Abstract
Acylhydrazones are still an important framework to the design of new bioactive compounds. As treatment of chronic pain represents a clinical challenge, we decided to modify the structure of LASSBio-1514 (1), previously described as anti-inflammatory and analgesic prototype. Applying the homologation as a strategy for molecular modification, we designed a series of cyclopentyl- (2a–e), cyclobutyl- (3a–e), and cyclopropylacylhydrazones (4a–e) that were synthetized and evaluated in murine models of inflammation and pain. A comparison of their in silico physicochemical and drug-like profile was conducted, as well as their anti-inflammatory and analgesic effect. Compounds 4a (LASSBio-1755) and 4e (LASSBio-1757) displayed excellent in silico drug-like profiles and were identified as new analgesic lead-candidates in acute and chronic model of pain, through oral administration.
Keywords: acylhydrazone, homologation, anti-inflammatory, analgesic, pain, in silico drug-like
1. Introduction
Acylhydrazone (R-CONHN=CHR1) is a versatile framework described as an important privileged structure. Inspired in this framework, several bioactive compounds have been designed and synthetized by modifying the nature of the substituents linked to the acyl (R-CO-) and/or imine (-N=CHR1) subunits [1,2,3,4,5,6,7]. Conformational and stereochemistry features of this framework have been studied, suggesting the s-trans amide conformer and the E geometrical isomers as the most prevalent species [8,9,10]. Recently, Thota and coworkers discussed the role of acylhydrazones as drugs and drug-candidates [11]. Owing to the fact that chronic pain is still an unmet medical need, the analgesic effect is one of the most recurrent activities ascribed to acylhydrazones [12,13].
Previously, our group has described the synthesis and pharmacological evaluation of cyclohexyl-N-acylhydrazones as orally active, analgesic, and anti-inflammatory lead-candidates. Among them, a compound bearing the 4-pyridinyl subunit (LASSBio-1514), linked to imine moiety, displayed important antihyperalgesic activity in a Spinal Nerve Ligation (SNL) model [14].
In an attempt to study the impact of homologation strategy [15] on the biological properties and drug-like profile of the cyclohexyl-N-acylhydrazones, we describe here the design, synthesis, pharmacological evaluation, and in silico drug-like prediction of cyclopolymethylenic homologous of compound LASSBio-1514 (1).
The design conception of the new cyclopolymethylene homologues series was proposed by decreasing size of the cyclohexyl ring of the prototype LASSBio-1514 (1), by the elimination of one, two, and three methylenes to generate the cyclopentyl (2a), cyclobutyl (3a), and cyclopropyl-N-acylhydrazone (4a), respectively. Further, isosteric ring replacement [16,17] was performed, allowing the substitution of the 4-pyridinyl moiety (a) by a phenyl (b), 2-thienyl (c), ferrocenyl (d), and 4-dimethylaminophenyl (e) ring (Figure 1).
Figure 1.
Design conception of the cyclopentyl-, cyclobutyl-, cyclopropyl-N-acylhydrazones (2a–e, 3a–e, and 4a–e) planned as inferior homologous of the anti-inflammatory and analgesic prototype LASSBio-1514 (1).
2. Results and Discussion
2.1. Chemistry
The target compounds were synthetized in two linear steps, exploring the cycloalkyl esters (5–7) as starting materials. The synthesis was based on the hydrazinolysis of the esters 5–7, followed by the condensation of the hydrazide intermediates (8–10) with aromatic and heteroaromatic aldehydes, using the methodology previously described by da Silva and coworkers, to obtain the desired cycloalkyl-acylhydrazones (2a–e, 3a–e, and 4a–e) in good yields [14] (Scheme 1).
Scheme 1.
Reagents and conditions: a) N2H4.H2O, EtOH, reflux, 48 h, 59–81%; b) Aromatic or heteroaromatic aldehyde, EtOH, HCl (cat), room temperature, 2 h, 22–80%.
Regarding the stereochemistry of imine double bond, all compounds (2a–e, 3a–e, and 4a–e) were obtained as E-isomers (Figures S1–S30). The duplication of the amide (CONH) and imine (N=CH) signals in the hydrogen nuclear magnetic resonance (1H-NMR) spectra suggested the presence of two rotamers in solution. Variable temperature 1H-NMR studies (25 °C, 60 °C, and 90 °C) were performed. At 90 °C, the two signals coalesced to a single peak, confirming the supposition of amide conformational isomers (s-cis and s-trans) (Figure S31).
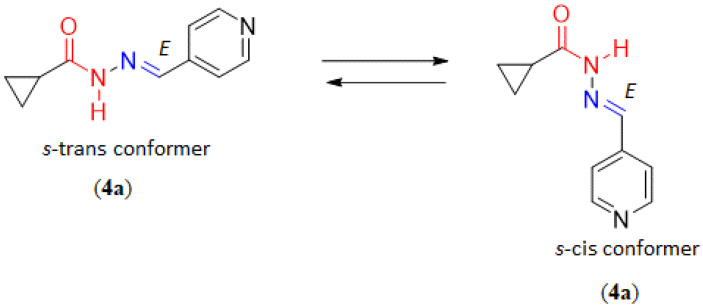
As previously synthesized and identified by Bastos and coworkers [18], compound 4a was assigned as the isomer E by X-ray powder diffraction studies. Unlike the mixture of amide conformers (s-cis and s-trans) identified in solution, the s-cis amide conformation was found by X-ray powder diffraction experiment [18] (Figure 2).
Figure 2.
The s-trans and s-cis conformation of the amide function of compound 4a.
The comparative physicochemical (Table 1) and drug-like (Table 2) profile of LASSBio-1514 (1) and their inferior homologous (2a–e, 3a–e, and 4a–e) was predicted using Percepta—a commercial Software of ACD/Labs [19]. With the exception of compounds 2d, 3d, and 4d which, due to their organometallic nature, cannot be predicted by Percepta, all other compounds comply with Lipinski’s rules [20] and Veber’ guidelines [21]. As depicted in Table 1, compounds were predicted as water soluble, except for compound 2b (solubility = 0.01 mg/mL). Similar to the prototype 1, the homologous series were foreseen as having an ideal partition coefficient, with logP values ranging from 1 to 3, with the exception of compound 4a, which was predicted to have the lowest lipophilicity. Based on the literature data, high lipophilicity is also expected for compounds 2d, 3d, and 4d compared to their isosteres 2b, 3b, and 4b, as ferrocene (Fc) is more lipophilic than benzene (Bz) (LogPFc = 3.54 compared to LogPBz = 2,13; πFc= 2.46 compared to πBz = 1.96) [22,23]. Considering the log of the acid dissociation constant (pKa) calculated for the compounds containing the 4-pyridine (a) and 4-dimethylaminophenyl (e) moieties, no significant change was found for the distribution coefficient (LogD) of the target compounds at pH 4.6 (duodenum) and pH 7.4 (blood).
Table 1.
