have been ongoing for a long time.
4.7. Development of New Boron Compounds
4.7.1. Problems with Existing Boron Compounds and New Drug Development
While general low-molecular-weight anticancer drugs show cell-killing effects at extremely low concentrations of 10−6 to 10−9 M in tumors, the concentration of boron agents that show therapeutic effects in BNCT is extremely high, at 10−3 M in the tumor (Figure 5c). Therefore, according to the guidelines for the development of BNCT irradiation systems, it is desirable to have a T/B ratio and a T/N ratio of ≥3.0, from the viewpoint of safety [192]. However, BPA, which is currently widely used in BNCT, is rapidly excreted from the tumor by transporters, and its retention in the tumor is low. In May 2020, steboronin was launched as the world’s first boron drug for BNCT in Japan, but its dosage is extremely high at 9000 mg/300 mL. In addition, to achieve excellent therapeutic effects in BNCT, it is important not only to maintain high concentrations of boron drugs in the tumor but also to distribute them homogeneously. Masunaga et al. have shown that quiescent cancer cells have reduced BPA uptake compared with proliferating cancer cells [193,194]. Since it has been reported that quiescent cells include hypoxic cells and CSCs that are resistant to various cancer therapies, including radiation [195,196,197], it can be inferred that the heterogeneous uptake distribution greatly affects the therapeutic effect of BPA-BNCT. Therefore, it is highly desirable to develop a delivery technology that enables tumor-selective and efficient delivery of boron drugs in BNCT therapy.
4.7.2. Drug Development Using Existing Boron Compounds
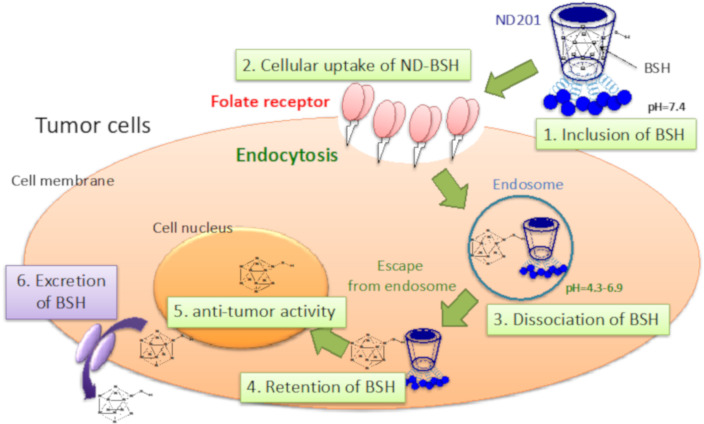
As a method for the highly efficient delivery of boron drugs, investigations using liposomes [198], antibody modification [199], and dendrimers [200] have been reported. Kueffer et al. reported that tumor accumulation could be improved by encapsulating BSH in liposomes and improving blood retention, but since BSH also accumulated in normal cells, a strategy to add tumor cell selectivity is necessary [201] Kang et al. reported that BSH was efficiently taken up by cancer cells by encapsulating it in liposomes modified with a peptide that has a high affinity for integrin αvβ3, which is highly expressed in neovascularization and GBM [202]. Maruyama et al. found that boron drugs encapsulated in PEGylated liposomes improved tumor retention and maintained tumor BPA levels at 30 μg/g tissue for at least 72 h after intravenous administration [203]. Furthermore, Nomoto et al. reported that BPA gel formed by mixing polyvinyl alcohol (PVA) and BPA could inhibit the excretion of BPA from tumors by allowing BPA to enter cells through endocytosis [204]. We also tried to improve the active accumulation of one of the existing boron compounds, BSH, to cancer using ND201, which is cyclodextrin modified with folic acid (Figure 6) [205]. As a result, ND201 induced active cancer accumulation and high retention of BSH depending on the expression level of folic acid receptors in cancer cells and succeeded in obtaining excellent antitumor effects using neutron irradiation.
Figure 6.
The mechanism of action of ND-BSH. BSH is included in the lumen of ND201 and recognizes cancer cells targeting the folate receptor.
4.7.3. Development of Next-Generation Boron Drug for Fusion with Drug Delivery System Technology
As mentioned above, a drug delivery system (DDS) that can control the intracellular uptake pathway and intracellular retention must be developed for BNCT. There are two concepts of DDS in solid tumor therapy: active targeting and passive targeting. Active targeting utilizes the specific binding ability of molecules for targeting. Passive targeting utilizes the characteristics of the tumor vascular system to achieve selective tumor accumulation. In general, macromolecules do not easily leak from normal blood vessels. However, in solid tumors, macromolecules tend to leak from tumor blood vessels because of characteristics, such as increased neovascularization and vascular permeability. In addition, because of the immaturity of the lymphatic system and other reasons, macromolecules that leak locally in the tumor remain there for a long time. As a result, high-molecular-weight anticancer drugs that are highly stable in the blood can be passively targeted [206,207]. This is the enhanced permeability and retention (EPR) effect. The EPR effect, first announced in 1986, has been accepted worldwide and has contributed to the development of methods to deliver anticancer drugs, nucleic acids, genes, and peptides to cancer tissues in polymeric polymers, liposome preparations, and micelle preparations at the animal experimental level.
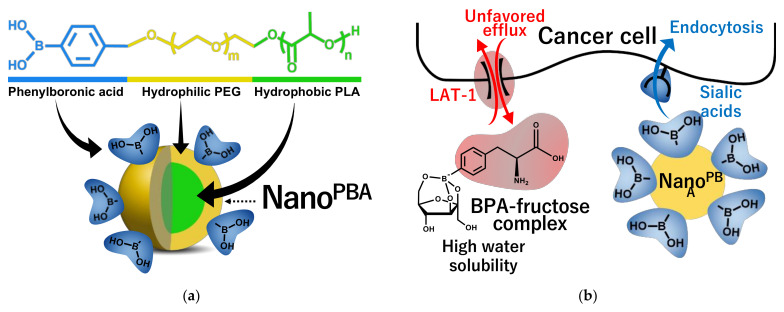
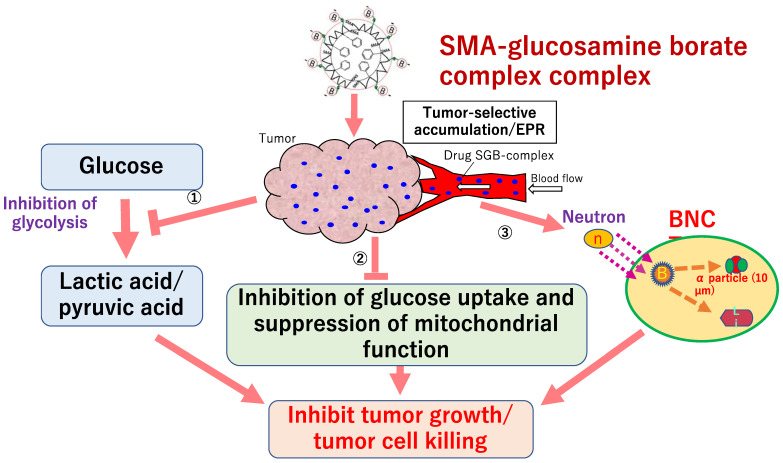
Nakamura et al. focused on the structure of lipids that form liposomes and developed boron lipids, distearoyl boron lipid (DSBL), and boron cholesterol with chlossodecaborate [208], and confirmed the long-term survival after thermal neutron irradiation in a mouse colon cancer CT26 cell transplantation model with an intratumor boron concentration of more than 170 ppm [209]. Nishiyama et al. synthesized PEG-b-P (Glu-SSH), a biodegradable poly(ethylene glycol)-poly(glutamic acid) block copolymer containing BSH disulfide bonded to mercaptoethylamine, and developed nanomicelles by self-assembly [210]. After administration of 50 mg B/kg to mice implanted with CT26 mouse colon tumor, the intratumor boron concentration reached 69 ppm after 24 h, indicating a T/B concentration ratio of more than 20 and a strong BNCT antitumor effect. In addition, Nakamura et al. developed a boron compound, maleimide-functionalized closo-dodecaborate (MID), which can be introduced into cysteine [211], and focused on the phenomenon that stained serum albumin accumulates in tumor tissue (EPR effect), maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC) was prepared by binding MID to serum albumin and verified the antitumor effect of BNCT using cancer transplant mice [212]. Nagasaki et al. developed nanomicelles (PM micelles) in which vinylcarborane was polymerized in biodegradable polyethylene glycol-polylactic acid block copolymer micelles [213]. In an experiment using tumor-bearing mice, PM micelles reached 14 ppm after 24 h of administration, and the tumors disappeared in two out of five mice after 25 days of thermal neutron irradiation. More recently, a collaboration between our group and the Nagasaki laboratory has led to the development of PBA-modified polymeric nanoparticles (NanoPBA) with sialic acid orientation as a boron preparation that has different targeting properties from LAT1 and can maintain 10B levels in tumors for a long time [214]. NanoPBA has a supramolecular structure consisting of a core and shell composed of poly(lactic acid) (PLA) and poly(ethylene glycol) (PEG) segments (Figure 7). PBA is located at the hydrophilic end of the polymer, and a large number of PBAs are exposed on the surface of the nanoparticles and bind to the membranes of cancer cells in multiple ways, resulting in a very strong targeting effect. In this study, the efficacy and safety of NanoPBA were verified by administering NanoPBA to a B16 melanoma-bearing mouse model that highly expresses sialic acid, followed by irradiation with thermal neutron radiation. The results showed that NanoPBA showed a longer intratumor accumulation time than BPA and showed a potent antitumor effect by efficient tumor targeting even at a low dose of 1/100. Furthermore, focusing on the anaerobic glycolytic system, which is characteristic of cancer, a collaboration between our group and Maeda laboratory has developed a multifunctional boron compound, styrene-maleic acid (SMA)-glucosamine borate complex, [215]. The complex is a novel boron compound with multiple functions that can induce changes in the metabolic system and suppress the growth of cancer by the contained boric acid and glucosamine and can also induce cell lethality by BNCT (Figure 8). A remarkable cancer growth inhibitory effect was confirmed by irradiating a cancer-bearing mouse model transplanted with Chinese hamster squamous cell SCCVII with thermal neutrons by administering 125 mg/kg of SMA-glucosamine borate complex. This was an antitumor effect equivalent to that of the 500 mg/kg BPA-administered group, and the SMA-glucosamine borate complex was able to show efficacy at 10B, which is 1/70 of that of BPA.
