To the Editor:
Acute respiratory distress syndrome (ARDS) occurred in ∼12% of hospitalized patients with coronavirus disease (COVID-19) in a recent New York City cohort and has a high mortality rate depending on the age decile (1). Pulmonary endothelial dysfunction, characterized by increased expression of inflammatory genes and increased permeability, is a major component of ARDS. Vascular leak results in the parenchymal accumulation of leukocytes, protein, and extravascular water, leading to pulmonary edema, ischemia, and activation of coagulation associated with COVID-19. Endothelial inflammation further contributes to an uncontrolled cytokine storm in ARDS (2). Recent studies have reported lung endothelial dysfunction in autopsy results of patients with COVID-19 (3, 4).
We have recently demonstrated that KLF2 (Krüppel-like factor 2), a transcription factor that promotes endothelial quiescence and monolayer integrity (5), is significantly reduced in experimental models of ARDS. Lung inflammation induced by influenza A H1N1 virus or LPS decreases KLF2, which drives pulmonary endothelial dysfunction and acute lung injury (6). High–tidal volume ventilation in rat models mimicking ventilator-induced lung injury also reduces KLF2 and drives lung injury (6). Mechanistically, we found that KLF2 is a potent transcriptional activator of RAPGEF3 (Rap guanine nucleotide exchange factor 3), which activates Rac1 (6), a key small GTPase that orchestrates and maintains vascular integrity by stabilizing cortical actin. Moreover, KLF2 regulates multiple genome-wide association study (GWAS)-implicated ARDS genes related to the cytokine storm, oxidation, and coagulation in the lung microvascular endothelium (6).
It is not known how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affects lung KLF2. Here, we report that endothelial KLF2 is significantly reduced in human lung autopsy samples from patients with COVID-19, supporting that ARDS due to SARS-CoV-2 is a vascular phenotype possibly attributable to KLF2 downregulation. Moreover, our in vitro results demonstrated that KLF2 is a major transcriptional activator of OAS1 (2′-5′ oligoadenylate synthetase 1) and OAS3, two antiviral genes recently implicated in COVID-19 severity by a GWAS (7). Some of the results of our studies have been previously reported in the form of a preprint (https://doi.org/10.1101/2021.01.15.426691).
Methods
Autopsies of patients with COVID-19 were performed within 24–72 hours of death, and lung tissue was stored in 10% neutral buffered formalin for at least 72 hours as per the University of Chicago protocol. Lung tissue from donors with COVID-19 were randomly selected and compared with control lungs that were obtained from organ donors whose lungs were nontransplantable and who did not have SARS-CoV-2 infection, with samples having been collected before the pandemic. Control lungs (obtained from the Gift of Hope Organ and Tissue Donor Network) were similarly processed between 24 and 72 hours of death and stored in 10% neutral buffered formalin for at least 72 hours. Lung tissues were then embedded in paraffin and sectioned. Tissue slices were then processed with conventional hematoxylin and eosin staining or immunofluorescence. Deparaffinization and rehydration was performed by using serial washes with decreasing amounts of ethanol and a final wash in water. Samples were then blocked with 1% BSA in PBS for 30 minutes followed by permeabilization in 1% BSA and 0.1% Triton X-100 for 1 hour at room temperature. Immunofluorescence was performed with overnight incubation with primary antibodies to KLF2 and VE-Cad (vascular endothelial cadherin; also known as CDH5), the latter of which was used to delineate endothelial cells. Samples were incubated with secondary anti–goat Alexa 488 and anti–mouse Alexa 647 antibodies for 1 hour at room temperature. Washes were performed with PBS. A confocal microscope was used to acquire fluorescent images.
Protocols of KLF2 knockdown by siRNAs and KLF2 overexpression by mRNA in human lung microvascular endothelial cells were described in our previous study (6). Absolute quantification of OAS1 or OAS3 mRNA was normalized to the geometric mean of β-actin, ubiquitin B, and GAPDH.
