Abstract
Surprisingly small peptide motifs can confer critical biological functions. One example is the WRPW tetrapeptide present in the Hairy family of transcriptional repressors, which mediates recruitment of the Groucho (Gro) corepressor to target promoters. We recently showed that Engrailed (En) is another repressor that requires association with Gro for its function. En lacks a WRPW motif; instead, it contains another short conserved sequence, the En homology region 1 (eh1)/GEH motif, that is likely to play a role in tethering Gro to the promoter. Here, we characterize a repressor domain from the Goosecoid (Gsc) developmental regulator that includes an eh1/GEH-like motif. We demonstrate that this domain (GscR) mediates efficient repression in Drosophila blastoderm embryos and that repression by GscR requires Gro function. GscR and Gro interact in vitro, and the eh1/GEH motif is necessary and sufficient for the interaction and for in vivo repression. Because WRPW- and eh1/GEH-like motifs are present in different proteins and in many organisms, the results suggest that interactions between short peptides and Gro represent a widespread mechanism of repression. Finally, we investigate whether Gro is part of a stable multiprotein complex in the nucleus. Our results indicate that Gro does not form stable associations with other proteins but that it may be able to assemble into homomultimeric complexes.
In recent years, it has become clear that transcriptional repression is used to regulate many aspects of development and cell differentiation. However, considerably more is understood about the mechanisms and factors that activate gene expression than on how repressors work. This is particularly true for a major class of repressors (so-called active repressors) that bind DNA regulatory sites and are thought to inhibit expression via protein-protein interactions with other factors at the promoter (reviewed in reference 29). Some active repressors can directly target components of the basal transcription machinery (52, 62). However, others are unable to repress transcription by themselves and need to recruit to the promoter accessory proteins (corepressors) that in turn effect repression. Corepressors are likely to act by a variety of mechanisms, including the modulation of chromatin organization mediated by histone deacetylation (reviewed in reference 27).
We have been studying the function and mechanism of action of the Hairy-related family of active repressors in Drosophila (44, 47, 63). These include the Hairy protein, a regulator of embryonic segmentation, and the Deadpan (Dpn) and Enhancer-of-split factors, which act during sex determination and neurogenesis, respectively (8, 16, 17, 37, 38, 50, 54, 68). Like most transcription factors, Hairy-related proteins have a modular structure: they contain a DNA-binding domain of the basic helix-loop-helix class and a separable repressor domain directly involved in mediating repression. This repressor domain includes a C-terminal tetrapeptide (WRPW) which is necessary for repression in vivo and can impose repressor activity on a heterologous DNA-binding domain in cultured cells (15, 23, 65).
The WRPW domain mediates repression via another protein, Groucho (Gro), a maternally contributed factor which contains multiple WD repeats but lacks a known DNA-binding domain (17, 30, 47). The WRPW motif binds specifically to Gro in vitro, and in vivo repression by this motif requires the presence of Gro (23, 33, 47). Thus, Gro behaves as a corepressor that is recruited to target promoters by interactions with the WRPW sequence. Once at the promoter, Gro mediates repression by an as yet unknown mechanism.
Recently, other repressors unrelated to Hairy, such as the homeodomain factor Engrailed (En) and the Rel domain protein Dorsal, have been shown to act via Gro (21, 33). Thus, an En repressor domain (EnR) binds to Gro in vitro, and repression by EnR requires Gro activity in vivo. Interaction with Gro and repressor activity requires a short (7- to 15-amino-acid) conserved sequence in En (En homology region 1 [eh1]; [33, 57]). This sequence does not have similarities with the WRPW motif, suggesting that Gro is a common effector for different classes of repressor domains.
The above studies indicate that the WRPW motif is necessary and sufficient for repression and interaction with Gro (15, 23, 33, 47, 65). The eh1 element is also necessary for these activities (33, 57), but it is not known whether it can act independently of other repressor sequences. The possibility that small protein motifs are sufficient to mediate repression is interesting because most repressor domains examined to date are relatively large, and little is known about their sequence requirements (reviewed in reference 29).
Protein motifs similar to the eh1 sequence are also present in other developmental regulators such as Goosecoid (Gsc), a homeodomain transcription factor. Gsc function has been mainly analyzed in Xenopus embryos, where it is expressed in the organizer region and contributes to specification of the prechordal plate (9, 11; reviewed in reference 18). Drosophila Gsc is implicated in formation of the somatogastric nervous system (26, 28). In this report, we present functional analyses of a Gsc domain (hereafter referred to as GscR) containing an eh1-like element (also known as the Gsc-En homology [GEH] element). We show that GscR mediates effective repression in Drosophila blastoderm embryos and that its activity depends on the eh1/GEH element. In addition, GscR requires Gro for repression in vivo, and the two proteins interact in vitro via the eh1/GEH motif. Evidence is presented that the eh1/GEH element is sufficient for repression and interaction with Gro. We also investigated the oligomeric status of Gro in the nucleus. Our results suggest that Gro is not recruited for repression as a preassembled complex with other proteins. We discuss the implications of these results in terms of the role of protein motifs in transcriptional regulation.
MATERIALS AND METHODS
DNA constructs.
Plasmid manipulations were carried out according to standard procedures (4, 51). Sequences encoding the HairyGsc derivative were assembled in pBluescript (Stratagene) by cloning a PCR fragment encoding a Drosophila Gsc domain (amino acids 102 to 216) as a BamHI-XbaI fragment downstream of the unique BamHI site in the hairy (h) cDNA. HairyGscΔGEH was made similarly, using as starting material for PCR a plasmid (kind gift of C. Mailhos and C. Desplan) in which the sequence corresponding to amino acids 117 to 118 of Gsc had been mutated to produce a BamHI site. As a result, Hairy1-268 was fused to a Gsc domain comprising amino acids 119 to 216 and lacking the eh1/GEH motif. To generate HairyGEH-17, a synthetic BamHI-XbaI linker encoding the Gsc eh1/GEH core element and its immediate flanking residues (amino acids 106 to 122) was cloned downstream of the BamHI site in h. HairyGEH-9, HairyGEH-9m, and HairyWRPW were made by following the same strategy and using linkers encoding the sequences LFTIDSILG, LETIDSILG, and GGQPWRPW, respectively.
Plasmids for fly transformation were made by recovering fusion sequences as BstEII-XbaI fragments and cloning them into a pCaSpeR4 plasmid carrying the hunchback (hb) promoter and h 3′ untranslated sequences (see references 33 and 46).
Expression vectors for glutathione S-transferase (GST) fusions were made by cloning in frame the relevant Hairy-derived sequences into pZEX, a modification of pGEX-2T (55) containing the following polylinker cloning sites: EcoRI, SmaI, BamHI, and XhoI. The GST-Hairy, GST-Hairy1-286, and pET-Gro plasmids were described previously (33, 47).