Figure 7.
(a) Schematic illustration of molecular design for PBA-decorated polymeric nanoparticle as a novel BNCT agent and (b) different accumulation mechanism from BPA-f.
Figure 8.
Multifunctional cancer growth inhibitory effect by SMA-glucosamine borate complex targeting treatment-resistant cancer cells.
4.7.4. Challenges in Conducting BNCT Research
At present, there are no clinical studies using these new compounds, except for phase II studies conducted on boronated porphyrin. In Japan, recurrent head and neck cancers are covered by public insurance, and the number of BNCT treatment facilities is expected to increase, as well as the number of applicable diseases and patients. As a result, the number of researchers involved in the development of new boron agents is expected to increase.
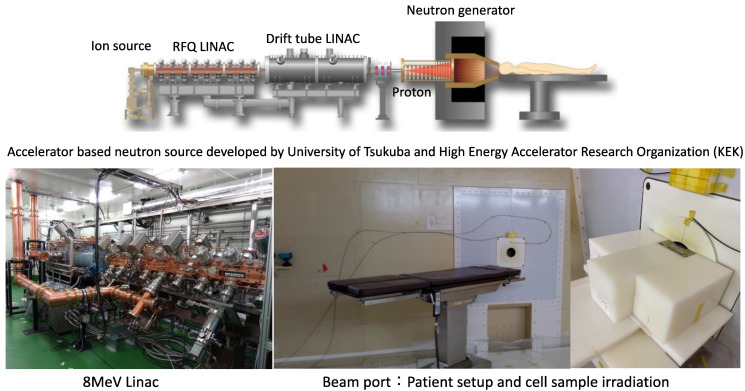
Further focus on the development of new drugs is desirable, with the understanding that the doses of boron compounds are orders of magnitude higher than those of conventional drugs, and that they do not need to have their drug effects, and therefore require tumor accumulation and more stringent safety evaluation. In other words, as mentioned above, it is clear that the development of new boron preparations is urgently needed in addition to the radiobiological research of BNCT to further expand the indications for BNCT. However, the number of neutron sources that can be used for BNCT is limited, and there are only a few facilities in the world where biological and chemical experiments using cells and especially laboratory animals are possible. We at the University of Tsukuba have been developing a new Linac accelerator BNCT device for several years and have already completed a device that can withstand the practical level (Figure 9). The facility also has a biological laboratory where basic research on cells and small animals (mouse and rat) irradiated with an accelerator BNCT device is possible, and animal experiments can be performed with a GLP-compliant grade. In the near future, we plan to publish a large number of research results on new boron drug candidates using this facility.
Figure 9.
Newly developed accelerator-based neutron source (Tsukuba model).
5. Concluding Remarks
The main purpose of combination therapy using existing therapies such as chemotherapy, molecular targeted agents, and radiation is to control the local area using radiation and to suppress metastasis, including micrometastasis, using chemotherapy and molecular targeted agents. In recent years, this trend has been changing, and combination protocols aimed at synergistically increasing the effect on the local area have been considered but have not been established. Owing to the limited number of facilities for particle therapy, there have been few results from both basic and clinical applications, and in principle, it has been considered to follow the conventional combination method in photon RT. However, with the recent remarkable development in the field of nanoparticles and biomaterials, the possibility of proposing new drugs and new combination therapies that better match the characteristics of particle RT is being demonstrated. In this review, we summarized the status of combination therapies in clinical practice with a focus on particle RT and summarized the latest findings on various combination therapies that are being clarified from basic biology, to provide an opportunity to consider combination therapies that should contribute to the next generation of particle RT for cancer.
The current particle therapy has achieved sufficient progress in the field of physical engineering, such as the miniaturization of accelerators and freedom of irradiation direction using gantries. However, the biological benefits are not yet fully understood, leaving the potential for clinical contributions. One of the reasons for this is that the uncertainty of biological problems is much greater than that of physical problems. Therefore, from a clinical point of view, in addition to the biological effects of particle beams, it is highly desirable to clarify the advantages and limitations of using particle beams in combination with other biological therapies. The expected benefits of combined RT include: (1) radiosensitization of tumor tissue (ideally tumor-specific), (2) protection of normal tissue, and (3) induction of bystander or abscopal effects in distant regions.
In PBT, combination therapy has been attempted based on the experience of photon RT, and the combination of radiation and cytotoxic chemotherapy has become the standard treatment for most locally advanced cancers. Because of its excellent dose distribution and mild biological effects, PBT can reduce the exposure dose of normal tissues, which may have side effects when used in combination with other therapies (Cox 2007). In contrast, the physical and biological properties of carbon ions are very different from those of photons, and while they can produce unexpected results, as in the immunotherapy described above, the risks are undeniably greater than expected.
Finally, BNCT, which is now covered by health insurance in Japan, is a different type of RT that must be used in combination with drugs. In recent years, researchers in the fields of drug development and drug delivery have become interested in radiation cancer therapy, probably because of the development and awareness of BNCT. In addition to the accelerator-based BNCT, our facility is equipped with experimental facilities for physics, chemistry, biology, and medicine, and we promise to make a significant contribution to future BNCT research. We hope that drug research in the field of BNCT will have a significant impact on the development of combination therapies for other types of particle therapies.
Acknowledgments
We wish to acknowledge the exceptional work of the Proton Medical Research Center at the University of Tsukuba in the field of particle radiobiology. The corresponding author would like to express special thanks to Hiroshi Maeda for giving him this opportunity to write a special issue about particle radiobiology.
Author Contributions
Y.M. wrote Section 1, Section 3, Section 4.7, and Section 5. N.F. wrote Section 2.3 and Section 2.4. H.I. wrote Section 2.1 and Section 2.2. K.N. wrote Section 4.1, Section 4.2, Section 4.3, Section 4.4, Section 4.5 and Section 4.6. H.S. supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, Grant Numbers JP15K09986 and JP18K07256, Takeda Science Foundation, Grant Number DLQC0004J, and Japan Agency for Medical Research and Development (AMED), Grant Number JP20lm0203010.
Institutional Review Board Statement
All animal experimental procedures were performed based on submitted animal experimental plans (approval number: 18-190, 19-154, 19-199 and 20-233), which were inspected and approved by the animal research committee of the University of Tsukuba and Kumamoto University.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings in this article are available from the corresponding author, Y.M., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson R.R. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence J.H., Tobias C.A., Born J.L., Mc C.R., Roberts J.E., Anger H.O., Low-Beer B.V., Huggins C.B. Pituitary irradiation with high-energy proton beams: A preliminary report. Cancer Res. 1958;18:121–134. [PubMed] [Google Scholar]
- 3.Particle Therapy Co-Operative Group. [(accessed on 22 July 2021)];2021 Available online: https://www.ptcog.ch/
- 4.Suit H., DeLaney T., Goldberg S., Paganetti H., Clasie B., Gerweck L., Niemierko A., Hall E., Flanz J., Hallman J., et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Mohan R., Grosshans D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lomax A. Intensity modulation methods for proton radiotherapy. Phys. Med. Biol. 1999;44:185–205. doi: 10.1088/0031-9155/44/1/014. [DOI] [PubMed] [Google Scholar]
- 7.Bert C., Durante M. Motion in radiotherapy: Particle therapy. Phys. Med. Biol. 2011;56:R113–R144. doi: 10.1088/0031-9155/56/16/R01. [DOI] [PubMed] [Google Scholar]
- 8.DeLaney T.F. Proton therapy in the clinic. Front. Radiat Oncol. 2011;43:465–485. doi: 10.1159/000322511. [DOI] [PubMed] [Google Scholar]
- 9.Engelsman M., Schwarz M., Dong L. Physics controversies in proton therapy. Semin. Radiat. Oncol. 2013;23:88–96. doi: 10.1016/j.semradonc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Hyer D.E., Hill P.M., Wang D., Smith B.R., Flynn R.T. A dynamic collimation system for penumbra reduction in spot-scanning proton therapy: Proof of concept. Med. Phys. 2014;41:091701. doi: 10.1118/1.4837155. [DOI] [PubMed] [Google Scholar]
- 11.Schippers J.M., Lomax A.J. Emerging technologies in proton therapy. Acta Oncol. 2011;50:838–850. doi: 10.3109/0284186X.2011.582513. [DOI] [PubMed] [Google Scholar]
- 12.Durante M., Debus J. Heavy charged particles: Does improved precision and higher biological effectiveness translate to better outcome in patients? Semin. Radiat. Oncol. 2018;28:160–167. doi: 10.1016/j.semradonc.2017.11.004. [DOI] [Google Scholar]
- 13.Weyrather W.K., Kraft G. RBE of carbon ions: Experimental data and the strategy of RBE calculation for treatment planning. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2004;73:S161. doi: 10.1016/S0167-8140(04)80041-0. [DOI] [PubMed] [Google Scholar]
