Abstract
Heat shock protein 70 (Hsp70) is thought to play a critical role in the thermotolerance of mammalian cells, presumably due to its chaperone activity. We examined the chaperone activity and cellular heat resistance of a clonal cell line in which overexpression of Hsp70 was transiently induced by means of the tetracycline-regulated gene expression system. This single-cell-line approach circumvents problems associated with clonal variation and indirect effects resulting from constitutive overexpression of Hsp70. The in vivo chaperone function of Hsp70 was quantitatively investigated by using firefly luciferase as a reporter protein. Chaperone activity was found to strictly correlate to the level of Hsp70 expression. In addition, we observed an Hsp70 concentration dependent increase in the cellular heat resistance. In order to study the contribution of the Hsp70 chaperone activity, heat resistance of cells that expressed tetracycline-regulated Hsp70 was compared to thermotolerant cells expressing the same level of Hsp70 plus all of the other heat shock proteins. Overexpression of Hsp70 alone was sufficient to induce a similar recovery of cytoplasmic luciferase activity, as does expression of all Hsps in thermotolerant cells. However, when the luciferase reporter protein was directed to the nucleus, expression of Hsp70 alone was not sufficient to yield the level of recovery observed in thermotolerant cells. In addition, cells expressing the same level of Hsp70 found in heat-induced thermotolerant cells containing additional Hsps showed increased resistance to thermal killing but were more sensitive than thermotolerant cells. These results suggest that the inducible form of Hsp70 contributes to the stress-tolerant state by increasing the chaperone activity in the cytoplasm. However, its expression alone is apparently insufficient for protection of other subcellular compartments to yield clonal heat resistance to the level observed in thermotolerant cells.
Cells exposed to nonlethal, elevated temperatures become transiently resistant to a subsequent heat shock. Thermotolerance development is paralleled by expression of heat shock proteins, which include members of the heat shock protein 70 (Hsp70) family (21, 29). In thermotolerant cells, endogenous proteins such as p68 kinase and exogenous reporter gene products such as β-galactosidase and firefly luciferase show increased resistance to heat damage (4, 24, 27, 31).
It is likely that Hsp70 contributes to the protection of cellular proteins by functioning as a molecular chaperone. Such a function is implied from in vitro studies in which purified members of the Hsp70 family are found to contribute to the heat protection of several substrate proteins. Hsp70 alone can protect topoisomerase I and DNA polymerase against heat inactivation and facilitate the reactivation of heat-inactivated topoisomerase I in a concentration-dependent fashion (3, 44). For reactivation of DNA polymerase, ATP is required (44). For the recovery of heat-denatured firefly luciferase and chemically denatured β-galactosidase, Hsp70 alone is not sufficient. Addition of Hsp40 or Hsp40 homologues plus ATP is required. Hsp40 is believed to stabilize binding of Hsp70 to the substrate by stimulating hydrolysis of Hsp70-bound ATP (25). Hsp70, Hsp40, and ATP either directly enhance refolding of heat-denatured firefly luciferase and chemically denatured β-galactosidase or can keep substrates in a folding-competent state at high temperatures (7, 35, 36). Subsequent incubation at normal temperatures leads to spontaneous refolding of β-galactosidase (7), while refolding of luciferase needs additional factors that are present in reticulocyte lysate (25). Furthermore, Hsp70 and cofactors can reactivate heat-inactivated substrates that are kept in a folding-competent conformation by other heat shock proteins such as Hsp90 or Hsp25 (5, 7).
In vivo, a chaperone function of Hsp70 is suggested from studies on heat effects on endogenous and exogenous proteins. Protection against heat-induced nuclear protein aggregation in thermotolerant HeLa S3 cells correlates with the expression level of Hsp70 (37). Overexpression of transfected Hsp70 in Rat-1 cells protects against nuclear protein aggregation, independent of the ATP binding domain (38, 39). In addition, cytoplasmic or nuclear firefly luciferase expressed in hamster fibroblasts is protected from inactivation when Hsp70 is constitutively coexpressed. Reactivation of the same proteins is facilitated in these cells, which is further enhanced when Hsp70 and Hsp40 are coexpressed (23).
Little is known about the specific contribution of Hsp70 and its chaperone activity to thermotolerance development. Constitutive overexpression of Hsp70 has been shown to increase heat resistance, and the level of this resistance correlates to the level of Hsp70 expression in individual clonal cell lines (20). Under normal growth conditions, however, Hsp70 has essential functions in protein folding, translocation, assembly, and disassembly (11). Furthermore, constitutive overexpression of Hsp70 was shown to reduce the rate of Drosophila cell growth (6). This might indirectly lead to an altered response to heat and therefore influence clonal thermoresistance. In our previous study on the in vivo chaperone activity of Hsp70, the effect on heat resistance could not be investigated because only a small fraction of the cells expressed the gene in the transient-transfection assay (23). In order to directly compare chaperone activity and heat resistance of cells overexpressing Hsp70 alone with thermotolerant cells expressing all heat-inducible factors, we used tetracycline-regulated gene expression (8). With this system, the heat-induced expression of Hsp70 in thermotolerant cells could be mimicked in the absence of induction of other stress proteins. Also, heat-induced expression of all Hsps, tetracycline-induced expression of Hsp70, and chronic expression of Hsp70 could be compared to the control situation within the same clonal cell line. In addition, a precise control of the level of expression enabled a quantitative analysis of the chaperone activity. The effect of Hsp70 expression on the in vivo heat inactivation and reactivation of firefly luciferase was taken as a measure of chaperone activity. Because Hsp70 has been shown to translocate and accumulate in the nucleus upon heat shock (30, 43), we examined its effect on cytoplasmic and nuclear firefly luciferase (24). These chaperone activities were finally related to protection against the cell-killing effect of heat.
Our data indicate that transient overexpression of Hsp70 alone increases the chaperone activity in the cytoplasm to the same extent as observed in heat-induced thermotolerant cells expressing all of the major Hsps. Although we also found some chaperone activity in the nucleus, expression of Hsp70 alone was insufficient to yield the same level of reactivation of nuclear luciferase as that observed in thermotolerant cells. In addition, Hsp70 alone was not able to confer the degree of resistance to heat killing seen with heat-induced thermotolerant cells. Thus, expression of the inducible form of Hsp70 in the absence of increased levels of other Hsps is apparently sufficient to achieve the level of chaperone activity in the cytoplasm found in thermotolerant cells. However, to obtain the same level of chaperone activity in the nucleus and the same level of clonogenic heat resistance as in thermotolerant cells, additional heat-inducible factors or activities are needed.
MATERIALS AND METHODS
Plasmids and constructs.
The plasmids pUHD15-1, pUHC13-3, and pHGR272 were kindly provided by H. Bujard (University of Heidelberg). pUHD15-1 encodes the tetracycline-responsive transactivator (tTA) under control of the human cytomegalovirus promoter 1E. pUHC13-3 encodes firefly luciferase under control of a tTA-dependent promoter (8). In pHGR272, a thymidine kinase minimal promoter from Herpes simplex virus controls a hygromycin resistance gene. pSV2neo (Clontech) encodes the neomycin resistance gene under the control of a simian virus 40 promoter. The plasmid pTBC70 was constructed by ligating a HindIII fragment from pEX2770, containing the human Hsp70 cDNA, downstream of the tTA-regulated promoter of pTBC-1 (a generous gift from H. Hauser, Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany) (16). pRSVLL/V encodes cytoplasmic firefly luciferase under the control of a Rous sarcoma virus long terminal repeat promoter (kindly provided by S. Subramani, University of California, San Diego, Calif.). Construction of pRSVnlsLL/V encoding firefly luciferase fused to a nuclear localization sequence has previously been described (24). pN3luc encodes firefly luciferase under the control of a stress-inducible hsp70 promoter (32).