Additional details on the construction of the plasmids and the sequences of the primers and linkers used in the cloning are available on request.
Transgenic flies.
Germ line transformation of hb constructs was performed as described previously (58) by DNA injection into y w embryos and selecting for rescue of w eyes. In general, two or more independent lines were analyzed for each construct. Insertions on the X chromosome were maintained in males by using an attached-X chromosome [C(1)M3]; autosomal insertions were kept as unbalanced stocks selecting for transformant males and nontransformant females. To analyze the effects of hb-hgsc on gro embryos, mosaic gro females (see below) were crossed to males carrying the hb construct on the X chromosome, so that all gro female embryos inherit the transgene. A similar strategy was used to examine the effects of the inactive hb-hgscΔGEH transgene in a wild-type background.
Germ line clones and embryo analysis.
Embryos deprived of maternal gro function were generated using the strong groE48 allele in combination with the ovoD-FLP-FRT system (12). In this system, all embryos derive from homozygous clones in the female germ line that have lost the dominant sterile mutation ovoD1. Briefly, males of the genotype hs-FLP1/Y; FRT[82B] ovoD1/Sb were crossed to FRT[82B] groE48/TM3 females, and 1- to 3-day-old progeny were heat shocked daily for 4 h at 37°C for the following 3 to 4 days. Eclosed hs-FLP1/+; FRT[82B] ovoD1/FRT[82B] groE48 virgin females (carrying homozygous groE48 clones) were crossed to males carrying the hb constructs. The progeny of these crosses was examined to confirm the expected lethality of all embryos laid due to the lack of maternal gro function.
For Sex-lethal (Sxl) stainings, embryos were dechorionated 130 to 190 min after egg laying, fixed for 12 to 15 min in heptane–4% formaldehyde–phosphate-buffered saline, and stained with a monoclonal antibody specific for the active form of Sxl derived from the early Sxl promoter (10). Signals were detected by using secondary antibodies coupled to alkaline phosphatase (Jackson Immunoresearch Laboratories); embryos were mounted in methacrylate (JB-4; Polyscience) and photographed under Nomarski optics.
In vitro binding assays.
GST fusions were expressed in Escherichia coli as described previously (47), using the protease-deficient strain SRP84 (gift of C. Higgins). Binding assays were performed by mixing equal amounts of fusion proteins (a total of 30 μl of glutathione-Sepharose beads normalized with beads from a blank bacterial extract), 20 to 30 μl of 35S-labeled Gro protein synthesized by using the TNT coupled rabbit reticulocyte lysate system (Promega), and 180 μl of binding buffer (20 mM HEPES-KOH [pH 7.9], 50 mM KCl, 2.5 mM MgCl2, 10% glycerol, 1 mM dithiothreitol, 0.2% Nonidet P-40 [NP-40]) supplemented with 3 μl of rabbit serum and 3 μl of a 100 mM phenylmethylsulfonyl fluoride stock. Binding reaction mixtures were rolled overnight at 4°C and rinsed four times with 1 ml of radioimmunoprecipitation assay buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.2% NP-40) at room temperature. The beads were boiled for 1 min in sample buffer, and aliquots were examined by electrophoresis, followed by Coomassie staining to confirm the integrity of GST fusions and autoradiography to detect bound Gro protein.
Analysis of embryonic Gro protein.
All protein procedures were at 4°C or on ice. Nuclear extracts were prepared from 0- to 12-h embryos as described previously (66). The final extract had a total protein concentration of about 18 mg/ml and was in HEMG.1 buffer (25 mM HEPES-KOH [pH 7.6], 0.1 mM EDTA, 12.5 mM MgCl2, 10% glycerol) containing 100 mM KCl, 0.01% NP-40, 1.5 mM dithiothreitol, 1 mM sodium metabisulfide, 0.2 mM aminoethylbenzenesulfonyl fluoride, leupeptin (2 μg/ml), and pepstatin (0.7 μg/ml). For glycerol gradient sedimentation, 100 μl of extract was loaded onto a 14-ml 15 to 35% glycerol gradient in HEMG.1 buffer. Fractions were collected after centrifugation of the gradients in an SW40 rotor for 17 h at 35,000 rpm. The molecular mass markers bovine serum albumin (BSA), aldolase, thyroglobulin, and dextran blue (Pharmacia) were sedimented in parallel gradients. Profiles of the standards were determined by the method of Bradford and by Coomassie staining. The gradient of crude nuclear extract was analyzed by protein immunoblotting with antibodies directed against Gro, Drosophila TAFII80, and Brahma.
Gel filtration analysis was performed on a Pharmacia HIPrep 16/60 S-300 Sephacryl column equilibrated and developed with HEMG.1 buffer on a Biologic HR system (Bio-Rad). The column was calibrated with native protein standards according to instructions provided by the supplier (Pharmacia). Either 250 μl of Drosophila nuclear extract or about 40 μg of recombinant Flag-tagged Gro (kindly provided by Katerina Katsani) purified from Spodoptera frugiperda SF9 cells infected with recombinant baculoviruses was loaded on the S-300 column. Fractions of 1 ml were collected throughout the runs, and 10 μl of each fraction was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western immunoblotting with antibodies directed against Gro.
RESULTS
The eh1/GEH motif in Gsc mediates Gro-dependent repression.
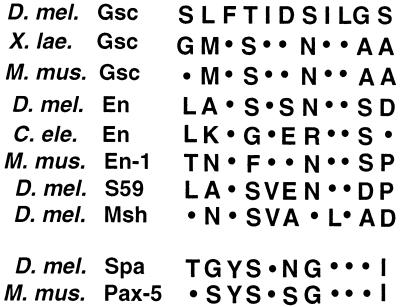
All known Gsc proteins contain a conserved 7- to 15-amino-acid eh1/GEH motif within the N-terminal half of the protein. This motif is present in several classes of homeodomain proteins and contains a highly conserved core of seven amino acids flanked by a variable number of residues that show weak homology within protein families (57). The eh1/GEH element has been shown to bind Gro and to be important for repression by the EnR in Drosophila embryos (33, 57). Figure 1 shows a comparison of the eh1/GEH core sequences present in selected proteins from different organisms.
FIG. 1.
Alignment of eh1/GEH and octapeptide motifs of different homeodomain proteins from Drosophila melanogaster (D. mel.), Xenopus laevis (X. lae.), mouse (Mus musculus [M. mus.]), and Caenorhabditis elegans (C. ele.). S59 is a protein expressed in muscle precursor cells (20), and Msh is involved in patterning the neuroectoderm and embryonic muscles (14, 32, 40). Note the presence of a strongly conserved seven-amino-acid core which always starts with a Phe residue (position 3 in the alignment). This Phe residue is not conserved in a related motif known as the octapeptide (reviewed in reference 43; see Discussion). Two octapeptide sequences from the Sparkling (Spa [25]) and Pax-5 (2) proteins are shown for comparison. See references 43 and 57 for more detailed sequence alignments.
