Abstract
A new coronavirus disease (COVID-19) has already affected millions of people in 213 countries. The possibilities of treatment have been reviewed in recent publications but there are many controversial results and conclusions. An analysis of the studies did not reveal a difference in mortality level between people treated with standard therapy, such as antiviral drugs and dexamethasone, and new antiviral drugs/additional immune therapy. However, most studies describe clinical improvement and a decrease in mortality among patients with severe and critical conditions, with the early initiation of additional immune therapy. Possible new targets based on viral life cycles were considered. Unfortunately, the data analysis on the efficacy of different medicine and therapy regimens among patients with COVID-19, showed little success in decreasing the mortality rate in all treatment methods. Some efficacy has been shown with an immunosuppressive therapy in small patient samples, but when a larger number of patients were analyzed the data did not differ significantly from the control groups.
Keywords: coronavirus infection, SARS-CoV-2, COVID-19, antiviral therapy, immune therapy, cytokines, plasma, intravenous immunoglobulin IgG
1. Introduction
The first novel coronavirus cases were officially recorded in Wuhan, Hubei Province, China (PRC) at the end of December 2019 [1,2]. At the end of 2019, the spread of the novel coronavirus caused by the SARS-CoV-2 virus led to the death of patients in 4–22% of cases [3,4], which were associated with severe manifestations of the disease, most often in adults with concomitant pathologies [5,6,7].
There is currently no etiological treatment for coronavirus infection, and a standard therapy is based on the pathogenesis of the disease. According to the pathogenesis established by Chinese scientists, the process can be divided into three stages [8]. Coronaviruses entering the mucosa of the upper respiratory tract are likely replicated in the cells of the ciliary epithelium [9] and cause rhinitis, glossitis, and a cough with possible systemic intoxication, manifested by fever and arthralgia [10]. When overcoming the upper respiratory tract barriers, the virus enters the lungs, where it binds to the angiotensin converting enzyme (ACE) using the receptor-binding domain (RBD) S1 of the subunit of the surface S (spike) protein, which initiates virion endocytosis in the cell [11,12,13]. From the lungs, the virus enters the systemic circulation known as the viremia phase. During this stage, the virus attacks cells that also express ACE: type 2 pneumocytes in the alveolar epithelium, heart, kidney, gastrointestinal tract cells, macrophages [14,15,16], as well as the endothelium of arterial and venous vessels, smooth muscle cells in the arteries [17]. The second stage is the acute phase, characterized by organ lesions due to infection. They can be explained by several mechanisms: the direct cytotoxic effect of the virus on cells, immune-mediated complications, vascular complications, and autoimmune side effects [8,18]. The SARS-CoV-2 virus induces a weak interferon response of types I, II and III and a strong activation of the interleukin IL-1β/IL-6 pathway [19]. In the lungs, infection of type II alveolar epithelial cells activates the inflammasome, which induces the production of IL-1β [20]. IL-1β induces the secretion of IL-6 by endothelial cells and vascular smooth muscle cells, which enhance the inflammatory response [21]. In the lungs immune-competent cells infiltrate the tissue and cause an additional alteration due to excessive secretion of proteases and active forms of oxygen [15,22]. The diffuse alteration of alveoli is characterized by the desquamation of alveolar cells, formation of hyaline membranes, development of lung edema and fibrosis [17,23]. It is important to note that the acute phase, characterized by the development of pneumonia, with adequate treatment and normal functioning of the immune system is followed by a stage three recovery. In risk groups (advanced age, the presence of concomitant diseases), the immune system cannot effectively control the course of the diseases. For this reason, serious life-threatening complications such as cytokine storm and massive thrombosis may occur. In such cases, patients end up in a very serious condition and need intensive care [8].
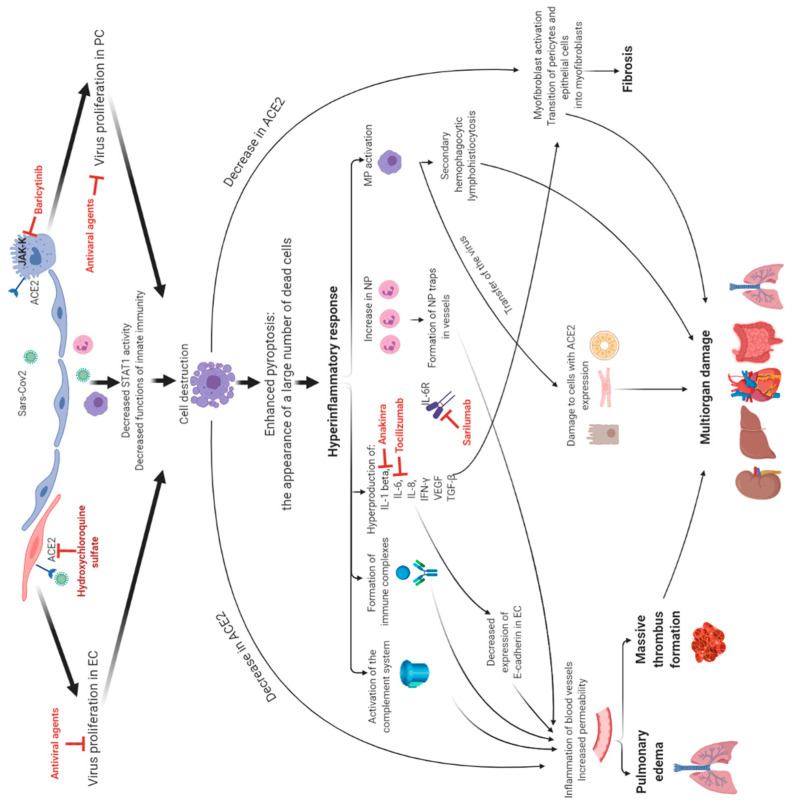
Therefore, existing therapy is aimed at inhibiting viral replication, as the binding to ACE2 and the activity of viral enzymes prevent the vascular and the immune complications from functioning (Figure 1). Despite the wide choice of drugs available, doubts about their efficacy, the most optimal prescription time, and patient selection criteria for certain drugs remain.