Cell culture and transfections.
O23 hamster fibroblasts (kindly provided by J. Landry, Quebec, Canada) were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (Life Technologies). A CaPO4 transfection procedure was used for all stable transfections. Lipofectamine (Life Technologies) was used for all transient transfections according to the procedure of the manufacturer. O23 cells were stably transfected with pUHD15-1 and pSV2Neo (80:1). Stable clones were selected in medium containing 1 mg of G418 (Life Technologies) and 3 μg of tetracycline (Sigma) per ml. Expression and activity of the tTA-protein was assayed by transient transfection with pUHC13-3. A clone that induced luciferase expression 30-fold after tetracycline withdrawal was subcloned and used for further experiments. This O23-tTA cell line (OT) was stably transfected with pTBC70 and pHGR272 (80:1). Stable clones were selected in medium containing 1 mg of G418, 1 mg of hygromycin (Boehringer Mannheim), and 3 μg of tetracycline per ml. A clone that was determined by Western blot analysis to show the highest induction of Hsp70 expression after the withdrawal of tetracycline was used for further experiments. Thermotolerance was induced by exposing the OT70 cells to a priming heat shock of 44°C for 20 min in medium containing 3 μg of tetracycline per ml followed by 16 h at 37°C. Expression of Hsp70 was induced by growing the cells in medium containing 0, 2.2, 4.4, 8.8, or 13.2 ng of tetracycline per ml for 24 h.
Western blotting, FACS, and immunofluorescence analysis.
Cells were suspended in phosphate-buffered saline, sonicated, lysed by the addition of 2× sample buffer (17), and boiled for 5 min prior to being loaded onto sodium dodecyl sulfate–12.5% polyacrylamide gels. After Western blotting (42), Hsp70, Hsp40, and Hsp25 were detected with anti-Hsp70 mouse monoclonal (Stressgen), anti-Hsp40 rabbit polyclonal (a generous gift from K. Ohtsuka, Nagoya, Japan), and anti-Hsp25 rabbit polyclonal (Stressgen) primary antibodies. Binding of anti-mouse or anti-rabbit immunoglobulin G (IgG) secondary antibodies (Amersham) was detected by enhanced chemiluminescence (ECL; Amersham). Recombinant Hsp70 was from Stressgen.
Fluorescence-activated cell-sorting (FACS) analysis was performed as described by Hang and Fox (10).
Hsp70 expression was detected with a primary anti-Hsp70 mouse monoclonal antibody and an anti-mouse IgG fluorescein isothiocyanate (FITC) conjugate (Dako). Immunolocalization of Hsp70 was performed as described previously (23). Images were obtained with a confocal scanning laser microscope (TCS 4D; Leica, Heidelberg, Germany) equipped with an argon-krypton laser and coupled to a Leitz DM IRB 9 (Leica) inverted microscope.
Luciferase activity assay.
Cells were transiently transfected with pRSVLL/V or pRSVnlsLL/V. After 24 h they were divided for Western analysis in culture flasks at a density of 2.5 × 105 cells per flask or for luciferase activity analysis in cell culture tubes at a density of 2.5 × 104 cells per tube. For heat inactivation and recovery experiments, the cells either were cultured for yet another 24 h in medium with different tetracycline concentrations or were made thermotolerant in medium containing 3 μg of tetracycline per ml. At 30 min before the cells were exposed to a challenging heat shock, the medium in the culture tubes was replaced by medium containing 20 μg of cycloheximide per ml and 20 mM MOPS (morpholinepropanesulfonic acid, pH 7.0; Sigma). After heat shock, the cells were incubated at 37°C for recovery, during which samples for luciferase activity measurements were taken at different time points. Cell lysis and luciferase measurements were performed as previously described (24).
Clonogenic survival assay.
Cells were cultured in T75 flasks at a density of 7.5 × 105 cells/flask. They either were cultured in medium with different tetracycline concentrations or were made thermotolerant. After trypsinization, the cells were heated in suspension at a concentration of 106 cells/ml. The clonogenic ability was determined by plating the cells at the appropriate dilutions. After 7 to 10 days, the colonies were fixed with 70% ethanol and stained with 0.5% crystal violet (Sigma).
RESULTS
Tetracycline-regulated expression of Hsp70 causes no general stress response.
O23 hamster fibroblasts were used as a model for examining the in vivo chaperone function of Hsp70. Under normal conditions, these cells do not express the inducible form of Hsp70. After a heat shock, these cells induce expression of Hsp70 along with the other heat shock proteins. In order to model the heat-induced expression of Hsp70 alone, the tetracycline-regulated tTA expression system was chosen (8).
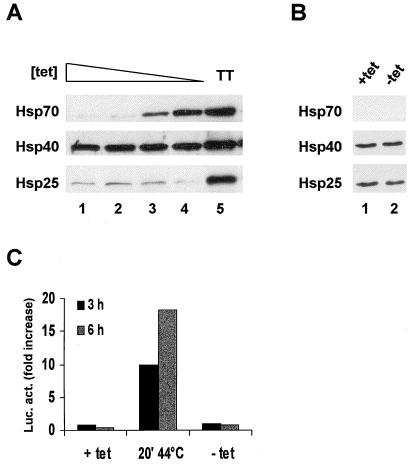
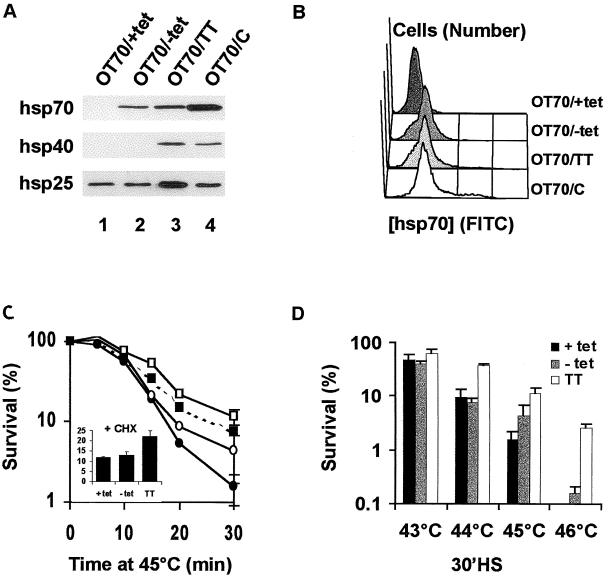
OT cells, which are O23 cells that expressed the tetracycline-responsive tTA protein, were stably transfected with a plasmid encoding Hsp70 under the control of a tTA-regulated promoter. In medium with decreasing concentrations of tetracycline, these OT70 cells expressed increasing amounts of Hsp70 (Fig. 1A, lanes 1 to 4). In the parental OT cells, no induction of Hsp70 could be observed in the absence of tetracycline (Fig. 1B, lanes 1 and 2). In addition, the expression of Hsp40 and Hsp25 was not visibly elevated in OT70 cells by the removal of tetracycline (Fig. 1A, lanes 1 to 4), in contrast to the heat-induced expression in thermotolerant cells (Fig. 1A, lane 5). Therefore, induction of Hsp70 expression in OT70 cells was not a result of a general stress response induced by changes in the tetracycline concentration. This was further verified by transient transfection of OT70 cells with a plasmid containing a firefly luciferase gene regulated by a stress-inducible Hsp70 promoter. After a priming heat shock, luciferase expression was highly induced from this plasmid, whereas no increased expression of luciferase could be observed after tetracycline withdrawal (Fig. 1C).
