Abstract
Background
Maternal stress is potentially a modifiable risk factor for spontaneous preterm birth (sPTB). However, Epidemiologic findings on the maternal stress - sPTB relationship have been inconsistent.
Methods
To investigate whether the maternal stress - sPTB associations may be modified by genetic susceptibility, we performed genome-wide gene×stress interaction analyses in 1,490 African-American women from the Boston Birth cohort who delivered term (n=1,033) or preterm (n=457) infants. Genotyping was performed using Illumina HumanOmni 2.5 array. Replication was performed using data from the NICHD genomic and Proteomic Network (GPN) for PTB Research.
Results
rs35331017, a T-allele insertion/deletion polymorphism in the protein-tyrosine phosphatase receptor Type D (PTPRD) gene, was the top hit that interacted significantly with maternal lifetime stress on risk of sPTB (PG×E =4.7×10−8). We revealed a dose-responsive association between degree of stress and risk of sPTB in mothers carrying the insertion/insertion genotype; but an inverse association was observed in mothers carrying the heterozygous or deletion/deletion genotypes. This interaction was replicated in African-American (PG×E =0.088) and Caucasian mothers (PG×E =0.023) from the GPN study.
Conclusion
We demonstrated a significant maternal PTPRD × stress interaction on sPTB risk. This finding, if further confirmed, may provide new insight into individual susceptibility to stress-induced sPTB.
Introduction
In the United States, more than 10% of all live births are preterm (defined as < 37 completed weeks of gestation). Preterm birth (PTB) remains a leading cause of infant mortality and morbidity in the US and the globe,(1, 2) and can lead to a cascade of health problems later in life.(3, 4) Of all PTBs, about 70% are spontaneous with either preterm labor (that is, having regular contractions and cervical changes at < 37 weeks of gestation) or preterm premature rupture of membranes, and the remaining PTBs are medically indicated that occur largely due to gestational complications. Women of African ancestry are known to bear a disproportionally high rate of PTB than women of other ethnicities.(5) However, risk factors of PTB that may underpin this disparity remain largely unknown.
Maternal perceived stress, in the form of acute, chronic, pregnancy-related and/or other life event-related stressors, is widespread in the general population and even more so among African Americans (AAs).(6) Maternal stress is potentially an important modifiable social determinant of maternal and child health in the US.(7) Although many epidemiologic studies have attempted to link maternal stress with risk of PTB, the results have been inconclusive.(7–14) Such inconsistencies may be due to multiple factors. Asides from study design or methodological issues, one possibility that contributes to this inconsistency is the effect modification by maternal genetic susceptibility. It is likely that women of different genetic backgrounds vary in their biological vulnerability to stress, leading to differences in stress - PTB associations. Boyce has proposed the “orchid vs dandelion” theory,(15) which also suggests that certain genetic variants can increase a person’s susceptibility to stressors. This plausibility was further supported by previous epidemiological studies that demonstrated a significant impact of the interaction between maternal genes and perceived stress on multiple child health outcomes. (16–18) Although there is an increasing number of genome-wide association studies (GWAS) of PTB (19, 20), few studies have been conducted to systematically investigate maternal gene × stress interactions on PTB, especially on a genome-wide scale.
To fill the aforementioned research gap, in this study, we performed genome-wide interaction analyses to explore common single nucleotide polymorphisms (SNPs) that may interact with maternal perceived stress (lifetime and during pregnancy) to affect PTB risk in African American women. We used a two-stage case-control study design (including the discovery and the replication stages) and focused on spontaneous PTB (sPTB) to reduce phenotypic heterogeneity. We further performed a meta-analysis combining the discovery and replication samples.
Materials and Methods
The study cohort and study population in the discovery stage
The study participants in the discovery stage were enrolled in the Boston Birth Cohort (BBC), an ongoing longitudinal, multi-ethnic, predominantly urban, low income minority birth cohort designed to identify gene-environment interactions associated with prematurity and other adverse birth outcomes.(21) Briefly, since 1998, mother-infant dyads have been recruited 1–3 days post-delivery and interviewed using a standard questionnaire at Boston Medical Center. Pregnancies resulting from in-vitro fertilization, multiple gestations, and/or pregnancies with fetal chromosomal abnormalities or major birth defects were excluded. After giving written informed consent, each enrolled mother was interviewed using a standard questionnaire to collect data on demographic variables, lifestyle and dietary intake. A maternal blood sample was obtained within 24–72 hours after delivery and an umbilical cord blood sample was obtained at delivery. The study protocol was approved by the Institutional Review Boards of Boston University Medical Center, and of the Johns Hopkins Bloomberg School of Public Health.