Figure 1.
The targets of drugs used in clinical practice and their influence on pathogenic processes. Abbreviations: EC are endotheliocytes, PC are pneumocytes, NP are neutrophils, and MP are macrophages.
The purpose of this review is to analyze the efficacy of antiviral and immunological treatments of COVID-19.
2. Results and Discussion
2.1. Antiviral Therapies
According to the presented data (Table 1), there is only an insignificant efficacy of hydroxychloroquine sulfate when used in conjunction with azithromycin and a low efficacy as a preventive monotherapy.
Table 1.
The results of the studies on the efficacy of antiviral drugs for COVID-19 treatment.
| N | Authors and Year of Publication | The Agent Studied | Mode of Drug Administration | Number of Patients/Control Group | Observation Time, Days (Median) | Comparison of Efficiency with Control Group (%) | Conclusions |
|---|---|---|---|---|---|---|---|
| 1 | Cao B. et al., 2020 [24] | lopinavir/ritonavir | 400/100 mg twice a day 14 days | 99/control (n = 100) | 28 | Mortality 19.2 vs. 25.0 |
No difference |
| 2 | Li Y et al., 2020 [25] | lopinavir/ritonavir umifenovir and hydrochloride monohidrate | 200/50 mg 2 times/day 7–14 days and 200 mg 3 t/day 7–14 days | 34 versus 35 and control (n = 17) |
21 | Efficacy: 85.3 vs. 91.4 vs 76.5 |
|
| 3 | Gautret Ph. et al., 2020 [26] | Hydroxychlo-roquine sulfate | 600 mg/day 10 days | 20/control (n = 16) | 14 | Efficacy: 57.1 vs. 12.5 |
|
| 4 | Gautret P, et al., 2020 [27] | hydroxychloroquine sulfate + azithromycin | 600 mg/day 10 days + 500 mg on 1-st day, further 250 mg 2nd–5th day | 80/no | ≥6 | Efficacy: 93.0 |
|
| 5 | Geleris J. et al., 2020 [28] | hydroxychloroquine sulfate | 600 mg on 1-st day, further 400 mg/day | 811/control (n = 565) | 22.5 | Efficacy: 45.8 (no data) |
|
| 6 | Grein J. et al., 2020 [29] |
remdesivir | 200 mg on 1-st day, further 100 mg 2nd–10th day | 53/no | 19 | Efficacy: 47.0 (no data) |
|
| 7 | WangY. et al., 2020 [30] | 200 mg on 1-st day, further 100 mg 2nd–10th day | 158/Placebo control (n = 79) |
28 | Efficacy: 65.0 vs. 58.0 |
||
| 8 | Beige JH. et al., 2020 [31] | 200 mg/day for 10 days | 538/placebo control 521 | 15 | Efficacy: 62.9 vs. 52.7 |
||
| 9 | Goldman JD.et al, 2020 [32] | 200 mg/day for 5 and 10 days | 200 (5 days)/197 (10 days) | 14 | Efficacy: 64.0 vs. 54.0 Mortality 8.0 vs 11.0 |
||
| 10 | Boulware D.R. et al., 2020 [33] | hydroxychloroquine sulfate (prophylactically) |
800 мг in a single dose, further 600 mg after 6 and 8 h, further 600 mg for 4 days | 414 patients with asympto-matic course/407 (placebo) |
14 | Got sick 11.8 vs. 14.3 |
|
| 11 | Freedberg ED et al., 2020 [34] | famotidine | 20 mg, 40 mg, 10 mg | 84/control 1536 | 5 | Mortality 10.0 vs. 22.0 |
|
| 12 | Horby P. at al, 2020 [35] | hydroxychloroquine sulfate | 800 мг in a single dose, further 400 mg after 12 h and 6 days | 1561/3155 (control) | n | Mortality 27.0 vs. 25.0 |
|
| 13 | Mather J et al., 2020 [36] | famotidine + hydroxychloroquine sulfate (n = 36) famotidine + azithromycin (n = 36) famotidine + corticosteroids (n = 48) |
20 mg, 40 mg 7 days | 83/689 (control group) | 36 | Mortality 21.6 vs. 39.7 |
A study of the efficacy of remdesivir in conjuction with COVID-19 was carried out in 53 patients with a confirmed SARS-CoV-2 virus carrier based on PCR and respiratory failure (an oxygen saturation of ≤94%/the need for oxygen support) [29]. In 68% of cases, there was an improvement in the oxygen support class, including 17 out of 30 patients who were on mechanical ventilation, and later extubated. The mortality level in the patient group who received invasive ventilation was 18% (6 out of 34) and 5% (1 out of 19) among those who did not need invasive ventilation. Furthermore, in a larger number of patients with COVID-19, another randomized trial was conducted and its findings indicated the results for the treatment of 538 patients and proved the effectiveness of the drug within 15 days of observation, compared with the control group who received a placebo (n = 521). However, the number of deaths in the groups did not significantly differ (7.1% versus 11.9%) [25].
One of the most significant studies with an analysis of a large number of clinical cases was devoted to the efficacy of dexamethasone in COVID-19 treatment [37]. A decrease of 10% in mortality rate was observed among patients with mechanical ventilation (29.3% vs. 41.4%). The analysis of the total mortality rate among COVID-19 patients with dexamethasone was not as significant (22.5% vs. 25.7%).
2.2. Immune Therapy
There are several directions that can be taken for the development of immune therapy for coronavirus infection [38]:
Monoclonal antibodies against cytokines and their receptors;
Kinase inhibitors;
Polyclonal antibodies by plasma therapy;
Intravenous immunoglobulin IgG (IVIG);
Polypeptide hormone for maturation of T cells.