FIG. 1.
Tetracycline-regulated expression of Hsp70. OT cells are cells that stably express the tTA protein and OT70 cells are OT cells that were stably transfected with a plasmid encoding Hsp70 behind a tTA-regulated promoter. (A) OT70 cells were grown in medium with decreasing concentrations of tetracycline (3,000 to 0 ng/ml) (lanes 1 to 4) or were made thermotolerant by a priming heat shock of 44°C for 20 min in medium containing 3,000 ng of tetracycline per ml followed by 16 h at 37°C (lane 5). Western analysis was performed with a monoclonal antibody to Hsp70 and polyclonal antibodies to Hsp40 and Hsp25. (B) OT cells were grown in medium with (lane 1) or without (lane 2) tetracycline (3,000 ng/ml). (C) OT70 cells were transiently transfected with a plasmid encoding luciferase behind a stress-inducible Hsp70 promoter. At 24 h after transfection the cells were either exposed to a priming heat shock of 44°C for 20 min or tetracycline was withdrawn from the medium. Luciferase activity was measured at the indicated time points and was expressed as the fold increase of basal activity.
Hsp70 expression increases cytoplasmic and nuclear chaperone activity in a temperature- and concentration-dependent fashion.
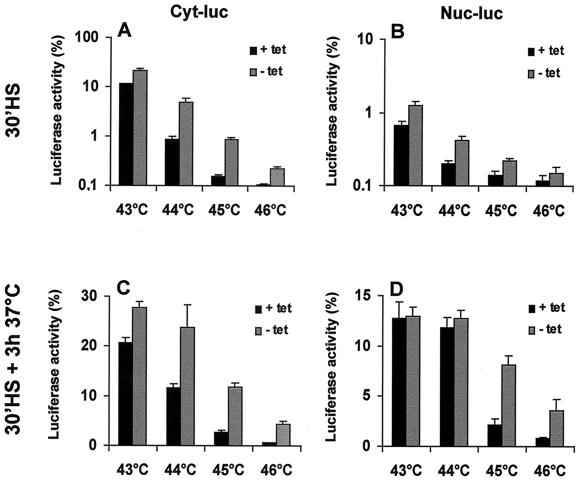
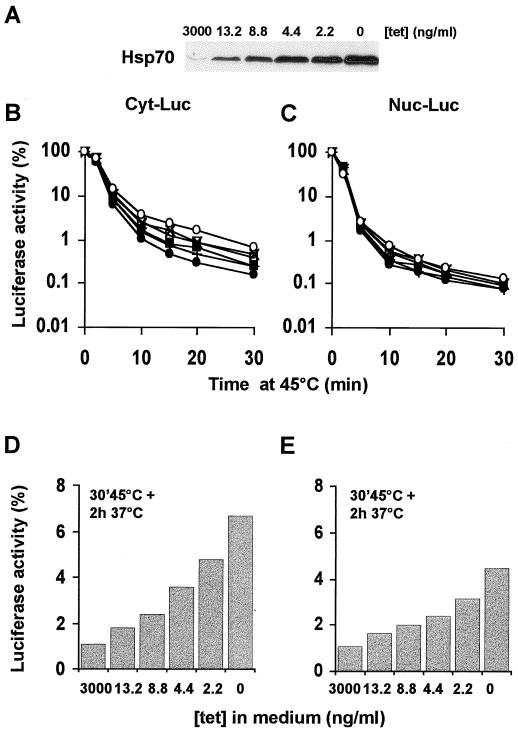
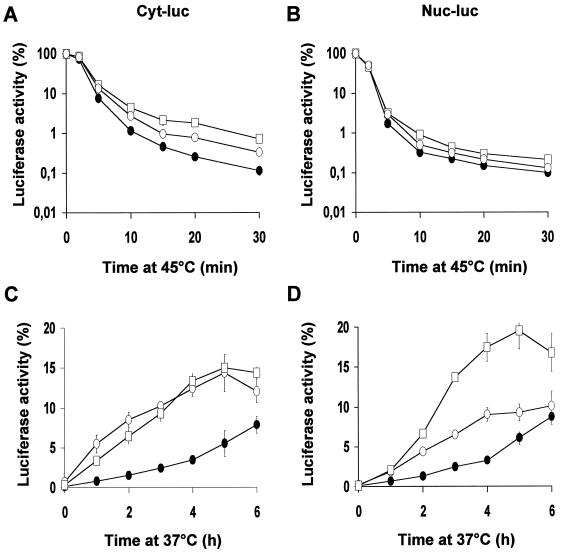
Previous research has shown that transient overexpression of Hsp70 protects nuclear and cytoplasmic firefly luciferase from heat inactivation and enhances recovery of the inactivated proteins (23). As can be seen in Fig. 2, similar results can be obtained by using the OT70 cells in which Hsp70 expression is induced after 24 h of growth in the absence of tetracycline. Both cytoplasmic and nuclear luciferase were protected against inactivation by 30-min treatments at 43 to 46°C (Fig. 2A and B). Also, more cytoplasmic luciferase activity was found to be recovered at 3 h after these heat shock treatments if Hsp70 was expressed (Fig. 2C). The extent of recovery of nuclear luciferase at 3 h after the heat shock, however, was not affected by Hsp70 expression after the milder (43 and 44°C) heat treatments. Expression of Hsp70 increased the recovered fraction of nuclear luciferase only after a 30-min heat shock at 45 or 46°C (Fig. 2C and D). We decided to use 45°C heat treatments for further experiments and examined whether the chaperone activity was related to the cellular concentration of Hsp70. To regulate the Hsp70 expression, OT70 cells were grown for 24 h in medium containing various concentrations of tetracycline (Fig. 3A). Hsp70 expression protected cytoplasmic luciferase during inactivation at 45°C in a concentration-dependent manner (Fig. 3A and B). In addition, the recovered fraction of cytoplasmic luciferase at 2 h after heat shock positively correlated with Hsp70 expression (r2 = 0.97, P < 0.002 [Fig. 3A and D]). The same result was observed for nuclear luciferase (r2 = 0.95, P < 0.005 [Fig. 3A and E]). Hsp70 expression did not influence the level of expression of luciferase (data not shown). This implies that the observed protective effects of Hsp70 are not due to alterations in the concentration of luciferase per cell.
FIG. 2.
