Abstract
The clinical outcome of patients with human epidermal growth factor receptor 2 (HER2) amplified breast carcinoma (BC) has improved with the development of anti-HER2 targeted therapies. However, patients can experience disease recurrence after curative intent and disease progression in the metastatic setting. In the current era of evolving immunotherapy agents, the understanding of the immune response against HER2 tumor cells developed by anti-HER2 antibodies (Abs) is rapidly evolving. Trastuzumab therapy promotes Natural Killer (NK) cell activation in patients with BC overexpressing HER2, indicating that the efficacy of short-term trastuzumab monotherapy, albeit direct inhibition of HER, could also be related with antibody-dependent cell-mediated cytotoxicity (ADCC). Currently, dual HER2 blockade using trastuzumab and pertuzumab is the standard of care in early and advanced disease as this combination could confer an additive effect in ADCC. In patients with disease relapse or progression, ADCC may be hampered by several factors such as FcγRIIIa polymorphism and an immunosuppressive environment, among others. Hence, new drug development strategies are being investigated aiming to boost the ADCC response triggered by anti-HER2 therapy. In this review, we summarize these strategies and the rationale, through mAbs engineering and combinatorial strategies, focusing on clinical results and ongoing trials.
Keywords: Anti-HER2 antibodies, Trastuzumab, ADCC, Margetuximab, NK cells
Highlights
-
•
Efficacy of short-term trastuzumab monotherapy could also be related with ADCC.
-
•
ADCC involves the activation of NK cells by Abs that bind to FcRs.
-
•
Different strategies to augment NK cell activation may be combined to enhance ADCC.
-
•
Margetuximab, an Fc optimized antiHER2 Ab, has a significant improvement in PFS.
-
•
Engineered mAbs are the most promising tools for tailored therapy in cancer treatment.
Abbreviations
- BC
breast carcinoma
- HER2
human epidermal growth factor receptor 2
- NK
Natural Killer
- Abs
antibodies
- mAb
monoclonal antibody
- bsAb
bispecific antibodies
- ADCC
antibody-dependent cell-mediated cytotoxicity
- FcRs
Fc receptors
- Ig
immunoglobulin
- ADCR
antibody-dependent cytokine release
- PBMC
peripheral blood mononuclear cells
- Ko
knockout
- pCR
pathological complete response
- ORR
objective response rate
- PFS
progression-free survival
- DFS
disease-free survival
- OS
overall survival
- APC
Antigen-presenting cells
- DCs
dendritic cells
- TAA
tumor antigens
- MHC
major histocompatibility complex
- KIRs
(Ig)-like receptors
- TILs
tumor-infiltrating lymphocytes
- DLTs
dose limiting toxicities
- IL:
Interleukin
- IL.2Rβ
IL-2 receptor beta
- Treg
regulatory T cells
- PEG
pegylated
- TNBC
triple negative breast cancer
- FAP
fibroblast activation protein-alpha
- IFN-γ:
Interferon gamma
- TKI
Tirosin kinase inhibitors
- CB
Clinical benefit
- Pts
patients
- GM-CSF
Granulocyte Macrophage Colony-Stimulating Factor
1. Introduction
HER2 amplification is found in 15–20% of tumors from patients with breast cancer (BC) and is related to more aggressive disease and worse prognosis. However, since the application of therapy directed against this receptor, the clinical panorama of patients has changed impressively, notably improving survival outcomes. Trastuzumab, the first approved monoclonal antibody (mAb) against the HER2 receptor in this tumor subtype, induces cell death through direct inhibition of HER2 signaling and may also invigorate the immune system to induce anti-tumor immune responses. Pertuzumab is a mAb that binds a different epitope of the HER2 extracellular domain and in combination with trastuzumab, enhances tumor cell death, conferring a significant improvement in the clinical outcomes of patients and preventing the emergence of resistance to anti-HER2 blockade.
In recent years, novel therapeutic agents have been and are currently in development, which, in addition to inhibiting proliferative pathways, contribute to increasing immunological activation and potentially long-term disease control. Given the evolving landscape of immunotherapy agents for cancer treatment, understanding the role of mAbs directed against tumor oncogenic drivers and how this can modulate different immune-cell is crucial. This review focuses on the immunological mechanisms of Ab mediated cellular cytotoxicity in HER2+ BC induced by anti-HER2 therapies, and the ongoing strategies in drug development and combinatorial therapies in this setting.
1.1. The ADCC mechanism
1.1.1. -Fc receptors (FcRs) in NK cells and other immune populations
Antibody-dependent cellular cytotoxicity (ADCC) is a cell-mediated immune response by which immune cells provoke cell death when specific antibodies (Abs) are attached to the cell membrane [1]. It is one of several mechanisms by which Abs, a major aspect of the humoral immune reaction, can confine and contain an infection [2]. Typically, ADCC involves the activation of Natural Killer (NK) cells by Abs that bind to FcRs. FcR receptors bind to the Fc part of an Ab. The most characterized FcR on the NK cell membrane is CD16 or FcƴRIII. Once the FcR binds to the Fc fragment of an IgG, NK cells release different cytotoxic molecules that provoke the death of the target cell [3]. ADCC can be induced by the infusion of therapeutic monoclonal Abs (mAbs) directed against specific antigens. Using this strategy, mAbs bind to cancer cells and are eventually linked to effector cells (leukocytes) through their FcƴRs [4]. The FcƴRs are composed of three distinct classes: FcγRI (CD64), FcγRII (CD32) FcRIIa and FcRIIb, and FcγRIII (CD16) FcRIIIa and FcRIIIb [5].
FcγRI is a high-affinity receptor that can bind to monomeric IgG. FcγRII and FcγRIII exhibit lower affinity with monomeric molecules and interact adequately with multimeric immune complexes. FcRIIbs induce inhibitory signals on monocytes/macrophages and polymorphonucleates. Conversely, FcγRIIa and FcγRIIIa are activating FcRs expressed on monocytes, macrophages, and NK cells. Albeit that most immune cells coexpress activating and inhibitory FcγRs, NK cells are unique as they solely constitutively express the activating low-affinity FcγRIIIa.
In another hand, multiple studies implicate the monocyte network as effector cells for Ab therapeutics. Specific FcγR or common γ-chain knockout mice support NK cells and the monocyte network in Ab-mediated effector function against tumors. Vermi et al. (2018) found in diffuse large B-cell lymphoma patients that peripheral blood slan+ monocytes, but not CD14+ monocytes, displayed highly efficient Rituximab-ADCC, almost equivalent to that exerted by NK cells [6]. Experiments performed in knockout mice support NK cells and the monocyte network in ab-mediated effector function against tumors, in particular in trastuzumab-mediated ADCC [7].
1.1.2. -Trastuzumab-mediated ADCC
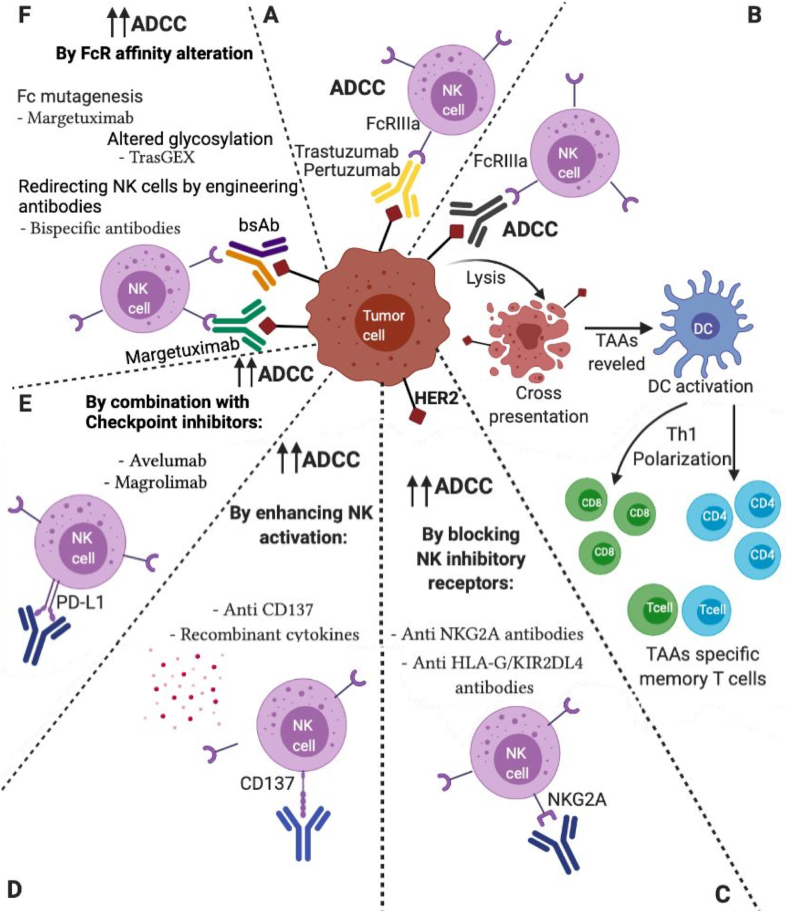
The role of ADCC as a therapeutic effect of various mAbs has been tested in preclinical models and validated in patients, and is currently known to be one of the main mechanisms that contribute to the effect of rituximab (anti-CD20), cetuximab (anti-EGFR), and trastuzumab, amongst others [8]. Along with the release of cytotoxic granules such as granzymes and perforins, NK cells liberate proinflammatory cytokines such as IFN-γ and TNF-α during ADCC, a mechanism known as Ab-dependent cytokine release (ADCR) [9]. Trastuzumab therapy promotes NK cell activation in patients with BC overexpressing HER2, indicating that the efficacy of trastuzumab alone is connected to ADCC mechanisms. The interaction between NK cells and tumor cells mediated by anti-HER2 abs, and the proinflammatory environment induced by ADCC can modulate other immune-cell populations, resulting in a “vaccination effect” by the therapeutic Abs [10,11] (Fig. 1).
