Abstract
The Mos protein kinase is a key regulator of vertebrate oocyte maturation. Oocyte-specific Mos protein expression is subject to translational control. In the frog Xenopus, the translation of Mos protein requires the progesterone-induced polyadenylation of the maternal Mos mRNA, which is present in the oocyte cytoplasm. Both the Xenopus p42 mitogen-activated protein kinase (MAPK) and maturation-promoting factor (MPF) signaling pathways have been proposed to mediate progesterone-stimulated oocyte maturation. In this study, we have determined the relative contributions of the MAPK and MPF signaling pathways to Mos mRNA polyadenylation. We report that progesterone-induced Mos mRNA polyadenylation was attenuated in oocytes expressing the MAPK phosphatase rVH6. Moreover, inhibition of MAPK signaling blocked progesterone-induced Mos protein accumulation. Activation of the MAPK pathway by injection of RNA encoding Mos was sufficient to induce both the polyadenylation of synthetic Mos mRNA substrates and the accumulation of endogenous Mos protein in the absence of MPF signaling. Activation of MPF, by injection of cyclin B1 RNA or purified cyclin B1 protein, also induced both Mos protein accumulation and Mos mRNA polyadenylation. However, this action of MPF required MAPK activity. By contrast, the cytoplasmic polyadenylation of maternal cyclin B1 mRNA was stimulated by MPF in a MAPK-independent manner, thus revealing a differential regulation of maternal mRNA polyadenylation by the MAPK and MPF signaling pathways. We propose that MAPK-stimulated Mos mRNA cytoplasmic polyadenylation is a key component of the positive-feedback loop, which contributes to the all-or-none process of oocyte maturation.
The germ cell-specific serine/threonine kinase Mos is a key regulator of oocyte maturation in both Xenopus and mouse. Mos is a direct activator of mitogen-activated protein kinase (MAPK) kinase (MEK), which in turn activates MAPK (61, 71). Unlike mammalian cells, which express both p42 and p44 MAPKs, Xenopus oocytes express only p42 (29, 60). MAPK has a diverse range of downstream targets in different cell types (7, 14). Mos has also been proposed to play a role in the activation of maturation-promoting factor (MPF) (reviewed in reference 73), which is composed of p34cdc2 and cyclin B1 (16, 17, 24). Mos activity is necessary for the completion of meiosis I in Xenopus and for maintenance of the metaphase II arrest in both unfertilized Xenopus and mouse eggs to prevent parthenogenetic activation (10, 12, 33, 67). Mos protein levels are tightly regulated in vivo during oocyte maturation. Progesterone stimulation of immature Xenopus oocytes induces Mos protein synthesis (35, 65, 66). Newly synthesized Mos protein is initially unstable (65, 81). Stable, hyperphosphorylated Mos is present in unfertilized eggs (81), implicating phosphorylation in the stabilization of Mos following germinal vesicle breakdown (GVBD). Mos protein levels are thus regulated initially at the level of translation and ultimately by controlling Mos protein stability.
During meiotic maturation, oocytes are transcriptionally repressed and all necessary proteins are translated from preexisting, maternally derived mRNAs (13). Mos translational control is exerted through the regulation of Mos mRNA cytoplasmic polyadenylation in both Xenopus (69, 70) and the mouse (27). Maternal Mos mRNA initially has a relatively short poly(A) tail in immature oocytes, to which further adenyl residues are added during oocyte maturation (20, 46, 58, 69). Cytoplasmic polyadenylation promotes the assembly of maternal mRNA into polysomes and stimulates translation (46, 57, 80). In addition to Mos, a number of other Xenopus maternal mRNAs are translationally regulated by cytoplasmic polyadenylation during progesterone-stimulated oocyte maturation. These mRNAs encode key regulators of cell cycle progression, including cdk2 (75), cyclin A1, cyclin B1, cyclin B2 (69, 74), the histone H1-like protein B4 (57, 58), and G10 (47). Interestingly, different maternal mRNAs exhibit temporally distinct patterns of cytoplasmic polyadenylation throughout the course of progesterone-stimulated oocyte maturation (4, 15, 69). Cytoplasmic polyadenylation is directed by two types of sequence-specific elements in the 3′ untranslated region (UTR) of these mRNAs: a uracil-rich cytoplasmic polyadenylation element (CPE) and the polyadenylation hexanucleotide sequence AAUAAA (reviewed in references 32, 40, 63, 76, and 82). It has been proposed that the temporal control of cytoplasmic polyadenylation may be regulated through the position of the CPEs within the 3′ UTR (15).
Progesterone-stimulated translational control may be divided into three processes: (i) progesterone-initiated signal transduction, (ii) signal amplification, and (iii) mRNA cytoplasmic polyadenylation and translation. Very little is known about the initial progesterone signaling pathway that triggers maturation, although progesterone does ultimately induce MAPK and MPF activation. The regulated components of the polyadenylation machinery have not been identified, although several candidates exist. The cytoplasmic element binding protein (CPEB) is required for oocyte maturation (74), but it has not been established whether CPEB acts as a translational repressor, a latent translational activator, or a “platform” protein to which polyadenylation accessory factors bind in a progesterone-dependent manner. Other potential targets for progesterone-directed regulation could include the cleavage and polyadenylation specificity factor, which interacts with the polyadenylation hexanucleotide sequence (6, 36, 52, 53), poly(A) polymerase (3, 9, 26, 78), or other components of the polyadenylation apparatus (37, 59).
The process of progesterone-triggered maternal mRNA polyadenylation is regulated chiefly through the signal amplification step (4). A positive-feedback loop has been proposed to function during oocyte maturation whereby Mos induces MAPK activation and MAPK stimulates MPF activation and synthesis of Mos protein (28, 30, 31, 34, 44, 45, 64, 79). This feedback loop may be critical for establishing the robust MPF activity necessary to complete meiosis I and to generate sufficient Mos activity (cytostatic factor) to arrest unfertilized eggs at meiotic metaphase II. The process by which either MPF or MAPK activity functions to stimulate de novo Mos synthesis has not been established. Modulation of Mos mRNA polyadenylation, initiation of mRNA translation, and enhanced Mos protein stability have all been proposed (28, 45).
In this study, we have used specific activators and inhibitors of the MAPK and MPF signaling pathways to dissect the mechanisms which regulate Mos mRNA cytoplasmic polyadenylation and translation during Xenopus oocyte maturation. We report that MAPK signaling stimulates Mos mRNA cytoplasmic polyadenylation and Mos protein translation. MPF also induces Mos mRNA polyadenylation, but we demonstrate that this effect of MPF required the activation of the MAPK signaling pathway. Our results provide a mechanistic explanation for the observed feedback loop, in which Mos induces the further accumulation of Mos protein. Mos mRNA cytoplasmic polyadenylation and translation leads to the activation of MEK and MAPK. We propose that MAPK, in turn, stimulates the cytoplasmic polyadenylation of additional Mos mRNA, resulting in an amplification of the signaling pathway. While MAPK also induces MPF activation, MPF did not directly stimulate Mos mRNA polyadenylation. Our data would position MPF outside the MAPK-mediated feedback amplification of Mos mRNA polyadenylation and translation. In contrast to the regulation of Mos mRNA polyadenylation, we report that MPF-induced cytoplasmic polyadenylation of the cyclin B1 mRNA did not require MAPK. Our results suggest that the cytoplasmic polyadenylation of maternal mRNAs is subject to differential regulation by the MAPK and MPF signaling pathways.
MATERIALS AND METHODS
Plasmid constructions and RNA synthesis. (i) Cyclin B1.
The pRF170 vector expressing Xenopus cyclin B1 was obtained from D. J. Donoghue (University of California, San Diego, Calif.) (21).
(ii) pGEMmos321.
The terminal 321 nucleotides of the Mos 3′ UTR was cloned from immature oocytes by reverse transcription-PCR. PCR primers were designed to include a 5′ BamHI restriction site [5′(+): CGCGG ATCCC CCGGG CACTA GTAGC CAGGA GTTCAT] and a 3′ XbaI restriction site [3′(−): GCGTC TAGAA GACAA ATCAA TTTCT TTATT]. The resulting PCR product was cloned into BamHI-XbaI-digested pGEM4Z (Promega) and designated pGEMmos321. The integrity of the Mos UTR was confirmed by DNA sequencing.
(iii) GST Mos.