Effects of transient expression of Hsp70 on thermal (43 to 46°C) inactivation and post-heat treatment reactivation of cytoplasmic and nuclear luciferase. OT70 cells were transiently transfected with a plasmid encoding either cytoplasmic luciferase (Cyt-luc) or nuclear luciferase (Nuc-luc). At 24 h after transfection, the cells were cultured in medium without (−tet) or with (+tet) 3,000 ng of tetracycline per ml for another 24 h. Cytoplasmic luciferase (A and C) and nuclear luciferase (B and D) activities were measured either immediately after a 43, 44, 45, or 46°C heat shock (A and B) or after a 3-h recovery period at 37°C (C and D). Cycloheximide (20 μg/ml) was added prior to heat shock and during the recovery period to prevent new protein synthesis. Luciferase activity is expressed as a percentage of the activity measured before heat inactivation. All data represent the average of three independent experiments. Error bars indicate the standard error of the mean.
FIG. 3.
Transient expression of Hsp70 protects luciferase against heat shock in a concentration-dependent fashion. OT70 cells were transiently transfected with a plasmid encoding either cytoplasmic luciferase (B and D) or nuclear luciferase (C and E). At 24 h after transfection, the cells were split into media containing decreasing concentrations of tetracycline and grown for another 24 h. (A) Western analysis of Hsp70 expression after 24 h of growth in media at the indicated concentrations of tetracycline. (B to E) Luciferase activity was measured either immediately after a 45°C heat shock for various exposure times (B and C) or at 2 h after a heat shock of 45°C for 30 min (D and E). Cycloheximide (20 μg/ml) was present during heat shock and recovery to prevent new luciferase protein synthesis. Luciferase activity is expressed as a percentage of the activity measured before heat inactivation. Cells were grown in medium containing 3,000 (●), 13.2 (+), 8.8 (■), 4.4 (▿), 2.2 (×), or 0 (○) ng of tetracycline/ml.
Removal of tetracycline from the parental OT cells did not affect the inactivation and reactivation of either form of luciferase, indicating that tetracycline withdrawal by itself did not influence these processes (data not shown). Enhanced reactivation of luciferase was also not caused by changes in the rate of degradation of luciferase due to Hsp70 overexpression. Figure 4 shows that no significant degradation of luciferase could be observed during the full course of the heating and recovery experiment. The results reveal that Hsp70 expression increases the cytoplasmic and nuclear chaperone activity in a concentration-dependent manner.
FIG. 4.
Transient expression of Hsp70 does not affect luciferase degradation after heat shock. OT70 cells were transiently transfected with a plasmid encoding either cytoplasmic luciferase (Cyt-luc) or nuclear luciferase (Nuc-luc). At 24 h after transfection, the cells were grown for another 24 h in medium containing 3,000 ng of tetracycline/ml (+tet) or no tetracycline (−tet). Next, they were either left unheated (C, lane 1) or were heated for 30 min at 45°C and incubated for 0 to 2 h at 37°C (lanes 2 to 5) as indicated. Cycloheximide (20 μg/ml) was present during the heat and recovery period. Samples were obtained at the indicated time points, and luciferase was detected by a polyclonal antibody to luciferase by Western blot analysis.
The mean molar ratio between Hsp70 and cytoplasmic luciferase was estimated by comparing the cellular levels of Hsp70 and luciferase with the titration curves of pure proteins obtained by using Western blotting. For estimation of the luciferase concentration per cell, corrections were made for the transfection efficiencies by calculating the fraction of cells stained with anti-luciferase antibodies by using immunofluorescence. Our analyses revealed that luciferase reactivation increased linearly at luciferase/Hsp70 molar ratios from 1:1 to 1:8. Changing the molar ratio by up to 1:40 by lowering the concentration of luciferase per cell at the maximum expression level of Hsp70 increased the reactivation of heat-inactivated luciferase even further, a finding in line with the reactivation at increasing concentrations of Hsp70. These results suggest that Hsp70 expression protects luciferase against irreversible heat damage in a concentration-dependent fashion over a wide range of molar ratios.
Transient overexpression of Hsp70 is sufficient to mimic the thermotolerant protection of cytoplasmic luciferase but not nuclear luciferase.
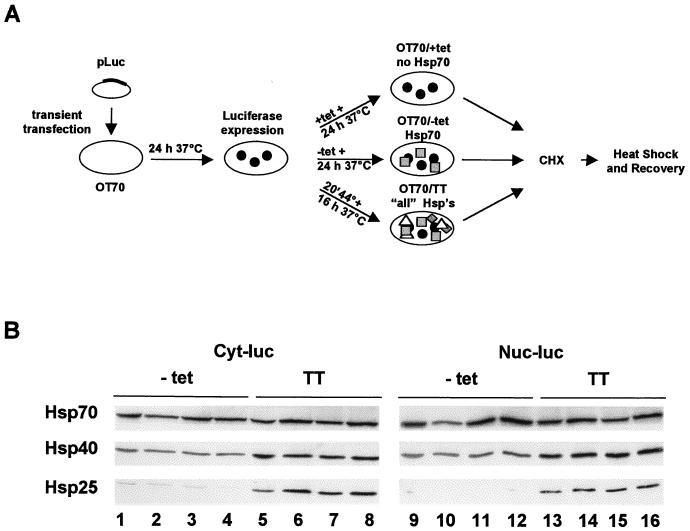
In thermotolerant cells, transiently expressing all heat shock proteins, cytoplasmic and nuclear forms of firefly luciferase are found to be protected during heat inactivation (24). We examined the contribution of Hsp70 alone both to this protection during heat inactivation and to the level of recovery of the initial luciferase activity in thermotolerant cells by using the experimental design depicted in Fig. 5A. In short, OT70 cells were transfected with plasmids encoding cytoplasmic or nuclear luciferase. Part of the transfected cells was exposed to a priming heat shock for the induction of thermotolerance. Another part of the cells was grown in medium without tetracycline for the induction of Hsp70 expression alone to the same level as was induced in the thermotolerant cells. After induction, the thermotolerant and Hsp70 expression OT70 cells were exposed to a challenging heat shock. Luciferase activity was measured immediately after the heat shock or upon post-heat treatment incubation at 37°C.
FIG. 5.
Protocol leading to equal expression levels of Hsp70 by the tetracycline-regulated system and by a heat shock used to induce thermotolerance. (A) Schematic representation of the experimental procedure for the experiments in Fig. 5B and 6 to 10. (B) Western analysis of Hsp27, Hsp40, and Hsp70 expression levels in OT70 as induced by the protocol described in panel A. OT70 cells were transiently transfected with a plasmid encoding either cytoplasmic luciferase (Cyt-luc, lanes 1 to 8) or nuclear luciferase (Nuc-luc, lanes 9 to 16). At 24 h after transfection, the cells were either grown for 24 h in medium without tetracycline (−tet) (lanes 1 to 4 and 9 to 12) or they were treated with a priming heat dose for 20 min at 44°C followed by 16 h of growth at 37°C in medium with 3,000 ng of tetracycline per ml (TT) (lanes 5 to 8 and 13 to 16) for Hsp induction. Each lane shows expression of Hsp70, Hsp40, and Hsp25 in 5 × 104 cells. Lanes 1 to 4, 5 to 8, 9 to 12, and 13 to 16 show expression of Hsp27, Hsp40, and Hsp70 from four independent experiments. Hsp expression levels (in gray values) were determined by densitometry and calculated as the average of four values with standard error of the mean. For Hsp70 these values were as follows: lanes 1 to 4, 2.7 ± 0.1; lanes 5 to 8, 2.9 ± 0.3; lanes 9 to 12, 3.2 ± 0.4; and lanes 13 to 16, 3.0 ± 0.2. The Hsp40 “gray” values were as follows: lanes 1 to 4, 1.4 ± 0.02; lanes 5 to 8, 2.4 ± 0.1; lanes 9 to 12, 1.4 ± 0.2; and lanes 13 to 16, 2.4 ± 0.3. For Hsp25 the values were as follows: lanes 1 to 4, 0.15 ± 0.06; lanes 5 to 8, 1.7 ± 0.6; lanes 9 to 12, 0.09 ± 0.02; and lanes 13 to 16, 1.3 ± 0.2.
