Abstract
In a newborn pig cystic fibrosis (CF) model, the ability of gland-containing airways to fight infection was affected by at least two major host-defense defects: impaired mucociliary transport and a lower airway surface liquid (ASL) pH. In the gland-containing airways, the ASL pH is balanced by CFTR (CF transmembrane conductance regulator) and ATP12A, which, respectively, control HCO3 − transport and proton secretion. We found that, although porcine small airway tissue expressed lower amounts of ATP12A, the ASL of epithelial cultures from CF distal small airways (diameter < 200 μm) were nevertheless more acidic (compared with non-CF airways). Therefore, we hypothesized that gland-containing airways and small airways control acidification using distinct mechanisms. Our microarray data suggested that small airway epithelia mediate proton secretion via ATP6V0D2, an isoform of the V0 d subunit of the H+-translocating plasma membrane V-type ATPase. Immunofluorescence of small airways verified the expression of the V0 d2 subunit isoform at the apical surface of Muc5B+ secretory cells, but not ciliated cells. Inhibiting the V-type ATPase with bafilomycin A1 elevated the ASL pH of small airway cultures, in the presence or absence of HCO3 −, and decreased ASL viscosity. These data suggest that, unlike large airways, which are acidified by ATP12A activity, small airways are acidified by V-type ATPase, thus identifying V-type ATPase as a novel therapeutic target for small airway diseases.
Keywords: cystic fibrosis, pig small airways, airway surface liquid, V-ATPase
Clinical Relevance
This work will open up novel avenues for treatment as well as applications to other diseases of the lungs that affect small airways, like asthma, chronic obstructive pulmonary disease, and interstitial lung disease.
Cystic fibrosis (CF) is caused by a defect in CFTR (CF transmembrane conductance regulator) (1), an anion channel permeable to both chloride and bicarbonate. In people with CF, airway infection and inflammation are the main causes of CF morbidity and mortality, and it is generally believed that the pathogenesis of early CF lung disease is driven by small airway abnormalities (2, 3). This hypothesis has been difficult to test because the small airways are not accessible for detailed mechanistic studies in people with CF, so an animal model that recapitulates the human condition is required. CFTR-knockout mice do not develop lung disease; thus, we study CF lung disease pathogenesis in a CFTR-knockout pig model. Using this CF pig model, we previously defined two host-defense defects in gland-containing airways: the mucociliary transport defect caused by anchored mucous strands in submucosal glands, and the bacterial killing defect caused by a low airway surface liquid (ASL) pH (4–6). Humans and pigs share a similar airway anatomy and physiology and our CFTR-knockout pigs spontaneously develop lung disease that closely mimics human CF (7).
In both species, as airways bifurcate, their diameter decreases but the combined total cross-sectional area increases. As a result, the resistance to air flow decreases in more distal regions of the lung. In dog and human lungs, small airways are operationally defined as airways of a diameter of 2 mm or less, where obstruction minimally increases the airway resistance (8). However, that definition does not account for the size of the lungs, age, or species. In newborn pigs, small airways can be identified by histology: the small airways have no submucosal glands, the epithelium is cuboidal, and the walls are surrounded by smooth muscle with little or no cartilage (9). This is the definition we used, and in young pigs, it corresponds to airways of less than ∼200 μm in diameter.
Previous in vitro methods for studying pig small airways (9) uncovered key differences in small versus large airway epithelia. In non-CF small airway epithelia, the ASL pH is higher than that in the large airway under cAMP-stimulated conditions. In CF small airway epithelia, both the large and small airways have a lower ASL pH than non-CF airways (9). In large airways, the ASL pH homeostasis is set by the balance between CFTR-mediated bicarbonate transport and ATP12A-mediated proton secretion (10). However, it is unknown whether the same mechanism controls pH in the small airway. To investigate this further, we used primary cell cultures of large and small airways to study ASL pH regulation.
Methods
Primary Cell Cultures of Pig Large and Small Airway Epithelia
All animal studies were approved by the University of Iowa Animal Care and Use Committee and conducted in accordance with specified guidelines/regulations.
Generation of CFTR −/− pigs has been previously reported (11). Animals were produced by mating CFTR +/− male and female pigs. Newborn CFTR+/+ or CFTR+/− (non-CF), and CFTR−/− (CF) piglets were obtained from Exemplar Genetics. Newborn piglet lungs were excised and the whole airway tree was microdissected by carefully combing off the parenchymal tissue. Subsequently, vascular tissue was separated from the airway tree by blunt dissection. Proximal large airways, including the trachea, main-stem bronchi, and distal small airways (diameter of ∼200 μm) were dissected out separately from the airway tree. To isolate enough small airway epithelia to study in vitro, the entire airway tree was microdissected. Next, primary porcine airway epithelia were isolated according to a procedure adapted from one used to isolate tracheal airway cells. Primary epithelial cells were seeded onto collagen-coated, semipermeable membranes (catalog number 3470, Corning) at a density of 106 cells/cm2 and cultured at the air–liquid interface (ALI) at 37°C in a 5% CO2 atmosphere as previously described (12). For small airway epithelia, the typical yield per lung was 3–4 × 106 cells per newborn pig. Small airway epithelial cultures were maintained in Small Airway Growth Media (Lonza) supplemented with 5% FBS and 10 ng/ml KGF (keratinocyte growth factor). All experiments were performed ∼2 weeks after seeding on matched large and small airway epithelia that had been isolated from the same animal.
RNA Knockdown of ATP6V0D2
To knockdown pig ATP6V0D2 in cultured pig small airway epithelia, cells were transfected at the time of seeding with siRNA against pig ATP6V0D2 (IDT Corp.) or universal control siRNA (catalog number 51-01-19-09, IDT Corp.) as previously described (13). Briefly, cells were freshly dissociated and then reverse transfected with 300-nM siRNAs mixed with Lipofectamine RNAiMAX (Invitrogen). Transwells (0.4-μm pore) were precoated with human placental collagen Type IV (Sigma), and 200,000 freshly dissociated pig small airway epithelial cells, suspended in Small Airway Growth Media + 5% FBS, were added to each insert. After overnight incubation, complete media were added to the basolateral surface. Cells were cultured at the ALI beginning on Day 3 as described above and were used for experiments 2 weeks after seeding. The siRNAs targeting pig ATP6V0D2 and negative control scrambled siRNA were designed and synthesized by IDT Corp. The sequences were as follows: siRNA against pig ATP6V0D2: sense strand–5′ GGGAAAGCUAGAUAAUUCCAAAUCA, antisense strand–3′ AACCCUUUCGAUCUAUUAAGGUUUAGU; scrambled (negative control for siRNAs): sense strand–5′ pCUUCCUCUCUUUCUCUCCCUUGUGA, antisense strand–3′ UCACAAGGGAGAGAAAGAGAGGAAGGA.
Measuring ASL pH
As previously reported (9), ASL pH was measured using 70 kD of dextran-conjugated SNARF (Molecular Probes), a ratiometric fluorescent pH indicator. Briefly, SNARF–dextran was applied to the apical surface, whereas forskolin (10 μM) and IBMX (100 μM) were applied to the basolateral media. After a 2-hour incubation, epithelia were placed in a humidified chamber (5% CO2, 37°C) and examined by confocal microscopy (Zeiss 510 Meta nonlinear optical microscope at 580 nm and 640 nm). The pH was calculated as previously reported (9). In some experiments, we eliminated HCO3 − transport by replacing the basolateral culture media with a HEPES-buffered solution (135 mM NaCl, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 5 mM HEPES, and 10 mM glucose).