Standard PCR techniques were used to add a ClaI restriction site to the N-terminal end of the mRNA encoding Xenopus c-Mos. A 0.5-kb ClaI-BglII fragment was amplified from pRF146 (22), which was provided by D. J. Donoghue. A 0.6-kb BglII-BamHI fragment encoding the C-terminal domain of c-Mos was excised from the pRF146 vector. The glutathione S-transferase (GST) fusion vector pXen1 (43) was digested within the multiple-cloning site by using ClaI and BamHI and subsequently ligated to both the N-terminal Mos PCR product and the C-terminal Mos restriction fragment.
(iv) GST 107Wee.
The pAX-SV40 Xenopus Wee1 (pXe-Wee1) vector was obtained from P. R. Mueller (University of Chicago, Chicago, Ill.) (51). To create the mutant that has the first 107 amino acids deleted, primers were designed to include a 5′ BamHI restriction site [5′(+): GCGGG ATCCC TTTTG TACAA AACGC TTCCC TCT] and a 3′ XbaI restriction site [3′(−): GCGTC TAGAT TAATA CCCTC CGCAG GTGAA GCT]. The resulting PCR product was cloned into BamHI-XbaI-digested pXen2 (43).
(v) MKP-1.
The pSG5-3CH134-Myc vector was obtained from N. K. Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.) (77). For expression in oocytes, the EcoRI (blunt)-BamHI fragment containing MKP-1 (amino acids 1 to 314) and the Myc epitope tag was cloned into NcoI (blunt)-BamHI-digested pSPvRaf (54).
(vi) GST rVH6.
The NdeI-HindIII fragment of the T77rVH6His plasmid from J. E. Dixon (University of Michigan, Ann Arbor, Mich.) (50) was blunt ended and subcloned into pXen1 linearized with SmaI.
(vii) GST MEK*.
pGST MEK* has been described previously (41).
(viii) RNA synthesis.
For in vitro transcription, plasmids were linearized and transcribed with SP6 RNA polymerase (Promega), as previously described (48). The pGST Mos and pMKP-1 plasmids were linearized with EcoRI, pGST MEK* was linearized with XbaI, and pGST 107Wee and pGST rVH6 were linearized with BglI. pRF170 was digested with XhoI prior to in vitro transcription. Linearization of pGEMmos321 with XbaI prior to in vitro transcription generated the UTRX RNA template.
Oocyte culture and injections.
Oocytes were defolliculated by collagenase digestion (Sigma type II, 1 mg/ml) and placed in 1× modified Barth’s saline solution plus HEPES with bovine serum albumin (1 mg/ml), Ficoll 400 (1 mg/ml), and antibiotics (54). Dumont stage VI immature oocytes were isolated and injected with 5 to 10 ng of the appropriate RNA. Oocytes were stimulated with 2 μg of progesterone (Sigma) per ml and were typically harvested when 50% of the control, progesterone-treated oocytes had undergone GVBD (GVBD50). For all experiments, pools of 5 to 10 oocytes were harvested in proportion to the percent GVBD in each sample. The results shown are from representative experiments that were typically performed three times with similar results. The MEK inhibitor PD98059 (New England Biolabs) was added to oocyte culture media at a final concentration of 100 μM in 1% dimethyl sulfoxide (DMSO). A baculovirus expressing His6-tagged human Δ87cyclin B1 (Δcyclin B) was generously provided by P. R. Mueller (38), and Δcyclin B protein was purified by Ni-agarose chromatography from infected Sf9 cells, as specified by the manufacturer (Qiagen), and stored in aliquots at −80°C. Oocytes were each injected with 50 to 100 pg Δcyclin B protein.
Western blot analyses.
Oocytes were lysed in 10 μl of cold Nonidet P-40 (NP-40) buffer per oocyte (42), and insoluble material and lipid were separated by centrifugation at 13,000 × g for 10 min at 4°C. The lysates were normalized for the amount of total protein, separated on sodium dodecyl sulfate (SDS)–10% or 14% polyacrylamide gels (Novex), and transferred to a 0.2-μm-pore-size nitrocellulose filter (Protran; Midwest Scientific). The filter was blocked with 5% nonfat dried milk in TBST (10 mM Tris [pH 7.5], 150 mM NaCl, 0.1% [vol/vol] Tween 20). Filters were incubated with antibody and visualized with an appropriate horseradish peroxidase-linked secondary antibody by enhanced chemiluminescence (Amersham). Rabbit polyclonal antisera against GST and Xenopus c-Mos were obtained from Santa Cruz Biotechnology, Inc. MAPK activation was visualized with an antibody specific for the activated, phosphorylated form of the enzyme (New England Biolabs). The 9E10 monoclonal antibody (Santa Cruz Biotechnology) raised against human c-Myc was used to recognize the Myc epitope tag.
MEK activity assay.
His-tagged, kinase-negative MAPK (KN-MAPK)-expressing Escherichia coli was provided by Gary Johnson (National Jewish Center for Immunology and Respiratory Medicine, Denver, Colo.) and KN-MAPK purified as described previously (23). Protein lysates were prepared from treated oocytes. Total protein (30 μg) was incubated with KN-MAPK in a 40-μl MEK activity reaction mixture (20 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 10 mM MnCl2, 20 mM β-glycerophosphate, 50 μM ATP, 10 μCi of [γ-32P]ATP, 100 ng of KN-MAPK) for 30 min at room temperature. The samples were separated on a 10% polyacrylamide gel (Novex), and the phosphoproteins were visualized by autoradiography (41).
cdc2 activity assay.
To specifically assay the activity of cdc2, cyclin-dependent kinase complexes were affinity purified prior to the kinase assay. Total protein lysates (150 μg) were incubated with 15 μl of GST-p13suc1 beads (Upstate Biotech Inc., Lake Placid, N.Y.) in 500 μl of NP-40 lysis buffer with gentle agitation for 20 min at 4°C. The beads were washed twice with ice-cold NP-40 buffer and twice with histone kinase buffer (20 mM HEPES [pH 7.4], 1 mM dithiothreitol, 10 mM MgCl2) and then incubated with 35 μl of histone kinase buffer containing 100 μM ATP, 2 μg of histone H1, and 20 μCi of [γ-32P]ATP. Samples were resolved on SDS–10% polyacrylamide gels (Novex), and the phosphoproteins were visualized by autoradiography.
Wee1 activity assay.
GST Wee1 was affinity purified from 200 μg of total protein lysate with 30 μl of glutathione-Sepharose beads (Pharmacia) in 500 μl of NP-40 buffer for 15 min, 4°C with gentle agitation. The beads were washed twice with NP-40 buffer and twice with kinase buffer (50 mM Tris [pH 7.5], 10 mM MgCl2) and then incubated for 30 min at room temperature in 40 μl of kinase buffer containing 1 mM dithiothreitol, 50 μM ATP, 2 μl of cyclin B-cdc2 complex, and 20 μCi of [γ-32P]ATP (51). Samples were resolved on SDS–14% polyacrylamide gels (Novex), and the phosphoproteins were visualized by autoradiography.
Polyadenylation assay.
Immature oocytes were microinjected with 5 to 10 ng of in vitro-transcribed RNA encoding the UTRX synthetic Mos template. Following the indicated treatments, total RNA was extracted from pools of 10 oocytes with 800 μl of RNA-STAT 60 as specified by the manufacturer (Tel-Test B, Friendswood, Tex.) and an additional phenol-chloroform extraction. To obtain high-quality RNA, samples were reprecipitated with 8 M LiCl (as described in reference 68). The samples were resuspended in 22 μl of loading buffer (1× MOPS [0.04 M morpholinopropanesulfonic acid {pH 7.0}, 12.5 mM sodium acetate, 1 mM EDTA], 18% formaldehyde, 45% formamide, 36 μg of ethidium bromide per ml) and resolved on a 2.2% (3:1 Nusieve-agarose) formaldehyde Northern gel. They were then transferred to a 0.2-μm-pore-size Nytran membrane (Schleicher & Schuell) and probed with an [α-32P]dCTP-labeled probe (Pharmacia) specific for the Mos 3′ UTR. For cyclin B1 polyadenylation assays, samples of total RNA were run on a 1% (3:1 Nusieve-agarose) formaldehyde gel and hybridized with a cyclin B1-specific probe. Following overnight hybridization, the membranes were washed twice in 1× SSC (0.15 M NaCl, 0.015 M sodium citrate)–0.5% SDS for 10 min at room temperature and twice in 0.1× SSC–0.1% SDS for 30 min at 55°C and analyzed by autoradiography (68). To confirm that any increase in mRNA size was specifically due to polyadenylation, RNA samples were treated with RNase H and oligo(dT) to eliminate any poly(A) tail prior to gel analysis. RNase H reactions were performed as previously described (49) with the following modifications: 5 to 10 μg of total RNA was incubated with 1 μl of RNase H (1 U/μl; Boehringer Mannheim) in 40 μl of digestion buffer (25 mM KCl, 0.5 mM EDTA, 10 mM Tris, 10 mM MgCl2, 50 mM NaCl, 1 mM dithiothreitol), and the samples were incubated for 30 min at 37°C in the presence or absence of an anchored oligo(dT) primer (0.3 μg). Following digestion, the samples were extracted with phenol-chloroform and ethanol precipitated.