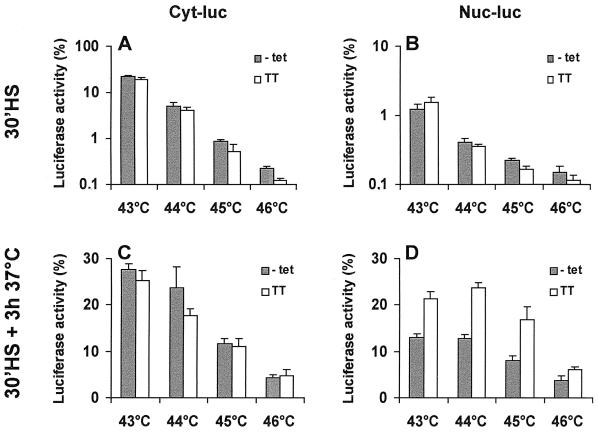
This protocol resulted in the induction of similar levels of Hsp70 in OT70 and thermotolerant cells, whereas the levels of other Hsps, such as Hsp25 and Hsp40, were only altered in the thermotolerant cells (Fig. 5B [see also Fig. 10A and B]). During inactivation, cytoplasmic luciferase was slightly better protected in cells that expressed Hsp70 alone than in thermotolerant cells. Interestingly, the level of recovery was the same in both cell types (Fig. 6A and C). This suggested that for this cytoplasmic chaperone activity, expression of Hsp70 alone is sufficient and that other heat-inducible factors or activities are dispensable. At 45°C, there was little or no effect of Hsp70 expression or thermotolerance on nuclear luciferase inactivation (Fig. 6B). Nuclear luciferase, however, was reactivated significantly more in thermotolerant cells than in cells expressing Hsp70 alone (Fig. 6D). A similar pattern was observed if the cells were heated for 30 min at 43 to 46°C (Fig. 7): whereas Hsp70 expression alone lead to a cytoplasmic chaperone activity similar to that in thermotolerant cells, it did not lead to the same level of nuclear chaperone activity as in thermotolerant cells.
FIG. 10.
Transient expression of Hsp70 alone is insufficient to account for thermotolerance at the clonogenic level. OT70 cells were grown in medium with 3,000 ng of tetracycline/ml (OT70/+tet, ●) or without tetracycline for 24 h (OT70/−tet, ○) or 7 days (OT70/C, ■) or were made thermotolerant (OT70/TT, □). (A) Western analysis of Hsp27, Hsp40, and Hsp70 expression levels. (B) Quantitative analysis of the level and distribution of Hsp70 expression as analyzed by flow cytometry. (C and D) Cell survival as determined by the clonogenic assay after treatments of the various cells for 0 to 30 min at 45°C (C) or for 30 minutes at 43 to 46°C (D). The inset in panel C shows the survival if cycloheximide (20 μg/ml) was added from 30 min before until 6 h after the 30-min 45°C heat shock to mimic the conditions used for the chaperone experiments. Data points represent the average of three independent experiments. Error bars represent the standard error of the mean.
FIG. 6.
Transient expression of Hsp70 alone is sufficient to account for the chaperone activity of cytoplasmic luciferase but not nuclear luciferase, as observed in thermotolerant cells. OT70 cells were transiently transfected with a plasmid encoding either cytoplasmic luciferase (A and C) or nuclear luciferase (B and D). At 24 h after transfection, the cells were split into medium without or with 3,000 ng of tetracycline/ml or were made thermotolerant. Luciferase activity was measured either immediately after a 45°C heat shock (A and B) or after 0 to 6 hours at 37°C after a challenging heat shock of 45°C for 30 min (C and D). Cycloheximide (20 μg/ml) was present during the heat and recovery period. Luciferase activity was measured at the indicated time points and expressed as a percentage of the activity measured before heat inactivation. Symbols: □, thermotolerant cells (increased expression of “all” Hsps); ●, cells grown in 3,000 ng of tetracycline per ml (no Hsp70 expression); ○, cells grown in the absence of tetracycline (expression of Hsp70 alone to the same level as in thermotolerant cells). Data points represent the mean of three independent experiments. Error bars indicate the standard error of the mean.
FIG. 7.
Comparison of the cytoplasmic and nuclear chaperone activity after 43 to 46°C heat treatments of cells transiently expressing Hsp70 alone and of thermotolerant cells. OT70 cells were transiently transfected with a plasmid encoding either cytoplasmic or nuclear luciferase. At 24 h after transfection, the cells were split into medium without tetracycline (−tet) or made thermotolerant in the presence of 3,000 ng of tetracycline (TT). Luciferase activity was measured either immediately after a 30-min heat shock at 43 to 46°C (A and B) or after 3 h at 37°C following a heat shock at 43 to 46°C for 30 min (C and D). Cycloheximide (20 μg/ml) was present during the heat and recovery period. Luciferase activity was measured at the indicated time points and expressed as a percentage of the activity measured before heat inactivation. The data represent the mean of three independent experiments. Error bars indicate the standard error of the mean.
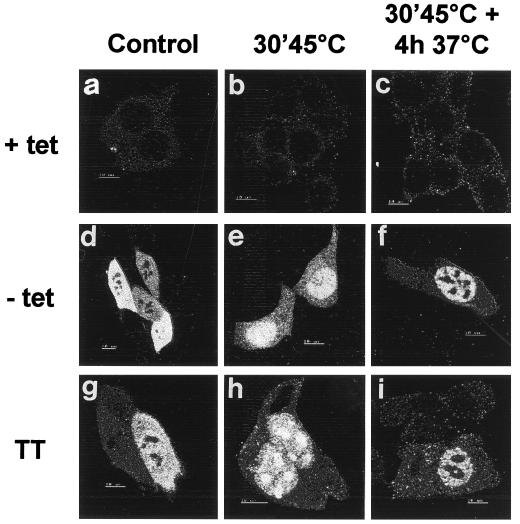
One explanation for the difference in nuclear protection could be a change in the ability Hsp70 to translocate in nonthermotolerant cells upon heat shock. We tested this possibility by using immunolocalization, which showed that Hsp70 alone was still able to translocate into the nucleus and nucleoli after heat shock and to relocalize from the nucleoli after recovery in a way similar to Hsp70 in thermotolerant cells (Fig. 8). Apparently, although the expression of Hsp70 alone can increase the nuclear chaperone activity, additional heat-inducible factors or activities appear to be needed to yield the same nuclear chaperone activity as that observed in thermotolerant cells.
FIG. 8.