- 14.Kanai T., Matsufuji N., Miyamoto T., Mizoe J., Kamada T., Tsuji H., Kato H., Baba M., Tsujii H. Examination of GyE system for HIMAC carbon therapy. Int. J. Radiat. Oncol. Biol. Phys. 2006;64:650–656. doi: 10.1016/j.ijrobp.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Ando K., Kase Y. Biological characteristics of carbon-ion therapy. Int. J. Radiat. Biol. 2009;85:715–728. doi: 10.1080/09553000903072470. [DOI] [PubMed] [Google Scholar]
- 16.Furusawa Y., Fukutsu K., Aoki M., Itsukaichi H., Eguchi-Kasai K., Ohara H., Yatagai F., Kanai T., Ando K. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat. Res. 2000;154:485–496. doi: 10.1667/0033-7587(2000)154[0485:IOAAHC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Gray L.H., Green F.O., Hawes C.A. Effect of nitric oxide on the radiosensitivity of tumour cells. Nature. 1958;182:952–953. doi: 10.1038/182952a0. [DOI] [PubMed] [Google Scholar]
- 18.Ito A., Nakano H., Kusano Y., Hirayama R., Furusawa Y., Murayama C., Mori T., Katsumura Y., Shinohara K. Contribution of indirect action to radiation-induced mammalian cell inactivation: Dependence on photon energy and heavy-ion LET. Radiat. Res. 2006;165:703–712. doi: 10.1667/RR3557.1. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama R., Ito A., Tomita M., Tsukada T., Yatagai F., Noguchi M., Matsumoto Y., Kase Y., Ando K., Okayasu R., et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat. Res. 2009;171:212–218. doi: 10.1667/RR1490.1. [DOI] [PubMed] [Google Scholar]
- 20.Aoki-Nakano M., Furusawa Y. Misrepair of DNA double-strand breaks after exposure to heavy-ion beams causes a peak in the LET-RBE relationship with respect to cell killing in DT40 cells. J. Radiat. Res. 2013;54:1029–1035. doi: 10.1093/jrr/rrt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi A., Kubo M., Ma H., Nakagawa A., Yoshida Y., Isono M., Kanai T., Ohno T., Furusawa Y., Funayama T., et al. Nonhomologous end-joining repair plays a more important role than homologous recombination repair in defining radiosensitivity after exposure to high-LET radiation. Radiat. Res. 2014;182:338–344. doi: 10.1667/RR13782.1. [DOI] [PubMed] [Google Scholar]
- 22.Blakely E.A., Tobias C.A., Yang T.C., Smith K.C., Lyman J.T. Inactivation of human kidney cells by high-energy monoenergetic heavy-ion beams. Radiat. Res. 1979;80:122–160. doi: 10.2307/3575121. [DOI] [PubMed] [Google Scholar]
- 23.Hirayama R., Furusawa Y., Fukawa T., Ando K. Repair kinetics of DNA-DSB induced by X-rays or carbon ions under oxic and hypoxic conditions. J. Radiat. Res. 2005;46:325–332. doi: 10.1269/jrr.46.325. [DOI] [PubMed] [Google Scholar]
- 24.Wenzl T., Wilkens J.J. Theoretical analysis of the dose dependence of the oxygen enhancement ratio and its relevance for clinical applications. Radiat. Oncol. 2011;6:171. doi: 10.1186/1748-717X-6-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 26.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa Y., Uno T., Oyama T., Kato K., Kato H., Kawakubo H., Kawamura O., Kusano M., Kuwano H., Takeuchi H., et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus. 2019;16:1–24. doi: 10.1007/s10388-018-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morota M., Gomi K., Kozuka T., Chin K., Matsuura M., Oguchi M., Ito H., Yamashita T. Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009;75:122–128. doi: 10.1016/j.ijrobp.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 29.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S., Bogart J., Hu C., Forster K., Magliocco A., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minsky B.D., Pajak T.F., Ginsberg R.J., Pisansky T.M., Martenson J., Komaki R., Okawara G., Rosenthal S.A., Kelsen D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 31.Kato K., Muro K., Minashi K., Ohtsu A., Ishikura S., Boku N., Takiuchi H., Komatsu Y., Miyata Y., Fukuda H., et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int. J. Radiat. Oncol. Biol. Phys. 2011;81:684–690. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Shirai K., Tamaki Y., Kitamoto Y., Murata K., Satoh Y., Higuchi K., Nonaka T., Ishikawa H., Katoh H., Takahashi T., et al. Dose-volume histogram parameters and clinical factors associated with pleural effusion after chemoradiotherapy in esophageal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:1002–1007. doi: 10.1016/j.ijrobp.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Wei X., Liu H.H., Tucker S.L., Wang S., Mohan R., Cox J.D., Komaki R., Liao Z. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Makishima H., Ishikawa H., Terunuma T., Hashimoto T., Yamanashi K., Sekiguchi T., Mizumoto M., Okumura T., Sakae T., Sakurai H. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J. Radiat. Res. 2015;56:568–576. doi: 10.1093/jrr/rrv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirano Y., Onozawa M., Hojo H., Motegi A., Zenda S., Hotta K., Moriya S., Tachibana H., Nakamura N., Kojima T., et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat. Oncol. 2018;13:23. doi: 10.1186/s13014-018-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizumoto M., Sugahara S., Nakayama H., Hashii H., Nakahara A., Terashima H., Okumura T., Tsuboi K., Tokuuye K., Sakurai H. Clinical results of proton-beam therapy for locoregionally advanced esophageal cancer. Strahlenther. Onkol. 2010;186:482–488. doi: 10.1007/s00066-010-2079-4. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa H., Hashimoto T., Moriwaki T., Hyodo I., Hisakura K., Terashima H., Ohkohchi N., Ohno T., Makishima H., Mizumoto M., et al. Proton beam therapy combined with concurrent chemotherapy for esophageal cancer. Anticancer Res. 2015;35:1757–1762. doi: 10.1016/j.ijrobp.2014.05.1129. [DOI] [PubMed] [Google Scholar]
- 38.Ono T., Wada H., Ishikawa H., Tamamura H., Tokumaru S. Clinical results of proton beam therapy for esophageal cancer: Multicenter retrospective study in Japan. Cancers. 2019;11:993. doi: 10.3390/cancers11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeCesaris C.M., Berger M., Choi J.I., Carr S.R., Burrows W.M., Regine W.F., Simone C.B., 2nd, Molitoris J.K. Pathologic complete response (pCR) rates and outcomes after neoadjuvant chemoradiotherapy with proton or photon radiation for adenocarcinomas of the esophagus and gastroesophageal junction. J. Gastrointest. Oncol. 2020;11:663–673. doi: 10.21037/jgo-20-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Palaskas N.L., Yusuf S.W., Abe J.I., Lopez-Mattei J., Banchs J., Gladish G.W., Lee P., Liao Z., Deswal A., et al. Incidence and onset of severe cardiac events after radiotherapy for esophageal cancer. J. Thorac. Oncol. 2020;15:1682–1690. doi: 10.1016/j.jtho.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Wei C., Tucker S.L., Myles B., Palmer M., Hofstetter W.L., Swisher S.G., Ajani J.A., Cox J.D., Komaki R., et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:885–891. doi: 10.1016/j.ijrobp.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi M., Xu C., Liao Z., Chang J.Y., Gomez D.R., Jeter M., Cox J.D., Komaki R., Mehran R., Blum M.A., et al. Comparative outcomes after definitive chemoradiotherapy using proton beam therapy versus intensity modulated radiation therapy for esophageal cancer: A retrospective, single-institutional analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017;99:667–676. doi: 10.1016/j.ijrobp.2017.06.2450. [DOI] [PubMed] [Google Scholar]
- 43.Lin S.H., Hobbs B.P., Verma V., Tidwell R.S., Smith G.L., Lei X., Corsini E.M., Mok I., Wei X., Yao L., et al. Randomized phase IIB trial of proton beam therapy versus intensity-modulated radiation therapy for locally advanced esophageal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:1569–1579. doi: 10.1200/JCO.19.02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davuluri R., Jiang W., Fang P., Xu C., Komaki R., Gomez D.R., Welsh J., Cox J.D., Crane C.H., Hsu C.C., et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017;99:128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Fang P., Shiraishi Y., Verma V., Jiang W., Song J., Hobbs B.P., Lin S.H. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int. J. Part. 2018;4:23–32. doi: 10.14338/IJPT-17-00033.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Routman D.M., Garant A., Lester S.C., Day C.N., Harmsen W.S., Sanheuza C.T., Yoon H.H., Neben-Wittich M.A., Martenson J.A., Haddock M.G., et al. A Comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv. Radiat. Oncol. 2019;4:63–69. doi: 10.1016/j.adro.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong T.S., Ryan D.P., Blaszkowsky L.S., Mamon H.J., Kwak E.L., Mino-Kenudson M., Adams J., Yeap B., Winrich B., DeLaney T.F., et al. Phase I study of preoperative short-course chemoradiation with proton beam therapy and capecitabine for resectable pancreatic ductal adenocarcinoma of the head. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:151–157. doi: 10.1016/j.ijrobp.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 48.Hong T.S., Ryan D.P., Borger D.R., Blaszkowsky L.S., Yeap B.Y., Ancukiewicz M., Deshpande V., Shinagare S., Wo J.Y., Boucher Y., et al. A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:830–838. doi: 10.1016/j.ijrobp.