We analyzed the transcriptional regulatory function of the eh1/GEH motif in Drosophila embryos, using an assay based on the regulation of the Sxl gene (46). Sxl is a key regulator of sex determination and dosage compensation whose transcription at the blastoderm stage occurs only in female embryos (7, 36). This control is partly dependent on the Hairy-related factor Dpn, which acts as a negative regulator of Sxl and ensures that its expression is not initiated in males (6, 68). Ectopic expression of Hairy at the time of sex determination mimics the negative effect of Dpn and leads to inappropriate repression of Sxl. Thus, premature Hairy expression in the anterior half of blastoderm embryos, driven by the hb gap gene promoter, represses Sxl in the anterior of female embryos and causes female-specific lethality (46). Repression by the hb-h transgene is Gro dependent, as it does not occur in embryos deprived of maternal Gro function (33).
We have previously shown that substitution of the C-terminal domain of Hairy by alternative repressor domains from other proteins generates chimeric Hairy derivatives that repress Sxl when expressed from the hb promoter (33). This repression is either dependent or independent of Gro, according to the chosen repressor domain. Conversely, a Hairy fusion containing the viral VP16 activation domain causes ectopic activation of Sxl in male embryos (34), showing that the hb-h assay is a useful strategy to test the activity of a transcriptional regulatory domain in vivo.
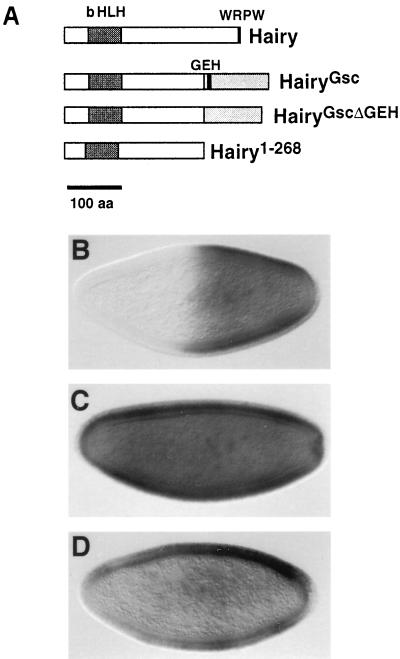
We used the hb-h assay to examine the function of a 114-amino-acid Gsc domain (GscR; see Materials and Methods) containing the eh1/GEH motif (Fig. 2A). We replaced the C-terminal 69 amino acids of Hairy by GscR and expressed the resulting chimera (HairyGsc) under the control of the hb promoter. hb-hgsc leads to efficient repression of Sxl in the anterior of female embryos (Fig. 2B). This repression depends on GscR, because the Hairy moiety lacking the C-terminal 69 amino acids (Hairy1-268) (and therefore the Gro-binding WRPW motif) is inactive in the assay (15, 33). In addition, hb-hgsc causes high levels of female lethality (>80% in several independent lines [Table 1]), as it is the case for hb-h (46). Thus, GscR behaves as a potent repression domain in blastoderm embryos.
FIG. 2.
GscR directs Gro-dependent repression via the eh1/GEH motif. (A) Diagram of Hairy derivatives expressed under the control of the hb promoter. The Gsc domains are represented by grey boxes; the position of the eh1/GEH (GEH) motif is indicated by a black box. (B to D) Effects on Sxl expression of hb-hgsc (B and C) and hb-hgscΔGEH (D) in wild-type (B and D) and gro mutant (C) embryos. Repression by HairyGsc requires the eh1/GEH motif and endogenous Gro activity.
TABLE 1.
Effects of Hairy derivatives on female viability
| Construct | No. of progeny from the cross +/+ females × hb-/Y males
|
|
|---|---|---|
| Females | Males | |
| hb-hgsc | 0 | 59 |
| hb-hgscΔGEH | 57 | 62 |
To test if the eh1/GEH motif is required for repression by GscR, we made a HairyGsc derivative containing a 15-amino-acid deletion of this motif (HairyGscΔGEH [Fig. 2A]). Expression of this protein under the control of the hb promoter causes neither efficient repression of Sxl (Fig. 2D) nor significant levels of female lethality (Table 1), showing that the eh1/GEH element is important for repression by GscR.
We also examined whether the activity of GscR depends on Gro. To this end, we assayed the effects of HairyGsc in embryos deprived of maternal gro function. Since homozygous gro females are lethal, these embryos were obtained by using the ovoD-FLP-FRT system (12), which generates clones of homozygous mutant cells in the germ line of heterozygous females (see Materials and Methods). As shown in Fig. 2C, hb-hgsc is unable to repress Sxl in groE48 embryos. In contrast, Hairy chimeras containing repressor domains from the Drosophila Snail, Even-skipped, and Krüppel regulators do repress Sxl in those embryos (33). These results indicate that GscR, and its eh1/GEH motif, act through Gro in vivo.
GscR interacts with Gro in vitro via the eh1/GEH element.
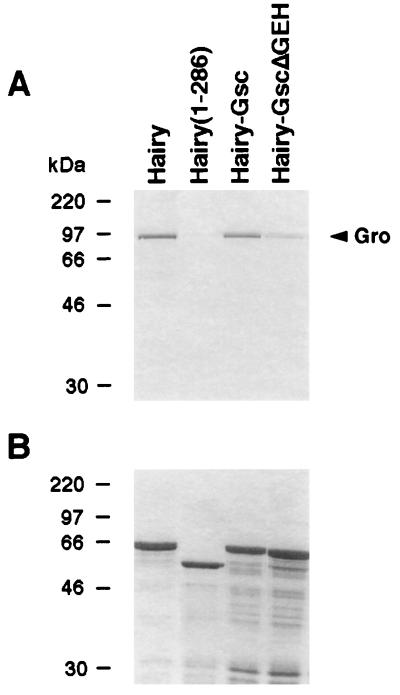
The preceding experiments indicate that GscR is a Gro-dependent repressor domain. To see whether this involves a physical interaction between GscR and Gro, we examined the ability of HairyGsc to bind to Gro in vitro. HairyGsc was expressed in bacteria as a GST fusion, purified with glutathione-Sepharose beads, and incubated with radiolabeled Gro protein. As shown in Fig. 3, strong binding between the two proteins is detected, whereas a Hairy truncation lacking GscR does not bind Gro. HairyGscΔGEH, lacking the eh1/GEH motif, binds much less effectively, showing that this motif plays a direct role in the interaction with Gro.
