Abstract
Vascular endothelial growth factor (VEGF) is a potent angiogenic inducer that stimulates the expression of tissue factor (TF), the major cellular initiator of blood coagulation. Here we show that signaling triggered by VEGF induced DNA-binding and transcriptional activities of nuclear factor of activated T cells (NFAT) and AP-1 in human umbilical vein endothelial cells (HUVECs). VEGF also induced TF mRNA expression and gene promoter activation by a cyclosporin A (CsA)-sensitive mechanism. As in lymphoid cells, NFAT was dephosphorylated and translocated to the nucleus upon activation of HUVECs, and these processes were blocked by CsA. NFAT was involved in the VEGF-mediated TF promoter activation as evidenced by cotransfection experiments with a dominant negative version of NFAT and site-directed mutagenesis of a newly identified NFAT site within the TF promoter that overlaps with a previously identified κB-like site. Strikingly, this site bound exclusively NFAT not only from nuclear extracts of HUVECs activated by VEGF, a stimulus that failed to induce NF-κB-binding activity, but also from extracts of cells activated with phorbol esters and calcium ionophore, a combination of stimuli that triggered the simultaneous activation of NFAT and NF-κB. These results implicate NFAT in the regulation of endothelial genes by physiological means and shed light on the mechanisms that switch on the gene expression program induced by VEGF and those regulating TF gene expression.
Angiogenesis, the sprouting of new capillaries from preexisting vascular beds, is a multistep program that involves the activation, proliferation, and migration of endothelial cells. Vascular endothelial growth factor (VEGF) is a potent angiogenic inducer that has been implicated in physiological and physiopathological conditions associated with angiogenesis (19). Thus, VEGF plays a major role in vasculogenesis and angiogenesis during embryonic development (6, 18), and enhanced expression of VEGF has been detected in processes associated with the menstrual cycle, pregnancy, rheumatoid arthritis, wound healing, diabetic retinopathy, and atherosclerosis (4, 19). In addition, there is considerable evidence to support a critical role of VEGF in tumorigenesis, which may be related to the neovascularization required for tumor growth and dissemination. Thus, VEGF mRNA is significantly enhanced in most tumors analyzed so far, and multiple cell lines have been found to synthesize and secrete VEGF. Furthermore, anti-VEGF antibodies inhibit the growth of tumors in vivo (4, 27, 63).
VEGF displays a potent mitogenic activity for endothelial cells and increases endothelial-cell permeability and migration (19). The biological effects of VEGF on endothelial cells are exerted through its binding to Flt-1 and Flk-1/KDR, two high-affinity tyrosine kinase receptors, both of which are related to the platelet-derived growth factor receptors (16, 40, 48). Signaling through such receptors initiates the activation of the intracellular signal transduction cascades that switch on the expression of genes that control the specific response to VEGF. Thus, VEGF induces the expression of the plasminogen activators (PA) uPA and tPA, the urokinase receptor (uPAR), and the metalloproteinase interstitial collagenase, facilitating the extracellular matrix degradation and further migration and sprouting of endothelial cells (19). VEGF also induces the expression of tissue factor (TF) (9), a glycoprotein expressed by monocytes and endothelial cells that functions as the high-affinity receptor and cofactor for the coagulation factors VII/VIIa and is the main initiator of the extrinsic pathway of the coagulation cascade. The induction of TF expression on the surface of monocytes and endothelial cells is upregulated upon activation with a number of stimuli including the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), the bacterial lipopolysaccharide (LPS), phorbol esters, and thrombin, as well as VEGF (34). TF is thought to play a major role in thrombogenic disorders in the setting of inflammation, septic shock, or cancer (34, 35, 52). Besides these properties, the targeted disruption of TF results in embryonic death due to a defective yolk sac vessel and abnormal vitelloembryonic circulation (7), a phenotype that in part resembles that found in VEGF-deficient embryos (6, 18).
Although many reports have addressed the biological responses of endothelial cells to VEGF, the intracellular transduction pathways that connect the signals between the cell membrane receptors and the nucleus, as well as the transcription factors that couple such signaling to the expression of the genes that regulate the cellular response to VEGF, are beginning to be understood. In this regard, a number of intracellular signaling components targeted by VEGF have been recently identified in different cell systems. VEGF induces tyrosine phosphorylation of molecules containing SH2 domains as well as other signaling molecules including phospholipase Cγ, phosphatidylinositol 3-kinase, GTPase-activating protein, p125FAK, paxillin, and the adapter proteins Nck and shc (1, 12, 21, 28, 56, 62). In addition, a number of recent reports have implicated VEGF in the activation of different members of the family of mitogen-activated protein kinases (MAPKs) including the extracellular signal-regulated kinases (ERKs), the stress-activated protein kinases/c-jun N-terminal kinases (SAPKs/JNKs), and the p38/HOG kinase (1, 12, 28, 30, 53, 56).
In accordance with a functional activation of phospholipase Cγ, VEGF has also been reported to induce turnover of inositol phosphates, diacylglycerol production, and elevation of intracellular Ca2+ concentrations (3, 45, 67). In different cell types, calcium signals lead to the activation of NFAT proteins, a family of transcription factors composed of at least four structurally related members, NFATp (NFAT1), NFATc (NFAT2), NFAT3, and NFAT4 (51). Calcineurin, a Ca2+/calmodulin-dependent phosphatase, regulates the processes of dephosphorylation and nuclear import of NFAT, both of which are blocked by the immunosuppressive drugs cyclosporin A (CsA) and FK506 (58, 59). Once in the nucleus, NFAT proteins can cooperate with transcription factors of the Fos and Jun families to regulate the inducible expression of a number of genes involved in the regulation and function of the immune response (50). Thus, NFAT proteins have been involved in the regulation of the gene expression of IL-2, IL-4, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, or CD40 and Fas ligands (11, 51). Recently, NFATp has been found in endothelial cells, where it participates in the inducible expression of the GM-CSF gene in response to pharmacological activation by phorbol myristate acetate (PMA) plus Ca2+ ionophore (10). In addition, during embriogenesis NFATc is expressed in the endocardium, a highly specialized endothelium that is involved in the morphogenesis of cardiac valves and septum, which are not developed in mice bearing a targeted disruption in the NFATc gene (13, 49). However, a functional role of NFAT proteins regulating the expression of endothelial genes in response to physiological stimuli has not yet been addressed.
Because of the involvement of MAPK cascades and increases in the intracellular calcium concentration in the signaling induced by VEGF, we here analyzed the role of NFAT and AP-1 in the activation of primary human endothelial cells by VEGF. We show that in human umbilical vein endothelial cells (HUVECs), VEGF triggers the dephosphorylation, translocation, and transcriptional activity of NFATp, which is accompanied by AP-1 activation. Further analysis involved NFAT in the regulation of the TF gene expression induced by VEGF. These results provide information on the mechanisms that mediate the gene expression program induced by VEGF and involve the transcription factors NFAT and AP-1 in the regulation of the expression of endothelial genes by physiological means. Moreover, the inhibitory effects of CsA on TF gene expression may be related to the beneficial effects displayed by this drug in pathological processes associated with a deregulation of TF expression.
MATERIALS AND METHODS
Cell culture and reagents.
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical veins as previously described (42) and routinely grown on 0.5% gelatin-coated tissue culture flasks in medium 199 (Biowhittaker) supplemented with 20% fetal calf serum (FCS), 50 μg of bovine brain extract per ml, and 100 μg of heparin per ml. The cells were used between passages 6 and 10. Jurkat cells were maintained in RPMI 1640 medium (Gibco-BRL) supplemented with 10% FCS. The recombinant human VEGF165 was purchased from Peprotech EC Ltd., (London, United Kingdom) or provided by H. Riese and I. Prieto (Pharmacia-Upjohn, Madrid, Spain). CsA was from Sandoz. TNF-α (3.2 × 107 U/mg) was from Wichem (Vienna, Austria). PMA, the calcium ionophore A23187, and actinomycin D were from Sigma Chemical Co. (St. Louis, Mo).
Immunofluorescence experiments.
HUVECs grown on 1% gelatin-coated coverslips in 24-well tissue culture plates were either left untreated or incubated for 2 h with CsA (200 ng/ml) before stimulation with VEGF (50 ng/ml), Ca2+ ionophore (1 μM), or TNF-α (25 ng/ml) for 20 min. The cells were then fixed with 3% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature and washed three times (5 min each) with washing buffer (PBS, 0.01% [vol/vol] Nonidet P-40 [NP-40]). After blocking for 30 min at 37°C with TNB buffer (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.5% blocking reagent [Boehringer Mannheim]), the coverslips were incubated for 45 min at 37°C with the anti-NFATp monospecific antiserum 67.1 (22) (0.01% [vol/vol] in TNB), provided by Anjana Rao. Unbound antibody was removed by rinsing twice with washing buffer (5 min at room temperature), and the coverslips were incubated for 20 min at 37°C with a fluorescein-conjugated anti-rabbit immunoglobulin (Ig; Amersham) (0.1% [vol/vol] in TNB), washed twice, and mounted in Mowiol mountant on glass slides. The cells were visualized with a Nikon Labophot-2 photomicroscope.