Transiently expressed Hsp70 shows no abnormal heat-induced intracellular (re)allocation pattern. Hsp70 localization was analyzed by immunofluorescence with a monoclonal antibody to Hsp70 and an FITC-labeled secondary antibody. Images were made by using confocal microscopy. Panels: A, B, and C, OT70 cells in medium with 3,000 ng of tetracycline per ml; D, E, and F, OT70 cells in medium without tetracycline; G, H, and I, thermotolerant OT70 cells in medium with 3,000 ng of tetracycline per ml. Images were obtained before heat shock (A, D, and G), immediately after heat shock (B, E, and H), and after 4 h of recovery after heat shock (C, F, and I).
Hsp70 expression enhances cell survival after heat shock in a concentration-dependent fashion.
The OT70 cell line appeared to be a well-defined system for determining the contribution of Hsp70 to the enhanced in vivo chaperone activity in thermotolerant cells. In order to learn whether this enhanced chaperone activity is related to the ability of cells to survive heat treatments, the effect of Hsp70 expression on cell survival after heat shock was investigated and quantitatively compared to thermotolerant cell survival. Like the chaperone activity, cell survival after 45°C increased with increasing expression levels of Hsp70 alone (Fig. 9). However, cells that transiently expressed Hsp70 alone at a level equal to the thermotolerant cells (Fig. 5B and Fig. 10A and B) were less resistant to heat-induced cell killing than were thermotolerant cells at all of the temperatures tested (Fig. 10C and D). Because the chaperone activities were determined in the presence of the protein synthesis inhibitor cycloheximide, we tested whether cycloheximide influenced the difference in thermoresistance. Although the overall resistance was higher after a heat shock of 45°C for 30 min and a subsequent recovery period of 6 h in the presence of cycloheximide, thermotolerant cells were still more heat resistant than were cells expressing Hsp70 alone (Fig. 10C, inset). Even if the cells were induced to constitutively express Hsp70 for 1 week, yielding expression levels of Hsp70 that were almost twofold higher than in thermotolerant cells (Fig. 10A and B), they still were less resistant to a 45°C heat shock than were thermotolerant cells (Fig. 10C).
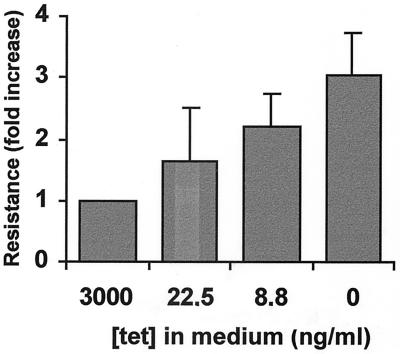
FIG. 9.
Transient expression of Hsp70 alone confers clonogenic heat resistance in a concentration-dependent fashion. OT70 cells were grown for 24 h in medium containing 3,000, 22.5, 8.8, or 0 ng of tetracycline/ml. After exposure to a challenging heat shock of 45°C for 30 min, they were tested for their ability to form colonies. The magnitude of resistance is expressed as the colony-forming ability relative to that of cells grown in medium containing 3,000 ng of tetracycline/ml in which Hsp70 was not induced (set at 1.0) and is the average of three independent experiments. Error bars represent the standard error of the mean.
As demonstrated by FACS analysis, heterogeneity in Hsp70 expression within the cell populations could not account for the difference. Not only were the mean expression levels of Hsp70 the same but also the expression profiles of the cell populations were the same for both Hsp70-expressing and thermotolerant OT70 cells (Fig. 10B).
Taken together, the results suggest that the observed similarity in cytoplasmic chaperone activity between Hsp70-expressing and thermotolerant cells (Fig. 6A and C and 7A and C) could not account for the similar thermoresistance. Furthermore, they indicate that the overexpression of Hsp70 alone can induce some level of thermoresistance. However, for a given level of expression, Hsp70 alone is not sufficient to account for all of the heat resistance at the level of cell survival seen in thermotolerant cells.
DISCUSSION
This study demonstrates that transient overexpression of Hsp70 increases both the level of cellular chaperone activity and the clonogenic thermoresistance in a concentration-dependent fashion. However, whereas overexpression of Hsp70 alone is sufficient to mimic cytoplasmic chaperone activity of thermotolerant cells, it is not sufficient for providing the same magnitude of nuclear chaperone activity or full thermotolerance, as measured by clonogenic survival. Apparently, additional heat-inducible factors or activities are needed for the latter two processes.
Our findings are in agreement with previous studies showing that constitutive overexpression of Hsp70 increases thermoresistance and that the level of this resistance correlates to the level of Hsp70 expression in different clones (20). In these earlier studies, however, Rat-1 cells that constitutively overexpressed Hsp70 at the level of the thermotolerant cells were as thermoresistant as the thermotolerant cells (38). In contrast to this, we found that transient overexpression of just Hsp70 to the same level as in the preheated cells did not protect cells against heat killing to the same extent as thermotolerance. In line with our data, Mosser et al. (26) also showed that the constitutive overexpression of Hsp70 led to higher levels of resistance against heat-induced apoptosis than did transient expression of Hsp70 to the same levels. Since Hsp70 has essential functions under physiological conditions (11) and since constitutive overexpression of this protein can influence cell growth (6), secondary changes might influence clonal thermoresistance. Therefore, clonally derived cells that constitutively overexpress Hsp70 might not be directly comparable to thermotolerant cells that transiently overexpress Hsp70. In this study, potential side effects and clonal variation were circumvented because we compared transient overexpression of Hsp70 to thermotolerance in the same cell line. Our data indicate that additional heat-inducible factors or activities are needed to achieve the level of heat resistance shown by thermotolerant cells.
As was suggested by Mosser et al. (26), cellular protection by Hsp70 may be due specifically to interference of Hsp70 with the pathway leading to apoptosis at some point downstream of SAPK-JNK activation. The cell lines used in our current and previous (37–39) studies, however, do not show any significant evidence of heat-induced apoptosis, as judged by flow cytometric analysis and trypan blue exclusion (data not shown). Hence, rather than a direct interference of Hsp70 with the apoptotic program, we propose that Hsp70-mediated cellular protection is due to a more global increase in intracellular chaperone capacity. Such may reduce the trigger for cell death, irrespective of necrosis or apoptosis. Consequently, for cells that do die through an apoptotic pathway, all downstream effects of SAPK-JNK activation such as poly(ADP-ribose)polymerase cleavage and caspase activation are attenuated (26).
Consistent with in vitro experiments (3, 7, 25, 35, 44), we found that transient overexpression of Hsp70 indeed increased the chaperone activity in both the cytoplasm and the nucleus of the cell in a concentration-dependent fashion. This finding did correlate to some level of heat resistance at the level of clonogenic cell survival. In addition, in mammalian cell lines constitutively over expressing Hsp70 the level of clonogenic resistance is related to the ability of Hsp70 to act as a chaperone in vivo (37–39). Our data should not be taken as evidence that rules out a more specific role of Hsp70 in the apoptotic pathway. In fact, it was recently demonstrated that the BAG-1 protein, which interacts with Bcl-2 to prevent apoptosis, can bind to Hsc70-Hsp70 and modulate its ATPase activity (14, 40). In vitro BAG-1 binding to Hsc70 or Hsp70 leads to a dominant negative effect of the Hsp70 chaperone activity (40). How this interaction in vivo may relate to the Hsp70 chaperone activity on the one hand and the antiapoptosis function of BAG-1 on the other yet remains elusive.