Results
Detailed clinical information was available for all patients with COVID-19 (Table 1). At diagnosis, the patients had severe acute hypoxemic respiratory failure and required either a simple nasal cannula or a high-flow nasal cannula. Some patients were mechanically ventilated and met Berlin criteria for ARDS. Chest X-rays showed multifocal bilateral alveolar opacities (data not shown). Pathological findings are also detailed in Table 1. Autopsy results in patients with COVID-19 demonstrate pathological findings consistent with those of ARDS (diffuse alveolar damage) as well as a significant component of superimposed pneumonia. Remarkably, only two samples demonstrated thrombosis or microthrombi.
Table 1.
Clinical and Pathological Characteristics of COVID-19 and Control Samples.
| Case No. | Age | Sex | Race | Smoking Status | Length of Hospital Stay | Immunosuppression | Ventilatory Support | Duration of MV | P/F Ratio | Lung Autopsy Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 36 | M | Black | Yes | 13 | Tocilizumab, hydrocortisone | HFNC, MV | 9 d | 64 | Acute bronchopneumonia and few microthrombi |
| C2 | 84 | F | Black | Former | 4 | None | NC | none | 191 | Lungs with acute and chronic aspiration pneumonia, focal bronchopneumonia |
| C3 | 84 | M | Black | N/A | 1 | None | HFNC | none | 65 | Reactive pneumocytes and fibrin thrombi with bilateral bronchopneumonia |
| C4 | 77 | F | Black | N/A | 15 | Tocilizumab | HFNC | none | NA | Hyaline membrane formation and focal superimposed bronchopneumonia |
| C5 | 51 | F | Black | Yes | 12 | Hydrocortisone | HFNC, MV | 7 d | 64 | Diffuse alveolar damage and mucous plugging |
| C6 | 82 | F | Black | Former | 13 | Hydrocortisone | HFNC, MV | 13 d | 63 | Diffuse alveolar damage, hemorrhage, aspiration pneumonia |
| C7 | 84 | F | Black | No | 5 | Hydrocortisone | NC | none | N/A | Acute and chronic aspiration pneumonia |
| C8 | 40 | M | Black | No | 25 | Tocilizumab | MV | 22 d | 62 | Diffuse alveolar damage, thromboemboli, areas of infarction |
| C9 | 80 | F | Black | Former | 1 | None | NC | none | N/A | Diffuse acute pneumonia and focal platelet thrombi |
| C10 | 78 | F | Black | Former | 5 | None | MV | 6 d | 141 | Diffuse alveolar damage and intraalveolar hemorrhage |
| R1 | 58 | M | N/A | Yes | N/A | N/A | MV | N/A | N/A | Recent hemorrhage (trauma) |
| R2 | 59 | F | N/A | Yes | N/A | N/A | MV | N/A | N/A | Patchy nonspecific interstitial fibrosis |
| R3 | 53 | M | N/A | Former | N/A | N/A | MV | N/A | N/A | Emphysema |
| R4 | 35 | M | N/A | Yes | N/A | N/A | MV | N/A | N/A | Emphysema, patchy nonspecific interstitial fibrosis |
| R5 | 59 | F | N/A | Yes | N/A | N/A | MV | N/A | N/A | Patchy nonspecific interstitial fibrosis |
Definition of abbreviations: C = COVID-19 case number; COVID-19 = coronavirus disease; HFNC = high-flow NC; MV = mechanical ventilation; N/A = not applicable; NC = nasal cannula; R = Gift of Hope case number.
As shown in Figure 1A, control lungs (n = 5, obtained from Gift of Hope) expressed significantly higher amounts of KLF2 protein than lungs from patients with COVID-19 (n = 10). KLF2 expression largely colocalized with VE-Cad. Only KLF2 protein, and not VE-Cad, was reduced in the SARS-CoV-2–infected lungs, suggesting that KLF2 reduction in patients with COVID-19 is not due to endothelial loss. Representative immunofluorescence images with histological counterparts are provided. Six random fields per sample were analyzed, with quantification being blinded to the condition.
Figure 1.
