Abstract
The irreversible G1 arrest in senescent human diploid fibroblasts is probably caused by inactivation of the G1 cyclin–cyclin-dependent kinase (Cdk) complexes responsible for phosphorylation of the retinoblastoma protein (pRb). We show that the Cdk inhibitor p21Sdi1,Cip1,Waf1, which accumulates progressively in aging cells, binds to and inactivates all cyclin E-Cdk2 complexes in senescent cells, whereas in young cells only p21-free Cdk2 complexes are active. Furthermore, the senescent-cell-cycle arrest occurs prior to the accumulation of the Cdk4-Cdk6 inhibitor p16Ink4a, suggesting that p21 may be sufficient for this event. Accordingly, cyclin D1-associated phosphorylation of pRb at Ser-780 is lacking even in newly senescent fibroblasts that have a low amount of p16. Instead, the cyclin D1-Cdk4 and cyclin D1-Cdk6 complexes in these cells are associated with an increased amount of p21, suggesting that p21 may be responsible for inactivation of both cyclin E- and cyclin D1-associated kinase activity at the early stage of senescence. Moreover, even in the late stage of senescence when p16 is high, cyclin D1-Cdk4 complexes are persistent, albeit reduced by ≤50% compared to young cells. We also provide new evidence that p21 may play a role in inactivation of the DNA replication factor proliferating cell nuclear antigen during early senescence. Finally, because p16 accumulates in parallel with the increases in senescence-associated β-Gal activity and cell volume that characterize the senescent phenotype, we suggest that p16 upregulation may be part of a differentiation program that is turned on in senescent cells. Since p21 decreases after senescence is achieved, this upregulation of p16 may be essential for maintenance of the senescent-cell-cycle arrest.
Human diploid fibroblasts (HDF) have a finite proliferative lifespan, at the end of which they are unable to enter S phase in response to mitogenic stimuli. Senescent HDF are also enlarged and flattened and synthesize an altered repertoire of cell-type-specific proteins, suggesting that they have differentiated as well as aged (5). Serum-stimulated senescent HDF fail to phosphorylate the retinoblastoma protein (pRb) (51), an event that is necessary for the release of E2F transcription factors that promote the expression of late G1 genes whose products are required for S-phase initiation and progression (39, 55). The inhibition of DNA synthesis in senescent nuclei can be overcome by factors or treatments that block or inactivate the inhibitory activity of pRb and its family of related proteins. For example, transfection or microinjection of simian virus 40 (SV40) T antigen into senescent HDF induces DNA synthesis, but this effect is lost when SV40 T antigen deficient in pRb binding is used (22). These data suggest that failure to phosphorylate pRb is a key mechanism for the cell cycle arrest of senescent cells.
Phosphorylation of pRb during G1 phase is carried out by cyclin D-Cdk4 and cyclin D-Cdk6 (cyclin D-Cdk4/6) and cyclin E-Cdk2 complexes (44, 50, 55). In quiescent young HDF, cyclin D1 and cyclin E are present in low amounts, but upon serum stimulation both their expression and their associated kinase activities increase during the mid and late G1 phases, respectively (14, 50). In contrast, we have shown previously that although serum-stimulated senescent HDF (IMR90) have abundant cyclin E-Cdk2 complexes, they lack cyclin E-associated kinase activity, a finding consistent with their failure to phosphorylate pRb (14). Regulation of Cdk2 activity by activating (Thr-160) and inhibiting phosphorylations (Thr-14, Tyr-15) did not account for the lack of cyclin E-Cdk2 kinase activity in senescent cells, i.e., even though approximately one-half of the cyclin E-associated Cdk2 was phosphorylated on Thr-160, treatment with Cdc25 phosphatase to dephosphorylate Thr-14 and Tyr-15 did not increase activity. The finding that senescent HDF contain elevated amounts of the ubiquitously acting cyclin-dependent kinase inhibitor (CKI) p21Sdi1,Cip1,Waf1 (p21) (40) suggested instead that cyclin E-Cdk2 complexes in senescent cells might be inactivated by increased binding of p21. However, this hypothesis was not tested directly.
In contrast to cyclin E-Cdk2, formal evidence that cyclin D1-Cdk4/6 complexes are inactive in senescent HDF is still missing. Although the absence of phosphorylated pRb (as based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] mobility shift assays supported this notion, new data imply that cyclin D1 mediates pRb hypophosphorylation, which cannot be monitored by SDS-PAGE (18). This raised the possibility that cyclin D1-Cdk4/6 might still be active in senescent cells. On the other hand, recent results indicate that p16Ink4a (p16), which acts as an inhibitor of cyclin D-associated kinase activity by sequestering Cdk4/6 (47), increases dramatically at the end of the lifespan in HDF, concomitant with a decline of p21 (1, 21). Because most of the Cdk4/6 in senescent HDF was associated with p16 rather than p21, these results suggested that p16 might be the principal inhibitor of Cdk4/6-associated kinase activity. However, the actual effect of elevated p16 on cyclin D-Cdk4/6 complex formation was not examined.
Both p21 and cyclin D interact with proliferating cell nuclear antigen (PCNA), an auxiliary factor for DNA polymerases δ and ɛ that is essential for DNA replication and repair (58). In vitro, p21 binds to PCNA and inhibits DNA replication (54), but this effect was not seen in vivo in cells induced to express p21 (36). In contrast, ectopic expression of a p21 mutant defective in Cdk2 binding resulted in G1 and G2 arrest provided that p21 retained the ability to bind PCNA (6). Acute overexpression of cyclin D1 also blocks cells in G1 phase. This inhibition could be reversed by increasing the amount of PCNA, suggesting that cyclin D1 acts as an inhibitor by binding to PCNA and keeping it in an inactive form (42). Taken together, these data raise the possibility that inhibition of PCNA by p21 and/or cyclin D1 may block entry into S phase.
To understand better the role of p21 and p16 in cellular senescence, we addressed the following questions. (i) Is binding to p21 sufficient to account for the inactivation of cyclin E-Cdk2 complexes in senescent HDF? (ii) Do senescent HDF also lack cyclin D-associated kinase activity? (iii) Is p16 accumulation sufficient to prevent cyclin D1-Cdk4/6 complex formation in senescent cells or does p21 also play a role in the inactivation of these complexes? (iv) Finally, do p21 and/or cyclin D1 bind to PCNA in senescent cells? We also investigated how aging affects the mechanism for quiescence in HDF and whether the elevation of p16 in senescent cells could be part of a differentiation program that is turned on at senescence.