As we reported previously(22), 698 biologically-unrelated AA mothers of preterm babies (PTB, < 36.8 weeks of gestation) and 1,035 AA mothers of term babies (TB, > 37 weeks of gestation) who are frequency matched with the cases on maternal country of origin (Haitian or non-Haitian), maternal age at delivery (+/− 5 years), parity and year of delivery, were successfully genotyped for the GWAS of PTB in the BBC. After removing 237 mothers with medically-indicated PTB and 6 mothers with missing data for maternal perceived stress, this study included 457 mothers of sPTB (cases) and 1033 mothers of TBs (controls) as the discovery sample.
Phenotype definition and covariates
Gestational age was assessed by early (<20 weeks) prenatal ultrasound and/or the first day of the last menstrual period. Spontaneous PTB (sPTB) was defined as a birth occurring secondary to documented active preterm labor (uterine contractions with cervical effacement and dilation at <37 weeks) or premature rupture of membranes at <37 weeks without uterine contractions or both. Early sPTB was defined as a birth occurring <33 weeks of gestation, and late sPTB as a birth occurring from 33 to 366/7 weeks.
Maternal perceived lifetime stress and stress during pregnancy were self-reported by mothers using a standard questionnaire interview, with the following two questions: (1) “how would you characterize the amount of stress in your life in general?” and (2) “How would you characterize the amount of stress in your life during this pregnancy?”. The response options for both questions included: 0 = not stressful (or low), 1 = average, or 2 = very stressful (or high). Other maternal characteristic variables were also collected through a questionnaire interview,(23) including: smoking during pregnancy, which was classified as “never smoker” (did not smoke cigarettes throughout the index pregnancy), “former smoker” (only smoked in the 3 months before pregnancy or during the first trimester), or “continuous smoker” (smoked continuously from pre-pregnancy to delivery); and, social support from the baby’s father, which was classified as “none”, “a little”, “a good amount”, or “an excellent amount”. Pre-pregnancy body mass index (BMI) was calculated as self-reported pre-pregnancy weight (kg) divided by height squared (m2).
Genome-wide genotyping and genetic ancestry estimation
DNA samples from the proposed mothers were quantified and then genotyped by the Center for Inherited Disease Research using the Illumina HumanOmni 2.5 array. The raw genome-wide genotyping and phenotypic data from the BBC have been deposited in the NIH dbGaP database (entry # phs000332.V3.P2). Detailed information on genotyping and quality control steps can be found in our previous publication.(22) A total of 2,160,368 SNPs from 1,733 biologically unrelated mothers passed the quality control steps and were available for SNP imputation. Phasing was performed using SHAPEIT(24) and SNP imputation was done using IMPUTE2(25) software, with all individuals in the 1000 Genomes Project (1000GP) as the reference panel.
Genetic ancestry for each subject was then computed by principal component analysis (PCA) using Eigenstrat (26), with all individuals in the 1000GP as the reference. Those mothers (n=9) whose estimated genetic ancestry was inconsistent with self-reported African American ancestry were removed from the subsequent data analyses, as we previously reported. (22) The estimated genetic ancestry, represented by the first three principal components from PCAs, was then included as three covariates in subsequent analyses.
Statistical analyses in the discovery stage
Population characteristics in the PTB case and control groups were compared using t-tests for continuous variables or chi-square tests for categorical variables. The genome-wide genotyped SNP × stress interactions were tested using the conventional 1-degree of freedom (df) interaction test. We added each SNP (under an additive genetic model, all with minor allele frequency [MAF] > 2%), maternal perceived stress (lifetime or during pregnancy, coded as 0=low, 1=average, 2=high, and treated as an ordered discrete variable) and their interaction term into a logistic regression model in PLINK(v1.07) (27), with adjustment of covariates including genotyping batch, maternal genetic ancestry, age at delivery, marital status, parity, social support from the baby’s father and newborn sex. The genome-wide suggestive and significance thresholds were set as P <5.0×10−7 and P <5.0×10−8, respectively. Manhattan and quantile-quantile (Q-Q) plots were generated using the R package GWASTools (28) to present genome-wide interaction associations. For any genomic loci having significant or suggestive interactions with maternal stress, imputed SNPs nearby (+/− 1Mb), with MAF > 0.02, were further analyzed for their interactions with maternal stress on risk of sPTB using the logistic regression model as described above. The LocusZoom plot was then generated using a published web tool(29) to locate the most significant locus or SNP.