Subcellular fractionation and Western blot analysis.
After the different treatments, confluent HUVECs grown in 35-mm culture dishes (one dish per condition) were washed with cold PBS and lysed in 100 μl of hypotonic buffer (10 mM Tris-HCl [pH 7.5] containing 10 mM NaCl, 3 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol [DTT], 0.1 mM EGTA, 2 μM leupeptin, 1 μg of aprotinin per ml, and 0.05% NP-40). The supernatant containing the cytosolic extracts was removed and resuspended in Laemmli buffer, and the nuclei were washed twice in hypotonic buffer without detergent and resuspended in Laemmli buffer. Whole-cell extracts from Jurkat cells were prepared by direct lysis in Laemmli buffer of PBS-washed cells collected after centrifugation. To determine the effect of RNA synthesis inhibition on the nuclear export of NFATp, HUVECs were pretreated with actinomycin D (5 μg/ml) for 4 h, washed, and lysed 5 min, 1 h, and 4 h after activation with VEGF.
The different extracts were boiled and resolved, under reducing conditions, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (6 or 12% polyacrylamide) for NFATp or proliferating-cell nuclear antigen detection, respectively. The gels were transferred to nitrocellulose membranes and blocked with a 5% (wt/vol) skim milk solution in Tris-buffered saline (TBS) buffer at 4°C overnight. After being washed twice in TBS-T (TBS, 0.05% Tween 20), the membranes were incubated for 2 h at room temperature with 0.03% or 0.01% (vol/vol) 67.1 and anti-PCNA (clone PC10; Dako) monoclonal antibody (64), respectively, in TBS-T. The membranes were washed five times for 5 min in TBS-T, incubated for 2 h at room temperature with the corresponding secondary antibody (peroxidase-labeled goat anti-rabbit IgG or anti-mouse IgG plus IgM [Pierce]), washed three times with TBS-T, and briefly rinsed in distilled H2O. Bound antibodies were detected with the ECL Western blotting analysis kit (Amersham).
Nuclear extracts and EMSAs.
For nuclear protein extraction, attached HUVECs grown in 150-mm dishes were lysed with 2 ml of hypotonic buffer containing 0.05% NP-40 (described above), except that 0.75 mM spermidine, 0.15 mM spermine, 10 mM Na2MoO4, and pepstatin (1 μg/ml) were also included. Nuclei were detached from the plates, collected, incubated in buffer C (20 mM HEPES [pH 7.6], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 10 mM Na2MoO4, 1 μg of pepstatin per ml, 4 μg of leupeptin per ml, 4 μg of aprotinin per ml) for 30 min in a rocking platform, and centrifuged at 15,000 × g for 10 min and the supernatants, containing the nuclear extracts, were frozen immediately in liquid nitrogen and stored at −80°C. The protein concentration was quantified by the Bradford procedure.
Electrophoretic mobility shift assays (EMSAs) were performed by incubating the nuclear proteins (1.5 to 2 μg) with 1 μg of poly(dI-dC) and 4 μl of 5× DNA binding buffer (10% [wt/vol] polivinylethanol, 12.5% [vol/vol] glycerol, 50 mM Tris [pH 8], 2.5 mM EDTA, 2.5 mM DTT) in a final volume of 18 μl on ice for 10 min. Then 2 μl (1 ng/μl) of 32P-labeled double-stranded oligonucleotide (5 × 107 to 1 × 108 cpm/μg), was added to the reaction mixture, which was incubated at 4°C for an additional 30 min. For competition experiments, a 30-fold molar excess of unlabeled oligonucleotide was added before the addition of the probe. When indicated, nuclear extracts were incubated with 0.5 μl of either a preimmune serum, the anti-NFATp antiserum 67.1, or the 796 anti-all NFAT antiserum (33) or with 1 μl of the antiserum 1226 raised against the p65 NF-κB subunit. Antisera were incubated for 15 min at 4°C before the addition of the probe. DNA-protein complexes were resolved by electrophoresis in 4% nondenaturing polyacrylamide gels. The sequences of the oligonucleotides (5′ to 3′) used in these experiments were as follows: gatcATAAAATTTTCCAATGTAAAC (mouse NFAT P sequence −60 to −80 of the murine IL-4 promoter), gatcGTAGACCGTGATTCAAGCTTAGC (human AP-1 −284 site of the ICAM-1 promoter), gatcGGGATTTCACCT (NF-κB-binding site of the human IL-2 promoter), ctagCCGGAGTTTCCTACC (nucleotides −183 to −197 of the human TF promoter [positions −186 to −191 boldface]), ctagCCGGAGGAATTCACC (nucleotides −183 to −197 of the human TF promoter containing mutated bases at positions −186 to −191 [boldface]), and AGTTGAGGGGACTTTCCCAGGC (oligonucleotide including the prototypic NF-κB site of the murine κ light-chain enhancer).
Plasmid constructs and transient-transfection assays.
The luciferase reporter plasmid NFAT-Luc, containing three tandem copies of the distal NFAT-binding site of the IL-2 gene promoter coupled to the IL-2 minimal promoter, and the pSH102CΔ418 NFATc expression plasmid, a derivative of plasmid pBJ5 that encodes a dominant negative truncated version of NFAT, have been previously described (17, 43) and were provided by G. Crabtree. The reporter plasmids pL1 (−2106 to +121) and pL4 (−278 to +121), containing deletion fragments of the TF promoter inserted into the cloning site of the p19luc luciferase vector (37), were provided by N. Mackman. The pCMV-TAM67, a derivative of pCMV plasmid that encodes a c-Jun dominant negative version (47), was a gift from C. M. Zacharchuk. The AP-1-dependent reporter plasmid −73 Col Luc containing the −73 to +63 region of the human collagenase promoter coupled to the luciferase gene (15) was provided by M. Karin. The AP-1-dependent luciferase construct driven by the −36 to +37 rat prolactin minimal promoter under the control of four tandem copies of the TPA-responsive element (TRE) consensus motif TGACTCA (AP-1 PROL Luc) and the parental vector (PROL Luc) were provided by M. Rincón.
For transient-transfection experiments, HUVECs were plated in 100-mm tissue culture dishes (1.5 × 106 cells/plate) the day before transfection. The cells were transfected in 4 ml of Dulbecco’s minimal essential medium plus 0.5% FCS using 10 μg of the indicated luciferase reporter plasmids, by the calcium phosphate procedure as previously described (42). Briefly, HUVECs were incubated with precipitated DNA for 4.5 h, washed twice with PBS, and detached with trypsin from the 100-mm plates. After centrifugation (1,200 rpm for 5 min in a Sorvall H1000B rotor), the cells were resuspended in OPTI-MEM (Life Technologies) supplemented with 0.5% FCS and split among six-well (35-mm) tissue culture plates (2.5 35-mm dishes were plated from one 100-mm plate) precoated with 0.5% of gelatin. Transfected cells were incubated at 37°C for 16 h and exposed to different stimuli for an additional 6 h unless otherwise specified. Then the cells were lysed and luciferase activity was measured in a Lumat LB 9501 luminometer (Berthold, Wildbad, Germany) as specified in the instructions of a Luciferase system kit (Promega). In cotransfection experiments, 5 μg of pBJ5 and pSH102CΔ418 or 1.7 μg of pCMV-TAM67 and pCMV-β-galactosidase expression plasmids were coprecipitated together with the reporter vector. Transfection experiments were performed in triplicate. The data presented are expressed as the mean and standard deviation of the determinations performed in triplicate. A representative experiment is shown in the reporter assays in all cases. The expression of Renilla luciferase was used as an internal control to normalize the values obtained with the firefly luciferase constructs. A total of 0.5 μg of the Renilla luciferase expression vector pRLCMV (Promega) was used in cotransfection experiments. In these experiments, 1/10 of cells cotransfected with both types of luciferase plasmids were plated in 24-well tissue culture plates, incubated at 37°C for 24 h, and lysed with passive lysis buffer. Renilla luciferase activity was measured by using the Dual luciferase assay kit (Promega), as specified by the manufacturer, to discriminate the activity of the two types of luciferases.
Northern blot analysis.
After different treatments, total RNA was isolated from attached HUVECs by using the Ultraspect system (Biotecx Laboratories, Inc.). Denatured RNA (20 μg per sample) was electrophoresed on 1% formaldehyde–agarose gels and blotted onto a nitrocellulose membrane. After UV cross-linking, the membranes were hybridized overnight at 42°C with specific probes. A tissue factor probe spanning positions 95 to 925 of the TF cDNA was generated by PCR and cloned into the pCR2.1 plasmid (Invitrogen) by using the oligonucleotides and conditions previously reported for this purpose (23). The resulting plasmid was digested with EcoRI and the fragment of the TF cDNA from 95 to 853 was used as a probe. For c-fos and c-jun mRNA detection, the 0.8-kb BglII-NcoI fragment of the c-fos cDNA and a 0.8-kb HindIII-PstI fragment of c-jun cDNA were used.
Site-directed mutagenesis.