MATERIALS AND METHODS
Cell culture and markers of the senescent phenotype.
IMR90 human fetal lung fibroblasts were seeded at 6,700 cells/cm2 in EF medium, which is a 1:1 mixture of Eagle minimal essential medium and F-12 plus 10% serum (53), fed after 7 days, and analyzed after 14 days. For experiments involving serum stimulation with EF plus 10% serum, quiescent and senescent cells were preincubated for 4 days in EF–0.1% serum. DNA synthesis (percent labeled nuclei) was determined by autoradiography of cells labeled with [3H]thymidine for 24 h. Relative cell volume was calculated from the volume of the pellet formed by 4 × 106 freshly trypsinized cells centrifuged at 100 × g for 5 min. Results were consistent with the cell volume calculated on the basis of cell diameter in a hemacytometer. Senescence-associated β-galactosidase activity was determined by the method of Dimri et al. (13).
Immunoprecipitations and immunoblot analysis.
Preparation of whole-cell lysates from frozen cell pellets, conditions for immunoprecipitation, histone H1 kinase assays, and immunoblotting have all been described previously (14). For p21 depletion, total cell extracts (usually 200 μg) were incubated with saturating amounts of p21-specific antibodies (2 h), whereas mock samples were incubated with protein A-Sepharose beads only. The resulting supernatants were analyzed by immunoblotting and/or used for further immunoprecipitation with cyclin-specific antibodies (see Fig. 2B). When both immunoprecipitating and immunoblotting antibodies were generated in the same species, the immunocomplexes were not boiled but only incubated in the Laemmli SDS-PAGE sample buffer at 37°C (15 min), and horseradish peroxidase (HRP)-conjugated ImmunoPure protein A/G (Pierce) was used for detection. Proteins were visualized by enhanced chemiluminescence (ECL; Amersham) and quantitated by densitometry (Shimadzu CS-930 scanner; Adobe Photoshop software).
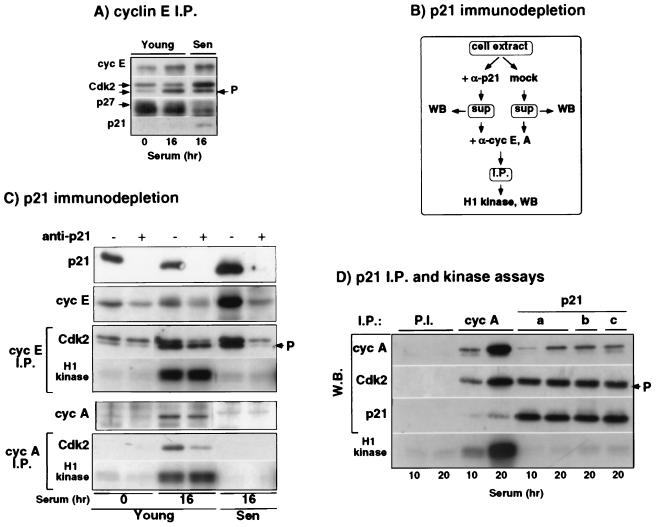
FIG. 2.
All in vivo p21-bound cyclin-Cdk2 complexes are inactive. Extracts were prepared from young quiescent IMR90 (0 h) and serum-stimulated young and senescent IMR90 (16 h). (A) Western blot analysis of cyclin E, Cdk2, p27, and p21, in cyclin E immunoprecipitates (I.P.) from total cell extracts. Arrows indicate isoforms of Cdk2. (B and C) Protocol and results, respectively. Cell extracts were immunodepleted of p21 by incubation with saturating amounts of p21-specific antibodies (+) or mock-treated with protein A-Sepharose (−). Supernatants, mock-treated and depleted of p21, were immunoblotted for p21, cyclin E, and cyclin A and used for the preparation of cyclin E and cyclin A immunoprecipitates, which were analyzed for their Cdk2 content and histone H1 kinase activity. (D) Histone H1 kinase activity of cyclin A and p21 immunocomplexes. Nonspecific antisera, cyclin A, and p21 immunoprecipitates were assayed for both histone H1 kinase activity and cyclin A, Cdk2, and p21 content as described in Materials and Methods. The arrow labeled “P” denotes the T160-phosphorylated (activated) Cdk2 isoform. Note that cyclin E binds to both forms of Cdk2, whereas cyclin A binds only the activated form of Cdk2.
In some cases (see Fig. 2) the same immunoprecipitates were used both for the determination of histone H1 kinase activity and for the immunoblot detection of various components. This was accomplished by a controlled partial transfer of SDS–12% PAGE gels. The resulting membrane was then used for immunoblot analysis with the indicated antibodies, whereas the gel was stained with Coomassie blue and dried and the histone H1 bands were excised and Cerenkov counted as described previously (14).
Antibodies.
Most of the primary antibodies used in this study were described previously (15, 16). In addition, we have used anti-Cdk4 (sc-601 and sc-260), anti-Cdk6 (sc-177), anti-p16Ink4a (sc-468), and anti p57Kip2 (sc-1040) from Santa Cruz Biotechnologies; anti-p21Cip1 rabbit antiserum generated against bacterially produced p21 (16), generously provided by Steve Reed’s laboratory (The Scripps Research Institute, La Jolla, Calif.); anti-p27Kip1 (K25020; Transduction Laboratories); anti-PCNA (ab-1; Oncogene Science); and anti-pRb-P-Ser780 (30), generously provided by Yoichi Taya (National Cancer Center Research Institute, Tokyo, Japan). Secondary antibodies were anti-mouse and anti-rabbit immunoglobulin G HRP conjugates (Promega).
RESULTS AND DISCUSSION
Effect of aging on the mechanism for quiescence in IMR90 HDF.
