Cystic fibrosis (CF) lung disease, caused by abnormal ion transport because of deficient CFTR (cystic fibrosis transmembrane conductance regulator) function, is characterized by a dehydrated, hyperconcentrated mucus layer, leading to persistent bacterial infection (1). The conducting airways of the lung are composed of two distinct regions. The large proximal bronchial airways contain cartilage and submucosal glands, whereas the glandless small distal bronchiolar airways constitute the major conducting airway surface area. Although defective mucociliary transport and abnormal airway surface liquid (ASL) pH have been described in CF pig large airways (2–4), differences in mechanisms regulating ASL homeostasis in the small airways are poorly understood. On the basis of combined data from pathology (5), pulmonary function (6), imaging (7), and direct lung sampling (8) studies, the small airways are likely crucial contributors to the development of mucus-obstructive airway diseases, including CF, raising the question of why small airways are so vulnerable. On the basis of structural differences in each airway region, coupled with different epithelial cell type compositions lining the two airway regions (9, 10), it is proposed that ASL homeostasis may be differentially regulated. However, much of the work focusing on the effects of the absence of CFTR anion transport in the airway has used ex vivo large airways, including their use as a source for primary cell cultures (11). The pathogenesis and progression of mucus occlusion, inflammation, and infection in small airways remains understudied because of the limited accessibility of human small airways, lack of animal models recapitulating human small airways, and limited availability of well-characterized in vitro models. In this issue of the Journal, Li and colleagues (pp. 146–156) describe how they tackled these hurdles using ex vivo pig small airway explants and in vitro pig small airway cell cultures (12). Their studies reveal a small airway–specific mechanism regulating ASL pH via a vacuolar type H+ adenosine triphosphatase, ATP6V0D2 (Figure 1). In particular, these studies focus on mechanisms of aberrant mucus in CF small airways and how these changes may initiate lung pathology.
Figure 1.
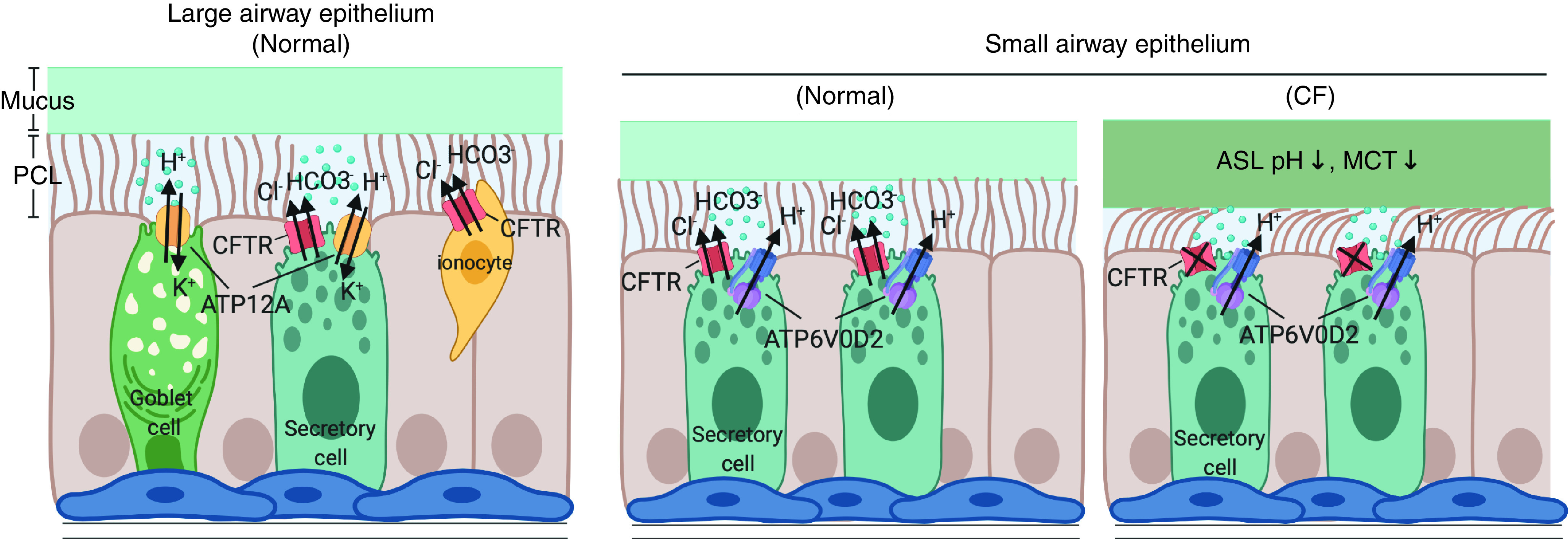
Differential regulation of airway surface liquid (ASL) pH in the pig large and small airway epithelium. The diagram shows proposed differential mechanisms regulating ASL pH in the large and small airways mediated by ATP12A and ATP6V0D2, respectively, balanced with CFTR (cystic fibrosis transmembrane conductance regulator). In CF pig small airways, defective anion transport into the airway lumen (i.e., Cl− and HCO3−), mediated by CFTR, results in reduced ASL pH and impaired mucociliary transport. CF = cystic fibrosis; MCT = mucociliary transport; PCL = periciliary layer.