Fig. 1.
Schematic diagram of different therapeutic approaches targeting HER2+ tumor cells. A) Trastuzumab/Pertuzumab therapy promotes ADCC. B) Vaccine effect of Anti-HER2 Antibodies. Strategies to potentiate anti-HER2 ADCC: C) By blocking NK inhibitory receptors. D) By enhancing NK activation. E) By combination with Checkpoints inhibitors F) By FcR affinity alteration. Created with BioRender.com. NK cell: Natural Killer cell; ADDC: Antibody-dependent cellular cytotoxicity; TAAs: Tumor-associated antigens; HER2bsAb: HER2 Bispecific Antibodies.
Given that activation of HER2 signaling requires receptor homodimerization or heterodimerization with other members of the HER family of receptors, a second anti-HER2 Ab pertuzumab was developed to prevented ligand-induced dimerization of HER2 [12]. The combination of pertuzumab and trastuzumab conveyed a synergistic induction of tumor regression in HER2 amplified BC xenograft models. In vitro evaluation of ADCC showed that both trastuzumab and pertuzumab, applied as single agents, effectively activated ADCC with the same potency. However, this study did not observe an increase in ADCC when both agents were used in combination [13]. More recently, in vivo studies showed that a combination of trastuzumab and pertuzumab increased migration of NK cells to tumors delaying trastuzumab resistance in BC xenograft models. Compared to monotherapy, the combination of both agents conferred an additive effect in ADCC at sub-saturation doses. In vitro experiments using trastuzumab-sensitive cells also supported the additive effect of this combination which could translate into enhanced ADCC in patients, with the expected clinical benefit [14]. Ab drug conjugates based on trastuzumab structure (trastuzumab-emtansine or trastuzumab-deruxtecan) retain the mechanisms of action of unconjugated trastuzumab, including ADCC activity [15,16].
1.2. FcƴR polymorphism in early-stage and metastatic HER2 positive breast cancer
FcƴRIIIa gene encodes two variants that differ at position 158, either a Val (V158) or a Phe (F158) [17]. This genetic polymorphism greatly influences the affinity of IgG1 to the Fcƴ receptor [18,19]. The V/V and V/F haplotypes are associated with a higher affinity of IgG1 with FcγR and consequently enhanced capacity to perform ADCC. The association between FcγRIIIa polymorphism and the therapeutic efficacy of monoclonal Abs has been tested in various models. Patients with the F/V and V/F genotypes have a better clinical response when treated with rituximab and cetuximab, respectively. However, there are controversial data regarding association with FcγRIIIa polymorphism and trastuzumab activity [[20], [21], [22], [23], [24], [25]].
2. Strategies to potentiate anti-HER ADCC
The activity of NK cells is known to be handled by a balance of signals generated from the cell surface inhibitor and activator receptors. Different strategies to augment NK cell activation may be combined to enhance their ADCC properties. Since NK cells can also trigger other immune processes through the release of cytokines, they provide a link to initiate additional immune responses. Several observations support the rationale for combinatorial approaches with cytokines, immunotherapy and Abs targeting NK cell receptors or co-receptors with activating and inhibitory functions (Fig. 1).
2.1. Enhancing ADCC
2.1.1. Blocking inhibitory signals of NK cells
Unlike T cells, NK cell recognition is not regulated by high-resolution antigen specificity but is mediated by signals conveyed through numerous activating and inhibitory receptors. This balance determines the NK cell activation or inhibition. NK cells have an intricate arrangement of inhibitory receptors. Killer immunoglobulin (Ig)-like receptors (KIRs), recognize different allelic groups of HLA-A/B/C molecules [26,27], whereas CD94-NKG2A recognizes HLA-E [28], a non-polymorphic molecule belonging to HLA-Ib (thoroughly reviewed in Ref. [29]). NK cells sense major histocompatibility complex (MHC) class I molecules by superficial receptors that promote NK inactivation. Hence, loss of MHC-I molecules and antigen presentation, which often occurs in cancer cells, induce NK cell-mediated lysis of target cells [26].
-
i)
Anti NKG2A antibodies
Monalizumab (IPH2201) is a first-in-class immune checkpoint inhibitor targeting NKG2A receptors expressed on NK cells and tumor infiltrating cytotoxic CD8+ T cells. Monalizumab may reinstate a broad anti-tumor response mediated by these cells, blocking the interaction between NKG2A and HLA-E [30], frequently overexpressed in several malignant tumors [[31], [32], [33]]. Consequently, it enhances the cytotoxic potential of other therapeutic Abs. Monalizumab can act simultaneously on T and NK cells, also acting on tumor-infiltrating immune cells. Given the encouraging results of the combination of this Ab with cetuximab [34], the MIMOSA phase II clinical trial is assessing the efficacy of combining monalizumab and trastuzumab in patients with metastatic or locally incurable HER2 positive BC.
-
ii)
Anti HLA-G/KIR2DL4 blockade
The HLA-G expression in tumor cells has been identified as a pivotal mediator of BC resistance to trastuzumab. Unless engaged by HLA-G, KIR2DL4 promotes ADCC and forms a regulatory circuit with the IFN-γ production pathway, in which IFN-γ upregulates KIR2DL4 via JAK2/STAT1 signaling, and then KIR2DL4 synergizes with the Fcγ receptor to increase IFN-γ secretion by NK cells. The effect of blocking the HLA-G/KIR2DL4 interaction improves the vulnerability of HER2-positive BC to trastuzumab treatment in vivo [35].
2.1.2. Targeting NK cell activation
-
i)
CD137/4-1BB
4-1BB, also known as CD137, is a costimulatory immune receptor, a member of the TNF receptor superfamily, predominantly expressed on activated CD4+ and CD8+ T cells, activated B cells, and NK cells [36,37]. 4-1BB plays an important role in immune response modulation and is considered a promising target for cancer immunotherapy given that it is expressed on TILs. Subsequent to pathway activation, it promotes enhanced proliferation, cytokine production and cytolytic activity of T and NK cells [36,38].
Utomilumab is a fully humanized IgG2 mAb that binds 4-1BB, impeding attachment to endogenous 4-1BBL [39]. A trial assessing the dosing and safety of utomilumab with trastuzumab emtansine or trastuzumab in patients with HER2 positive BC (NCT03364348) is ongoing. Its combination with other drugs is also being evaluated in the AVIATOR study (NCT03414658) studying the combination of trastuzumab and vinorelbine with avelumab or avelumab and utomilumab in Advanced HER2+ BC.
-
ii)
Recombinant cytokines
NK cells have a repertoire of cytokine receptors that contribute to their development, homeostasis and function [40]. ADCC may also be intensified by modulating NK cells through recombinant cytokines.
- IL-2
Interleukin-2 is an immune-stimulatory cytokine shown to enhance NK cell responses [41,42]. Administration of subcutaneous IL-2 increases the absolute number of circulating NK cells by approximately 10-fold and heightens their activation against several BC targets [43]. However, the nonspecific nature of this immune activation has not proven a clinical benefit so far. Another study showed that NK cells can be safely augmented in vivo with outpatient IL-2 therapy and that trastuzumab increases NK-cell killing of BC targets in a HER2-specific manner. Nevertheless, no correlation between biological endpoints, including NK cell expansion or degree of ADCC activity and clinical response or toxicity was observed [44,45].
Bempegaldesleukin (NKTR-214) is a CD122-preferential IL-2 pathway agonist designed to trigger T cells and NK cell populations by targeting CD122 specific receptors. CD122, also known as the IL-2 receptor beta (IL.2Rβ) subunit, is a crucial signaling receptor known to increase the expansion of these effector cells. A first-in-human multicenter phase I study including 2 patients with BC provided insights of clinical activity including tumor reduction and sustained disease stabilization in heavily pretreated patients [46]. A study combining NKTR-214 with the anti PD-1 Ab nivolumab in patients with metastatic triple negative BC (TNBC) showed encouraging results [47], but data on patients with HER2 positive BC is still lacking.