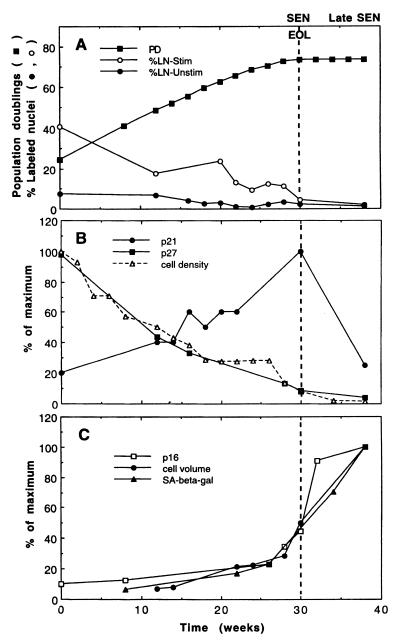
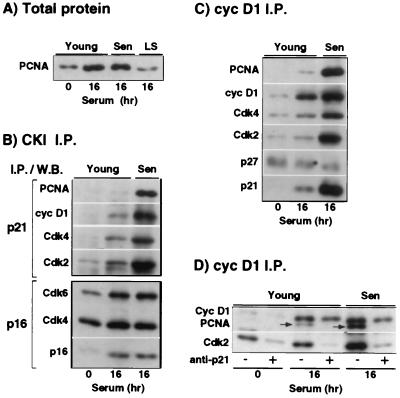
To understand how normal HDF make the transition from a reversible quiescent arrest to an irreversible senescent arrest, we aged IMR90 HDF according to a set protocol, whereby they were seeded at a constant cell density, fed after 1 week, and harvested after 2 weeks. Regardless of their age, the cells were arrested at the end of the 2-week period (Fig. 1A). Initially, the cells were reversibly arrested at high cell density with a high amount of the p27Kip1 inhibitor of CKIs, as expected from the known association of p27 with high-cell-density arrest (9). As the cells aged, their density at quiescence declined and consequently so did their amount of p27 (Fig. 1B). In contrast, the related CKI p21Sdi1,Cip1,Waf1 increased with age (1, 40). (The third related CKI, p57Kip2, is not readily detectable in IMR90 [data not shown]). Because p21 and p27 can bind to and inactivate common G1 cyclin-Cdk targets, their reciprocal relationship in aging HDF suggests that as p21 increases, less p27 is required for the cells to become arrested, and consequently they achieve quiescence at progressively lower cell densities. Thus, we are proposing that the decline in p27 and cell density at quiescence are joint consequences of the age-related increase in p21. In addition, because the accumulation of p27 at quiescence is reversible by subcultivation or serum stimulation (see Fig. 3), whereas the age-related increase in p21 is not, the quiescent cell cycle arrest becomes progressively less reversible (Fig. 1A, %LN-Stim) as the p21 level increases and the p27 level declines. Hence, the mechanism for the quiescent cell cycle arrest is constantly changing as the cells age until they become irreversibly arrested with high p21 and low p27 levels.
FIG. 1.
Age-related changes in CKIs and putative markers of differentiation in IMR90. HDF were analyzed at 14 days after each subcultivation. (A) Cumulative population doublings (PD) and the percentage of [3H]thymidine-labeled nuclei (LN) before and after serum stimulation. (B) Cell density at quiescence and the amount of p21 and p27 determined by immunoblotting and densitometry, such that each CKI is quantitated relative to its own maximum in this experiment. (C) Cell volume and p16 relative to the maximum achieved for each of these parameters and the percentage of cells that were SA–β-Gal positive. Abbreviations: EOL, end of lifespan, no further population doublings; SEN, senescent, %LN-Stim of <5%; late SEN, elevated p16.
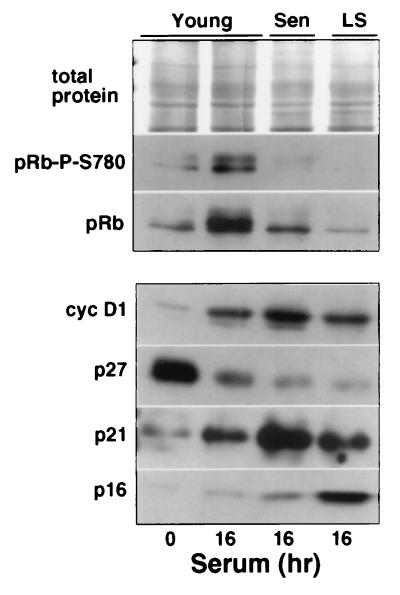
FIG. 3.
Cyclin D1-Cdk complexes are inactive in senescent HDF. Young, senescent, and late senescent (LS) IMR90 were harvested before (0 h) and/or after serum stimulation (16 h). A Western blot analysis of total cell lysates, probed for cyclin D1-dependent pRb phosphorylation at Ser-780 (30), is shown. The total protein demonstrates equal loading, and an analysis of pRb, cyclin D1, p27, p21, and p16 confirms the young, senescent, and late senescent status of the cells.
p21 and p16 exhibit different dynamics of accumulation in senescent HDF, but G1 arrest correlates with p21 accumulation.
Although senescence of HDF is strongly correlated with the accumulation of p21, this is probably not the only factor involved in the cell cycle arrest because the amount of p21 declines after the cells achieve senescence, whereas the amount of the CKI p16Ink4a increases and remains elevated for at least 2 months (Fig. 1B and C [1, 21]). Thus, senescence is not a static condition, particularly in its early phases, which is important to know when results from different experiments are compared. This may explain why we observed variable amounts of p21 and p16 in our early senescent cultures (cf. Fig. 1B with Fig. 3, 4, and 6). Nevertheless, when IMR90 were harvested at the first signs of senescence and their labeling index was <5% after serum stimulation, these cells had an exceptionally high amount of p21 but almost no increase in p16 (see Fig. 3, Sen), implying that an increase of p21 alone may be sufficient to arrest IMR90 in the early stage of senescence. Moreover, this result is consistent with the known ability of a high p21 level to arrest cells in G1 phase after DNA damage (15, 17).
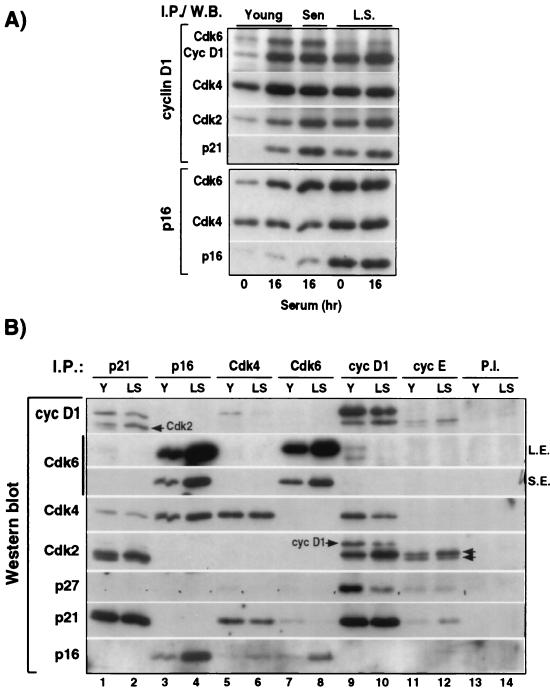
FIG. 4.