Calculated physicochemical properties (molecular weight (MW), log of partition coefficient (LogP), log of distribution coefficient (LogD), log of the acid dissociation constant (pKa) and aqueous solubility of compound 1 and its inferior homologous (2a–e, 3a–e, and 4a–e) by ACD/Percepta Software.
| Compound | Physicochemical Properties | ||||||
|---|---|---|---|---|---|---|---|
| M.W. g/mol |
LogP | LogD4,6 | LogD7,4 | pKa | TPSA | Solubility mg/mL |
|
| 1 | 231.30 | 1.95 | 1.93 | 1.95 | 11.5 ± 0.4 5.2 ± 0.8 |
54.35 | 0.18 |
| 2a | 217.27 | 1.48 | 1.46 | 1.48 | 11.5 ± 0.4 5.2 ± 0.8 |
54.35 | 0.25 |
| 2b | 216.28 | 2.78 | 2.78 | 2.78 | 11.5 ± 0.4 | 41.46 | 0.01 |
| 2c | 222.31 | 2.70 | 2.70 | 2.70 | 11.5 ± 0.4 | 69.70 | 0.21 |
| 2d | 324.21 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 2e | 259.35 | 3.05 | 3.02 | 3.05 | 11.5 ± 0.4 3.4 ± 0.4 |
44.70 | 0.27 |
| 3a | 203.24 | 1.23 | 1.21 | 1.23 | 11.5 ± 0.4 5.2 ± 0.4 |
54.35 | 1.31 |
| 3b | 202.25 | 2.09 | 2.09 | 2.09 | 11.5 ± 0.4 | 41.46 | 0.13 |
| 3c | 208.28 | 2.00 | 2.00 | 2.00 | 11.5 ± 0.4 | 69.70 | 0.35 |
| 3d | 310.18 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3e | 245.32 | 2.52 | 2.52 | 2.52 | 11.5 ± 0.4 3.4 ± 0.4 |
44.70 | 0.13 |
| 4a | 189.21 | 0.68 | 0.66 | 0.68 | 11.1 ± 0.5 5.2 ± 0.8 |
54.35 | 1.02 |
| 4b | 188.23 | 1.70 | 1.70 | 1.70 | 11.1 ± 0.5 | 41.46 | 0.12 |
| 4c | 194.25 | 1.64 | 1.64 | 1.64 | 11.1 ± 0.5 | 69.70 | 0.31 |
| 4d | 296.15 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4e | 231.29 | 2.16 | 2.13 | 2.16 | 11.1 ± 0.5 3.4 ± 0.4 |
44.70 | 0.12 |
Table 2.
Calculated drug-like properties (permeability (Caco-2), plasma protein binding (PPB), volume of distribution (Vd), ability to penetrate CNS (CNS), oral bioavailability (Foral), stability in human liver microsomes (HLM), mutagenic potential (AMES), and hERG inhibition potential (hERG)) of compound 1 and its inferior homologous (2a–e, 3a–e and 4a–e) by ACD/Percepta Software.
| Compound | ADMET Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| Caco-2 (cm/s) |
PPB (%) |
Vd (L/Kg) |
CNS | Foral (%) |
HLM | AMES | hErg | |
| 1 | 187 × 10−6 | 81 | 1.6 | −2.45 | 99 | 0.48 | 0.44 | 0.39 |
| 2a | 155 × 10−6 | 70 | 1.5 | −2.30 | 99 | 0.47 | 0.45 | 0.39 |
| 2b | 224 × 10−6 | 84 | 1.9 | −2.14 | 80 | 0.44 | 0.37 | 0.38 |
| 2c | 223 × 10−6 | 85 | 2.0 | −2.10 | 98 | 0.50 | 0.43 | 0.35 |
| 2d | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 2e | 229 × 10−6 | 87 | 2.0 | −2.17 | 97 | 0.51 | 0.48 | 0.40 |
| 3a | 135 × 10−6 | 61 | 1.4 | −2.27 | 99 | 0.49 | 0.48 | 0.39 |
| 3b | 198 × 10−6 | 77 | 1.8 | −2.02 | 99 | 0.48 | 0.43 | 0.38 |
| 3c | 193 × 10−6 | 85 | 1.9 | −2.30 | 99 | 0.50 | 0.46 | 0.35 |
| 3d | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3e | 215 × 10−6 | 72 | 1.9 | −1.97 | 99 | 0.49 | 0.48 | 0.39 |
| 4a | 91 × 10−6 | 57 | 1.3 | −2.46 | 99 | 0.46 | 0.55 | 0.37 |
| 4b | 174 × 10−6 | 74 | 1.6 | −2.18 | 96 | 0.47 | 0.58 | 0.37 |
| 4c | 171 × 10−6 | 86 | 1.6 | −2.43 | 99 | 0.49 | 0.57 | 0.34 |
| 4d | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4e | 200 × 10−6 | 74 | 1.6 | −2.11 | 98 | 0.47 | 0.57 | 0.39 |
The in-silico drug-like properties of 2a–c, 3a–c, 4a–c, and 2–4e revealed that all compounds have highly permeable profiles in Caco-2 cells, and were predicted as able to penetrate the central nervous system. They were foreseen to have moderate to high plasma protein binding ability (PPB ranging from 57% to 87%) and volume of distribution between 1.3 to 2 L. Given the good in silico aqueous solubility and good permeability, the compounds were projected to have high oral bioavailability (80–99%). Unfortunately, the software was not able to determine the metabolic stability (HLM), mutagenic (AMES), and cardiotoxic potential of the target compounds. The score values obtained fell into the method’s undefined range (0.33–0.67).
2.2. Pharmacological Activities
Acetic acid- and formalin-induced pain protocols were used in order to investigate the peripheral analgesic profile of the cycloalkyl-acylhydrazones (2a–e, 3a–e, and 4a–e) [24,25]. Table 3 shows the analgesic effect observed after oral administration of the target compounds, indomethacin, dipyrone, and LASSBio-1514 (100 µmol/kg). Cyclobutyl (3a) and cyclopropyl (4a) analogues demonstrated similar inhibitory effect on acetic acid-induced pain, or slightly better than the prototype LASSBio-1514. Inhibition of abdominal writhing induced by acetic acid of 75.4 ± 8.1, 94.3 ± 2.0, 79.4 ± 3.3, 70.8 ± 1.9, 77.8 ± 7.6, and 72.3 ± 0.9% for the homologous 2e, 3b, 3c, 4b, 4c, and 4d, respectively, confirmed the intense analgesic activity (Table 3). To reinforce the analgesic profile of 2a–e, 3a–e, and 4a–e, they were evaluated in formalin-induced pain test in mice. Unlike the cyclohexyl (1), cyclopentyl (2a–d) and cyclopropylacylhydrazones (4a, 4d–e), the cyclobutyl derivatives (3a–e) were unable to display activity at neurogenic phase of formalin test. However, in the early phase of formalin test, compounds 4a and 4e inhibited a hypernociceptive response greater than 60%, indicating a possible central analgesic activity. The assumption was not confirmed by hot plate test (data not shown). Cyclopentyl homologous 2a–e were the most active in the late phase of the formalin test, indicating their great effect in inflammatory pain (Table 3).
Table 3.
Comparative analgesic effect of LASSBio-1514 (100 μmol/kg, p.o) and its inferior homologous (2a–e, 3a–e, and 4a–e; 100 μmol/kg, p.o) on acetic acid- and formalin-induced pain assays, using dipyrone or indomethacin as standard drugs (100 μmol/kg, p.o).
| Analgesic Activity (% Inhibition) | |||
|---|---|---|---|
| Compounds | Acetic Acid-Induced Pain | Formalin-Induced Pain | |
| 1st Phase | 2nd Phase | ||
| Indomethacin | N.D. | 23.5 ± 7.9 | 57.4 ± 5.8 ** |
| Dipyrone | 82.5 ± 6.2 ** | N.D. | N.D. |
| LASSBio1514 (1) | 65.7 ± 9.5 ** | 36.9 ± 8.5 | 43.0 ± 5.7 ** |
| 2a | 39.5 ± 3.6 ** | 37.4 ± 4.9 ** | 51.7 ± 6.8 ** |
| 2b | 51.6 ± 8.7 ** | 45.8 ± 12.1 ** | 36.2 ± 2.1 ** |
| 2c | 51.6 ± 8.6 ** | 47.6 ± 12.6 ** | 57.6 ± 8.7 ** |
| 2d | 60.5 ± 7.0 ** | 46.9 ± 3.5 ** | 38.7 ± 5.8 ** |
| 2e | 75.4 ± 8.1 ** | 31.5 ± 13.7 | 63.9 ± 4.5 ** |
| 3a | 66.7 ± 10.4 ** | 15.4 ± 7.3 | 35.5 ± 8.3 * |
| 3b | 94.3 ± 2.0 ** | 9.4 ± 4.1 | 31.8 ± 8.5 |
| 3c | 79.4 ± 3.3 ** | 9.5 ± 6.4 | 43.4 ± 2.6 ** |
| 3d | 65.7 ± 6.3 ** | 29.6 ± 8.6 | 18.4 ± 11.7 |
| 3e | 54.0 ± 5.2 ** | 5.7 ± 4.7 | 22.4 ± 9.5 |
| 4a | 71.8 ± 0.8 ** | 76.3 ± 3.3 ** | 34.9 ± 3.8 * |
| 4b | 70.8 ± 1.9 ** | 36.1 ± 1.8 | 13.1 ± 4.9 |
| 4c | 77.8 ± 7.6 ** | 9.5 ± 6.5 | 0.6 ± 6.1 |
| 4d | 72.3 ± 0.9 ** | 42.3 ± 12.9 * | 43.8 ± 7.9 ** |
| 4e | 51.1 ± 3.8 ** | 64.9 ± 6.2 ** | 37.1 ± 11.7 ** |
Data represent the mean and the standard error of the mean (* p < 0.05, ** p < 0.01 in the ANOVA test followed by Dunnettt).