RO6874281 is a recombinant fusion protein composed of a human mAb directed against fibroblast activation protein-alpha (FAP) coupled to an engineered variant form of IL-2 (IL-2v) with potential immunostimulating and antineoplastic activities. FAP is a cell surface protein expressed on a wide variety of cancer cells. Upon administration of RO6874281, the mAb moiety recognizes and attaches to FAP, thereby focalizing IL-2 in FAP-expressing tumor tissue. Subsequently, the IL-2 moiety of this fusion protein may activate a local immune response and stimulate NK cells. IL-2v cannot bind to IL-2 Rα and does not activate regulatory Tregs. The BP29842 study is evaluating the safety, tolerability and early signs of activity of RO6874281 in combination with trastuzumab in patients with HER2 positive metastatic or recurrent BC following pretreatment with obinutuzumab. RO6874281 is currently being investigated as a single agent in patients with advanced and/or metastatic solid tumors in part A of the study. Preliminary results from part A show, as expected when avoiding IL-2Rα binding, rapid expansion of effector T and NK cells but not Tregs, in both blood and tumor samples [48].
- IL-12
Interleukin-12 (IL-12) was first named NK cell stimulating factor and is essentially produced by APC cells, such as DCs, monocytes and macrophages [49]. It acts by increasing the production of IFN-γ, the most potent mediator of IL-12 actions in NK and T cells; stimulating maturation and cytotoxicity of activated NK cells, CD8+ and CD4+ T cells; and enhancing ADCC against tumor cells [[50], [51], [52]]. Low-dose IL-12 can decrease the fluorescence intensity of CD56 and prompt the expansion of more mature CD56dimCD16+ and CD56dimKIR+ NK cells [53]. Previous studies showed that human NK cells, costimulated with trastuzumab-coated tumor cells and IL-12, secreted 10-fold higher amounts of IFN-γ compared to NK cells stimulated with either agent alone [50]. In a phase I trial, trastuzumab was administered with IL-12 in patients with HER2+ tumors and positive clinical outcomes were associated with NK cell production of IFN-γ [54]. A follow-up phase I trial of trastuzumab, IL-12 and paclitaxel confirmed those results [55]. In contrast, clinical outcomes did not correlate with ADCC in patients enrolled in these studies. This finding might reveal the inability of in vitro assays to seize the true extent of NK cell cytotoxic activity taking place in the tumor microenvironment in human subjects. Despite high expectations, initial IL-12 clinical studies did not grant sufficient results. IL-12 injections after initial stimulation with IFN-γ led to an adaptive response and gradual decline of IL-12 IFN-γ induction [56,57].
- IL-15
Interleukin-15 (IL-15) presents a stimulatory function similar to IL-2 and is critical for NK cell development and role [58]. The β and γ chain receptor subunits are shared by IL-15 and IL-2, differing only in the α chain. However, IL-15Rα alone binds to IL-15 with affinity akin to that of the binding of IL-2Rαβγ to IL-2 [59]. This strong affinity allows NK cell activation at relatively low concentrations. After this interaction, NK cells are sensitized to secondary stimuli, known as “priming”, resulting in magnified responses [60]. IL-15 boosts NK cells to be fully prepared to respond through fast production of granzymes and perforin [61] and NK cell cytolytic capabilities are retained after cytokine withdrawal [62]. Concurrent IL-15 administration with trastuzumab elicited immune response in mice models that derived in tumor eradication and prevention of the development of metastasis, stressing the significance of immunosurveillance as a decisive mechanism for efficient tumor cell eradication, especially during therapeutic trastuzumab treatment [63]. NKTR-255 is a polymer-conjugated human IL-15 designed to activate the IL-15 pathway, expand NK cells and promote the survival and development of memory CD8+ T cells without Treg induction. Through engagement of the IL-15Rα/IL-2Rβγ receptor complex, it intensifies the formation of long-term immunological memory leading to sustained anti-tumor immune response. NKTR-255 overcomes the challenges faced with recombinant IL-15, rapid clearance and frequent high dosing administration, which limits its utility due to toxicity and logistics. NKTR-255 enhances trastuzumab tumor growth inhibition activity in human tumor xenograft models in mice, showing that it may potentially be applied in certain cancer therapies to enhance the ADCC-dependent therapeutic activity of tumor-targeting mAbs in solid tumors [64]. Although there are no clinical trials with this compound including patients with BC, the preclinical evidence may prompt a future development for HER2+ BC.
ALT-803 (N803) is an IL-15 superagonist comprising an IL-15Ra fused to IgG1Fc bound to IL-15 mutein (N72D). It was designed to simulate the typical trans-presentation of IL-15 with a prolonged half-life (25 h vs. < 40 min), with the intention to activate ADCC [65]. In a phase 1 trial, total lymphocyte and CD8+ T cell expansion were discreet with this drug; however, NK cell counts were increased substantially [66]. This data, together with compelling evidence of synergy in preclinical and clinical studies provide the rationale for combining it with agents already available in clinical practice, such as anti-HER2 Abs ± Avelumab.
-
iii)Combination with Checkpoint inhibitors
- Avelumab
Avelumab is a fully human IgG1 anti PD-L1 mAb able to trigger ADCC. The JAVELIN Solid Tumor phase I trial tested avelumab as monotherapy in patients with heavily pretreated metastatic BC showing a low ORR of 3.0% [67]. This response was higher in patients whose tumors had infiltrating immune cells expressing PD-L1+ with a 10% staining cutoff (ORR 16.7% versus 1.6%) and were surprisingly long lasting in a subset of TNBC patients. Nevertheless, no responses were observed among patients with HER2 positive metastatic BC treated with avelumab (n: 26). Unlike other anti-PD-L1 Abs, avelumab retains its Fc fraction and capacity to induce ADCC through binding CD16. This mechanism has been demonstrated in several human tumor cell lines in vitro [68], specifically in BC cell lines. Juliá and col. Showed that this effect was associated with PD-L1 expression using 5 TNBC cell lines [69]. The mechanism underlying this intrinsic resistance of immune cells to avelumab mediated ADCC is unknown but may be related to IFN-γ induced MHC-I on the lymphocyte surface, a known negative regulator of NK cell function [68]. As described previously, the AVIATOR study (NCT03414658) evaluating trastuzumab and Vinorelbine with avelumab or avelumab plus utomilumab will provide more information on the role of avelumab in advanced HER2 positive BC.
- Magrolimab
Magrolimab is a humanized mAb that blocks CD47 interaction with signal regulatory protein-α (SIRPα), thereby diminishing the inhibition of macrophages by cancer cells [70]. When Hu5F9-G4 (magrolimab) blocks the CD47 “don't eat me” signal, it facilitates macrophage-mediated phagocytosis. A very recent preclinical study demonstrates that the combination of magrolimab and trastuzumab eliminated HER2+ BC cells with increased efficacy due to the enhancement of Ab-dependent cellular phagocytosis by macrophages [71].
-
iv)
Enhancing ADCC by FcγR affinity alteration
Worldwide, FcγRIII 158 V homozygotes are found in 10–20% and FcγRIIa 131H homozygotes in 25% of Caucasians and Africans and 50–60% of Asian populations [72,73]. Most responsive FcγR genotypes occur in a minor fraction of the population. Therefore, research efforts should be focused on ADCC enhancement through drug development of new anti-HER2 compounds or future combinations of anti-HER2 mAbs and Tyrosine kinase inhibitors (TKI). This could improve the interaction of trastuzumab with low-binding alleles of activating FcγRs and increased affinity for both isoforms of FcγRIIIa. One of the proposed strategies is by modifying the Fc domain to expand anti-HER2 response, potentially benefiting patients’ outcomes regardless of FcγR genotype [74].
- Fc Mutagenesis
- Margetuximab development
Margetuximab is a mAb derived from 4D5, the precursor in which trastuzumab was developed. Margetuximab (MGAH22) binds to the same HER2 epitope as trastuzumab with equal affinity and exhibits a similar anti-proliferative activity as trastuzumab in vitro [75]. Using engineering technology platforms, five amino acids were modified into the margetuximab IgG1 Fc domain. These changes increased the binding of this new compound with activating isoforms of FcγRIIIa and reduced its interaction with inhibitory FcγRIIb.
In xenograft studies, Margetuximab demonstrated increased activity against HER2 expressing tumors in transgenic mice with the human FcγRIIIa 158F, the low-binding allele. These studies were conducted in wild type murine FcγR, in mice lacking murine FcγRIII or in mice lacking murine FcγRIII but transgenic for human FcγRIIIa 158F [75]. Therefore, MGAH22 may also be active in low HER2 expressing tumors. Furthermore, in transgenic mice expressing the lower affinity human FcγRIIIa 158F, margetuximab exerted activity in JIMT-1 cells, a cell line resistant to growth inhibition by anti-HER2 Abs like trastuzumab [76].