2.2.1. Monoclonal Antibodies against Cytokines and Their Receptors
According to the pathogenesis of hyperinflammation in COVID-19, the main participants are IL-1β and IL-6; therefore, the focus of clinical research was to study drugs which can block the signaling pathways of these molecules [19].
The results of 15 different studies using drugs to block the signaling pathways of IL-1 beta and IL-6 in patients with COVID-19 of varying levels of severity, are presented in Table 2. We can see that employing the described drugs had a beneficial effect in reducing the severity of the disease; however, in most cases, the summary indicators (survival/mortality) were similar to the control group.
Table 2.
The results of the studies on the efficacy of the drugs blocking the IL-1β and IL-6 signaling pathways for COVID-19 treatment.
| № | Authors, Year | The Type of the Study | The Drug | The Treatment Characteristics of Patients |
Conclusions | |
|---|---|---|---|---|---|---|
| Studied Group | Comparison Group | |||||
| 1 | Cavalli G et al. [39] | Retrospective cohort study | Anakinra (block IL-1 beta R) | Patients (aged ≥18 years) with COVID-19, moderate-to-severe ARDS, and hyperinflammation (n = 29) Standard treatment + Anankinra dose 5 mg/kg twice a day 100 mg subcutaneously 21 days |
COVID-19, ARDS, and hyperinflammation Standard treatment | Decreased mortality |
| 2 | Pontali E. et al. [40] | Uncontrolled cohort study | 5 patients with severe/moderate COVID-19 100 mg IV every 8 h n = 5 |
- | Faster de-escalation of the intensity of care | |
| 3 | Ucciferri C et al. [41] | Retrospective cohort study | Canakinumab (block IL-1β) | 300 mg subcutaneously n = 10 |
- | Faster de-escalation of the intensity of care |
| 4 | Xu X et al. [42] | Retrospective cohort study | Tocilizumab (block IL-6) | Severe or critical COVID-19 n = 21 4–8 mg/kg, recommended dose–400–800 mg singly 21 days |
- | Faster de-escalation of the intensity of care |
| 5 | Malekzadeha R et al. [43] | Multicenter, prospective, open-label, uncontrolled | Adult patients with severe and critical COVID-19 n = 126 324 mg (<100 kg bodyweight) or 486 mg (≥100 kg bodyweight). 40 days |
- | Faster de-escalation of the intensity of care | |
| 6 | Stone JH et al. [44] | A randomized, double-blind, placebo-controlled trial | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hyperinflammatory states n = 161 4–8 mg/kg, recommended dose–400–800 mg singly 14 and 28 days |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hyperinflammatory states n = 81 Standard treatment |
No difference | |
| 7 | Alattar R et al. [45] | Retrospective cohort study | severe COVID-19 n = 25 4–8 mg/kg, recommended dose–400–800 mg singly 14 and 28 days |
n | No difference | |
| 8 | Tsai A et al. [46] | A single-center propensity-score matched cohort study | Severe COVID-19 n = 66 8 mg/kg, recommended dose–400–800 mg singly |
Severe COVID-19 n = 66 Standard treatment |
No difference | |
| 9 | Klopfenstein T et al. [47] | a retrospective case-control study | Severe COVID-19 n = 20 tocilizumab (1 or 2 doses) |
Severe COVID-19 n = 25 Standard treatment |
Decreased mortality | |
| 10 | Toniati P et al. [48] | severe COVID-19 n = 100 8 mg/kg by two consecutive intravenous infusions 12 h apart |
- | Faster de-escalation of the intensity of care | ||
| 11 | Guaraldi G et al. [49] | Retrospective, observational cohort study |
n = 179 intravenously at 8 mg/kg bodyweight (up to a maximum of 800 mg) in two infusions, 12 h apart, or subcutaneously at 162 mg administered in two simultaneous doses, one in each thigh (ie, 324 mg in total) |
Adults (≥18 years) with severe COVID-19 n = 365 Standard treatment |
Decreased mortality | |
| 12 | Potere N. et al. [50] | Retrospective case–control study | severe COVID-19 n = 40 324 mg, given as two concomitant subcutaneous injections |
Severe COVID-19 n = 40 Standard treatment (SOC) |
Faster de-escalation of the intensity of care | |
| 13 | Rojas-Marte G. et al. [51] | a Retrospective, case–control, Single-center study | severe to critical COVID-19 n = 96 4–8 mg/kg, recommended dose–400–800 mg singly 15 and 17 days |
severe to critical COVID-19 n = 97 Standard treatment |
Decreased mortality | |
| 14 | Colaneri M et al. [52] | Prospective study | 8 mg/kg, recommended dose–400–800 mg singly 7 days n = 21 |
n = 91 Standard treatment |
No difference | |
| 15 | Tarrytown NY. et al. [53] | Randomized Phase 2 | Sarilumab (block IL-6 R) | Critical, severe COVID-19 n = 281 136 (200 mg)/145 (400 mg) |
Critical, severe COVID-19 n = 77 placebo |
No difference |
It can be assumed that using cytokine inhibitors is the most appropriate way to treat patients with severe disease and hyperinflammation, where extensive organ damage is not evident and mechanical ventilation support is not needed. Other researchers have come to similar conclusions. Tocilizumab has been described as reducing fever and systemic inflammation within 5–7 days, was associated with improved oxygenation rates within 48–72 h, and also delayed the risk of intubation or mortality [54]. Analysis of clinical trials (RCT-TCZ-COVID-19, CORIMUNO-19-TOCI-1, BACC Bay Tocilizumab, and STOP-COVID-19) showed that mortality can be reduced by early medication of tocilizumab [55].