Mutation of the NFAT binding site localized at positions −186 to −194 of the TF promoter was performed by PCR with the pL4 plasmid as a template. Primer pL4 HindIII sense (5′ tttaagcttGGGCAACTAGACCCGCCTGC) and pL4 EcoRI antisense (5′ tttgaattcCTCCGGGACCCTGCAAGGG 3′) were used to generate PCR fragment A. Primer Mut EcoRI sense (5′ tttgaattcACCGGGAGGAGGCGGGGC) and TF XmaI antisense (5′ CCGGCCCGGGTCACTTGCC) were used to generate fragment B. Fragments A and B were subjected to 35 cycles of amplification with the following thermal cycle: 94°C for 30 s, 64°C for 30 s, and 72°C for 90 s. Primers pL4 EcoRI antisense and Mut EcoRI sense were partially complementary and carried point mutations that transform the NFAT core-binding site into an EcoRI restriction site. PCR fragments A and B were digested with HindIII and EcoRI or with EcoRI and XmaI, respectively, and the resulting fragments were ligated into the HindIII-XmaI-digested pL4 vector to generate the pL4 mut (−186 to −194) plasmid. The nucleotide sequence of the mutant was confirmed by DNA sequencing.
RESULTS
VEGF triggers NFATp dephosphorylation and nuclear translocation in HUVECs by a CsA-sensitive mechanism.
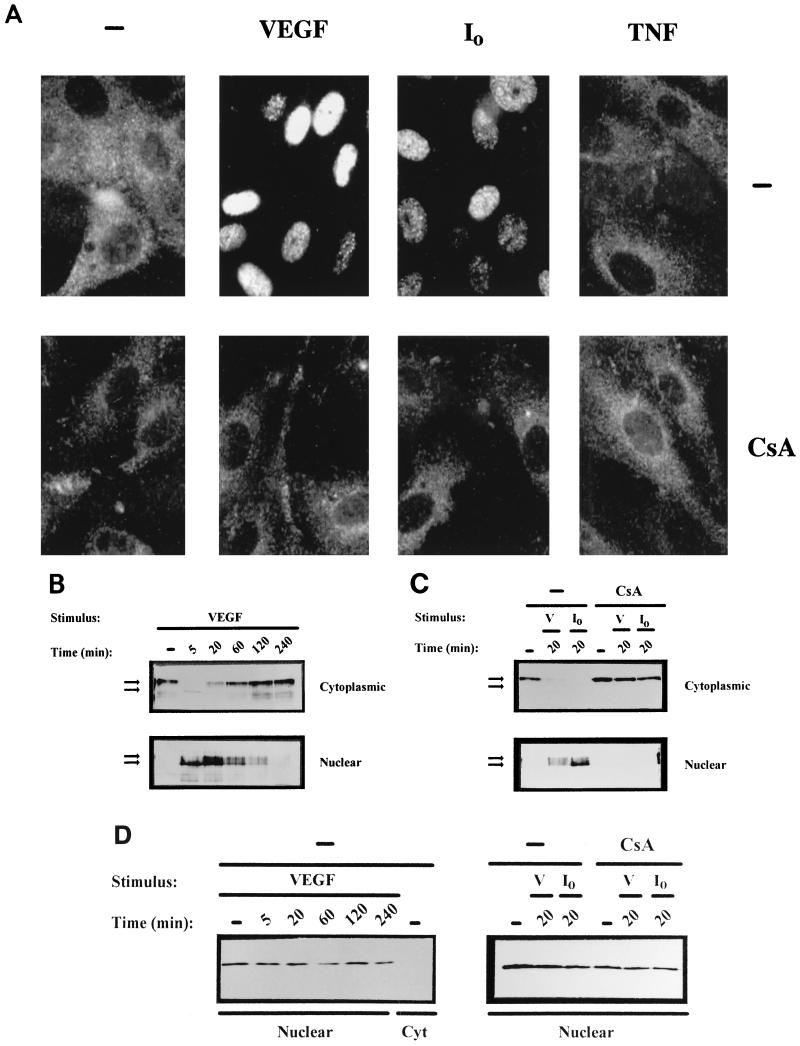
To determine whether NFAT could mediate transcriptional responses in endothelial cells stimulated with VEGF, we first performed immunocytochemical analysis with HUVECs by using a specific anti-NFAT1/NFATp antiserum. As shown in Fig. 1A, endogenous NFATp was present in the cytoplasm of resting cells and translocated to the nucleus upon activation with calcium ionophore. Strikingly, stimulation by VEGF also resulted in complete translocation of NFATp to the nucleus whereas TNF-α failed to modify the cytoplasmic localization of the transcription factor. Furthermore, preincubation with pharmacological concentrations of CsA resulted in the total prevention of the nuclear translocation of NFATp triggered by both VEGF or calcium ionophore (Fig. 1A, bottom panels).
FIG. 1.
VEGF induces dephosphorylation and nuclear localization of NFATp in HUVECs. (A) Immunocytochemical staining of HUVECs unstimulated (−) or treated with VEGF (50 ng/ml), the calcium ionophore A23187 (1 μM) (I0), or 25 ng of TNF-α per ml for 20 min. The cells were either untreated (top panels) or incubated with 200 ng of CsA per ml (bottom panels) for 2 h prior to the addition of the stimuli. After stimulation, the cells were fixed and stained with the anti-NFATp antiserum 67.1. (B and C) HUVECs, either pretreated or not (−) with CsA (200 ng/ml) for 2 h, were left untreated (−) or stimulated with VEGF (50 ng/ml) or calcium ionophore (I0) (1 μM) for the indicated times. Fractionated cytoplasmic or nuclear extracts were analyzed by Western blotting with the 67.1 antiserum for NFATp detection. The mobilities of the upper and lower bands, corresponding to phosphorylated and dephosphorylated forms of NFATp, respectively, are indicated by arrows. (D) As a control for the fractionation process, aliquots of the extracts used for NFATp detection were analyzed by Western blotting for the presence of nuclear PCNA. Cyt, cytoplasmic; V, VEGF.
Since, CsA targets the phosphatase activity of calcineurin, precluding the dephosphorylation and translocation of NFAT, in other cell systems (29, 55), we designed Western blot experiments to analyze the phosphorylation status of NFAT in fractionated cellular extracts prepared at different time points after activation with VEGF. These experiments revealed that exposure of HUVECs to VEGF induced a rapid dephosphorylation and nuclear translocation of NFATp that was complete as early as 5 min upon activation. Although dephosphorylated NFATp was present in the nucleus for at least 2 h, after 20 min of treatment the presence in the nucleus of phosphorylated NFATp was already evident and the amount of nuclear NFATp was declining to undetectable levels by 4 h. Conversely, phosphorylated NFATp was progressively reappearing in the cytosolic fractions to reach maximal levels by 2 to 4 h (Fig. 1B). The dephosphorylation, nuclear translocation, and further expression of NFATp in the cytoplasm of VEGF-treated cells were also observed when the RNA synthesis of HUVECs was inhibited by actinomycin D (data not shown). Parallel Western blot analysis indicated that both the dephosphorylation and nuclear translocation of NFATp induced by either VEGF or calcium ionophore were prevented by CsA (Fig. 1C). Control experiments showed that the upper and lower bands of NFATp detected in HUVECs displayed identical mobility to that of the phosphorylated and dephosphorylated NFATp forms (data not shown) previously characterized in T cells (33, 39, 46). In addition, the subcellular fractionation process of the extracts used to analyze the import and export of NFATp was controlled by Western blots that revealed the presence of the proliferating-cell nuclear antigen (64) in nuclear extracts but not in the cytosolic fractions of VEGF-treated cells (Fig. 1D). These results, on the one hand, suggest that NFATp imported to the nucleus is rapidly phosphorylated and further exported to the cytoplasm after 1 to 2 h of activation with VEGF and, on the other hand, indicate that the dephosphorylation and translocation of NFATp in endothelial cells were sensitive to the calcineurin inhibitor CsA. Therefore, the nuclear import and export of NFATp induced by VEGF in endothelial cells appears to be regulated in a similar fashion to that demonstrated for lymphocytes activated by different stimuli.
VEGF induces NFAT DNA-binding activity.