RESULTS
MAPK signaling is necessary for Mos protein accumulation.
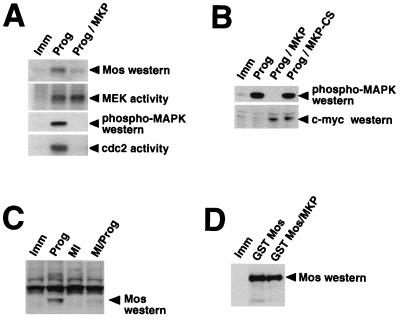
To determine the role of MAPK signaling in the regulation of Mos protein accumulation, we made use of a MAPK-specific phosphatase, MKP-1 (also called CL100), which has been previously shown to inhibit progesterone-stimulated MAPK activation and oocyte maturation (28). Immature oocytes were microinjected with RNA encoding a Myc-tagged form of MKP-1 and incubated for 14 h to allow protein expression from the injected RNA. MKP-injected oocytes or control uninjected oocytes were then stimulated with progesterone, and maturation was assessed by the appearance of a white spot on the animal pole (which is indicative of GVBD [73]). MKP-1 expression caused 97.3% ± 0.612% inhibition of progesterone-induced oocyte maturation (mean ± standard error of the mean; n = 3) when control samples exceeded 90% maturation (GVBD90). At the biochemical level, MKP-1 expression blocked progesterone-induced MAPK activation and MPF activation and dramatically attenuated Mos protein accumulation (Fig. 1A). Of note, there was always some, albeit variable, level of MEK activation over background in the presence of MKP-1. This activation may be due to incomplete inhibition of Mos synthesis, since low levels of Mos protein were detected in MKP-1-expressing oocytes. Interestingly, we observed complete inhibition of progesterone-stimulated cdc2 activity in MKP-1-injected oocytes, in agreement with previous reports, which have suggested that MPF activation is downstream of the MAPK pathway (28, 34, 66). As a control for specificity, an inactive mutant form of MKP-1 (MKP-CS) (77) failed to block progesterone-stimulated oocyte maturation or MAPK activation (Fig. 1B).
FIG. 1.
MAPK signaling is required for Mos protein accumulation. (A) Immature oocytes were injected with MKP RNA, as indicated, and left for 14 h to express the protein. The oocytes were then stimulated with progesterone (Prog) or left untreated (Imm). Protein lysates were prepared when the progesterone controls reached GVBD50. The same pooled lysate preparations were used for the following analyses: Mos protein accumulation was measured by Western blotting with Mos antisera; the MEK activity assay was performed as described in Materials and Methods with KN-MAPK as substrate; MAPK activation was measured with phospho-MAPK antisera; cdc2 activity assay was measured as described in Materials and Methods with histone H1 as the substrate. (B) Immature oocytes were preinjected with MKP or MKP-CS RNA, as indicated, and left for 14 h to express the protein. The oocytes were stimulated with progesterone (Prog) or left untreated (Imm). Oocyte lysates were used to measure MAPK activation (as described for panel A) and to measure the expression of myc-tagged MKP and MKP-CS by immunoblotting with anti-c-myc antibody. (C) Immature oocytes in medium containing 1% DMSO (MEK inhibitor solvent) were pretreated for 1 h with 100 μM MEK inhibitor (MI) as indicated. The oocytes were then stimulated with progesterone (Prog) or left untreated (Imm). Protein lysates were prepared when progesterone-treated samples reached GVBD50. Mos protein accumulation was analyzed as described for panel A. A nonspecific band, larger than Mos, was also detected by the antibody in all lanes. (D) Immature oocytes were injected with MKP RNA, as indicated, and left for 14 h to express the protein. The oocytes were then injected with RNA encoding GST Mos or left untreated (Imm). Protein lysates were prepared 8 h later, and GST Mos accumulation was measured by Western blotting with Mos antibodies.
To further substantiate the involvement of the MAPK pathway in Mos protein accumulation, we used a specific MEK inhibitor (PD98059) (2, 11). We found that maximal inhibition of oocyte maturation was observed at 100 μM MEK inhibitor, and this concentration was used for all subsequent experiments. Greater than 80% inhibition (81.2% ± 5.05%; n = 6) of progesterone-stimulated maturation was achieved at 100 μM MEK inhibitor, when control samples reached GVBD50. Of note, the MEK inhibitor lost efficacy after about 4 h in aqueous solution. While efficiently inhibiting maturation at early time points, the MEK inhibitor delayed rather than abolished maturation at later time points. The MEK inhibitor dramatically reduced Mos protein accumulation in response to progesterone (Fig. 1C), consistent with the effect of MKP-1 (Fig. 1A). The MEK inhibitor also significantly reduced the activation of MEK, MAPK, and MPF in response to progesterone (data not shown). The inhibition of progesterone-stimulated maturation cannot be simply attributed to MKP-1 or the MEK inhibitor acting as general inhibitors of protein translation in oocytes, since neither treatment blocked the synthesis of GST Mos protein from coinjected mRNA (Fig. 1D and data not shown). We conclude that MAPK signaling is necessary to establish significant Mos protein accumulation in response to progesterone stimulation of immature oocytes.
Mos mRNA cytoplasmic polyadenylation and protein accumulation are stimulated by the MAPK signaling pathway.
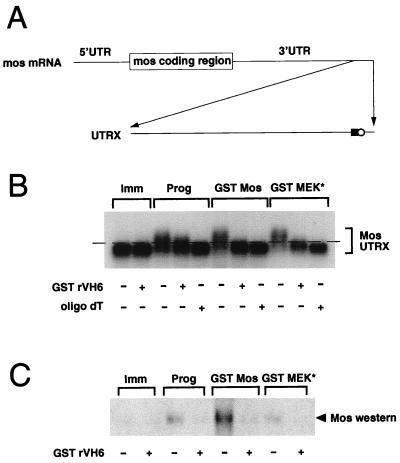
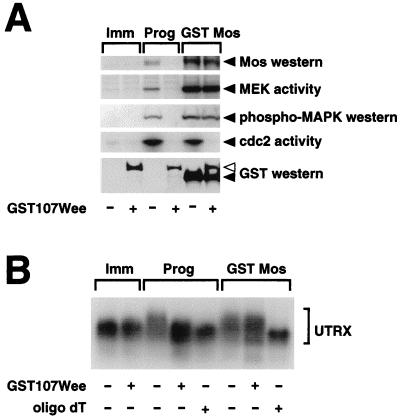
To investigate the mechanism by which the MAPK pathway mediates Mos protein accumulation, we manipulated the MAPK signaling pathway in vivo by injection of RNA encoding a constitutively active MEK mutant (GST MEK*) or a stable form of Mos (GST Mos) into immature oocytes. Injection of GST MEK* or GST Mos RNA induced the maturation of immature oocytes in the absence of exogenous progesterone. Previous studies have demonstrated that Mos protein synthesis in Xenopus oocytes is regulated by the selective cytoplasmic polyadenylation of Mos mRNA in response to progesterone (69, 70). To determine if MAPK activation induced Mos mRNA cytoplasmic polyadenylation, we used a synthetic RNA template specifying the terminal 321 nucleotides from the Mos mRNA 3′ untranslated region (UTR) (Fig. 2A). Use of this template increased the resolution of poly(A) tail length differences on Northern gels. The Mos template, UTRX, specifies all the cis-regulatory elements required to confer cytoplasmic polyadenylation and translational regulation to a luciferase reporter construct (69). The UTRX template, along with RNA encoding GST Mos or GST MEK*, was injected into immature oocytes, and total RNA was extracted from the injected oocytes at GVBD50. The poly(A) tail length of the recovered UTRX templates was analyzed by Northern blotting with a Mos-specific probe (Fig. 2B). Progesterone stimulation of immature oocytes induced an increase in the size of the UTRX RNA (Prog, Fig. 2B) compared to that of RNA prepared from untreated oocytes (Imm, Fig. 2B). Coinjection of UTRX with RNA encoding GST Mos or GST MEK* to activate the MAPK pathway also induced an increase in the size of the UTRX RNA, to a size comparable to that induced by progesterone treatment (Fig. 2B). The increase in size of the UTRX template following each treatment was specifically due to an increase in polyadenylation, since addition of oligo(dT) and RNase H reduced the UTRX mobility back to that observed in immature oocytes (oligo dT +, Fig. 2B). Interestingly, when RNA encoding a GST-tagged form of the MAPK phosphatase rVH6 (50) was coinjected with GST Mos or GST MEK*, UTRX polyadenylation was abolished. This result directly implicates MAPK as a crucial mediator of UTRX polyadenylation and rules out a contribution from a GST Mos-stimulated, MEK-independent pathway. Moreover, GST rVH6 expression attenuated the proportion of UTRX transcripts that were fully polyadenylated in response to progesterone stimulation. Since it has been established that the Mos mRNA must receive a poly(A) tail of sufficient length to stimulate efficient protein translation (5), our results would suggest that abrogation of the MAPK signaling pathway blocks progesterone-stimulated Mos protein accumulation through attenuation of Mos mRNA polyadenylation. Consistent with this hypothesis, an analysis of Mos protein accumulation in samples that were duplicates of those analyzed for UTRX polyadenylation revealed that no significant Mos protein accumulation occurred in GST rVH6-expressing oocytes in response to progesterone, GST Mos, or GST MEK* (Fig. 2C). We note that the rVH6 MAPK phosphatase, rather than MKP-1, was used in these experiments. While both GST MKP-1 and GST rVH6 were expressed to similar levels in oocytes and effectively blocked progesterone-stimulated oocyte maturation, GST MKP-1 was less effective than GST rVH6 at inhibiting a robust MAPK-activating signal generated by GST Mos. Since MKP-1 is localized primarily to the nucleus in mammalian cells and rVH6 is cytoplasmic (8, 50), the effectiveness of the two enzymes could reflect different subcellular targeting within the oocyte. Alternatively, MKP-1 and rVH6 may have different specific activities toward Xenopus MAPK.