2.2.2. The Kinase Inhibitors
Janus kinase inhibitors (JAK) downregulate the phosphorylation of the signal transducer and transcriptional activator (STAT) of several inflammatory proteins. Blocking the JAK inhibits the activation of the immune system and the development of inflammation (for example, the cellular response to proinflammatory cytokines such as interleukin IL-6) [56,57].
Baricitinib, a Janus kinase (JAK) inhibitor of JAK1 and JAK2 kinases, and Bruton’s tyrosine kinase (BTK), a B-cell antigen receptor signaling molecule, are currently being investigated in clinical trials. The data of the studies (n = 4) are shown in Table 3.
Table 3.
The results of the studies on efficacy of Janus kinase inhibitors for COVID-19 treatment.
| № | Authors, Year | The Type of the Study | The Drug | The Treatment Characteristics of Patients |
Conclusions | |
|---|---|---|---|---|---|---|
| Studied Group | Comparison Group | |||||
| 1 | Cantini F et al. [58] | Pilot study with open-label design, with no randomization and a low number of treated patients’ | Baricytinib (block JAK-k) | Moderate COVID-19 4 mg/day 14 days n = 24 |
Moderate COVID-19 n = 24 |
Faster de-escalation of the intensity of care |
| 2 | Kalil AC et al. [59] | Multicenter. A randomized, double-blind ACTT-2 trial | Moderate to severe COVID-19 4 mg daily (for up to 14 days or until hospital discharge), n = 515 |
Moderate to severe COVID-19 n = 518 placebo |
Dereased mortality | |
| 3 | Cao Y et al. [60] | Small, single-blind, randomized, controlled Phase 2 trial | Ruxolitinib (block JAK-k) | Severe COVID-19 n = 20 5 mg orally twice daily |
Severe COVID-19 n = 21 Placebo (vitamin C 100 mg) |
No statistical difference was observed. |
| 4 | Roschewski M et al. [61] | Retrospective case series | Acalabrutinib (Bruton’s Tyrosine Kinase Inhibitors) | Severe COVID-19 n = 19 |
- | Faster de-escalation of the intensity of care |
According to the analysis of these studies, the use of Janus kinase inhibitors (JAK) was associated with a clinical improvement; however, a reduction in mortality was not achieved. It should be noted that most results were obtained from small numbers of patients with differing degrees of severity which means that, for more accurate results, additional double-controlled studies with stricter inclusion criteria, a larger number of patients, and the presence of comparison groups were needed.
2.2.3. Intravenous Immunoglobulin IgG (IVIG)
According to some studies, IVIG (intravenous immunoglobulin) [62,63] also achieves some efficacy in the treatment of COVID-19. Some information about clinical studies with the use of IVIG are shown in Table 4.
Table 4.
The results of the studies on efficacy of IVIG for COVID-19 treatment.
| № | Authors, Year | The Type of the Study | Treatment Patient Characteristics |
Conclusions | |
|---|---|---|---|---|---|
| Studied Group | Comparison Group | ||||
| 1 | Shao Z et al. [64] | Multicenter retrospective cohort study | Critical COVID-19 n = 174 human Immunoglobulin (pH4) for intravenous injection 28 and 60 days |
Critical COVID-19 n = 151 |
No difference |
| 2 | Zhou Z-G et al. [63] |
n = 10 Short-term moderate-dose corticosteroid (160 mg/d) plus immunoglobulin (20 g/d) |
- | Faster de-escalation of the intensity of care | |
| 3 | Xie Y et al. [62] | Retrospective study | Severe or critical illness due to COVID-19 n = 58 |
- | Faster de-escalation of the intensity of care |
The obtained data are insufficient for making accurate conclusions; however, it can be noted that the use of IVIG during early stages of the disease is associated with an improvement in the clinical parameters of patients and the prognosis of the disease.
2.2.4. Convalescent Plasma Transfusion
Plasma transfusion can eradicate pathogens from the circulation and neutralize ferritin and cytokines [65,66]. Convalescent plasma generated a lot of enthusiasm during early days of the COVID-19 pandemic due to its plausible mechanism of action and its easy availability from donors [67]. Information about clinical research, which was carried out by studying the efficacy of plasma convalescents with the new coronavirus infection. is provided in Table 5.
Table 5.
The results of the studies on efficacy of the use of convalescent plasma for COVID-19 treatment.
| № | Authors, Year | The Type of Research | Treatment Patients Characteristic, n |
Conclusions | |
|---|---|---|---|---|---|
| Studied Group | Comparison Group | ||||
| 1 | Simonovich VA et al. [68] | Double-blind, placebo-controlled, multicenter tria | Severe COVID-19 n = 228 Early administration of convalescent plasma (median titer of 1:3200 of total SARS-CoV-2 antibodies) |
Severe COVID-19 n = 105 placebo |
No difference |
| 2 | Libster R et al. [69] | A randomized, double-blind, placebo-controlled trial | Mildly ill infected older adults n = 80 Early administration of high-titer convalescent plasma 250 mL (IgG titer greater than 1:1000 against SARS-CoV-2 spike) |
Mildly ill infected older adults n = 80 placebo |
No statistical difference reduced the progression of COVID-19 |
| 3 | Salazar E et al. [70] | Prospective, ongoing study | Severe and/or life-threatening COVID-19 n = 136 600 mL plasma was collected from each donor 7 and 14 days |
Severe and/or life-threatening COVID-19 n = 251 |
Decreaesd mortality |
| 4 | Khamis F et al. [71] | Single-center, case series study |
n = 11 Early therapeutic plasma exchange (TPE), 14, 28 days |
Critical COVID-19 n = 20 |
Decreased mortality |
| 5 | Li L et al. [72] | Open-label, multicenter, randomized clinical trial | Severe or life-threatening COVID-19 n = 52 specific IgG titer ≥ 1:640; 200 mL of plasma 28 days |
Severe or life-threatening COVID-19 n = 51 |
No difference |
| 6 | Gharbharan A et al. [73] | A randomized trial |
n = 43 ≥1:80; 300 mL 15 days |
n = 43 | No statistical difference Mortality 14.0 vs. 26.0 |
| 7 | Agarwal A at al [74] | Open label, parallel arm, phase II, multicentre, randomised controlled trial. | Moderate COIVD-19 n = 235 2 doses of 200 mL CP |
n = 229 | No statistical difference Mortality: 14.5 vs. 13.5 |
| 8 | Joyner MJ et al. [75] | Open-label, Expanded Access Program (EAP) for the treatment of COVID-19 patients with human convalescent plasma. | Severe critical COVID-19 n = 35 150–200 mL 30 days |
n = 322 | Decreased mortality |
| 9 | Liu STH et al. [76] | Retrospective, propensity score-matched case-control study | Severe or life-threatening COVID-19 2 units of CP; 1:320 14 days n = 39 |
Severe or life-threatening COVID-19 n = 156 |
No diference |
The greatest efficacy of the therapy is observed with the early use of plasma (up to 72 h) among patients with a severe level of the disease. A failure response of plasma therapy was noted in a review by Pathak et al. [67].
