Abstract
Extracellular vesicles (EVs) have been recognized as a universal method of cellular communications and are reportedly produced in bacteria, archaea, and eukaryotes. Bacterial EVs are often called “Outer Membrane Vesicles” (OMVs) as they were the result of a controlled blebbing of the outer membrane of gram-negative bacteria such as Porphyromonas gingivalis (P. gingivalis). Bacterial EVs are natural messengers, implicated in intra- and inter-species cell-to-cell communication among microorganism populations present in microbiota. Bacteria can incorporate their pathogens into OMVs; the content of OMVs differs, depending on the type of bacteria. The production of distinct types of OMVs can be mediated by different factors and routes. A recent study highlighted OMVs ability to carry crucial molecules implicated in immune modulation, and, nowadays, they are considered as a way to communicate and transfer messages from the bacteria to the host and vice versa. This review article focuses on the current understanding of OMVs produced from major oral bacteria, P. gingivalis: generation, characteristics, and contents as well as the involvement in signal transduction of host cells and systemic diseases. Our recent study regarding the action of P. gingivalis OMVs in the living body is also summarized.
Keywords: Extracellular vesicles, Outer membrane vesicles, Porphyromonas gingivalis, Host cell interaction, In vivo imaging
1. Introduction
Signal transduction between cells, tissues, and organs is meticulously regulated and is involved in cellular development, growth, and diseases. These communications are initiated by direct or indirect interactions. In the case of indirect interaction, “cytokines” or “hormones,” released from immune cells and endocrine cells, are involved respectively in cell survival, proliferation, differentiation, and activity.
More recently, a novel intercellular indirect communication system is gathering the attention of scientists. In this system, cells enclose various substances (information) in small particles and then release them to be transferred to other cells. The cargo in which cells contain packaged substances is called extracellular vesicles (EVs); they encompass cell-specific molecules such as nucleic acids, proteins, sugars, and lipids. Over the last decade, research in this area has exponentially expanded — its role has uncovered various insights into the importance of areas such as cancer and immunity. Under physiological or pathological conditions, eukaryotic cells secrete various sizes of vesicles wrapped in plasma or intracellular membrane. These extracellular vesicles are disseminated in fluids – blood, saliva, urine, breast milk, amniotic fluid, etc. – and play an important role in various stages such as development, differentiation, immune response, and aging. Since EVs are presumably released from all cells, they become critical to every evolutionary process in both unicellular and multicellular organisms.
In contrast to the well-established various roles of EVs in eukaryotic cell biology, outer membrane vesicles (OMVs), produced via blebbing of prokaryotic membranes, have frequently been regarded as cell debris or microscopy artificial products. Over the past decades, the role of OMVs produced gram-negative bacteria have been analyzed. OMVs nowadays have been considered a very sophisticated mechanism for transporting many molecular effectors, cell-cell interactions, nutrients, interaction, host cell immune dysregulation, modulation, bacterial aggregation, biofilm formation, etc. Some of OMVs-releasing species reported include Porphyromonas gingivalis (P. gingivalis), Actinobacillus actinomycetemcomitans, Borrelia burgdorferi, and Pseudomonas aeruginosa. [[1], [2], [3], [4]].
Periodontitis is among the most common human infection, initiated by specific species of bacteria: Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Treponema denticola, and P. gingivalis [5]. The pathogenesis of periodontitis is mediated through interactions between these microbial factors and the host cells. In particular, P. gingivalis has been frequently associated with specific types of periodontitis and possesses various mechanisms that favor the pathogenic process. P. gingivalis has been observed within gingival tissues in vivo, suggesting that P. gingivalis may invade deeper structures of the connective tissue [6]. On the other hand, P. gingivalis has been reported to be heavily involved in a wide variety of systemic diseases including cardiology, rheumatology, diabetology, oncology, immunology, and neurology [7]. Several mechanisms have been reported to associate P. gingivalis with these systemic diseases. P. gingivalis affects endothelial cells, platelets, leucocytes (mainly monocytes and macrophages), cardiomyocytes, and smooth muscle cells to aggravate cardiovascular diseases such as atherosclerotic cardiovascular diseases and myocardial infarction [[8], [9], [10]]. P. gingivalis enhances oxidative stress, inflammatory and prothrombotic responses in endothelial cells [[11], [12], [13]]. P. gingivalis fimbria and lipopolysaccharide (LPS) increase the expression of adhesion molecules in endothelial cells, such as vascular cell adhesion molecule-1, intercellular adhesion molecule-1, and monocyte chemoattractant protein (P-seletin and E-selectin) [14,15]. P. gingivalis fimbria and LPS also support monocyte to migrate and infiltrate into endothelial cells, which differentiate monocyte into pro-inflammatory macrophages [16,17]. More recently, P. gingivalis W83 strain was reported to invade into dermal microvascular cells via Intercellular Adhesion Molecule 1 (ICAM-1) and inhibit the formation of vascular networks [18]. Autoantibody against citrullinated proteins is considered an important pathological base of rheumatoid arthritis (RA). Regarding rheumatology, circulating bacterial antibodies and the anti-cyclic citrullinated peptide induced by periodontal pathogens such as P. gingivalis seems to be involved in the progression of RA [19]. P. gingivalis is the only microorganism to express the enzyme mediating protein citrullination called peptidylarginine deiminases (PAD) [20]. P. gingivalis PAD is proposed to break immunotolerance to citrullinated proteins, leading to the occurrence of RA [20]. Among the reported P. gingivalis-elicited mechanisms involved in diabetes, insulin resistance seems to be the most significant. P. gingivalis infection stimulates inflammation and elevates inflammatory markers, such as CRP and IL-6, leading to insulin resistance [21]. More recently, it was reported that P. gingivalis causes insulin resistance by the phosphorylation of insulin receptor through activation of mammalian target of rapamycin and related genes in the mice fed with high-fat diet [22]. P. gingivalis LPS also is involved in obesity-associated insulin resistance by inducing pro-inflammatory adipokine production and oxidative stress in adipocytes [23]. P. gingivalis, reportedly, raises the development of oral squamous cell carcinoma, esophageal and pancreatic cancers through epithelial-mesenchymal transition (EMT) of oral epithelial cells, the prevention of epithelial cell apoptosis, and the promotion of immune evasion, etc [8,[24], [25], [26]]. P. gingivalis modulates EMT by regulating zinc-finger homeobox proteins (ZEB1 and Zeb2) and GSK-3β/β-catenin/forkhead box-O1 (FOXO1) [27]. Furthermore, P. gingivalis controls epithelial apoptosis many apoptotic/anti-apoptotic pathways including PI3K/Akt and JAK/Stat pathways as well as mitochondrial apoptosis-related factors (Bad, Bcl-2, and Bax) [8,26,28]. In the field of neurology, P. gingivalis infection has been suggested to be the risk of Alzheimaer’s disease (AD) and depression by several epidemiological studies [29,30]. Animal experiments also support the possible relevance of P. gingivalis in AD pathogenesis characterized as microglia-mediated neuroinflammation and β-amyloid (Aβ) accumulation in neurons, which impaires cognitive function and reduces in learning and memory [8,31,32]. P. gingivalis and its LPS are thought to be involved in the progression of AD via cathepsin B (CatB), a crucial mediator for Aβ deposition and neuroinflammation [31,33]. Inflammatory mediators - TNF-α, IL-1, IL-6, and IL-8 - released from host cells infected with P. gingivalis are also reported to associate with the progression of AD by reaching central nervous system via hematoencephalic barieer-free area [34,35].