FIG. 2.
MAPK signaling stimulates Mos RNA polyadenylation. (A) Schematic diagram of the synthetic Mos UTRX template. The terminal 321 bp of the Mos 3′ UTR was cloned into pGEM4Z by reverse transcription-PCR, as described in Materials and Methods. The positions of the CPE (solid square) and the nuclear polyadenylation element (AAUAAA) (open circle) are indicated. Prior to in vitro transcription, linearization of the construct with XbaI generated the 321-nucleotide RNA (UTRX), encoding both the CPE and AAUAAA elements. (B and C) Immature oocytes were injected with RNA encoding GST rVH6, as indicated, and left for 14 h to express the protein. The oocytes were then injected with the Mos UTRX and stimulated with progesterone (Prog), coinjected with GST Mos or GST MEK* RNA, or left untreated (Imm). When each separate treatment had reached GVBD70–90 (4 h for progesterone, 9 h for GST Mos, and 23 h for GST MEK*), pools of 10 oocytes were taken for both protein lysates and RNA extraction. (B) A 5-μg portion of total RNA was incubated with RNase H in the presence or absence of oligo(dT) as indicated. RNAs were separated on an agarose gel, and the extent of polyadenylation was analyzed by Northern blotting with a Mos UTR-specific probe as described in Materials and Methods. The dashed line acts as a reference point: RNAs migrating under the line are not polyadenylated, and RNAs migrating above the line are polyadenylated. (C) Endogenous Mos protein accumulation was analyzed as described in the legend to Fig. 1.
MPF-induced Mos protein accumulation is mediated by the MAPK pathway.
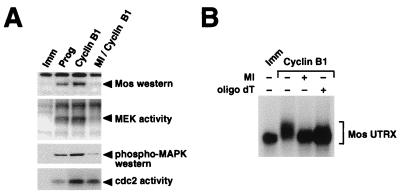
MPF (cdc2-cyclin B) has been implicated in the induction of mRNA cytoplasmic polyadenylation (4, 15, 47, 58). Since MPF appears to be downstream of MAPK signaling, MAPK-induced Mos mRNA cytoplasmic polyadenylation (Fig. 2A) could be mediated by MPF. We wished to determine the specific contribution of MPF to Mos accumulation in the absence of MAPK signaling. Oocytes were injected with RNA encoding cyclin B1 to activate endogenous cdc2. Overexpression of cyclin B1 can induce meiotic maturation of Xenopus oocytes in the absence of progesterone (21). Consistent with previous reports (29, 72, 79), we demonstrate that microinjection of cyclin B1 RNA induced the activation of MPF (Fig. 3A, bottom). Cyclin B1 RNA injection also induced the activation of MEK and MAPK (Fig. 3A, middle) and stimulated the expression of Mos protein (Fig. 3A, top).
FIG. 3.
MPF requires MAPK activity to induce Mos protein accumulation and Mos UTRX polyadenylation. Immature oocytes were preincubated with 100 μM MEK inhibitor (MI), as indicated, prior to injection with RNA encoding cyclin B1. When cyclin and progesterone-stimulated control oocytes had reached GVBD50, pools of oocytes were harvested and both protein lysates and total RNA were prepared. (A) Protein lysates were analyzed for Mos protein expression (top panel) and kinase activation (lower panels) as described in the legend to Fig. 1. (B) RNA samples were incubated with RNase H, with or without oligo(dT) as indicated, and Mos UTRX polyadenylation was analyzed as described in the legend to Fig. 2B.
Pretreatment of oocytes with the MEK inhibitor dramatically reduced the ability of cyclin B1 RNA to induce maturation and Mos synthesis. The MEK inhibitor blocked cyclin B1-induced oocyte maturation (78% ± 7% inhibition; n = 3). Western blot analysis showed that the MEK inhibitor blocked the ability of cyclin B1 to stimulate Mos protein accumulation (Fig. 3A, top). These oocyte lysates were analyzed for MEK and MAPK activities. Pretreatment of the oocytes with MEK inhibitor significantly blocked the activation of both MEK and MAPK in response to cyclin B1 (Fig. 3A, middle), while MPF activity was only partially reduced (Fig. 3A, bottom). Because the positive-feedback loop could contribute to the total levels of MPF activity, inhibition of MAPK signaling may attenuate MPF activation by this mechanism. However, the activity of MPF in the MEK inhibitor-treated oocytes was still maintained at a higher level than that observed in progesterone-stimulated oocytes. Since Mos protein was efficiently synthesized in progesterone-stimulated oocytes but not in the cyclin B1-MEK inhibitor-treated oocytes, we conclude that MPF activity is not sufficient for Mos protein accumulation in the absence of MEK and MAPK activities.
To determine the role of MPF in Mos mRNA cytoplasmic polyadenylation, we coinjected cyclin B1 RNA and the UTRX template. Polyadenylation of the UTRX template in cyclin B1-injected oocytes was comparable to that observed with progesterone treatment (Fig. 3B, compare lanes Imm and cyclin B1). The ability of cyclin B1 to induce Mos UTRX polyadenylation was blocked in the presence of MEK inhibitor (Fig. 3B, MI +), consistent with our data demonstrating that the MEK inhibitor blocked cyclin B1-induced Mos protein synthesis (Fig. 3A). Similar results were obtained when MKP-1 was used instead of the MEK inhibitor. Taken together, these findings indicate that MPF-stimulated Mos mRNA polyadenylation and protein accumulation require a functional MAPK pathway.
MAPK signaling stimulates Mos mRNA polyadenylation and translation in the absence of MPF activity.
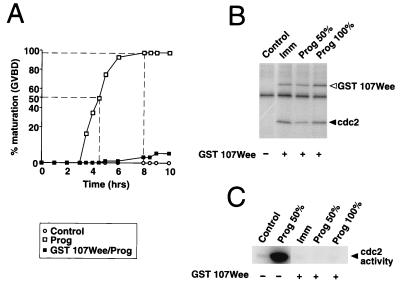
We generated an N-terminally truncated, constitutively active version of the Xenopus cdc2 kinase inhibitor Wee1 (51) to specifically inhibit MPF activity in maturing oocytes. In this construct, the first 107 amino acids of the wild-type Wee1 protein have been deleted and replaced with a GST epitope tag (GST 107Wee). This deletion removes 8 of 11 potential cdc2 phosphorylation sites which may negatively regulate Wee1 activity (51).