Persistence of cyclin D1-Cdk4/6 complexes in senescent HDF. (A) Western blot analysis of cyclin D1, Cdk2, Cdk4, Cdk6, p21, and/or p16 in cyclin D1 and p16 immunoprecipitates from young, senescent, and late senescent IMR90 harvested before (0 h) and/or after serum stimulation (16 h). (B) Differential association between various CKI, Cdk, and G1 cyclins in young and late senescent HDF at 16 h after serum stimulation. Western blot analysis of seven proteins (cyclin D1, Cdk6, Cdk4, Cdk2, p27, p21, and p16) in p21, p16, Cdk4, Cdk6, cyclin D1, cyclin E, and nonspecific (P.I.) complexes immunoprecipitated from equal amounts (150 mg) of cell extracts prepared from serum-stimulated young (Y) and late senescent (LS) IMR90 fibroblasts. Except in the case of Cdk4 (75% removal) and cyclin E (60% removal), the remaining supernatants were virtually depleted for the indicated protein. Prolonged ECL exposure was necessary to detect the presence of Cdk6 in p21 and cyclin D1 immunocomplexes (S.E., short exposure; L.E., long exposure). Because the Western blots were probed sequentially with different antibodies, the arrows indicate residual Cdk2 and cyclin D1 signals that remained after their “stripping.” Note that cyclin D1 binds only the inactive, unphosphorylated form of Cdk2, whereas cyclin E binds both forms of Cdk2 (parallel arrows).
FIG. 6.
Accumulation of PCNA-p21-cyclin D1-Cdk complexes in early senescent HDF. Young, senescent, and late senescent (LS) IMR90 were harvested before (0 h) and/or after serum stimulation (16 h). (A) PCNA levels in total cell extracts. (B and C) Western blot analysis of p21 and p16 immunoprecipitates (I.P.) (B) and cyclin D1 immunoprecipitates (C). In both p21 and cyclin D1 immunoprecipitates, the strong PCNA accumulation correlates with increased Cdk2. (D) p21-dependent association of PCNA with cyclin D1-Cdk2 complexes, as assessed by Western blot analysis of cyclin D1 immunoprecipitates prior to (−) and after (+) p21 immunodepletion (see Fig. 2B). Note that the senescent cells used in this figure show no increase in p16, indicating that they were harvested at a very early senescent stage, where cyclin D1 is usually at its highest level. The combination of high cyclin D1, high p21 (to stabilize cyclin D1-Cdk complexes), and low p16 may account for the high amounts of cyclin D1-associated Cdks in the cyclin D1 immunoprecipitates.
Coordinate increase of p16 and markers of senescent cell differentiation.
When HDF become senescent, they also become differentiated in a number of ways, including dramatic changes in their morphology and size (2), expression of a neutral senescence-associated β-galactosidase activity (SA–β-Gal) (13), and altered expression of genes that affect the production and remodeling of the extracellular matrix, such as fibronectin, procollagen α1(I) and α2(I), collagenase, stromelysin, and the tissue inhibitor of metalloproteinases (31, 37, 56). If these changes were induced by an exogenous agent rather than by aging, the simplest interpretation would be that the cells had been induced to differentiate. Because p16 also increases abruptly once the cells become senescent, we hypothesized that its accumulation might be part of a putative differentiation program that is turned on in senescent cells. To test this possibility, we examined two of the differentiated characteristics of senescent HDF, cell volume and SA–β-Gal activity, and found that they increased coordinately with each other and with p16 at the end of the lifespan (Fig. 1C). Thus, these data support the possibility that the upregulation of p16 in senescent cells is part of their differentiated phenotype. Interestingly, p16 is also increased in association with the terminal differentiation of neuronal cells (34). To simplify further discussion of the mechanisms for senescence, we will refer to cells with significantly elevated amounts of p16 as being in the late stage of senescence (LS in figures).
A senescence-like arrest and differentiation of young HDF can be induced by ectopic expression of p16, p15Ink4b, p21, or p27 (29, 35), as well as by treatment with various DNA-damaging agents that cause a dramatic increase in p21 (8, 12, 15, 17, 45). These results suggest that a sustained G1 arrest in conjunction with mitogenic stimulation may induce the senescent phenotype regardless of which CKI is used to accomplish the cell cycle arrest. Although ectopically expressed p16 is elevated in young HDF before other markers of senescence are induced (35), this does not argue against our hypothesis that in unperturbed cells, the accumulation of p16 may be coregulated with other markers of senescence-like differentiation. Likewise, the induction of senescence through ectopic expression of a single CKI does not mean that endogenous expression of another CKI is not also necessary. To learn more about the relative contributions of p21 and p16 to the senescent cell cycle arrest, we carried out a detailed analysis of the effects of these two CKIs on the G1 cyclin-Cdks in senescent cells.
p21 inhibits cyclin E-Cdk2 kinase activity in senescent HDF.
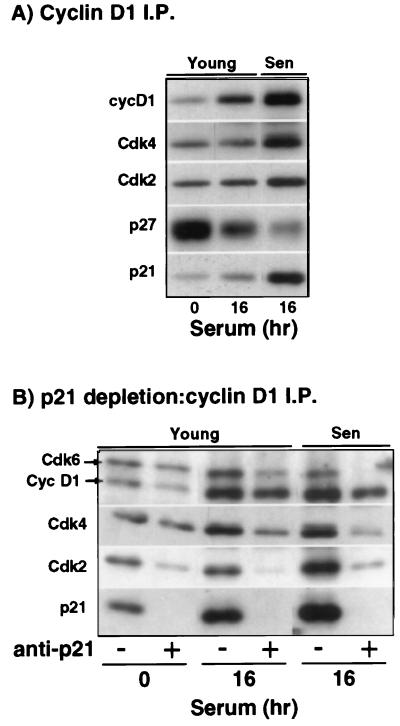
We have shown previously that senescent IMR90 accumulate inactive cyclin E-Cdk2 complexes (14). Consequently, we investigated whether increased binding of p21 to cyclin E-Cdk2 accounts for the lack of activity of these complexes. Figure 2A shows that cyclin E immunoprecipitates from serum-stimulated senescent HDF do indeed contain more p21 than do cyclin E complexes from comparably treated young HDF. Next, we wanted to determine whether the age-dependent p21 accumulation results in an increase in the fraction of cyclin E-Cdk2 associated with p21, thereby inactivating these complexes, or whether additional p21 binds to already existing p21-cyclin E-Cdk2 complexes leading to their inactivation, as implied by the stoichiometric theory (57). To this end, we incubated cell lysates with p21-specific antibodies to deplete them of p21 and its associated proteins (Fig. 2B). In senescent cells, almost all of the cyclin E and cyclin E-bound Cdk2 were removed by p21 depletion, implying that they were associated with p21 (Fig. 2C). In contrast, in young serum-stimulated cells, one-half of the cyclin E-bound Cdk2 was not removed by p21 depletion. Most importantly, p21 depletion did not affect the amount of histone H1 kinase activity in cyclin E immunoprecipitates (Fig. 2C), implying that all cyclin E-Cdk2 kinase activity resides solely in complexes that are “p21 free.” Since all of the potentially active cyclin E-Cdk2 complexes in serum-stimulated senescent HDF are bound to p21, these data show clearly that binding to p21 is sufficient to account for their inactivation.