Interestingly, in addition to these mechanisms described above, several reports demonstrated the involvement of P. gingivalis OMVs in the etiology of systemic diseases including diabetes and vascular calcification. P. gingivalis OMVs translocates to the liver and attenuates insulin sensitivity and hepatic glycogen synthesis by downregulating the Akt, -GSK3β-FOXO1 pathway [36,37]. P. gingivalis OMV induces vascular smooth muscle cell calcification through activation of ERK1/2-Runx2 [38]. These observations lead us to the advanced interpretation that P. gingivalis-involved diseases, including periodontitis, are not only caused by direct local infection and transfer of virulence factors into host cells, but also by strategic indirect communication devices of P. gingivalis. Among the several factors associated with P. gingivalis-involved diseases, increasing evidence suggests that OMVs contribute to the pathogenesis of this bacterium. Therefore, the function of OMVs synthesized from P. gingivalis will be reviewed and discussed here.
2. P. gingivalis OMVs generation
Based on their membrane properties, bacteria are classified as Gram-negative or Gram-positive. Gram-negative bacteria are characterized by a double plasma membrane layer separated by the periplasm. Most EVs produced by Gram-negative are thought to be OMVs, which are composed of a single bilayer membrane derived from an outer membrane and contain periplasmic contents such as lipids, outer membrane proteins, lipoproteins, and lipids [39,40]. P. gingivalis, a gram-negative bacterium, has been observed to produce vesicles on its cell surface and release them to its environments [41,42].
The mechanism of OMVs biogenesis has not been fully elucidated, despite their biological importance. The curvature of the OMVs membrane is approximately 14 times that of the extracellular membrane, suggesting that OMVs generation requires energy expense due to the prominent curvature of the outer membrane [40]. These observations indicate that P. gingivalis OMVs generation of could be completed by increasing the production of specific inner or outer leaflet lipids. This is carried out by anionic lipopolysaccharide (A-LPS) on the outer surface and its related C-terminal domain (CTD) family proteins [40].
Various factors have been reported to be involved in the formation of OMVs. Autolysins, endogenous murein hydrolases that cleave covalent bonds in the peptidoglycan of the cell wall, play an important role, physiologically, in bacterial growth, cell division, cell wall remodeling, peptidoglycan turnover, and recycling [43]. Autolysin is involved in the release of P. gingivalis OMVs [44]. There are three types of gingipains in P. gingivalis: lysine-specific gingipain (Kgp), arginine-specific gingipain A (RgpA), and arginine-specific gingipain B (RgpB) [45,46]. These gingipains are encoded by the rgpA, rgpB, and kgp, genes respectively. In contrast to the mature RgpB, which only possesses a catalytic domain, RgpA and Kgp have homologous adhesin/hemagglutinin domains as a part of their C-terminal extension [47]. Among them, the deletion of RgpA results in a reduction of OMVs [48]. P. gingivalis LPS is the major component of the outer membrane of the bacteria that stimulates the innate immune system and is a key virulence factor which induce the production of cytokines [49]. P. gingivalis has A-LPS, consisting of phosphorylated branched mannan repeating unit attached to the lipid A core [50]. A-LPS and PG0027, outer membrane proteins, are also involved in the formation of OMVs [51]. PG0027 dephosphorylates lipid A of A-LPS; this process may be necessary for optimal OMVs formation. Dephosphorylation of lipid A of A-LPS, controlled by PG0027, induces the destabilization of the outer membrane, resulting in blebbing and the generation of OMVs [51].
The surrounding environment affects the generation of P. gingivalis OMVs. P. gingivalis can adapt to the surrounding environment, and its gene expression is controlled by extracytoplasmic function (ECF) sigma factors. SigP is one of these factors and mutation of sigP increases the level of OMVs formed on the P. gingivalis cell surface [52]. SigP is thought to be involved in activities – gingipain activity, autoaggregation, hemagglutinination, vesicle formation, antimicrobial susceptibility – associated with the bacterial surface [52]. In addition, under hemin-limitation, the gravimetric yield of OMVs increased by 2.5-folds, although cell yield did not changed. Growth in hemin-excess conditions resulted in increased hemin-binding capacities of OMVs [53].
3. P. gingivalis OMVs characteristics
There are several cases that P. gingivalis bacterial cells are not detected in distant tissues or organs although P. gingivalis DNA is present. Compared with P. gingivalis bacterial cells, P. gingivalis OMVs have various functional advantages. For instance, P. gingivalis OMVs possess some well-known bacterial virulence compared to levels found in P. gingivalis bacterial cells. There are approximately 3–5 fold increase in gingipain levels in P. gingivalis OMVs compared with the levels in surface extracts of the original bacterial cells [54]. P. gingivalis OMVs, moreover, show the increased antigenicity, which may result from the more concentrated immune-responsive factors on the vesicles compared with the surface of bacterial cells [55,56]. Additionally, P. gingivalis OMVs bring bacterial DNA and signal molecules to distant target organs without degradation. In the mice applied with P. gingivalis into gingival sulcus, DNA of P. gingivalis was detected in the liver by PCR. However, the bacterial cells was not observed in the liver under strict examination by a transmission electron microscope [57]. It was also reported that in RA patients P. gingivalis organisms are not observed in joint fluid although its DNA was detected [58]. P. gingivalis OMVs are thought to be an important carrier of P. gingivalis DNA to distant organs without the direct translocation of P. gingivalis bacteria cells.
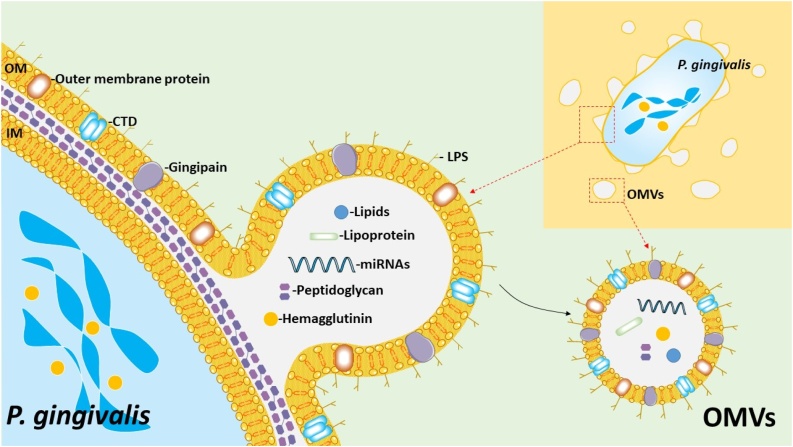
Fig. 1 shows a schematic summary of the structure and representative components of P. gingivalis OMVs. P. gingivalis OMVs were originally reported in the 1980s; however, the biological pathogenic functions were not immediately recognized. Similar to that of other gram-negative bacterial vesicles, the diameter of P. gingivalis OMVs is approximately 50−250 nm but is most commonly around 50 nm [59]. OMVs are not only just a part of a bacterial component, but also include a toxic complex of LPS and proteolytic enzymes (proteases). Bacteria discharges vesicles like missiles as a mechanism for survival and also to create an environment for growth and proliferation. LPS and the proteases associated with P. gingivalis OMVs are likely to represent the heat-stable and the heat-unstable components, respectively [60].
Fig. 1.
Schematic summary of the P. gingivalis OMVs generation.
OMVs are composed of a single bilayer membrane derived from an outer membrane and contain periplasmic contents such as lipids, outer membrane proteins, lipoproteins, and lipids. The generation of OMVs is considered to be carried out by anionic lipopolysaccharide (A-LPS) on the outer surface and its related C-terminal domain (CTD) family proteins. Dephosphorylation of lipid A of A-LPS by PG0027 induces the destabilization of the outer membrane, resulting in blebbing and the generation of OMVs. LptO is essential for the O-deacylation of LPS and for the coordinated secretion and attachment of A-LPS and CTD proteins. OM: outer membrane; IM: inner membrane.
The major outer membrane proteins Pgm6/7, which are homologous to the OmpA protein in Escherichia coli, are important for maintaining the integrity of the outer membrane. The loss of Pgm6/7 induced wavy and irregular OM and increased the number of vesicles and the rate of gingipain activity [61]. PGN_1251 (gtfB) is also involved in the transition of gingipain to the cell surface. PGN_1251 (gtfB) shares homology with genes encoding for glycosyltransferase 1 with several bacteria. Both LPSs containing O side chain polysaccharide (O-LPS) and anionic polysaccharide (A-LPS) were not synthesized in gftB mutant bacteria, resulting in a complete loss of surface-associated proteins including gingipain [62].