To establish that the GST 107Wee construct inhibited MPF in vivo, immature oocytes were injected with RNA encoding GST 107Wee and incubated for 14 h to allow GST 107Wee protein expression. The oocytes were then split into pools and either left untreated or stimulated by the addition of progesterone. Figure 4A shows that GST 107Wee prevented oocyte maturation in response to progesterone. Indeed, only 10% of oocytes that were injected with GST 107Wee matured even after 10 h of culture. Figure 4B shows that affinity-purified GST 107Wee was constitutively active in both unstimulated oocytes (Fig. 4B, Imm) and oocytes that have been treated with progesterone. We confirmed that GST 107Wee inhibited endogenous cdc2 activity in these lysates (Fig. 4C). When 50% of control uninjected progesterone-stimulated oocytes had matured, there was a high level of cdc2 kinase activation. However, progesterone-stimulated cdc2 activity was completely abolished in oocytes expressing GST 107Wee. These data show that GST 107Wee effectively inhibits MPF activity.
FIG. 4.
GST 107Wee inhibits oocyte maturation and cdc2 activity. (A) Immature oocytes were injected with RNA encoding GST 107Wee, as indicated, and left for 14 h to express the protein. The oocytes were then stimulated with progesterone (open squares, and solid squares) or left untreated (open circles). At the times shown after progesterone stimulation, maturation was scored by the appearance of a white spot (GVBD). (B and C) Immature oocytes were preinjected with RNA encoding GST 107 Wee, as indicated, and left for 14 h to express the protein. The oocytes were then stimulated with progesterone or left untreated (Imm). Pools of oocytes were taken when the progesterone controls had reached GVBD50 (Prog 50%) and again at GVBD100 (Prog 100%). The control samples are untreated, immature oocytes. The same lysates were used for panels B and C. (B) GST 107Wee was affinity purified with glutathione-Sepharose beads, and its activity was measured in an in vitro kinase reaction, as described in Materials and Methods, with cyclin B-cdc2 complex as the substrate. The solid and open arrowheads show the positions of cdc2 and GST 107Wee autophosphorylation, respectively. (C) Endogenous cdc2 activity in the same lysates was measured as described in the legend to Fig. 1.
To determine whether the MAPK pathway could stimulate Mos protein accumulation in the absence of MPF activity, immature oocytes were injected with GST 107Wee and left for 14 h to express the protein as described above. The oocytes were then either left untreated or stimulated with progesterone or by injection of GST Mos RNA to activate the MAPK pathway. GST 107Wee prevented GST Mos-stimulated maturation of oocytes by 92% when GST Mos controls reached GVBD50. Lysates were prepared, and the levels of expression of GST 107Wee and GST Mos were analyzed by Western blotting. Equivalent levels of GST 107Wee and GST Mos were expressed in either singly or doubly injected oocytes (Fig. 5A, bottom). We verified that expression of GST 107Wee prevented the activation of MPF in response to progesterone or GST Mos in these lysates (Fig. 5A, cdc2 activity). Injection of RNA encoding GST Mos induced the accumulation of endogenous Mos protein, and this was not inhibited by coinjection of GST 107Wee. In contrast, when oocytes were stimulated by progesterone, GST 107Wee did inhibit Mos protein accumulation (Fig. 5A, top). The levels of MAPK activation stimulated by GST Mos were approximately equivalent to those induced by progesterone. GST 107Wee did not prevent stimulation of MEK and MAPK activities in response to injected GST Mos RNA. This data suggests that MPF activity is not necessary for Mos protein accumulation under conditions of robust MAPK activation. However, GST 107Wee did inhibit progesterone-induced MEK and MAPK activities (Fig. 5A, middle panels), suggesting that progesterone-stimulated MAPK activation is not sustainable in the absence of MPF.
FIG. 5.
MAPK signaling stimulates Mos mRNA polyadenylation and translation in the absence of MPF activity. Immature oocytes were injected with RNA encoding GST 107Wee, as indicated, and left for 14 h to express the protein. The oocytes were then either stimulated with progesterone (Prog) or injected with RNA encoding GST Mos. Pools of oocytes were taken when the controls were at GVBD50 (progesterone at 4.5 h and GST Mos at 8 h). Protein lysates and total RNA were prepared from the same experiment. (A) Mos protein accumulation (top panel) and kinase activities (middle panels) are as described in the legend to Fig. 1. Expression of the GST-tagged proteins (bottom panel) was verified by Western blotting with GST antisera. The open arrowhead shows the position of GST 107Wee, and the solid arrowhead shows the position of GST Mos. (B) UTRX polyadenylation was analyzed as described in the legend to Fig. 2.
To determine if a robust MAPK signal could stimulate Mos UTRX polyadenylation in the absence of MPF activity, oocytes were preinjected with UTRX RNA followed by appropriate combinations of GST 107Wee and GST Mos as described above. We found that GST Mos-induced UTRX polyadenylation still occurred in GST 107Wee-expressing oocytes (Fig. 5B). This data suggests that MPF activity is not necessary for MAPK-induced Mos mRNA polyadenylation. Consistent with the findings above, expression of GST 107Wee did inhibit polyadenylation of the Mos UTRX template when the oocytes were stimulated with progesterone.
MPF stimulates cyclin B1 mRNA cytoplasmic polyadenylation in the absence of MAPK signaling.
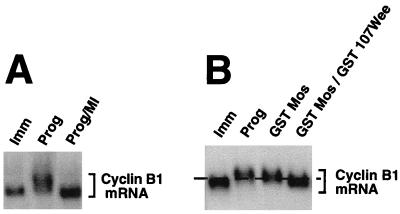
Since our data indicate that MAPK signaling can mediate the cytoplasmic polyadenylation of Mos mRNA, we wanted to know if the polyadenylation of other maternal mRNAs required MAPK signaling. We investigated the requirements for MAPK and MPF signaling on cyclin B1 RNA polyadenylation. It has been previously demonstrated that the cytoplasmic polyadenylation of cyclin B1 mRNA occurs later in maturation than that of Mos mRNA (69) and that cyclin B1 mRNA polyadenylation requires the prior polyadenylation of Mos mRNA (4). Although MPF-stimulated cyclin B1 mRNA polyadenylation has been shown to occur in the absence of detectable MAPK activation (15), the requirement for MAPK has not been directly tested. To determine whether MAPK activity was necessary for cyclin B1 mRNA polyadenylation, oocytes were pretreated with the MEK inhibitor and then stimulated with progesterone. Total RNA was analyzed by Northern blotting, and the endogenous cyclin mRNA was detected with a cyclin B1-specific probe. Because the cytoplasmic polyadenylation of the cyclin B1 mRNA results in the addition of 200 to 300 adenyl residues, the size difference between the nonpolyadenylated and polyadenylated mRNA can be readily analyzed by probing Northern blots for endogenous cyclin B1 mRNA without the need for a short UTR construct. Figure 6A shows that the MEK inhibitor blocked progesterone-stimulated cyclin B1 polyadenylation, implicating MAPK signaling in this process. We next tested if MAPK signaling was sufficient for cyclin B1 mRNA polyadenylation in the absence of MPF activity. Oocytes were preinjected with GST 107Wee RNA and cultured overnight. These and control oocytes were injected with GST Mos RNA to generate a robust MAPK signal. When the control oocytes were fully matured, total RNA was prepared from pools of oocytes and Northern blots were analyzed as described above. Figure 6B shows that expression of GST 107Wee attenuated cyclin B1 mRNA polyadenylation induced by GST Mos. Some polyadenylation of the cyclin B1 mRNA did occur in the oocytes coinjected with GST Mos plus GST 107Wee, but GST 107Wee expression dramatically reduced the proportion of mRNA which received a full-length poly(A) tail (Fig. 6B, compare lanes GST Mos/GST Wee to GST Mos and Prog). Our data suggest that MAPK is not sufficient and MPF activity is required to generate fully polyadenylated cyclin B1 mRNA.
FIG. 6.
MAPK signaling is not sufficient to stimulate endogenous cyclin B1 mRNA polyadenylation in the absence of MPF activity. (A) Immature oocytes in 1% DMSO were pretreated for 1 h with 100 μM MEK inhibitor (MI), as indicated, and then stimulated with progesterone or left untreated (Imm). When the oocytes had reached GVBD50, pools were taken and total RNA was extracted. Endogenous cyclin B1 mRNA polyadenylation was analyzed by separating total RNA on a 1% agarose gel and hybridizing with a probe specific for cyclin B1. The migration of endogenous cyclin B1 mRNA is shown by the bracket. (B) Immature oocytes were injected with GST 107Wee RNA, as indicated, and left for 14 h to express the protein. The oocytes were then either stimulated with progesterone (Prog) or injected with RNA encoding GST Mos. Pools of oocytes were harvested when the progesterone and GST Mos-injected oocytes were fully mature (GVBD>90), and total RNA was extracted. Polyadenylation of endogenous cyclin B1 mRNA was analyzed as described above. The dashed line acts as a reference point: RNAs migrating under the line are not polyadenylated, and RNAs migrating above the line are polyadenylated.