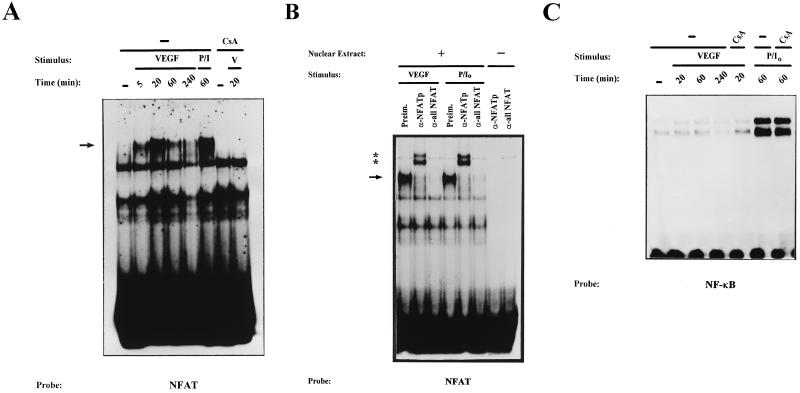
We further analyzed the effect of VEGF on the activation of NFAT by determining the binding of the transcription factor to an NFAT site of the IL-4 promoter that has been shown to bind NFAT independently of AP-1 transcription factor (65). In agreement with the results of the Western blot experiments, EMSAs with nuclear extracts from HUVECs exposed to VEGF for different periods showed that NFAT-binding activity was significantly induced as early as 5 min and was maximal after 20 min of treatment. High levels of binding were still found after 60 min of treatment and markedly declined by 4 h (Fig. 2A). Similarly, pharmacological activation of HUVECs with the combination of PMA with calcium ionophore, a very potent stimulus for NFAT activation in T lymphocytes, also resulted in a strong increase in NFAT binding activity. In both cases, the specific bands were competed with an excess of cold oligonucleotide and CsA blocked the induction of NFAT DNA-binding activity (Fig. 2A and data not shown). The identity of the nuclear factor(s) responsible for the retardation of the DNA-protein complexes generated with the NFAT probe was further characterized by EMSAs in the presence of anti-NFAT antisera. These assays detected the sole presence of NFATp (NFAT1) in the nuclear extracts of activated HUVECs. Thus, the addition of specific anti-NFATp antiserum 67.1 (22) completely supershifted the NFAT complex generated with nuclear extracts from HUVECs stimulated with both VEGF and PMA plus ionophore. In addition, NFAT complex formation was inhibited by incubation with the 796 anti-all-NFAT antiserum (33) directed against a conserved sequence among the NFAT proteins located in the DNA-binding domain (Fig. 2B). We also performed control experiments by analyzing the effect of VEGF and PMA plus ionophore on NF-κB-binding activity by using aliquots of the nuclear extracts where we detected induction of NFAT binding. These EMSAs showed a clear induction of NF-κB in nuclear extracts from HUVECs treated with PMA plus ionophore that was not affected by CsA, whereas no substantial differences were found in the binding to the NF-κB probe of cells treated or not with VEGF for different periods (Fig. 2C).
FIG. 2.
Kinetic analysis and serological characterization of the NFAT DNA-binding complexes induced by VEGF. Nuclear extracts from HUVECs stimulated for the indicated times with VEGF (50 ng/ml) (V) or a combination of PMA (20 ng/ml) plus ionophore (1 μM) (P/I0) or pretreated with CsA (200 ng/ml) for 2 h before stimulation were analyzed by EMSA. (A) Analysis of the DNA-binding activity to the NFAT probe of the IL-4 promoter in nuclear extracts from HUVECs activated for different times with VEGF (VEGF or V). EMSAs with extracts from VEGF-activated cells pretreated with CsA as well as control extracts from PMA- plus ionophore-treated cells are shown. The mobility of the specific VEGF-induced (CsA-sensitive) complex is indicated by an arrow. (B) Serological characterization of the NFAT DNA-binding complexes. EMSAs were performed in the presence (+) or absence (−) of nuclear extracts from cells activated with VEGF or PMA plus ionophore that were incubated with 0.5 μl of either preimmune serum (Preim.), anti-NFATp antiserum 67.1, or the anti-NFAT family antiserum 796 for 15 min prior to the addition of the labeled probe. The VEGF-induced NFAT complex and the supershifted complexes induced by the anti-NFATp 67.1 are indicated by an arrow and asterisks, respectively. (C) Nuclear extracts from HUVECs treated as in panel A were analyzed for NF-κB binding with the κB site of the IL-2 promoter as a probe.
VEGF activates NFAT and AP-1-dependent transcription and increases c-fos and c-jun mRNA levels.
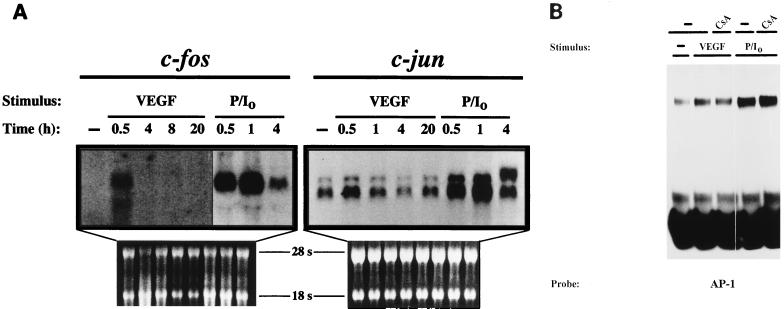
NFAT has been shown to cooperate with AP-1 transcription factor in transactivation and DNA binding to mediate the transcriptional activation of a number of promoter elements (50). In addition, signaling induced by VEGF results in the activation of MAPK cascades that can integrate different signals at the AP-1 level (5, 26). Therefore, we next analyzed whether AP-1 was a target in the activation of VEGF that could cooperate with NFAT to regulate VEGF-dependent transcription. For this purpose, we first performed Northern blot experiments to determine the effect of VEGF on the mRNA levels of c-fos and c-jun AP-1 components. As shown in Fig. 3A, c-fos mRNA steady-state levels, undetected in untreated HUVECs, were induced after 30 min of treatment with VEGF. This induction was transient and markedly declined after 1 h of activation (data not shown), whereas stimulation with the phorbol ester PMA plus calcium ionophore yielded a stronger induction of c-fos that peaked after 1 h of stimulation and could be still detected after 4 h of treatment. In contrast, c-jun mRNA levels were already detected in unstimulated HUVECs, and VEGF induced a small increase over the baseline mRNA levels by 30 min of activation. Parallel control experiments showed that c-jun mRNA levels were strongly upregulated by PMA plus ionophore, displaying similar kinetics to that exhibited by c-fos. The effect of VEGF on AP-1 DNA binding activity was examined by EMSAs, and a moderate increase in the DNA-binding activity to an AP-1 site was reproducibly found when different nuclear extracts from HUVECs treated with VEGF were used. The complex generated with the AP-1 probe was supershifted in the presence of anti-Fos and anti-Jun family antisera, and the DNA binding was higher with extracts from cells stimulated with PMA plus ionophore and was not inhibited by CsA (Fig. 3B and data not shown).
FIG. 3.
Effects of VEGF on the mRNA levels of c-fos and c-jun and on the DNA-binding activity of AP-1. (A) Northern blot analysis with total RNA from HUVECs untreated or activated for the indicated times with VEGF or a combination of PMA plus ionophore (P/I0). After isolation, RNA was separated by agarose gel electrophoresis, blotted onto a nitrocellulose membrane, and hybridized with specific probes for c-fos and c-jun. RNA controls of the corresponding blots are shown. A sixfold-longer exposure of the autoradiograph is presented for the control and VEGF points of c-fos. (B) The AP-1 DNA-binding activity displayed by nuclear extracts from HUVECs activated with VEGF or PMA plus ionophore (P/I0) for 1 h was tested with a specific probe encompassing the −284 AP-1 site of the ICAM-1 promoter. Extracts from CsA-treated cells were obtained after pretreatment of 2 h with 200 ng of CsA per ml and a further 1-h treatment with the stimuli. VEGF (50 ng/ml), PMA (20 ng/ml), and A23187 calcium ionophore (1 μM) were used at the same doses in single or combined treatments in panels A and B.
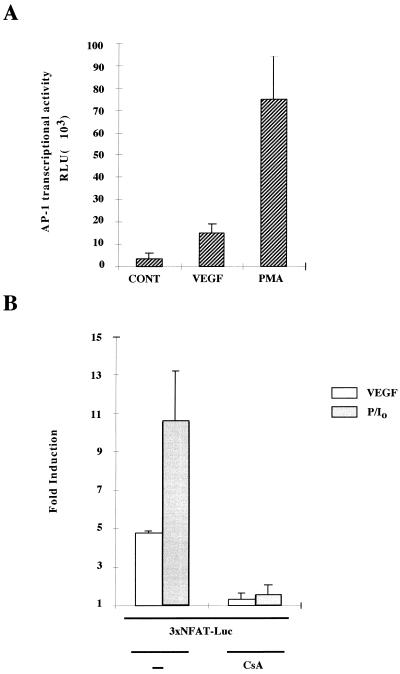
To further analyze the effect of VEGF on AP-1 activation, we carried out transfection experiments in HUVECs with the −73 Col Luc AP-1-dependent reporter plasmid. These experiments revealed that both VEGF and PMA (included as a positive control) stimulated the transcriptional activity of the AP-1 reporter plasmid (Fig. 4A). Similar results were obtained with a different AP-1-dependent construct driven by the rat prolactin minimal promoter under the control of four TRE tandem copies (data not shown). Given that the effects of VEGF on translocation and DNA-binding activity of NFATp were concomitant with the activation of AP-1, we next determined whether activation by VEGF resulted in the functional activation of the distal NFAT motif of the IL-2 promoter, a site that has been shown to require the binding of AP-1 and NFAT to be functionally active (11, 50). Therefore, we transfected HUVECs with a luciferase reporter construct driven by three tandem copies of the NFAT–AP-1 composite site and found that the transcriptional activity of this construct was significantly induced in response to VEGF or PMA plus calcium ionophore in a CsA-sensitive fashion (Fig. 4B).