In order to investigate the anti-inflammatory profile of compound 1 and their inferior homologous (100 μmol/kg, p.o), they were evaluated at carrageenan-induced peritonitis model [26]. In this assay, all compounds inhibited leukocyte infiltration, revealing their anti-inflammatory activity (Table 4)
Table 4.
Comparative anti-inflammatory profile of indomethacin (100 μmol/kg, p.o), LASSBio-1514 (100 μmol/kg, p.o) and its inferior homologous (2a–e, 3a–e, and 4a–e; 100 μmol/kg, p.o) on carrageenan induced peritonitis in mice.
| Carrageenan Induced Peritonitis | |
|---|---|
| Compounds | % Leukocyte Infiltration Inhibition |
| Indomethacin | 65.06 ± 4.0 ** |
| LASSBio1514 (1) | 81.9 ± 2.2 ** |
| 2a | 42.7 ± 2.1 ** |
| 2b | 54.9 ± 5.3 ** |
| 2c | 47.6 ± 8.9 ** |
| 2d | 44.2 ± 3.2 ** |
| 2e | 57.7 ± 7.1 ** |
| 3a | 45.6 ± 5.6 ** |
| 3b | 72.9 ± 5.8 ** |
| 3c | 61.4 ± 4.2 ** |
| 3d | 58.4 ± 4.2 ** |
| 3e | 62.1 ± 7.9 ** |
| 4a | 41.6 ± 3.4 ** |
| 4b | 31.7 ± 3.5 ** |
| 4c | 55.4 ± 3.9 ** |
| 4d | 35.5 ± 3.8 ** |
| 4e | 49.2 ± 4.3 ** |
Data represent the mean and the standard error of the mean (** p < 0.01 in the ANOVA test followed by Dunnettt).
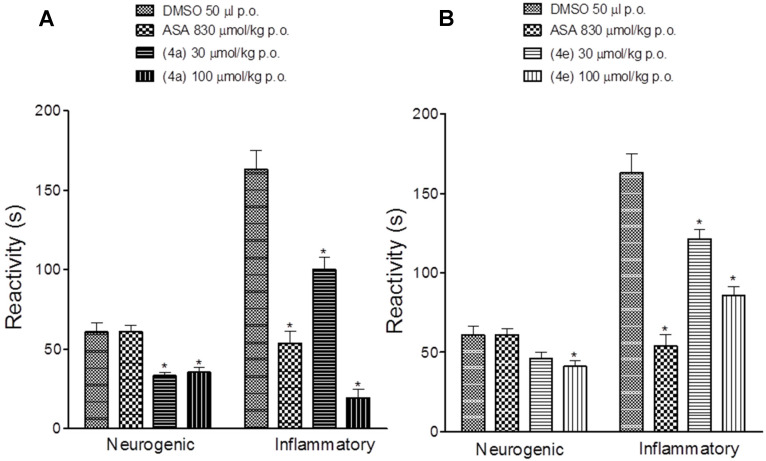
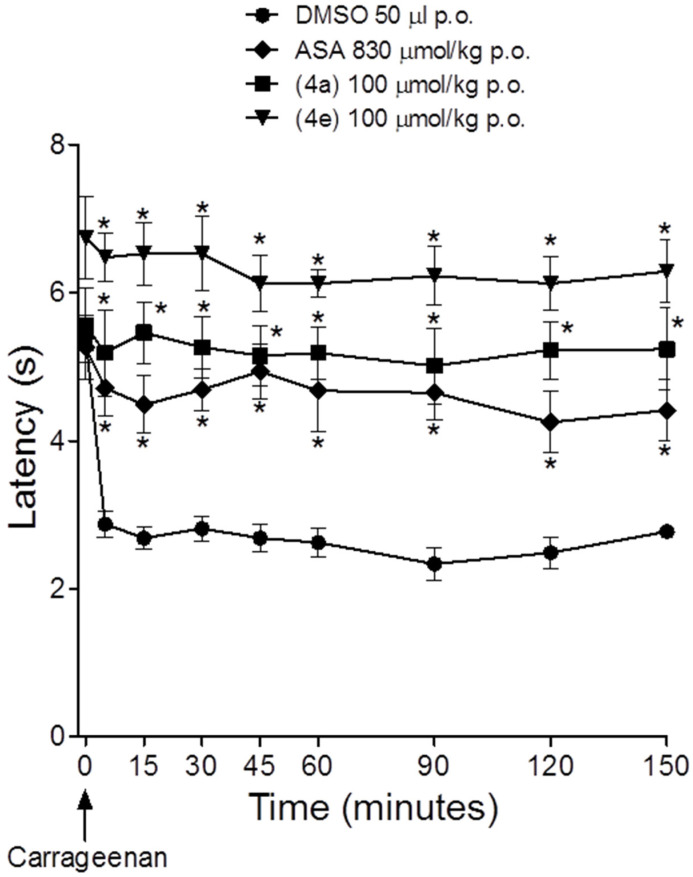
Considering the great analgesic profile of compounds 4a (LASSBio-1755) and 4e (LASSBio-1757) and their anti-inflammatory activity, associated to their in silico drug-like profile, they were selected for further investigation. As shown in Figure 3, both compounds showed a reduction in pain induced by the formalin in a dose-dependent manner. In the inflammatory phase, LASSBio-1755 (4a) reduced the reactivity to 100.1 ± 7.4 s and 19.4 ± 5.3 s after oral administration of 30 and 100 umol/kg, respectively. The compounds were more effective than acetylsalicylic acid (ASA) in reducing the hyperalgesic response during the neurogenic phase of formalin test. Figure 4 shows the effect of LASSBio-1755 (4a) and LASSBio-1757 (4e) on the hyperalgesic response induced by carrageenan. Both compounds reduced the hyperalgesic response at a dose eight times lower than ASA (Figure 4).
Figure 3.
Antihyperalgesic activity of acetylsalicylic acid (ASA), LASSBio-1755 (A), and LASSBio-1757 (B) in formalin induced nociception in mice. Data represent mean ± SEM (n = 8). * p < 0.05 compared to DMSO.
Figure 4.
Comparative antihyperalgesic activity of acetylsalicylic acid (ASA), LASSBio-1755 (4a), and LASSBio-1757 (4e) in carrageenan-induced pain in mice. The points represent the mean ± SEM (n = 8). * p < 0.05 versus DMSO.
Further, it was evaluated the antinociceptive effect of the cyclopropyl-N-acylhydrazones 4a and 4e in a chronic pain model (Figure 5). The compounds reduced the thermal hyperalgesia and mechanical allodynia induced by the spinal nerve ligation (SNL) in rats, recovering the withdrawal latency from 7.2 ± 0.3 to 11.2 ± 0.3 s, and threshold from 24.0 ± 1.1 to 38.0 ± 0.5 g after 14 days of oral administration of 4a (100 umol/kg). Similar results were observed for 4e (100 umol/kg, p.o.).
Figure 5.
Reduction in hyperalgesia and allodynia by LASSBio-1755 (4a) and LASSBio-1757 (4e) in animals submitted to spinal nerve ligation (SNL). The compounds were orally administered (gavage) daily over 7 days. (A) Withdrawal latency in response to thermal stimulation; (B) withdrawal threshold in response to mechanical stimulation applied to the paw of rats. Data represent mean ± SEM (n = 6). * p <0.05 compared to day 0. # p < 0.05 compared to day 7 in the same group.