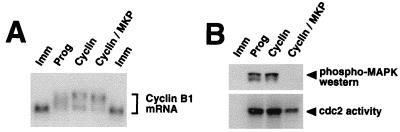
MPF activity has been previously implicated in the control of cyclin B1 mRNA polyadenylation (4, 15). We next asked if MPF activity was sufficient for cyclin B1 RNA polyadenylation in the absence of MAPK signaling. To this end, MKP-expressing oocytes were injected with purified cyclin B1 protein to activate MPF. In these experiments, we injected cyclin protein rather than cyclin B1 RNA, since the injected cyclin B1 RNA and the endogenous cyclin B1 mRNA are the same size and complicated the interpretation of subsequent Northern blots. Pools of oocytes were taken when 50 to 60% had undergone GVBD, and total RNA or protein lysates were prepared from duplicate samples. Figure 7A shows that injected cyclin protein induced the polyadenylation of endogenous cyclin B1 mRNA. MKP-1 expression did not prevent MPF-induced cyclin B1 mRNA polyadenylation. Analysis of the oocyte lysates (Fig. 7B) showed that expression of MKP-1 prevented the activation of MAPK following the injection of cyclin B protein (Fig. 7B, phospho-MAPK). Endogenous MPF is activated by cyclin protein in MKP-1-expressing oocytes, albeit to levels below those in cyclin-injected, control oocytes (Fig. 7B, cdc2 activity). This reduced MPF activity may reflect the contribution of the positive-feedback loop, which would be blocked in MKP-1 expressing oocytes, analogous to the effect of the MEK inhibitor (Fig. 3A). Despite the lower MPF activity levels, endogenous cyclin B1 mRNA polyadenylation was observed in oocytes coinjected with cyclin plus MKP-1 (Fig. 7A), indicating that attenuated levels of MPF are sufficient for cyclin B1 mRNA polyadenylation in the absence of MAPK signaling. This MAPK independence is in contrast to our analysis of Mos UTRX, where MPF-stimulated Mos UTRX polyadenylation required MAPK activity (Fig. 3). These results indicate that the cytoplasmic polyadenylation of Mos and cyclin B1 mRNAs are differentially regulated by MAPK and MPF signaling pathways.
FIG. 7.
MPF activity stimulates endogenous cyclin B1 mRNA polyadenylation in the absence of MAPK activity. Immature oocytes were injected with RNA encoding MKP, as indicated, and left for 14 h to express the protein. The oocytes were then either stimulated with progesterone (Prog), injected with cyclin B1 protein, or left untreated (Imm). Pools of oocytes were taken when the controls had reached GVBD50, and both RNA and protein lysates were prepared. (A) Endogenous cyclin B1 mRNA polyadenylation was analyzed as described in the legend to Fig. 6. (B) Kinase activities are as described in the legend to Fig. 1.
DISCUSSION
The stimulation of Mos protein accumulation is regulated at a translational level through the cytoplasmic polyadenylation of the maternal Mos mRNA. The identity of the signaling pathways which contribute to Mos mRNA cytoplasmic polyadenylation is of considerable interest, given the importance of the Mos protein for oocyte maturation and the prevention of parthenogenetic activation. Previous studies had demonstrated that both MAPK and MPF participate in progesterone-stimulated oocyte maturation and Mos protein accumulation. However, the exact role of MAPK and MPF in the induction of Mos translation had not been characterized. In this study, we have identified a novel role for MAPK-dependent signaling in the regulation of maternal mRNA translation. We demonstrate that the MAPK signaling pathway stimulates Mos mRNA cytoplasmic polyadenylation and translation of Mos protein in Xenopus oocytes. When the MAPK pathway was activated in the absence of MPF, Mos mRNA polyadenylation and translation were stimulated. In contrast, when MPF was activated in the absence of MAPK signaling, no polyadenylation or translation of Mos mRNA was observed. We conclude that MAPK-dependent signaling, rather than MPF, mediates Mos mRNA cytoplasmic polyadenylation. Although both MAPK and MPF contribute to oocyte maturation, the dramatic reduction of Mos protein levels that we observed in MEK inhibitor-treated or MAPK phosphatase-expressing oocytes suggests that the MAPK pathway controls the overall accumulation of Mos protein in response to progesterone.
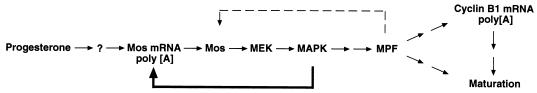
A signal transduction feedback loop involving Mos, MAPK, and MPF has been proposed to operate during oocyte maturation and is dependent upon protein synthesis. This feedback loop contributes significantly to the ability of progesterone-stimulated oocytes to switch in an “all-or-none” manner from the immature to the fully matured state, irrespective of the absolute level of the initiating progesterone stimulus (19). Our findings, which implicate the MAPK pathway in the control of Mos mRNA cytoplasmic polyadenylation, provide a mechanistic basis for this feedback control. We propose a model to illustrate the relative roles of MAPK and MPF during progesterone-stimulated oocyte maturation. In this model (Fig. 8), progesterone triggers the initial polyadenylation and translation of Mos mRNA by an as yet undefined pathway. This initial polyadenylation of Mos mRNA could be mediated by MAPK signaling but would necessitate a MEK activator distinct from Mos, since Mos protein translation is dependent on Mos mRNA polyadenylation (69, 70). While there is some evidence for additional MAPK activators in Xenopus oocytes (72), our data do not preclude the existence of a MAPK-independent trigger pathway contributing to the initial polyadenylation of Mos mRNA (4). Consistent with a MAPK-independent pathway, we observed low levels of Mos protein following progesterone stimulation in the absence of detectable MAPK activation (Fig. 1). Some Mos UTRX polyadenylation did occur in the absence of detectable MAPK signaling, but the poly(A) tails were significantly shorter in GST rVH6-expressing oocytes than in the control samples (Fig. 2B). Since Mos mRNA translational efficiency is governed by the absolute length of the poly(A) tail (5), we conclude that these transcripts would not be effectively translated. However, these transcripts could nonetheless contribute to the very low levels of Mos protein detected in GST rVH6- and MKP-expressing, progesterone-treated oocytes. Regardless of the initial trigger pathway, translation of Mos protein subsequently activates the MAPK pathway. As we report in this paper, MAPK stimulates Mos mRNA cytoplasmic polyadenylation and translation (Fig. 2 and 5). MAPK-mediated stimulation of Mos mRNA polyadenylation would establish a positive-feedback loop (Fig. 8), resulting in the translation of additional Mos protein and consequently in the further activation of MAPK signaling. MAPK also induces MPF activation (28, 34, 66). The mechanism by which MAPK induces MPF activity is not known but could involve the polo-like kinase (39, 62), cdc25 phosphatase (18, 25), and the inactivation of a cdc2-inhibitory kinase (1, 56). Since we have clearly demonstrated that MPF was unable to stimulate Mos mRNA polyadenylation in the absence of MAPK signaling (Fig. 3B), MPF is positioned outside the Mos mRNA polyadenylation and MAPK-signaling feedback loop in our model (Fig. 8).
FIG. 8.
Model to illustrate the role of MAPK signaling in mediating Mos mRNA polyadenylation. Progesterone stimulation of immature oocytes induces Mos mRNA polyadenylation. The initial progesterone-stimulated signaling mechanism that “triggers” Mos mRNA polyadenylation remains to be determined. Mos mRNA translation would then result in MEK and MAPK activation. We propose that activation of MAPK signaling stimulates Mos mRNA polyadenylation, resulting in positive feedback (bold arrow) while independently inducing MPF activation. Since MPF is not able to stimulate Mos mRNA polyadenylation in the absence of MAPK signaling, we have positioned MPF outside the MAPK-Mos mRNA polyadenylation feedback loop. MPF may functionally synergize with the feedback loop by acting to stabilize newly synthesized Mos protein (55) (dashed line), although this requirement can be overcome under conditions of robust MAPK activation (Fig. 5). MPF in turn, can induce the polyadenylation of additional maternal mRNAs (15), including cyclin B1.