FIG. 3.
Binding of Hairy derivatives to Gro in vitro. (A) GST-Hairy fusions were immobilized on glutathione-Sepharose beads and incubated with 35S-labeled Gro protein. After the beads were washed, the bound Gro protein was detected by SDS-PAGE and autoradiography. Hairy and HairyGsc bind Gro with high affinity. In contrast, little or no binding is detected with Hairy1-286 (which lacks the C-terminal 51 amino acids of the protein) and the HairyGscΔGEH chimera. (B) Coomassie staining of the gel shown in panel A, demonstrating the integrity of the different GST fusions after the binding reaction.
The eh1/GEH and WRPW motifs are sufficient for Gro-mediated repression.
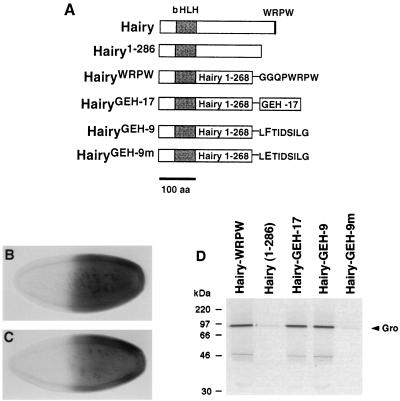
Short conserved protein motifs are necessary for Gro binding and Gro-mediated repression. We examined whether they are also sufficient for such binding by fusing them to the Hairy1-268 truncation. First, we tested if the WRPW tetrapeptide is sufficient for repression in the hb assay, by examining the activity of HairyWRPW, a fusion of Hairy1-268 with the six C-terminal amino acids of Hairy, including the WRPW sequence (Fig. 4A and Materials and Methods). hb-hWRPW has effects indistinguishable from those of hb-h: it causes high levels of female lethality (>90% in most lines) and clear repression of Sxl (Fig. 4B and data not shown). These results support the work of Fisher et al. (23), who showed that the WRPW tetrapeptide is sufficient to bind Gro in vitro and acts as a portable repressor domain in transfected cells. Our results suggest that this motif is also sufficient for repression in the embryo (see Discussion).
FIG. 4.
The WRPW and eh1/GEH motifs are sufficient for repression in vivo and binding to Gro in vitro. (A) Diagram of the HairyWRPW and HairyGEH derivatives. (B and C) Expression of HairyWRPW (B) and HairyGEH-17 (C) under the control of the hb promoter causes efficient repression of Sxl. (D) Binding of GST-Hairy derivatives to Gro in vitro. HairyWRPW, HairyGEH-17, and HairyGEH-9 bind to Gro with similar efficiencies; in contrast, HairyGEH-9m, which carries a mutation in a conserved Phe residue within the eh1/GEH element, does not interact with Gro.
We next tested whether the eh1/GEH element in GscR is sufficient to mediate transcriptional repression. We fused Hairy1-268 to a 17-amino-acid sequence including the Gsc eh1/GEH motif and its immediate flanking residues (Fig. 4A; see Materials and Methods). Expression of this chimera (HairyGEH-17) driven by the hb promoter leads to variable levels of female lethality depending on the line tested, but at its strongest, this lethality is >90% (data not shown). In addition, the construct causes efficient repression of Sxl at the anterior of female embryos (Fig. 4C). These results argue that the eh1/GEH sequence from Gsc acts as a minimal repressor domain.
The ability of HairyGEH and HairyWRPW to repress Sxl predicts that both proteins should be able to associate with Gro. Indeed, GST fusions of these derivatives bind Gro in vitro with affinities similar to that of GST-Hairy (Fig. 4D), suggesting that the GEH and WRPW sequences are not only necessary but also sufficient for binding to Gro (see also reference 23).
We also tested a shorter eh1/GEH peptide from Gsc for binding to Gro in vitro. We made a GST-Hairy derivative containing a nine-amino-acid eh1/GEH sequence (LFTIDSILG [Fig. 1]) at its C terminus. This fusion protein, HairyGEH-9, binds to Gro as efficiently as the HairyGEH-17 and HairyGsc chimeras (Fig. 4D), indicating that the nine-amino-acid motif is sufficient for the interaction with Gro. Finally, we assayed an equivalent HairyGEH derivative carrying a mutation in the highly conserved Phe residue of the eh1/GEH motif (HairyGEH-9m [Fig. 1]). The same mutation (Phe to Glu) has been shown to cause a strong reduction in the ability of En to repress transcription in vivo (57). As shown in Fig. 4D, the mutation largely abolishes the binding to Gro. Taken together, the results suggest that this Phe residue is important for recruitment of Gro to target promoters.
Gro does not form a stable complex with other proteins in the nucleus.
As a corepressor, Gro is expected to associate with other proteins. These interactions could be relatively transient, occurring only at target promoters, or may involve stable complexes which are preassembled before Gro is recruited to promoters. The ability to form stable nuclear complexes is a typical feature of proteins of the general transcriptional machinery and other transcriptional cofactors. Recently, several corepressors have been shown to be part of multimeric complexes that survive methods of biochemical purification. One of the best-characterized examples is the yeast Tup1 protein. Tup1 is a WD-containing protein, like Gro, and exerts its function as part of a multimeric complex consisting of several molecules of Tup1 and the Ssn6 protein (35, 49, 64, 67). This complex is thought to be recruited to target promoters by specific repressors such as the α2 regulator (35), presumably through relatively transient interactions. Thus, it is possible that repressors such as Gsc and Hairy do not recruit a single molecule of Gro to target genes but, instead, recruit a Gro-containing complex. Indeed, recent studies have suggested that Gro is assembled into oligomeric complexes of various sizes (e.g., ∼170 and 240 kDa [45]).