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terashima K., Demizu Y., Hashimoto N., Jin D., Mima M., Fujii O., Niwa Y., Takatori K., Kitajima N., Sirakawa S., et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012;103:25–31. doi: 10.1016/j.radonc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 50.Kim T.H., Lee W.J., Woo S.M., Kim H., Oh E.S., Lee J.H., Han S.S., Park S.J., Suh Y.G., Moon S.H., et al. Effectiveness and safety of simultaneous integrated boost-proton beam therapy for localized pancreatic cancer. Technol. Cancer Res. Treat. 2018;17:1533033818783879. doi: 10.1177/1533033818783879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jethwa K.R., Tryggestad E.J., Whitaker T.J., Giffey B.T., Kazemba B.D., Neben-Wittich M.A., Merrell K.W., Haddock M.G., Hallemeier C.L. Initial experience with intensity modulated proton therapy for intact, clinically localized pancreas cancer: Clinical implementation, dosimetric analysis, acute treatment-related adverse events, and patient-reported outcomes. Adv. Radiat. Oncol. 2018;3:314–321. doi: 10.1016/j.adro.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiroshima Y., Fukumitsu N., Saito T., Numajiri H., Murofushi K.N., Ohnishi K., Nonaka T., Ishikawa H., Okumura T., Sakurai H. Concurrent chemoradiotherapy using proton beams for unresectable locally advanced pancreatic cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019;136:37–43. doi: 10.1016/j.radonc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Maemura K., Mataki Y., Kurahara H., Kawasaki Y., Iino S., Sakoda M., Ueno S., Arimura T., Higashi R., Yoshiura T., et al. Comparison of proton beam radiotherapy and hyper-fractionated accelerated chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2017;17:833–838. doi: 10.1016/j.pan.2017.07.191. [DOI] [PubMed] [Google Scholar]
- 54.Kawashiro S., Yamada S., Okamoto M., Ohno T., Nakano T., Shinoto M., Shioyama Y., Nemoto K., Isozaki Y., Tsuji H., et al. Multi-institutional study of carbon-ion radiotherapy for locally advanced pancreatic cancer: Japan carbon-ion radiation oncology study group (J-CROS) study 1403 pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2018;101:1212–1221. doi: 10.1016/j.ijrobp.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 55.Sai S., Wakai T., Vares G., Yamada S., Kamijo T., Kamada T., Shirai T. Combination of carbon ion beam and gemcitabine causes irreparable DNA damage and death of radioresistant pancreatic cancer stem-like cells in vitro and in vivo. Oncotarget. 2015;6:5517–5535. doi: 10.18632/oncotarget.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhan H.X., Xu J.W., Wu D., Wu Z.Y., Wang L., Hu S.Y., Zhang G.Y. Neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6:1201–1219. doi: 10.1002/cam4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blazer M., Wu C., Goldberg R.M., Phillips G., Schmidt C., Muscarella P., Wuthrick E., Williams T.M., Reardon J., Ellison E.C., et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann. Surg. Oncol. 2015;22:1153–1159. doi: 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vitolo V., Cobianchi L., Brugnatelli S., Barcellini A., Peloso A., Facoetti A., Vanoli A., Delfanti S., Preda L., Molinelli S., et al. Preoperative chemotherapy and carbon ions therapy for treatment of resectable and borderline resectable pancreatic adenocarcinoma: A prospective, phase II, multicentre, single-arm study. BMC Cancer. 2019;19:922. doi: 10.1186/s12885-019-6108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukumitsu N., Okumura T., Hiroshima Y., Ishida T., Numajiri H., Murofushi K.N., Ohnishi K., Aihara T., Ishikawa H., Tsuboi K., et al. Simulation study of dosimetric effect in proton beam therapy using concomitant boost technique for unresectable pancreatic cancers. Jpn. J. Radiol. 2018;36:456–461. doi: 10.1007/s11604-018-0743-2. [DOI] [PubMed] [Google Scholar]
- 61.Chuong M., Badiyan S.N., Yam M., Li Z., Langen K., Regine W., Morris C., Snider J., 3rd, Mehta M., Huh S., et al. Pencil beam scanning versus passively scattered proton therapy for unresectable pancreatic cancer. J. Gastrointest. Oncol. 2018;9:687–693. doi: 10.21037/jgo.2018.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori S., Shinoto M., Yamada S. Four-dimensional treatment planning in layer-stacking boost irradiation for carbon-ion pancreatic therapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014;111:258–263. doi: 10.1016/j.radonc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center; Tokyo, Japan: 2021. (Vital Statistics of Japan) [Google Scholar]
- 64.Fowler J.F., Ritter M.A., Chappell R.J., Brenner D.J. What hypofractionated protocols should be tested for prostate cancer? Int. J. Radiat. Oncol. Biol. Phys. 2003;56:1093–1104. doi: 10.1016/S0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 65.Michalski J.M., Yan Y., Watkins-Bruner D., Bosch W.R., Winter K., Galvin J.M., Bahary J.P., Morton G.C., Parliament M.B., Sandler H.M. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fellin G., Mirri M.A., Santoro L., Jereczek-Fossa B.A., Divan C., Mussari S., Ziglio F., La Face B., Barbera F., Buglione M., et al. Low dose rate brachytherapy (LDR-BT) as monotherapy for early stage prostate cancer in Italy: Practice and outcome analysis in a series of 2237 patients from 11 institutions. Br. J. Radiol. 2016;89:20150981. doi: 10.1259/bjr.20150981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shipley W.U., Verhey L.J., Munzenrider J.E., Suit H.D., Urie M.M., McManus P.L., Young R.H., Shipley J.W., Zietman A.L., Biggs P.J., et al. Advanced prostate cancer: The results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int. J. Radiat. Oncol. Biol. Phys. 1995;32:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 68.Roach M., 3rd, DeSilvio M., Valicenti R., Grignon D., Asbell S.O., Lawton C., Thomas C.R., Jr., Shipley W.U. Whole-pelvis, “mini-pelvis”, or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:647–653. doi: 10.1016/j.ijrobp.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 69.Zietman A.L., Bae K., Slater J.D., Shipley W.U., Efstathiou J.A., Coen J.J., Bush D.A., Lunt M., Spiegel D.Y., Skowronski R., et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from proton radiation oncology group/american college of radiology 95-09. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuban D.A., Tucker S.L., Dong L., Starkschall G., Huang E.H., Cheung M.R., Lee A.K., Pollack A. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 71.D’Amico A.V., Manola J., Loffredo M., Renshaw A.A., DellaCroce A., Kantoff P.W. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 72.Schulte R.W., Slater J.D., Rossi C.J., Jr., Slater J.M. Value and perspectives of proton radiation therapy for limited stage prostate cancer. Strahlenther. Onkol. 2000;176:3–8. doi: 10.1007/PL00002302. [DOI] [PubMed] [Google Scholar]
- 73.Ishikawa H., Tsuji H., Kamada T., Akakura K., Suzuki H., Shimazaki J., Tsujii H., Working Group for Genitourinary Tumors Carbon-ion radiation therapy for prostate cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2012;19:296–305. doi: 10.1111/j.1442-2042.2012.02961.x. [DOI] [PubMed] [Google Scholar]
- 74.Murakami M., Ishikawa H., Shimizu S., Iwata H., Okimoto T., Takagi M., Murayama S., Akimoto T., Wada H., Arimura T., et al. Optimal androgen deprivation therapy combined with proton beam therapy for prostate cancer: Results from a multi-institutional study of the japanese radiation oncology study group. Cancers. 2020;12:1690. doi: 10.3390/cancers12061690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanders J.E., Hawley J., Levy W., Gooley T., Buckner C.D., Deeg H.J., Doney K., Storb R., Sullivan K., Witherspoon R., et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–3052. doi: 10.1182/blood.V87.7.3045.bloodjournal8773045. [DOI] [PubMed] [Google Scholar]
- 76.Green D.M., Whitton J.A., Stovall M., Mertens A.C., Donaldson S.S., Ruymann F.B., Pendergrass T.W., Robison L.L. Pregnancy outcome of female survivors of childhood cancer: A report from the childhood cancer survivor study. Am. J. Obs. Gynecol. 2002;187:1070–1080. doi: 10.1067/mob.2002.126643. [DOI] [PubMed] [Google Scholar]
- 77.Mertens A.C., Liu Q., Neglia J.P., Wasilewski K., Leisenring W., Armstrong G.T., Robison L.L., Yasui Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eaton B.R., Esiashvili N., Kim S., Weyman E.A., Thornton L.T., Mazewski C., MacDonald T., Ebb D., MacDonald S.M., Tarbell N.J., et al. Clinical Outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: A comparison of disease control and overall survival. Int. J. Radiat. Oncol. Biol. Phys. 2016;94:133–138. doi: 10.1016/j.ijrobp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eaton B.R., Esiashvili N., Kim S., Patterson B., Weyman E.A., Thornton L.T., Mazewski C., MacDonald T.J., Ebb D., MacDonald S.M., et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro-Oncol. 2016;18:881–887. doi: 10.1093/neuonc/nov302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cotter S.E., Herrup D.A., Friedmann A., Macdonald S.M., Pieretti R.V., Robinson G., Adams J., Tarbell N.J., Yock T.I. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: Clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1367–1373. doi: 10.1016/j.ijrobp.2010.07.1989. [DOI] [PubMed] [Google Scholar]