Our results suggesting that MAPK signaling mediates the stimulation of Mos mRNA polyadenylation are particularly interesting in light of previous studies which have implicated MPF in the regulation of maternal mRNA cytoplasmic polyadenylation (4, 15). MPF-induced cytoplasmic polyadenylation of cyclin B1 mRNA occurred in the presence of an antisense Mos oligonucleotide, where significant MAPK activation was not detected (15). We have extended these observations and demonstrated that MPF-stimulated cyclin B1 polyadenylation does not require MAPK activity. Our data thus reveal a differential regulation of the cytoplasmic polyadenylation of specific maternal mRNAs by the MAPK and MPF signaling pathways.
Interestingly, inhibition of either MAPK or MPF blocked Mos accumulation in response to progesterone, suggesting that both signaling pathways contribute to Mos mRNA polyadenylation in vivo. Since we observed no stimulation of Mos mRNA cytoplasmic polyadenylation by MPF in the absence of MAPK activity, our data would argue against a direct role for MPF in stimulating Mos mRNA polyadenylation. Our data would be more consistent with the notion that MPF contributes to progesterone-stimulated Mos protein accumulation through an indirect route. Indeed, it has been reported that MPF activity is not required for the initial translation of Mos but, rather, is required for enhancing Mos protein stability (Fig. 8) (55). Similarly, inhibition of either MAPK or MPF blocked progesterone-stimulated cyclin B1 mRNA polyadenylation. Our data is most consistent with a model where MPF is the predominant regulator of cyclin B1 mRNA polyadenylation. While our data do not preclude a contribution of MAPK signaling to the stimulation of cyclin B1 mRNA polyadenylation, robust activation of the MAPK pathway was not sufficient for generation of fully polyadenylated cyclin B1 mRNA in the absence of MPF signaling (Fig. 6). The requirement for progesterone-stimulated MAPK activity in stimulating cyclin B1 mRNA polyadenylation may therefore be indirect and may involve the MAPK-dependent activation of MPF via the positive-feedback loop.
It has been demonstrated that the cytoplasmic polyadenylation of different maternal mRNAs follows a temporal order during progesterone-stimulated Xenopus oocyte maturation (4, 15, 69). Our results suggest that the cytoplasmic polyadenylation of maternal mRNAs is subject to differential regulation by the MAPK and MPF signaling pathways. This differential control may contribute to the temporally orchestrated pattern of maternal mRNA translation during oocyte maturation. One prediction of our model is that mRNAs which undergo polyadenylation later during maturation (e.g., cyclin B1) may require the positive-feedback loop to generate sufficient levels of MAPK and MPF activity before the cytoplasmic polyadenylation of these mRNAs is initiated. Further work should elucidate the molecular mechanisms which underscore the differential control and temporal order of maternal mRNA translation during early vertebrate development.
ACKNOWLEDGMENTS
His-tagged KN-MAPK-expressing E. coli was generously provided by Gary Johnson (National Jewish Center for Immunology and Respiratory Medicine, Denver, Colo.). MEK* was provided by Natalie Ahn. Plasmid constructs encoding Xenopus cyclin B1 and Mos were provided by D. J. Donoghue (University of California, San Diego, Calif.). The baculovirus encoding Δcyclin B, purified cdc2-cyclin B complexes, and a plasmid encoding Xenopus Wee1 were generous gifts from Paul Mueller (University of Chicago). A plasmid construct encoding MKP-1 was provided by N. K. Tonks (Cold Spring Harbor Laboratory). rVH6 was provided by Jack E. Dixon (University of Michigan, Ann Arbor, Mich.). We thank Hon Ip, Ed Morrisey, Paul Mueller, David Straus, and Mike Denney for helpful discussions and Lisa Fitzgerald for technical assistance. We thank Tim Karr and Melanie MacNicol for critical reading of the manuscript.
E.L.H. was supported in part by NRSA training grant 5 T32 HL07381-17. A.M.M. was supported by a Young Investigator Award from the Cancer Research Foundation and the Charlotte Geyer Foundation.
E.L.H. and A.C. contributed equally to this work.
REFERENCES
- 1.Abrieu A, Doree M, Picard A. Mitogen-activated protein kinase activation down-regulates a mechanism that inactivates cyclin B-cdc2 kinase in G2-arrested oocytes. Mol Biol Cell. 1997;8:249–261. doi: 10.1091/mbc.8.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne S, Bilger A, Astrom J, Virtanen A, Wickens M. Poly (A) polymerases in the nucleus and cytoplasm of frog oocytes: dynamic changes during oocyte maturation and early development. RNA. 1995;1:64–78. [PMC free article] [PubMed] [Google Scholar]
- 4.Ballantyne S, Daniel D L, Wickens M. A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and Cdk1 activation. Mol Biol Cell. 1997;8:1633–1648. doi: 10.1091/mbc.8.8.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkoff A, Ballantyne S, Wickens M. Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. EMBO J. 1998;17:3168–3175. doi: 10.1093/emboj/17.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bienroth S, Wahle E, Suter C C, Keller W. Purification of the cleavage and polyadenylation factor involved in the 3′-processing of messenger RNA precursors. J Biol Chem. 1991;266:19768–19776. [PubMed] [Google Scholar]
- 7.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brondello J M, McKenzie F R, Sun H, Tonks N K, Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. [PubMed] [Google Scholar]
- 9.Colgan D F, Murthy K G, Prives C, Manley J L. Cell-cycle related regulation of poly(A) polymerase by phosphorylation. Nature. 1996;384:282–285. doi: 10.1038/384282a0. [DOI] [PubMed] [Google Scholar]
- 10.Colledge W H, Carlton M B, Udy G B, Evans M J. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370:65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- 11.Cross D A, Smythe C. PD 98059 prevents establishment of the spindle assembly checkpoint and inhibits the G2-M transition in meiotic but not mitotic cell cycles in Xenopus. Exp Cell Res. 1998;241:12–22. doi: 10.1006/excr.1998.4023. [DOI] [PubMed] [Google Scholar]
- 12.Daar I, Paules R S, Vande Woude G F. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991;114:329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson E H. Gene activity in early development. 3rd ed. London, United Kingdom: Academic Press; 1986. [Google Scholar]
- 14.Davis R J. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 15.de Moor C H, Richter J D. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol Cell Biol. 1997;17:6419–6426. doi: 10.1128/mcb.17.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draetta G, Luca F, Westendorf J, Brizuela L, Ruderman J, Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989;56:829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- 17.Dunphy W G, Brizuela L, Beach D, Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988;54:423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- 18.Dunphy W G, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- 19.Ferrell J J, Machleder E M. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 20.Fox C A, Sheets M D, Wickens M P. Poly(A) addition during maturation of frog oocytes: distinct nuclear and cytoplasmic activities and regulation by the sequence UUUUUAU. Genes Dev. 1989;3:2151–2162. doi: 10.1101/gad.3.12b.2151. [DOI] [PubMed] [Google Scholar]
- 21.Freeman R S, Ballantyne S M, Donoghue D J. Meiotic induction by Xenopus cyclin B is accelerated by coexpression with mosXe. Mol Cell Biol. 1991;11:1713–1717. doi: 10.1128/mcb.11.3.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman R S, Pickham K M, Kanki J P, Lee B A, Pena S V, Donoghue D J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci USA. 1989;86:5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner A M, Lange C C, Vaillancourt R R, Johnson G L. Measuring activation of kinases in mitogen-activated protein kinase regulator network. Methods Enzymol. 1994;238:258–270. doi: 10.1016/0076-6879(94)38024-4. [DOI] [PubMed] [Google Scholar]
- 24.Gautier J, Norbury C, Lohka M, Nurse P, Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+ Cell. 1988;54:433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- 25.Gautier J, Solomon M J, Booher R N, Bazan J F, Kirschner M W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 26.Gebauer F, Richter J D. Cloning and characterization of a Xenopus poly(A) polymerase. Mol Cell Biol. 1995;15:1422–1430. doi: 10.1128/mcb.15.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebauer F, Xu W, Cooper G M, Richter J D. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994;13:5712–5720. doi: 10.1002/j.1460-2075.1994.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotoh Y, Masuyama N, Dell K, Shirakabe K, Nishida E. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein cascade. J Biol Chem. 1995;270:25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- 29.Gotoh Y, Moriyama K, Matsuda S, Okumura E, Kishimoto T, Kawasaki H, Suzuki K, Yahara I, Sakai H, Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991;10:2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotoh Y, Nishida E, Matsuda S, Shiina N, Kosako H, Shiokawa K, Akiyama T, Ohta K, Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991;349:251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- 31.Haccard O, Lewellyn A, Hartley R S, Erikson E, Maller J L. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- 32.Hake L E, Richter J D. Translational regulation of maternal mRNA. Biochim Biophys Acta. 1997;1332:M31–M38. doi: 10.1016/s0304-419x(96)00039-x. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- 34.Huang C Y, Ferrell J J. Dependence of Mos-induced Cdc2 activation on MAP kinase function in a cell-free system. EMBO J. 1996;15:2169–2173. [PMC free article] [PubMed] [Google Scholar]