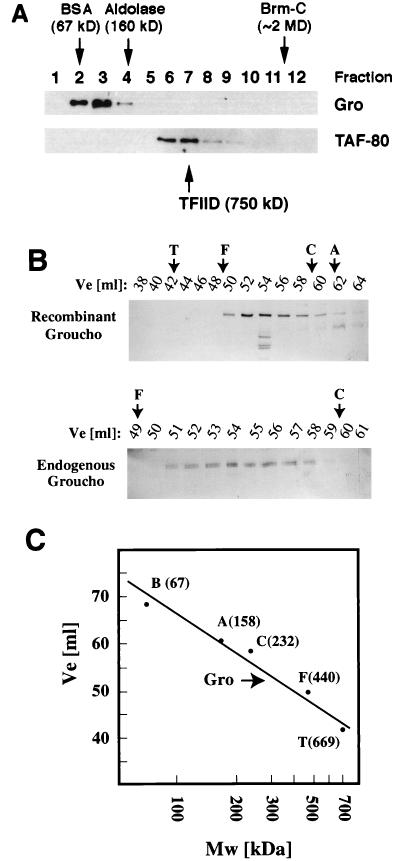
We examined the issue of complex formation by Gro by determining the apparent size of the native protein during glycerol gradient sedimentation. Crude nuclear extracts were prepared from 0- to 12-h Drosophila embryos, and Gro was readily detected in these preparations with a monoclonal antibody directed against this protein (17). The extracts were centrifuged through a glycerol gradient under very mild conditions known to favor complex formation, and different fractions were collected and analyzed for the presence of Gro protein by Western blotting (see Materials and Methods). Gro migrates with an apparent molecular mass of ∼80 kDa, similar to its predicted molecular mass (Fig. 5A). In contrast, analysis of the same fractions with a monoclonal antibody against the TAF-80 transcription factor (another WD protein, similar in size to Gro, which is normally part of the TFIID complex [22, 39]) shows that this 80-kDa protein migrates with an apparent mass of about 600 to 700 kDa, compatible with that of TFIID. Likewise, the 180-kDa Brahma protein, a member of the SWI/SNF family of proteins that form large multiprotein complexes, migrates as a ∼2-MDa complex, in agreement with previously published results (Fig. 5A and data not shown [see reference 19]). Thus, the conditions used in these experiments do not disrupt known multiprotein complexes. Nevertheless, Gro behaves as a free monomer and, unlike Tup1, does not appear to be part of a stable multiprotein complex.
FIG. 5.
Gro does not form oligomeric complexes. Embryo nuclear extracts were centrifuged through a glycerol gradient, and the different fractions collected were examined for the presence of Gro and TAF-80 proteins. The migration of markers (BSA and aldolase) and known endogenous complexes (TFIID and the Brahma complex [Brm-C]) is depicted by arrows. Gro migrates with an apparent size similar to its predicted molecular mass (80 kDa). In contrast, TAF-80 (which also has a molecular mass of 80 kDa) migrates with an apparent mass of ∼600 to 700 kDa because it forms part of the TFIID complex. (B) Gel filtration analysis of endogenous and purified recombinant Gro by Sephacryl S-300 chromatography. Fractions collected were examined for the presence of Gro by SDS-PAGE and Western blot analysis with an antibody directed against Gro. The elution volume (Ve) is indicated. Only the gels that contained Gro are shown. Positions of the protein standards are indicated as follows: T, thyroglobulin; F, ferritin; C, catalase; A, aldolase, B, BSA. (C) The position of the approximate Gro peak is indicated on the calibration curve.
To investigate this issue further, we also analyzed Gro by gel filtration chromatography (Fig. 5B). In this assay, endogenous Gro present in nuclear extracts migrates between the 232- and 440-kDa standards, showing an apparent molecular mass significantly larger than its monomeric molecular mass. Purified, recombinant Gro behaves similarly (Fig. 5), raising the possibility that Gro is not a globular protein but, instead, has an extended conformation. Alternatively, Gro may form multimers; the mobility on the size exclusion column is consistent with the presence of a Gro tetramer (Fig. 5C; see Discussion). In any case, Gro in the nuclear extracts is clearly not part of a large stable complex with other factors. Thus, potential functional interactions of Gro with other proteins in the nucleus are likely to be transient.
DISCUSSION
An increasing number of studies are currently addressing the mechanism by which active repressors inhibit gene expression. Several repressor domains, distinct from DNA-binding regions, have been identified in different proteins, but their molecular targets are usually not yet known. In this report, we characterize a conserved repressor domain from the Gsc developmental regulator (the eh1/GEH motif) and show that its activity depends on association with the Gro corepressor. We find that the eh1/GEH element is not only necessary but also sufficient to mediate binding to Gro and transcriptional repression. This functional association appears to represent a widespread mechanism of repression, since several other regulators (e.g., the Drosophila En, S59, and Msh proteins [Fig. 1 and reference 57]) contain versions of the eh1/GEH motif. Consistent with this idea, Drosophila Gro is ubiquitously expressed and appears to be involved in many developmental processes. In addition, these interactions may be highly conserved during evolution, since a wide variety of organisms, including nematodes and vertebrates, have both eh1/GEH-containing factors and Gro homologues (42, 48, 53, 57, 59), and the eh1/GEH domain is necessary for Gsc rescue of UV-ventralized Xenopus embryos (41). Furthermore, the Drosophila EnR is functional in vertebrate cell and embryonic systems and on a wide variety of promoters (5, 13).
Association of Gro with small repressor motifs.
Previous work showed that the WRPW motif is sufficient to mediate significant (three- to fourfold) repression in cultured cells and to interact with Gro in vitro (23). A WRPW-like (WRPY) motif present in the Drosophila Runt protein, a regulator of segmentation and sex determination, has been shown to be important for repression in vivo and sufficient for binding to Gro in yeast (3). Our experiments indicate that the WRPW motif is able to repress the Sxl promoter completely (Fig. 4B), and we present evidence that the eh1/GEH motif is sufficient for repression in vivo and binding to Gro in vitro (Fig. 4C and D). Thus, the WRP(W/Y) and eh1/GEH elements represent minimal repressor motifs that can recruit Gro independently of other protein domains. The chimeric constructs that we have used contain the N-terminal portion of Hairy (Hairy1-268) which includes the basic helix-loop-helix region and the so-called Orange domain. Dawson et al. (15) suggested that these domains can mediate a separable mode of repression that could contribute to the ability of the WRPW and eh1/GEH motifs to repress Sxl. However, only the latter motifs appear capable to associate with Gro (references 23, 33, and 47; this report), arguing that this activity is independent of other protein domains. Indeed, recent experiments with the intracellular portion of the Notch receptor argue that this tetrapeptide can act as an autonomous repressor element (1). This autonomous function is also evident in the case of the GEH motif, which can confer repression on protein sequences (Hairy1-268) to which it is not normally associated.
The eh1/GEH sequence that we have tested in vivo is 17 amino acids long and includes a 7-amino-acid core and ∼10 flanking residues that are partly conserved among Gsc proteins but do not show significant similarities with the equivalent region of other eh1/GEH-containing proteins. Thus, the seven-amino-acid core is likely to be the active sequence capable of binding to Gro. Indeed, we find that a nine-amino-acid eh1/GEH sequence mediates binding to Gro in vitro and that a highly conserved Phe residue in this sequence which is essential for repression in vivo is also required for the interaction with Gro in vitro. Interestingly, this Phe residue distinguishes the eh1/GEH motif from a related sequence known as the octapeptide, which is present in several paired-domain and homeodomain proteins (reviewed in reference 43) (Fig. 1). A domain containing an octapeptide motif behaves as a weak repressor domain in the Sxl assay (unpublished data), but proteins containing this motif have not yet been shown to act normally as active repressors. Thus, the eh1/GEH and octapeptide motifs may have a common ancestry but are likely to interact with different factors.