The identified interactions in the discovery sample were validated in the replication samples as describe below. For the validated gene × stress interactions, additional analyses were also performed to test whether the identified interaction varied by different PTB subtypes (i.e., early vs late sPTB) or by newborn sex.
Replication Studies
The replication study was conducted using the epidemiological and GWAS data from the NICHD Genomic and Proteomic Network for Preterm Birth Research (the GPN study, dbGaP entry #phs000714.v1.p1). The GPN study, as reported previously by Zhang et al (30), is to investigate genome-wide associations of sPTB in multi-ethnic populations. Mothers of sPTB (defined as birth at 20 – 336/7 weeks) and of TB controls (defined as birth at 39 – 416/7 weeks) were matched on race/ethnicity, maternal age, and parity, and genotyping was performed using Affymetrix Genome-wide Human SNP Array 6.0. After data cleaning using similar criteria as in the BBC samples and removing individuals with missing data on maternal stress or on the targeted SNP, the GPN study had 337 AA mothers and 738 Caucasian mothers for replication. Maternal stress was self-reported based on questionnaire interview, with the question about “felt nervous and stressed?”. Due to the limited number of AA mothers, we recoded maternal stress into three categories: “low” (if the mothers reported “never” or “almost never” stressed), “average”(if mothers reported “sometimes” or “fairly often”),” or “high” (if mothers reported “very often”). The gene × stress interactions were analyzed using the conventional 1-d.f. test based on the logistic regression model, with the adjustment of genetic ancestry, maternal age at enrollment, parity, marital status, and newborn sex. For the imputed SNP rs35331017, the best-guessed genotype was applied for interaction tests.
Results
Population characteristics of the discovery sample
After data cleaning steps (see “Materials and Methods”), the discovery sample of this study included 457 AA mothers of sPTB (cases) and 1033 AA mothers delivering at term (controls) from the BBC. Their population characteristics are presented in Table 1. Compared to controls, mothers of sPTB were more likely to have high lifetime stress (16.2% vs 10.2%, P=0.001), to be unmarried (72.9% vs 66.4%, P=0.013) and to smoke during pregnancy (17.1% vs 9.4%, P <0.001); and mothers of sPTB were less likely to receive substantial support from the baby’s father (P=0.001) or from other family members/friends (P =0.003). In comparison, maternal stress during pregnancy was only marginally different between these two groups (23.6% vs 18.1%, P=0.071).
Table 1.
Population characteristics of 1,490 African-American mothers from the Boston Birth Cohort
| Maternal characteristics a | Mothers of TB (Controls) | Mothers of sPTB (Cases) | P-value b | |
|---|---|---|---|---|
|
| ||||
| n | 1033 | 457 | ||
| Age at delivery (years), mean ± SD | 28.3±0.20 | 28.5±0.32 | 0.603 | |
| Gestational age at delivery (weeks), mean ± SD | 39.6±0.04 | 33.3±0.17 | <0.001 | |
| Age at delivery (years), n (%) | 0.400 | |||
| <20 | 115 (11.1) | 53 (11.6) | ||
| 20–29.9 | 503 (48.7) | 208 (45.5) | ||
| 30–34.9 | 239 (23.1) | 102 (22.3) | ||
| ≥ 35.0 | 176 (17.1) | 94 (20.6) | ||
| Pre-pregnancy BMI (kg/m2), n (%) | ||||
| <18.5 | 36 (3.5) | 18 (3.9) | 0.189 | |
| 18.5–24.9 | 445 (43.1) | 192 (42.0) | ||
| 25.0–29.9 | 261 (25.3) | 136 (29.8) | ||
| ≥ 30 | 233 (22.6) | 82 (17.9) | ||
| Missing | 58 (5.6) | 29 (6.3) | ||
| Marital status, n (%) | Married | 347 (33.6) | 124 (27.1) | 0.013 |
| Parity, n (%) | 0 | 439 (42.4) | 191 (41.8) | 0.096 |
| 1 | 297 (28.8) | 112 (24.5) | ||
| ≥ 2 | 297 (28.8) | 154 (33.7) | ||
| Education level, n (%) | College or above | 406 (39.3) | 160 (35.1) | 0.130 |
| Smoking during pregnancy, n (%) | ||||
| Never | 853 (82.6) | 337 (73.7) | <0.001 | |
| Former smoker | 76 (7.4) | 41 (9.0) | ||
| Current smoker | 97 (9.4) | 78 (17.1) | ||
| Missing | 7 (0.7) | 1 (0.2) | ||
| Alcohol drinking during pregnancy | 74 (7.2) | 43 (9.4) | 0.062 | |
| Maternal lifetime stress | Low | 395 (38.2) | 144 (31.5) | 0.001 |
| Average | 533 (51.6) | 239 (52.3) | ||
| High | 105 (10.2) | 74 (16.2) | ||
| Maternal stress during pregnancy | ||||
| Low | 384 (37.2) | 148 (32.4) | 0.071 | |
| Average | 459 (44.4) | 200 (43.8) | ||
| High | 187 (18.1) | 108 (23.6) | ||
| Missing | 3 (0.3) | 1 (0.2) | ||
| Support from the baby’s father | No or little | 177 (17.1) | 102 (22.3) | 0.001 |
| Fairy or more | 791 (76.6) | 310 (67.8) | ||
| Missing | 65 (6.3) | 45 (9.8) | ||
| Support from family/friends | No or little | 76 (7.3) | 40 (8.8) | 0.003 |
| Fairy or more | 910 (88.2) | 377 (82.4) | ||
| Missing | 47 (4.5) | 40 (8.8) | ||
| Newborn sex: Male | 526 (50.9) | 238 (52.1) | 0.680 | |
TB: Term birth; sPTB: Spontaneous preterm birth; BMI: Body mass index; SD: Standard deviation
n (%) are shown in the table, if not specified.