FIG. 4.
VEGF activates AP-1- and NFAT-dependent transcription- (A) HUVECs were transfected by the calcium phosphate procedure with the AP-1-responsive (−73 to +63) region of the collagenase promoter plasmid for 4.5 h and stimulated or left untreated for an additional 16 h, and then the luciferase activity was determined. The data is expressed as the mean and standard deviation (error bars) of determinations performed in triplicate. Results of a representative experiment are shown. Four different experiments yielded similar results. (B) HUVECs were transfected with an NFAT-dependent luciferase reporter plasmid. At 16 h after transfection, the cells were pretreated or not (−) with CsA (200 ng/ml) for 2 h, and then left untreated or further stimulated for an additional 6 h with VEGF or a combination of PMA and calcium ionophore (P/I0). The results are expressed as the relative fold induction over the relative luciferase units (RLU) displayed by the corresponding transfected unstimulated cells. Results of a representative experiment of five are shown. VEGF (50 ng/ml), PMA (20 ng/ml), and A23187 calcium ionophore (1 μM) were used at the same doses in single or combined treatments in panels A and B.
Identification of an NFAT-binding site within the TF promoter regulated by VEGF.
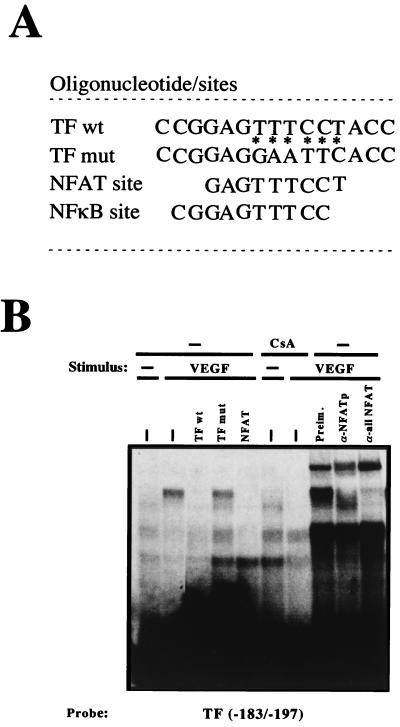
To evaluate the functional relevance of the activation of NFAT in the expression of endothelial genes regulated by VEGF, we carried out a database search for NFAT motifs within the promoters of several genes that have been shown to be induced by VEGF. These analyses revealed the presence of an NFAT consensus site at positions −186 to −194 within the TF promoter region (Fig. 5A). Therefore, we next determined whether this site was able to bind NFAT by performing EMSAs with synthetic oligonucleotides spanning positions −183 to −197 of the TF promoter that included the NFAT site. These experiments demonstrated the ability of this site to efficiently bind NFAT from nuclear extracts of VEGF-treated HUVECs in a CsA-sensitive manner. The specificity of this binding was further confirmed by addition of an excess of TF homologous oligonucleotide or an excess of a heterologous oligonucleotide including the NFAT site of the IL-4 promoter that abolished the specific binding to the probe but not by the corresponding TF mut oligonucleotide carrying several nucleotide substitutions within the NFAT sequence that failed to compete the binding (Fig. 5). Furthermore, the addition of anti-NFATp or anti-all NFAT antisera resulted in supershift or inhibition of the NFAT-DNA complex, respectively (Fig. 5B).
FIG. 5.
NFATp binds to the −183 to −197 region within the TF promoter. (A) The sequences (5′ to 3′) of the oligonucleotides including the −183 to −197 region of the TF promoter (TF wt) and that of the TF mut are shown. Base pair substitutions incorporated in the TF mut are indicated by asterisks. The partially overlapping nucleotides corresponding to the κB-like and the NFAT sites are indicated. (B) Nuclear extracts from HUVECs pretreated or not with 200 ng of CsA per ml and then exposed to VEGF (50 ng/ml) were analyzed by EMSA with a probe spanning positions −183 to −197 of the TF promoter. A 30-fold molar excess of unlabeled TF −183 to −197, the corresponding oligonucleotide mutated at positions −186 to −194 (TF mut), or an NFAT consensus site of the IL-4 promoter oligonucleotides was added to the binding-reaction mixtures to detect the specific binding. Serological identification of the complexes was performed by addition of 0.5 μl of either preimmune serum (Preim.), anti-NFATp antiserum (67.1), or anti-NFAT family antiserum (796). Antisera and cold oligonucleotides were added to the binding-reaction mixture prior to addition of the probe.
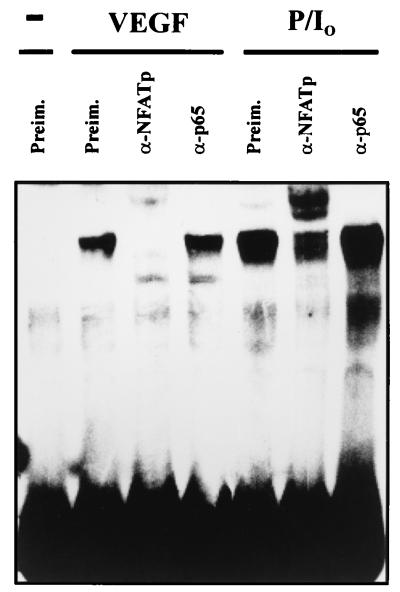
Since the TF oligonucleotide from −183 to −197 included a previously identified κB-like site reported to bind NF-κB in response to TNF-α or LPS (34, 41, 51), we performed gel retardation experiments with this TF probe and nuclear extracts from HUVECs treated with PMA plus ionophore, a combination of stimuli that triggered the DNA-binding and transcriptional activity of both NF-κB and NFAT (Fig. 2 and 4 and data not shown). Strikingly, as occurred with the complex induced by VEGF, the inducible complex generated with nuclear extracts from HUVECs exposed to PMA plus ionophore was supershifted by the anti-NFATp antiserum whereas no supershift or inhibition of the binding was detected with antisera directed against the p65 NF-κB subunit (Fig. 6). Parallel control experiments with aliquots of the nuclear extracts of cells activated with PMA plus ionophore and the prototypic κB probe of the Igκ light-chain enhancer revealed the presence of an inducible NF-κB complex supershifted by the anti-p65 antiserum (data not shown). Hence, the −183 to −197 region of the TF promoter, including a previously identified κB site that overlapped with the NFAT-binding site, bound exclusively to NFAT from nuclear extracts of HUVECs activated by VEGF. Similarly, the activation of these cells with PMA plus ionophore, which triggered both NFAT and NF-κB binding to different consensus probes, induced predominant NFAT binding to the TF probe.
FIG. 6.
Serological analysis of the binding to the TF/NFAT site induced by VEGF and PMA plus ionophore. The DNA binding to the −183 to −197 region of the TF promoter was analyzed by using nuclear extracts from HUVECs treated with VEGF (50 ng/ml) or with PMA (20 ng/ml) plus A23187 calcium ionophore (1 μM) for 1 h. Anti-NFATp antiserum 67.1 (0.5 μl) or 1 μl of anti-p65 antisera was added to the binding-reaction mixture before the addition of the probe.
VEGF regulates TF gene expression by mechanisms involving NFAT in a CsA-sensitive fashion.
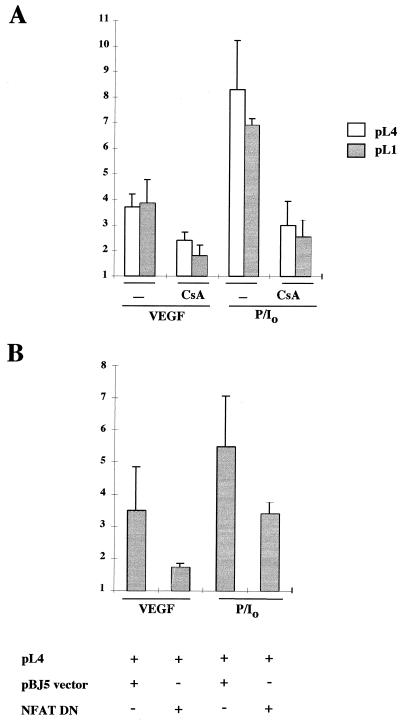
Because of the presence of the newly identified NFAT-binding site within the TF promoter, we analyzed whether NFAT could play a role in the regulation of TF gene expression induced by VEGF. We initially determined whether the induction described for TF protein in response to VEGF (9) was also reflected at the promoter level. For this purpose, we transfected HUVECs with the pL1 and pL4 plasmids containing the −2106 to +121 and the −278 to +121 upstream regulatory regions of the TF gene fused to the luciferase reporter gene, respectively (37). These experiments showed that VEGF induced the transcriptional activity of the TF promoter reporter plasmids by three- to fivefold. This activation was lower than that displayed by PMA plus ionophore, which appeared to be a very potent stimulus for TF promoter activation, and was partially inhibited by CsA (Fig. 7A and data not shown).
FIG. 7.