- 35.Kanki J P, Donoghue D J. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mos protooncogene. Proc Natl Acad Sci USA. 1991;88:5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller W, Bienroth S, Lang K M, Christofori G. Cleavage and polyadenylation factor CPF specifically interacts with the pre-mRNA 3′ processing signal AAUAAA. EMBO J. 1991;10:4241–4249. doi: 10.1002/j.1460-2075.1991.tb05002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuge H, Richter J D. Cytoplasmic 3′ poly(A) addition induces 5′ cap ribose methylation: implications for translational control of maternal mRNA. EMBO J. 1995;14:6301–6310. doi: 10.1002/j.1460-2075.1995.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumagai A, Dunphy W G. Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumagai A, Dunphy W G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald P M, Smibert C A. Translational regulation of maternal mRNAs. Curr Opin Genet Dev. 1996;6:403–407. doi: 10.1016/s0959-437x(96)80060-8. [DOI] [PubMed] [Google Scholar]
- 41.MacNicol A M, Muslin A J, Howard E L, Kikuchi A, MacNicol M C, Williams L T. Regulation of Raf-1-dependent signaling during early Xenopus development. Mol Cell Biol. 1995;15:6686–6693. doi: 10.1128/mcb.15.12.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacNicol A M, Muslin A J, Williams L T. Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell. 1993;73:571–583. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- 43.MacNicol M C, Pott D, MacNicol A M. pXen, a utility vector for the expression and purification of GST-fusion proteins in Xenopus oocytes and embryos. Gene. 1997;196:25–29. doi: 10.1016/s0378-1119(97)00171-6. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda S, Kosako H, Takenaka K, Moriyama K, Sakai H, Akiyama T, Gotoh Y, Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992;11:973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matten W T, Copeland T D, Ahn N G, Vande Woude G F. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179:485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 46.McGrew L L, Dworkin R E, Dworkin M B, Richter J D. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989;3:803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- 47.McGrew L L, Richter J D. Translational control by cytoplasmic polyadenylation during Xenopus oocyte maturation: characterization of cis and trans elements and regulation by cyclin/MPF. EMBO J. 1990;9:3743–3751. doi: 10.1002/j.1460-2075.1990.tb07587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melton D A, Krieg P A, Rebagliati M R, Maniatis T, Zinn K, Green M R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercer J F, Wake S A. An analysis of the rate of metallothionein mRNA poly(A)-shortening using RNA blot hybridization. Nucleic Acids Res. 1985;13:7929–7943. doi: 10.1093/nar/13.22.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mourey R J, Vega Q C, Campbell J S, Wenderoth M P, Hauschka S D, Krebs E G, Dixon J E. A novel cytoplasmic dual specificity protein tyrosine phosphatase implicated in muscle and neuronal differentiation. J Biol Chem. 1996;271:3795–3802. doi: 10.1074/jbc.271.7.3795. [DOI] [PubMed] [Google Scholar]
- 51.Mueller P R, Coleman T R, Dunphy W G. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthy K G, Manley J L. The 160-kD subunit of human cleavage-polyadenylation specificity factor coordinates pre-mRNA 3′-end formation. Genes Dev. 1995;9:2672–2683. doi: 10.1101/gad.9.21.2672. [DOI] [PubMed] [Google Scholar]
- 53.Murthy K G, Manley J L. Characterization of the multisubunit cleavage-polyadenylation specificity factor from calf thymus. J Biol Chem. 1992;267:14804–14811. [PubMed] [Google Scholar]
- 54.Muslin A J, MacNicol A M, Williams L T. Raf-1 protein kinase is important for progesterone-induced Xenopus oocyte maturation and acts downstream of mos. Mol Cell Biol. 1993;13:4197–4202. doi: 10.1128/mcb.13.7.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nebreda A R, Gannon J V, Hunt T. Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 1995;14:5597–5607. doi: 10.1002/j.1460-2075.1995.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer A, Gavin A-C, Nebreda A R. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paris J, Philippe M. Poly(A) metabolism and polysomal recruitment of maternal mRNAs during early Xenopus development. Dev Biol. 1990;140:221–224. doi: 10.1016/0012-1606(90)90070-y. [DOI] [PubMed] [Google Scholar]
- 58.Paris J, Swenson K, Piwnica W H, Richter J D. Maturation-specific polyadenylation: in vitro activation by p34cdc2 and phosphorylation of a 58-kD CPE-binding protein. Genes Dev. 1991;5:1697–1708. doi: 10.1101/gad.5.9.1697. [DOI] [PubMed] [Google Scholar]
- 59.Pause A, Belsham G J, Gingras A C, Donze O, Lin T A, Lawrence J J, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 60.Posada J, Sanghera J, Pelech S, Aebersold R, Cooper J A. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991;11:2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posada J, Yew N, Ahn N G, Vande Woude G, Cooper J A. Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian Y-W, Erikson E, Li C, Maller J L. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richter J D. Dynamics of poly(A) addition and removal during development. In: Hershey J, Mathews M, Sonenberg N, editors. Translational control. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 481–503. [Google Scholar]
- 64.Roy L M, Haccard O, Izumi T, Lattes B G, Lewellyn A L, Maller J L. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12:2203–2211. [PubMed] [Google Scholar]
- 65.Sagata N, Daar I, Oskarsson M, Showalter S D, Vande Woude G. The product of the mos proto-oncogene as a candidate “initiator” for oocyte maturation. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- 66.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude G. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 67.Sagata N, Watanabe N, Vande Woude G, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 68.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 69.Sheets M D, Fox C A, Hunt T, Vande Woude G, Wickens M. The 3′-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994;8:926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- 70.Sheets M D, Wu M, Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995;374:511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- 71.Shibuya E K, Morris J, Rapp U R, Ruderman J V. Activation of the Xenopus oocyte mitogen-activated protein kinase pathway by Mos is independent of Raf. Cell Growth Differ. 1996;7:235–241. [PubMed] [Google Scholar]
- 72.Shibuya E K, Polverino A J, Chang E, Wigler M, Ruderman J V. Oncogenic ras triggers the activation of 42-kDa mitogen-activated protein kinase in extracts of quiescent Xenopus oocytes. Proc Natl Acad Sci USA. 1992;89:9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith L D. The induction of oocyte maturation: transmembrane signaling events and regulation of the cell cycle. Development. 1989;107:685–699. doi: 10.1242/dev.107.4.685. [DOI] [PubMed] [Google Scholar]
- 74.Stebbins-Boaz B, Hake L E, Richter J D. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 1996;15:2582–2592. [PMC free article] [PubMed] [Google Scholar]
- 75.Stebbins-Boaz B, Richter J D. Multiple sequence elements and a maternal mRNA product control cdk2 RNA polyadenylation and translation during early Xenopus development. Mol Cell Biol. 1994;14:5870–5880. doi: 10.1128/mcb.14.9.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stebbins-Boaz B, Richter J D. Translational control during early development. Crit Rev Eukaryot Gene Expression. 1997;7:73–94. doi: 10.1615/critreveukargeneexpr.v7.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 77.Sun H, Charles C H, Lau L F, Tonks N K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 78.Thuresson A C, Astrom J, Astrom A, Gronvik K O, Virtanen A. Multiple forms of poly(A) polymerases in human cells. Proc Natl Acad Sci USA. 1994;91:979–983. doi: 10.1073/pnas.91.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.VanRenterghem B, Gibbs J B, Maller J L. Reconstitution of p21ras-dependent and -independent mitogen-activated protein kinase activation in a cell-free system. J Biol Chem. 1993;268:19935–19938. [PubMed] [Google Scholar]
- 80.Vassalli J D, Huarte J, Belin D, Gubler P, Vassalli A, O’Connell M L, Parton L A, Rickles R J, Strickland S. Regulated polyadenylation controls mRNA translation during meiotic maturation of mouse oocytes. Genes Dev. 1989;3:2163–2171. doi: 10.1101/gad.3.12b.2163. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe N, Vande Woude G, Ikawa Y, Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989;342:505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- 82.Wickens M, Kimble J, Strickland S. Translational control of developmental decisions. In: Hershey J, Mathews M, Sonenberg N, editors. Translational control. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 411–450. [Google Scholar]