3. Experimental Section
3.1. Chemistry
3.1.1. General Methods
NMR spectra were determined in deuterated chloroform or dimethyl sulfoxide containing ca. 1% tetramethylsilane as an internal standard, using a 200/50 MHz Bruker DPX-200, 300/75 MHz Varian Unity-300, and 400 MHz Varian—MR spectrometer. The progress of all reactions was monitored by thin layer chromatography, which was performed on 2.0 cm × 6.0 cm aluminum sheets pre-coated with silica gel 60 (HF-254, Merck, Darmstadt, Germany) to a thickness of 0.25 mm. The developed chromatograms were viewed under ultraviolet light at 254 nm. Merck silica gel (230–400 mesh) was used for column chromatography. Elemental analyses were carried out on a Thermo Scientific Flash EA 1112 Series CHN-Analyzer. Melting points were determined with a Quimis Q340.23 apparatus and are uncorrected. All described products showed 1H and 13C NMR spectra according to the assigned structures. All organic solutions were dried over anhydrous sodium sulfate, and all organic solvents were removed under reduced pressure in rotatory evaporator. HPLC for purity determinations were conducted using Shimadzu LC-20AD with a Kromasil 100-5C18 (4.6 mm × 250 mm) and a Shimadzu SPD-M20A detector at 254 nm wavelength. The solvent system for HPLC purity analyses was 70:30 acetonitrile:phosphate buffer solution at pH 7. The isocratic HPLC mode was used, and the flow rate was 1.0 mL/min.
3.1.2. General Procedure for Preparation of Cycloalkyl-hydrazides 8–10
Hydrazine hydrate (80%, 4 equivalents) was added to a solution of methyl cycloalkyl esters 5–7 (1.00 g) in absolute ethanol (4 mL). The reaction mixture was kept under reflux for 48 h. Then, the solvent was evaporated under reduced pressure and ice was added to the residue, resulting in a precipitate formation. The precipitate was filtered to give compounds 8–10 in good yields.
Cyclopentanecarbohydrazide (8)
Compound 8 was obtained in 81% yield as a white powder. Its melting point is 111–113 °C. The data for this compound are in agreement with previous reports (117–118 °C) [27].
Cyclobutanecarbohydrazide (9)
Compound 9 was obtained in 63% yield as a white powder. Its melting point is 79–81 °C. The data for this compound are in agreement with previous reports (79–80 °C) [28].
Cyclopropanecarbohydrazide (10)
Compound 10 was obtained in 59% yield as a white powder. Its melting point is 74–76 °C. The data for this compound are in agreement with previous reports (98–99 °C) [29].
General Procedure for Preparation of cycloalkyllacylhydrazones 2a–e, 3a–e, and 4a–e
The corresponding aromatic or heteroaromatic aldehyde (2 mmol) was added to a solution of cycloalkylcarbohydrazides (8–10, 2 mmol) in absolute ethanol (10 mL). The mixture was stirred for 2h at room temperature. At the end of the reaction, the volume of ethanol was partially concentrated at reduced pressure, and the resulting mixture was poured into cold water. The precipitate was filtered out, dried under vacuum, and then the solid was washed with n-hexane and/or recrystallized from ethanol to give the target cycloalkyl-acylhydrazones (2a–e, 3a–e and 4a–e).
(E)-N’-(pyridin-4-ylmethylene)cyclopentanecarbohydrazide (LASSBio-1521, 2a)
Compound 2a was obtained as a white powder in 59% yield by condensation of cyclopentane-carbohydrazide (8) with isonicotinaldehyde, m.p. 139–140 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.60/11.46 (s,1H, CONH-), 8,62 (d, 1H, J = 4 Hz, H8/H12) 8.18/7.95 (s, 1H, N=CH), 7,61 (d, 1H, J = 4 Hz, H9/H11), 3.51/2.50 (m, 1H, H1), 1.61 (m, 8H, cycloalkyl).13C-NMR (50 MHz) DMSO-d6 (ppm): 178,07/172.87 (NC=O), 150.75 (2C, C9/C11), 143.92/140.30 (1C, N=CH), 142.23 (1C, C7), 121.41 (2C, C8/C12), 43.72 (1C, C1), 30.47/29.83 (2C, C-2/C5), 26.24 (2C, C3/C4). IR (KBr) νmax (cm−1): 3179 (NH); 2952 (CH3); 1669 (C=O); 1595 (C=N); 1406 (Pyridine). % purity = 96.55% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 4.75 min).
(E)-N’-benzylidenecyclopentanecarbohydrazide (LASSBio-1519, 2b)
Compound 2b was obtained as a white powder in 80% yield by condensation of cyclopentane-carbohydrazide (8) with benzaldehyde, m.p. 160–161 °C. The data for this compound are in agreement with previous reports (158–160 °C) [5].
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.35/11.16 (s, 1H, CONH-), 8.18/7.98 (s, 1H, N=CH), 7.64 (m, 2H, H8 e H12), 7.41 (m, 3H, H9/H10/H11), 3.49/2.64 (m, 1H), 1.64 (m, 8H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 177.73/172.54 (NC=O), 146/142 (N=CH), 134,01 (1C, C7), 130,33 (2C, C8/C12), 127.03 (3C, C9/C10/C12), 43.43 (1C, C1), 29–31 (2C, C-2/C5), 26,25 (2C, C3/C4). IR [KBr] νmax (cm−1): 3187 (NH); 2953 (CH3); 1649 (C=O); 1562 (N=C). Anal. Calcd. for C13H16N2O: C, 72.19; H, 7.46; N, 12.95. Found: C, 72.11; H, 7.44; N 12.75.
(E)-N’-(thiophen-2-ylmethylene)cyclopentanecarbohydrazide (LASSBio-1520, 2c)
Compound 2c was obtained as a white powder in 71% yield by condensation of cyclopentane-carbohydrazide (8) with thiophene-2-carbaldehyde, m.p. 182–184 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.30/11.13 (s,1H, CONH-), 8.39/8.15 (s, 1H, N=CH), 7,63 (d, 1H, J = 5 Hz, H8), 7,46 (d, 1H, J = 5 Hz, H10), 7,34 (m, 1H, H9), 2,61 (m, 1H), 1.61 (m, 8H, cycloalkyl). 13C (50 MHz) DMSO-d6 (ppm): 176.99/171.98 (NC=O), 141.22 (1C, C7), 139.38 (1C, C8), 137.49 (1C, C10), 130.53 (1C, C9), 127.95 (N=CH), 43.24 (1C, C1), 30.06/29.30 (2C, C-2/C5), 25.78 (2C, C3/C4).IR [KBr] νmax (cm−1): 3180(NH); 2948(CH3); 1653(C=O). Anal. Calcd. for C11H14N2O: C, 59.43; H, 6.35;N, 12.44. Found: C, 59.20; H, 6.30; N 12.44.
(E)-N’-(E)-N’-Ferrocenylcyclopentanecarbohydrazide (LASSBio-1522, 2d)
Compound 2d was obtained as a white powder in 69% yield by condensation of cyclopentane-carbohydrazide (8) with ferrocenecarboxaldehyde m.p. 204–205 °C.
1H- NMR (200 MHz) DMSO-d6 (ppm): 11.02/10.83 (s,1H, CONH-), 8.00/7.79 (s, 1H, N=CH), 4.59 (s, 2H, H7/H11), 4.40 (s, 2H, H9/H10), 4.20 (s, 5H), 3.51/2.50 (m, 1H, H1), 1.61 (m, 8H). 13C (50 MHz) DMSO-d6 (ppm): 176.94/169.81 (NC=O), 148.24/143.28 (1C, N=CH), 79.84 (1C, C7), 70.72 (2C, C8/C11), 69.24 (2C, C9/C10), 68.07 (5C), 43.91 (1C, C1), 30.56/29.83 (2C, C-2/C5), 26.70 (2C, C3/C4). IR [KBr] νmax (cm−1): 3181 (NH); 2953 (CH3); 1649 (C=O); 1578 (C=N). Anal. Calcd. for C17H20FeN2O: C, 62.; 98H, 6.22;N, 8.64. Found: C, 61.59; H, 6.12; N 8.60.