Furthermore, P. gingivalis possesses a unique CTD system essential for secretion and attachment to the cell surface. The attachment of PgpB proteinases to the outer membrane is associated with the presence of a conserved CTD of approximately 70-amino-acid residues in the encoded sequences [63]. The outer membrane protein LptO is essential for the O-deacylation of LPS and for the coordinated secretion and attachment of A-LPS and CTD proteins [64]. It was also reported that OMVs are enriched in high molecular weight A-LPS molecules. Sorting factors within P. gingivalis OMVs is selective, and LPS plays an important role [65].
Lo A, et al. demonstrate that both FimR and FimS are involved in P. gingivalis biofilm formation, including the regulation of genes associated with fimbriation (Lo et al., 2010). Mantri et al. compared the protein components of P. gingivalis OMVs using a fimbriated strain (33277) and P. gingivalis of an afimbriated strain (W83) [54]. Gingipain and hemagglutinin were contained in the OMVs produced from both bacterial strains. FimC, FimD and FimE were found in the OMVs of 33277 strain, but not in W83 vesicles, indicating that different species contain different factors in their own OMVs.
P. gingivalis OMVs have been demonstrated to enter cells such as human oral keratinocytes and gingival fibroblasts more effectively than originating bacterial cells [55]. FimA, a well-known adhesion responsible factor, is considered important for the adhesion and invasion of P. gingivalis into the host cells. However, the OMVs derived from the mutant strain of FimA were able to penetrate the cells [54]. On the other hand, in the case of OMVs produced FimR-deleted P. gingivalis, the penetration rate was significantly reduced. Thus, FimR is most likely involved in the invasion of P. gingivalis OMVs into host cells, not FimA.
4. Supporting other microorganisms
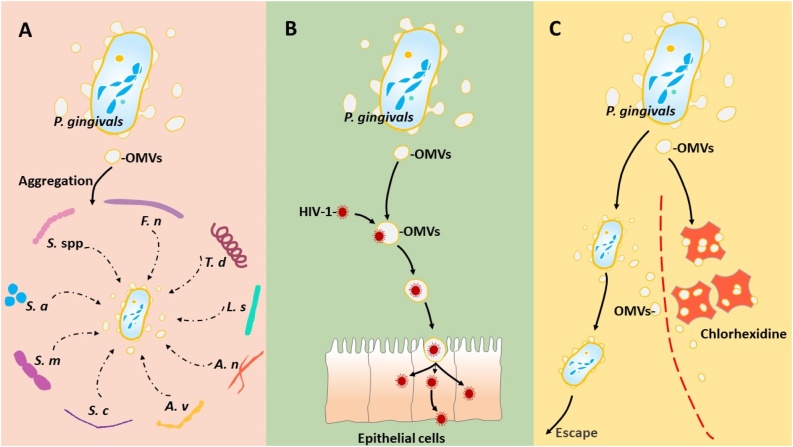
Fig. 2 shows various functions of P. gingivalis OMVs. P. gingivalis OMVs may protect other bacterial species from complement action; thus, favoring the pathogenic process of periodontitis. P. gingivalis OMVs can aggregate a wide range of Streptococcus spp., Fusobacterium nucleatum, T. denticola, L. saburreum, Actinomyces naeslundii, and Actinomyces viscosus [66,67]. Also, P. gingivalis OMVs are capable of attaching to various molecules [68], which supports the aggregation of S. cricetus and S. mutans as well as the attachment of bacteria such as A. viscosus to the tooth surface [69]. Autoaggregation of S. aureus in the presence of P. gingivalis OMVs is inhibited by l-arginine, l-lysine, and l-cysteine, suggesting that gingipains are involved in these effects.
Fig. 2.
Involvement of P. gingivalis OMVs in aggregation of other microorganisms.
(A), P. gingivalis OMVs aggregate Streptococcus spp (S. spp), Fusobacterium nucleatum (F. n), T. denticola (T. d), L. saburreum (L. s), Actinomyces naeslundii (A. n), and Actinomyces viscosus (A. v), S. cricetus (S. c), and S. mutans (S. m). Gingipains of P. gingivalis OMVs are involved in autoaggregation of S. aureus (S. a). P. gingivalis OMVs supports viral entry into oral epitherial cells. (B), P. gingivalis OMVs supports viral entry into oral epitherial cells. (C), P. gingivalis OMVs protect themselves and other species of oral bacteria by binding to the antimicrobial chlorhexidine.
It has been found that the receptor-independent invasion of human immunodeficiency virus-1 (HIV-1) into epithelial cells is mediated by P. gingivalis. P. gingivalis OMVs promote mucosal transmission of HIV-1. Dynabeads technology showed that a specific interaction between HIV-1 and P. gingivalis OMVs supports viral entry into oral epithelial cells [70]. Subsequently, HIV-1 was reverse-transcribed and viral DNA was integrated into the genome of these cells. OMVs released from P. gingivalis are thought to be vehicles for HIV-1 and promote infection to the mucous membranes.
P. gingivalis OMVs bind to the antimicrobial chlorhexidine via LPS and protect themselves and other species of oral bacteria. In such a way, the interaction between bacteria in the oral cavity affects the sensitivity of microbes to drugs [71].
5. Systemic diseases
P. gingivalis exerts its pathogen to different organs (sometimes to distant organs), manipulates the invasion of other cells, disrupts phagocytosis, affects complement-related factors, and induces pro-inflammatory signaling cascades. Other review articles have already described the effects of P. gingivalis bacteria itself on systemic diseases such as atherosclerosis and AD as well as its involvement in signal transduction in disease-related cells [72]. Therefore, in this review, the role of P. gingivalis OMVs in these systemic diseases is highlighted.
5.1. Arteriosclerosis
Atherosclerosis, a chronic inflammatory disease of the blood vessels, is one of the most common causes of morbidity and mortality worldwide. The association between atherosclerosis and periodontitis has been epidemiologically suggested. In addition, there is research to elucidate this by molecular biological strategy. Bacteria and their products in dental plaque enter the bloodstream from the infected site, and become involved in arteriosclerosis and thromboembolic events [[73], [74], [75], [76]]. Platelet aggregation is considered important for atherosclerotic plaque formation. P. gingivalis OMVs possess powerful platelet aggregation activity [77], implying that P. gingivalis is most causative agent for arterial infarction.
Foam cells, fat-storage macrophages indicating signs of plaque formation or atherosclerosis, are generally associated with an increased risk of heart attack and stroke. It was also demonstrated that P. gingivalis OMVs stimulates foam cell formation in murine macrophage cells [78]. In addition, several studies reported that P. gingivalis OMVs induced LDL aggregation, which then induced murine macrophage to form foam cells [79,80]. Thus, P. gingivalis OMVs released from periodontitis into the circulation may deliver virulence factors to the arterial wall to initiate or promote foam cell formation in macrophages and contribute to atheroma development. P. gingivalis OMVs are more potent inducers of the inflammatory response associated with the development of atherosclerosis [81].
5.2. Alzheimer’s disease
Research concerning AD is also being conducted with the focus on the oral cavity and the brain. According to various key evaluations of prospective and retrospective population-based data, the presence of chronic inflammation for over 10 years increases the risk of developing sporadic AD by more than two times [82]. In in vivo experiments, P. gingivalis infection induces pathological phenomena such as inclusive of extracellular amyloid plaques and enhances phosphorylation of tau, characteristics of AD-like phenotype. Other studies have also investigated the decline in cognitive functions in AD patients with untreated periodontitis [82]. It seems that P. gingivalis OMVs are being used effectively as a trigger for inflammation and inflammatory mediators as a result of OMVs attack, which may lead to organic and functional changes that define inflammation and impaired cognitive abilities typical of AD.