Effects of CsA and NFAT dominant negative plasmid on the transcription of the TF promoter by VEGF. (A) The transcriptional activity of the TF promoter was tested by transfection of HUVECs. The pL4 and pL1 luciferase reporter plasmids (10 μg) were transfected by calcium phosphate precipitation for 4.5 h. At 16 h posttransfection, cells were preincubated or not (−) with CsA for 2 h, and further stimulated for 6 h with VEGF, PMA plus ionophore (P/I0), or vehicle. Results of a representative experiment of three independent experiments performed are shown. (B) The pL4 promoter construct (5 μg) was cotransfected with 5 μg of either the NFAT dominant negative pSH102CΔ418 expression vector or its parental empty vector (pBJ5). At 16 h after transfection, the cells were stimulated with VEGF, PMA plus ionophore (P/I0), or vehicle for 6 h. Results of a representative experiment of three independent ones are presented. The results are expressed as fold induction over the expression of pL4 and pL1 plasmids in the absence of stimuli in both panels. Experiments were performed in triplicate. VEGF (50 ng/ml), PMA (20 ng/ml), CsA (200 ng/ml), and A23187 calcium ionophore (1 μM) were used at the same doses in single and combined treatments.
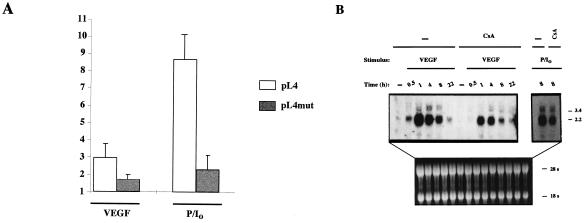
The involvement of NFAT in the regulation of TF promoter by VEGF was analyzed in cotransfection experiments by testing the effect of the expression of an NFAT dominant negative plasmid (43) on the transcriptional activity of the pL4 TF promoter reporter construct. As shown in Fig. 7B, the transfection of the NFAT dominant negative expression construct resulted in a clear although partial inhibition of the TF promoter activity induced by VEGF or PMA plus ionophore. Since the TF mut oligonucleotide carrying a 5-bp substitution in the core region of the NFAT-binding site failed to compete for the NFAT complex (Fig. 5B), we evaluated the functional contribution of the identified NFAT site to the overall transcriptional response of the TF promoter by performing site-directed mutagenesis to introduce the same 5-bp substitution into the pL4 TF reporter construct. Consistent with the results of the cotransfection experiments with the NFAT dominant negative construct and the TF promoter, the inducibility of the mutated TF promoter was significantly reduced in response to both VEGF and PMA plus ionophore (Fig. 8A).
FIG. 8.
Inhibition of VEGF-induced TF expression by CsA. (A) HUVECs were transfected by calcium phosphate precipitation with 10 μg of the parental pL4 luciferase plasmid or with the pL4mut-derived plasmid mutated in the NFAT site for 4.5 h. At 16 h posttransfection, the cells were stimulated for 6 h with VEGF (50 ng/ml) or PMA (20 ng/ml) plus ionophore (1 μM). Experiments were performed in triplicate, and the results are expressed as fold induction over the baseline levels of transfected unstimulated cells. Results of a representative experiment of three performed are shown. (B) Northern blot analysis of HUVECs pretreated or not (−) with CsA (200 ng/ml) for 2 h and further stimulated with VEGF (50 ng/ml) or a combination of PMA (20 ng/ml) plus ionophore (1 μM) (P/I0) for the time points indicated. Total RNA was electrophoresed, blotted onto a nitrocellulose membrane, and hybridized with a TF cDNA probe. Autoradiographs corresponding to exposures for VEGF and PMA-plus-ionophore treatments of 1 week and 16 h, respectively, and RNA controls of the blot are presented.
To analyze whether the effects exerted by VEGF on the activation of TF at the promoter level correlated with those detected on the TF mRNA steady-state levels, we performed Northern blot experiments of HUVECs exposed to VEGF or PMA plus ionophore in the presence or absence of CsA. These experiments revealed that TF mRNA levels, undetected in resting HUVECs, were upregulated after treatment with VEGF or PMA plus ionophore. Exposure of cells to VEGF resulted in maximal expression of TF mRNA after 1 h of treatment and a progressive decline between 4 and 8 h. Furthermore, the induction of TF mRNA levels induced by both VEGF and PMA plus ionophore was markedly inhibited by CsA at the different time points analyzed (Fig. 8B).
Taken together, these results suggest that VEGF regulates TF gene expression in endothelial cells by transcriptional mechanisms involving the activation of NFAT, and they identify a functional NFAT site required for full inducibility of the TF promoter that is likely to account for the partial inhibition of TF gene expression displayed by CsA.
DISCUSSION
VEGF is a multifunctional cytokine that plays a pivotal role in the regulation of angiogenesis and is required for the development and differentiation of the vascular system (6, 18). Despite extensive research focused on the biological functions of VEGF and its role in the physiological and pathological angiogenic processes, the molecular mechanisms of signal transduction and the transcription factors that couple such signals to the gene expression programs induced by VEGF are still largely unknown. In this study, we show that VEGF triggers the activation of NFAT and AP-1, two transcription factors that are rapidly mobilized in cell activation processes to regulate gene transcription in response to a large number of stimuli (26, 51). To our knowledge, this is the first report demonstrating the activation of NFAT by a physiological stimulus in endothelial cells. In addition, we have found that the VEGF-induced expression of TF involves the activation of NFAT and identified a functional NFAT-binding site within the TF promoter that overlaps with a previously identified κB-like element.
Exposure of HUVECs to VEGF increases the intracellular calcium concentration (12, 45). In T lymphocytes and fibroblasts, elevations of intracellular calcium levels result in calcineurin activation and subsequent activation and nuclear localization of NFAT proteins (32, 54, 59, 60). Similarly, we have demonstrated that the dephosphorylation and translocation of NFATp triggered by both VEGF and calcium ionophore were blocked by the calcineurin inhibitor CsA in endothelial cells. Once in the nucleus, the imported NFAT was detected displaying forms with the mobility of phosphorylated NFATp at times when the transcription factor was progressively found in the cytoplasm of activated cells, and this process was independent of RNA synthesis. These data suggest that the activation of the calcium-calcineurin pathway in HUVECs by VEGF leads to the translocation of NFATp to the nucleus, where it is phosphorylated and then exported to the cytoplasm. Therefore, NFATp activation appears to be regulated in HUVECs by mechanisms similar to those operating in T cells or fibroblasts.
The major role that endothelial cells play in angiogenesis as well as in inflammatory and immune reactions is mediated largely by its ability to produce and respond to cytokines (38). In a previous report, NFATp has been shown to be activated in endothelial cells treated with PMA plus calcium ionophore and bind to an NFAT site of the GM-CSF enhancer. Furthermore, in these activated cells, the induction of both the transcriptional activity of a GM-CSF enhancer-promoter reporter construct and protein synthesis of GM-CSF were partially inhibited by CsA (10). Although NFAT proteins are the major targets of the calcineurin inhibitors CsA and FK506, these drugs have been shown to affect the activation of other transcription factors that can also be regulated by calcineurin (23, 25, 61, 66). Since the expression of other cytokines produced by endothelial cells including IL-1, IL-6, and IL-8 is inhibited by CsA in different cell types (51), the possible involvement of NFAT in the transcriptional regulation of these cytokine genes in endothelial cells will be an important issue for future studies.
We have detected by serological analysis of the NFAT-binding complex generated with nuclear extracts from HUVECs activated with VEGF the presence of only the NFAT1 (NFATp) protein. Likewise, NFATp was the only member detected in the DNA-binding complexes formed with the GM-CSF probe in endothelial cells activated with PMA plus ionophore (10). Hence, of the four NFAT family members identified, only NFATp protein has so far been found to be expressed by endothelial cells in the adult. However, using specific oligonucleotides of the different members, as well as degenerate oligonucleotides of sequences within the DNA-binding domain of NFAT members, we have amplified by reverse transcription-PCR the cDNAs of NFAT1 (NFATp), NFAT2 (NFATc), NFAT3, and NFATx from mRNA of HUVECs (data not shown). Although the presence of mRNAs of the different forms does not imply the presence or the functional involvement of these proteins, if other NFAT proteins are actually expressed in these endothelial cells, they could be potentially involved in the regulation of TF or GM-CSF gene expression by other stimuli or in the control of other, so far unidentified, NFAT-regulated endothelial genes. In this regard, it is important to note the recent identification of NFATc in endocardial cells during embryonic heart development, where it has been found to be required for the formation of the aortic and pulmonary valves (13, 49). Endocardial cells are specialized endothelial cells of the heart that give rise to the cardiac valves and septum. It is noteworthy that the translocation of NFATc to the nucleus of these cells can be inhibited by CsA or FK506 in cultured whole embryos (13, 49), suggesting the involvement of the calcium-calcineurin signaling pathway in the morphogenesis of cardiac valves and septum. Since the ligand(s) that triggers the translocation of NFATc in endocardial cells during development has not so far been identified, it will be important to determine whether VEGF could play a role in this important process.