To test the effect of ATP12A and V-type-ATPase inhibitors, we used ouabain and bafilomycin, respectively. To apply ouabain, 1 μl of a 10-μM solution was applied to the apical cell surface for an estimated 10-μM final concentration. For bafilomycin, the drug was added to the culture media at 200 nM. For both inhibitors, the epithelia were incubated for 2 hours at 37°C before measuring the ASL pH. The ASL pH was measured before and after intervention and compared with the ASL pH of vehicle-treated epithelia.
V-ATPase Translocation Assay
To determine if the localization of V-ATPase (vacuolar type H+ ATPase) in small airway epithelia is also regulated by different luminal pH levels, the apical surface of small airway epithelia was artificially set to 6.8 or 7.4 with HEPES-buffered solutions in which the pH had been previously adjusted to 6.8 and 7.4. The basolateral media was also replaced with HEPES-buffered solution with an adjusted pH of 7.4. The epithelia were incubated in a no-CO2 environment at 37°C for 30 minutes and then immediately fixed.
Immunohistochemistry
For human studies, airway epithelial cells were obtained from human trachea and bronchi specimens acquired from the Iowa Donor Network, either as postmortem specimens or from tissue deemed not fit for transplant. Studies were approved by the University of Iowa Institutional Review Board. Informed consent was obtained when required, and all appropriate guidelines and regulations were followed.
Pig and human primary large and small airway epithelia were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 (Pierce). To quench endogenous peroxidase activity, cells were exposed to 3% hydrogen peroxide for 8 minutes, and nonspecific background binding was blocked by 30 minutes of incubation with Background Buster (catalog number NB306; Innovex Biosciences). Primary antibody (anti-ATP12A rabbit polyclonal antibody, HPA039526, Sigma-Aldrich) was applied to the epithelia (1:50 dilution, 1 h, room temperature). Signal was visualized using the Envision System Reagent (DAKO). Harris hematoxylin was used to counterstain nuclei (Surgipath/Leica Biosystems).
Immunofluorescence
The epithelia were fixed and permeabilized for immunohistochemistry as above, and then the epithelia were probed with four primary antibodies: 1:300 rabbit anti–acetylated α-tubulin (Sigma); 1:100 mouse anti-ATP6V0D2 (Abcam); 1:100 rabbit anti-Muc5B (Santa Cruz); 1:50 Phalloidin conjugated with Alexa 647 (Thermo Fisher Scientific). Secondary antibodies, goat anti–mouse-Alexa-Fluor-488 and goat anti–rabbit-Alexa-Fluor-568, both at 1:500, (Invitrogen) were used as previously described (14). Nuclei were counterstained with DAPI. The signal was visualized using an Olympus Fluoview FV1000 confocal microscope with a UPLSAPO 60X oil lens.
Western Blot
Primary large and small airway epithelia were lysed as previously described (3). The protein concentration of the lysate was measured using a Bradford assay (Pierce). Twenty-five to 50 μg of total protein was resolved by 4–20% PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (BioRad). Blots were probed with a 1:500 dilution of ATP12A rabbit polyclonal antibody (catalog number HPA03952, Sigma-Aldrich) and 1:1,000 GAPDH and were then incubated with HRP-conjugated secondary antibodies. The signal was visualized by using enhanced chemiluminescence (ECL) (Pierce). Densitometry of relative expression was measured using ImageJ.
Gene Expression Profiles
Primary large and small airway epithelia were expanded to passage 4, and total RNA was extracted from them with TRIzol Reagent (Invitrogen). Using 5 μg of total RNA, biotinylated complementary RNA was synthesized (Affymetrix Gene Chip one-cycle target labeling kit) and probed with the Affymetrix Porcine GeneChip (23,937 probe sets that interrogate ∼23,256 transcripts from 20,201 Sus scrofa genes). All samples were hybridized in parallel. Array output was scanned using the Affymetrix Model 450 Fluidics Station and Affymetrix Model 3,000 Scanner. GeneChip Operating Software, version 1.4, was used to collect the data which was then analyzed with Partek Genomics Suite Software (Partek).
ASL Viscosity Measurement
ASL viscosity was assayed using fluorescence recovery after photobleaching as previously described (9).
Tracking Fluorescent Microsphere Movement across the Surface of Small Airways Ex Vivo
Small airways were removed from newborn pigs and immediately placed in PBS or in Krebs-Ringer saline and stored at 4°C for 1–12 hours. Before microscopy, excised small airways were transferred to a HEPES-buffered Krebs-Ringer solution (pH = 7.35) and incubated at 37°C for 30 minutes. After incubation, small airways were cut longitudinally and pinned to a dish layered with dental wax. Red fluorescent carboxylate–modified microspheres (1-μm diameter FluoSpheres, Molecular Probes) were added and sonicated in buffer to a final dilution of 1: 20,000. The bead slurry was applied to the pinned airway: ∼20 ml of microsphere-containing solution, to a depth of ∼500 μm, was used to cover the airway tissue surface. To track movement of fluorescent microspheres, they were visualized using 4× dry, 10× dry, or 25× water-immersion objectives on an upright, Nikon A1R resonant scanning confocal microscope. In some preparations, the small airway morphology was simultaneously visualized with reflected light. The tissue chamber stage was maintained at 37°C for experiments. Particle movement was visually tracked by imaging via a 25× objective and video confocal microscopy (1 frame per second [fps]). One-millimolar ATP was added for 30 minutes to stimulate mucin secretion (15). Reconstructions of microsphere movement across the tissue surface were processed as surface renderings of reflected light and red microsphere fluorescence. The Imaris software (Imaris, version 7.6.4; Bitplane) can track each microsphere across small airways and generate a trace for each microsphere. For each trace, the software can calculate the maximum velocity (max). We plotted all the max values obtained under the same conditions and generated the relative frequency of distribution using Prism software. We collected the max from 111 microspheres, and the max for each particle ranged from 1.1 to 24.59591 mm/min. We used a bin center of 2.9 to generate the max frequency of distribution. We collected those data from three non-CF pigs and three CF pigs. We pooled the max from three pigs and generated the max distribution frequency.
Statistics
Data are expressed as the mean ± SEM. For analyses that compared large and small airways from the same animal, we used a nonparametric Wilcoxon signed-rank test. For the statistical analysis of the distribution of the microspheres’ movement, we used the Kolmogorov-Smirnov test. P values are presented in figure legends and in the results section.
All analyses were done using Prism version 7.0 (GraphPad Software).