(E)-N’-(4-(dimethylamino)benzylidene)cyclopentanecarbohydrazide (LASSBio-1518, 2e)
Compound 2e was obtained as a white powder in 71% yield by condensation of cyclopentane-carbohydrazide (8) with 4-(dimethylamino)benzaldehyde, m.p. 178–180 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11/11.86 (s,1H, CONH-), 8.02/7.84 (s, 1H, N=CH), 7,49 (d, 2H, J =8 Hz, H11/H9), 6.75 (d, 2H, J= 8Hz, H8/H12), 3.34/2.51 (m, 1H), 2.96 (s, 6H), 1.71 (m, 8H, cycloalkyl). 13C-NMR (50 MHz) CDCl3 (ppm): 160 (NC=O), 147 (N=CH), 128.73 (2C, C11/C9), 122.33 (2C, C7 e C10), 112.45 (2C, C8/C12), 41.34 (1C, C1), 30.57/29.81 (2C, C2/C5), 26,25 (2C, C3/C4). IR (KBr) νmax (cm−1): 3201 (NH); 2926 (CH3); 1663 (C=O); 1600 (N=C). Anal. Calcd. for C15H21FeN3O: C, 64.90; H, 8.43; N, 15.15. Found: C, 64.88; H, 8.43; N 14.93.
(E)-N’-(pyridin-4-ylmethylene)cyclobutanecarbohydrazide (LASSBio-1686, 3a)
Compound 3a was obtained as a white powder in 22% yield by condensation of cyclobutanecarbohydrazide (9) with isonicotinaldehyde, m.p. 121–123 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.44/11.36 (s, 1H, CONH-), 8.52 (d, 2H, J = 4 Hz, H8/H9), 8.08/7.83 (s, 1H, N=CH), 7.51 (d, 2H, J = 4 Hz, H7 e H10), 3.71/3.05 (m, 1H, H1), 2.15–1.65 (m, 6H). 13C-NMR (50 MHz ) DMSO-d6 (ppm):176.58/171.38 (NC=O), 150.74 (2C, C8 e C9), 144.11/140.38 (s, N=CH), 142.12 (1C, C6), 121.41 (2C, C7 e C10), 36.66 (1C, C1), 24.95 (2C, C2 e C4), 18.48 (1C, C3). IR [KBr] νmax (cm−1): 3185 (NH); 2936 (CH3); 1671 (C=O); 1593 (C=N); 1406/807 (4-Pyridine). % purity = 98.97% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 2.97 min)
(E)-N’-benzylidenecyclobutanecarbohydrazide (LASSBio-1687, 3b)
Compound 3b was obtained as a white powder in 62% yield by condensation of cyclobutanecarbohydrazide (9) with benzaldehyde, m.p. 124–126 °C.
1H (200 MHz) DMSO-d6 (ppm): 11.20/11.14 (s, 1H, CONH-), 8.16/7.93 (s, 1H, N=CH), 7.64 (m, 2H, H7 e H11), 7.42 (m, 3H, H8/H9/H10), 4.40–3.11 (m, 1H, H1), 2.17–1.65 (m, 6H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176 (NC=O), 142.22 (s, N=CH), 134.11 (1C, C6), 129.47 (2C, C7/C11), 126.50 (3C, C8/C9/C10), 37.08 (1C, C1), 24.36 (2C, C2/C4), 17.91(2C, C3). IR [KBr] νmax (cm−1): 3185 (NH); 2929 (CH3); 1651(C=O); 758/695 (phenyl). Anal. Calcd. for C12H14N2O: C, 7126.; H, 6.98; N, 13.85. Found: C, 71.00; H, 6.93; N 13.57.
(E)-N’-(thiophen-2-ylmethylene)cyclobutanecarbohydrazide (LASSBio-1685, 3c)
Compound 3c was obtained as a white powder in 55% yield by condensation of cyclobutanecarbohydrazide (9) with thiophene-2-carbaldehyde, m.p. 128–130 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.15/11.13 (s, 1H, CONH-), 8.40/8.10 (s, 1H, N=CH), 7.63 (d, 2H, J = 5 Hz, H9), 7.39 (d, 2H, J = 5Hz, H7), 7.13 (d, 1H, J = 5Hz, H9), 3.81/3.05 (m, 1H, H1), 2.15–1.65 (m, 6H, cycloalkyl). 13C-NMR (50 MHz) DMSO-d6 (ppm): 176.58/171.38 (NC=O), 150.74 (2C, C7), 144.11/140.38 (s, N=CH), 142.12 (1C, C6), 121.41 (2C, C8 e C9), 36.66 (1C, C1), 24.95 (2C, C2 e C4), 18.48 (1C, C3). IR [KBr] νmax (cm−1): 3167 (NH); 2949 (CH3); 1663 (C=O); 1593 (C=N); 1441 (2-thiophene). % purity = 99.41% by HPLC-C18. (acetonitrile/water 6:4) (Rt = 9.74 min).
(E)-N’-Ferrocenylcyclobutanecarbohydrazide (LASSBio-1683, 3d)
Compound 3d was obtained as a white powder in 60% yield by condensation of cyclobutanecarbohydrazide (9) with ferrocenecarboxaldehyde, m.p. 92–94 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 10.87/10.82 (s,1H, CONH-), 8.00/7.74 (s, 1H, N=CH), 4.58 (s, 2H, H6/H9), 4.40 (s, 2H, H7/H8), 141 4.20 (s, 5H, H10), 3.67/3.02 (m, 1H, H1), 2.15–1.65 (m, 6H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 174.92/169.54 (NC=O), 147.31/142.73 (s, N=CH), 69.91 (2C, C6/C9), 68.81 (2C, C7/C8), 67.08 (5C, C10), 36.11 (1C, C1), 24.41 (2C, C2 e C4), 17.92 (1C, C3). % purity = 99.17% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 5.19 min).
IR [KBr] νmax (cm−1): 3440 (NH); 3130 (CH3); 1660 (C=O). % purity = 99.17% by HPLC-C18(acetonitrile/water 7:3) (Rt = 5.19 min)
(E)-N’-(4-(dimethylamino)benzylidene)cyclobutanecarbohydrazide (LASSBio-1684, 3e)
Compound 3e was obtained as a white powder in 50% yield by condensation of cyclobutanecarbohydrazide (9) with 4-(dimethylamino)benzaldehyde, m.p. 186–188 °C.
1H (200 MHz) CDCl3 (ppm): 9.75/8.95 (s, 1H, CONH-), 8.04/8.10 (s, 1H, N=CH), 7.59 (d, 2H, J = 8 Hz, H7/H10), 6.72 (d, 2H, J = 8 Hz, H7 e H11), 3.01 (s, 6H, CH3), 3.81/3.05 (m, 1H, H1), 2.15–1.65 (m, 6H). 13C-NMR (50 MHz) CDCl3 (ppm): 177.35 (NC=O), 151.56 (1C, C8/C9), 143.99 (s, N=CH), 129.22 (2C, C7/C11), 122.10 (2C, C7), 111.75 (2C, C8/C10), 40.29 (2C, CH3), 36.88 (1C, C1), 25.06 (2C, C2 e C4), 18.48 (1C, C3). IR [KBr] νmax (cm−1): 3200 (NH); 2937 (CH3); 1665 (C=O); 1599 (C=N); 1367 (ArN(CH3)2). Anal. Calcd. for C14H19N3O: C, 63.84; H,7.23; N, 15.96. Found: C, 64.02; H, 7.88; N 15.82.
(E)-N’-(pyridin-4-ylmethylene)cyclopropanecarbohydrazide (LASSBio-1755, 4a)
Compound 4a was obtained as a white powder in 42% yield by condensation of cyclopropanecarbohydrazide (10) with isonicotinaldehyde, m.p. 193–194 °C, as previously described by Bastos and coworkers (2017) [18].