Initially, it was surprising to find that only p21-free cyclin E-Cdk2 complexes are active because earlier studies reported that p21 is associated with both active and inactive Cdk2 complexes from replicating cells (24, 57). However, two other studies have found recently that only p21-free cyclin E-Cdk2 complexes are active, lending support to our conclusions (3, 43).
All p21-bound cyclin A-Cdk2 complexes are inactive.
The aforementioned p21-associated Cdk2 kinase activity could reflect only the activity of cyclin A-Cdk2, which is considerably greater than the activity of cyclin E-Cdk2 (14). To test this possibility, we investigated whether association with p21 also results in inactivation of cyclin A-Cdk2. As shown in Fig. 2C (lower panel), complete p21 depletion removes a significant fraction of cyclin A-Cdk2 complexes in serum-stimulated young cells, but it does not diminish the amount of associated histone H1 kinase activity, indicating again that only p21-free complexes are active. In agreement with this, p21 immunoprecipitates prepared from stimulated young IMR90 at 10 h (mid G1) and 20 h (S-G2) had little or no histone H1 kinase activity, a finding comparable to precipitates obtained with nonspecific preimmune antisera and much less (<70-fold) than cyclin A immunoprecipitates (Fig. 2D). To strengthen the validity of this conclusion, three different p21-specific antisera, which precipitated p21 equally well, were employed, and the resulting immunoprecipitates were shown to contain almost as much Cdk2 as the cyclin A immunoprecipitates (Fig. 2D). Furthermore, the kinase activity was measured during the linear part of the reaction. Recently, we also showed that cyclin A-associated kinase activity from Hs68 HDF in late G2 phase is the same both before and after p21 depletion (16). In summary, our data imply that both cyclin A-Cdk2 and cyclin E-Cdk2 complexes are invariably inactive when associated with p21 and thus disagree with the earlier results showing that p21 can be associated with active Cdk2 complexes in vivo (24, 57).
Senescent HDF lack cyclin D1-Cdk4 mediated phosphorylation of pRb–Ser-780.
Although activity of cyclin E-Cdk2 is necessary for cells to enter S phase (41), cyclin D1-Cdk4 appears to be the critical kinase responsible for pRb phosphorylation in mid G1, prior to the activation of cyclin E-Cdk2 (44). Therefore, it is important to ascertain whether this kinase is still active in senescent HDF. Because in vitro-measurable cyclin D1-associated pRb kinase activity is very low even in extracts of young HDF (unpublished results), we could not address this question directly, and so far no one has reported a decrease in senescent cells based on this assay.
Our previous results implied that cyclin D1-Cdk4/6 is inactive in serum-stimulated senescent cells because they lack phosphorylated pRb, as judged by SDS-PAGE mobility shift assays (51). However, Ezhevsky et al. (18) reported recently that during early G1, cyclin D1-Cdk4/6 complexes can hypophosphorylate pRb in a manner that does not cause a mobility shift. Consequently, we took advantage of an antibody that specifically recognizes pRb phosphorylated at Ser-780 to further assess cyclin D-associated kinase activity in senescent cells. In vitro, the pRb–Ser-780 site is phosphorylated exclusively by cyclin D1-Cdk4/6 kinase complexes, and in vivo the same site is phosphorylated in early G1, coincident with the upregulation of Cdk4 and prior to the activation of Cdk2 (30). Moreover, Kitagawa et al. (30) showed that E2F1 immunoprecipitates do not contain any pRb phosphorylated on Ser-780, suggesting that phosphorylation at this site may be necessary for the release of E2F1. Although other sites in pRb (especially Ser-795 and Ser-788) are also phosphorylated preferentially by cyclin D1-Cdk4 complexes (10), phosphorylation of pRb–Ser-780 should be indicative of the presence of cyclin D1-associated kinase activity. Indeed, our data indicate that although phosphorylated pRb–Ser-780 is abundant in serum-stimulated young cells, it is completely absent in comparably treated senescent cells, implying that they lack cyclin D1-associated kinase activity (Fig. 3). This occurs in spite of an abundance of cyclin D1 in both the early (high p21, low p16) and late (high p16, moderate p21) stages of senescence.
Interestingly, we have repeatedly observed that late senescent cells have significantly less pRb than early senescent cells or unstimulated young cells (Fig. 3). This may correlate with the rise in p16 in late senescent cells, since other studies have suggested that pRb negatively regulates p16 expression (33).
p16 accumulation in late senescent HDF affects predominantly formation of cyclin D1-Cdk6 complexes.
In contrast to p21 and p27, which bind to cyclin D-Cdk complexes, p16 binds exclusively to Cdk4/6, thus preventing their association with cyclin D (47, 50). Therefore, low cyclin D1-associated kinase activity in senescent HDF could result from a diminution of cyclin D1-Cdk4/6 complexes owing to the drastic p16 accumulation, as proposed earlier by Alcorta et al. (1). However, these authors did not directly analyze cyclin D1 complexes. Therefore, we investigated whether increased binding of Cdk4 and Cdk6 by p16 actually results in a decrease in the amount of cyclin D1-Cdk4/6 in late senescent IMR90. After serum stimulation, the amount of Cdk6 complexed to cyclin D1 is approximately equal in young and early senescent cells, but it is greatly decreased in late senescent cells, which have high amounts of p16 (Fig. 4A, Cyc D1 I.P.). Concurrently, the amount of Cdk6 associated with p16 is highest in the late senescent cells (Fig. 4A, p16 I.P.). Thus, in these cells, elevated p16 strongly inhibits cyclin D1-Cdk6 association. However, it is important to note that early senescent cells, which still have abundant cyclin D1-Cdk6 complexes, are arrested in the cell cycle and lack the cyclin-D1-mediated phosphorylation of pRb.
In contrast to the dramatic decrease in cyclin D1-Cdk6 complex formation, cyclin D1-Cdk4 complexes are still relatively abundant in late senescent cells. In spite of the strong accumulation of p16, there was only a twofold increase of p16-bound Cdk4 in late senescent versus early senescent cells, and cyclin D1-Cdk4 complexes decreased by only 20 to 50% (cf. Fig. 4A and B). Hence, our data indicate that elevated p16 only partially prevents cyclin D1-Cdk4 association and therefore is not sufficient to account for a lack of Cdk4 kinase activity, even in late senescent IMR90.
To understand better the quantitative relationships between cyclin D1, Cdk4, Cdk6, p16, and p21 in young versus late senescent cells, we examined what proportion of the total pool of each of these proteins was associated with each of the other components. (Because p21 can also associate with cyclin E-Cdk2, we included these molecules in our analysis as well and found that only a small fraction of total p21 is present in cyclin E immunoprecipitates [Fig. 4B]). To accomplish this goal, we immunoprecipitated p21, p16, Cdk4, Cdk6, cyclin D1, and cyclin E complexes (separately, not sequentially) from equal amounts (150 μg) of cell extracts prepared from serum-stimulated young (Y) and late senescent (LS) IMR90 cells and analyzed all of the immunoprecipitates simultaneously by immunoblotting (Fig. 4B). In this way, the intensity of the bands produced in the horizontal strips blotted with a given antiserum are proportional to the same amount of starting material in each lane. Thus, by examining the cyclin D1 Western blot we see both the total amount of cyclin D1 (lanes 9 and 10 in Y and LS cells, respectively) and also what proportion of that total is associated with p21 (lanes 1 and 2), p16 (lanes 3 and 4), Cdk4 (lanes 5 and 6), and Cdk6 (lanes 7 and 8) in those cells. Since this analysis assumes that each protein was efficiently precipitated by its own antiserum, we carried out a parallel Western blot analysis of the resulting supernatants (data not shown) and found efficiencies of >95%, except in the cases of Cdk4 (75%) and cyclin E (60%).
Figure 4B clarifies the following issues regarding the role of p16 in senescence. (i) The Cdk4 immunoblot shows that in young cells similar amounts of Cdk4 are bound to cyclin D1 and p16, whereas in late senescent cells more Cdk4 is associated with p16 than with cyclin D1. Nevertheless, 25 to 35% of the total Cdk4 is still associated with cyclin D1 in late senescent cells versus approximately 50% in young cells, even though p16 is increased at least fourfold in the late senescent cells. (ii) In contrast, the Cdk6 immunoblot shows that even in young cells almost all of the Cdk6 is associated with p16. Consequently, only a small fraction of total Cdk6 is associated with cyclin D1 in young cells. In late senescent cells, there is more Cdk6, but it is virtually all sequestered by p16, and thus there is a negligible amount of Cdk6 complexed to cyclin D1. (iii) The p16 immunoblot shows further that less than one-half of the total p16 in LS cells is associated with either Cdk4 or Cdk6. These results suggest that either p16 binds Cdk4 inefficiently in vivo or that 16 has another function in senescent cells which competes with its ability to sequester Cdk4. Although p19Ink4d, a CKI related to p16, can form ternary p19-cyclin D-Cdk4 complexes in vitro (26), we find no evidence for comparable p16-cyclin D1-Cdk4 in vivo, i.e., there is no association between cyclin D1 and p16 in young or late senescent cells. Thus, our data indicate clearly that the strong p16 increase in late senescent cells only partially affects cyclin D1-Cdk4 association.
The persistence of cyclin D1-Cdk4 complexes in senescent HDF suggests that additional mechanisms are required for their inactivation in both early and late senescent cells. Interestingly, Serrano et al. (49) showed that ectopic expression of oncogenic ras (V12) in human fibroblasts led to a senescence-like arrest that included an increase of both p16 and p21, and yet coexpression of cyclin D1 and a p16-insensitive form of Cdk4 did not prevent this ras-induced arrest. Thus, the latter cells achieved a senescence-like arrest even though they presumably contained abundant cyclin D1-Cdk4 complexes.
p21 is associated with almost all cyclin D1-Cdk4/6 complexes in senescent HDF.
Because senescent IMR90 have abundant, but apparently inactive, cyclin D1-Cdk4 complexes, we investigated the possibility that these complexes are inhibited by p21. Indeed, the relative amount of p21 associated with cyclin D1 is increased in senescent cells, whereas p27 is decreased (Fig. 5A). In addition, p21 depletion experiments demonstrate that the majority of cyclin D1-Cdk4 and all cyclin D1-Cdk6 complexes in senescent cells are associated with p21 (Fig. 5B). In contrast, “p21-free” cyclin D1-Cdk4/6 complexes are more abundant in serum-stimulated young cells. Although these results suggest that p21-free cyclin D1-Cdk4/6 complexes may be responsible for cyclin D1-associated pRb kinase activity, other studies have indicated that cyclin D1-Cdk4/6 complexes associated with one molecule of p21 are active, whereas binding an additional molecule(s) of p21 inhibits activity (32, 57). In either case, our data show that relatively more p21 is bound to cyclin D1-Cdk4/6 complexes in senescent cells than in young cells, suggesting that p21 probably plays a role in inhibiting cyclin D1-Cdk4/6 kinase activity in senescent cells. This result is consistent with the recent finding that HDF completely lacking p21 have an extended lifespan that ends in crisis rather than senescence in spite of elevated p16 levels (4).
FIG. 5.
Increased association of p21 with cyclin D1-Cdk4/6 complexes in senescent HDF. (A) Western blot analysis of cyclin D1, Cdk2, Cdk4, p27, and p21 in cyclin D1 immunoprecipitates. (B) Western blot analysis of cyclin D1, Cdk2, Cdk4, Cdk6, and p21 in cyclin D1-immunoprecipitates after p21 immunodepletion as described in Fig. 2B.
Early senescent cells accumulate p21-PCNA-cyclin D1-Cdk complexes.