Perhaps the conflicting results can be explained by the lack of standards and methods for the screening of donor plasma, in search of the presence of binding and neutralizing antibodies to SARS-CoV-2, which could lead to use of plasma with a low level of antibodies [75].
2.2.5. Polypeptide Hormone for Maturation of T Cells
One of the most underexplored fields of research is immunomodulation using thymosin, a polypeptide hormone used for T-cell maturation. Only two clinical trials (ChiCTR2000029541 and ChiCTR2000029806) used thymosin in combination with a standard therapy [77].
At this moment in time, it is difficult to draw conclusions on the efficacy of the immune therapy of COVID-19. However, most studies describe a clinical improvement and deacrease in mortality among patients in a severe and critical condition with the early initiation of additional immune therapy. These findings are supported by the study by Alessia Alunno et al., according to which none of the many immunomodulators had an impact on the mortality of patients; however, there is currently no final decision regarding the use of tocilizumab [78].
It is necessary to carry out a comparative analysis on the efficacy of each type of immune therapy in order to determine the effectiveness predictive factors for certain categories of patients, depending on the severity of the disease, age, concomitant diseases, and the time since the onset of symptoms.
3. Possible Therapy Targets for COVID Treatment
The present therapy has some advantages in its metabolic characteristics, dosages used, and potential efficacy, but “broad-spectrum” medicaments and their side effects should not be underestimated. Therefore, the research on new therapeutic targets and drugs continues to be conducted. The therapies that act on the coronavirus can be divided into several categories based on the specific pathways [79]:
Enzymes or functional proteins for RNA synthesis and replication, for example: Nsp3 (Nsp3b, e Papain-like proteinase (PLpro)), Nsp7*Nsp8 complex, Nsp9eNsp10, Nsp14eNsp16, Nsp5 (3CLpro), Nsp12 (RdRp), Nsp13 (Helicase);
Structural proteins for binding to human cell receptors, for example: Spike protein, E-channel, C-terminal RNA binding domain (CRBD), N-terminal RNA binding domain (NRBD);
Virulence factors damaging the host’s innate immunity, for example: Nsp1, Nsp3c, ORF7a;
The host’s specific receptors or enzymes, for example: TMPRSSS2, ACE2.
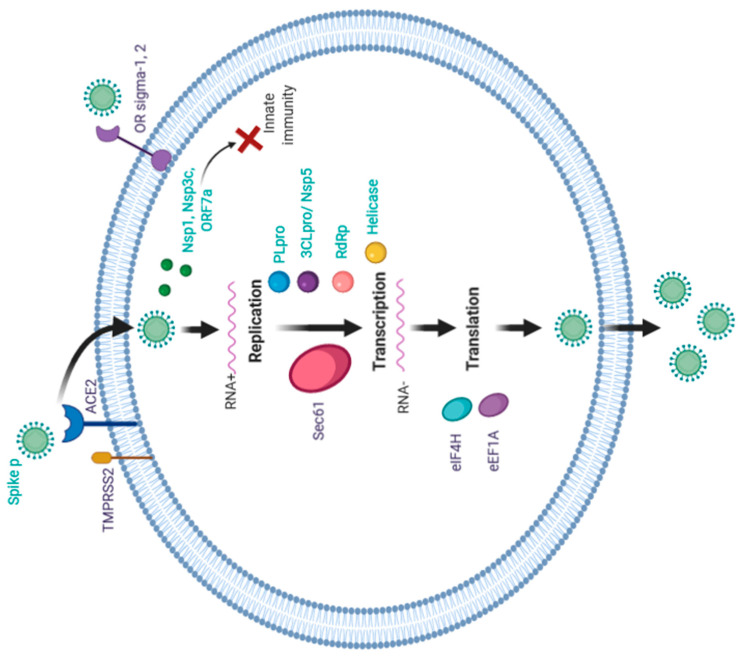
Wu C. et al. [80] conducted a virtual screening of ligands based on 21 targets (including two human targets) and selected molecules capable of inhibiting them. Gordon et al. [81] identified two classes of molecules and experimentally demonstrated their antiviral efficacy: inhibitors of protein biogenesis (zotifine, ternatin-4, and PS3061) and ligands of sigma-1 and sigma-2 receptors (haloperidol, PB28, PD-144418 and hydroxychloroquine, which is undergoing clinical trials). The authors noted the importance of the discovery of antiviral activity in sigma-1 and sigma-2 opioid receptor subtype inhibitors. It is possible that these molecules also contribute to the penetration of the virus into the cells, which may explain some neurological symptoms, in particular anosmia, because the olfactory bulb is rich in these proteins. Possible targets and their role in the life cycle of the virus are shown in the Figure 2.
Figure 2.