The ability of the eh1/GEH and WRPW motifs to mediate specific protein-protein interactions is striking, as it has often been assumed that such interactions depend on larger domains. However, recent studies have provided other examples of very small peptides that direct critical regulatory protein-protein associations. Thus, a five-amino-acid motif present in several transcriptional cofactors such as RIP-140 and CBP is necessary and sufficient for binding of these proteins to nuclear receptors (31, 60). Similarly, the evolutionarily conserved cofactor HCF binds several regulatory proteins which share only a tetrapeptide motif essential for those interactions (24). These results argue that characteristic short peptide motifs play more widespread roles in mediating physical associations between proteins than has been previously suspected.
Many repressor domains identified so far are considerably larger (>50 amino acids) than the eh1/GEH and WRPW motifs used in our experiments. However, most of these domains have not been dissected in detail, and it is difficult to compare their function with that of the small Gro-dependent motifs. For example, some repressor domains contain relatively long stretches of Ala residues whose role is not understood (29). Indeed, the initial GscR used in our experiments (Fig. 2) also includes two such Ala-rich sequences, but these seem to play a relatively minor role in repression compared with the eh1/GEH motif (Fig. 2 and 4; see also reference 57). Thus, it is possible that detailed analyses of large repressor domains will reveal the presence of short subdomains bearing most of the activity.
Gro is not present as a stable complex with other proteins.
An important aspect of the function of Gro is whether it forms stable nuclear complexes with other corepressor proteins. The Tup1 protein, which has served as a paradigm for the role of Gro, appears to form a large multimeric complex in the yeast nucleus (49, 64, 67). However, our results suggest that the great majority of Gro protein is not stably associated with other repressor proteins: Gro protein from crude nuclear extracts migrates through glycerol gradients with an apparent size compatible with its monomeric molecular mass (Fig. 5).
During gel filtration, endogenous and recombinant Gro migrate with very similar mobilities, again indicating that Gro is not stably associated with other proteins. However, its mobility during gel filtration suggests a molecular mass of around 350 kDa. This result could be due to an extended protein conformation or to formation of a weak homomultimeric complex that is stable to gel filtration chromatography but not to glycerol gradient sedimentation. The first possibility is supported by the glycerol gradient experiment, which used mild conditions of extraction and sedimentation that appear unlikely to disrupt structural complexes. Indeed, our positive controls demonstrate the integrity of complexes formed by TAF-80, Brahma (Fig. 5A), and several other protein complexes (data not shown) under identical conditions, and Tup1 complexes survive similar sedimentation techniques (49, 64). However, the second possibility is consistent with recently published protein cross-linking experiments that suggest Gro oligomerization, possibly via a putative dimerization domain at the N terminus of Gro family proteins (45). Also, the Stokes radius determined by gel filtration implies a calculated axial ratio of almost 20 for a putative Gro monomer. Such an extended conformation of Gro seems unlikely and supports the notion that Gro forms a multimer that survives gel filtration chromatography but not glycerol gradient sedimentation.
We suggest that Gro is not present in a highly stable complex with other proteins in the nucleus. This would represent a functional difference from Tup1, which forms structural complexes with Ssn6 that may be critical for efficient targeting to promoters (56, 61). Instead, our results suggest that the interactions of Gro with transcriptional repressors are sufficient for its recruitment to target promoters (see reference 33 for a discussion of the role of WD repeats in this process). Additional protein-protein interactions involving Gro probably take place after its recruitment to the promoter and may involve chromatin components (45). Alternatively, once tethered by a repressor, Gro may directly bind to a component of the basal machinery to block transcription.
Conclusion.
Our data suggest that the conserved eh1/GEH element present in Gsc and other developmental regulators acts as an interaction motif that is sufficient to recruit Gro to target promoters. A similar conclusion applies for the WRP(W/Y) motifs present in Hairy-related factors and members of the Runt family (this report; references 3 and 23). Together, these results suggest that interactions between very short peptides and Gro represent ancient functional associations that have been employed repeatedly and in several contexts during evolution. Gro may be the common node in an evolutionarily conserved network of interactions that includes different classes of transcription factors and which serves to repress gene expression in a wide variety of developmental processes.
ACKNOWLEDGMENTS
We thank members of our laboratories for their support and encouragement; in particular we are indebted to S. Pinchin for her help with the experiments shown in Fig. 2 and to Katerina Katsani for the gift of purified recombinant Gro. We are also grateful to Z. Paroush for many helpful discussions, to C. Desplan, C. Mailhos, and J. Jaynes for communicating unpublished results, and to C. Mailhos and C. Desplan for Gsc plasmids.
G.J. was supported by the EC Human Capital and Mobility Programme, the Imperial Cancer Research Fund, and EMBO. This work was supported by the Imperial Cancer Research Fund and a grant from the Howard Hughes Medical Institute through the International Research Scholars Program.