Results
Large versus Small Airways Express Different Amounts of ATP12A
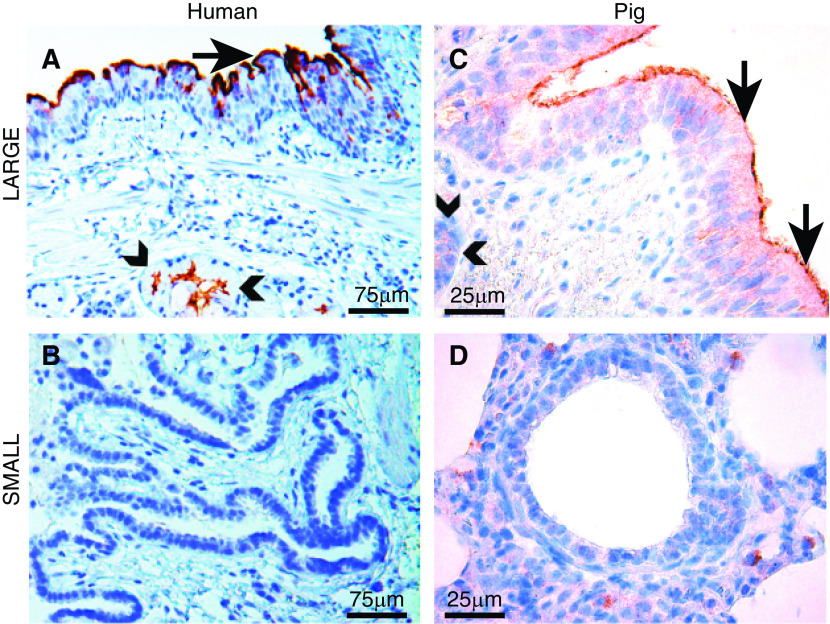
CF is caused by mutations in the gene that encodes the CFTR anion channel. In humans and pigs, the loss of CFTR in large airways impairs respiratory host defenses, causing airway infection (7). CF mice do not develop CF lung disease. This is believed to be due to the lack of nongastric H+/K+ adenosine triphosphatase (ATP12A) proton secretion, leading to an ASL pH that is not acidic compared with that of non-CF mice (10). As mouse airway sizes (external tracheal diameter around 1.5 mm) are small, compared with human and pig airway sizes (16), we hypothesized that the ATP12A concentration is correlative with airway size. Therefore, human and pig small airways would have a lower amount of ATP12A expression which is similar to mouse airways. To test this hypothesis, we evaluated the expression of ATP12A in tissue sections from large versus small airways with immunohistochemistry for both human and pig lungs. Consistent with previous studies, ATP12A was highly expressed on the surface epithelia (Figures 1A and 1C) and in the submucosal glands in both human and pig large airways. However, no ATP12A was detected in human and pig small airways (Figures 1B and 1D). Therefore, we hypothesized that proton secretion is driven by a different mechanism in small airways.
Figure 1.
Large (Lg) airways express high amounts of ATP12A but small (Sm) airways express lower amounts of ATP12A. (A and C) ATP12A immunostaining (brown coloration) of human and pig Lg airway shows intense staining at apical surface of epithelial cells (arrows) and immunostaining is also seen in submucosal glands (arrowheads). Scale bars: A, 75 μm; C, 25 μm. (B and D) ATP12A immunostaining is absent in Sm airways. Scale bars: B, 75 μm; D, 25 μm.
Primary Cell Cultures of CF Small Airway Epithelia Have an Abnormally Acidic ASL pH
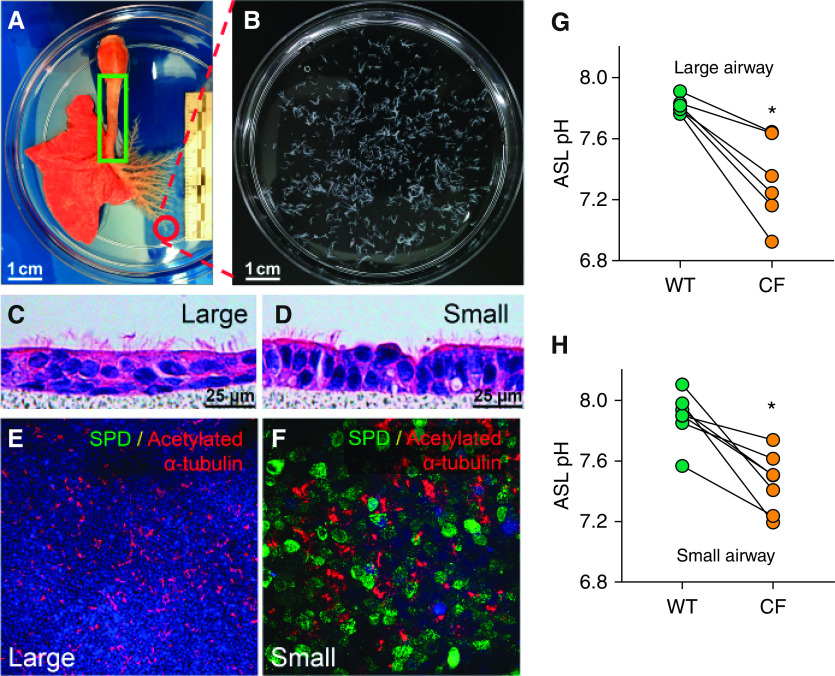
To further investigate whether CF small airway ASL pH is acidic, we developed methods to culture primary small airway epithelial cells. To obtain enough primary small airway cells, we manually microdissected most of the small airways from the whole airway tree (as shown in Figures 2A and 2B) and isolated epithelial cells. After culture at the ALI for 2 weeks, primary large and small airway epithelia were examined for cellular morphology by hematoxylin and eosin staining, confirming that the cultures mimicked native tissue, including development of cilia (Figures 2C and 2D) and SPD (surfactant protein D) expression, exclusively in small airway epithelia (Figures 2E and 2F).
Figure 2.
The airway surface liquid (ASL) pH is lower in cystic fibrosis (CF) airway epithelium. (A) The entire airway from the left lung was manually dissected. Sm airways were defined as having a diameter of <200 μm and as lacking cartilage rings and submucosal glands. The green box indicates the region from which Lg airway cells were isolated. Circled region indicates Sm airways, which are shown in B. Scale bars, 1 cm. (C and D) Hematoxylin and eosin staining of well-differentiated, primary, porcine airway epithelial cells. Airway primary cultures were grown on a polycarbonate filter (pore size of 0.4 μm). Note the differentiated epithelium with cilia on the apical surface. Scale bars, 25 μm. (E and F) SPD (surfactant protein D) is detected by immunostaining in cultured primary Sm airway epithelia (F) but not in Lg airway epithelia (E). SPD is stained in green, acetylated α-tubulin is stained in red, and DAPI is shown in blue. Scale bars: E, 100 μm; F, 25 μm. (G and H) The ASL pH was measured using a ratiometric fluorescent dye (SNARF–dextran), which showed that the ASL pH in CF cell cultures from Lg (C) and Sm (D) airways was lower than that in analogous non-CF cell cultures. Each dot represents the measurement taken from a single pig. Data are expressed as the mean ± SE. N = 7 for Sm airway cultures, and N = 6 for Lg airway cultures. *P < 0.05 by Student’s t test. WT = wild type.
After establishing the primary cell culture model, we measured the ASL pH by using the ratiometric pH-sensitive dye SNARF–dextran. The ASL pH was more acidic in CF primary cultures, in both large and small airways (Figures 2G and 2H). These results are consistent with our previous study in which we used conditional reprogramming to expand large and small airway cells (9).
Pig Small Airway ASL pH Is Not Set by ATP12A
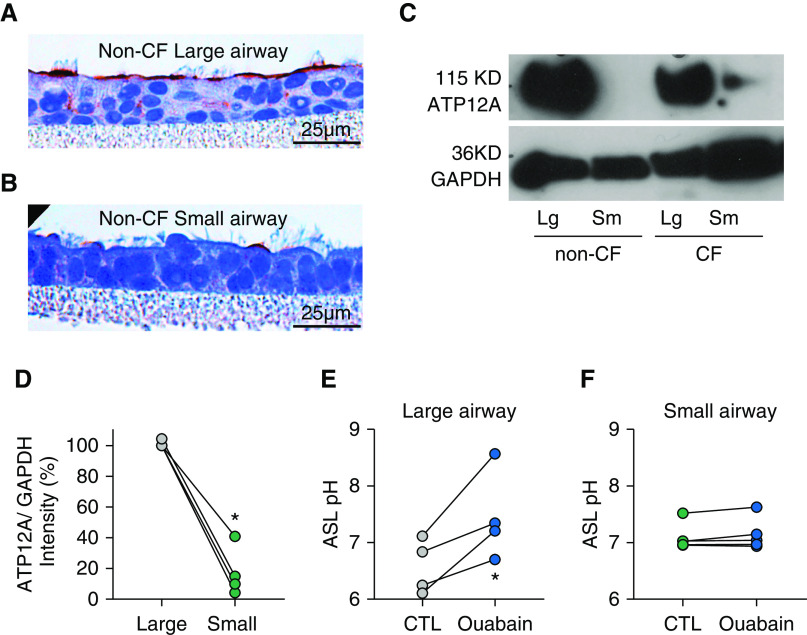
Previous studies showed that gland-containing airways express ATP12A to acidify the ASL pH (10, 17–21). To test whether the same mechanism operates in small airways, we examined whether small airway cultures express ATP12A. Robust ATP12A expression was detected in large airway epithelia but was less evident in small airways (Figures 3A and 3B). IB of cultured large and small airways confirmed this expression pattern (Figures 3C and 3D). These results are consistent with the expression pattern of ATP12A and SPD in native tissue immunostaining, suggesting that primary cell cultures maintained native tissue properties.