Possible therapeutic targets and their role in the life cycle of the SARS-CoV-2.
According to these studies, the most promising drugs might be anti-bacterial (Chloramphenicol, Cefamandole, Tigecycline, Lymecycline, Demeclocycline, Doxycycline, Oxytetracycline, Novobiocin, Gallstonedissolving, Drug Chenodeoxycholic Acid, Cefsulodine, Rolitetracyclin, Sulfasalazine, Azlocillin, Penicillin, Pivampicillin, Hetacillin, Cefoperazone, Clindamycin, Cefmenoxime, Piperacillin, Cefpiramide, Streptomycin, Lymecycline, Tetracycline); anti-viral (Ribavirin, Saquinavir, Valganciclovir, Thymidine), anti-tumor (Idarubicin, Zotatifin, Ternatin-4, Ps3061); and anti-hypertensive (Nicardipine, Rescinnamine, Losartan, Conivaptan, Telmisartan, Iloprost, Prazosin). More detailed information is presented in Table 6.
Table 6.
Possible therapeutic targets and drugs for COVID-19 treatment.
| The Group of Therapeutic Target | The Target | The Inhibiting Molecule |
|---|---|---|
| Blocking replication | Papain-like proteinase (PLpro) |
anti-virus drugs (ribavirin, valganciclovir, thymidine) anti-bacterial drugs (chloramphenicol, cefamandole, tigecycline) muscle relaxant drug (chlorphenesin carbamate) anti-tussive drug (levodropropizine) |
| 3C-like main protease (3CLpro/Nsp5) |
anti-bacterial drugs (lymecycline, demeclocycline, doxycycline, oxytetracycline) anti-hypertensive drugs (nicardipine, telmisartan, conivaptan) |
|
| RNA-dependent RNA polymerase (RdRp) |
antifungal drug itraconazole anti-bacterial drug novobiocin gallstone dissolving drug chenodeoxycholic acid anti-allergic drug cortisone anti-tumor drug idarubicin hepatoprotective drug silybin muscle relaxant drug pancuronium bromide anticoagulant drug dabigatran |
|
| Helicase |
anti-bacterial drug (lymecycline, cefsulodine, rolitetracycline) anti-fungal drug itraconazole anti-HIV1 drug saquinavir anti-coagulant drug dabigatran diuretic drug canrenoic acid |
|
| Restoring host’s innate immunity | Nsp1, Nsp3c, ORF7a |
anti-bacterial drugs (piperacillin, cefpiramide, streptomycin, lymecycline, tetracycline) |
| Blocking viral structural proteins | Spike protein |
antihypertensive drugs (rescinnamine, iloprost, prazosin) antifungal drugs (posaconazole, itraconazole) anti-bacterial drug (sulfasalazine, azlocillin, penicillin, cefsulodin) anti-coagulant drug dabigatran etexilate |
| Interface between Spike and ACE2 |
Hesperidin | |
| Blocking host‘s proteins | ACE2 protein |
antidiabetes drug troglitazone anti-hypertensive drug losartan analgesia drug ergotamine anti-bacterial drug cefmenoxime hepatoprotective drug silybin phyllaemblicin |
| TMPRSS2 | anti-bacterial drugs (pivampicillin, hetacillin, cefoperazone, clindamycin) | |
| Ligands of the sigma-1,2 receptors | Haloperidol, PB28, PD-144418 and hydroxychloroquine | |
| Eukaryotic Translation Initiation Factor 4H (eIF4H) | Zotatifin | |
| Elongation factor-1A (eEF1A) | Ternatin-4 | |
| Sec61 translocon | PS3061 |
Additionally, the scientists revealed some natural compounds (catechin compounds with an antioxidant effect and xanthones with an insecticide effect) that can block the viral life cycle in different phases. Some of these are presented in Table 7.
Table 7.
Some of the natural compounds considered to have an inhibiting effect on the SARS-CoV-2 life cycle.
| The Plant | Scutellaria Baicalensis | Cassine Xylocarpa | Swertia Genus | Citrus Aurantium | Phyllanthus Emblica |
|---|---|---|---|---|---|
| Molecules inhibiting Sars-Cov-2 | Baicalin Chrysin-7-o-b-glucuronide Wogonoside Cosmosiin |
Betulonal Etexilate betulonal | Deacetylcentapicrin Triptexanthoside D 1,7-dihydroxy-3- methoxyxanthone Kouitchenside I, D | Neohesperidin Hesperidin | Phyllaemblinol Phyllaemblicin B, G7 |
4. Conclusions
Unfortunately, the data analysis on the efficacy of different medicines and therapy regimens among patients with COVID-19 showed little success in decreasing the mortality rate through all methods. Some efficacy was shown with immunosuppressive therapy in a small number of patients, but when a larger number of patients was analyzed, the data did not differ significantly from the control groups. Furthermore, initial hopeful results concerning plasma application were ineffective in larger studies. This analysis led us to postulate that there is no evidence for the effective treatment of COVID-19 patients. Despite the presence of a great number of agents and studies conducted, no effective treatment methods were revealed.
Studies on virtual ligand screening and affinity-purification mass spectrometry revealed a wide spectrum of anti-SARS-COV2 molecules, mostly human proteins (opioid like receptors, factors of replication and translation) involved in the virus life cycle, and the molecules that inhibit them.
The most important aspect is the analysis of data on the use of various types of COVID-19 therapy in patients with a severe, critical course of the disease, especially in older age groups.
The analysis showed that, to date, there is no effective antiviral agent for the treatment of COVID-19. According to the previously obtained data, it was demonstrated that the administration of hydroxychloroquine for the treatment and prevention of coronavirus infection is not effective. On the other hand, the use of remdesevir in some patients, including those who were on invasive ventilation, showed an improvement in the course of the disease without a significant effect on mortality.