- 81.Ladra M.M., Szymonifka J.D., Mahajan A., Friedmann A.M., Yong Yeap B., Goebel C.P., MacDonald S.M., Grosshans D.R., Rodriguez-Galindo C., Marcus K.J., et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:3762–3770. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weber D.C., Ares C., Albertini F., Frei-Welte M., Niggli F.K., Schneider R., Lomax A.J. Pencil beam scanning proton therapy for pediatric parameningeal rhabdomyosarcomas: Clinical outcome of patients treated at the paul scherrer institute. Pediatr. Blood Cancer. 2016;63:1731–1736. doi: 10.1002/pbc.25864. [DOI] [PubMed] [Google Scholar]
- 83.Mizumoto M., Murayama S., Akimoto T., Demizu Y., Fukushima T., Ishida Y., Oshiro Y., Numajiri H., Fuji H., Okumura T., et al. Preliminary results of proton radiotherapy for pediatric rhabdomyosarcoma: A multi-institutional study in Japan. Cancer Med. 2018;7:1870–1874. doi: 10.1002/cam4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pignon J.P., Bourhis J., Domenge C., Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. doi: 10.1016/S0140-6736(00)90011-4. [DOI] [PubMed] [Google Scholar]
- 85.Schlaich F., Brons S., Haberer T., Debus J., Combs S.E., Weber K.J. Comparison of the effects of photon versus carbon ion irradiation when combined with chemotherapy in vitro. Radiat. Oncol. 2013;8:260. doi: 10.1186/1748-717X-8-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrabi S., Combs S.E., Brons S., Haberer T., Debus J., Weber K.J. Temozolomide in combination with carbon ion or photon irradiation in glioblastoma multiforme cell lines—Does scheduling matter? Int. J. Radiat. Biol. 2013;89:692–697. doi: 10.3109/09553002.2013.791406. [DOI] [PubMed] [Google Scholar]
- 87.Harrabi S.B., Adeberg S., Winter M., Haberer T., Debus J., Weber K.J. S-phase-specific radiosensitization by gemcitabine for therapeutic carbon ion exposure in vitro. J. Radiat. Res. 2016;57:110–114. doi: 10.1093/jrr/rrv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carter R.J., Nickson C.M., Thompson J.M., Kacperek A., Hill M.A., Parsons J.L. Complex DNA damage induced by high linear energy transfer alpha-particles and protons triggers a specific cellular DNA damage response. Int. J. Radiat. Oncol. Biol. Phys. 2018;100:776–784. doi: 10.1016/j.ijrobp.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luhr A., von Neubeck C., Krause M., Troost E.G.C. Relative biological effectiveness in proton beam therapy—Current knowledge and future challenges. Clin. Transl. Radiat. Oncol. 2018;9:35–41. doi: 10.1016/j.ctro.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grosse N., Fontana A.O., Hug E.B., Lomax A., Coray A., Augsburger M., Paganetti H., Sartori A.A., Pruschy M. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:175–181. doi: 10.1016/j.ijrobp.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 91.Matsumoto Y., Ando K., Kato T.A., Sekino Y., Ishikawa H., Sakae T., Tsuboi K., Sakurai H. Difference in degree of sub-lethal damage recovery between clinical proton beams and X-Rays. Radiat. Prot. Dosim. 2019;183:93–97. doi: 10.1093/rpd/ncy270. [DOI] [PubMed] [Google Scholar]
- 92.Iwata H., Shuto T., Kamei S., Omachi K., Moriuchi M., Omachi C., Toshito T., Hashimoto S., Nakajima K., Sugie C., et al. Combined effects of cisplatin and photon or proton irradiation in cultured cells: Radiosensitization, patterns of cell death and cell cycle distribution. J. Radiat. Res. 2020;61:832–841. doi: 10.1093/jrr/rraa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krause M., Zips D., Thames H.D., Kummermehr J., Baumann M. Preclinical evaluation of molecular-targeted anticancer agents for radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2006;80:112–122. doi: 10.1016/j.radonc.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 94.Chae Y.K., Pan A.P., Davis A.A., Patel S.P., Carneiro B.A., Kurzrock R., Giles F.J. Path toward precision oncology: Review of targeted therapy studies and tools to aid in defining “actionability” of a molecular lesion and patient management support. Mol. Cancer. 2017;16:2645–2655. doi: 10.1158/1535-7163.MCT-17-0597. [DOI] [PubMed] [Google Scholar]
- 95.Falzone L., Salomone S., Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharm. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koricanac L.B., Zakula J.J., Petrovic I.M., Valastro L.M., Cirrone G.A., Cuttone G., Ristic-Fira A.M. Anti-tumour activity of fotemustine and protons in combination with bevacizumab. Chemotherapy. 2010;56:214–222. doi: 10.1159/000316333. [DOI] [PubMed] [Google Scholar]
- 97.Park H.J., Oh J.S., Chang J.W., Hwang S.G., Kim J.S. Proton irradiation sensitizes radioresistant non-small cell lung cancer cells by modulating epidermal growth factor receptor-mediated DNA repair. Anticancer. Res. 2016;36:205–212. [PubMed] [Google Scholar]
- 98.Moncharmont C., Guy J.B., Wozny A.S., Gilormini M., Battiston-Montagne P., Ardail D., Beuve M., Alphonse G., Simoens X., Rancoule C., et al. Carbon ion irradiation withstands cancer stem cells’ migration/invasion process in Head and Neck Squamous Cell Carcinoma (HNSCC) Oncotarget. 2016;7:47738–47749. doi: 10.18632/oncotarget.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirai T., Saito S., Fujimori H., Matsushita K., Nishio T., Okayasu R., Masutani M. Radiosensitization by PARP inhibition to proton beam irradiation in cancer cells. Biochem. Biophys. Res. Commun. 2016;478:234–240. doi: 10.1016/j.bbrc.2016.07.062. [DOI] [PubMed] [Google Scholar]
- 100.Wera A.C., Lobbens A., Stoyanov M., Lucas S., Michiels C. Radiation-induced synthetic lethality: Combination of poly(ADP-ribose) polymerase and RAD51 inhibitors to sensitize cells to proton irradiation. Cell Cycle. 2019;18:1770–1783. doi: 10.1080/15384101.2019.1632640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kageyama S.I., Junyan D., Hojo H., Motegi A., Nakamura M., Tsuchihara K., Akimoto T. PARP inhibitor olaparib sensitizes esophageal carcinoma cells to fractionated proton irradiation. J. Radiat. Res. 2020;61:177–186. doi: 10.1093/jrr/rrz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang L., Cao J., Wang X., Lin E., Wang Z., Li Y., Li Y., Chen M., Wang X., Jiang B., et al. Proton and photon radiosensitization effects of niraparib, a PARP-1/-2 inhibitor, on human head and neck cancer cells. Head Neck. 2020;42:2244–2256. doi: 10.1002/hed.26155. [DOI] [PubMed] [Google Scholar]
- 103.Hirai T., Shirai H., Fujimori H., Okayasu R., Sasai K., Masutani M. Radiosensitization effect of poly(ADP-ribose) polymerase inhibition in cells exposed to low and high liner energy transfer radiation. Cancer Sci. 2012;103:1045–1050. doi: 10.1111/j.1349-7006.2012.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lesueur P., Chevalier F., El-Habr E.A., Junier M.P., Chneiweiss H., Castera L., Muller E., Stefan D., Saintigny Y. Radiosensitization effect of talazoparib, a parp inhibitor, on glioblastoma stem cells exposed to low and high linear energy transfer radiation. Sci. Rep. 2018;8:3664. doi: 10.1038/s41598-018-22022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirakawa H., Fujisawa H., Masaoka A., Noguchi M., Hirayama R., Takahashi M., Fujimori A., Okayasu R. The combination of Hsp90 inhibitor 17AAG and heavy-ion irradiation provides effective tumor control in human lung cancer cells. Cancer Med. 2015;4:426–436. doi: 10.1002/cam4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H.K., Matsumoto Y., Furusawa Y., Kamada T. PU-H71, a novel Hsp90 inhibitor, as a potential cancer-specific sensitizer to carbon-ion beam therapy. J. Radiat. Res. 2016;57:572–575. doi: 10.1093/jrr/rrw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu J.I., Choi C., Shin S.W., Son A., Lee G.H., Kim S.Y., Park H.C. Valproic acid sensitizes hepatocellular carcinoma cells to proton therapy by suppressing NRF2 activation. Sci. Rep. 2017;7:14986. doi: 10.1038/s41598-017-15165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerelchuluun A., Maeda J., Manabe E., Brents C.A., Sakae T., Fujimori A., Chen D.J., Tsuboi K., Kato T.A. Histone deacetylase inhibitor induced radiation sensitization effects on human cancer cells after photon and hadron radiation exposure. Int. J. Mol. Sci. 2018;19:496. doi: 10.3390/ijms19020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosa S., Connolly C., Schettino G., Butterworth K.T., Prise K.M. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8:2. doi: 10.1186/s12645-017-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H., Mu X., He H., Zhang X.D. Cancer radiosensitizers. Trends Pharm. Sci. 2018;39:24–48. doi: 10.1016/j.tips.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Klebowski B., Depciuch J., Parlinska-Wojtan M., Baran J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018;19:4031. doi: 10.3390/ijms19124031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeremic B., Aguerri A.R., Filipovic N. Radiosensitization by gold nanoparticles. Clin. Transl. Oncol. 2013;15:593–601. doi: 10.1007/s12094-013-1003-7. [DOI] [PubMed] [Google Scholar]
- 113.Schuemann J., Bagley A.F., Berbeco R., Bromma K., Butterworth K.T., Byrne H.L., Chithrani B.D., Cho S.H., Cook J.R., Favaudon V., et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020;65:21RM02. doi: 10.1088/1361-6560/ab9159. [DOI] [PubMed] [Google Scholar]