Recently, the results of the open-label phase III trial of margetuximab were presented [77]. SOPHIA trial (NCT02492711) enrolled 538 patients with advanced HER2 positive metastatic BC and randomly assigned in a 1:1 fashion to margetuximab (15 mg/kg intravenously every 3 weeks) plus chemotherapy or trastuzumab (6 mg/kg [8-mg/kg loading dose]) plus chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) given every 3 weeks. All patients had received trastuzumab and pertuzumab, and over 90% had also received T-DM1. Most patients had received a taxane, more than 40% had received an anthracycline, and almost half had received an endocrine agent. Margetuximab improved primary PFS over trastuzumab with 24% relative risk reduction (p = 0.03) with a median PFS of 5.8 vs 4.9 months. ORR was higher with margetuximab: 25.2% vs 13.7%, improving the clinical benefit rate from 35.6% with trastuzumab to 48.1% with margetuximab (p = 0.0025). The median duration of response was similar in the two arms. In the planned exploratory analysis by FcRIII genotype, the benefit was enhanced in patients with low-affinity FcγIIIa genotypes containing a 158F allele, in which disease progression was reduced by 32%. In addition, in this population, the ORR was higher with margetuximab/chemotherapy compared to trastuzumab/chemotherapy, 22.1% vs 16.0% (p = 0.060), as was the clinical benefit rate, 36.6% vs 24.8% (p = 0.003). The median PFS with margetuximab/chemotherapy versus trastuzumab/chemotherapy in the various allele subsets was: 6.9 vs 5.1 months (HR = 0.68; p = 0.005) for the F/F or F/V genotype, 4.8 vs 5.6 months (HR = 1.78; P = 0.110) for patients with the V/V genotype, 8.2 vs 5.6 months (HR = 0.69; p = 0.080) in the F/F genotype (n = 192) and 6.3 vs 4.3 months (HR = 0.71; p = 0.055) in the F/V genotype. In patients who were homozygous for the high-affinity VV allele, the effects of margetuximab and trastuzumab were relatively similar, but in the 85% who carried at least one F allele, the progression-free benefit was enhanced, and further enhanced in patients who were homozygous for the F allele. Median OS at the second interim analysis was prolonged by 4.3 months in the margetuximab arm for those who carried the FcIIIa 158F allele (85% of participants): 23.7 months vs 19.4 months with trastuzumab (P = 0.087). Patients who were homozygous for the FcIIIa 158 V/V allele (15% of participants) had no benefit with margetuximab. The results may have been affected by confounding factors like a higher proportion of visceral metastasis, brain metastasis and older age in patients randomly assigned to margetuximab. Safety profiles and treatment discontinuation rates were comparable in the margetuximab and trastuzumab arms [78].
- Altered glycosylation
For several different abs, it has also been reported that alterations of the glycosylation patterns can be used to increase affinity for activating FcγR to boost ADCC activity. Of the oligosaccharides attached to the Fc domain, fucose sugar units appear to play the largest role in determining binding to FcγRIIIA. These results led to the development of methods for producing mAbs that lacked fucose in their Fc region. In Ref. [79] authors deeply describe different strategies to produce afucosylated Abs.
Junttila et al. demonstrated that removing fucose from trastuzumab increased its binding to FcγRIIIa, enhanced ADCC, and more than doubled the median PFS when compared with conventional trastuzumab in treating preclinical models of HER2-amplified BC [80].
- TrasGEX
TrasGEX is a second-generation mAb of trastuzumab, glyco-optimized to enhance ADCC while fully retaining trastuzumab's antigen-binding properties to HER2. A phase I dose-escalation study was conducted to establish the optimal TrasGEX dose and regimen for phase II studies and to define the safety, pharmacokinetics (PK) and preliminary antitumor activity of TrasGEX. TrasGEX was safe, well-tolerated and showed antitumor activity in 50% of evaluable patients, all with progressive disease at study entry (NCT01409343) [79].
-
v)Redirecting NK cells by engineering antibodies
- Bispecific antibodies
Bispecific Abs (bsAbs) are engineering mAbs with two Fab arms contained within a single molecule that targets two different antigens [47,81]. BsAbs are also subclassified into two main groups: 1) IgG-like with traditional mAb structure with one Fc region and two Fabs (called trifunctional Ab) [82] or 2) non-IgG-like with lack of Fc fragment and chemically linked Fabs consisting of only the Fab regions and various types of bivalent and trivalent single-chain variable fragments or fusion proteins mimicking the variable domains of two Abs [82,83]. These Abs have the ability to selectively trigger a distinct activating FcγR with high affinity and allow recruitment of FcγR expressing effector cells redirecting to the tumor-site. These new drugs have raised great interest as powerful cancer immunotherapy strategies.
The development of bsAbs like blinatumomab and catumaxomab has been tested clinically, recruiting T cells to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia and malignant ascites, respectively [[84], [85], [86]]. Several bsAbs are being evaluated in clinical trials in the setting of HER2+ BC (Table 1) [87,88].
Table 1.
Bispecific antibodies.
| Therapeutic agents | Molecular/Immune cell targets | Immune response | Notes | Ref. |
|---|---|---|---|---|
| MCLA-128 | NK cells, monocytes, macrophages, DCs HER2, HER3 FcγR |
TCMC, ADCC, enhanced HER2 uptake by DCs | IgG-like BsAb. Phase I/II study CBR 70%, good safety profile. Ongoing phase II trial: cohort 1 MCL-128 + trastuzumab/CT in HER2+; cohort 2 MCL-128 + ET in ER+/low HER2 BC [NCT03321981] | [[89], [90], [91], [92]] |
| ZW25 | NK cells, monocytes, macrophages, DCs HER2-ECD4, HER2-ECD2 FcγRs, |
TCMC, ADCC, enhanced HER2 uptake by DCs, | IgG-like BsAb. Phase I trial in pretreated HER2+ tumors. CBR: 50% | [93,111] |
| GBR1302 | HER2, CD3/T cells | TCMC | HER2 x CD3 BITE. Ongoing phase I trial in pretreated HER2+ tumors. Most common AEs: IRR/CRS | [95] |
| p95HER2xCD3 BsAb T cell | p95HER2 CD3 T cells |
TCMC | In vitro evidence. No toxicity on normal cells |
[96] |
| Tribody [(HER2)2xCD16] | HER2, FcγRIIIa NK cells, γδ T cells, monocytes, macrophages, DCs |
ADCC enhanced HER2 uptake by DCs, TCMC | Tribody mAb modification of the HER2 single-chain variable fragment domain producing an antiHER2 scFv and IFNγ fusion protein. In vitro evidence. | [[97], [98], [99], [100]] |
| HER2bsFab | HER2-ECD4, FcγRIIIa NK cells, monocytes, macrophages, DCs |
ADCC enhanced HER2 uptake by DCs, TCMC | BsAb linking the pertuzumab Fab to an FcRIIIA single domain Ab In vitro evidence | [101] |
| Ertumaxo Mab |
HER2, CD3, FcγRs NK cells, monocytes, macrophages, DCs |
ADCC enhanced HER2 uptake by DCs, TCMC | Trifunctional bsAb. Phase I trials ORR 30%. Well tolerated, grade 1 flu syndrome | [82,102,103] |
| HER2/CD3 BsAb | CD3 T Cells HER2 |
TCMC | Fc domain silenced to avoid CRS. In vitro evidence | [104] |
| MM-111 | HER2/HER3 heterodimer, HER3 ligand |
AntiHER | bsAb fusion protein. Phase I trial of MM- 111 + trastuzumab or lapatinib. Well tolerated. CBR: 55%. Phase 2 ongoing |
[105,106] |
| BsPD-L1xrErbB2 | HER2, FcγRs, PD-L1/NK cells, monocytes, macrophages, DCs, T cells | ADCC enhanced HER2 uptake by DCs, TCMC | Mouse IgG2a bsAb. In vitro evidence. | [107] |
| HER2Bi-aATCs | HER2 CD3 T cells/macrophages |
TCMC | Mouse IgG2a Fc backbone targeting PD-L1 and rat Her2. Phase I trial CBR: 59.1%, mOS 36,2 | [108] |
| MDX-210 | HER2, FcγRI/monocytes, |
ADCC | Phase I trial. Well tolerated. 10 pts, 1 PR | [109] |
Adapted from Musolino et al. Role of Innate and Adaptive Immunity in the Efficacy of anti-HER2 Monoclonal Antibodies for HER2-positive Breast Cancer. Critical Reviews in Oncology/Hematology (2020), doi: https://doi.org/10.1016/j.critrevonc.2020.102927 [112].