5.3. Rheumatoid arthritis
P. gingivalis has been recently hailed as a potential etiology of autoimmune disease RA. In particular, PAD of P. gingivalis is involved in the citrullination of bacterial or host cell proteins. PAD then induces the production of anticitrullinated protein antibodies, leading to the loss of tolerance to citrullinated proteins in RA patients. PAD exists in the form of two variants: the outer-membrane-bound state and the soluble secreted state [83,84]. This different feature of PAD is closely related to the transport system for PAD excretion, and PAD binds to the outer membrane via A-LPS anchoring [85]. As a result, in many cases, PAD is present in OMVs secretion (less common in the soluble state). Interestingly, some P. gingivalis strains showed a reduced PAD binding to OMVs, which was closely related to the substitution of 373 Glutamine for lysine residues [86]. It is strongly suggested that the glutamine residue in this part is involved in the association between PAD and OMVs. Recently, a comparative analysis of wild type P. gingivalis and PAD deficient strains was performed [87]. In this report, 51 citrullinated proteins (51 of 78) of wild type OMVs were identified, of which most were assumed to have been translocated to the bacterial surface. PAD transferred to P. gingivalis OMVs seems to be strongly involved in the pathogenesis of RA.
6. The effects of OMVs on host cells
6.1. Immune cells
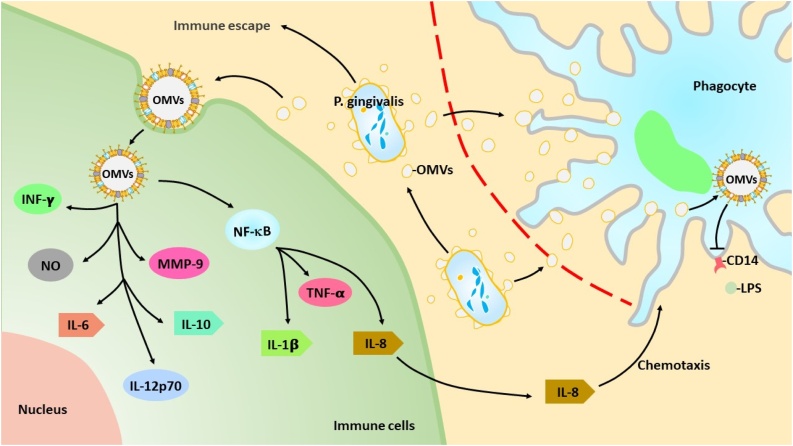
Fig. 3 shows a schematic summary of the effects of P. gingivalis OMVs on host cells. P. gingivalis OMVs can be used offensively as delivery systems for virulence factors and defensively to aid in the survival of the bacterium in hostile environments. P. gingivalis OMVs can enter cells more effectively than originating bacterial cells [55]. P. gingivalis OMVs seem to contribute to tissue destruction by disrupting the immune system and regulating cellular responses involved in inflammation and acquired immunity.
Fig. 3.
Schematic summary of the effects of P. gingivalis OMVs on host cells.
P. gingivalis OMVs can effectively enter the host cells and destroy the tissues by disrupting the immune system and regulating cellular responses involved in inflammation and acquired immunity.
OMVs play a role in local immune evasion strategies that promote monocyte unresponsiveness and make microbial detection difficult [72]. P. gingivalis OMVs have been reported to contribute to the loss of LPS receptor, a membrane-bound CD14 receptor. Such a phenomenon results in a hyporesponsiveness of macrophages to LPS stimulation, which may contribute to an increased virulence capacity of P. gingivalis. Furthermore, some strains of P. gingivalis can raise matrix metalloproteinase 9 (MMP9) activity in macrophages and may be involved in plaque disruption. In the destructive periodontitis, P. gingivalis stimulates monocytes primed with IFNγ to release IL-12, thereby enhancing IFNγ accumulation in T-cell populations [88]. Gingipain on the surface of P. gingivalis OMVs membrane induces IL-8 cleavage and activation, leading to the recruitment of neutrophils [89]. P. gingivalis OMVs induce nitric oxide (NO) production in RAW264.7 cells [90]. Nakao et al. investigated the effect of P. gingivalis OMVs on host immune response and tissue destruction during P. gingivalis infection. P. gingivalis OMVs had high antigenic, and absorption of patient sera with OMVs greatly reduced reactivity with whole cells of P. gingivalis [91]. Interestingly, P. gingivalis OMVs suppress monocyte unresponsiveness to living P. gingivalis but maintain bacterial responsiveness to DNA. TLR2 plays a central role in the innate immunity of P. gingivalis, but TLR4 appears to have a selective role in the response to P. gingivalis OMVs [92].
Some reports reveal the immense ability of P. gingivalis OMVs to induce inflammation compared with that of P. gingivalis bacteria itself. Treatment of P. gingivalis OMVs immensely stimulates the production of TNFa, IL-12p70, IL-6, IL-10, IFNb, and NO in macrophages, whereas direct infection of P. gingivalis shows a significantly lower effect [93]. P. gingivalis OMVs stimulation causes a metabolic shift in macrophage and increases the expression of genes important for glycolysis and decreases the expression of genes related to the TCA cycle. P. gingivalis OMVs, without the direct infection of P. gingivalis, induces inflammasome activation (activation of Caspase-1 and production of IL-ib and IL-18). These findings indicate that P. gingivalis and P. gingivalis OMVs have different effects on macrophage inflammatory phenotype, mitochondria function, inflammasome activation, etc.
Cecil et al. investigated immuno-modulatory effects of P. gingivalis OMVs on monocytes and differentiated macrophages [94]. P. gingivalis OMVs were phagocytosed into monocytes and M (naïve) and M (IFNg) macrophages. P. gingivalis OMVs induced NF-kB activation and cytokine secretion such as TNFa, IL-8, and IL-1b. P. gingivalis OMVs also induced anti-inflammatory IL-10 secretion [94,95]. These observations indicate that P. gingivalis OMVs interfere with the reaction of host immune cells and may contribute to local immune evasion.
6.2. Non-immune cells
P. gingivalis OMVs affect non-immune cell function and elicit signal transduction. P. gingivalis OMVs significantly inhibit the proliferation of human gingival fibroblasts capillary tube formation in cultured human umbilical vein endothelial cells (HUVEC) [96]. P. gingivalis OMVs enter gingival epithelial cells via lipid rafts through the actin filament assembly in phosphatidylinositol 3-kinase and Rac1 dependently. After entry, OMVs-associated gingipains degrade cellular proteins, which are essential for intracellular transferrin and cellular migration, leading to their functional impairment [97]. P. gingivalis OMVs induce the expression and activity of eNOS in HUVEC through Rho kinase (ROCK)-stimulated ERK1 / 2 and p38 MAPK [98]. P. gingivalis OMVs promote calcification of vascular smooth muscle cells, implying the involvement in arteriosclerosis. P. gingivalis OMVs enhance the expression of markers of osteoblast differentiation and calcification in vascular smooth muscle cells and calcification via ERK1 / 2-Runx2 [38]. In addition, P. gingivalis OMVs induce oral squamous epithelial cell detachment, which is inhibited by preincubating P. gingivalis OMVs with anti-gingipain serum. E-selectin and ICAM-1 are important factors for the adhesion of leukocytes to endothelial cells. P. gingivalis OMVs induced the expression of E-selectin and ICAM-1 on the surfaces of vascular endothelial cells. These findings demonstrate that P. gingivalis OMVs are capable of inducing acute inflammation, which is characterized by the accumulation of large numbers of neutrophils in connective tissues [99].