REFERENCES
- 1.Adachi A, Struhl G. Nuclear access and action of Notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 2.Adams B, Dörfler P, Aguzzi A, Kozmik Z, Urbánek P, Maurer-Fogy I, Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992;6:1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- 3.Aronson B D, Fisher A L, Blechman K, Caudy M, Gergen J P. Groucho-dependent and -independent repression activities of Runt domain proteins. Mol Cell Biol. 1997;17:5581–5587. doi: 10.1128/mcb.17.9.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F J, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1987–1997. [Google Scholar]
- 5.Badiani P, Corbella P, Kioussis D, Marvel J, Weston K. Dominant interfering alleles define a role for c-Myb in T-cell development. Genes Dev. 1994;8:770–782. doi: 10.1101/gad.8.7.770. [DOI] [PubMed] [Google Scholar]
- 6.Barbash D A, Cline T W. Genetic and molecular analysis of the autosomal component of the primary sex determination signal of Drosophila melanogaster. Genetics. 1995;141:1451–1471. doi: 10.1093/genetics/141.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell L R, Maine E M, Schedl P, Cline T W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;65:229–239. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 8.Bier E, Vässin H, Younger-Shepherd S, Jan L Y, Jan Y N. deadpan, an essential pan-neural gene in Drosophila, encodes a helix-loop-helix protein similar to the hairy gene product. Genes Dev. 1992;6:2137–2151. doi: 10.1101/gad.6.11.2137. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg B, Wright C V E, De Robertis E M, Cho K W Y. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991;253:194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- 10.Bopp D, Bell L R, Cline T W, Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991;5:403–415. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- 11.Cho K W Y, Blumberg B, Steinbeisser H, De Robertis E M. Molecular nature of the Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou T B, Noll E, Perrimon N. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development. 1993;119:1359–1369. doi: 10.1242/dev.119.4.1359. [DOI] [PubMed] [Google Scholar]
- 13.Conlon F L, Sedgwick S G, Weston K M, Smith J C. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development. 1996;122:2427–2435. doi: 10.1242/dev.122.8.2427. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessio M, Frasch M. msh may play a conserved role in dorsoventral patterning of the neuroectoderm and mesoderm. Mech Dev. 1996;58:217–231. doi: 10.1016/s0925-4773(96)00583-7. [DOI] [PubMed] [Google Scholar]
- 15.Dawson S R, Turner D L, Weintraub H, Parkhurst S M. Specificity for the Hairy/Enhancer of split basic helix-loop-helix (bHLH) proteins maps outside the bHLH domain and suggests two separable modes of transcriptional repression. Mol Cell Biol. 1995;15:6923–6931. doi: 10.1128/mcb.15.12.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delidakis C, Artavanis-Tsakonas S. The Enhancer-of-split [E(spl)] locus of Drosophila encodes seven independent helix-loop-helix proteins. Proc Natl Acad Sci USA. 1992;89:8731–8735. doi: 10.1073/pnas.89.18.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delidakis C, Preiss A, Hartley D A, Artavanis-Tsakonas S. Two genetically and molecularly distinct functions involved in early neurogenesis reside within the Enhancer-of-split locus of Drosophila melanogaster. Genetics. 1991;129:803–823. doi: 10.1093/genetics/129.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Robertis E M, Faindsod A, Gont L K, Steinbeisser H. The evolution of vertebrate gastrulation. In: Akam M, Holland P, Ingham P, Wray G, editors. The evolution of developmental mechanisms. Development Supplement. England: Cambridge; 1994. pp. 117–124. [PubMed] [Google Scholar]
- 19.Dingwall A K, Beek S J, McCallum C M, Tamkun J W, Kalpana G V, Goff S P, Scott M P. The Drosophila snr1 and brm proteins are related to yeast SWI/SNF proteins and are components of a large protein complex. Mol Biol Cell. 1995;6:777–791. doi: 10.1091/mbc.6.7.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohrmann C, Azpiazu N, Frasch M. A new Drosophila homeobox gene is expressed in mesodermal precursor cells of distinct muscles during embryogenesis. Genes Dev. 1990;4:2098–2111. doi: 10.1101/gad.4.12a.2098. [DOI] [PubMed] [Google Scholar]
- 21.Dubnicoff T, Valentine S A, Chen G, Shi T, Lengyel J A, Paroush Z, Courey A J. Conversion of Dorsal from an activator to a repressor by the global corepressor Groucho. Genes Dev. 1997;11:2952–2957. doi: 10.1101/gad.11.22.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dynlacht B D, Weinzierl R O, Admon A, Tjian R. The dTAFII80 subunit of Drosophila TFIID contains β-transducin repeats. Nature. 1993;363:176–179. doi: 10.1038/363176a0. [DOI] [PubMed] [Google Scholar]
- 23.Fisher A L, Ohsako S, Caudy M. The WRPW motif of the Hairy-related basic helix-loop-helix repressor proteins acts as a four-amino-acid transcription repression and protein-protein interaction domain. Mol Cell Biol. 1996;16:2670–2677. doi: 10.1128/mcb.16.6.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freiman R N, Herr W. Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev. 1997;11:3122–3127. doi: 10.1101/gad.11.23.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu W, Noll M. The Pax2 homolog sparkling is required for development of cone and pigment cells in the Drosophila eye. Genes Dev. 1997;11:2066–2078. doi: 10.1101/gad.11.16.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goriely A, Stella M, Coffinier C, Kessler D, Mailhos C, Dessain S, Desplan C. A functional homologue of goosecoid in Drosophila. Development. 1996;122:1641–1650. doi: 10.1242/dev.122.5.1641. [DOI] [PubMed] [Google Scholar]
- 27.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 28.Hahn M, Jäckle H. Drosophila goosecoid participates in neural development but not in body axis formation. EMBO J. 1996;15:3077–3084. [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna-Rose W, Hansen U. Active repression mechanisms of eukaryotic transcription repressors. Trends Genet. 1996;12:229–234. doi: 10.1016/0168-9525(96)10022-6. [DOI] [PubMed] [Google Scholar]
- 30.Hartley D A, Preiss A, Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, Enhancer-of-split, shows homology to mammalian G-protein beta subunit. Cell. 1988;55:785–795. doi: 10.1016/0092-8674(88)90134-1. [DOI] [PubMed] [Google Scholar]
- 31.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 32.Isshiki T, Takeichi M, Nose A. The role of the msh homeobox gene during Drosophila neurogenesis: implication for the dorsoventral specification of the neuroectoderm. Development. 1997;124:3099–3109. doi: 10.1242/dev.124.16.3099. [DOI] [PubMed] [Google Scholar]
- 33.Jiménez G, Paroush Z, Ish-Horowicz D. Groucho acts as a corepressor for a subset of negative regulators, including Hairy and Engrailed. Genes Dev. 1997;11:3072–3082. doi: 10.1101/gad.11.22.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiménez G, Pinchin S M, Ish-Horowicz D. In vivo interactions of the Drosophila Hairy and Runt transcriptional repressors with target promoters. EMBO J. 1996;15:7088–7098. [PMC free article] [PubMed] [Google Scholar]