Abbreviations: FcγR, Fc gamma receptor; NK, natural killer, MØ, macrophages; DCs, dendritic cell; ADCC, antibody dependent cell-mediated cytotoxicity; TCMC, T cell-mediated cytotoxicity; BC, breast cancer; Fc, fragment crystallizable; MBC, metastatic breast cancer; ORR, overall response rate; ADC, antibody-drug conjugate; SDR: stable disease rate; w/, with; w/o, without; PFS, progression-free survival; T-DM1, Trastuzumab emtansine; SAE, serious adverse event; ECD, extracellular domain; BsAb, bispecific antibody; DCR: disease control rate (percentage of patients who achieved complete response, partial response, and stable disease to a therapeutic intervention); Fab, fragment antigen-binding; CD16; FcγRIIIa; scFv, single-chain variable fragment; AE, adverse event; BiTE, bispecific T cell engager; IRR, infusion-related reaction; CRS, cytokine release syndrome; p95HER2, truncated form of HER2; SoC, standard of care; CBR, clinical benefit rate (same definition as DCR); HER2Bi-aATCs, activated T cells armed with anti-HER2 bispecific antibody.
MCLA-128 (Merus) is a bispecific humanized full-length IgG1Ab with enhanced ADCC activity developed to inhibit HER2:HER3-driven cell growth and to overcome HER3-mediated resistance (primary or acquired) and/or relapse in HER2-or EGFR-targeted therapies. This bsAb employs a ‘dock and block’ mechanism. Based on X-ray crystal structure, MCLA-128 docks to the HER2 domain I which orients the HER3 binding arm to block the HER3 domain III (putative histidine rich glycoprotein domain), thereby blocking oncogenic signaling via the HER2:HER3 heterodimer [89]. In vitro, MCLA-128 demonstrated superior preclinical activity over trastuzumab with or without pertuzumab and anti-HER3 Abs [90]. Additionally, MCLA-128 presented better ADCC enhancement in cell lines with low HER2 expression and in effector cells with low-affinity FcγRIII [91].
Preliminary results of a phase I/II study in solid tumors (NCT 02912949) were reported including eight patients with heavily pretreated metastatic BC, with three or more previous lines of anti-HER2-directed therapies [92]. With a median of 4.5 cycles of MCLA-128, 1 patient had a partial response, and five patients had stable disease. Overall, the clinical benefit rate was 70%. MCLA-128 was well tolerated and the most common adverse effects were grade 1–2, mainly infusion related reactions, diarrhea, rash, and fatigue and no cardiac toxicity was observed. A phase II study (NCT03321981) is ongoing: Cohort 1 assesses a combination of MCLA-128 with trastuzumab plus chemotherapy in HER2 positive patients; cohort 2 evaluates MCLA-128 with endocrine therapy in ER positive/low-HER2 expression.
Zanidatamab - ZW25 (Zymeworks Inc.) is an IgG-like HER2-targeted bsAb that binds to two different epitopes on the extracellular domain of HER2. By using the Azymetric bispecific platform, this IgG is transformed from monospecific Abs to bispecific Abs. ZW25 is biparatopic since it is capable of binding two HER2 epitopes: the trastuzumab binding domain ECD4 and the pertuzumab binding domain ECD2. As a result of these modifications, ZW25 shows increased tumor cell binding, blockade of ligand-dependent and independent growth and improved receptor internalization and downregulation compared to trastuzumab. It also conveys antitumor activity in HER2-low expression cell lines models [93]. In vitro studies show that ZW25 elicits concentration-dependent ADCC with maximal lysis up to 52% on TNBC cell lines expressing HER2 at a 0/1+ level. ZW25 also exhibits synergy and additivity with multiple chemotherapeutic agents including platinums, taxanes, microtubule inhibitors, and DNA synthesis inhibitors in various HER2 expressing tumor cell lines [94].
In a phase I basket trial (NCT02892123) 42 patients were enrolled. 71% had received prior antiHER2 therapy median of five HER2-targeted regimens for metastatic disease, and 20 patients had BC. Single-agent ZW25 had antitumoral activity and a good safety profile, without dose-limiting toxicity. Overall, the partial response rate was 33% (6 pts of 18) and disease control rate was 50%. The most common toxicity was grade 1–2 diarrhea and infusion reaction [93]. A study of zanidatamab with palbociclib plus fulvestrant in patients with HER2+/HR+ advanced BC is ongoing (NCT 04224272). FDA has granted breakthrough designation for this molecule for HER2 amplified biliary tract carcinoma.
GBR1302 (Glenmark Pharmaceuticals) is a HER2xCD3 bsAb developed to direct T-cells to HER2-expressing tumor cells; it binds sites to the invariant CD3 chain of the T-cell receptor and tumor associated antigens. This yields crosslinking of T-cell receptor and lymphocyte activation with antitumor activity, although on-target off-tumor effects caused by redirected lymphocytes can result in severe adverse events. GBR1302 antitumor activity was demonstrated in preclinical studies also suppressing the growth of JIMT-1 cells, a cell line insensitive to inhibition by trastuzumab. The GBR1302 dose required to induce cardiomyocytes death with normal HER2 levels was up to 1000 times greater than the dose required to lyse HER2 positive tumor cell lines. A phase 1 trial (first-in-human study) of GBR1302 monotherapy in advanced HER2 positive solid tumors with antiHer2 therapy progression is ongoing (NCT02829372). Interim study analysis provided biomarker data suggesting modulation of peripheral T-cell populations and cytokines with this agent [95].
P95HER. p95HER2 is a truncated form of HER2, a tumor antigen expressed by 40% of HER2 positive tumors; this protein is not expressed in normal tissues. P95HER2 is a T cell bsAb against p95HER2 that has demonstrated in vitro and in vivo evidence of antitumoral effect in HER2 positive BC [96].
Tribody [(HER2)2xFcRIII]. This tribody mAb enhances antitumor activity by modification of the HER2 single-chain variable fragment domain, producing an antiHER2 scFv and IFN-γ fusion protein [97]. The increased efficacy of [(HER2)2xCD16] is in part produced by an increase in immune cell degranulation. Preliminary data informed an antitumoral effect in BC xenograft models, demonstrating higher antitumor activity than the 4D5 HER2-directed Ab [98].
Tribody encompasses two HER2-specific single chain fragment variables fused to a fragment antigen binding directed to the FcγRIII antigen expressed on γδ T cells and NK cells [99]. It also demonstrated superiority in triggering γδ T cell and NK cell-mediated lysis of HER2-expressing tumor cells, such as pancreatic ductal adenocarcinoma, BC, and primary ovarian tumors [100].
BsAb, HER2(Per)-S-Fab. Her2(Per)-S-Fab bsAb activity is developed by linking the pertuzumab Fab to a FcγRIIIA single domain Ab. Preclinical evidence demonstrated potent cytotoxicity against HER2 positive tumor cells [101]; in vitro and in vivo studies also reported that this Ab may offer clinical activity in tumors with low or mild HER2 expression and could overcome tumor resistance to trastuzumab.
Ertumaxomab. This trifunctional bsAb binds HER2, CD3, FcγRI, FcγRIIA and FcγRIII. Consequently, ertumaxomab activity induced a ternary complex between lymphoid T cells, tumor cells and stromal cells [82]. This bsAb joins another epitope on the extracellular surface of HER2 (unlike trastuzumab and pertuzumab), which could be an important issue for antitumor effects against low HER2 expression cells. A phase I clinical trial with ertumaxomab in HER2 positive tumors showed a 30% antitumor response rate and safety profile [102]. Haense and col. Studied the activity of ertumaxomab in fourteen heavily pretreated patients with different tumor types. In this small study, one partial response and two stable diseases were achieved in patients. All patients had AE; however, they were transient and reversible, most frequently fatigue, headache, chills, nausea, fever, emesis and diarrhea [103].
HER2/CD3 bispecific Ab. This bsAb represents an IgG single-chain variable fragment with monovalent binding to CD3 and bivalent binding to HER2. HER2/CD3 bsAb preserved the trastuzumab antitumor effect and enhanced ADCC activity by recruiting nonspecific circulating T-cells and promoting T cell tumor infiltration. To reduce adverse events and cytokine release syndrome the Fc domain is silenced. In vitro and in vivo studies demonstrated superior antitumor activity of this compound compared to trastuzumab. Moreover, in vitro experiments demonstrated that HER2-bsAb-mediated T cell cytotoxicity was relatively insensitive to PD-L1 expression on the tumor targets or PD-1 expression on T cells [104].
MM-111. MM-111 is a bsAb fusion protein that specifically binds the HER2/HER3 heterodimer and avoids the HER-3 ligand link. In HER2 cell lines, MM-111 showed efficacy in cells with resistance to currently existing anti-HER2 therapies. In a multi-arm phase I trial including 86 patients with different tumors, the combination of MM-111 with trastuzumab or lapatinib was well tolerated. Overall, the clinical benefit rate was 55% [105]. This evidence supported the design of a phase II trial, now ongoing [106].
sPD-L1xrErbB2. This bsAb is engineered with a mouse IgG2a Fc backbone targeting PD-L1 and Her2 which lead the antitumor immune response in HER2-overexpressing tumor cells. BsPD-L1xrErbB2 also presents IFN-γ activity, enhancing ADCC effect and avoiding the PD-1/PD-L1 link, augmenting intratumor CD8+ T lymphocyte infiltration [107].