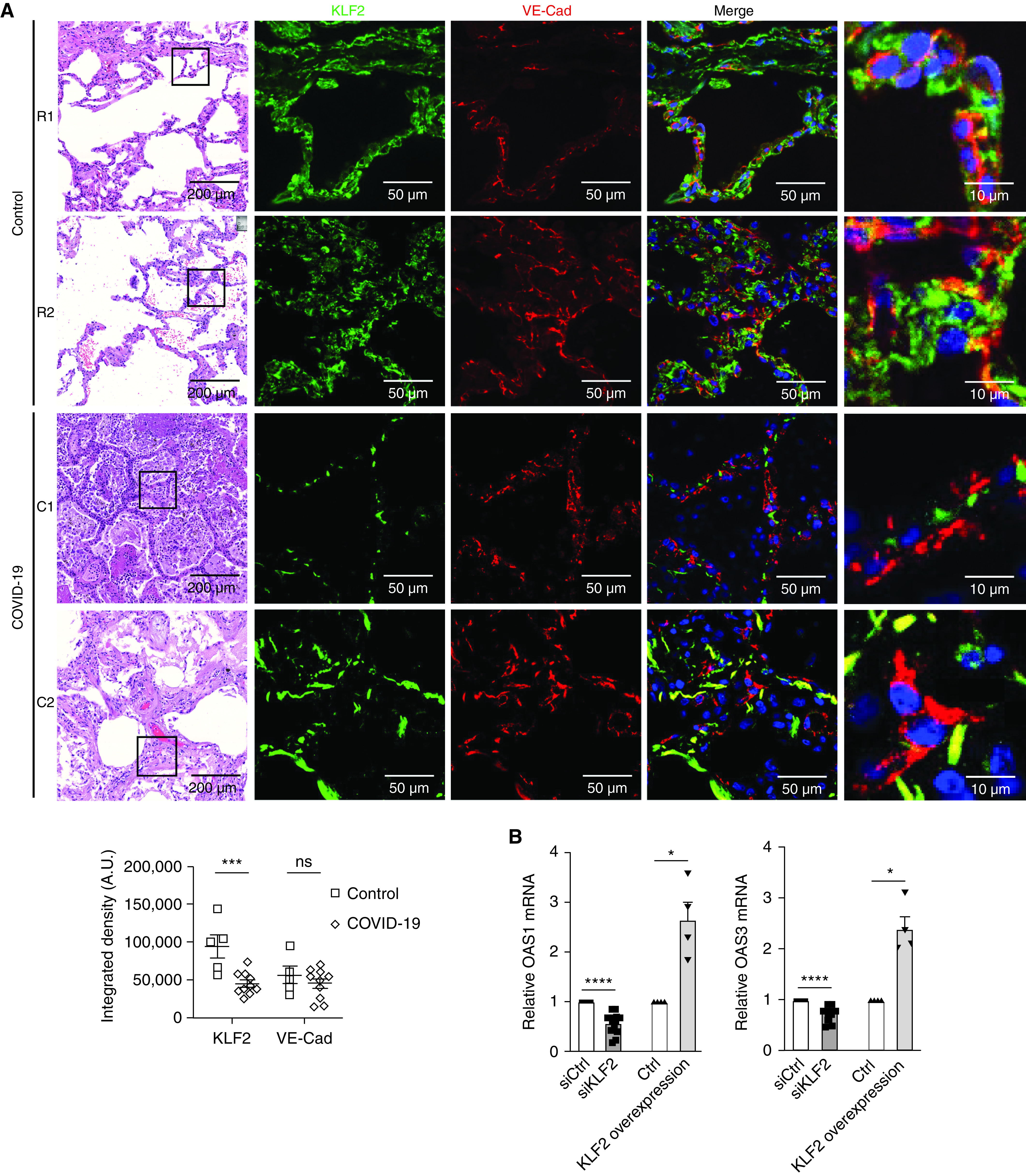
Endothelial KLF2 (Krüppel-like factor 2) is reduced in coronavirus disease (COVID-19) lung autopsy samples and increases OAS1 (2′-5′ oligoadenylate synthetase 1) and OAS3 mRNA. (A) Both control (Ctrl) and COVID-19 lung autopsy samples demonstrate distorted architecture on histology. Immunofluorescence is performed for KLF2 and VE-Cad (vascular endothelial cadherin) and is quantified below the images. In Ctrl lungs, the KLF2 signal (green) colocalized with VE-Cad (red), which highlights pulmonary vasculature (n = 5), is shown. In COVID-19 samples, the KLF2 signal is significantly reduced when compared with that of Ctrl lungs (n = 10). There is no significant difference in VE-Cad between Ctrl and COVID-19 samples. Scale bars: first column, 200 μm; second and third columns, 50 μm; fourth column 10 μm. (B) Human lung microvascular endothelial cells treated with siKLF2 (siRNA against KLF2) had reduced OAS1 and OAS3 compared with Ctrl cells and had increased OAS1 and OAS3 when transfected with KLF2 transcripts. Error bars show the SEM. Statistical significance was determined by using a Student’s t test. *P < 0.05, ***P < 0.001, and ****P < 0.0001. A.U. = arbitrary units; C = COVID-19 sample number; ns = not significant; R = Ctrl sample number; siCtrl = control siRNA.