7. Vaccination
Periodontitis is the most prevalent infectious disease and is related to oral and systemic health; therefore, novel prophylaxis to prevent the disease is highly desirable. There are several attempts to develop novel vaccines using P. gingivalis OMVs and some desirable results have been obtained. Nasally or percutaneously administered 40-kDa outer membrane protein of P. gingivalis elicits specific antibodies, which inhibits the co-aggregation activity of P. gingivalis, implying that this fragmented outer membrane protein is useful for vaccination against chronic periodontitis [[100], [101], [102]]. A few years later, Nakao et al. assessed the capacity of P. gingivalis OMVs as a vaccine antigen by intranasal immunization to BALB/c mice. They proposed that P. gingivalis OMVs are an intriguing immunogen for the development of a periodontitis vaccine [56]. Recently, their group performed sub-immunoproteome analysis using P. gingivalis OMV-immunized mouse serum samples to identify immunodominant antigens. As a result, it has been reported that LPS and A-LPS-modified proteins in P. gingivalis OMVs are immunodominant determinants that lead to the production of P. gingivalis-specific antibodies in mice [103].
P. gingivalis OMVs are stable structurally and functionally, resistant to proteinase K, able to withstand long-term storage, and advantageous in delivering components to host immune cells. The challenge to generate antibodies specific to P. gingivalis is proceeded by using intranasal vaccination of P. gingivalis OMVs [104].
8. Contents of P. gingivalis OMVs and movement in the host body
Recent studies using genetic, proteomic, and morphological tools have demonstrated that P. gingivalis OMVs include a variety of virulence factors provided by parental cells [70]. As described above, P. gingivalis OMVs possess various components of outer membrane constituents, including LPS, muramic acid, a capsule, fimbriae, and gingipains [41,42]. P. gingivalis OMVs are potent vehicles for transmitting virulence factors into the host cells and are involved in the etiology of periodontitis.
The proteins in P. gingivalis OMVs were examined by LC–MS/MS analysis [105]. Most proteins are derived from outer membrane or periplasm, which are distributed on the vesicle surface, membrane, lumen, etc. Numerous virulence factors including all CTD proteins are shown to be preferentially packaged into P. gingivalis OMVs.
Small RNAs are also incorporated in P. gingivalis OMVs. P. gingivalis has also been shown to have small RNAs similar in size to eukaryote microRNAs (miRNAs). Small RNA, similar in size to miRNAs (miRNA-size, small RNAs or msRNA), has a length of 15–25 nucleotides, and its precursor is assumed to be the secondary structure of a hairpin loop. Deep sequencing reveals that msRNA expressed on P. gingivalis are secreted via OMVs [106]. P. gingivalis OMVs are also found to be capable of transporting RNAs to eukaryotic cells. These exogenous msRNA are reported to suppress cytokines in Jurkat cells [106]. msRNA may be a new bacterial signaling molecule that connects bacteria to humans.
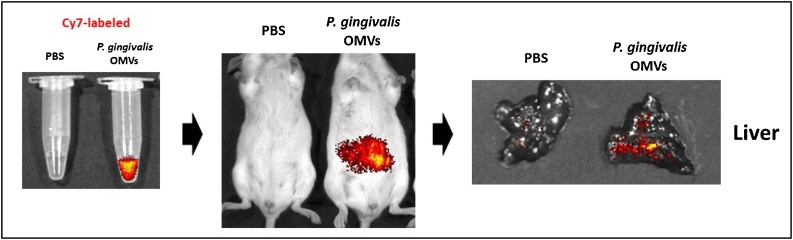
As far as we know, no reports have directly analyzed the existence and pathogenetic properties of P. gingivalis OMVs in human clinical isolates. Gabarrini et al. primitively tested P. gingivalis’s peptidylarginine deiminase using unfiltered growth medium fractions of 93 clinical isolates, which contain both OMV-associated proteins [86]. In recent years, we showed that P. gingivalis OMVs were equipped with P. gingivalis-derived proteases gingipains and translocated to the liver in mice (Fig. 4). In these mice, the hepatic glycogen synthesis decreased in response to insulin, and thus, high blood glucose levels were maintained. P. gingivalis OMVs also attenuated the insulin-induced Akt/glycogen synthase kinase-3 β (GSK-3β) signaling in a gingipain-dependent fashion in hepatic HepG2 cells [57].
Fig. 4.
Experimental strategy for tracking the movement of P. gingivalis OMVs in vivo.
We established a novel tracking system of P. gingivalis OMVs by in vivo imaging. P. gingivalis OMVs were incubated with Cy7 Mono NHS Ester and Cy7-labeled P. gingivalis OMVs were injected intraperitoneally to mice. Cy7 fluorescence was detected in the liver by in vivo imaging system (IVIS) Spectrum.
9. Future direction
Small particles secreted from oral bacteria, which was initially thought to be debris or artificial products, are now considered the most sophisticated tools conducted by oral bacteria. Oral bacteria can use these tools to deliver their nucleic acids and proteins to local and distant organs and tissues. In recent years, our preliminary study has shown that P. gingivalis invades macrophages and hides pathogenic factors in EVs released by the macrophages. The released EVs in turn can reach distant organs such as the brain and the lungs more efficiently. What tools should we utilize to cope with such a tricky oral bacterial strategy? The communication between oral bacteria and the host cells via small vesicles is still unknown to a large extent, and further clarification is strongly required.
Acknowledgments
This study was supported by grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan (19H0405111, HO), Astellas Academic support (HO), Novartis foundation (HO), Shionogi Academic support (HO), Bayer Academic support (HO), and Daiichi Sankyo Academic support (HO).
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Nowotny A., Behling U.H., Hammond B., Lai C.H., Listgarten M., Pham P.H. Release of toxic microvesicles by Actinobacillus actinomycetemcomitans. Infect Immun. 1982;37:151–154. doi: 10.1128/iai.37.1.151-154.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoberg R.J., Thomas D.D. Specific adherence of Borrelia burgdorferi extracellular vesicles to human endothelial cells in culture. Infect Immun. 1993;61:3892–3900. doi: 10.1128/iai.61.9.3892-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadurugamuwa J.L., Beveridge T.J. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou L., Srisatjaluk R., Justus D.E., Doyle R.J. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol Lett. 1998;163:223–228. doi: 10.1111/j.1574-6968.1998.tb13049.x. [DOI] [PubMed] [Google Scholar]
- 5.Amano A., Chen C., Honma K., Li C., Settem R.P., Sharma A. Genetic characteristics and pathogenic mechanisms of periodontal pathogens. Adv Dent Res. 2014;26:15–22. doi: 10.1177/0022034514526237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saglie F.R., Marfany A., Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol. 1988;59:259–265. doi: 10.1902/jop.1988.59.4.259. [DOI] [PubMed] [Google Scholar]
- 7.Fiorillo L., Cervino G., Laino L., D’Amico C., Mauceri R., Tozum T.F. Porphyromonas gingivalis, periodontal and systemic implications: a systematic review. Dent J (Basel) 2019;7 doi: 10.3390/dj7040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei F., Xie M., Huang X., Long Y., Lu X., Wang X. Porphyromonas gingivalis and its systemic impact: current status. Pathogens. 2020;9 doi: 10.3390/pathogens9110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues P.H., Reyes L., Chadda A.S., Bélanger M., Wallet S.M., Akin D. Porphyromonas gingivalis strain specific interactions with human coronary artery endothelial cells: a comparative study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama K. Porphyromonas gingivalis cell-induced hemagglutination and platelet aggregation. Periodontol 2000. 2010;54:45–52. doi: 10.1111/j.1600-0757.2010.00351.x. [DOI] [PubMed] [Google Scholar]
- 11.Bélanger M., Rodrigues P.H., Dunn W.A., Jr., Progulske-Fox A. Autophagy: a highway for Porphyromonas gingivalis in endothelial cells. Autophagy. 2006;2:165–170. doi: 10.4161/auto.2828. [DOI] [PubMed] [Google Scholar]