- 114.Peukert D., Kempson I., Douglass M., Bezak E. Metallic nanoparticle radiosensitisation of ion radiotherapy: A review. Phys. Med. 2018;47:121–128. doi: 10.1016/j.ejmp.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 115.Lacombe S., Porcel E., Scifoni E. Particle therapy and nanomedicine: State of art and research perspectives. Cancer Nanotechnol. 2017;8:9. doi: 10.1186/s12645-017-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen L., Fujisawa N., Takanohashi M., Najmina M., Uto K., Ebara M. A smart hyperthermia nanofiber-platform-enabled sustained release of doxorubicin and 17AAG for synergistic cancer therapy. Int. J. Mol. Sci. 2021;22:2542. doi: 10.3390/ijms22052542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen W., Wu Z., Yang H., Guo S., Li D., Cheng L. In vitro and in vivo evaluation of injectable implants for intratumoral delivery of 5-fluorouracil. Pharm. Dev. Technol. 2014;19:223–231. doi: 10.3109/10837450.2013.769568. [DOI] [PubMed] [Google Scholar]
- 118.Kim Y.J., Ebara M., Aoyagi T. Temperature-responsive electrospun nanofibers for ‘on-off’ switchable release of dextran. Sci. Technol. Adv. Mater. 2012;13:064203. doi: 10.1088/1468-6996/13/6/064203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y., Kotsuchibashi Y., Liu Y., Narain R. Study of bacterial adhesion on biomimetic temperature responsive glycopolymer surfaces. ACS Appl. Mater. Interfaces. 2015;7:1652–1661. doi: 10.1021/am508792k. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y., Kotsuchibashi Y., Uto K., Ebara M., Aoyagi T., Liu Y., Narain R. pH and glucose responsive nanofibers for the reversible capture and release of lectins. Biomater. Sci. 2015;3:152–162. doi: 10.1039/C4BM00269E. [DOI] [PubMed] [Google Scholar]
- 121.Okada T., Niiyama E., Uto K., Aoyagi T., Ebara M. Inactivated sendai virus (HVJ-E) immobilized electrospun nanofiber for cancer therapy. Materials. 2015;9:12. doi: 10.3390/ma9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki K., Tanaka H., Ebara M., Uto K., Matsuoka H., Nishimoto S., Okada K., Murase T., Yoshikawa H. Electrospun nanofiber sheets incorporating methylcobalamin promote nerve regeneration and functional recovery in a rat sciatic nerve crush injury model. Acta Biomater. 2017;53:250–259. doi: 10.1016/j.actbio.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 123.Niiyama E., Uto K., Lee C.M., Sakura K., Ebara M. Hyperthermia nanofiber platform synergized by sustained release of paclitaxel to improve antitumor efficiency. Adv. Healthc Mater. 2019;8:e1900102. doi: 10.1002/adhm.201900102. [DOI] [PubMed] [Google Scholar]
- 124.Niiyama E., Uto K., Lee C.M., Sakura K., Ebara M. Alternating magnetic field-triggered switchable nanofiber mesh for cancer thermo-chemotherapy. Polymers. 2018;10:1018. doi: 10.3390/polym10091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fujisawa N., Takanohashi M., Chen L., Uto K., Matsumoto Y., Takeuchi M., Ebara M. A Diels-Alder polymer platform for thermally enhanced drug release toward efficient local cancer chemotherapy. Sci. Technol. Adv. Mater. 2021;22:522–531. doi: 10.1080/14686996.2021.1939152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Formenti S.C., Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haikerwal S.J., Hagekyriakou J., MacManus M., Martin O.A., Haynes N.M. Building immunity to cancer with radiation therapy. Cancer Lett. 2015;368:198–208. doi: 10.1016/j.canlet.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 128.Scheithauer H., Belka C., Lauber K., Gaipl U.S. Immunological aspects of radiotherapy. Radiat. Oncol. 2014;9:185. doi: 10.1186/1748-717X-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 130.Grass G.D., Krishna N., Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer. 2016;40:10–24. doi: 10.1016/j.currproblcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 131.Girdhani S., Lamont C., Hahnfeldt P., Abdollahi A., Hlatky L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat. Res. 2012;178:33–45. doi: 10.1667/RR2724.1. [DOI] [PubMed] [Google Scholar]
- 132.Gameiro S.R., Malamas A.S., Bernstein M.B., Tsang K.Y., Vassantachart A., Sahoo N., Tailor R., Pidikiti R., Guha C.P., Hahn S.M., et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int. J. Radiat. Oncol. Biol. Phys. 2016;95:120–130. doi: 10.1016/j.ijrobp.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Girdhani S., Sachs R., Hlatky L. Biological effects of proton radiation: What we know and don’t know. Radiat. Res. 2013;179:257–272. doi: 10.1667/RR2839.1. [DOI] [PubMed] [Google Scholar]
- 134.Ando K., Fujita H., Hosoi A., Ma L., Wakatsuki M., Seino K.I., Kakimi K., Imai T., Shimokawa T., Nakano T. Intravenous dendritic cell administration enhances suppression of lung metastasis induced by carbon-ion irradiation. J. Radiat. Res. 2017;58:446–455. doi: 10.1093/jrr/rrx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ogata T., Teshima T., Kagawa K., Hishikawa Y., Takahashi Y., Kawaguchi A., Suzumoto Y., Nojima K., Furusawa Y., Matsuura N. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005;65:113–120. [PubMed] [Google Scholar]
- 136.Ohkubo Y., Iwakawa M., Seino K., Nakawatari M., Wada H., Kamijuku H., Nakamura E., Nakano T., Imai T. Combining carbon ion radiotherapy and local injection of alpha-galactosylceramide-pulsed dendritic cells inhibits lung metastases in an in vivo murine model. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:1524–1531. doi: 10.1016/j.ijrobp.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 137.Durante M., Brenner D.J., Formenti S.C. Does heavy ion therapy work through the immune system? Int. J. Radiat. Oncol. Biol. Phys. 2016;96:934–936. doi: 10.1016/j.ijrobp.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 138.Durante M., Reppingen N., Held K.D. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol. Med. 2013;19:565–582. doi: 10.1016/j.molmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 139.Abei M., Okumura T., Fukuda K., Hashimoto T., Araki M., Ishige K., Hyodo I., Kanemoto A., Numajiri H., Mizumoto M., et al. A phase I study on combined therapy with proton-beam radiotherapy and in situ tumor vaccination for locally advanced recurrent hepatocellular carcinoma. Radiat. Oncol. 2013;8:239. doi: 10.1186/1748-717X-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taylor H.J., Goldhaber M. Detection of nuclear disintegration in a photographic emulsion. Nature. 1935;135:341. doi: 10.1038/135341a0. [DOI] [Google Scholar]
- 141.GL L. Biological effects and therapeutic possiblilities of neutron. Am. J. Roentgenol. 1936;36:1–13. [Google Scholar]
- 142.Farr L.E., Sweet W.H., Robertson J.S., Foster C.G., Locksley H.B., Sutherland D.L., Mendelsohn M.L., Stickley E.E. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am. J. Roentgenol. Radium Nucl. Med. 1954;71:279–293. [PubMed] [Google Scholar]
- 143.Slatkin D.N. A history of boron neutron capture therapy of brain tumours. Postulation of a brain radiation dose tolerance limit. Brain. 1991;114:1609–1629. doi: 10.1093/brain/114.4.1609. [DOI] [PubMed] [Google Scholar]
- 144.Sweet W.H. Early history of development of boron neutron capture therapy of tumors. J. Neurooncol. 1997;33:19–26. doi: 10.1023/A:1005752827194. [DOI] [PubMed] [Google Scholar]
- 145.Hatanaka H., Nakagawa Y. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int. J. Radiat. Oncol. Biol. Phys. 1994;28:1061–1066. doi: 10.1016/0360-3016(94)90479-0. [DOI] [PubMed] [Google Scholar]
- 146.Mishima Y., Honda C., Ichihashi M., Obara H., Hiratsuka J., Fukuda H., Karashima H., Kobayashi T., Kanda K., Yoshino K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound. Lancet. 1989;2:388–389. doi: 10.1016/S0140-6736(89)90567-9. [DOI] [PubMed] [Google Scholar]
- 147.Sauerwein W., Zurlo A., Group E.B.N.C.T. The EORTC Boron Neutron Capture Therapy (BNCT) Group: Achievements and future projects. Eur. J. Cancer. 2002;38:S31–S34. doi: 10.1016/S0959-8049(01)00452-X. [DOI] [PubMed] [Google Scholar]
- 148.Wittig A., Moss R.L., Stecher-Rasmussen F., Appelman K., Rassow J., Roca A., Sauerwein W. Neutron activation of patients following boron neutron capture therapy of brain tumors at the high flux reactor (HFR) Petten (EORTC Trials 11961 and 11011) Strahlenther. Onkol. 2005;181:774–782. doi: 10.1007/s00066-005-1433-4. [DOI] [PubMed] [Google Scholar]
- 149.Sakamoto S., Kiger W.S., 3rd, Harling O.K. Sensitivity studies of beam directionality, beam size, and neutron spectrum for a fission converter-based epithermal neutron beam for boron neutron capture therapy. Med. Phys. 1999;26:1979–1988. doi: 10.1118/1.598784. [DOI] [PubMed] [Google Scholar]
- 150.Kobayashi T., Kanda K., Ujeno Y., Ishida M.R. Biomedical irradiation system for boron neutron capture therapy at the Kyoto University Reactor. Basic Life Sci. 1990;54:321–339. doi: 10.1007/978-1-4684-5802-2_25. [DOI] [PubMed] [Google Scholar]
- 151.Auterinen I., Kotiluoto P., Hippelainen E., Kortesniemi M., Seppala T., Seren T., Mannila V., Poyry P., Kankaanranta L., Collan J., et al. Design and construction of shoulder recesses into the beam aperture shields for improved patient positioning at the FiR 1 BNCT facility. Appl. Radiat. Isot. 2004;61:799–803. doi: 10.1016/j.apradiso.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 152.Nakamura T., Horiguchi H., Kishi T., Motohashi J., Sasajima F., Kumada H. Resumption of JRR-4 and characteristics of neutron beam for BNCT. Appl. Radiat. Isot. 2011;69:1932–1935. doi: 10.1016/j.apradiso.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 153.Wang C.K., Blue T.E., Gahbauer R.A. A design study of an accelerator-based epithermal neutron source for boron neutron capture therapy. Strahlenther. Onkol. 1989;165:75–78. [PubMed] [Google Scholar]