HER2Bi-aATCs. This bsAb is developed from activated T cells generated from PBMC expanded with anti-CD3 mAb and IL-2 and sheltered with a CD3xHER2 bsAb. This target T cell therapy was assessed in a phase I trial. HER2Bi-aATCs with IL-2 and granulocyte macrophage colony-stimulating factor (GM-CSF) was evaluated in 23 patients with advanced BC. The combination was well tolerated (no dose limiting adverse event) and the clinical benefit rate was 59.1%. Overall, the median OS was 36.2 months [108].
MDX-210. MDX-210 is a bsAb that concurrently links FcγRI and HER2; in this way, it activates effector cells such as monocytes and macrophages and promotes antitumor activity against overexpressing HER2 cells. MDX-210 was evaluated in a Phase Ia/lb trial in patients with advanced BC or ovarian carcinoma overexpressing HER2. This bsAb had a good safety profile; the main toxicities were fever, asthenia and low blood pressure (mostly grade 1 or 2). Among patients with evaluable disease (10 pts), one partial response and one mixed tumor response were observed [109].
3. Concluding remarks
Immunotherapy has clearly been established as a pillar of cancer treatment in recent years. Understanding the paramount role of ADCC in native and adaptive cancer immune response to mAbs will allow us to rationally combine these types of treatments in the setting of HER2 amplified BC. This can be further enhanced by cancer therapies such as chemotherapy, radiotherapy or surgery. Several other promising combination strategies aimed at boosting NK cell ADCC responses to tumors are now being developed pre-clinically and clinically, as mAb + matrix metalloproteases inhibitor, Bruton's tyrosine kinase inhibitor, etc [110]. However, none of these have been tested yet in combination with anti-HER abs, and for that reason, they are not part of this review. In this sense, engineered mAbs are the most promising tools to treat cancer. They will likely allow us to move a new step forward in the near future.
Funding
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) PICT1866-2018, Fundación Sales, Fundación Cáncer, Fundación Pedro F. Mosoteguy, Argentina. EL is a member of CONICET.
Declaration of competing interest
Authors have NO affiliations with or involvement in any organization with any financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgments
We thank Hollyday Cartar for her assistance with abstract submission.
References
- 1.Ravetch J v, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 2.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson M.J., Ritz J. Biology and clinical relevance of human natural killer cells. J Am Soc Echocardiogr. 2008;21:1–2. doi: 10.1016/j.echo.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Fanger M.W., Shen L., Graziano R.F., Guyre P.M. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989;10:92–99. doi: 10.1016/0167-5699(89)90234-X. [DOI] [PubMed] [Google Scholar]
- 5.Patel K.R., Roberts J.T., Barb A.W. Multiple variables at the leukocyte cell surface impact Fc γ receptor-dependent mechanisms. Front Immunol. 2019;10:223. doi: 10.3389/fimmu.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermi W., Micheletti A., Finotti G., Tecchio C., Calzetti F., Costa S. Slan+ monocytes and macrophages mediate CD20-dependent b-cell lymphoma elimination via ADCC and ADCP. Canc Res. 2018;78:3544–3559. doi: 10.1158/0008-5472.CAN-17-2344. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y., Fan X., Deng H., Brezski R.J., Rycyzyn M., Jordan R.E. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J Immunol. 2015;194:4379–4386. doi: 10.4049/jimmunol.1402891. [DOI] [PubMed] [Google Scholar]
- 8.Zahavi D., Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. 2020;12:1–20. doi: 10.3390/antib9030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassatella M.A., Anegón I., Cuturi M.C., Griskey P., Trinchieri G., Perussia B. Fc gamma R(CD16) interaction with ligand induces Ca2+ mobilization and phosphoinositide turnover in human natural killer cells. Role of Ca2+ in Fc gamma R(CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989;169:549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gall V.A., Philips A.V., Qiao N., Clise-Dwyer K., Perakis A.A., Zhang M. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Canc Res. 2017;77:5374–5383. doi: 10.1158/0008-5472.CAN-16-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abès R., Teillaud J.L. Modulation of tumor immunity by therapeutic monoclonal antibodies. Canc Metastasis Rev. 2011;30:111–124. doi: 10.1007/s10555-011-9282-3. [DOI] [PubMed] [Google Scholar]
- 12.Frankling M., Carey K., Vajdos F., Leahy D., de Vos A., Sliwkowski M. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Canc Cell. 2004;5:317–328. doi: 10.1177/002193470003000503. [DOI] [PubMed] [Google Scholar]
- 13.Scheuer W., Friess T., Burtscher H., Bossenmaier B., Endl J., Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Canc Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 14.Tóth G., Szöőr Á., Simon L., Yarden Y., Szöllősi J., Vereb G. The combination of trastuzumab and pertuzumab administered at approved doses may delay development of trastuzumab resistance by additively enhancing antibody-dependent cell-mediated cytotoxicity. mAbs. 2016;8:1361–1370. doi: 10.1080/19420862.2016.1204503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junttila T.T., Li G., Parsons K., Phillips G.L., Sliwkowski M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Canc Res Treat. 2011;128:347–356. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- 16.Ogitani Y., Aida T., Hagihara K., Yamaguchi J., Ishii C., Harada N. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Canc Res. 2016;22 doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 17.Lehrnbecher T., Foster C.B., Zhu S., Leitman S.F., Goldin L.R., Huppi K. Variant genotypes of the low-affinity FcgReceptors in two control populationsand a review of low-affinity FcgReceptor polymorphisms in controland disease populations. J Biol Chem. 1999;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 18.Koene B.H.R., Kleijer M., Algra J., Roos D., Kr A.E.G., Borne V. FcgRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell FcgRIIIa, independently of the FcgRIIIa-48l/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 19.Shields R.L., Namenuk A.K., Hong K., Meng Y.G., Rae J., Briggs J. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 20.Musolino A., Naldi N., Bortesi B., Pezzuolo D., Capelletti M., Missale G. Immunoglobulin g fragment c receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 21.Tamura K., Shimizu C., Hojo T., Akashi-Tanaka S., Kinoshita T., Yonemori K. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22:1302–1307. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- 22.Hurvitz S.A., Betting D.J., Stern H.M., Quinaux E., Stinson J., Seshagiri S. Analysis of Fc receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Canc Res. 2012;18:3478–3486. doi: 10.1158/1078-0432.CCR-11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton N., Olson R.M., Pegram M., Tenner K., Ballman K.V., Clynes R. Association studies of Fcγ receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Canc. Immunol. Res. 2014;2:962–969. doi: 10.1158/2326-6066.CIR-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavin P.G., Song N., Kim S.R., Lipchik C., Johnson N.L., Bandos H. Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2–positive breast cancer. JAMA Oncol. 2017;3:335. doi: 10.1001/jamaoncol.2016.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahaweni N.M., Olieslagers T.I., Rivas I.O., Molenbroeck S.J.J., Groeneweg M., Bos G.M.J. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-34258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moretta A., Bottino C., Vitale M., Pende D., Biassoni R., Mingari M.C. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 27.Lanier L.L., Cortiss B.C., Wu J., Leong C., Phillips J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 28.Lee N., Llano M., Carretero M., Ishitani A., Navarro F., Lopez-Botet, Miguel, Geraghty D.E. HLA-E is a major ligand for the natural killer inhibitory receptor. Proc Natl Acad Sci USA. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pende D., Falco M., Vitale M., Cantoni C., Vitale C., Munari E. Killer Ig-like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.André P., Denis C., Soulas C., Bourbon-Caillet C., Lopez J., Arnoux T. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–1743. doi: 10.1016/j.cell.2018.10.014. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gooden M., Lampen M., Jordanova E.S., Leffers N., Trimbos J.B. HLA-E expression by gynecological cancers restrains tumor-in fi ltrating CD8 + T lymphocytes. Proc Natl Acad Sci U S A. 2011;108:10656–10661. doi: 10.1073/pnas.1100354108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1100354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy E.M., Bianchini M., Von Euw E.M., Barrio M.M., Bravo A.I., Furman D. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol. 2008;32:633–641. doi: 10.3892/ijo.32.3.633. [DOI] [PubMed] [Google Scholar]