Each variable was compared between sPTB cases and TB controls, using chi-squared and t-test, respectively, for categorical and continuous variables.
Genome-wide screening for maternal SNP × maternal perceived stress interactions
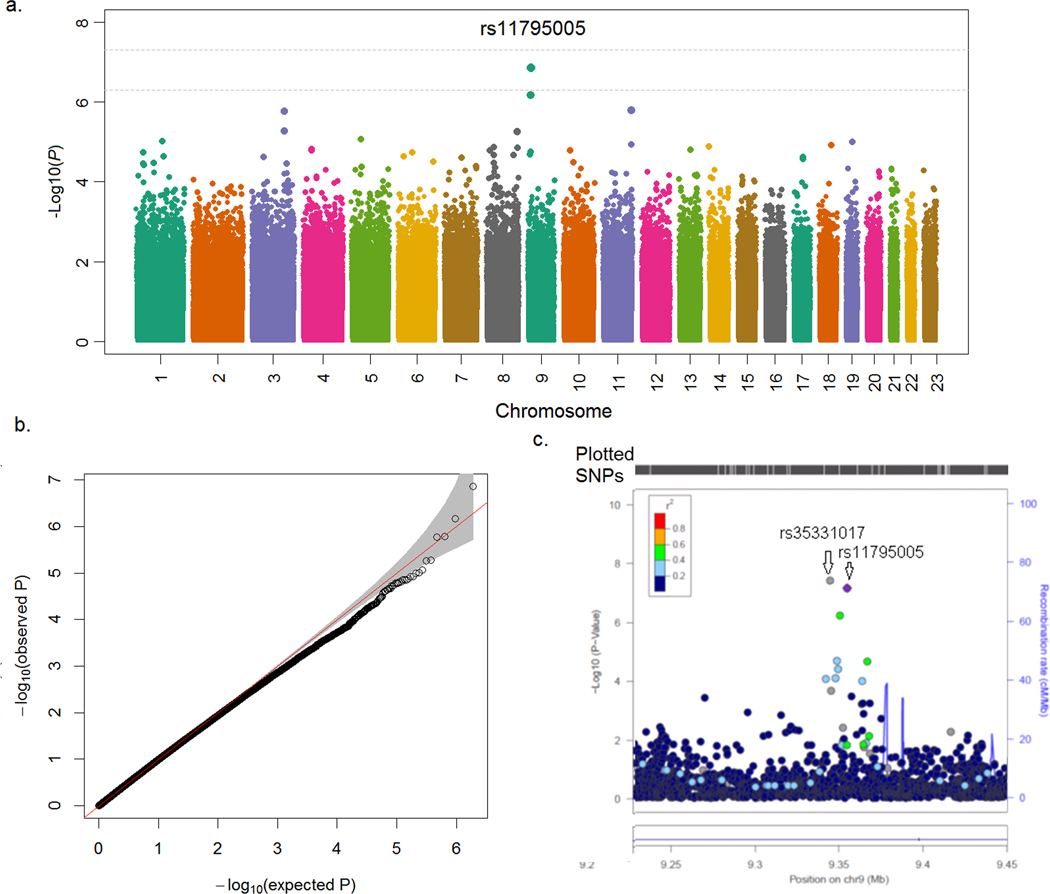
At the discovery stage, we analyzed genome-wide SNP interactions with maternal lifetime stress and with maternal stress during pregnancy, separately, on risk of sPTB. After adjustment for covariates (see “Materials and Methods”), we found a suggestive genome-wide interaction between rs11795005 at 9p24.1-p23 and maternal lifetime stress on risk of sPTB (PG×E =1.4×10−7, Figure 1a). There was no evidence of genomic inflation in our data analyses, as demonstrated by the Q-Q plot (Figure 1b). The identified SNP, rs11795005, is located within an intronic region of the protein-tyrosine phosphatase receptor Type D (PTPRD) gene. Following SNP imputation of this genomic region, we identified another SNP, rs35331017, that demonstrated a genome-wide significant interaction with maternal lifetime stress (PG×E =4.7×10−8, Fig. 1c) on risk of sPTB. Of note, rs35331017 and rs11795005 are in significant linkage disequilibrium (r=0.97). In comparison, we did not identify any genomic significant or suggestive regions interacting with maternal stress during pregnancy on risk of sPTB (Supplementary Fig S1).