- 35.Keleher C A, Redd M J, Schultz J, Carlson M, Johnson A D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992;68:709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- 36.Keyes L N, Cline T W, Schedl P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell. 1992;68:933–943. doi: 10.1016/0092-8674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- 37.Klämbt C, Knust E, Tietze K, Campos-Ortega J A. Closely related transcripts encoded by the neurogenic gene complex Enhancer of split of Drosophila melanogaster. EMBO J. 1989;8:203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knust E, Schrons H, Grawe F, Campos-Ortega J A. Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics. 1992;132:505–518. doi: 10.1093/genetics/132.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokubo T, Gong D W, Yamashita S, Takada R, Roeder R G, Horikoshi M, Nakatani Y. Molecular cloning, expression, and characterization of the Drosophila 85-kilodalton TFIID subunit. Mol Cell Biol. 1993;13:7859–7863. doi: 10.1128/mcb.13.12.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lord P C W, Lin M H, Hales K H, Storti R V. Normal expression and the effects of ectopic expression of the Drosophila muscle segment homeobox (msh) gene suggest a role in differentiation and patterning of embryonic muscles. Dev Biol. 1995;171:627–640. doi: 10.1006/dbio.1995.1310. [DOI] [PubMed] [Google Scholar]
- 41.Mailhos C, André S, Mollereau B, Goriely A, Hemmati-Brivanlou A, Desplan C. Drosophila Goosecoid requires a conserved heptapeptide for repression of Paired-class homeoprotein activators. Development. 1998;125:937–947. doi: 10.1242/dev.125.5.937. [DOI] [PubMed] [Google Scholar]
- 42.Mallo M, Steingrimsson E, Copeland N G, Jenkins N A, Gridley T. Genomic organization, alternative polyadenylation, and chromosomal localization of Grg, a mouse gene related to the groucho transcript of the Drosophila Enhancer-of-split complex. Genomics. 1994;21:194–201. doi: 10.1006/geno.1994.1242. [DOI] [PubMed] [Google Scholar]
- 43.Noll M. Evolution and role of Pax genes. Curr Opin Gen Dev. 1993;3:595–605. doi: 10.1016/0959-437x(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 44.Ohsako S, Hyer J, Panganiban G, Oliver I, Caudy M. Hairy Function as a DNA-binding helix-loop-helix repressor of Drosophila sensory organ formation. Genes Dev. 1994;8:2743–2755. doi: 10.1101/gad.8.22.2743. [DOI] [PubMed] [Google Scholar]
- 45.Palaparti A, Baratz A, Stifani S. The Groucho/Transducin-like Enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3. J Biol Chem. 1997;272:26604–26610. doi: 10.1074/jbc.272.42.26604. [DOI] [PubMed] [Google Scholar]
- 46.Parkhurst S M, Bopp D, Ish-Horowicz D. X:A ratio, the primary sex determining signal in Drosophila, is transduced by helix-loop-helix proteins. Cell. 1990;63:1179–1191. doi: 10.1016/0092-8674(90)90414-a. [DOI] [PubMed] [Google Scholar]
- 47.Paroush Z, Finley R L J, Kidd T, Wainwright S M, Ingham P W, Brent R, Ish-Horowicz D. Groucho is required for Drosophila neurogenesis, segmentation and sex-determination, and interacts directly with Hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 48.Pflugrad A, Meir J Y-J, Barnes T M, Miller D M., III The Groucho-like transcription factor UNC-37 functions with the neural specificity gene unc-4 to govern motor neuron identity in C. elegans. Development. 1997;124:1699–1709. doi: 10.1242/dev.124.9.1699. [DOI] [PubMed] [Google Scholar]
- 49.Reed M J, Arnaud M B, Johnson A D. A complex composed of Tup1 and Ssn6 represses transcription in vitro. J Biol Chem. 1997;272:11193–11197. doi: 10.1074/jbc.272.17.11193. [DOI] [PubMed] [Google Scholar]
- 50.Rushlow C A, Hogan A, Pinchin S M, Howe K R, Lardelli M T, Ish-Horowicz D. The Drosophila hairy protein acts in both segmentation and bristle patterning and shows homology to N-myc. EMBO J. 1989;8:3095–3103. doi: 10.1002/j.1460-2075.1989.tb08461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 52.Sauer F, Fondell J D, Ohkuma Y, Roeder R G, Jäckle H. Control of transcription by Krüppel through interactions with TFIIB and TFIIE β. Nature. 1995;375:162–164. doi: 10.1038/375162a0. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt C J, Sladek T E. A rat homolog of the Drosophila Enhancer-of-split (groucho) locus lacking WD-40 repeats. J Biol Chem. 1993;268:25681–25686. [PubMed] [Google Scholar]
- 54.Schrons H, Knust E, Campos-Ortega J A. The Enhancer of split complex and adjacent genes in the 96F region of Drosophila melanogaster are required for segregation of neural and epidermal progenitor cells. Genetics. 1992;132:481–503. doi: 10.1093/genetics/132.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith D, Johnston K. Single-step purification of polypeptides expressed in E. coli as fusions with glutathione S-transferase. Gene. 1988;76:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 56.Smith R L, Reed M J, Johnson A D. The tetratricopeptide repeats of Ssn6 interact with the homeo domain of α2. Genes Dev. 1995;9:2903–2910. doi: 10.1101/gad.9.23.2903. [DOI] [PubMed] [Google Scholar]
- 57.Smith S T, Jaynes J B. A conserved region of Engrailed, shared among all En-, Gsc-, Nk1-, Nk2- and Msh-class homeoproteins, mediates active transcriptional repression in vivo. Development. 1996;122:3141–3150. doi: 10.1242/dev.122.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spradling A C. P element-mediated transformation, 175–197. In: Roberts D B, editor. Drosophila: a practical approach. Oxford, England: IRL Press; 1986. [Google Scholar]
- 59.Stifani S, Blaumueller C M, Redhead N J, Hill R E, Artavanis-Tsakonas S. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet. 1992;2:119–127. doi: 10.1038/ng1092-119. [DOI] [PubMed] [Google Scholar]
- 60.Torchia J, Rose D W, Inostroza J, Kamel Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 61.Tzamarias D, Struhl K. Functional dissection of the yeast Cyc8-Tup1 transcriptional co-repressor complex. Nature. 1994;369:758–761. doi: 10.1038/369758a0. [DOI] [PubMed] [Google Scholar]
- 62.Um M, Li C, Manley J L. The transcriptional repressor Even-skipped interacts directly with TATA-binding protein. Mol Cell Biol. 1995;15:5007–5016. doi: 10.1128/mcb.15.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Doren M, Bailey A M, Esnayra J, Ede K, Posakony J W. Negative regulation of proneural gene activity: hairy is a direct transcriptional repressor of achaete. Genes Dev. 1994;8:2729–2742. doi: 10.1101/gad.8.22.2729. [DOI] [PubMed] [Google Scholar]
- 64.Varanasi U S, Klis M, Mikesell P B, Trumbly R J. The Cyc8 (Ssn6)-Tup1 corepressor complex is composed of one Cyc8 and four Tup1 subunits. Mol Cell Biol. 1996;16:6707–6714. doi: 10.1128/mcb.16.12.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wainwright S M, Ish-Horowicz D. Point mutations in the Drosophila hairy gene demonstrate in vivo requirements for basic, helix-loop-helix, and WRPW domains. Mol Cell Biol. 1992;12:2475–2483. doi: 10.1128/mcb.12.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wampler S L, Tyree C M, Kadonaga J T. Fractionation of the general RNA polymerase II transcription factors from Drosophila embryos. J Biol Chem. 1990;265:21223–21231. [PubMed] [Google Scholar]
- 67.Williams F E, Varanasi U, Trumbly R J. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol Cell Biol. 1991;11:3307–3316. doi: 10.1128/mcb.11.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Younger-Shepherd S, Vässin H, Bier E, Jan L Y, Jan Y N. deadpan, an essential pan-neural gene encoding an HLH protein, acts as a denominator in Drosophila sex determination. Cell. 1992;70:911–922. doi: 10.1016/0092-8674(92)90242-5. [DOI] [PubMed] [Google Scholar]