KLF2 maintains the endothelial expression of OAS1 and OAS3, which are antiviral genes encoding OASs that activate RNases to degrade double-stranded RNA, an intermediate in coronaviruses (8). A GWAS recently reported a significant association between genetic variants in human OAS genes and COVID-19 severity (7). OAS1 variants were previously implicated in susceptibility to SARS-CoV in Vietnam and China (9, 10). In Figure 1B, we demonstrate that silencing KLF2 (with siKLF2 [siRNA against KLF2]) in human lung microvascular endothelial cells significantly reduces OAS1 and OAS3, whereas overexpression of KLF2 significantly increases OAS1 and OAS3 expression. These results implicate lung vasculature KLF2 in antiviral protection against coronaviruses in addition to its homeostatic functions.
Discussion
Lung microvascular dysfunction is instrumental to the breakdown of the alveolar–vascular barrier, resulting in edema and neutrophil recruitment, which are followed by radiographic opacities and hypoxemia due to shunt formation. Endothelial KLF2 is a major regulator of microvascular quiescence and integrity; here, we show that endothelial KLF2 also maintains OAS1 and OAS3 expression. Our data demonstrate that lung endothelial KLF2 is significantly downregulated with SARS-CoV-2 infection, suggesting that endotheliitis and vascular dysfunction contribute to COVID-19 pneumonia and ARDS.
COVID-19 has generated lay controversy because of an apparent disproportion of hypoxia to the work of breathing; patients may have hypoxemia but relatively mild dyspnea. It is unknown whether dysfunctional pulmonary blood flow itself can cause hypoxia in COVID-19 pneumonia. One proposed mechanism is dysregulated nitric oxide synthesis, which under normal conditions provides appropriate ventilation–perfusion matching. Capillary flow maintains expression of endothelial KLF2, which is a direct transcriptional activator of eNOS (endothelial nitric oxide synthase; also known as NOS3). Thus, poor blood flow in pneumonia may itself reduce nitric oxide release, independently of alveolar filling or the presence of virus. KLF2 also maintains blood fluidity by inhibiting vascular thrombosis (11), another postulated mechanism for hypoxia in COVID-19. Interestingly, statins, potent activators of endothelial KLF2, is associated with reduced mortality of patients with COVID-19 (12), although this was a retrospective study. Given that statins increase endothelial KLF2 to promote nitric oxide production, which is antithrombotic, strengthens the vascular barrier, and promotes antiviral activity, we believe that a randomized clinical trial studying statins in COVID-19 pneumonia would be clinically useful. Another proposed mechanism is that reduction of KLF2 may impair antiviral defenses through OAS1 and OAS3, increasing susceptibility to viral survival, although this has to be verified in future mechanistic studies. One limitation is that the controls were nontransplantable donor lungs that had also undergone mechanical ventilation for an undetermined length of time. Furthermore, a comparison with other respiratory viruses, such as influenza, would be necessary to assess the specificity of vasculopathy in COVID-19 or whether vasculopathy is a general phenomenon of virally induced lung injury. Lastly, KLF2 is implicated in the development of metabolic diseases such as obesity and coronary artery disease (13, 14) and may regulate innate immunity in humans (15), but whether the diseases themselves regulate KLF2 is unknown.
