To the Editor: We read with great interest the case series by Sidlow et al1 describing the urticarial allergic dermatitis secondary to SARS-CoV-2 vaccination. With mass vaccination unfolding, it is imperative that providers and patients are aware of this benign reaction to receive proper treatment and reassurance. We report 4 cases of cutaneous hypersensitivity reactions following Moderna messenger RNA (mRNA)-1273 vaccination that presented to a small community practice.
Case 1
A 63-year-old man with no medical conditions presented to the clinic 1 week after receiving his second dose of Moderna mRNA-1273 vaccine with a pruritic rash involving his arms and legs. Physical examination revealed erythematous papules on the bilateral extremities (Fig 1). The eruption resolved spontaneously over the next 2 weeks without further intervention.
Fig 1.
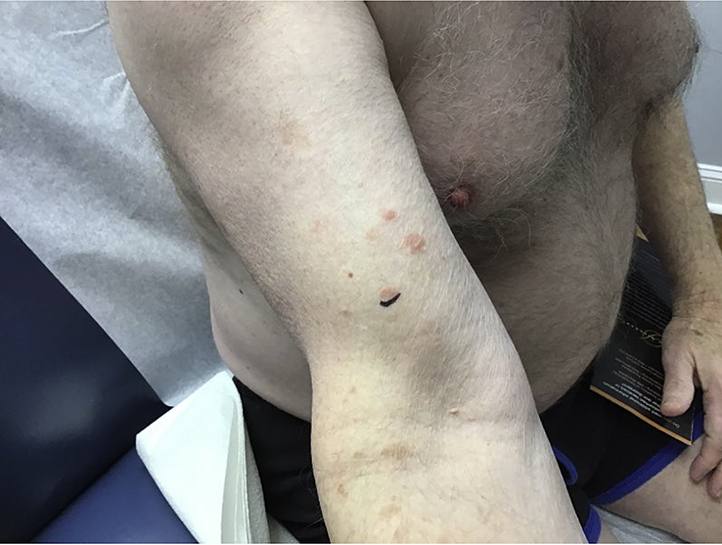
Patient 1. Erythematous papules on the right arm.
Case 2
A 73-year-old woman with no significant past medical history presented 3 days after receiving her first dose of Moderna mRNA-1273 vaccine with an erythematous rectangular patch in the area of the vaccination site (Fig 2). She first noticed the reaction 2 days after vaccination, and it continued to worsen. She endorsed localized symptoms of a burning sensation and her arm feeling hot. There were no associated fevers, chills, or other systemic symptoms. The rash gradually subsided over the next 5 days in response to betamethasone 0.05% ointment twice daily.
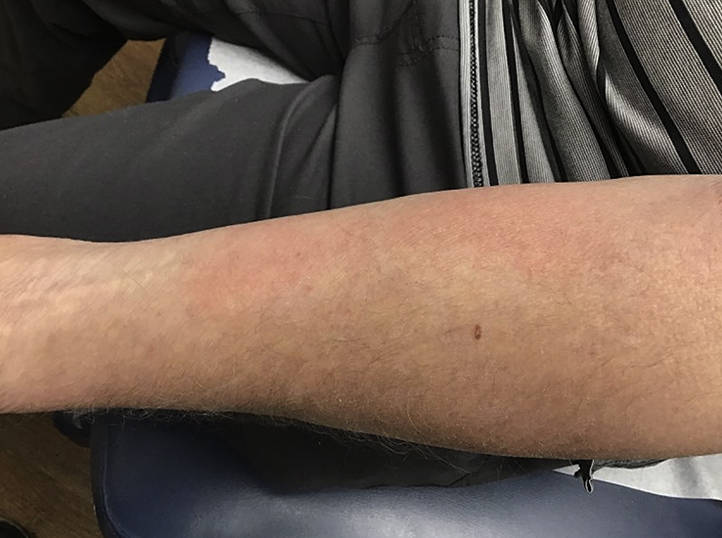
Fig 2.
Patient 2. Erythematous rectangular patch localized to the vaccination site.
Case 3
A 79-year-old woman with no significant past medical history presented to the clinic 4 weeks after receiving the Moderna mRNA-1273 vaccine with severe morbilliform eruption involving her torso and extremities (Fig 3). The eruption started 3 weeks after her first vaccination. Pathology demonstrated vacuolar interface dermatitis with the differential diagnosis of viral exanthema versus drug eruption. She did not have any associated fever, chills, or other systemic symptoms. The rash slowly improved with the administration of methylprednisolone 4-mg dose pack and continued levocetirizine 5 mg over a period of 4 weeks. The rash has mostly resolved with mild residual erythema, more prominent after a warm shower. The patient chose to proceed with her second vaccine dose 4 weeks later and did not experience any recurrence or flareup of the skin reaction.
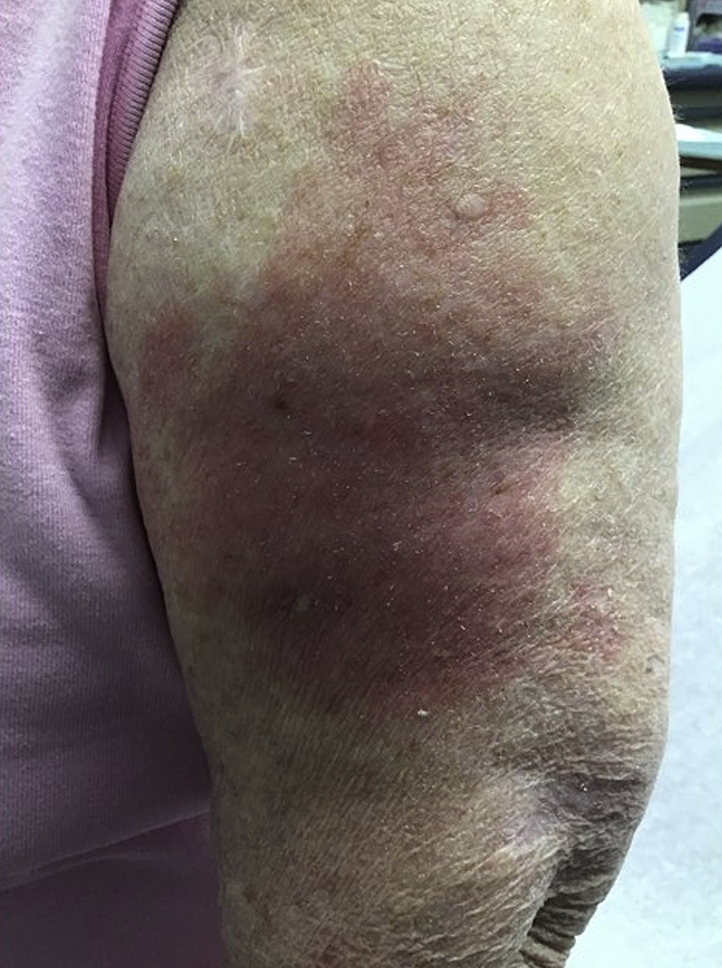
Fig 3.
Patient 3. Severe morbilliform eruption involving both arms.
Case 4
A 67-year-old man with no medical conditions presented with a bilateral rash involving his arms 4 days after receiving his second dose of Moderna mRNA-1273 vaccine. The patient endorsed pruritus but denied fever, pain, chills, and other systemic symptoms. Physical examination revealed bilateral erythematous exanthem on his arms (Fig 4). The rash responded to treatment with triamcinolone 0.1% cream twice daily and resolved after 2 weeks.
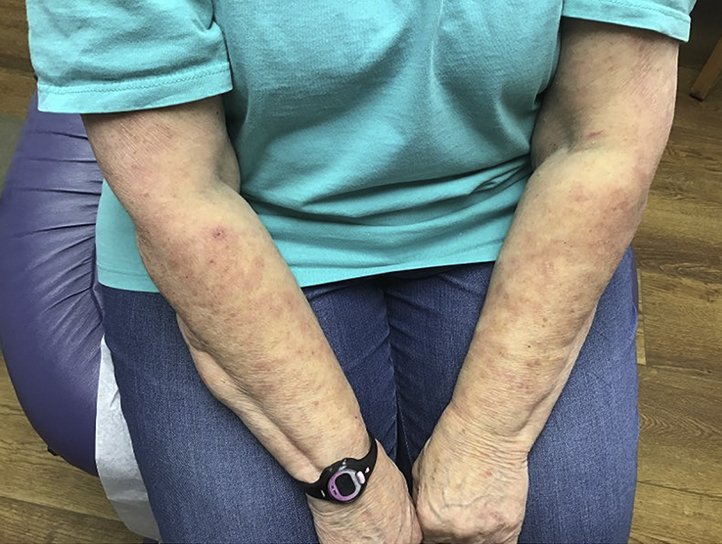
Fig 4.
Patient 4. Erythematous patch involving the left arm.
Vaccine reactions are increasingly being reported as mass vaccination continues to unfold. Mcmahon et al2 found 414 patients with hypersensitivity reactions after COVID-19 vaccination, with 83% of these reactions occurring from the Moderna mRNA-1273 vaccine. The reactions varied from localized to morbilliform reactions and resolved with topical corticosteroids, oral antihistamine drugs, and spontaneously without treatment. However, some patients were given antibiotics out of concern for cellulitis.
With multiple cases of vaccine reactions, in a limited patient population, it is possible that cutaneous hypersensitivity reactions may be more common following COVID-19 vaccination than initially realized. Increased awareness will lead to a reduction of antibiotics use for the benign, transient reactions following vaccination.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Sidlow J.S., Reichel M., Lowenstein E.J. Localized and generalized urticarial allergic dermatitis secondary to SARS-CoV-2 vaccination in a series of 6 patients. JAAD Case Rep. 2021;14:13–16. doi: 10.1016/j.jdcr.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMahon D.E., Amerson E., Rosenbach M. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]