Figure 3.
Sm airway pH is not orchestrated by ATP12A. (A and B) Compared with the Lg airway (A), the Sm airway (B) has rare apical epithelial ATP12A immunostaining (brown coloration). Scale bars, 25 μm. (C) Western blotting shows expression of ATP12A (115 kD) and GAPDH (36 kD) in Lg versus Sm and CF versus non-CF airway epithelia. (D) Quantification of the band intensity of ATP12A and GAPDH. Data are shown as the ratio of ATP12A to GAPDH band intensity (set as 1.0 for the Lg airway) as measured by ImageJ. N = 4 piglets and lines plus connected dots indicate experimental pairs. (E) The ATP12A inhibitor, ouabain, increases the pH in the Lg airway, in bicarbonate-free solutions. (F) In contrast, in the Sm airway, ouabain has little effect under the same conditions, showing that Sm airway pH is set by another mechanism. For Lg airway experiments, N = 4, and for Sm airway experiments, N = 5. Lines plus connected dots indicate experimental pairs. *P < 0.05 by paired t test. CTL = control.
To test the function of ATP12A in large and small airways, the ATP12A inhibitor ouabain was applied to the apical surface of cultured cells in bicarbonate-free conditions. Consistent with previously reported ATP12A activity (10, 22), apical ouabain increased the ASL pH in the large airway epithelia (Figure 3E). In contrast, apical ouabain had little effect in small airway epithelia (Figure 3F), suggesting that although CF pig small airways have an acidic ASL pH, the proton secretion is not mediated by ATP12A.
Small Airways Express ATP6V0D2
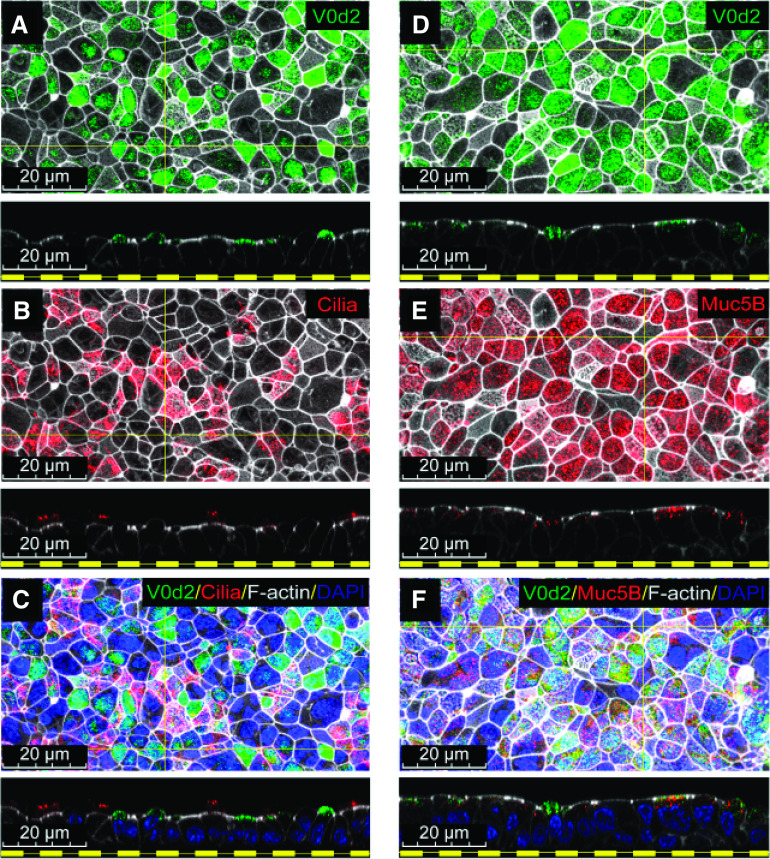
To identify a candidate mechanism for H+ secretion, expression profiles were compared in paired small and large airway epithelia using a microarray. Large airway epithelia expressed less CFTR and more ATP12A than small airway epithelia. We found that ATP6V0D2 was differentially expressed at a higher concentration in small airways, about ∼50-fold more than in large airways (see Figure E1 data supplement). ATP6V0D2 is the gene that encodes the d2 subunit isoform of V-ATPase, whereas the other 12 subunits of V-ATPase are expressed at similar amounts in both large and small airways (Figure E1). To confirm the microarray data, the ATP6V0D2 expression pattern in pig small airways was determined by immunocytochemistry, and its expression was localized to the apical membrane of small airway epithelia. Because not all the cells in primary cell cultures of small airways express ATP6V0D2, we hypothesized that ATP6V0D2 is expressed in a ciliated, goblet, or “novel” airway epithelial cell type. To test which cell type(s) express ATP6V0D2, we immunostained ciliated and secretory cells of the small airways and found that ciliated cells did not express ATP6V0D2 but that MUC5B+ secretory cells did (Figures 4A–4F).
Figure 4.
Non-CF Sm airway secretory cells express ATP6V0D2 on the apical plasma membrane. (A–C) Immunostaining of cultured Sm airway epithelial cells for expression of ATP6V0D2 (green), acetylated α-tubulin (red), F-actin (white), and DAPI (blue) shows that V0D2 is absent from ciliated cells. (D–F) Immunostaining of cultured Sm airway epithelial cells for expression for V0D2 (green), Muc5B (red), F-actin (white), and DAPI (blue) shows that V0D2 is expressed in Muc5B+ cells. Scale bars, 20 μm.
V-ATPase Regulates Small Airway ASL pH and Viscosity
To test whether the V-ATPase secretes H+ across small airway epithelia, we treated the epithelia with bafilomycin A1, a V-ATPase inhibitor. When the epithelia were bathed in either HCO3 −-containing or HCO3 −-free media, the ASL pH increased significantly with bafilomycin treatment (Figures 5A and 5B).
Figure 5.