- 33.Yazdi M.T., Riet S van, van Schadewijk A., Fiocco M., van Hall T., Taube C. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget. 2016;7:3477–3488. doi: 10.18632/oncotarget.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen R.B., Bauman J.R., Salas S., Colevas A.D., Even C., Cupissol D. Combination of monalizumab and cetuximab in recurrent or metastatic head and neck cancer patients previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors. J Clin Oncol. 2020;38 doi: 10.1200/jco.2020.38.15_suppl.6516. 6516–6516. [DOI] [Google Scholar]
- 35.Zheng G., Guo Z., Li W., Xi W., Zuo B., Zhang R. Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer resistance to trastuzumab. Signal Transduct. Targeted Ther. 2021;6 doi: 10.1038/s41392-021-00629-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin W., Voskens C.J., Zhang X., Schindler D.G., Wood A., Burch E. Fc-dependent expression of CD137 on human NK cells : insights into “ agonistic ” effects of anti-CD137 monoclonal antibodies. Blood. 2008;112:699–707. doi: 10.1182/blood-2007-11-122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melero I., Murillo O., Dubrot J., Hervás-Stubbs S., Perez-Gracia J.L. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Melero I., Johnston J.V., Shufford W.W., Mittler R.S., Chen L. NK1 . 1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal. Antibodies. 1998;172:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 39.Fisher T.S., Kamperschroer C., Oliphant T., Love V.A., Lira P.D., Doyonnas R. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Canc Immunol Immunother. 2012;61:1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abel A.M., Yang C., Thakar M.S., Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1–23. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyman O., Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 42.Christopher S.H., Kagemasa K., Donald E.K., Steven G. IL-2 augments NK cell activity. Nature. 1981;291:335–338. [Google Scholar]
- 43.Burns L.J., Weisdorf D.J., DeFor T.E., Vesole D.H., Repka T.L., Blazar B.R. IL-2-based immunotherapy after authologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 44.Repka T., Chiorean E.G., Gay J., Herwig K.E., Kohl V.K., Yee D. Interleukln (IL) 15 is a novel cytoklne that activates human natural killer cells via components of the IL-2 receptor. Clin Canc Res. 2003;9:2440–2446. [PubMed] [Google Scholar]
- 45.Fleming G.F., Meropol N.J., Rosner G.L., Hollis D.R., Carson W.E., Caligiuri M. A Phase I trial of escalating doses of trastuzumab combined with daily subcutaneous interleukin 2: report of cancer and leukemia group B 9661. Clin Canc Res. 2002;8:3718–3727. [PubMed] [Google Scholar]
- 46.Bentebibel S.E., Hurwitz M.E., Bernatchez C., Haymaker C., Hudgens C.W., Kluger H.M. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Clin Canc Res. 2012;9:443–446. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 47.Pernas S., Tolaney S.M. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Therapeut. Adv. Med. Oncol. 2019;11:1–16. doi: 10.1177/1758835919833519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soerensen M.M., Ros W., Rodriguez-Ruiz M.E., Robbrecht D., Rohrberg K.S., Martin-Liberal J. Safety, PK/PD, and anti-tumor activity of RO6874281, an engineered variant of interleukin-2 (IL-2v) targeted to tumor-associated fibroblasts via binding to fibroblast activation protein (FAP) J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.e15155. e15155–e15155. [DOI] [Google Scholar]
- 49.Macatonia S.E., Hosken N.A., Litton M., Vieira P., Hsieh C.S., Culpepper J.A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071. [PubMed] [Google Scholar]
- 50.Parihar R., Dierksheide J., Hu Y., Carson W.E. IL-12 enhances the natural killer cell cytokine response to Ab-coated tumor cells. J Clin Invest. 2002;110:983–992. doi: 10.1172/JCI200215950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luedke E., Jaime-Ramirez A.C., Bhave N., Roda J., Choudhary M.M., Kumar B. Cetuximab therapy in head and neck cancer: immune modulation with interleukin-12 and other natural killer cell-activating cytokines. Surgery. 2012;152:431–440. doi: 10.1016/j.surg.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinchieri G., Wysocka M., D'Andrea A., Rengaraju M., Aste-Amezaga M., Kubin M. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-B. [DOI] [PubMed] [Google Scholar]
- 53.Lehmann D., Spanholtz J., Sturtzel C., Tordoir M., Schlechta B., Groenewegen D. IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential. PloS One. 2014;9 doi: 10.1371/journal.pone.0087131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roda J.M., Parihar R., Magro C., Nuovo G.J., Tridandapani S., Carson W.E. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Canc Res. 2006;66:517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 55.Bekaii-Saab T.S., Roda J.M., Guenterberg K.D., Ramaswamy B., Young D.C., Ferketich A.K. A phase I trial of paclitaxel and trastuzumab in combination with interleukin-12 in patients with HER2/neu-expressing malignancies. Mol Canc Therapeut. 2009;8:2983–2991. doi: 10.1158/1535-7163.MCT-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Portielje J.E.A., Lamers C.H.J., Kruit W.H.J., Sparreboom A., Bolhuis R.L.H., Stoter G. Repeated administrations of interleukin (IL)-12 are associated with persistently elevated plasma levels of IL-10 and declining IFN-γ, tumor necrosis factor-α, IL-6, and IL-8 responses. Clin Canc Res. 2003;9:76–83. [PubMed] [Google Scholar]
- 57.Becker J.C., Andersen M.H., Schrama D., Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Canc Immunol Immunother. 2013;62:1137–1148. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carson W.E., Giri J.G., Lindemann M.J., Linett M.L., Ahdieh M., Paxton R. 1994. Interleukln (IL) 15 is a novel cytoklne that activates human natural killer cells via components of the IL-2 receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giri J.G., Kumaki S., Ahdieh M., Friend D.J., Loomis A., Shanebeck K. Identification and cloning of a novel IL-15 binding protein that is structurally related to the α chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fehniger T.A., Cai S.F., Cao X., Bredemeyer A.J., Presti R.M., French A.R.R. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 62.Mao Y., Van Hoef V., Zhang X., Wennerberg E., Lorent J., Witt K. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood. 2016;128:1475–1489. doi: 10.1182/blood-2016-02-698027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wege A.K., Weber F., Kroemer A., Ortmann O., Nimmerjahn F., Brockhoff G. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM) Oncotarget. 2017;8:2731–2744. doi: 10.18632/oncotarget.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kivimae S., Kivimae S., Miyazaki T., Pena R., Wildaliz N., Moffett A. NKTR-255, a polymer-conjugated IL-15 receptor agonist, enhances effiency of therapeutic monoclonal antibodies with ADCC activity in solid tumor models. 34th Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2019): part 2 : national Harbor, MD, USA. 10 November 2019. J. Immunother. Canc. 2019;7:283. doi: 10.1186/s40425-019-0764-0. [DOI] [Google Scholar]
- 65.Wong H.C., Jeng E.K., Rhode P.R. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8+ T cells into innate-like effector cells with antitumor activity. OncoImmunology. 2013;2:9–12. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Margolin K., Morishima C., Velcheti V., Miller J.S., Lee S.M., Silk A.W. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Canc Res. 2018;24:5552–5561. doi: 10.1158/1078-0432.CCR-18-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dirix L.Y., Takacs I., Jerusalem G., Nikolinakos P., Arkenau H.T., Forero-Torres A. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Canc Res Treat. 2018;167:671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyerinas B., Jochems C., Fantini M., Heery C.R., Gulley J.L., Tsang K.Y. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Canc. Immunol. Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juliá E.P., Amante A., Pampena M.B., Mordoh J., Levy E.M. Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front Immunol. 2018;9:1–12. doi: 10.3389/fimmu.2018.02140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Wang L., Zhao F., Tseng S., Narayanan C., Shura L. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0137345.eCollection2015. Sep. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Upton R., Banuelos A., Feng D., Biswas T., Kao K., Mckenna K. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc Natl Acad Sci Unit States Am. 2021;118:1–8. doi: 10.1073/pnas.2026849118/-/DCSupplemental.Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sullivan K.E., Jawad A.F., Piliero L.M., Kim N., Luan X., Goldman D. Analysis of polymorphisms affecting immune complex handling in systemic lupus erythematosus. Rheumatology. 2003;42:446–452. doi: 10.1093/rheumatology/keg157. [DOI] [PubMed] [Google Scholar]
- 73.Kyogoku C., Dijstelbloem H.M., Tsuchiya N., Hatta Y., Kato H., Yamaguchi A. Fcγ receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–1254. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 74.Gianni L. The “other” signaling of trastuzumab: antibodies are immunocompetent drugs. J Clin Oncol. 2008;26:1778–1780. doi: 10.1200/JCO.2007.15.7404. [DOI] [PubMed] [Google Scholar]