It was shown that early use of immunosuppressive agents (e.g., tocilizumab, JAK kinase inhibitors, IVIG) may have affected the severity of clinical manifestations, but did not impact mortality.
The first inspiring data on the transfusion of plasma convalescents to patients with COVID-19 were not confirmed. However, subsequent studies of this method with the formation of common standards may improve these results.
The data which demonstrated potential therapeutic targets (mainly from antiviral, antibacterial, and antihypertensive drugs, as well as human proteins involved in the virus life cycle and the molecules that inhibit them) are encouraging, but at the present moment, they are of scientific rather than practical interest.
Thus, according to the results of this analysis, none of the considered methods of COVID-19 therapy showed a significantly positive effect on mortality or a significant effectiveness in comparison with other methods, indicating the need for further research.
Acknowledgments
This work is supported by the grant of the Government of the Russian Federation for the state support of scientific research carried out under the supervision of leading scientists, agreement 14.W03.31.0009.
Author Contributions
A.S.—analysis of the materials, coordinator of the project, writing of the manuscript; A.M.—writing of the manuscript; U.Z.—analysis of the materials; D.K.—writing of the manuscript; A.G.—writing of the manuscript; I.D.—analysis of the materials; P.Y.—analysis of the materials, coordinator of the project.; Y.S.—analysis of the materials, coordinator of the project. All authors have read and agreed to the published version of the manuscript.
Funding
Government funding was obtained from Almazov National Medical Research Centre of the Ministry of Health of Russian Federation and St. Petersburg Scientific Research Institute of Phthisiopulmonology of the Ministry of Health of Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . Emergency Use ICD Codes for COVID-19 Disease Outbreak. WHO; Geneva, Switzerland: 2020. [Google Scholar]
- 2.WHO . Coronavirus Disease (COVID-19) Pandemic. WHO; Geneva, Switzerland: 2020. [(accessed on 5 May 2021)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 3.Tagarro A., Epalza C., Santos M., Sanz-Santaeufemia F.J., Otheo E., Moraleda C., Calvo C. Screening and Severity of Coronavirus Disease 2019 (COVID-19) in Children in Madrid, Spain. JAMA Pediatr. 2021;175:316–317. doi: 10.1001/jamapediatrics.2020.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Liang X., Bi Z., Ren J., Wang B., Li L. Consideration on the strategies during epidemic stage changing from emergency response to continuous prevention and control. Chin. J. Endem. 2020;41:297–300. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe Acute Respiratory Syndrome Coronavirus Infection of Human Ciliated Airway Epithelia: Role of Ciliated Cells in Viral Spread in the Conducting Airways of the Lungs. J. Virol. 2005;79:15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., et al. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [Google Scholar]
- 11.Babcock G.J., Esshaki D.J., Thomas W.D., Ambrosino D.M. Amino Acids 270 to 510 of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein Are Required for Interaction with Receptor. J. Virol. 2004;78:4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-Amino Acid Fragment of the SARS Coronavirus S Protein Efficiently Binds Angiotensin-converting Enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/S0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 15.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giani M., Seminati D., Lucchini A., Foti G., Pagni F. Exuberant Plasmocytosis in Bronchoalveolar Lavage Specimen of the First Patient Requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe. J. Thorac. Oncol. 2020;15:e65–e66. doi: 10.1016/j.jtho.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., Alijotas-Reig J., Zinserling V., Semenova N., Amital H., et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loppnow H., Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J. Clin. Investig. 1990;85:731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalli E., Petralia M.C., Basile M.S., Bramanti A., Bramanti P., Nicoletti F., Spandidos D.A., Shoenfeld Y., Fagone P. Transcriptomic analysis of covid-19 lungs and bronchoalveolar lavage fluid samples reveals predominant b cell activation responses to infection. Int. J. Mol. Med. 2020;46:1266–1273. doi: 10.3892/ijmm.2020.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solun B., Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med. Drug Discov. 2020;7:100052. doi: 10.1016/j.medidd.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., Mo X., Wang J., Wang Y., Peng P., et al. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med. 2020;1:105–113. doi: 10.1016/j.medj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. medRxiv. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J.G.V. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr G., et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman J.D., Lye C.B.D., Hui S.D., Marks M.K., Bruno R., Montejano R., Spinner D.C., Galli M., Ahn M.-Y., Nahass G.R., et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for COVID-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedberg D.E., Conigliaro J., Wang T.C., Tracey K.J., Callahan M.V., Abrams J.A. Famotidine Research Group. Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horby P.W., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 doi: 10.1101/2020.07.15.20151852. [DOI] [Google Scholar]
- 36.Mather J.F., Seip R.L., McKay R.G. Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19. Am. J. Gastroenterol. 2020;115:1617–1623. doi: 10.14309/ajg.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.AminJafari A., Ghasemi S. The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol. 2020:83. doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Din C.T., Boffini N., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pontali E., Volpi S., Signori A., Antonucci G., Castellaneta M., Buzzi D., Montale A., Bustaffa M., Angelelli A., Caorsi R., et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J. Allergy Clin. Immunol. 2021;147:1217–1225. doi: 10.1016/j.jaci.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ucciferri C., Auricchio A., Di Nicola M., Potere N., Abbate A., Cipollone F., Vechhiet J., Falasca K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020;2:e457–e458. doi: 10.1016/S2665-9913(20)30167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malekzadeh R., Abedini A., Mohsenpour B., Sharifipour E., Ghasemian R., Javad-Mousavi S.A., Khodashahi R., Darban M., Kalantari S., Abdollahi N., et al. Subcutaneous tocilizumab in adults with severe and critical COVID-19: A prospective open-label uncontrolled multicenter trial. Int. Immunopharmacol. 2020;89:107102. doi: 10.1016/j.intimp.2020.