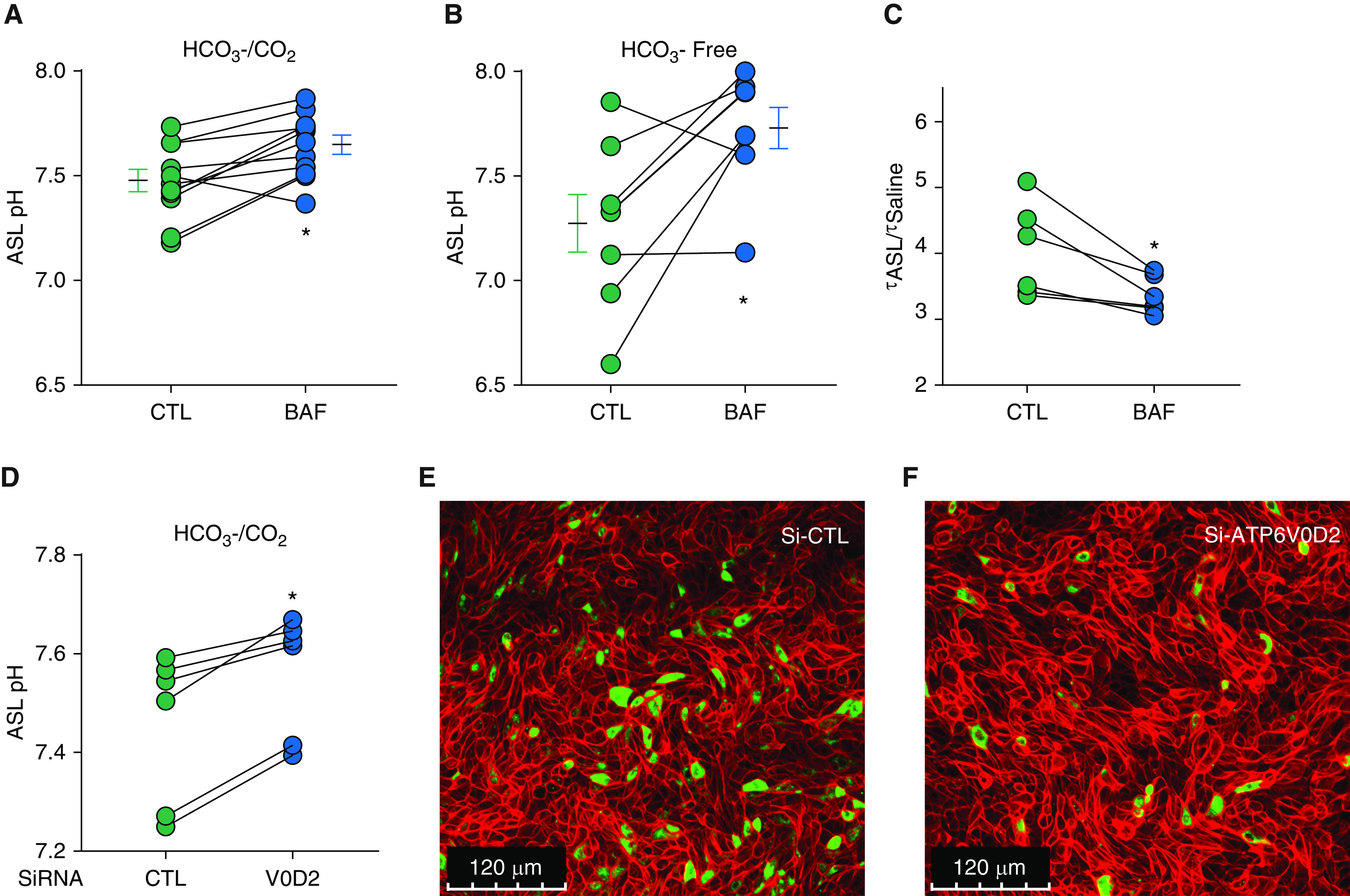
Inhibition of the V-type ATPase increases the ASL pH and decreases ASL viscosity in porcine non-CF Sm airways. ASL pH was measured using a ratiometric fluorescent dye (SNARF–dextran), which showed that the pH increased with bafilomycin treatment under both (A) bicarbonate-containing conditions (N = 11) and (B) bicarbonate-free conditions (N = 7). Lines plus connected dots indicate experimental pairs. *P < 0.05 by paired t test. (C) ASL viscosity was assayed using fluorescence recovery after photobleaching, showing that viscosity in Sm airways is controlled by pH, which is set by V-type ATPase. Lines plus connected dots indicate experimental pairs (N = 6). (D) siRNA knockdown of V0 d2 in cultured Sm airway epithelial cells increases the ASL pH. (E and F) Sm airway cells immunostained for V0D2, cells treated with Si-CTL versus Si-ATP6V0D2. Green indicates positive staining for V0D2, whereas red indicates F-actin staining. Scale bars, 120 μm. *P < 0.05 by paired t test. Si-ATP6V0D2 = siRNA 1 targeting V0D2 transcripts; Si-CTL = scrambled siRNA CTL.
To investigate the role of the ATP6V0 d2 subunit in regulating the ASL pH, ATP6V0 d2 expression was blocked in small airway epithelia by siRNA knockdown. As expected, ASL pH increased in the epithelia treated with ATP6V0 d2 siRNA (Figure 5D). To validate siRNA knockdown, the epithelia were immunostained for ATP6V0 d2 protein expression; the epithelia treated with siRNA targeting ATP6V0D2 expressed less ATP6V0 d2 than those treated with the control siRNA (Figures 5E and 5F and E4).
Because, in large airways, the pH has a large effect on ASL viscosity (17), we tested whether V-ATPase regulates ASL viscosity in small airway epithelia. We applied bafilomycin to the epithelia for 2 hours and measured the ASL viscosity by fluorescence recovery after photobleaching, as described (9). Consistent with our prediction, the ASL viscosity of the small airway cultures decreased after treatment with bafilomycin (Figure 5C).
pH-dependent Localization of V-type ATPase in Small Airway Epithelia
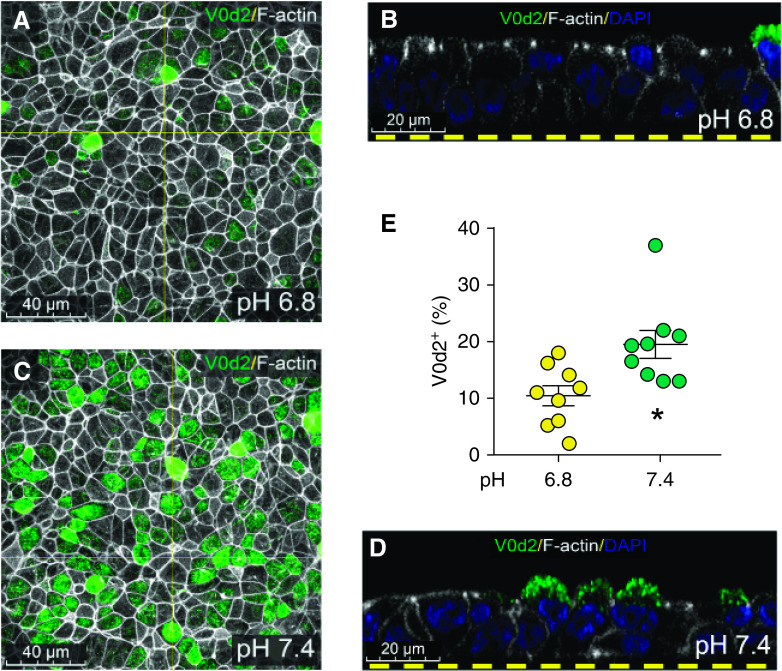
Renal epithelia have a feedback mechanism that controls V-type ATPase translocation from cytoplasm to membrane, in response to luminal pH (23, 24). To test whether a similar mechanism operates in the small airway, we artificially clamped the ASL pH to 6.8 or 7.4 and monitored localization of V-type ATPase by immunostaining. When the ASL pH was artificially set at a pH of 6.8, the V-type ATPase localized to the cytoplasm of small airway epithelial cells (Figures 6A and 6B), and when the ASL pH was set to 7.4, the V-type ATPase translocated to the apical membrane (Figures 6C and 6D). When the ASL pH was set at 6.8, the ATP6V0 d2+ cells were ∼11% of cells. However, when the ASL pH was set at 7.4, the ATP6V0 d2+ cells were increased to ∼20% of cells (Figure 6E). These changes in localization suggest a feedback mechanism operating in the small airways.