1H-NMR (200 MHz) DMSO-d6 (ppm): 8.60 (d, 2H, J = 4 Hz, H7/H8), 8.16/8.00 (s, 1H, N=CH), 7.60 (d, 2H, J = 4 Hz, H6/H9), 2.67/1.64 (m, 1H, H1), 0.99–0.81 (m, 4H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 175.00/169.83 (NC=O), 159.63/142.98 (s, N=CH), 150.31/150.16 (1C, C7/C8), 142.98/141.65 (1C, C5), 122.81 (2C, C6/C9), 126.89 (2C, C7/C9), 12.94/9.72 (1C, C1), 8.12/7.25 (2C, C2/C3). IR [KBr] νmax (cm−1): 3176 (NH); 2952 (CH3); 1663(C=O); 1595 (C=N). % purity = 99.95% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 2.23 min).
(E)-N’-benzylidenecyclopropanecarbohydrazide (LASSBio-1753, 4b)
Compound 4b was obtained as a white powder in 55% yield by condensation of cyclopropanecarbohydrazide (10) with benzaldehyde, m.p. 152–154 °C. The data for this compound are in agreement with previous reports (152 °C) [6].
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.63/11.37 (s, 1H, CONH-), 8.18/8.04 (s, 1H, N=CH), 7.70 (m, 2H, H6/H10), 7.42 (m, 3H, H7/H8/H9), 2.73/1.67 (m, 1H, H1), 1.09–0.65 (m, 4H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 174.57/169.32 (NC=O), 145.36/142.77 (s, N=CH), 134.32 (1C, C5), 129.76 (1C, C8), 128.73 (2C, C6/C10), 126.89 (2C, C7/C9), 12.83/9.70 (1C, C1), 7.81/6.89 (2C, C2/C3). IR [KBr] νmax (cm−1): 3189 (NH); 3013 (CH3); 1645 (C=O); 757/692 (phenyl). % purity = 99.80% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 3.73 min).
(E)-N’-(thiophen-2-ylmethylene)cyclopropanecarbohydrazide (LASSBio-1756, 4c)
Compound 4c was obtained as a white powder in 53% yield by condensation of cyclopropanecarbohydrazide (10) with thiophene-2-carbaldehyde, m.p. 137–140 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.57/11.35 (s, 1H, CONH-), 8.40/8.22 (s, 1H, N=CH), 7.63 (d, 1H, J = 5 Hz, H8), 7.43 (d, 1H, J = 5 Hz, 144 H6), 7.14 (dd, 1H, J = 5 Hz, J = 2 Hz, H7), 2.51/1.65 (m, 1H, H1), 0.99–0.77 (m, 4H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 174.23/169.18 (NC=O), 140.66/138.01 (s, N=CH), 139.19/139.06 (1C, C5), 130.49/129.94 (1C, C8), 128.48/127.99 (1C, C6), 127.84/127.74 (1C, C7), 12.86/9.53 (1C, C1), 7.86/6.94 (2C, C2/C3). IR [KBr] νmax (cm−1): 3188 (NH); 2939 (CH3); 1660 (C=O). % purity = 98.45% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 3.53 min).
(E)-N’-Ferrocenylcyclopropanecarbohydrazide (LASSBio-1754, 4d)
Compound 4d was obtained as a white powder in 56% yield by condensation of cyclopropanecarbohydrazide (10) with ferrocenecarboxaldehyde, m.p. 223–225 °C. The data for this compound are not in agreement with previous reports (208–209 °C) [6].
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.30/11.05 (s, 1H, CONH-), 8.01/7.85 (s, 1H, N=CH), 4.69 (s, 2H, H5/H8), 4.31 (s, 2H, H6/H7), 4.07 (s, 5H, H9), 2.73/1.57 (m, 1H, H1), 1.09–0.65 (m, 4H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 69.93 (2C, C6/C7), 68.86(2C, C5/C8), 66.08 (5C, C9), 12.03/9.27 (1C, C1), 7.81/6.89 (2C, C2/C3). IR [KBr] νmax (cm−1): 3181 (NH); 3005 (CH3); 1653 (C=O). % = 95.39% by HPLC-C18.(acetonitrile/water 7:3) (Rt = 4.37 min).
(E)-N’-(4-(dimethylamino)benzylidene)cyclopropanecarbohydrazide (LASSBio-1757, 4e)
Compound 4e was obtained as a white powder in 47% yield by condensation of cyclobutanecarbohydrazide (10) with 4-(dimethylamino)benzaldehyde, m.p. 184–186 °C.
1H-NMR (200 MHz) DMSO-d6 (ppm): 11.31/11.07 (s,1H, CONH-), 8.50/8.03 (s, 1H, N=CH), 7.67 (d, 2H, J = 8Hz, H6/H10), 6.74 (d, 2H, J = 8Hz, H8/H9), 3.38 (s, 6H, CH3) 2.67/1.63 (m, 1H, H1), 0.85–0.73 (m, 4H). 13C-NMR (50 MHz) DMSO-d6 (ppm): 174.01/168.69 (NC=O), 151.35/151.20 (1C, C8), 146.20/143.66 (s, N=CH), 128.21/127.88 (2C, C6/C10), 121.76/121.70 (1C, C5), 111.81 (2C, C7/C9), 40.29 (2C, CH3), 12.78/9.69 (1C, C1), 7.59/6.59 (2C, C2/C3). IR [KBr] νmax (cm−1): 3190 (NH); 2924 (CH3); 1656 (C=O); 1611 (C=N). % purity = 95.87% by HPLC-C18. (acetonitrile/water 7:3) (Rt = 2,67 min).
3.2. Antinociceptive and Anti-Inflammatory Pharmacological Evaluation
3.2.1. Animals
Male Swiss mice (20–30 g) were obtained from Federal University of Rio de Janeiro and BIOCEN-UFAL, and male Wistar rats (180–220 g) were obtained from Federal University of Rio de Janeiro. Animals was housed in group cages and maintained on a 12 h light/12 h dark cycle. The animals had free access to food and water at all times. Experiments were carried out according to a protocol approved by the Animal Welfare Committee of Federal University of Alagoas (UFAL) (Protocol Number: 026681/2009-23), and Institutional Animal Care and Use Committee at Universidade Federal of Rio de Janeiro (UFRJ) and in accordance with the ethical guidelines for investigation of experimental pain in conscious animals.
3.2.2. Reagents
Acetic acid (Merck, Rio de Janeiro, Brasil), gum arabic (Sigma-Aldrich, Rio de Janeiro, RJ, Brasil), dipyrone (Sigma-Aldrich), and indomethacin (Sigma-Aldrich) were obtained from commercial sources. A solution of formalin 2.5% was prepared with formaldehyde (Merck) in saline (NaCl 0.9%). Dimethyl sulfoxide (DMSO, used as vehicle) and tramadol were donated by Cristália Produtos Químicos e Farmacêuticos Ltd.a (Itapira, SP, Brazil).
3.2.3. Carrageenan-Induced Peritonitis
Peritoneal inflammation was induced according to the method described by Ferrandiz and Alcaraz [20]. A solution of carrageenan 1% (Sigma-Aldrich) was prepared in saline (NaCl 0.9%) and injected into the peritoneal cavity of mice (250 μL/animal). After 4h of carrageenan injection, the animals were euthanized by cervical dislocation and the peritoneal cavity was washed with 3 mL of cold Hank’s. Compounds and indomethacin were administered at the dose of 100 μmol/kg (p.o.) 30 min before carrageenan injection. The control group received 10 mL/kg of the vehicle (gum arabic, p.o.). The number of cells was quantified by optical microscope, using 100× lens.
3.2.4. Acetic Acid-Induced Writhing Test
Mice received intraperitoneally (i.p.) administered acetic acid (0.6%, v/v, 0.1 mL/10 g), as previously reported [24,30]. The number of writhes, a response consisting of contraction of an abdominal wall, pelvic rotation followed by hind limb extension, was counted during continuous observation for 20 min beginning from 5 min after the acetic acid injection. Dipyrone and compounds (all 100 μmol/kg, oral administration) were administered 60 min before the acetic acid injection. Antinociceptive activity was expressed as inhibition percent of the usual amount of writhing observed in control animals.