Immune suppression via corticosteroids is now used to treat severe COVID-19 pneumonia (16). Theoretically, corticosteroids suppress the immune cell–mediated cytokine storm, which causes widespread organ dysfunction and hypotension. In the endothelium, glucocorticoids were also shown to inhibit major proinflammatory cytokines and chemokines (17), which are also suppressed by KLF2. Thus, corticosteroids may also dampen vascular inflammation associated with the cytokine storm in ARDS. Further investigation into vascular dysfunction in ARDS, especially in COVID-19, may yield mechanistic insight and therapeutic benefit with vessel-targeted therapy.
Footnotes
Supported by National Heart, Lung. and Blood Institute, National Institutes of Health, grants K99HL145113 (D.W.), K08HL153955 (N.S.), K23HL146942 (A.A.), R01HL138223 (Y.F.), and HL136765 (Y.F.); National Institute of Environmental Health Sciences, National institutes of Health, grant ES015024 (G.M.M.); U.S. Department of Defense grant W81XWH-16-1-0711; the Chicago Biomedical Consortium; and American Heart Association grant 20TPA35490401 (Y.F.).
Author Contributions: T.-H.L. performed experiments, analyzed and interpreted data, and edited the manuscript. R.-T.H. performed experiments, analyzed and interpreted data, and edited the manuscript. R.G. performed sample collection and processing, analyzed and interpreted data, and edited the manuscript. N.S. and A.A. analyzed and interpreted data and edited the manuscript. A.S., J.M., and A.H. performed sample collection, processed and interpreted the data, and edited the manuscript. D.W., G.M.M., and Y.F. designed experiments, analyzed and interpreted data, and wrote and edited the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0564LE on May 10, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, et al. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang RT, Wu D, Meliton A, Oh MJ, Krause M, Lloyd JA, et al. Experimental lung injury reduces Krüppel-like factor 2 to increase endothelial permeability via regulation of RAPGEF3-Rac1 signaling. Am J Respir Crit Care Med. 2017;195:639–651. doi: 10.1164/rccm.201604-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. GenOMICC Investigators; ISARIC4C Investigators; COVID-19 Human Genetics Initiative; 23andMe Investigators; BRACOVID Investigators; Gen-COVID Investigators. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 8. Choi UY, Kang JS, Hwang YS, Kim YJ. Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases. Exp Mol Med. 2015;47:e144. doi: 10.1038/emm.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamano E, Hijikata M, Itoyama S, Quy T, Phi NC, Long HT, et al. Polymorphisms of interferon-inducible genes OAS-1 and MxA associated with SARS in the Vietnamese population. Biochem Biophys Res Commun. 2005;329:1234–1239. doi: 10.1016/j.bbrc.2005.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He J, Feng D, de Vlas SJ, Wang H, Fontanet A, Zhang P, et al. Association of SARS susceptibility with single nucleic acid polymorphisms of OAS1 and MxA genes: a case-control study. BMC Infect Dis. 2006;6:106. doi: 10.1186/1471-2334-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 12. Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187, e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sweet DR, Vasudevan NT, Fan L, Booth CE, Keerthy KS, Liao X, et al. Myeloid Krüppel-like factor 2 is a critical regulator of metabolic inflammation. Nat Commun. 2020;11:5872. doi: 10.1038/s41467-020-19760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Das H, Kumar A, Lin Z, Patino WD, Hwang PM, Feinberg MW, et al. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci USA. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahabeleshwar GH, Kawanami D, Sharma N, Takami Y, Zhou G, Shi H, et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34:715–728. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zielińska KA, Van Moortel L, Opdenakker G, De Bosscher K, Van den Steen PE. Endothelial response to glucocorticoids in inflammatory diseases. Front Immunol. 2016;7:592. doi: 10.3389/fimmu.2016.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]