Figure 6.
Localization of V-type ATPase is pH-dependent in non-CF Sm airway epithelia. (A and B) When the ASL pH is artificially set at 6.8, the V-type ATPase localizes to the cytoplasm of Sm airway epithelial cells. (C and D) When the ASL pH is set to 7.4, the V-type ATPase translocates to the apical membrane. Quantification of total ATP6V0 d2+ cells (E). Scale bars: A and C, 40 μm; B and D, 20 μm. *P < 0.05 by paired t test.
Mucociliary Transport Is Defective in CF Small Airways
To assess if there is a mucociliary transport impairment in CF small airways, as we detected acidic ASL pH in CF small airway, we developed an ex vivo assay using freshly excised small airways. Small airways were dissected from CF and non-CF newborn pigs and the airways were then pinned flat in a HEPES-buffered solution containing fluorescent microspheres (Figure 7A). Maximal speeds of fluorescent spheres crossing the surface of the small airway were tracked (Figure 7B).
Figure 7.
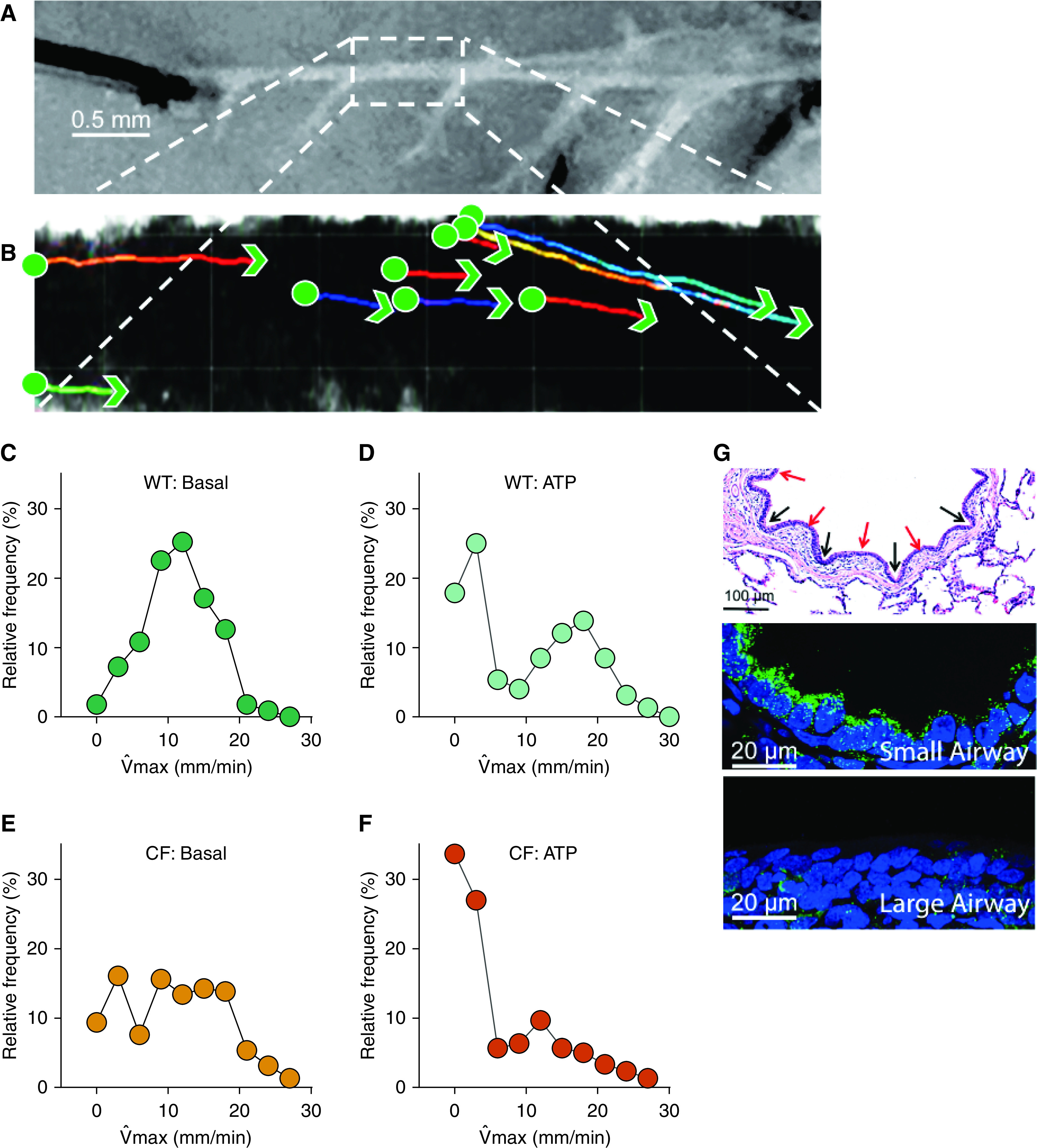
The CF Sm airway is defective in mucociliary transport. (A and B) Representative experiment showing a histogram from pooled data of three non-CF pigs and three CF pigs. (A) The particles are moving across the surface of the Sm airway. Scale bar, 0.5 mm. Sm airways were removed from newborn pigs and maintained in solution ex vivo, cut longitudinally, and pinned to a dish layered with dental wax. (B) Red fluorescent carboxylate–modified microspheres (1-μm diameter FluoSpheres, Molecular Probes) were added, and particle movement was visually tracked by 25× objective and video confocal microscopy (1 frame per second [fps]). To stimulate mucin secretion, the tissue preparation was incubated with 1 mM ATP for 30 minutes. (C–F) The maximum speed distribution, under each condition, was plotted using Prism software. Green circles represent non-CF samples, and orange circles represent CF samples. (G) Hematoxylin and eosin stain of a Sm airway from a non-CF pig (top). The image shows mucosal furrows (black arrows) and ridges (red arrows). Scale bar, 100 μm. Immunofluorescence for ATP6V0D2 (green) and nuclei (blue) in Sm airway epithelia (middle panel) and Lg airway epithelia (bottom panel). Scale bars, 20 μm. max = maximal velocity.
Under basal conditions, for non-CF small airways, the max of beads showed a normal distribution. The proportion of beads that did not move was 1.8%; the mode of max equaled 11.5 mm/min (Figure 7C). For CF small airways, the max of beads showed a bimodal distribution. Moving beads were transported with a mode of 10 mm/min, similar to non-CF small airway. However, the proportion of beads that did not move was 9.3% (Figure 7E). We also investigated the effect of ATP on the speed of beads as ATP can stimulate mucin release from secretory cells and increase ciliary beat frequency (Figure E3). Under ATP-stimulated conditions, in non-CF pigs, the distribution of max became bimodal. The fast-moving beads, defined as particles moving >10 mm/min, increased the mode of max. The mode for the faster proportion was around 20 mm/min whereas ∼17.8% of the beads became still and failed to move (Figure 7D). In contrast, in CF small airways under ATP-stimulated conditions, the proportion of beads that did not move was 33.6%; and the moving beads only slightly increased the mode of max from 9 mm/minute to 12 mm/minute (Figure 7F). At baseline there was not a statistically significant difference between the cumulative distribution between non-CF and CF pigs (P = 0.3). However, both the cumulative distributions of non-CF baseline versus ATP treatment, and non-CF with ATP versus CF with ATP were statistically significant (P < 0.001). The data suggest that ATP induces mucus secretion and microspheres get stuck on the mucus. We found similar findings in large airways, where cholinergic stimulation of CF pigs caused micro disks to become stuck (6).