In the large airway, impaired epithelial chloride and bicarbonate secretion causes mucus dehydration and reduces ASL pH, resulting in defective mucus transport and reduced bacterial killing at the airway surface (2, 3, 13, 14). Decreased ASL pH has been detected in multiple experimental models of CF (15, 16), although lower pH has not been found by direct in vivo measurement in the human CF lung (17). In addition to CFTR bicarbonate transport in the large airway, ASL pH is regulated by epithelial proton transporters, notably ATP12A, the α-subunit of the nongastric H+/K+ adenosine triphosphatase, which secretes H+ into the lumen to regulate pH homeostasis in pig and human airways (13). Inhibiting ATP12A function in these tissues normalizes CF ASL pH and reduces bacterial proliferation (18), identifying it as an important contributor to ASL homeostasis.
Although the effects of ATP12A and reduced pH on airway biology have been studied in large airways, it is not known how these factors affect small airways. Li and colleagues set out to answer this question using epithelial cells cultured from microdissected small airways from normal and CF pigs. Although ASL pH in CF pig small airway epithelial cell cultures was reduced compared with non-CF cultures, similar to results obtained in large airways, ATP12A expression was not detected in the small airways. The small airway epithelial cells also did not respond to ouabain, an ATP12A inhibitor, confirming the absence of this transporter. Rather, the authors detected the presence of a different transporter, ATP6V0D2, expressed in secretory but not ciliated cells. Blocking ATP6V0D2, thereby increasing pH, decreased ASL viscosity in the small airway cultures. Li and colleagues also detected upregulation of ATP6V0D2 when pH was artificially increased, suggesting that expression can be regulated to adjust pH when necessary, presumably in response to an insult.
These studies make several important contributions. ATP6V0D2 appears to be a small airway proxy for ATP12A, marking a distinction in mechanisms regulating ASL pH in each airway region. CF pig small airway explants responded abnormally to ATP stimulation with reduced mucus transport. The tunability of small airway pH is likely important to maintain homeostasis, especially in response to mucus secretion, and could be a significant factor preventing small airway mucus occlusion. The presence of enriched ATP6V0D2 expression on secretory cells may indicate a unique role of this cell type to maintain normal small airway ASL physiology. The specificity of proton transporter expression may enable targeting of small airways to address early CF lung disease, an approach that is currently limited in CF and may be applicable to CFTR variants that are nonresponsive to currently available modulators. Thus, not only have Li and colleagues shown for the first time that mucociliary transport is decreased in CF small airways, as has been long known in CF large airways (1), but they have also illustrated potentially important small airway therapeutic targets.
Like many other important studies, these experiments raise additional questions. The authors did not use ATP6V0D2 inhibition in excised CF small airways to examine pH after ATP administration and the effects on ASL viscosity or mucus transport. More precise and in-depth measures of mucin secretion, concentration, and conformation would add to our understanding of the impact of altered small airway pH. In addition, because pH differences in the human lung in vivo have yet to be established, it is critically important to test whether the findings in the pig small airway demonstrated here are replicated in the human small airway. These questions must be addressed before the full impact of ATP6V0D2 on human small airway physiology is understood. In summary, the work of Li and colleagues adds to the growing data supporting the importance of the small airways in muco-obstructive diseases and offers a potential therapeutic target. ▪
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2021-0070ED on April 8, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA. 2014;111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62:153–161. doi: 10.1136/thx.2006.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ranganathan SC, Stocks J, Dezateux C, Bush A, Wade A, Carr S, et al. The evolution of airway function in early childhood following clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 2004;169:928–933. doi: 10.1164/rccm.200309-1344OC. [DOI] [PubMed] [Google Scholar]
- 7. Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 8. Esther CR, Jr, Muhlebach MS, Ehre C, Hill DB, Wolfgang MC, Kesimer M, et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med. 2019;11:eaav3488. doi: 10.1126/scitranslmed.aav3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okuda K, Chen G, Subramani DB, Wolf M, Gilmore RC, Kato T, et al. Localization of secretory mucins MUC5AC and MUC5B in normal/healthy human airways. Am J Respir Crit Care Med. 2019;199:715–727. doi: 10.1164/rccm.201804-0734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, Zoso A, et al. Animal and model systems for studying cystic fibrosis. J Cyst Fibros. 2018;17:S28–S34. doi: 10.1016/j.jcf.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Villacreses R, Thornell IM, Noriega J, Mather S, Brommel CM, et al. V-type ATPase mediates airway surface liquid acidification in pig small airway epithelial cells. Am J Respir Cell Mol Biol. 2021 doi: 10.1165/rcmb.2020-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Birket SE, Davis JM, Fernandez CM, Tuggle KL, Oden AM, Chu KK, et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight. 2018;3:97199. doi: 10.1172/jci.insight.97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scudieri P, Musante I, Caci E, Venturini A, Morelli P, Walter C, et al. Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight. 2018;3:123616. doi: 10.1172/jci.insight.123616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, et al. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun. 2017;8:1409. doi: 10.1038/s41467-017-00532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simonin J, Bille E, Crambert G, Noel S, Dreano E, Edwards A, et al. Airway surface liquid acidification initiates host defense abnormalities in cystic fibrosis. Sci Rep. 2019;9:6516. doi: 10.1038/s41598-019-42751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