3.2.5. Formalin Induced Nociception
The procedure was performed as described by Sudo et al. (2015) [30] in mice to investigate the antinociceptive effects of LASSBio-1755 (4a) and LASSBio-1757 (4e) on neurogenic and inflammatory pain. The formalin (2.5%, 20 µL) was injected into the right hind paw 15 min after oral administration of DMSO (50 µL, vehicle), acetylsalicylic acid (150 mg/kg, reference drug), LASSBio-1755 (30 and 100 µmol/kg) and LASSBio-1757 (30 and 100 µmol/kg). Evaluation started immediately after formalin injection, being evaluated during 0–5 min (neurogenic pain response) and 15–30 min (inflammatory pain response). The duration of licking and biting of the injected paw was analyzed.
3.2.6. Carrageenan-Induced Nociception
The thermal hyperalgesia induced by carrageenan (1%, 20 µL) was evaluated in mice according to the method described by Mendes et al. (2009) [31]. Peripheral inflammation was caused by intraplantar injection of carrageenan into the right hind paw in mice. The latency of each animal to the thermal stimuli was assessed at different times: before (control) and after carrageenan injection. Saline, acetylsalicylic acid (830 µmol/kg), and LASSBio-1755 (4a) (100 µmol/kg) and LASSBio-1757 (4e) (100 µmol/kg) were orally administered 15 min before carrageenan. Heat stimulus under light applied on hind paws until the paw withdrawal was studied; the time between thermal stimulus and paw withdrawal defined as latency. A cut-off time of 15 s used to avoid tissue damage. Evaluation was made using a plantar analgesia meter (ITC Inc. model 33).
3.2.7. Neuropathic Pain Model Induced by Spinal Nerve Ligation
The peripheral neuropathy model was induced by spinal nerve ligation (SNL), according with Kim and Chung (1992) [26]. Wistar rats (200–220 g) were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.), and endured an incision of approximately 1 cm at the level of spinal L5 to S1. The L6 transverse process was partially removed to allow identification of L4 and L5 spinal nerves. The right L5 spinal nerve was isolated and ligated with 6.0 silk. The false-operated animals (SHAM) used for control were submitted to the same surgery procedure without the nerve ligation.
Evaluation of the Thermal Hyperalgesia and Mechanical Allodynia Induced by SNL
Thermal hyperalgesia and mechanical hypersensitivity signals were defined as a significant reduction in the paw withdrawal threshold and latency, respectively, in SNL animals compared to SHAM. After the development of these signals, the animals were orally treated for 7 days with LASSBio-1755 (100 µmol/kg), LASSBio-1757 (100 µmol/kg), tramadol (33 µmol/kg) or DMSO (100 µL, vehicle). For measurement of paw withdrawal latency [32], we used a radiant heat source (Ugo Basile model 37,370), applied the plantar surface of the hind paws, taking three measurements with a cut-off of 30 s to avoid tissue injury. However, the withdrawal threshold was evaluated using a digital version of Von Frey filaments (Analgesymeter Digital Device, model EFF301) [24,30]. Mechanical stimuli were applied to plantar region of the hind paw, and the withdrawal threshold was taken in five measurements with a 120 g cut-off to avoid tissue injury.
Statistical Analysis
Results are expressed as means ± standard error of means (SEM). The values of reactivity in the formalin test, paw withdrawal latency, and threshold were compared by Analysis of Variance One-Way followed by Newman–Keuls test. Two-way ANOVA, followed by Bonferroni post hoc was used to analyze carrageenan-induced paw withdrawal, using GraphPad Prism (version 5.0; GraphPad Software, Inc., San Diego, CA, USA). p values of <0.05 were considered significant.
4. Conclusions
Taken together, a systematic inferior homologation was applied at the structure of the previous prototype 1 (LASSBio-1514), yielding the design and synthesis of the cyclopentyl-, cyclobutyl- and cyclopropylacylhydrazones, which showed similar in silico physicochemical and drug-like profiles to the parent compound 1. Compounds revealed a good in vivo anti-inflammatory and antinociceptive profile, allowing the identification of compounds 4e (LASSBio-1757) and 4a (LASSBio-1755, previously synthetized and characterized by Bastos and coworkers [18]), as new analgesic lead-candidates, active by oral administration in acute and chronic model of pain. Additionally, our data demonstrate the success of the homologation strategy in the design of bioactive compounds.
Acknowledgments
The authors would like to thank CNPq (BR), FAPERJ (BR), CAPES (BR, Finance Code 001) and INCT-INOFAR (BR, 573.564/2008-6 and E-26/170.020/2008) for fellowship and financial support.
Supplementary Materials
The following are available online, Figure S1. 1H NMR spectrum of 2a (DMSO-d6, 200 MHz); Figure S2. 13C NMR spectrum of 2a (DMSO-d6, 50 MHz); Figure S3. 1H NMR spectrum of 2b (DMSO-d6, 200 MHz); Figure S4. 13C NMR spectrum of 2b (DMSO-d6, 50 MHz); Figure S5. 1H NMR spectrum of 2c (DMSO-d6, 200 MHz); Figure S6. 13C NMR spectrum of 2c (DMSO-d6, 50 MHz); Figure S7. 1H NMR spectrum of 2d (DMSO-d6, 200 MHz); Figure S8. 13C NMR spectrum of 2d (DMSO-d6, 50 MHz); Figure S9. 1H NMR spectrum of 2e (DMSO-d6, 200 MHz); Figure S10. 13C NMR spectrum of 2e (CDCl3, 50 MHz); Figure S11. 1H NMR spectrum of 3a (DMSO-d6, 200 MHz); Figure S12. 13C NMR spectrum of 3a (DMSO-d6, 50 MHz); Figure S13. 1H NMR spectrum of 3b (DMSO-d6, 400 MHz); Figure S14. 13C NMR spectrum of 3b (DMSO-d6, 50 MHz); Figure S15. 1H NMR spectrum of 3c (DMSO-d6, 200 MHz); Figure S16. 13C NMR spectrum of 3c (DMSO-d6, 50 MHz); Figure S17. 1H NMR spectrum of 3d (DMSO-d6, 200 MHz); Figure S18. 13C NMR spectrum of 3d (DMSO-d6, 50 MHz); Figure S19. 1H NMR spectrum of 3e (CDCl3, 200 MHz); Figure S20. 13C NMR spectrum of 3e (CDCl3, 50 MHz); Figure S21. 1H NMR spectrum of 4a (DMSO-d6, 200 MHz); Figure S22. 13C NMR spectrum of 4a (DMSO-d6, 50 MHz); Figure S23. 1H NMR spectrum of 4b (DMSO-d6, 200 MHz); Figure S24. 13C NMR spectrum of 4b (DMSO-d6, 50 MHz); Figure S25. 1H NMR spectrum of 4c (DMSO-d6, 200 MHz); Figure S26. 13C NMR spectrum of 4c (DMSO-d6, 50 MHz); Figure S27. 1H NMR spectrum of 4d (DMSO-d6, 200 MHz); Figure S28. 13C NMR spectrum of 4d (DMSO-d6, 50 MHz); Figure S29. 1H NMR spectrum of 4e (DMSO-d6, 200 MHz); Figure S30. 13C NMR spectrum of 4e (DMSO-d6, 50 MHz); Figure S31. 1H NMR of 4c at different temperatures (DMSO-d6, 300 MHz).
Author Contributions
Conceptualization: L.M.L. and E.J.B.; methodology: T.F.d.S., W.B.J., A.C.d.Q. and C.E.d.S.M.; software: L.M.L. and C.A.-S.; formal analysis: T.F.d.S., W.B.J., C.E.d.S.M., A.C.d.Q. and C.A.-S.; investigation: T.F.d.S., W.B.J., C.E.d.S.M. and A.C.d.Q.; resources: E.J.B., L.M.L., G.Z.-S. and M.S.A.-M.; writing—original draft preparation: L.M.L., C.A.-S., C.E.d.S.M.; writing—review and editing: L.M.L., E.J.B., G.Z.-S., M.S.A.-M.; supervision: L.M.L., E.J.B., G.Z.-S., M.S.A.-M.; funding acquisition: E.J.B., L.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
INCT-INOFAR (BR, 573.564/2008-6 and E-26/170.020/2008); CAPES (BR, Finance Code 001); CNPq (#573.564/2008-6); FAPERJ (E-26/170.020/2008 and E-26/202.676/2019).