The effects of VEGF activating pathways that involve the MAPKs ERKs (12, 53, 56), JNKs/SAPKs (30), and p38/HOG (53) in endothelial cells are consistent with the activation of AP-1 by VEGF that we show here. These MAPKs are components of the signal transduction cascades that regulate the AP-1 family members at the transcriptional and posttranscriptional levels (20, 26). Although we observed a clear induction of the human collagenase AP-1-dependent reporter construct and the mRNA levels of c-fos, both the AP-1 DNA-binding activity and the c-jun mRNA levels were only weakly induced in response to VEGF. The lack of correlation between AP-1 DNA-binding and transcriptional activation has been previously noted in other cell systems, and the reason for this disagreement appears to be related to the changes in the composition of the AP-1 complexes during the activation processes. Thus, various members of the Fos family can associate with Jun proteins to form heterodimers displaying similar DNA-binding activities but different transactivating capabilities. In addition, the phosphorylation of Fos and Jun proteins has been reported to stimulate their transactivating efficacy without altering the DNA-binding activities (14, 26). The effect of VEGF on the activation of AP-1 is evidenced not only by the stimulation of transcriptional activity of the AP-1 dependent reporter construct −73 Col Luc but also by the activation exerted on the reporter construct directed by the NFAT–AP-1 composite site of the IL-2 promoter, whose transcriptional induction requires the activation of both NFAT and AP-1 transcription factors (47). Furthermore, we have confirmed the involvement of the AP-1 transcription factor in the VEGF-mediated endothelial activation by using the TAM-67 c-jun dominant negative plasmid, whose expression basically blocked the transcriptional activity of the TF promoter in response to VEGF (data not shown). Whether this blocking effect of TAM-67 reflects the relevance of the AP-1-binding sites of the promoter in response to VEGF or the importance of cooperative interactions of AP-1 with other transcription factors (or both) remains to be determined.
TF deficiency results in embryonic death due to defective yolk sac vessel and abnormal vitelloembryonic circulation (7). Since the abnormal yolk sac vasculature resembles in part the phenotype found in VEGF-deficient embryos (6, 18), the possibility that the functions of VEGF and TF are interrelated has been suggested (8). In fact, the induction of TF by VEGF leads to the generation of fibrin and the sequential activation of tPA and plasmin, which stimulates the degradation of matrix proteins, a critical step for the migration and sprouting of endothelial cells during angiogenesis (57). Experiments aimed to establish a functional link between VEGF-induced gene expression and the activation of NFAT and AP-1 transcription factors led us to analyze the TF gene promoter. TF expression in monocytes and endothelial cells is induced by a variety of proinflammatory agents, including LPS, IL-1β, and TNF-α, through transcriptional and posttranscriptional mechanisms (34). Previous reports have identified Sp1–Egr-1 sites required for basal expression of the gene and NFκB and AP-1 sites involved in the inducible response to LPS and cytokines, and a concerted action of the transcription factors that bind to these sites has been proposed to regulate TF promoter in monocytes and endothelial cells (34, 36, 41, 44). We have shown that VEGF induced the expression of TF mRNA levels and the transcriptional activation of the TF gene promoter involving the activation of NFAT. This role of NFAT was evidenced by the expression of the NFAT dominant negative construct that resulted in significant inhibition of the TF promoter transcription by VEGF, and it correlated with the defective inducibility displayed by the TF promoter construct carrying the NFAT mutated site. In both cases, the inhibitory effect observed was partial, as occurred with that displayed by CsA on both the TF promoter activation and mRNA expression triggered by VEGF. Since we have also demonstrated that the effects of CsA precluding the translocation and transcriptional activation of NFAT are also operative in VEGF-activated endothelial cells, these data suggest that the inhibition of TF gene expression by CsA is exerted through the inhibition of NFAT activation. Given the role that AP-1 appears to play in the activation of TF promoter, it is likely that AP-1 could account for the promoter activity that was refractory to the inhibition by CsA or to the expression of the NFAT dominant negative construct.
The presence of an NFAT site overlapping with a previously identified κB-like functional site within the TF promoter is noteworthy. Since previous studies have demonstrated the ability of this site to bind NF-κB proteins in response to LPS or TNF-α (34, 41, 44), our data indicate that the NFAT–NF-κB motif (CGGAGTTTCCT) may represent one example of the κB-like sites such as those of the CD28RE of the IL-2 and GM-CSF promoters, which also include the TTCC sequence, thought to be critical for the binding of either NFAT or NF-κB proteins but not both simultaneously (51). However, stimulation of HUVECs with PMA plus ionophore, which resulted in the activation of NF-κB-binding activity to the IL-2 or Igκ light-chain κB probes, strongly stimulated NFAT binding to the TF probe but failed to induce detectable κB binding activity to this probe. Therefore, NFAT binding to this NFAT–NF-κB motif appears to be predominant (or exclusive) when cells are activated by a stimulus that triggers the simultaneous activation of NFAT and NF-κB. The comparative analysis of the murine, porcine, and human sequences of the TF promoter (41) revealed that the NFAT sequence (GAGTTTCCT) is completely conserved in all three species whereas the partially overlapping NF-κB sequence ([C/T]GGAGTTTCC) displays a 9-of-10-base match among the species. The high degree of conservation of this region is consistent with the critical role reported in the regulation of the TF gene by proinflammatory stimuli through NF-κB (34) and also with its functional role in the response to VEGF or other stimuli that could induce the TF gene by triggering the activation of NFAT.
The inhibition of TF gene expression by CsA that we have detected suggests a putative role of this drug interfering with the procoagulant activity of VEGF described in monocytes and endothelial cells (9). CsA has also been reported to reduce the activation of TF transcription and NF-κB-binding activity to the TF-κB site in LPS-activated monocytes as well as in monocytes from cardiac transplant recipients (23, 24). TF is thought to be involved in fibrin deposition and thrombogenic processes associated with a number of disorders, including atherosclerosis, septic shock, and cancer (34, 52). Therefore, it is conceivable that part of the beneficial effects described for CsA in some of these clinical disorders, such as those reported for experimental transplant atherosclerosis (2), are related not only to the reported ability of the drug to interfere with the activation of TF expression by stimuli that trigger NFκB activation but also to those that induce NFAT activation in nonlymphoid cells such as VEGF. On the other hand, given the major role of VEGF in physiological and pathological angiogenesis, the identification of NFAT and AP-1 as transcription factors that couple VEGF signaling to the transcriptional gene response may help to localize therapeutic targets to antagonize or modulate the angiogenic process and to further delineate the upstream signaling pathways and the specific gene expression program triggered by VEGF in endothelial cells.
ACKNOWLEDGMENTS
We are very grateful to G. Crabtree, A. García-Martín, M. Karin, N. Mackman, A. Rao, N. Rice, M. Rincón, and C. Zacharchuk for providing plasmids and antibodies, which made this work possible. We thank H. Riese, G. Márquez, I. Prieto, and Pharmacia Upjohn, Madrid, Spain, for the gift of recombinant VEGF 165. We also thank M. López Cabrera and S. Lamas for critical reading of the manuscript, R. Tejedor for helping us with the immunofluorescence experiments, L. Horrillo and Charo Martin for excellent secretarial assistance, and the members of the Servicio de Inmunología of the Hospital de la Princesa (Madrid) for their continual help and support.
This work was supported by a grant from the Ministerio de Educación y Cultura (MEC) of Spain (PM96-0076) and grants from the Comunidad Autónoma de Madrid (CAM) 07/046/96 and 8.3/011/97 to J.M.R. A.L.A. was supported by grants from the Comunidad Autónoma de Madrid. The Centro de Biología Molecular S.O. is supported by a grant from the Fundación Ramón Areces. P.G.A. was supported by an FPI fellowship from the CAM. E.L.A. and S.M.M. were supported by FPI fellowships from the MEC.