Discussion
The findings reported here identify a key difference in the mechanisms that control the ASL pH of large versus small airways, with implications for a CF therapy that normalizes ASL pH. Our previous studies showed that, in large airways, ASL pH influences mucociliary transport and bacterial killing (4–6), and that ASL pH is balanced by CFTR-mediated HCO3 − secretion and ATP12A-mediated proton secretion (10, 22). In contrast, we determined that, in small airway epithelia, proton secretion is controlled by V-type ATPase. Like ATP12A, which is not expressed in small airways, V-type ATPase regulates pH and therefore ASL viscosity in small airways. Thus, in the case of CF, where CFTR function is compromised and cannot balance proton secretion, restoring ASL homeostasis could potentially be achieved by targeting these two sources of protons. Recent publications showing that ionocytes of human airway epithelial cells also express V0 d2 (25), suggest that V-ATPase may also play a role in the regulation of pH in large airways and warrants further investigation.
In mammals, V-ATPases are ubiquitously expressed, two-part, multisubunit proton pumps, composed of a transmembrane domain (V0) and a cytosolic domain (V1) (26–28). The V1 domain has eight subunits (A-H) and is responsible for the hydrolysis of ATP which provides the energy for the rotary function of the pump; the V0 domain contains five subunits (a, d, e, c, c”) and is responsible for proton translocation (27). V-ATPases acidify intracellular vesicles (vacuoles, endosomes, lysosomes, trans-Golgi network) and regulate extracellular acidification in epithelia and osteoclasts (29, 30). Human mutations and targeted deletions of various subunits result in renal tubular acidosis, deafness, anosmia, and abnormal sperm due to lack of acidification in the epididymis (31–34). V-ATPase intracellular localization, tissue distribution, and function are controlled by expression of different isoforms (e.g., isoforms B1, a3, a4, and d2 which are expressed in epithelia and osteoclasts) (35). In mice, the promoter of the V-ATPase V1B1 subunit can drive EGFP expression in intercalated cells in the kidney, clear cells in the epididymis, and club cells of the airway (36). Here, we show that the V0 d2 isoform is expressed in mucin-containing epithelia of the small airways, where it regulates ASL pH. Our data suggest that the V-ATPase containing the ATP6V0D2 isoform confers apical localization to the V-ATPase in small airways, a situation similar to that of the collecting duct and epididymis (24, 27).
Small airway acidification of ASL is controlled by V-type ATPase, which is in contrast to ATP12A-driven acidification in large airway epithelia, revealing a new mechanism for maintaining pH airway homeostasis. It is unknown why the two regions use a different proton pump to acidify the same milieu. Because changes in the ASL pH alter viscoelastic properties (17), we speculate that, in the small airways, the ASL pH requires a constant pH to optimize the antimicrobial properties of antimicrobial peptides and the viscoelastic properties of mucus. It has also been reported that low pH decreases ciliary function and disrupts the morphology of epithelial cells in cow trachea (37). We also speculate that small airways might be more susceptible than large airways to low ASL pH values, so they would require a more constant (less acidic) ASL pH to maintain their normal function, which in part could be achieved by regulating V-ATPase.
The dissected small airways show similar mucociliary transport (MCT) in CF and non-CF. Upon ATP stimulation, we found a clear difference between CF and non-CF. In non-CF, moving particles appear to speed up, consistent with an increase in ciliary beat frequency. However, many particles get stuck in the CF airway. At this point, we can only speculate on what explains the difference between CF and non-CF. Because the buffer used likely clamps the pH in the lumen of both CF and non-CF small airways, we suspect that the mucins secreted in the “furrows” (Figure 7G, top) are affected by changes in the pH of the microenvironment, similar to what we see in the submucosal glands in the large airways. In a recent publication, in a microfluidic model of submucosal glands, release of mucin from banana slugs under an acidic pH resulted in abnormal biophysical properties that failed to correct once the pH was increased (38).
The role of luminal acidification by V-ATPase in airways has been controversial. Various studies of the large airways demonstrated no significant change when bafilomycin was applied (10, 39, 40). However, the baseline ASL pH in these studies was less than 7.0, a condition in which V-ATPase is internalized. In contrast to studies of the large airways, a previous study has shown the role of luminal acidification by V-ATPase in pig distal airways (41). Interestingly, the rate of acidification (proton secretion) was higher when the starting luminal pH was higher. It decreased substantially when it reached a pH level close to 7, and this effect was blocked by bafilomycin A1. Although these pH changes are likely due to a complex interaction of buffering capacity of the ASL, pumps, channels, and paracellular transport, we speculate that V-ATPase translocation plays an important role that could explain these findings.
Other mechanisms reportedly regulate V-type ATPase activity. In clear cells of the vas deferens, V-ATPases are regulated by apical pH (24, 42), where alkalinizing the lumen translocates V-ATPase to the apical membrane and acidifying the lumen has the opposite effect. In addition, ANGII translocated V-ATPase from an intracellular compartment to the apical membrane (Figure E2) (43). We hypothesize that the ASL pH likewise regulates the localization of the V-ATPase in the small airway epithelia. In the small airway, the proton concentration regulates localization (Figure 6), as shown by our experiments performed in the absence of bicarbonate. In contrast, in kidney cells, the bicarbonate concentration on the apical side regulates V-type ATPase localization. Thus, it has not been clear what mechanism controls translocation of V-ATPase in the small airway. Previous studies were able to regulate V-ATPase activity in the renal collecting duct and vas deferens (44), where treatment with 1 μM ANG II (angiotensin II) caused V-ATPase containing the ATP6V0D2 isoform to translocate to the apical membrane (43). Likewise, we found that exposing porcine small airway epithelia to ANG II similarly triggered a marked translocation of V-ATPase V0D2 to the apical membrane (Figures E2A and E2B). These mechanisms are shared by the kidney and airway.
The ASL pH is a key element of CF lung disease because it affects mucociliary transport and host defense against infection. Our results imply that targeting ATP6V0D2 might offer a way to raise the ASL pH and provide a therapeutic benefit for CF. Nevertheless, using bafilomycin to increase the ASL pH in small airways is not practical because it inhibits a broad range of V-ATPase isoforms that control essential functions (e.g., endocytosis and exocytosis pathways).
To inhibit the V-type ATPase therapeutically would require targeting of particular, nonubiquitous subunit isoforms. Because the V0 d2 subunit is expressed in a cell type–specific manner and determines intracellular localization, it likely would make an excellent therapeutic target. An obvious next step will be to design a drug screen to select specific apical V-type ATPase inhibitors. Our in vitro system could be miniaturized and automated for this purpose. Notably, because other small airway diseases such as chronic obstructive pulmonary disease, asthma, and bronchiolitis obliterans show similar symptoms (e.g., mucus plugging, coughing, and susceptibility to infection), they might also respond to inhibition of the V-type ATPase.
Acknowledgments
Acknowledgment
The authors thank Dr. Michael Welsh, Dr. Lynda Ostedgaard, Dr. Anthony Fischer, Dr. Mahmoud Abou Alaiwa, Peter Taft and Linda Powers for excellent technical assistance, valuable suggestions and advice. They thank the University of Iowa In Vitro Models and Cell Culture Core and DNA Core Facility for providing equipment and necessary materials to perform these experiments.