Institutional Review Board Statement
Ethics and Research Committee of the Federal University of Alagoas (N° 026681/2009-23) and Committee on Ethics in the Use of Animals (CEUA) in Scientific Experimentation at Federal University of Rio de Janeiro (UFRJ #144/15).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
No conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh N., Ranjana R., Kumari M., Kumar B. A Review on Biological Activities of Hydrazone Derivatives. Intern. J. Pharmac. Clin. Res. 2016;8:162–166. [Google Scholar]
- 2.Maia R.C., Tesch R., Fraga C.A.M. Acylhydrazone derivatives: A patent review. Expert Opin. Ther. Pat. 2014;24:1161–1170. doi: 10.1517/13543776.2014.959491. [DOI] [PubMed] [Google Scholar]
- 3.Verma G., Marella A., Shaquiquzzaman M., Akhtar M., Rahmat Ali M., Alam M.M. A review exploring biological activities of hydrazones. J. Pharm. Biol. Sci. 2014;6:69–80. doi: 10.4103/0975-7406.129170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rollas M., Küçükgüzel S.G. Biological Activities of Hydrazone Derivatives. Molecules. 2007;12:1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popiołek Ł., Stefańska J., Kiełczykowska M., Musik I., Biernasiuk A., Malm A., Wujec M. Synthesis, Dissociation Constants, and Antimicrobial Activity of Novel 2,3-Disubstituted-1,3-thiazolidin-4-one Derivatives. J. Heterocycl. Chem. 2015;53:393–402. doi: 10.1002/jhet.2418. [DOI] [Google Scholar]
- 6.Popp F.D., Kirby J.A. Ferrocenylidene Hydrazides. J. Chem. Eng. Data. 1963;8:604. doi: 10.1021/je60019a041. [DOI] [Google Scholar]
- 7.Rector D.L., Conder G.A., Folz S.D. Anthelmintic Pyridinyl Acylhydrazones, Method of Use and Compositions. Publication Date: 14 August 1986. [(accessed on 31 July 2021)]; Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1986004582.
- 8.Palla G., Predieri G., Domiano P., Vignali C., Turner W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron. 1986;42:3649–3654. doi: 10.1016/S0040-4020(01)87332-4. [DOI] [Google Scholar]
- 9.Kumar P., Kadyan K., Duhan M., Sindhu J., Singh V., Saharan B.S. Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents. Chem. Cent. J. 2017;11:115. doi: 10.1186/s13065-017-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu C., Htan B., Ma C., Liao R.-Z., Gan Q. Acylhydrazone Switches: E/Z Stability Reversed by Indroduction of Hydrogen Bonds. Eur. J. Org. Chem. 2018;48:7046–7050. doi: 10.1002/ejoc.201801466. [DOI] [Google Scholar]
- 11.Thota S., Rodrigues D.A., Pinheiro P.S.M., Lima L.M., Fraga C.A.M., Barreiro E.J.B. N-Acylhydrazones as drugs. Bioorg. Med. Chem. Lett. 2018;28:2797–2806. doi: 10.1016/j.bmcl.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Kress H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur. J. Pain. 2009;13:11–15. doi: 10.1016/S1754-3207(09)70004-5. [DOI] [PubMed] [Google Scholar]
- 13.Karra R., Rossing S.H., Mohammed D., Parmeggiani L., Heine M., Namnún O.C. Unmet needs in the management of functional impairment in patients with chronic pain: A multinational survey. Pain Manag. 2021;11:303–314. doi: 10.2217/pmt-2020-0098. [DOI] [PubMed] [Google Scholar]
- 14.Silva T.F., Júnior W.B., Moreira M.S.A., Costa F.N., Monteiro C.E.S., Ferreira F.F., Barroso R.C.R., Noël F., Sudo R.T., Sudo G.Z., et al. Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives. Molecules. 2015;20:3067–3088. doi: 10.3390/molecules20023067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lima L.M., Alves M.A., Amaral D.N. Homologation: A Versatile Molecular Modification Strategy to Drug Discovery. Cur. Top. Med. Chem. 2019;19:1734–1750. doi: 10.2174/1568026619666190808145235. [DOI] [PubMed] [Google Scholar]
- 16.Lima L.M., Barreiro E.J. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design. Cur. Med. Chem. 2005;12:23–49. doi: 10.2174/0929867053363540. [DOI] [PubMed] [Google Scholar]
- 17.Lima L.M., Barreiro E.J. Comprehensive Medicinal Chemistry. III. 3rd ed. Elsevier; 2017. [(accessed on 9 August 2021)]. Beyond Bioisosterism: New Concepts in Drug Discovery; pp. 186–210. eBook; v.1. Available online: https://www.elsevier.com/books/comprehensive-medicinal-chemistry-iii/chackalamannil/978-0-12-803200-8. [Google Scholar]
- 18.Bastos I.T.S., Costa F.N., Silva T.F., Barreiro E.J., Lima L.M., Braz D., Lombardo G.M., Punzo F., Ferreira F.F., Barroso R.C. A combined experimental and in silico characterization to highlight additional structural features and properties of a potentially new drug. J. Mol. Struct. 2017;1146:735–743. doi: 10.1016/j.molstruc.2017.06.061. [DOI] [Google Scholar]
- 19. [(accessed on 31 July 2021)]; Available online: https://www.acdlabs.com/products/percepta/
- 20.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 21.Veber D.F., Johnson S.R., Cheng H.Y., Smith M.R., Ward K.W., Kopple K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 22.Patra M., Gasser G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017;1:0066. doi: 10.1038/s41570-017-0066. [DOI] [Google Scholar]
- 23.Abraham M.H., Benjelloun-Dakhama N., Gola J.M.R., Acree W.E., Jr., Cain W.S., Enrique Cometto-Muniz J. Solvation descriptors for ferrocene, and the estimation of some physicochemical and biochemical properties. New J. Chem. 2000;24:825–829. doi: 10.1039/b004291i. [DOI] [Google Scholar]
- 24.Collier H.O., Dinneen L.C., Johnson C.A., Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunskaar S., Hole K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 26.Ferrandiz M.L., Alcaraz M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions. 1991;32:283–288. doi: 10.1007/BF01980887. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz E., Stark A., Hörig C. Cyclische Diazoverbindungen, V: Ein Diazoketon mit Dreiringstruktur. Eur. J. In. Chem. 1965;98:2509–2515. doi: 10.1002/cber.19650980812. [DOI] [Google Scholar]
- 28.Martin W.B., Swett M.L.R. Isopropylhydrazine Derivatives. US2928875A. 1960 Mar 15;
- 29.Liu X.H. High Throughput Receptor-Based Virtual Screening under ZINC Database, Synthesis, and Biological Evaluation of Ketol-Acid Reductoisomerase Inhibitors. Chem. Biol. Drug Des. 2010;75:228–232. doi: 10.1111/j.1747-0285.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 30.Sudo R.T., Neto M.L., Monteiro C.E., Amaral R.V., Resende A.C., Souza P.J., ZapataSudo G., Moura R.S. Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Acai) in a rodent model of acute and neuropathic pain. BMC Complement. Altern. Med. 2015;15:208. doi: 10.1186/s12906-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendes T.C., Raimundo J.M., Nascimento-Junior N.M., Fraga C.A., Barreiro E.J., Sudo R.T., Zapata-Sudo G. Sedation and antinociception induced by a new pyrazolo [3,4-b]pyrrolo[3,4-d]pyridine derivative (LASSBio-873) is modulated by activation of muscarinic receptors. Pharmacol. Biochem. Behav. 2009;94:70–74. doi: 10.1016/j.pbb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.H., Chung J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.