REFERENCES
- 1.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- 2.Andersen H O, Madsen G, Nordestgaard B G, Hansen B F, Kjeldsen K, Stender S. Cyclosporin suppresses transplant arteriosclerosis in the aorta-allografted, cholesterol-clamped rabbit. Suppression preceded by decrease in arterial lipoprotein permeability. Arterioscler Thromb. 1994;14:944–950. doi: 10.1161/01.atv.14.6.944. [DOI] [PubMed] [Google Scholar]
- 3.Brock T A, Dvorak H F, Senger D R. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol. 1991;138:213–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Brown L F, Detmar M, Claffey K, Nagy J A, Feng D, Dvorak A M, Dvorak H F. Vascular permeabilty factor/vascular endothelial growth factor: A multifunctional angiogenic cytokine. In: Rosen G A, editor. Regulation of angiogenesis. Basel, Switzerland: Birkhauser Verlag; 1997. pp. 233–269. [DOI] [PubMed] [Google Scholar]
- 5.Cahill M A, Janknecht R, Nordheim A. Signalling pathways: jack of all cascades. Curr Biol. 1996;6:16–19. doi: 10.1016/s0960-9822(02)00410-4. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van V I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Moons L, Dewerchin M, Mackman N, Luther T, Breier G, Ploplis V, Muller M, Nagy A, Plow E, Gerard R, Edgington T, Risau W, Collen D. Insights in vessel development and vascular disorders using targeted inactivation and transfer of vascular endothelial growth factor, the tissue factor receptor, and the plasminogen system. Ann N Y Acad Sci. 1997;811:191–206. doi: 10.1111/j.1749-6632.1997.tb52002.x. [DOI] [PubMed] [Google Scholar]
- 9.Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti P C, Pan Y C, Olander J V, Connolly D T, Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockerill G W, Bert A G, Ryan G R, Gamble J R, Vadas M A, Cockerill P N. Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporin A and the T-cell transcription factor NFAT. Blood. 1995;86:2689–2698. [PubMed] [Google Scholar]
- 11.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 12.D’Angelo G, Struman I, Martial J, Weiner R I. Activation of mitogen-activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kDa N-terminal fragment of prolactin. Proc Natl Acad Sci USA. 1995;92:6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Pompa J L, Timmerman L A, Takimoto H, Yoshida H, Elia A J, Samper E, Potter J, Wakeham A, Marengere L, Langille B L, Crabtree G R, Mak T W. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 14.Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–175. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng T, Karin M. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes Dev. 1993;7:479–490. doi: 10.1101/gad.7.3.479. [DOI] [PubMed] [Google Scholar]
- 16.de Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 17.Durand D B, Shaw J P, Bush M R, Replogle R E, Belagaje R, Crabtree G R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrara N, Carver M K, Chen H, Dowd M, Lu L, O’Shea K S, Powell B L, Hillan K J, Moore M W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N, Davis S T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 20.Gómez del Arco P, Martínez-Martínez S, Calvo V, Armesilla A L, Redondo J M. Antioxidants and AP-1 activation: a brief overview. Immunobiology. 1997;198:273–278. doi: 10.1016/S0171-2985(97)80047-2. [DOI] [PubMed] [Google Scholar]
- 21.Guo D, Jia Q, Song H, Warren R S, Donner D B. Vascular endothelial growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. J Biol Chem. 1995;12:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- 22.Ho A M, Jain J, Rao A, Hogan P G. Expression of the transcription factor NFATp in a neuronal cell line and in the murine nervous system. J Biol Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 23.Holschermann H, Durfeld F, Maus U, Bierhaus A, Heidinger K, Lohmeyer J, Nawroth P P, Tillmanns H, Haberbosch W. Cyclosporin A inhibits tissue factor expression in monocytes/macrophages. Blood. 1996;88:3837–3845. [PubMed] [Google Scholar]
- 24.Holschermann H, Kohl O, Maus U, Durfeld F, Bierhaus A, Nawroth P P, Lohmeyer J, Tillmanns H, Haberbosch W. Cyclosporin A inhibits monocyte tissue factor activation in cardiac transplant recipients. Circulation. 1997;96:4232–4238. doi: 10.1161/01.cir.96.12.4232. [DOI] [PubMed] [Google Scholar]
- 25.Jain J, Burgeon E, Badalian T M, Hogan P G, Rao A. A similar DNA-binding motif in NFAT family proteins and the Rel homology region. J Biol Chem. 1995;270:4138–4145. [PubMed] [Google Scholar]
- 26.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 27.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 28.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 29.Liu J. FK506 and cyclosporin A, molecular probes for studying intracellular signal transduction. Immunol Today. 1993;14:290–295. doi: 10.1016/0167-5699(93)90048-P. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z Y, Ganju R K, Wang J F, Schweitzer K, Weksler B, Avraham S, Groopman J E. Characterization of signal transduction pathways in human bone marrow endothelial cells. Blood. 1997;90:2253–2259. [PubMed] [Google Scholar]
- 31.Loh C, Carew J A, Kim J, Hogan P G, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–3954. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loh C, Shaw K T, Carew J, Viola J P, Luo C, Perrino B A, Rao A. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J Biol Chem. 1996;271:10884–10891. doi: 10.1074/jbc.271.18.10884. [DOI] [PubMed] [Google Scholar]
- 33.Lyakh L, Ghosh P, Rice N R. Expression of NFAT-family proteins in normal human T cells. Mol Cell Biol. 1997;17:2475–2484. doi: 10.1128/mcb.17.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackman N. Regulation of the tissue factor gene. FASEB J. 1995;9:883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- 35.Mackman N. Regulation of the tissue factor gene. Thromb Haemost. 1997;78:747–754. [PubMed] [Google Scholar]
- 36.Mackman N, Brand K, Edgington T S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991;174:1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackman N, Fowler B J, Edgington T S, Morrissey J H. Functional analysis of the human tissue factor promoter and induction by serum. Proc Natl Acad Sci USA. 1990;87:2254–2258. doi: 10.1073/pnas.87.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani A, Bussolino F, Introna M. Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today. 1997;18:231–240. doi: 10.1016/s0167-5699(97)81662-3. [DOI] [PubMed] [Google Scholar]
- 39.Martínez-Martínez S, Gómez del Arco P, Armesilla A L, Aramburu J, Luo C, Rao A, Redondo J M. Blockade of T-cell activation by dithiocarbamates involves novel mechanisms of inhibition of nuclear factor of activated T cells. Mol Cell Biol. 1997;17:6437–6447. doi: 10.1128/mcb.17.11.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millauer B, Wizigmann V S, Schnurch H, Martínez R, Moller N P, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 41.Moll T, Czyz M, Holzmuller H, Hofer W R, Wagner E, Winkler H, Bach F H, Hofer E. Regulation of the tissue factor promoter in endothelial cells. Binding of NF kappa B-, AP-1-, and Sp1-like transcription factors. J Biol Chem. 1995;270:3849–3857. doi: 10.1074/jbc.270.8.3849. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz C, Castellanos M C, Alfranca A, Vara A, Esteban M A, Redondo J M, de Landázuri M O. Transcriptional up-regulation of intracellular adhesion molecule-1 in human endothelial cells by the antioxidant pyrrolidine dithiocarbamate involves the activation of activating protein-1. J Immunol. 1996;157:3587–3597. [PubMed] [Google Scholar]
- 43.Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 44.Oeth P A, Parry G C, Kunsch C, Nantermet P, Rosen C A, Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol Cell Biol. 1994;14:3772–3781. doi: 10.1128/mcb.14.6.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papapetropoulos A, García C G, Madri J A, Sessa W C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Yaseen N R, Hogan P G, Rao A, Sharma S. Phosphorylation of the transcription factor NFATp inhibits its DNA binding activity in cyclosporin A-treated human B and T cells. J Biol Chem. 1995;270:20653–20659. doi: 10.1074/jbc.270.35.20653. [DOI] [PubMed] [Google Scholar]
- 47.Petrak D, Memon S A, Birrer M J, Ashwell J D, Zacharchuk C M. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J Immunol. 1994;153:2046–2051. [PubMed] [Google Scholar]
- 48.Quinn T P, Peters K G, De V C, Ferrara N, Williams L T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranger A M, Grusby M J, Hodge M R, Gravallese E M, De la Brousse F C, Hoey T, Mickanin C, Baldwin H S, Glimcher L H. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–190. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 50.Rao A. NF-ATp: a transcription factor required for the co-ordinate induction of several cytokine genes. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 51.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 52.Rao L V. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev. 1992;11:249–266. doi: 10.1007/BF01307181. [DOI] [PubMed] [Google Scholar]
- 53.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 54.Ruff V A, Leach K L. Direct demonstration of NFATp dephosphorylation and nuclear localization in activated HT-2 cells using a specific NFATp polyclonal antibody. J Biol Chem. 1995;270:22602–22607. doi: 10.1074/jbc.270.38.22602. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber S L, Crabtree G R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 56.Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- 57.Senger D R. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am J Pathol. 1996;149:1–7. [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw K T, Ho A M, Raghavan A, Kim J, Jain J, Park J, Sharma S, Rao A, Hogan P G. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci USA. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibasaki F, Price E R, Milan D, McKeon F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature. 1996;382:370–373. doi: 10.1038/382370a0. [DOI] [PubMed] [Google Scholar]
- 60.Timmerman L A, Clipstone N A, Ho S N, Northrop J P, Crabtree G R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 61.Venkataraman L, Burakoff S J, Sen R. FK506 inhibits antigen receptor-mediated induction of c-rel in B and T lymphoid cells. J Exp Med. 1995;181:1091–1099. doi: 10.1084/jem.181.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waltenberger J, Claesson W L, Siegbahn A, Shibuya M, Heldin C H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 63.Warren R S, Yuan H, Matli M R, Gillett N A, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waseem N H, Lane D P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96:121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- 65.Weiss D L, Hural J, Tara D, Timmerman L A, Henkel G, Brown M A. Nuclear factor of activated T cells is associated with a mast cell interleukin 4 transcription complex. Mol Cell Biol. 1996;16:228–235. doi: 10.1128/mcb.16.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woronicz J D, Lina A, Calnan B J, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol Cell Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia P, Aiello L P, Ishii H, Jiang Z Y, Park D J, Robinson G S, Takagi H, Newsome W P, Jirousek M R, King G L. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]