- 154.Kreiner A.J., Baldo M., Bergueiro J.R., Cartelli D., Castell W., Thatar Vento V., Gomez Asoia J., Mercuri D., Padulo J., Suarez Sandin J.C., et al. Accelerator-based BNCT. Appl. Radiat. Isot. 2014;88:185–189. doi: 10.1016/j.apradiso.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 155.Kumada H., Kurihara T., Yoshioka M., Kobayashi H., Matsumoto H., Sugano T., Sakurai H., Sakae T., Matsumura A. development of beryllium-based neutron target system with three-layer structure for accelerator-based neutron source for boron neutron capture therapy. Appl. Radiat. Isot. 2015;106:78–83. doi: 10.1016/j.apradiso.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 156.Taskaev S., Berendeev E., Bikchurina M., Bykov T., Kasatov D., Kolesnikov I., Koshkarev A., Makarov A., Ostreinov G., Porosev V., et al. Neutron source based on vacuum insulated tandem accelerator and lithium target. Biology. 2021;10:350. doi: 10.3390/biology10050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hirose K., Konno A., Hiratsuka J., Yoshimoto S., Kato T., Ono K., Otsuki N., Hatazawa J., Tanaka H., Takayama K., et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan ((10)B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021;155:182–187. doi: 10.1016/j.radonc.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 158.Kanno H., Nagata H., Ishiguro A., Tsuzuranuki S., Nakano S., Nonaka T., Kiyohara K., Kimura T., Sugawara A., Okazaki Y., et al. Designation products: Boron neutron capture therapy for head and neck carcinoma. Oncologist. 2021;26:e1250–e1255. doi: 10.1002/onco.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miyatake S.I., Wanibuchi M., Hu N., Ono K. Boron neutron capture therapy for malignant brain tumors. J. Neurooncol. 2020;149:1–11. doi: 10.1007/s11060-020-03586-6. [DOI] [PubMed] [Google Scholar]
- 160.Provenzano L., Koivunoro H., Postuma I., Longhino J.M., Boggio E.F., Farias R.O., Bortolussi S., Gonzalez S.J. The essential role of radiobiological figures of merit for the assessment and comparison of beam performances in boron neutron capture therapy. Phys. Med. 2019;67:9–19. doi: 10.1016/j.ejmp.2019.09.235. [DOI] [PubMed] [Google Scholar]
- 161.Lee P.Y., Tang X., Geng C., Liu Y.H. A bi-tapered and air-gapped beam shaping assembly used for AB-BNCT. Appl. Radiat. Isot. 2021;167:109392. doi: 10.1016/j.apradiso.2020.109392. [DOI] [PubMed] [Google Scholar]
- 162.Yamamoto T., Matsumura A., Yamamoto K., Kumada H., Shibata Y., Nose T. In-phantom two-dimensional thermal neutron distribution for intraoperative boron neutron capture therapy of brain tumours. Phys. Med. Biol. 2002;47:2387–2396. doi: 10.1088/0031-9155/47/14/302. [DOI] [PubMed] [Google Scholar]
- 163.Kumada H., Yamamoto K., Matsumura A., Yamamoto T., Nakagawa Y., Nakai K., Kageji T. Verification of the computational dosimetry system in JAERI (JCDS) for boron neutron capture therapy. Phys. Med. Biol. 2004;49:3353–3365. doi: 10.1088/0031-9155/49/15/003. [DOI] [PubMed] [Google Scholar]
- 164.Wojnecki C., Green S. A preliminary comparative study of two treatment planning systems developed for boron neutron capture therapy: MacNCTPlan and SERA. Med. Phys. 2002;29:1710–1715. doi: 10.1118/1.1485058. [DOI] [PubMed] [Google Scholar]
- 165.Koivunoro H., Kumada H., Seppala T., Kotiluoto P., Auterinen I., Kankaanranta L., Savolainen S. Comparative study of dose calculations with SERA and JCDS treatment planning systems. Appl. Radiat. Isot. 2009;67:S126–S129. doi: 10.1016/j.apradiso.2009.03.092. [DOI] [PubMed] [Google Scholar]
- 166.Li H.S., Liu Y.W., Lee C.Y., Lin T.Y., Hsu F.Y. Verification of the accuracy of BNCT treatment planning system THORplan. Appl. Radiat. Isot. 2009;67:S122–S125. doi: 10.1016/j.apradiso.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 167.Kumada H., Yamamoto K., Yamamoto T., Nakai K., Nakagawa Y., Kageji T., Matsumura A. Improvement of dose calculation accuracy for BNCT dosimetry by the multi-voxel method in JCDS. Appl. Radiat. Isot. 2004;61:1045–1050. doi: 10.1016/j.apradiso.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 168.Nakagawa Y., Pooh K., Kobayashi T., Kageji T., Uyama S., Matsumura A., Kumada H. Clinical review of the Japanese experience with boron neutron capture therapy and a proposed strategy using epithermal neutron beams. J. Neurooncol. 2003;62:87–99. doi: 10.1007/BF02699936. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe K., Yoshihashi S., Ishikawa A., Honda S., Yamazaki A., Tsurita Y., Uritani A., Tsuchida K., Kiyanagi Y. First experimental verification of the neutron field of Nagoya University Accelerator-driven neutron source for boron neutron capture therapy. Appl. Radiat. Isot. 2021;168:109553. doi: 10.1016/j.apradiso.2020.109553. [DOI] [PubMed] [Google Scholar]
- 170.Nakamura S., Igaki H., Ito M., Imamichi S., Kashihara T., Okamoto H., Nishioka S., Iijima K., Chiba T., Nakayama H., et al. Neutron flux evaluation model provided in the accelerator-based boron neutron capture therapy system employing a solid-state lithium target. Sci. Rep. 2021;11:8090. doi: 10.1038/s41598-021-87627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kato I., Ono K., Sakurai Y., Ohmae M., Maruhashi A., Imahori Y., Kirihata M., Nakazawa M., Yura Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004;61:1069–1073. doi: 10.1016/j.apradiso.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 172.Kato I., Fujita Y., Maruhashi A., Kumada H., Ohmae M., Kirihata M., Imahori Y., Suzuki M., Sakrai Y., Sumi T., et al. Effectiveness of boron neutron capture therapy for recurrent head and neck malignancies. Appl. Radiat. Isot. 2009;67:S37–S42. doi: 10.1016/j.apradiso.2009.03.103. [DOI] [PubMed] [Google Scholar]
- 173.Aihara T., Morita N., Kamitani N., Kumada H., Ono K., Hiratsuka J., Harada T. BNCT for advanced or recurrent head and neck cancer. Appl. Radiat. Isot. 2014;88:12–15. doi: 10.1016/j.apradiso.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 174.Kankaanranta L., Seppala T., Koivunoro H., Saarilahti K., Atula T., Collan J., Salli E., Kortesniemi M., Uusi-Simola J., Valimaki P., et al. Boron neutron capture therapy in the treatment of locally recurred head-and-neck cancer: Final analysis of a phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:e67–e75. doi: 10.1016/j.ijrobp.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 175.Wang L.W., Wang S.J., Chu P.Y., Ho C.Y., Jiang S.H., Liu Y.W., Liu Y.H., Liu H.M., Peir J.J., Chou F.I., et al. BNCT for locally recurrent head and neck cancer: Preliminary clinical experience from a phase I/II trial at Tsing Hua Open-Pool Reactor. Appl. Radiat. Isot. 2011;69:1803–1806. doi: 10.1016/j.apradiso.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 176.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 177.Barth R.F., Yang W., Rotaru J.H., Moeschberger M.L., Boesel C.P., Soloway A.H., Joel D.D., Nawrocky M.M., Ono K., Goodman J.H. Boron neutron capture therapy of brain tumors: Enhanced survival and cure following blood-brain barrier disruption and intracarotid injection of sodium borocaptate and boronophenylalanine. Int. J. Radiat. Oncol. Biol. Phys. 2000;47:209–218. doi: 10.1016/S0360-3016(00)00421-1. [DOI] [PubMed] [Google Scholar]
- 178.Yamamoto T., Nakai K., Kageji T., Kumada H., Endo K., Matsuda M., Shibata Y., Matsumura A. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009;91:80–84. doi: 10.1016/j.radonc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 179.Takai S., Wanibuchi M., Kawabata S., Takeuchi K., Sakurai Y., Suzuki M., Ono K., Miyatake S.I. Reactor-based boron neutron capture therapy for 44 cases of recurrent and refractory high-grade meningiomas with long-term follow-up. Neuro-Oncol. 2021 doi: 10.1093/neuonc/noab108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kageji T., Nagahiro S., Matsuzaki K., Mizobuchi Y., Toi H., Nakagawa Y., Kumada H. Boron neutron capture therapy using mixed epithermal and thermal neutron beams in patients with malignant glioma-correlation between radiation dose and radiation injury and clinical outcome. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:1446–1455. doi: 10.1016/j.ijrobp.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 181.Kageji T., Mizobuchi Y., Nagahiro S., Nakagawa Y., Kumada H. Clinical results of boron neutron capture therapy (BNCT) for glioblastoma. Appl. Radiat. Isot. 2011;69:1823–1825. doi: 10.1016/j.apradiso.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 182.Miyatake S., Kawabata S., Kajimoto Y., Aoki A., Yokoyama K., Yamada M., Kuroiwa T., Tsuji M., Imahori Y., Kirihata M., et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: An efficacy study based on findings on neuroimages. J. Neurosurg. 2005;103:1000–1009. doi: 10.3171/jns.2005.103.6.1000. [DOI] [PubMed] [Google Scholar]