Transient overexpression of Hsp70 alone to the level observed in thermotolerant cells was sufficient to mimic the level of cytoplasmic chaperone activity observed in thermotolerant cells. Expression of Hsp70 alone resulted in the same extent of reactivation of the cytoplasmic luciferase as in thermotolerant cells. Interestingly, an excellent correlation was obtained between the effect of Hsp70 expression on initial inactivation and reactivation of cytoplasmic luciferase under all conditions tested (r2 = 0.959, P < 0.0001 [data not shown]). This would suggest that the main action of Hsp70 in vivo is to protect the substrate against irreversible damage and be consistent with our previous data on insolubilization and resolubilization of endogenous nuclear proteins (37–39). Moreover, the data suggest that Hsp70 is a rate-limiting factor for chaperoning cytoplasmic luciferase. In vitro, Hsp70 is found to protect a wide variety of substrates (3, 5, 7, 44) and to selectively recognize and bind peptide sequences specific for non-native proteins (34). Therefore, if we assume that Hsp70 not only protects luciferase but also protects other heat-sensitive cytoplasmic proteins, our data imply that sufficient cofactors are present under physiological conditions and other heat-inducible factors were dispensable for cytoplasmic chaperone activity to a level observed in thermotolerant cells. This cytoplasmic chaperone activity then appears not to be sufficient for thermotolerant survival protection.
In contrast to cytoplasmic luciferase, the level of nuclear chaperone activity observed in thermotolerant cells could not be fully accounted for by transient overexpression of just Hsp70. In fact, this is mainly because thermotolerant cells show more reactivation of nuclear luciferase for a given level of inactivation than was observed for all of the other conditions and for cytoplasmic luciferase. Apparently, the priming heat shock to induce thermotolerance results in events, besides the elevated expression of Hsp70 alone, that are specifically aimed to increase the chaperone activity in the nucleus. It is tempting to speculate that this activity is specifically required to protect against the lethal effects of subsequent heat shocks. Such would be in line with findings that the nuclear proteins are the most heat sensitive (18) and that the aggregation of nuclear proteins closely correlates with the extent of heat-induced cell killing (15, 33). The absence of sufficient nuclear chaperone activity in cells expressing just Hsp70 would then be consistent with the absence of resistance against heat killing. In any case, for nuclear chaperone activity, additional events other than just the expression of Hsp70 appear to be required. Although we found that Hsp70 was still able to change localization upon heating in a way similar to the manner observed in thermotolerant cells, we did not study the kinetics of the changes. Therefore, it cannot be excluded that additional heat-inducible factors or activities in the cytoplasm alter or enhance the kinetics of translocation in thermotolerant cells, which might be necessary for full chaperone activity in the nucleus. Alternatively, limiting heat-inducible factors might influence the reactivation of heat-inactivated luciferase in the nucleus. These could include both factors that can hold denatured proteins in a folding-competent conformation for Hsp70-mediated refolding and modulators of the activity of Hsp70 itself. Factors that can sequester heat-denatured proteins in a conformation of which Hsp70 can facilitate the reactivation in vitro include, for example, Hsp90 (7) and Hsp27/25 (5). Hsp27/25 and Hsp90 have been found to be transiently overexpressed in thermotolerant cells and to translocate to the nucleus upon heat shock (1, 2). Therefore, they might also be limiting for the reactivation of heat-inactivated nuclear luciferase to the level of thermotolerant cells.
Factors that have been shown to interact with Hsp70 and modulate its activity in vitro include Hsp40 (25), Hip (13), RF-Hsp70 (9), p16 (19), and BAG-1 (14, 40). Several of the above-mentioned proteins have been found to interact with heat-denatured luciferase during its reactivation in reticulocyte lysates (41). Of these various factors, Hsp40 has been found to colocalize with Hsp70 into the nucleus after heat shock (12). In vitro, Hsp40 is essential for Hsp70-mediated protection of luciferase (25). In our cells, however, luciferase was protected against heat by an increased level of Hsp70 alone. This indicated that either Hsp40 can be omitted for this protection or that the cells expressed a basal level of Hsp40 that was sufficient for assisting Hsp70 in this process. Using titration curves with pure proteins, we calculated the Hsp70/Hsp40 molar ratio. If, in nonpreheated cells, Hsp70 was induced at a level that was similar to that of the preheated cells, this ratio was 1:2. This finding is in agreement with what is required for luciferase protection in vitro (25) and therefore did not exclude the need for Hsp40 in the Hsp70-mediated heat protection of luciferase in vivo. Yet in thermotolerant cells, the Hsp70/Hsp40 ratio was raised to 1:3, suggesting a greater availability of Hsp40 (data not shown). Indeed, increasing the expression level of Hsp40 enhanced Hsp70-mediated reactivation of heat-inactivated luciferase, which was most prominent in the nucleus (23). Whether increasing the level of expression of Hsp40 to the level found in thermotolerant cells results in full thermotolerant protection of nuclear luciferase and full clonogenic heat resistance remains to be investigated. Interestingly, if the constitutive expression of Hsp70 was mimicked by turning on the tTA-system for 7 days, cells were more thermoresistant than if they transiently expressed Hsp70 for only 24 h. Besides a higher expression level of Hsp70, these cells also seemed to express a higher level of Hsp40, whereas expression of Hsp25 seemed not to be influenced (see Fig. 10A).
In summary, although transient overexpression of Hsp70 alone to the same level observed in heat-induced thermotolerant cells did increase the chaperone activity in the cell and did protect from loss of clonogenicity after heat shock, the level of this protection was far less than that observed in thermotolerant cells. In yeast cells, the transient overexpression of just one Hsp, Hsp104, is sufficient for thermotolerance (22). Like Hsp70, Hsp104 functions as a chaperone in the protection of heat-inactivated proteins (29). Apparently, in contrast to Hsp104 in yeast cells, overexpression of Hsp70 alone is not sufficient to protect all of the critical targets in mammalian cells.
ACKNOWLEDGMENTS
We thank Geert Mesander of the Flow Cytometry Unit, University Hospital Groningen, and Willy Lemstra for technical assistance.
This work was supported by grant RUG 94-830 from the Dutch Cancer Society, Amsterdam, The Netherlands. Lee A. Weber was supported by grant GM43167 from the National Institutes of Health.