Footnotes
Supported by the U.S. National Institutes of Health (HL091842 and HL51670 [D.A.S. and J.Z.]) and by The Cystic Fibrosis Foundation (LIX16G0 [X.L.] and The Iowa Cystic Fibrosis Foundation Research Development Program). I.M.T. is supported by the Gilead Sciences Research Program in Cystic Fibrosis.
Data Availability: The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions: X.L., R.V., D.A.S., and J.Z.: conception and design of research. X.L., R.V., I.M.T., J.N., S.M., C.M.B., L.L., A.Z., A.E., and D.K.M.: performance of experiments and data acquisition. X.L., R.V., and J.Z.: analysis of data. X.L., R.V., I.M.T., D.A.S., and J.Z.: interpretation of results of experiments. X.L., R.V., D.A.S., and J.Z.: figure preparation. X.L. and R.V.: drafting of manuscript. X.L., R.V., I.M.T., J.N., S.M., C.M.B., L.L., A.Z., A.E., D.K.M., and D.A.S.: editing, reviewing, and approval of the final version to be published.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0349OC on March 31, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ramsey BW, Banks-Schlegel S, Accurso FJ, Boucher RC, Cutting GR, Engelhardt JF, et al. Future directions in early cystic fibrosis lung disease research: an NHLBI workshop report. Am J Respir Crit Care Med. 2012;185:887–892. doi: 10.1164/rccm.201111-2068WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quinton PM. Both ways at once: keeping small airways clean. Physiology (Bethesda) 2017;32:380–390. doi: 10.1152/physiol.00013.2017. [DOI] [PubMed] [Google Scholar]
- 3. Tiddens HA, Donaldson SH, Rosenfeld M, Paré PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol. 2010;45:107–117. doi: 10.1002/ppul.21154. [DOI] [PubMed] [Google Scholar]
- 4. Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA. 2014;111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 9. Li X, Tang XX, Vargas Buonfiglio LG, Comellas AP, Thornell IM, Ramachandran S, et al. Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense. Am J Physiol Lung Cell Mol Physiol. 2016;310:L670–L679. doi: 10.1152/ajplung.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zabner J, Wadsworth SC, Smith AE, Welsh MJ. Adenovirus-mediated generation of cAMP-stimulated Cl- transport in cystic fibrosis airway epithelia in vitro: effect of promoter and administration method. Gene Ther. 1996;3:458–465. [PubMed] [Google Scholar]
- 13. Ramachandran S, Krishnamurthy S, Jacobi AM, Wohlford-Lenane C, Behlke MA, Davidson BL, et al. Efficient delivery of RNA interference oligonucleotides to polarized airway epithelia in vitro . Am J Physiol Lung Cell Mol Physiol. 2013;305:L23–L32. doi: 10.1152/ajplung.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Comellas AP, Karp PH, Ernst SE, Moninger TO, Gansemer ND, et al. CFTR is required for maximal transepithelial liquid transport in pig alveolar epithelia. Am J Physiol Lung Cell Mol Physiol. 2012;303:L152–L160. doi: 10.1152/ajplung.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. Br J Pharmacol. 1991;103:1053–1056. doi: 10.1111/j.1476-5381.1991.tb12299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonvin E, Le Rouzic P, Bernaudin JF, Cottart CH, Vandebrouck C, Crié A, et al. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J Physiol. 2008;586:3231–3243. doi: 10.1113/jphysiol.2008.150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah VS, Ernst S, Tang XX, Karp PH, Parker CP, Ostedgaard LS, et al. Relationships among CFTR expression, HCO3- secretion, and host defense may inform gene- and cell-based cystic fibrosis therapies. Proc Natl Acad Sci USA. 2016;113:5382–5387. doi: 10.1073/pnas.1604905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abou Alaiwa MH, Launspach JL, Sheets KA, Rivera JA, Gansemer ND, Taft PJ, et al. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight. 2016;1:e87535. doi: 10.1172/jci.insight.87535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cooney AL, Abou Alaiwa MH, Shah VS, Bouzek DC, Stroik MR, Powers LS, et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight. 2016;1:e88730. doi: 10.1172/jci.insight.88730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abou Alaiwa MH, Beer AM, Pezzulo AA, Launspach JL, Horan RA, Stoltz DA, et al. Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. J Cyst Fibros. 2014;13:373–377. doi: 10.1016/j.jcf.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scudieri P, Musante I, Caci E, Venturini A, Morelli P, Walter C, et al. Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight. 2018;3:123616. doi: 10.1172/jci.insight.123616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol. 2009;212:1762–1772. doi: 10.1242/jeb.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 2013;28:318–329. doi: 10.1152/physiol.00007.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cotter K, Stransky L, McGuire C, Forgac M. Recent insights into the structure, regulation, and function of the v-atpases. Trends Biochem Sci. 2015;40:611–622. doi: 10.1016/j.tibs.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 28. Stransky L, Cotter K, Forgac M. The function of V-ATPases in cancer. Physiol Rev. 2016;96:1071–1091. doi: 10.1152/physrev.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith AN, Jouret F, Bord S, Borthwick KJ, Al-Lamki RS, Wagner CA, et al. Vacuolar H+-ATPase d2 subunit: molecular characterization, developmental regulation, and localization to specialized proton pumps in kidney and bone. J Am Soc Nephrol. 2005;16:1245–1256. doi: 10.1681/ASN.2004090761. [DOI] [PubMed] [Google Scholar]
- 30. Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, et al. V-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med. 2006;12:1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- 31. Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, et al. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest. 2004;113:1560–1570. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith AN, Borthwick KJ, Karet FE. Molecular cloning and characterization of novel tissue-specific isoforms of the human vacuolar H+-ATPase C, G and d subunits, and their evaluation in autosomal recessive distal renal tubular acidosis. Gene. 2002;297:169–177. doi: 10.1016/s0378-1119(02)00884-3. [DOI] [PubMed] [Google Scholar]
- 33. Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21:84–90. doi: 10.1038/5022. [DOI] [PubMed] [Google Scholar]
- 34. Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerbäck S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H+-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS One. 2009;4:e4471. doi: 10.1371/journal.pone.0004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, et al. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod. 2006;74:185–194. doi: 10.1095/biolreprod.105.043752. [DOI] [PubMed] [Google Scholar]
- 36. Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, et al. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol. 2005;288:C1134–C1144. doi: 10.1152/ajpcell.00084.2004. [DOI] [PubMed] [Google Scholar]
- 37. Holma B, Lindegren M, Andersen JM. pH effects on ciliomotility and morphology of respiratory mucosa. Arch Environ Health. 1977;32:216–226. doi: 10.1080/00039896.1977.10667285. [DOI] [PubMed] [Google Scholar]
- 38. Xie Y, Lu L, Tang XX, Moninger TO, Huang TJ, Stoltz DA, et al. Acidic submucosal gland pH and elevated protein concentration produce abnormal cystic fibrosis mucus. Dev Cell. 2020;54:488–500, e5. doi: 10.1016/j.devcel.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. Am J Physiol Cell Physiol. 2002;282:C736–C743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- 40. Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290:C741–C749. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 41. Inglis SK, Wilson SM, Olver RE. Secretion of acid and base equivalents by intact distal airways. Am J Physiol Lung Cell Mol Physiol. 2003;284:L855–L862. doi: 10.1152/ajplung.00348.2002. [DOI] [PubMed] [Google Scholar]
- 42. Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wagner CA, Mohebbi N, Uhlig U, Giebisch GH, Breton S, Brown D, et al. Angiotensin II stimulates H+-ATPase activity in intercalated cells from isolated mouse connecting tubules and cortical collecting ducts. Cell Physiol Biochem. 2011;28:513–520. doi: 10.1159/000335112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–1117. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]