- 75.Nordstrom J.L., Gorlatov S., Zhang W., Yang Y., Huang L., Burke S. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Canc Res. 2011;13:1–14. doi: 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanner M., Kapanen A.I., Junttila T., Raheem O., Grenman S., Elo J. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Canc Therapeut. 2004;3:1585–1592. [PubMed] [Google Scholar]
- 77.Rugo H.S., Im S.-A., Wright G.L.S., Escriva-de-Romani S., DeLaurentiis M., Cortes J. SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx) J Clin Oncol. 2019;37:1000. doi: 10.1200/JCO.2019.37.15_suppl.1000. [DOI] [Google Scholar]
- 78.Rugo H.S., Im S.A., Cardoso F., Cortés J., Curigliano G., Musolino A. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira N.A., Chan K.F., Lin P.C., Song Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs. 2018;10:693–711. doi: 10.1080/19420862.2018.1466767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Junttila T.T., Parsons K., Olsson C., Lu Y., Xin Y., Theriault J. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Canc Res. 2010;70:4481–4489. doi: 10.1158/0008-5472.CAN-09-3704. [DOI] [PubMed] [Google Scholar]
- 81.Gligorov J., Richard S., Todorovic V. New anti-HER2 agents: from second-generation tyrosine kinases inhibitors to bifunctional antibodies. Curr Opin Oncol. 2017;29:405–410. doi: 10.1097/CCO.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 82.Yu S., Liu Q., Han X., Qin S., Zhao W., Li A. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:1–15. doi: 10.1186/s40164-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buie L.W., Pecoraro J.J., Horvat T.Z., Daley R.J. Blinatumomab: a first-in-class bispecific T-cell engager for precursor B-cell acute lymphoblastic leukemia. Ann Pharmacother. 2015;49:1057–1067. doi: 10.1177/1060028015588555. [DOI] [PubMed] [Google Scholar]
- 84.Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 85.Topp M.S., Gökbuget N., Zugmaier G., Klappers P., Stelljes M., Neumann S. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 86.Jäger M., Schoberth A., Ruf P., Hess J., Hennig M., Schmalfeldt B. Immunomonitoring results of a phase II/III study of malignant ascites patients treated with the trifunctional antibody catumaxomab (Anti-EpCAM x anti-CD3) Canc Res. 2012;72:24–32. doi: 10.1158/0008-5472.CAN-11-2235. [DOI] [PubMed] [Google Scholar]
- 87.Ishiguro T., Sano Y., Komatsu S.I., Kamata-Sakurai M., Kaneko A., Kinoshita Y. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017;9:1–14. doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 88.Han X., Yu S., Li A., Liu Q., Yuan X., Xu H. Recent advances of bispecific antibodies in solid tumors. J Hematol Oncol. 2017;10:1–16. doi: 10.1186/s13045-017-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maussang-Detaille D., Nardis C de, Hendriks L., Bartelink-Clements C., Rovers E., Gallenne T. Abstract 33: the binding mode of the bispecific anti-HER2xHER3 antibody MCLA-128 is responsible for its potent inhibition of HRG-driven tumorigenesis. 2017. 33–33. 2. [DOI]
- 90.Calvo E., Alsina M., Schellens J.H.M., Huitema A.D.R., Tabernero J., de Vries-Schultink A. Abstract CT050: a phase I/II study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3, in patients with solid tumors. Canc Res. 2016;76 doi: 10.1158/1538-7445.AM2016-CT050. CT050 LP-CT050. [DOI] [Google Scholar]
- 91.Geuijen C., Rovers E., Nijhuis R., den Blanken-Smit R., Visser T., Bartelink W. Preclinical activity of MCLA-128, an ADCC enhanced bispecific IgG1 antibody targeting the HER2:HER3 heterodimer. J Clin Oncol. 2014;32:560. doi: 10.1200/jco.2014.32.15_suppl.560. [DOI] [Google Scholar]
- 92.Alsina M., Boni V., Schellens J.H.M., Moreno V., Bol K., Westendorp M. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC) J Clin Oncol. 2017;35 doi: 10.1200/jco.2017.35.15_suppl.2522. 2522–2522. [DOI] [Google Scholar]
- 93.ZW25 Effective in HER2-Positive Cancers Canc Discov. 2019;9:8. doi: 10.1158/2159-8290.CD-NB2018-162. [DOI] [PubMed] [Google Scholar]
- 94.Weisser N., Wickman G., Davies R., Rowse G. Abstract 31: preclinical development of a novel biparatopic HER2 antibody with activity in low to high HER2 expressing cancers. Canc Res. 2017;77:31. doi: 10.1158/1538-7445.AM2017-31. LP – 31. [DOI] [Google Scholar]
- 95.Wermke M., Alt J., Kauh J.S., Back J., Salhi Y., Reddy V. Preliminary biomarker and pharmacodynamic data from a phase I study of single-agent bispecific antibody T-cell engager GBR 1302 in subjects with HER2-positive cancers. J Clin Oncol. 2018;36:69. doi: 10.1200/JCO.2018.36.5_suppl.69. [DOI] [Google Scholar]
- 96.Ruiz I.R., Vicario R., Morancho B., Morales C.B., Arenas E.J., Herter S. P95HER2–T cell bispecific antibody for breast cancer treatment. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai Z., Fu T., Nagai Y., Lam L., Yee M., Zhu Z. scFv-based “grababody” as a general strategy to improve recruitment of immune effector cells to antibody-targeted tumors. Canc Res. 2013;73:2619–2627. doi: 10.1158/0008-5472.CAN-12-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H., Lam L., Nagai Y., Zhu Z., Chen X., Ji M.Q. A targeted immunotherapy approach for HER2/neu transformed tumors by coupling an engineered effector domain with interferon-γ. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2017.1300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glorius P., Baerenwaldt A., Kellner C., Staudinger M., Dechant M., Stauch M. The novel tribody [(CD20)2 xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia. 2013;27:190–201. doi: 10.1038/leu.2012.150. [DOI] [PubMed] [Google Scholar]
- 100.Oberg H.H., Kellner C., Gonnermann D., Sebens S., Bauerschlag D., Gramatzki M. Tribody [(HER2)2xCD16] is more effective than trastuzumab in enhancing γδ T cell and natural killer cell cytotoxicity against HER2-expressing cancer cells. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deng W., Liu J., Pan H., Li L., Zhou C., Wang X. A bispecific antibody based on pertuzumab Fab has potent antitumor activity. J Immunother. 2018;41:1–8. doi: 10.1097/CJI.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 102.Kiewe P., Hasmüller S., Kahlert S., Heinrigs M., Rack B., Marmé A. Phase I trial of the trifunctional anti-HER2 × anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Canc Res. 2006;12:3085–3091. doi: 10.1158/1078-0432.CCR-05-2436. [DOI] [PubMed] [Google Scholar]
- 103.Haense N., Atmaca A., Pauligk C., Steinmetz K., Marmé F., Haag G.M. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Canc. 2016;16:1–10. doi: 10.1186/s12885-016-2449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lopez-Albaitero A., Xu H., Guo H., Wang L., Wu Z., Tran H. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. OncoImmunology. 2017;6:1–12. doi: 10.1080/2162402X.2016.1267891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richards D.A., Braiteh F.S., Garcia A.A., Denlinger C.S., Conkling P.R., Edenfield W.J. A phase 1 study of MM-111, a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2-positive solid tumors. J Clin Oncol. 2014;32:651. doi: 10.1200/jco.2014.32.15_suppl.651. [DOI] [Google Scholar]
- 106.Higgins M.J., Gabrail N.Y., Miller K., Agresta S.V., Sharma S., McDonagh C. A phase I/II study of MM-111, a novel bispecific antibody that targets the ErB2/ErB3 heterodimer, in combination with trastuzumab in advanced refractory HER2-positive breast cancer. J Clin Oncol. 2011;29 doi: 10.1200/jco.2011.29.15_suppl.tps119. TPS119–TPS119. [DOI] [Google Scholar]
- 107.Mittal D., Vijayan D., Neijssen J., Kreijtz J., Habraken M.M.J.M., Van Eenennaam H. Blockade of ErbB2 and PD-L1 using a bispecific antibody to improve targeted anti-ErbB2 therapy. OncoImmunology. 2019;8 doi: 10.1080/2162402X.2019.1648171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lum L.G., Thakur A., Al-Kadhimi Z., Colvin G.A., Cummings F.J., Legare R.D. Targeted T-cell therapy in stage IV breast cancer: a phase i clinical trial. Clin Canc Res. 2015;21:2305–2314. doi: 10.1158/1078-0432.CCR-14-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Valone F.H., Kaufman P.A., Guyre P.M., Lewis L.D., Memoli V., Deo Y. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol. 1995;13:2281–2292. doi: 10.1200/JCO.1995.13.9.2281. [DOI] [PubMed] [Google Scholar]
- 110.lo Nigro C., Macagno M., Sangiolo D., Bertolaccini L., Aglietta M., Merlano M.C. NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives. Ann Transl Med. 2019;7 doi: 10.21037/atm.2019.01.42. 105–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meric-Bernstam F., Beeram M., Mayordomo J.I., Hanna D.L., Ajani J.A., Blum Murphy M.A. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol. 2018;36:2500. doi: 10.1200/JCO.2018.36.15_suppl.2500. [DOI] [Google Scholar]
- 112.Musolino A., Boggiani D., Pellegrino B., Zanoni D., Sikokis A., Missale G. Role of innate and adaptive immunity in the efficacy of anti-HER2 monoclonal antibodies for HER2-positive breast cancer. Crit Rev Oncol Hematol. 2020;149:102927. doi: 10.1016/j.critrevonc.2020.102927. [DOI] [PubMed] [Google Scholar]