In contrast to an earlier report that senescent HDF lack PCNA mRNA (7), we found that early senescent IMR90 contain as much PCNA protein as young stimulated cells (Fig. 6A). On the other hand, the amount of PCNA protein is decreased substantially in late senescent cells, a finding consistent with a decline in PCNA mRNA at that stage of senescence. As a unifying hypothesis, we suggest that PCNA may decline in senescent HDF as part of the same putative differentiation program that leads to an upregulation of p16 and SA–β-Gal activity and increasing cell volume.
Since previous experiments suggested that both p21 and cyclin D1 may be involved in inactivation of PCNA (42, 54), we sought to determine whether this could be the case in the early stage of senescence, i.e., before the rise of p16. Indeed, Western blot analysis of both p21 and cyclin D1 immunoprecipitates revealed a dramatic increase in the amount of PCNA associated with these proteins in newly senescent cells (Fig. 6B and C). After p21 depletion, PCNA could not be detected in cyclin D1 immunoprecipitates, suggesting that p21 is present in all PCNA-cyclin D1 complexes (Fig. 6D). Consistent with this, the amount of PCNA immunoprecipitated with cyclin D1 and p21 is approximately equal (data not shown). If PCNA is indeed inactivated by association with p21 and cyclin D1 (42, 54), then this event occurs to a much greater extent in senescent cells than in young cells, providing another mechanism that can contribute to the G1 arrest in these cells. To our knowledge, this is the first report describing a physiological situation where p21-PCNA-cyclin D1 complexes accumulate.
Although both Cdk4 and Cdk2 are present in cyclin D1 and p21 immunoprecipitates, the strong PCNA accumulation correlates primarily with increased Cdk2 (Fig. 6B and C). Cdk4 depletion experiments confirmed this interpretation (data not shown). Even though cyclin D1-Cdk2 complexes are abundant in senescent HDF, little is known about their function since they are constitutively inactive (14, 25). Our data suggest that cyclin D1-Cdk2 may contribute to the regulation of PCNA by p21.
Concluding remarks.
The bypass of senescence by p21−/− HDF and the death of these cells at the end of an extended lifespan imply that p21 is essential for the senescent cell cycle arrest (4). Furthermore, we have shown here that p21 is sufficient to account for the lack of cyclin E-associated kinase activity in senescent IMR90. We have also found that senescent HDF lack cyclin D1-Cdk4-mediated phosphorylation of pRb at a time that p21, but not p16, is dramatically elevated in these cells, implying that p21 may also be the critical inhibitor of cyclin D1-Cdk4 complexes in early senescent cells. Thus, p21 may be both necessary and sufficient for the initial senescent cell cycle arrest.
In contrast, there are several reasons to think that p21 may not be sufficient for the long-term maintenance of the senescent arrest state. First, after senescence is achieved, p21 declines considerably to an amount that was consistent with a reversible arrest earlier in the lifespan. Second, p16 increases as p21 declines and reduces the number of targets for p21 through its inhibitory effect on cyclin D1-Cdk4/6 complex formation. Third, loss of p16 accompanied spontaneous immortalization of Li-Fraumeni fibroblasts (46). Likewise, mouse embryo fibroblasts with a targeted disruption of the INK4A locus fail to senesce (48), but this could result solely from abrogation of the alternate reading frame protein p19ARF, whose loss alone has the same effect (28, 48). Thus, it remains to be determined whether loss of p16 per se will permit mouse embryo fibroblasts to escape from senescence. Finally, p16, rather than p21, is frequently mutated in immortalized human tumor cells (27). Thus, a number of observations are consistent with the hypothesis that elevated p16 may be critical to maintain the senescent cell cycle arrest as p21 declines from its maximum at the initiation of senescence.
Because p21 and p16 have very different age-related patterns of accumulation in HDF, we propose that replicative senescence in HDF comprises two events: an increase in p21 that is driven by the “mitotic clock” and an upregulation of p16 as part of a program of differentiation that is turned on in senescent cells. First, the progressive age-dependent accumulation of p21 suggests that it occurs as a consequence of replication-related processes such as telomere shortening (23), DNA demethylation (19), and the effects of DNA damage (8, 20). It results in inactivation of all G1 cyclin-Cdks, such that pRb fails to be phosphorylated, E2F transcription factors are not released, late-G1 genes necessary for DNA synthesis are not expressed, and the cells become irreversibly arrested in G1 phase, as suggested by a number of studies (reviewed in reference 52). In parallel, an efficient G1 block may also be assured by inactivation of PCNA by association with p21 and cyclin D1. Second, we hypothesize that at senescence a program of differentiation is initiated that involves the accumulation of p16, as well as changes in the morphology, size, and functional attributes of the cells (1, 2, 13, 21, 31, 37, 38, 56). The concomitant decline of p21 from its peak in early senescence could occur owing to decay of the replication-related signals that drove its increase as the cells were aging, or p21 might be downregulated as a necessary part of the putative differentiation program, as was shown recently for the terminal differentiation of primary mouse keratinocytes (11). Consequently, in late senescent cells Cdk inactivation and the cell cycle arrest are maintained through the combined effect of p16 and p21. Thus, if p16 is indeed upregulated as an integral part of the age-induced differentiation of senescent HDF, as suggested by our model, then this process may be necessary to ensure the irreversibility of the senescent cell cycle arrest.
ACKNOWLEDGMENTS
We thank Annick Péléraux, Dan Fisher, Marcel Dorée, and Jacques Piette, (Centre de Recherches de Biochemie Macromoléculaire and IGM, Montpellier, France) and Anita K. Miller, Lauren Sompayrac, and Carol Alexander (University of Colorado, Boulder, Colo.) for critical comments on the manuscript. We are also grateful to Yoichi Taya (National Cancer Center Research Institute, Tokyo, Japan) for his gift of antibody to phosphorylated pRb-Ser780 and to Steve Reed (Scripps Institute, La Jolla, Calif.) for cyclin A and p21-specific antisera.
This work was supported by Public Health Service grant AG00947 from the National Institute on Aging (G.H.S.) and l’Association pour la Recherche sur le Cancer (ARC-6852) and ATIPE (V.D.).