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., et al. Efficacy of Tocilizumab in Patients Hospitalized with COVID-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alattar R., Ibrahim T.B.N., Shaar S.H., Abdalla S.A., Shukri K., Daghfal J.N., Khatib M.Y., Abukhattab M., Hussam A., Alsoub H.A., et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020;92:2042–2049. doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai A., Diawara O., Nahass R.G., Brunetti L. Impact of tocilizumab administration on mortality in severe COVID-19. medRxiv. 2020 doi: 10.1038/s41598-020-76187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klopfenstein T., Zayet S., Lohse A., Balblanc J.C., Badie J., Royer P.Y., Toko L., Mezher C., Kadiane-Oussou N.J., Bossert M., et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med. Mal. Infect. 2020;50:397–400. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.-A., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19:102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., et al. Tocilizumab in patients with severe COVID-19: A retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potere N., Di Nisio M., Cibelli D., Scurti R., Frattari A., Porreca E., Abbate A., Parruti G. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: A case-control study. Ann. Rheum. Dis. 2021;80:271–272. doi: 10.1136/annrheumdis-2020-218243. [DOI] [PubMed] [Google Scholar]
- 51.Rojas-Marte G., Khalid M., Mukhtar O., Hashmi A.T., Waheed M.A., Ehrlich S., Aslam A., Siddiqui S., Agarwal C., Malyshev Y., et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: A case-controlled study. QJM Int. J. Med. 2020;113:546–550. doi: 10.1093/qjmed/hcaa206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F., Montecucco C., Mojoli F., Giusti E.M., Bruno R., et al. Tocilizumab for treatment of severe COVID-19 patients: Preliminary results from smatteo COVID19 registry (smacore) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarrytown N.Y. Regeneron and Sanofi Provide Update on U.S. Phase 2/3 Adaptive-Designed Trial of Kevzara® (Sarilumab) in Hospitalized COVID-19 Patients. [(accessed on 5 July 2021)];Regen. Pharm. Inc. 2020 Available online: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive/ [Google Scholar]
- 54.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., Bonora S., Calcagno A., Cecchi I., Cinnirella G., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin. Exp. Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 55.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., Bruzzi P., Boni F., Braglia L., Turrà C., et al. Effect of Tocilizumab vs. Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babon J.J., Lucet I.S., Murphy J.M., Nicola N.A., Varghese L.N. The molecular regulation of Janus kinase (JAK) activation. Biochem. J. 2014;462:1–13. doi: 10.1042/BJ20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bousoik E., Montazeri Aliabadi H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018;8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Meng F., Huang L., Wang N., Zhou X., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A., et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020:5. doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., Yang L., Fu S., Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020;81:318. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Z.-G., Xie S.-M., Zhang J., Zheng F., Jiang D.-X., Li K.-Y., Liu L.-H., Cai C.-L., Zhang L. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. 2020 Preprints. [Google Scholar]
- 64.Shao Z., Feng Y., Zhong L., Xie Q., Lei M., Liu Z., Wang C., Ji J., Liu H., Gu Z., et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: A multicenter retrospective cohort study. Clin. Transl. Immunol. 2020:9. doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., Grazzini G. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rojas M., Rodriguez Y., Monsalve D.M., Acrosta-Ampudia Y., Camacho B., Gallo J.E., Tojas-Villarraga A., Ramirez-Santana C., Diaz-Coronado J.C., Maniriqu R., et al. Convalescent plasma in COVID-19: Possible mechanisms of action. Autoimmun. Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pathak E.B. Convalescent plasma is ineffective for COVID-19. BMJ. 2020:371. doi: 10.1136/bmj.m4072. [DOI] [PubMed] [Google Scholar]
- 68.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy C., Giunta D.H., Pérez L.G., Gamarnik A.V., et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N. Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., et al. Early High-Titer Plasma Therapy to Prevent Severe COVID-19 in Older Adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salazar E., Christensen P.A., Graviss E.A., Nguyen D.T., Castillo B., Chen J., Lopez B.V., Eagar T.N., Yi X., Zhao P., et al. Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am. J. Pathol. 2020;190:2290–2303. doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khamis F., Al-Zakwani I., Al-Hashmi S., Al-Dowaiki S., Al-Bahrani M., Pandak N., Al-Khalili H.M.Z. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int. J. Infect. Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gharbharan A., Jordans C.C.E., Geurtsvankessel K.G., den Hollander G.J., Femke K.F.P.N., Mollema F.P.N., Stalenhoef-Schukken M.J.E., Dofferhoff A., Ludwig I., Koster A., et al. Convalescent plasma for COVID-19: A randomized clinical trial. medRxiv. :2020. doi: 10.1038/s41467-021-23469-2. [DOI] [Google Scholar]
- 74.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial) BMJ. 2020 doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joyner M.J., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., Wiggins C.C., Bruno K.A., Klompas A.M., Lesser E.R., et al. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv. 2020 doi: 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 76.Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F., Rodriguez D., Tandon P., Bassily-Marcus A., Bander J., et al. Convalescent plasma treatment of severe COVID-19: A propensity score–matched control study. Nat. Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q., Wang Y., Qi C., Shen L., Li J. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020;92:540–545. doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alunno A., Najm A., Machado P.M., Bertheussen H., Burmester G.R., Carubbi F., Marco G.D., Giacomelli R., Hermine O., Isaacs J.D., et al. EULAR points to consider on pathophysiology and use of immunomodulatory therapies in COVID-19. Ann. Rheum. Dis. 2021;80:698–706. doi: 10.1136/annrheumdis-2020-219724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarkar K., Sil P.C., Nabavi S.F., Berindan-Neagoe I., Cismaru C.A., Nabavi S.M., Habtemariam S. Possible Targets and Therapies of SARS-CoV-2 Infection. Mini Rev. Med. Chem. 2020;20:1900–1907. doi: 10.2174/1389557520666200807131855. [DOI] [PubMed] [Google Scholar]
- 80.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository.