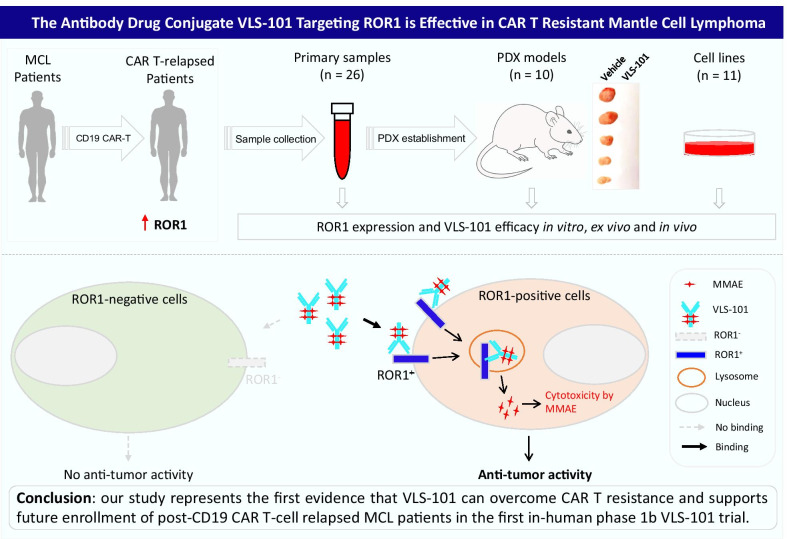
Abstract
Mantle cell lymphoma (MCL) is a rare, aggressive and incurable subtype of non-Hodgkin’s B-cell lymphoma. The principal barrier is frequent clinical relapse to multiple lines of therapies, including new FDA-approved biologics and cell therapy. Brexucabtagene autoleucel, the first and only FDA approved chimeric antigen receptor (CAR) T product in MCL, demonstrated unprecedented efficacy in overcoming resistance to Bruton’s tyrosine kinase inhibitors. However, relapses have inevitably occurred and once relapsed these patients display a very poor clinical outcome. Currently, there is no optional therapy specifically designed for these patients. The development of tailored and more efficacious therapies is therefore critical and represents a new medical need. We found that while the receptor tyrosine kinase-like orphan receptor 1 (ROR1) is expressed across most of the MCL cells, it is significantly elevated in CAR T-relapsed MCL tumors. To see whether this aberrant ROR1 expression contributed to CAR T resistance, we targeted ROR1 using VLS-101, a monomethyl auristatin E conjugated anti-ROR1 antibody. VLS-101 showed potent anti-MCL activity in vitro in ROR1-expressing MCL cell lines and ex vivo in primary patient samples. Importantly, VLS-101 safely induced tumor regression in PDX models resistant to CAR T-cell therapy, ibrutinib and/or venetoclax. These data advocate for targeting ROR1 as a viable approach in the treatment of ROR1-positive MCL tumors, especially those with failure to prior therapies. These data also provide strong evidence for future enrollment of post-CD19 CAR T-cell relapsed MCL patients in a first in-human phase 1b VLS-101 trial. The upcoming testing in a clinical setting will provide important insights on this new therapeutic development aiming to overcome the CAR T resistance via targeting ROR1, which is a rising unmet clinical need in MCL.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-021-01143-w.
Keywords: Mantle cell lymphoma, ROR1, VLS-101, CAR T resistance
To the Editor
Brexucabtagene autoleucel (BA) [1], is the first and only chimeric antigen receptor (CAR) T product approved by FDA for relapsed or refractory (R/R) mantle cell lymphoma (MCL) based on its recently reported safety and unprecedented efficacy (ORR 93%) [2]. Unfortunately, relapses do occur; relapsed patients display a median survival of only 4.1 months[3]. This rapidly growing clinical challenge warrants mechanistic decoding of CAR T resistance and new therapeutic developments to overcome it.
Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is an embryo-oncogenic transmembrane receptor with tightly controlled expression in normal tissues [4]. Its aberrant expression was shown in many cancer types including MCL [4, 5]. High ROR1 expression associates with aggressive disease, poor survival and therapeutic resistance [6, 7]. Interestingly, ROR1 forms a functional complex with CD19 on cell surface that activates various signaling pathways to promote MCL cell proliferation in a B-cell receptor/BTK signaling-independent manner [8].
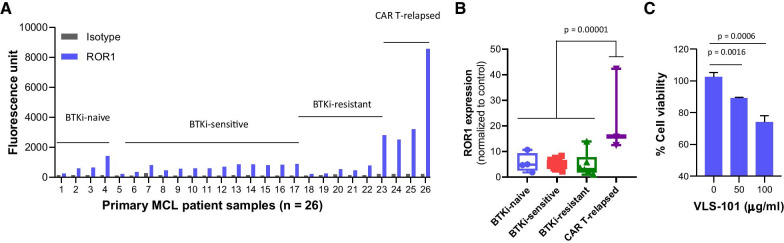
We detected ROR1 expression in 92% (24/26) MCL samples collected (non-preferentially) by either apheresis or by excisional biopsy as per physician’s directions (Fig. 1a). ROR1 was positive in all patient-derived xenograft (PDX) models (n = 10) and MCL cell lines (9/11, 82%) (Additional file 1: Figure S1A and S2A). Interestingly, ROR1 expression was highest in BA-relapsed samples (n = 3) (p = 0.00001) (Fig. 1b), suggesting that ROR1 may be a driver of BA resistance in MCL and targeting ROR1 may overcome it.
Fig. 1.
ROR1 expression and targeting in primary MCL patient samples, including CAR T-resistant MCL patient cells. (a) ROR1 expression detected by flow cytometry in primary MCL patient samples (n = 26), classified into four groups: BTKi-naïve (n = 4), BTKi-sensitive (n = 13), BTKi-resistant (n = 6) and CAR T-relapsed (n = 3). (b) Cell surface ROR1 expression of the four groups in (a) normalized to controls. (c) Dose-dependent inhibition of cell viability by MMAE-conjugated ROR1 antibody VLS-101. ROR1 targeting by VLS-101 is shown in a CAR T-relapsed MCL patient sample. Student t test was used to calculate statistical significance
UC-961 is a first-in-class humanized monoclonal specific anti-ROR1 antibody, shown to be safe in preclinical and clinical trials [9, 10]. To increase its anti-tumor activity, VLS-101 was created by conjugating UC-961 to monomethyl auristatin E (MMAE) via a cleavable linker [11]. VLS-101 retains UC-961 specificity in targeting ROR1 on cell surface and induces a target-antibody complex internalization which facilitates MMAE release in the lysosome to kill cells [12].
Ex vivo treatment with VLS-101 induced dose-dependent cytotoxicity in ROR1+ primary MCL cells collected from a BA-relapsed patient (Fig. 1c). Consistently, VLS-101 treatment resulted in potent cytotoxicity in ROR1+ but not in ROR1− MCL cell lines at a low IC50 range (4.0–23.3 µg/ml, at 72 h) and in a dose- and time-dependent manner (Additional file 1: Figure S1B–E). VLS-101 induced cell apoptosis and cell cycle arrest at G2/M (Additional file 1: Figure S1F-G) likely due to intracellular release of MMAE upon ROR1 engagement and internalization [12].
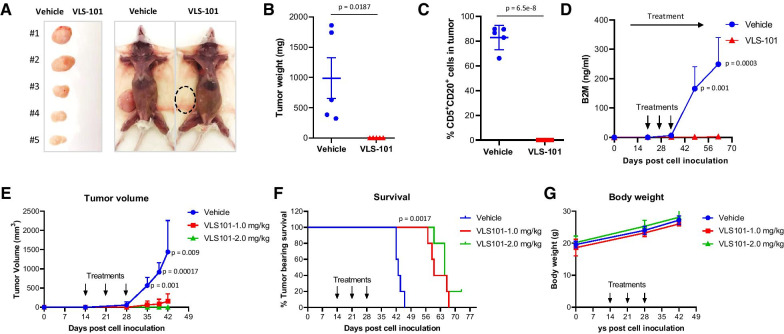
To determine whether VLS-101 can overcome therapeutic resistance, patient-derived xenograft (PDX) models were successfully established in immunodeficient NSG mice. Importantly, ROR1 expression retains from patients to PDX (p = 0.76, Additional file 1: Figure S2B). VLS-101 treatment at 2.5 mg/kg (weekly × 3, intravenously) eliminated subcutaneous tumor growth of BA-resistant PDX model (PDX-1) (Fig. 2a–c). This was confirmed by the production of serum beta-2-microglobulin (B2M), a systemic tumor load marker (Fig. 2d). We repeated the experiment with lower doses to further check its potency; the tumors were barely palpable (mean = 160 mm3) and completely regressed when treated with VLS-101 at 1 and 2 mg/kg, respectively. In contrast, vehicle-treated tumors reached a mean volume of 1440 mm3 (Fig. 2e). In addition, VLS-101 treatment significantly prolonged tumor bearing mouse survival (60 and 65 days, respectively), compared to vehicle-treated group (43 days, p = 0.0017) (Fig. 2f). Importantly, VLS-101 treatment at 1, 2 and 2.5 mg/kg was well-tolerated as determined by gross observation and body weight measurements (Fig. 2g). Consistent VLS-101 efficacy was also observed in the disseminated PDX model (PDX-2) derived from an ibrutinib-venetoclax dual-resistant patient tumor with positive ROR1 expression (Additional file 1: Figure S2B). Treatment with VLS-101 at 2.5 mg/kg eradicated the tumors in the spleen (p = 0.0001), and significantly inhibited tumor growth in the liver (p = 0.002), compared to vehicle-treated group (Additional file 1: Figure S3A–C).
Fig. 2.
VLS-101 is potent in targeting ROR1-expressing PDX models with dual resistance to ibrutinib and CAR T. A PDX model was established from one of the ibrutinib-CAR T dual resistant MCL patient in immunodeficient NSG mice. (a–d) Freshly isolated PDX cells from previous generation were inoculated subcutaneously into NSG mice. When tumors became palpable (at day 20 post cell inoculation), the mice (n = 5) were treated intravenously (IV) with vehicle (n = 5) or VLS-101 (n = 5) at 2.5 mg/kg (QW × 3). When the tumors in the vehicle groups reached 15 mm in diameter, the subcutaneous tumors were dissected, imaged (a) and weighted (b). The percentage of MCL cells in the subcutaneous tumors were detected by flow cytometry with fluorescence conjugated CD5 and CD20 antibodies (c). B2M levels were detected by B2M ELISA kits using the mouse serum collected every two weeks (d). (e–g) The same model was used to pass on to the next generation and the mice were treated with VLS-101 at lower doses 1 and 2 mg/kg (QW × 3, IV) (n = 5 per group) when tumors were palpable (at day 14 post cell inoculation). Tumor volume (e), mice survival (f), and body weight (g) were monitored and plotted. We used the 15 mm tumor size as humane end points for animal survival analysis. Student t test was used to calculate statistical significance
Collectively, we demonstrated that BA-relapsed patients express aberrantly higher ROR1 compared to other MCL tumors and that VLS-101 is highly effective in resistant MCL preclinical models. Most importantly, VLS-101 treatment was safe and efficient in eradicating BA-resistant tumors in subcutaneous PDX models and ibrutinib-venetoclax resistant tumors in disseminated PDX models. At the 2020 American Hematology Society annual meeting, VLS-101 was reported to be safe and effective in overcoming ibrutinib resistance (ORR 47%) in a first in-human phase 1b trial [13]. A cohort expansion of post-CD19 CAR T-cell relapsed MCL patients is being considered based on our study. Translational and mechanistic studies are currently ongoing to understand why post-CD19 CAR T-cell relapsed tumors are hypersensitive to VLS-101 targeting embryo-oncogenic ROR1 in MCL.
Supplementary Information
Additional file 1. Supplemental Figures.
Acknowledgements
We thank the patients and their families who contributed to this research study. This work was partially supported by a grant from the VelosBio Inc and philanthropy from Steve and Nancy Fox Cancer Research Fund. VelosBio Inc had no role in the experimental design or data acquisition; VLS-101 was provided by VelosBio Inc at no cost to us. Thanks to Dr. Lei Nie for manuscript revision and Dr. Liana Adam for editing and submitting the manuscript.