- 12.Xie M., Tang Q., Nie J., Zhang C., Zhou X., Yu S. BMAL1-Downregulation aggravates Porphyromonas gingivalis-induced atherosclerosis by encouraging oxidative stress. Circ Res. 2020;126:e15–e29. doi: 10.1161/CIRCRESAHA.119.315502. [DOI] [PubMed] [Google Scholar]
- 13.Roth G.A., Moser B., Huang S.J., Brandt J.S., Huang Y., Papapanou P.N. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J Thromb Haemost. 2006;4:2256–2261. doi: 10.1111/j.1538-7836.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu B., Cheng L., Liu D., Wang J., Zhang X., Shu R. Role of p38 mitogen-activated protein kinase pathway in Porphyromonas gingivalis lipopolysaccharide-induced VCAM-1 expression in human aortic endothelial cells. J Periodontol. 2012;83:955–962. doi: 10.1902/jop.2011.110406. [DOI] [PubMed] [Google Scholar]
- 15.Khlgatian M., Nassar H., Chou H.H., Gibson F.C., 3rd, Genco C.A. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect Immun. 2002;70:257–267. doi: 10.1128/IAI.70.1.257-267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollreisz A., Huang Y., Roth G.A., Cheng B., Kebschull M., Papapanou P.N. Enhanced monocyte migration and pro-inflammatory cytokine production by Porphyromonas gingivalis infection. J Periodontal Res. 2010;45:239–245. doi: 10.1111/j.1600-0765.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- 17.Li X.Y., Wang C., Xiang X.R., Chen F.C., Yang C.M., Wu J. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages. Oncol Rep. 2013;30:1329–1336. doi: 10.3892/or.2013.2600. [DOI] [PubMed] [Google Scholar]
- 18.Reyes L., Getachew H., Dunn W.A., Progulske-Fox A. Porphyromonas gingivalis W83 traffics via ICAM1 in microvascular endothelial cells and alters capillary organization in vivo. J Oral Microbiol. 2020;12 doi: 10.1080/20002297.2020.1742528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher J.U., Bretz W.A., Abramson S.B. Periodontal disease and subgingival microbiota as contributors for rheumatoid arthritis pathogenesis: modifiable risk factors? Curr Opin Rheumatol. 2014;26:424–429. doi: 10.1097/BOR.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koziel J., Mydel P., Potempa J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep. 2014;16:408. doi: 10.1007/s11926-014-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradhan A.D., Manson J.E., Rossouw J.E., Siscovick D.S., Mouton C.P., Rifai N. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. Jama. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 22.Tian J., Liu C., Zheng X., Jia X., Peng X., Yang R. Porphyromonas gingivalis induces insulin resistance by increasing BCAA levels in mice. J Dent Res. 2020;99:839–846. doi: 10.1177/0022034520911037. [DOI] [PubMed] [Google Scholar]
- 23.Le Sage F., Meilhac O., Gonthier M.P. Porphyromonas gingivalis lipopolysaccharide induces pro-inflammatory adipokine secretion and oxidative stress by regulating toll-like receptor-mediated signaling pathways and redox enzymes in adipocytes. Mol Cell Endocrinol. 2017;446:102–110. doi: 10.1016/j.mce.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Wen L., Mu W., Lu H., Wang X., Fang J., Jia Y. Porphyromonas gingivalis promotes oral squamous cell carcinoma progression in an immune microenvironment. J Dent Res. 2020;99:666–675. doi: 10.1177/0022034520909312. [DOI] [PubMed] [Google Scholar]
- 25.Lee J., Roberts J.S., Atanasova K.R., Chowdhury N., Han K., Yilmaz Ö. Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. doi: 10.3389/fcimb.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao S., Park Y., Hasegawa Y., Tribble G.D., James C.E., Handfield M. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohshima J., Wang Q., Fitzsimonds Z.R., Miller D.P., Sztukowska M.N., Jung Y.J. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc Natl Acad Sci U S A. 2019;116:8544–8553. doi: 10.1073/pnas.1900101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao L., Jermanus C., Barbetta B., Choi C., Verbeke P., Ojcius D.M. Porphyromonas gingivalis infection sequesters pro-apoptotic bad through Akt in primary gingival epithelial cells. Mol Oral Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C.K., Wu Y.T., Chang Y.C. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res Ther. 2017;9:56. doi: 10.1186/s13195-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu C.C., Hsu Y.C., Chen H.J., Lin C.C., Chang K.H., Lee C.Y. Association of periodontitis and subsequent depression: a nationwide population-based study. Medicine (Baltimore) 2015;94:e2347. doi: 10.1097/MD.0000000000002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z., Ni J., Liu Y., Teeling J.L., Takayama F., Collcutt A. Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav Immun. 2017;65:350–361. doi: 10.1016/j.bbi.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Yu C., Zhang X., Chen H., Dong J., Lu W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 2018;15:37. doi: 10.1186/s12974-017-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie R., Wu Z., Ni J., Zeng F., Yu W., Zhang Y. Porphyromonas gingivalis infection induces amyloid-β accumulation in monocytes/macrophages. J Alzheimers Dis. 2019;72:479–494. doi: 10.3233/JAD-190298. [DOI] [PubMed] [Google Scholar]
- 34.Amano A., Nakagawa I., Okahashi N., Hamada N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J Periodontal Res. 2004;39:136–142. doi: 10.1111/j.1600-0765.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- 35.Al-Obaidi M.M.J., Desa M.N.M. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol. 2018;38:1349–1368. doi: 10.1007/s10571-018-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa M., Yoshida K., Okamura H., Ochiai K., Takamura H., Fujiwara N. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3β signaling pathway. Biochim Biophys Acta. 2013;1832:2035–2043. doi: 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Takamura H., Yoshida K., Okamura H., Fujiwara N., Ozaki K. Porphyromonas gingivalis attenuates the insulin-induced phosphorylation and translocation of forkhead box protein O1 in human hepatocytes. Arch Oral Biol. 2016;69:19–24. doi: 10.1016/j.archoralbio.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Yang W.W., Guo B., Jia W.Y., Jia Y. Porphyromonas gingivalis-derived outer membrane vesicles promote calcification of vascular smooth muscle cells through ERK1/2-RUNX2. FEBS Open Bio. 2016;6:1310–1319. doi: 10.1002/2211-5463.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaparakis-Liaskos M., Ferrero R.L. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 40.Gui M.J., Dashper S.G., Slakeski N., Chen Y.Y., Reynolds E.C. Spheres of influence: Porphyromonas gingivalis outer membrane vesicles. Mol Oral Microbiol. 2016;31:365–378. doi: 10.1111/omi.12134. [DOI] [PubMed] [Google Scholar]
- 41.Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987;55:111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayrand D., Grenier D. Biological activities of outer membrane vesicles. Can J Microbiol. 1989;35:607–613. doi: 10.1139/m89-097. [DOI] [PubMed] [Google Scholar]
- 43.Uehara T., Bernhardt T.G. More than just lysins: peptidoglycan hydrolases tailor the cell wall. Curr Opin Microbiol. 2011;14:698–703. doi: 10.1016/j.mib.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi J., Hamada N., Kuramitsu H.K. The autolysin of Porphyromonas gingivalis is involved in outer membrane vesicle release. FEMS Microbiol Lett. 2002;216:217–222. doi: 10.1111/j.1574-6968.2002.tb11438.x. [DOI] [PubMed] [Google Scholar]
- 45.Curtis M.A., Kuramitsu H.K., Lantz M., Macrina F.L., Nakayama K., Potempa J. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodontal Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 46.Eichinger A., Beisel H.G., Jacob U., Huber R., Medrano F.J., Banbula A. Crystal structure of gingipain R: an Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 1999;18:5453–5462. doi: 10.1093/emboj/18.20.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N., Collyer C.A. Gingipains from Porphyromonas gingivalis — complex domain structures confer diverse functions. Eur J Microbiol Immunol (Bp) 2011;1:41–58. doi: 10.1556/EuJMI.1.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang R., Yang J., Wu J., Sun W.B., Liu Y. Effect of deletion of the rgpA gene on selected virulence of Porphyromonas gingivalis. J Dent Sci. 2016;11:279–286. doi: 10.1016/j.jds.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diya Z., Lili C., Shenglai L., Zhiyuan G., Jie Y. Lipopolysaccharide (LPS) of Porphyromonas gingivalis induces IL-1beta, TNF-alpha and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immun. 2008;14:99–107. doi: 10.1177/1753425907088244. [DOI] [PubMed] [Google Scholar]
- 50.Rangarajan M., Aduse-Opoku J., Paramonov N., Hashim A., Bostanci N., Fraser O.P. Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J Bacteriol. 2008;190:2920–2932. doi: 10.1128/JB.01868-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rangarajan M., Aduse-Opoku J., Hashim A., McPhail G., Luklinska Z., Haurat M.F. LptO (PG0027) is required for lipid A 1-phosphatase activity in Porphyromonas gingivalis W50. J Bacteriol. 2017;199 doi: 10.1128/JB.00751-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujise K., Kikuchi Y., Kokubu E., Okamoto-Shibayama K., Ishihara K. Effect of extracytoplasmic function sigma factors on autoaggregation, hemagglutination, and cell surface properties of Porphyromonas gingivalis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smalley J.W., Birss A.J., McKee A.S., Marsh P.D. Haemin-restriction influences haemin-binding, haemagglutination and protease activity of cells and extracellular membrane vesicles of Porphyromonas gingivalis W50. FEMS Microbiol Lett. 1991;69:63–67. doi: 10.1016/0378-1097(91)90647-s. [DOI] [PubMed] [Google Scholar]
- 54.Mantri C.K., Chen C.H., Dong X., Goodwin J.S., Pratap S., Paromov V. Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiologyopen. 2015;4:53–65. doi: 10.1002/mbo3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho M.H., Chen C.H., Goodwin J.S., Wang B.Y., Xie H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakao R., Hasegawa H., Ochiai K., Takashiba S., Ainai A., Ohnishi M. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seyama M., Yoshida K., Fujiwara N., Ono K., Eguchi T., Kawai H. Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim Biophys Acta Mol Basis Dis. 2020 doi: 10.1016/j.bbadis.2020.165731. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Martinez R.E., Abud-Mendoza C., Patiño-Marin N. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 2009;36:1004–1010. doi: 10.1111/j.1600-051X.2009.01496.x. [DOI] [PubMed] [Google Scholar]
- 59.Xie H. Biogenesis and function of Porphyromonas gingivalis outer membrane vesicles. Future Microbiol. 2015;10:1517–1527. doi: 10.2217/fmb.15.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grenier D., Belanger M. Protective effect of Porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun. 1991;59:3004–3008. doi: 10.1128/iai.59.9.3004-3008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwami J., Murakami Y., Nagano K., Nakamura H., Yoshimura F. Further evidence that major outer membrane proteins homologous to OmpA in Porphyromonas gingivalis stabilize bacterial cells. Oral Microbiol Immunol. 2007;22:356–360. doi: 10.1111/j.1399-302X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi M., Sato K., Yukitake H., Noiri Y., Ebisu S., Nakayama K. A Porphyromonas gingivalis mutant defective in a putative glycosyltransferase exhibits defective biosynthesis of the polysaccharide portions of lipopolysaccharide, decreased gingipain activities, strong autoaggregation, and increased biofilm formation. Infect Immun. 2010;78:3801–3812. doi: 10.1128/IAI.00071-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seers C.A., Slakeski N., Veith P.D., Nikolof T., Chen Y.Y., Dashper S.G. The RgpB C-terminal domain has a role in attachment of RgpB to the outer membrane and belongs to a novel C-terminal-domain family found in Porphyromonas gingivalis. J Bacteriol. 2006;188:6376–6386. doi: 10.1128/JB.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y.Y., Peng B., Yang Q., Glew M.D., Veith P.D., Cross K.J. The outer membrane protein LptO is essential for the O-deacylation of LPS and the co-ordinated secretion and attachment of A-LPS and CTD proteins in Porphyromonas gingivalis. Mol Microbiol. 2011;79:1380–1401. doi: 10.1111/j.1365-2958.2010.07530.x. [DOI] [PubMed] [Google Scholar]
- 65.Haurat M.F., Aduse-Opoku J., Rangarajan M., Dorobantu L., Gray M.R., Curtis M.A. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem. 2011;286:1269–1276. doi: 10.1074/jbc.M110.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamaguchi A., Nakayama K., Ichiyama S., Nakamura R., Watanabe T., Ohta M. Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol. 2003;47:485–491. doi: 10.1007/s00284-003-4069-6. [DOI] [PubMed] [Google Scholar]
- 67.Grenier D. Porphyromonas gingivalis outer membrane vesicles mediate coaggregation and piggybacking of Treponema denticola and Lachnoanaerobaculum saburreum. Int J Dent. 2013;2013 doi: 10.1155/2013/305476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duchesne P., Grenier D., Mayrand D. Demonstration of adherence properties of Porphyromonas gingivalis outer membrane vesicles using a new microassay. Oral Microbiol Immunol. 1995;10:76–80. doi: 10.1111/j.1399-302x.1995.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 69.Hiratsuka K., Abiko Y., Hayakawa M., Ito T., Sasahara H., Takiguchi H. Role of Porphyromonas gingivalis 40-kDa outer membrane protein in the aggregation of P. gingivalis vesicles and Actinomyces viscosus. Arch Oral Biol. 1992;37:717–724. doi: 10.1016/0003-9969(92)90078-m. [DOI] [PubMed] [Google Scholar]
- 70.Dong X.H., Ho M.H., Liu B., Hildreth J., Dash C., Goodwin J.S. Role of Porphyromonas gingivalis outer membrane vesicles in oral mucosal transmission of HIV. Sci Rep. 2018;8:8812. doi: 10.1038/s41598-018-27284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grenier D., Bertrand J., Mayrand D. Porphyromonas gingivalis outer membrane vesicles promote bacterial resistance to chlorhexidine. Oral Microbiol Immunol. 1995;10:319–320. doi: 10.1111/j.1399-302x.1995.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 72.Olsen I., Taubman M.A., Singhrao S.K. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer’s disease. J Oral Microbiol. 2016;8:33029. doi: 10.3402/jom.v8.33029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beck J., Garcia R., Heiss G., Vokonas P.S., Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 74.DeStefano F., Anda R.F., Kahn H.S., Williamson D.F., Russell C.M. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinane D.F. Periodontal diseases’ contributions to cardiovascular disease: an overview of potential mechanisms. Ann Periodontol. 1998;3:142–150. doi: 10.1902/annals.1998.3.1.142. [DOI] [PubMed] [Google Scholar]
- 76.Mealey B.L. Influence of periodontal infections on systemic health. Periodontol 2000. 1999;21:197–209. doi: 10.1111/j.1600-0757.1999.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 77.Sharma A., Novak E.K., Sojar H.T., Swank R.T., Kuramitsu H.K., Genco R.J. Porphyromonas gingivalis platelet aggregation activity: outer membrane vesicles are potent activators of murine platelets. Oral Microbiol Immunol. 2000;15:393–396. doi: 10.1034/j.1399-302x.2000.150610.x. [DOI] [PubMed] [Google Scholar]
- 78.Kuramitsu H.K., Qi M., Kang I.C., Chen W. Role for periodontal bacteria in cardiovascular diseases. Ann Periodontol. 2001;6:41–47. doi: 10.1902/annals.2001.6.1.41. [DOI] [PubMed] [Google Scholar]