REFERENCES
- 1.Akner G, Mossberg K, Sundqvist K G, Gustavsson J Å, Wikström A C. Evidence for reversible, non-microtubule and non-microfilament-dependent nuclear translocation of Hsp90 after heat shock in human fibroblasts. Eur J Cell Biol. 1992;58:356–364. [PubMed] [Google Scholar]
- 2.Arrigo A-P, Suhan J P, Welch W J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciavarra R P, Goldman C, Wen K-K, Tedeschi B, Castora F J. Heat stress induces hsc70/nuclear topoisomerase I complex formation in vivo: evidence for hsc70-mediated, TP-independent reactivation in vitro. Proc Natl Acad Sci USA. 1994;91:1751–1755. doi: 10.1073/pnas.91.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois M F, Hovanessian A G, Bensaude O. Heat-shock-induced denaturation of proteins. Characterization of the insolubilization of the interferon-induced p68 kinase. J Biol Chem. 1991;266:9707–9711. [PubMed] [Google Scholar]
- 5.Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feder J H, Rossi J M, Solomon J, Solomon N, Lindquist S. The consequences of expressing Hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- 7.Freeman B C, Morimoto R I. The human cytosolic molecular chaperones Hsp90, Hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 1996;15:2969–2979. [PMC free article] [PubMed] [Google Scholar]
- 8.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross M, Hessefort S. Purification and characterization of a 66-kDa protein from rabbit reticulocyte lysate which promotes the recycling of Hsp70. J Biol Chem. 1996;271:16833–16841. doi: 10.1074/jbc.271.28.16833. [DOI] [PubMed] [Google Scholar]
- 10.Hang H, Fox M H. Levels of 70-kDa heat shock protein through the cell cycle in several mammalian cell lines. Cytometry. 1995;25:367–373. doi: 10.1002/(SICI)1097-0320(19961201)25:4<367::AID-CYTO8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Hartl F-U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 12.Hattori H, Kaneda T, Lokeshwar B, Laszlo A, Ohtsuka K. A stress-inducible 40 kDa protein (Hsp40): purification by modified two-dimensional gel electrophoresis and co-localization with hsc70(p73) in heat-shocked HeLa cells. J Cell Sci. 1993;104:629–638. doi: 10.1242/jcs.104.3.629. [DOI] [PubMed] [Google Scholar]
- 13.Höhfeld J, Minami Y, Hartl H-U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 14.Höhfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kampinga H H, Turkel-Uygur N, Roti Roti J L, Konings A W T. The relationship of increased nuclear protein content induced by hyperthermia to killing of HeLa cells. Radiat Res. 1989;117:511–522. [PubMed] [Google Scholar]
- 16.Kirchhoff S, Schaper F, Hauser H. Interferon regulatory factor 1 (IRF-1) mediates cell growth inhibition by transactivation of downstream target genes. Nucleic Acids Res. 1993;21:2881–2889. doi: 10.1093/nar/21.12.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Lepock J R, Frey H E, Ritchie K P. Protein denaturation in intact hepatocytes and isolated organelles during heat shock. J Cell Biol. 1993;122:1267–1276. doi: 10.1083/jcb.122.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung S-M, Hightower L E. A 16-kDa protein functions as a new regulatory protein for Hsc70 molecular chaperone and is identified as a member of the Nm23/nucleoside diphosphate kinase family. J Biol Chem. 1996;272:2607–2614. doi: 10.1074/jbc.272.5.2607. [DOI] [PubMed] [Google Scholar]
- 20.Li G C, Li L, Liu R Y, Rehman M, Lee W M F. Heat shock protein Hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci USA. 1992;89:2036–2040. doi: 10.1073/pnas.89.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G C, Mivechi N F, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia. 1995;11:459–488. doi: 10.3109/02656739509022483. [DOI] [PubMed] [Google Scholar]
- 22.Lindquist S, Kim G. Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc Natl Acad Sci USA. 1996;93:5301–5306. doi: 10.1073/pnas.93.11.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michels A A, Kanon B, Bensaude O, Konings A W T, Kampinga H H. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J Biol Chem. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- 24.Michels A A, Nguyen V-T, Konings A W T, Kampinga H H, Bensaude O. Thermostability of a nuclear-targeted luciferase expressed in mammalian cells. Destabilizing influence of the intranuclear microenvironment. Eur J Biochem. 1995;234:382–389. doi: 10.1111/j.1432-1033.1995.382_b.x. [DOI] [PubMed] [Google Scholar]
- 25.Minami Y, Höhfeld J, Ohtsuka K, Hartl F-U. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homologue, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- 26.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein Hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen V-T, Morange M, Bensaude O. Protein denaturation during heat shock and related stress. Escherichia coli beta-galactosidase and Photinus pyralis luciferase inactivation in mouse cells. J Biol Chem. 1989;264:10487–10492. [PubMed] [Google Scholar]
- 28.Parsell D A, Kowal A S, Singer M A, Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 29.Parsell D A, Lindquist S. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1994. pp. 457–494. [Google Scholar]
- 30.Pelham H R B. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984;3:3095–3100. doi: 10.1002/j.1460-2075.1984.tb02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinto M, Morange M, Bensaude O. Denaturation of proteins during heat shock. In vivo recovery of solubility and activity of reporter enzymes. J Biol Chem. 1991;266:13941–13946. [PubMed] [Google Scholar]
- 32.Pirity M, Nguyen V T, Dubois M F, Bensaude O, Venetianer A. Decreased stress inducibility of the Hsp68 protein in a rat hepatoma variant clone. Eur J Biochem. 1992;210:793–800. doi: 10.1111/j.1432-1033.1992.tb17482.x. [DOI] [PubMed] [Google Scholar]
- 33.Roti Roti J L, Lazlo A. The effects of hyperthermia on cellular macromolecules. In: Urano M, editor. Hyperthermia and oncology. Vol. 1. Zeist, The Netherlands: VSP; 1988. pp. 13–56. [Google Scholar]
- 34.Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher R J, Hansen W J, Freeman B C, Alnemri E, Litwack G, Toft D. Cooperative action of Hsp70, Hsp90, and DnaJ proteins in protein renaturation. Biochemistry. 1996;35:14889–14898. doi: 10.1021/bi961825h. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher R J, Hurst R, Sullivan W P, McMahon N J, Toft D O, Matts R L. ATP-dependent chaperoning activity of reticulocyte lysate. J Biol Chem. 1994;269:9493–9499. [PubMed] [Google Scholar]
- 37.Stege G J J, Brunsting J F, Kampinga H H, Konings A W T. Thermotolerance and nuclear protein aggregation: protection against initial damage or better recovery? J Cell Physiol. 1995;164:573–586. doi: 10.1002/jcp.1041640316. [DOI] [PubMed] [Google Scholar]
- 38.Stege G J J, Li G C, Li L, Kampinga H H, Konings A W T. On the role of Hsp72 in heat-induced intranuclear protein aggregation. Int J Hyperthermia. 1994;10:659–674. doi: 10.3109/02656739409022446. [DOI] [PubMed] [Google Scholar]
- 39.Stege G J J, Li L, Kampinga H H, Konings A W T, Li G C. Importance of the ATP-binding domain and nucleolar localization domain of Hsp72 in the protection of nuclear proteins against heat-induced aggregation. Exp Cell Res. 1994;214:279–284. doi: 10.1006/excr.1994.1259. [DOI] [PubMed] [Google Scholar]
- 40.Takayama S, Bimston D N, Matsuzawa S-I, Freeman B C, Aime-Sempe C, Xie Z, Morimoto R I, Reed J C. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thulasiraman V, Matts R L. Effect of geldanamycin on the kinetics of chaperone-mediated renaturation of firefly luciferase in rabbit reticulocyte lysate. Biochemistry. 1996;35:13443–13450. doi: 10.1021/bi9615396. [DOI] [PubMed] [Google Scholar]
- 42.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch W J, Feramisco J R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984;259:4501–4513. [PubMed] [Google Scholar]
- 44.Ziemienowicz A, Zylicz M, Floth C, Hübscher U. Calf thymus Hsc70 protein protects and reactivates prokaryotic and eukaryotic enzymes. J Biol Chem. 1995;270:15479–15484. doi: 10.1074/jbc.270.26.15479. [DOI] [PubMed] [Google Scholar]