Abbreviations
- BA
Brexucabtagene autoleucel
- B2M
Beta-2-microglobulinBTK: Bruton’s tyrosine kinase
- BTKi
BTK inhibitor
- CAR
Chimeric antigen receptor
- ROR1
Receptor tyrosine kinase-like orphan receptor 1
- MCL
Mantle cell lymphoma
- MMAE
Monomethyl auristatin E
- PDX
Patient-derived xenograft
Authors' contributions
M.W. and V.C.J conceived the idea and designed the experiments; V.C.J, Y.L., A.J., J.M., YJ.L., Y.C., and W.W. performed the experiments; V.C.J, Y.L., K.A.J., B.J.L., and M.W. performed data analysis. V.C.J drafted the manuscript; V.C.J, K.A.J., B.J.L., and M.W. revised the manuscript. All authors read and approved the final manuscript.
Funding
M.W. has a grant from VelosBio Inc., and philanthropy from Steve and Nancy Fox Cancer Research Fund.
Declarations
Ethics approval and consent to participate
The patient samples were collected from peripheral blood, bone marrow, or apheresis after obtaining informed consent and approval from the Institutional Review Board at The University of Texas MD Anderson Cancer Center. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center.
Consent for publication
Not applicable.
Competing interests
M.W. has a grant from VelosBio Inc., and philanthropy from Steve and Nancy Fox Cancer Research Fund. K.A.J., and B.J.L., are fulltime employed by VelosBio Inc. All other authors declare no competing financial interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Footnotes
Presented in abstract form at the 62nd annual meeting of the American Society of Hematology, December 5, 2020.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jiang, V.C. et al. Targeting ROR1 using the antibody drug conjugate Vls-101 in aggressive mantle cell lymphoma. Blood. 2020;136
- 2.Wang MH, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. New Engl J Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain P, et al. Outcomes and management of patients with mantle cell lymphoma after progression on brexucabtagene autoleucel therapy. Brit J Haematol. 2021;192:E38–E42. doi: 10.1111/bjh.17197. [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan A, et al. Analysis of ROR1 protein expression in human cancer and normal tissues. Clin Cancer Res. 2017;23:3061–3071. doi: 10.1158/1078-0432.CCR-16-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, et al. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181:1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SP, et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci USA. 2019;116:1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui B, et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood. 2016;128:2931–2940. doi: 10.1182/blood-2016-04-712562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, et al. Cutting edge: ROR1/CD19 receptor complex promotes growth of mantle cell lymphoma cells independently of the B cell receptor-BTK signaling pathway. J Immunol. 2019;203:2043–2048. doi: 10.4049/jimmunol.1801327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi M, et al. Durable and specific inhibition of ROR1 signaling associates with prolonged progression free survival in patients with chronic lymphocytic leukemia treated with cirmtuzumab. Blood. 2017;130:829. doi: 10.1182/blood.V130.Suppl_1.829.829. [DOI] [Google Scholar]
- 10.Choi MY, et al. Phase I trial: cirmtuzumab inhibits ROR1 signaling and stemness signatures in patients with chronic lymphocytic leukemia. Cell Stem Cell. 2018;22:951–959 e953. doi: 10.1016/j.stem.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaisitti T, et al. Vls-101 is a novel therapeutic antibody-drug conjugate (ADC) targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1) in Richter's syndrome (RS) Blood. 2019;134:2856. doi: 10.1182/blood-2019-126827. [DOI] [Google Scholar]
- 12.Vaisitti T, et al. ROR1 targeting with the antibody-drug conjugate VLS-101 is effective in Richter syndrome patient-derived xenograft mouse models. Blood. 2021;137:3365–3377. doi: 10.1182/blood.2020008404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, et al. VLS-101, a ROR1-targeting antibody-drug conjugate, demonstrates a predictable safety profile and clinical efficacy in patients with heavily pretreated mantle cell lymphoma and diffuse large B-cell lymphoma. Blood. 2020;136:13–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Figures.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.