- 183.Kawabata S., Miyatake S., Kuroiwa T., Yokoyama K., Doi A., Iida K., Miyata S., Nonoguchi N., Michiue H., Takahashi M., et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J. Radiat. Res. 2009;50:51–60. doi: 10.1269/jrr.08043. [DOI] [PubMed] [Google Scholar]
- 184.Miyatake S., Kawabata S., Yokoyama K., Kuroiwa T., Michiue H., Sakurai Y., Kumada H., Suzuki M., Maruhashi A., Kirihata M., et al. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J. Neurooncol. 2009;91:199–206. doi: 10.1007/s11060-008-9699-x. [DOI] [PubMed] [Google Scholar]
- 185.Ono K., Masunaga S., Suzuki M., Kinashi Y., Takagaki M., Akaboshi M. The combined effect of boronophenylalanine and borocaptate in boron neutron capture therapy for SCCVII tumors in mice. Int. J. Radiat. Oncol. Biol. Phys. 1999;43:431–436. doi: 10.1016/S0360-3016(98)00421-0. [DOI] [PubMed] [Google Scholar]
- 186.Coderre J.A., Elowitz E.H., Chadha M., Bergland R., Capala J., Joel D.D., Liu H.B., Slatkin D.N., Chanana A.D. Boron neutron capture therapy for glioblastoma multiforme using p-boronophenylalanine and epithermal neutrons: Trial design and early clinical results. J. Neurooncol. 1997;33:141–152. doi: 10.1023/A:1005741919442. [DOI] [PubMed] [Google Scholar]
- 187.Finkel G.C., Poletti C.E., Fairchild R.G., Slatkin D.N., Sweet W.H. Distribution of 10B after infusion of Na210B12H11SH into a patient with malignant astrocytoma: Implications for boron neutron capture therapy. Neurosurgery. 1989;24:6–11. doi: 10.1227/00006123-198901000-00002. [DOI] [PubMed] [Google Scholar]
- 188.Kageji T., Nakagawa Y., Kitamura K., Matsumoto K., Hatanaka H. Pharmacokinetics and boron uptake of BSH (Na2B12H11SH) in patients with intracranial tumors. J. Neurooncol. 1997;33:117–130. doi: 10.1023/A:1005785718533. [DOI] [PubMed] [Google Scholar]
- 189.Imahori Y., Ueda S., Ohmori Y., Sakae K., Kusuki T., Kobayashi T., Takagaki M., Ono K., Ido T., Fujii R. Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: Part I. Clin. Cancer Res. 1998;4:1825–1832. [PubMed] [Google Scholar]
- 190.Imahori Y., Ueda S., Ohmori Y., Sakae K., Kusuki T., Kobayashi T., Takagaki M., Ono K., Ido T., Fujii R. Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: Part II. Clin. Cancer Res. 1998;4:1833–1841. [PubMed] [Google Scholar]
- 191.Aihara T., Hiratsuka J., Morita N., Uno M., Sakurai Y., Maruhashi A., Ono K., Harada T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck. 2006;28:850–855. doi: 10.1002/hed.20418. [DOI] [PubMed] [Google Scholar]
- 192.Guidebook on Accelerator BPA-BNCT. Japan Society for Neutron Capture Therapy, Osaka/Japan Society for Radiation Oncology; Tokyo, Japan: 2020. [Google Scholar]
- 193.Masunaga S., Ono K., Sakurai Y., Suzuki M., Takagaki M., Kobayashi T., Kinashi Y., Akaboshi M. Responses of total and quiescent cell populations in solid tumors to boron and gadolinium neutron capture reaction using neutrons with two different energy spectra. Jpn. J. Cancer Res. 1998;89:81–88. doi: 10.1111/j.1349-7006.1998.tb00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Masunaga S., Ono K., Sakurai Y., Takagaki M., Kobayashi T., Suzuki M., Kinashi Y., Akaboshi M. Response of quiescent and total tumor cells in solid tumors to neutrons with various cadmium ratios. Int. J. Radiat. Oncol. Biol. Phys. 1998;41:1163–1170. doi: 10.1016/S0360-3016(98)00149-7. [DOI] [PubMed] [Google Scholar]
- 195.Abad E., Samino S., Yanes O., Potesil D., Zdrahal Z., Lyakhovich A. Activation of glycogenolysis and glycolysis in breast cancer stem cell models. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165886. doi: 10.1016/j.bbadis.2020.165886. [DOI] [PubMed] [Google Scholar]
- 196.Bao B., Azmi A.S., Li Y., Ahmad A., Ali S., Banerjee S., Kong D., Sarkar F.H. Targeting CSCs in tumor microenvironment: The potential role of ROS-associated miRNAs in tumor aggressiveness. Curr. Stem. Cell Res. 2014;9:22–35. doi: 10.2174/1574888X113089990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luo M., Wicha M.S. Targeting cancer stem cell redox metabolism to enhance therapy responses. Semin. Radiat. Oncol. 2019;29:42–54. doi: 10.1016/j.semradonc.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakamura H. Boron lipid-based liposomal boron delivery system for neutron capture therapy: Recent development and future perspective. Future Med. Chem. 2013;5:715–730. doi: 10.4155/fmc.13.48. [DOI] [PubMed] [Google Scholar]
- 199.Nakase I., Aoki A., Sakai Y., Hirase S., Ishimura M., Takatani-Nakase T., Hattori Y., Kirihata M. Antibody-based receptor targeting using an fc-binding peptide-dodecaborate conjugate and macropinocytosis induction for boron neutron capture therapy. ACS Omega. 2020;5:22731–22738. doi: 10.1021/acsomega.0c01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamagami M., Tajima T., Ishimoto K., Miyake H., Michiue H., Takaguchi Y. Physical modification of carbon nanotubes with a dendrimer bearing terminal mercaptoundecahydrododecaborates (Na2B12H11S) Heteroat. Chem. 2018;29:e21467. doi: 10.1002/hc.21467. [DOI] [Google Scholar]
- 201.Kueffer P.J., Maitz C.A., Khan A.A., Schuster S.A., Shlyakhtina N.I., Jalisatgi S.S., Brockman J.D., Nigg D.W., Hawthorne M.F. Boron neutron capture therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc. Natl. Acad. Sci. USA. 2013;110:6512–6517. doi: 10.1073/pnas.1303437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kang W., Svirskis D., Sarojini V., McGregor A.L., Bevitt J., Wu Z. Cyclic-RGDyC functionalized liposomes for dual-targeting of tumor vasculature and cancer cells in glioblastoma: An in vitro boron neutron capture therapy study. Oncotarget. 2017;8:36614–36627. doi: 10.18632/oncotarget.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maruyama K., Ishida O., Kasaoka S., Takizawa T., Utoguchi N., Shinohara A., Chiba M., Kobayashi H., Eriguchi M., Yanagie H. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT) J. Control. Release. 2004;98:195–207. doi: 10.1016/j.jconrel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 204.Nomoto T., Inoue Y., Yao Y., Suzuki M., Kanamori K., Takemoto H., Matsui M., Tomoda K., Nishiyama N. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci. Adv. 2020;6:eaaz1722. doi: 10.1126/sciadv.aaz1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Matsumoto Y., Hattori K., Arima H., Motoyama K., Higashi T., Ishikawa H., Fukumitsu N., Aihara T., Nakai K., Kumada H., et al. Folate-appended cyclodextrin improves the intratumoral accumulation of existing boron compounds. Appl. Radiat. Isot. 2020;163:109201. doi: 10.1016/j.apradiso.2020.109201. [DOI] [PubMed] [Google Scholar]
- 206.Maeda H. The 35th anniversary of the discovery of EPR effect: A new wave of nanomedicines for tumor-targeted drug delivery-personal remarks and future prospects. J. Pers. Med. 2021;11:229. doi: 10.3390/jpm11030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 208.Koganei H., Ueno M., Tachikawa S., Tasaki L., Ban H.S., Suzuki M., Shiraishi K., Kawano K., Yokoyama M., Maitani Y., et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjug Chem. 2013;24:124–132. doi: 10.1021/bc300527n. [DOI] [PubMed] [Google Scholar]
- 209.Tachikawa S., Miyoshi T., Koganei H., El-Zaria M.E., Vinas C., Suzuki M., Ono K., Nakamura H. Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy. Chem. Commun. 2014;50:12325–12328. doi: 10.1039/C4CC04344H. [DOI] [PubMed] [Google Scholar]
- 210.Mi P., Yanagie H., Dewi N., Yen H.C., Liu X., Suzuki M., Sakurai Y., Ono K., Takahashi H., Cabral H., et al. Block copolymer-boron cluster conjugate for effective boron neutron capture therapy of solid tumors. J. Control. Release. 2017;254:1–9. doi: 10.1016/j.jconrel.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 211.Kikuchi S., Kanoh D., Sato S., Sakurai Y., Suzuki M., Nakamura H. Maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumor for neutron capture therapy. J. Control. Release. 2016;237:160–167. doi: 10.1016/j.jconrel.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 212.Ishii S., Sato S., Asami H., Hasegawa T., Kohno J.Y., Nakamura H. Design of S-S bond containing maleimide-conjugated closo-dodecaborate (SSMID): Identification of unique modification sites on albumin and investigation of intracellular uptake. Org. Biomol. Chem. 2019;17:5496–5499. doi: 10.1039/C9OB00584F. [DOI] [PubMed] [Google Scholar]
- 213.Sumitani S., Oishi M., Yaguchi T., Murotani H., Horiguchi Y., Suzuki M., Ono K., Yanagie H., Nagasaki Y. Pharmacokinetics of core-polymerized, boron-conjugated micelles designed for boron neutron capture therapy for cancer. Biomaterials. 2012;33:3568–3577. doi: 10.1016/j.biomaterials.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 214.Kim A., Suzuki M., Matsumoto Y., Fukumitsu N., Nagasaki Y. Non-isotope enriched phenylboronic acid-decorated dual-functional nano-assembles for an actively targeting BNCT drug. Biomaterials. 2021;268:120551. doi: 10.1016/j.biomaterials.2020.120551. [DOI] [PubMed] [Google Scholar]
- 215.Islam W., Matsumoto Y., Fang J., Harada A., Niidome T., Ono K., Tsutsuki H., Sawa T., Imamura T., Sakurai K., et al. Polymer-conjugated glucosamine complexed with boric acid shows tumor-selective accumulation and simultaneous inhibition of glycolysis. Biomaterials. 2021;269:120631. doi: 10.1016/j.biomaterials.2020.120631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings in this article are available from the corresponding author, Y.M., upon reasonable request.