REFERENCES
- 1.Alcorta D A, Xiong Y, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16 (Ink4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayreuther K, Rodemann H P, Hommel R, Dittmann K, Albiez M, Francz P I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci USA. 1988;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bresnahan W A, Boldogh I, Ma T, Albrecht T, Thompson E A. Cyclin E/Cdk2 activity is controlled by different mechanisms in the G0 and G1 phases of the cell cycle. Cell Growth Differ. 1996;7:1283–1290. [PubMed] [Google Scholar]
- 4.Brown J P, Wei W, Sedivy J M. Bypass of senescence after disruption of p21Cip1/Waf1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 5.Campisi J. The biology of replicative senescence. Eur J Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 6.Cayrol C, Kniebiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- 7.Chang C-D, Phillips P, Lipson K E, Cristofalo V J, Baserga R. Senescent human fibroblasts have a post-transcriptional block in the expression of the proliferating cell nuclear antigen gene. J Biol Chem. 1991;266:8663–8666. [PubMed] [Google Scholar]
- 8.Chen Q, Fischer A, Reagan J D, Yan L J, Ames B N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci USA. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 10.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 12.DiLeonardo A, Linke S P, Clarkin K, Wahl G M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 13.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O M, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dulić V, Drullinger L F, Lees E, Reed S I, Stein G H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulić V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 16.Dulić V, Stein G H, Far D F, Reed S I. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Deiry W S, Harper J W, O’Connor P M, Velculescu V E, Canman C E, Jackman J, Pietenpol J A, Burrell M, Hill D E, Wang Y, Wiman K G, Mercer W E, Kastan M, Kohn K W, Elledge S J, Kinzler K W, Vogelstein B. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;55:1169–1174. [PubMed] [Google Scholar]
- 18.Ezhevsky S A, Nagahara H, Vocero-Akbani A M, Gius D, Wei M C, Dowdy S F. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci USA. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairweather D S, Fox M, Margison G P. The in vitro lifespan of MRC-5 cells is shortened by 5-azacytidine-induced demethylation. Exp Cell Res. 1987;168:153–159. doi: 10.1016/0014-4827(87)90424-1. [DOI] [PubMed] [Google Scholar]
- 20.Fraga C G, Shigenaga M K, Park J W, Degan P, Ames B N. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara E, Uzman J A, Dimri G P, Nehlin J O, Testori A, Campisi J. The helix-loop-helix protein Id-1 and a retinoblastoma protein binding mutant of SV40 T antigen synergize to reactivate DNA synthesis in senescent human fibroblasts. Dev Genet. 1996;18:161–172. doi: 10.1002/(SICI)1520-6408(1996)18:2<161::AID-DVG9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Harley C B, Futcher A B, Greider C W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 24.Harper J W, Elledge S J, Keyomarsi K, Dynlacht B, Tsai L-H, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, Fox M P, Wei N. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi H, Suzuki-Takahashi I, Saitoh S, Segawa K, Taya Y, Okuyama A, Nishimura S, Kitagawa M. Cyclin-dependent kinase-2 (Cdk2) forms an inactive complex with cyclin D1 since Cdk2 associated with cyclin D1 is not phosphorylated by Cdk7-cyclin-H. Eur J Biochem. 1996;237:460–467. doi: 10.1111/j.1432-1033.1996.0460k.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirai H, Roussel M F, Kato J Y, Ashmun R A, Sherr C J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases Cdk4 and Cdk6. Mol Cell Biol. 1995;15:2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirama T, Koeffler H P. Role of the cyclin-dependent kinase inhibitors in the development of cancer. Blood. 1995;86:841–854. [PubMed] [Google Scholar]
- 28.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4α locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 29.Kato D, Miyazawa K, Ruas M, Starborg M, Wada I, Oka T, Sakai T, Peters G, Hara E. Features of replicative senescence induced by direct addition of antennapedia-p16Ink4A fusion protein to human diploid fibroblasts. FEBS Lett. 1998;427:203–208. doi: 10.1016/s0014-5793(98)00426-8. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 31.Kumazaki T, Robetorye R S, Robetorye S C, Smith J R. Fibronectin expression increases during in vitro cellular senescence: correlation with increased cell area. Exp Cell Res. 1991;195:13–19. doi: 10.1016/0014-4827(91)90494-f. [DOI] [PubMed] [Google Scholar]
- 32.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Nichols M A, Shay J W, Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994;54:6078–6082. [PubMed] [Google Scholar]
- 34.Lois A F, Cooper L T, Geng Y, Nobori T, Carson D. Expression of the p16 and p15 cyclin-dependent kinase inhibitors in lymphocyte activation and neuronal differentiation. Cancer Res. 1995;55:4010–4013. [PubMed] [Google Scholar]
- 35.McConnell B B, Starborg M, Brookes S, Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol. 1998;8:351–354. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- 36.Medema R H, Klompmaker R, Smits V A J, Rijksen G. p21waf1 can block cells at two points in the cell cycle, but does not interfere with processive DNA replication or stress-activated kinases. Oncogene. 1998;16:431–441. doi: 10.1038/sj.onc.1201558. [DOI] [PubMed] [Google Scholar]
- 37.Millis A J T, Hoyle M, McCue H M, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- 38.Mitsui Y, Schneider E L. Relationship between cell replication and volume in senescent human diploid fibroblasts. Mech Ageing Dev. 1976;5:45–56. doi: 10.1016/0047-6374(76)90007-5. [DOI] [PubMed] [Google Scholar]
- 39.Nevins J R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 40.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pagano M, Theodoras A M, Tam S W, Draetta G F. Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev. 1994;8:1627–1639. doi: 10.1101/gad.8.14.1627. [DOI] [PubMed] [Google Scholar]
- 43.Planas-Silva M D, Weinberg R A. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reznitsky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S phase transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodemann H P, Bayreuther K, Franz P I, Dittman K, Albiez M. Selective enrichment and biochemical characterization of seven human skin fibroblast cell types in vitro. Exp Cell Res. 1989;180:84–93. doi: 10.1016/0014-4827(89)90214-0. [DOI] [PubMed] [Google Scholar]
- 46.Rogan E M, Bryan T M, Hukku B, McLean K, Chang A C-M, Moy E L, Englezou A, Warneford S G, Dalla-Pozza L, Reddel R R. Alterations in p53 and p16Ink4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol Cell Biol. 1995;15:4745–4753. doi: 10.1128/mcb.15.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/Cdk4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 48.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho R A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 49.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 50.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 51.Stein G H, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249:666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- 52.Stein G H, Dulić V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 53.Stein G H, Yanishevsky R M, Gordon L, Beeson M. Carcinogen-transformed human cells are inhibited from entry into S phase by fusion to senescent cells but cells transformed by DNA tumor viruses overcome the inhibition. Proc Natl Acad Sci USA. 1982;79:5287–5291. doi: 10.1073/pnas.79.17.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waga S, Hannon G J, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 56.West M D, Pereira-Smith O M, Smith J R. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989;184:138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H, Hannon G J, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]