- 79.Miyakawa H., Honma K., Qi M., Kuramitsu H.K. Interaction of Porphyromonas gingivalis with low-density lipoproteins: implications for a role for periodontitis in atherosclerosis. J Periodontal Res. 2004;39:1–9. doi: 10.1111/j.1600-0765.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 80.Qi M., Miyakawa H., Kuramitsu H.K. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog. 2003;35:259–267. doi: 10.1016/j.micpath.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 81.Ho M.H., Guo Z.M., Chunga J., Goodwin J.S., Xie H. Characterization of innate immune responses of human endothelial cells induced by Porphyromonas gingivalis and their derived outer membrane vesicles. Front Cell Infect Microbiol. 2016;6:139. doi: 10.3389/fcimb.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singhrao S.K., Olsen I. Are Porphyromonas gingivalis outer membrane vesicles microbullets for sporadic Alzheimer’s disease manifestation? J Alzheimers Dis Rep. 2018;2:219–228. doi: 10.3233/ADR-180080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sato K., Yukitake H., Narita Y., Shoji M., Naito M., Nakayama K. Identification of Porphyromonas gingivalis proteins secreted by the Por secretion system. FEMS Microbiol Lett. 2013;338:68–76. doi: 10.1111/1574-6968.12028. [DOI] [PubMed] [Google Scholar]
- 84.Glew M.D., Veith P.D., Peng B., Chen Y.Y., Gorasia D.G., Yang Q. PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J Biol Chem. 2012;287:24605–24617. doi: 10.1074/jbc.M112.369223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gabarrini G., Heida R., van Ieperen N., Curtis M.A., van Winkelhoff A.J., van Dijl J.M. Dropping anchor: attachment of peptidylarginine deiminase via A-LPS to secreted outer membrane vesicles of Porphyromonas gingivalis. Sci Rep. 2018;8:8949. doi: 10.1038/s41598-018-27223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gabarrini G., Palma Medina L.M., Stobernack T., Prins R.C., du Teil Espina M., Kuipers J. There’s no place like OM: vesicular sorting and secretion of the peptidylarginine deiminase of Porphyromonas gingivalis. Virulence. 2018;9:456–464. doi: 10.1080/21505594.2017.1421827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larsen D.N., Mikkelsen C.E., Kierkegaard M., Bereta G.P., Nowakowska Z., Kaczmarek J.Z. Citrullinome of Porphyromonas gingivalis outer membrane vesicles: confident identification of citrullinated peptides. Mol Cell Proteomics. 2020;19:167–180. doi: 10.1074/mcp.RA119.001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yun P.L., DeCarlo A.A., Collyer C., Hunter N. Modulation of an interleukin-12 and gamma interferon synergistic feedback regulatory cycle of T-cell and monocyte cocultures by Porphyromonas gingivalis lipopolysaccharide in the absence or presence of cysteine proteinases. Infect Immun. 2002;70:5695–5705. doi: 10.1128/IAI.70.10.5695-5705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mikolajczyk-Pawlinska J., Travis J., Potempa J. Modulation of interleukin-8 activity by gingipains from Porphyromonas gingivalis: implications for pathogenicity of periodontal disease. FEBS Lett. 1998;440:282–286. doi: 10.1016/s0014-5793(98)01461-6. [DOI] [PubMed] [Google Scholar]
- 90.Imayoshi R., Cho T., Kaminishi H. NO production in RAW264 cells stimulated with Porphyromonas gingivalis extracellular vesicles. Oral Dis. 2011;17:83–89. doi: 10.1111/j.1601-0825.2010.01708.x. [DOI] [PubMed] [Google Scholar]
- 91.Nakao R., Takashiba S., Kosono S., Yoshida M., Watanabe H., Ohnishi M. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes Infect. 2014;16:6–16. doi: 10.1016/j.micinf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Waller T., Kesper L., Hirschfeld J., Dommisch H., Kolpin J., Oldenburg J. Porphyromonas gingivalis outer membrane vesicles induce selective tumor necrosis factor tolerance in a toll-like receptor 4- and mTOR-dependent manner. Infect Immun. 2016;84:1194–1204. doi: 10.1128/IAI.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fleetwood A.J., Lee M.K.S., Singleton W., Achuthan A., Lee M.C., O’Brien-Simpson N.M. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol. 2017;7:351. doi: 10.3389/fcimb.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cecil J.D., O’Brien-Simpson N.M., Lenzo J.C., Holden J.A., Singleton W., Perez-Gonzalez A. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Front Immunol. 2017;8:1017. doi: 10.3389/fimmu.2017.01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cecil J.D., Sirisaengtaksin N., O’Brien-Simpson N.M., Krachler A.M. Outer membrane vesicle-host cell interactions. Microbiol Spectr. 2019;7 doi: 10.1128/microbiolspec.PSIB-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartruff J.B., Yukna R.A., Layman D.L. Outer membrane vesicles from Porphyromonas gingivalis affect the growth and function of cultured human gingival fibroblasts and umbilical vein endothelial cells. J Periodontol. 2005;76:972–979. doi: 10.1902/jop.2005.76.6.972. [DOI] [PubMed] [Google Scholar]
- 97.Furuta N., Takeuchi H., Amano A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect Immun. 2009;77:4761–4770. doi: 10.1128/IAI.00841-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia Y., Guo B., Yang W., Zhao Q., Jia W., Wu Y. Rho kinase mediates Porphyromonas gingivalis outer membrane vesicle-induced suppression of endothelial nitric oxide synthase through ERK1/2 and p38 MAPK. Arch Oral Biol. 2015;60:488–495. doi: 10.1016/j.archoralbio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 99.Srisatjaluk R., Doyle R.J., Justus D.E. Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-gamma-mediated MHC class II expression by human vascular endothelial cells. Microb Pathog. 1999;27:81–91. doi: 10.1006/mpat.1999.0287. [DOI] [PubMed] [Google Scholar]
- 100.Namikoshi J., Otake S., Maeba S., Hayakawa M., Abiko Y., Yamamoto M. Specific antibodies induced by nasally administered 40-kDa outer membrane protein of Porphyromonas gingivalis inhibits coaggregation activity of P. gingivalis. Vaccine. 2003;22:250–256. doi: 10.1016/s0264-410x(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 101.Tagawa H., Kiyama-Kishikawa M., Lee S.Y., Abiko Y. Inhibition of hemagglutinating activity by monoclonal antibody against Porphyromonas gingivalis 40-kDa outer membrane protein. Hybrid Hybridomics. 2004;23:183–186. doi: 10.1089/1536859041224244. [DOI] [PubMed] [Google Scholar]
- 102.Maeba S., Otake S., Namikoshi J., Shibata Y., Hayakawa M., Abiko Y. Transcutaneous immunization with a 40-kDa outer membrane protein of Porphyromonas gingivalis induces specific antibodies which inhibit coaggregation by P. gingivalis. Vaccine. 2005;23:2513–2521. doi: 10.1016/j.vaccine.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 103.Bai D., Nakao R., Ito A., Uematsu H., Senpuku H. Immunoreactive antigens recognized in serum samples from mice intranasally immunized with Porphyromonas gingivalis outer membrane vesicles. Pathog Dis. 2015;73 doi: 10.1093/femspd/ftu006. [DOI] [PubMed] [Google Scholar]
- 104.Nakao R., Hasegawa H., Dongying B., Ohnishi M., Senpuku H. Assessment of outer membrane vesicles of periodontopathic bacterium Porphyromonas gingivalis as possible mucosal immunogen. Vaccine. 2016;34:4626–4634. doi: 10.1016/j.vaccine.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 105.Veith P.D., Chen Y.Y., Gorasia D.G., Chen D., Glew M.D., O’Brien-Simpson N.M. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 2014;13:2420–2432. doi: 10.1021/pr401227e. [DOI] [PubMed] [Google Scholar]
- 106.Choi J.W., Kim S.C., Hong S.H., Lee H.J. Secretable small RNAs via outer membrane vesicles in periodontal pathogens. J Dent Res. 2017;96:458–466. doi: 10.1177/0022034516685071. [DOI] [PubMed] [Google Scholar]