Figure 1. Manhattan, quantile-quantile (Q-Q), and locuszoom plot of the genome-wide interaction associations with maternal lifetime stress on spontaneous PTB, in 1,490 African-American mothers from the Boston Birth Cohort.
a-c manhattan plot, Q-Q plot and locuszoom plot, respectively, for the genome-wide interaction analyses performed using the conventional 1-degree of freedom interaction test based on the multiple logistic regression models, adjusted for genotyping batch, maternal genetic ancestry, age at delivery, parity, marital status, social support from the baby’s father and newborn sex.
Rs35331017 × maternal stress interaction and sensitivity analyses
SNP rs35331017 is a T-nucleotide insertion (I) / deletion (D) polymorphism with a minor allele (or D allele) frequency of 5% in the BBC. This variant was analyzed under a dominant model (the II genotype versus the ID/DD genotype) in subsequent analyses, given that only 4 women carried the DD genotype. Table 2 presents the odds ratios (ORs) of maternal lifetime stress on risk of sPTB, stratified by the rs35331017 genotypes. Among women carrying the rs35331017-II genotype, those reporting average and high lifetime stress had 1.5 (95% CI=1.1–1.9, P=0.007) and 2.1 times (95% CI=1.4– 3.1, P=0.0003) increased odds of sPTB, respectively, compared to mothers reporting low lifetime stress. However, in women carrying the rs35331017-ID/DD genotype, those reporting high lifetime stress demonstrated a reduction in the odds of sPTB compared to women reporting low lifetime stress (OR= 0.05, 95% CI= 0.01–0.32; P=0.001). The interacting effects of the rs35331017 genotype and maternal stress are presented in Figure 2, which further demonstrates a dose-response positive association between maternal lifetime stress and risk of sPTB only in mothers carrying the rs35331017-II genotype. We then carried out similar analyses to explore whether there was an interaction effect between the rs35331017 genotype and maternal stress during pregnancy on risk of sPTB. We found a similar pattern, although the effect size was relatively modest (Table 2 & Fig 2, PG×E = 1.2 ×10−5).
Table 2.
Stratified analysesa by genotypes of the PTPRD rs35331017 variant for the association between lifetime stress and spontaneous PTB in the mothers from the Boston Birth Cohort
| Maternal stress | Mothers carrying rs35331017 II genotype | Mothers carrying rs35331017-ID / DD genotype | Pint c | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| sPTB | TB | OR (95% CI)b | Pb | sPTB | TB | OR (95% CI)b | Pb | ||
|
| |||||||||
| Maternal lifetime stress | |||||||||
| Low a | 116 | 368 | 1.0 | Ref | 28 | 26 | 1.0 | Ref | |
| Average | 226 | 473 | 1.46 (1.11–1.93) | 0.007 | 11 | 57 | 0.10 (0.03–0.27) | 1.1×10−5 | |
| High | 72 | 91 | 2.09 (1.40–3.11) | 0.0003 | 2 | 14 | 0.05 (0.01–0.32) | 0.0014 | 4.7×10−8 |
|
| |||||||||
| Maternal stress during pregnancy | |||||||||
| Low a | 123 | 352 | 1.0 | Ref | 23 | 30 | 1.0 | Ref | |
| Average | 186 | 415 | 1.25 (0.94–1.65) | 0.123 | 12 | 41 | 0.26 (0.10–0.68) | 0.006 | |
| High | 104 | 163 | 1.60 (1.14–2.26) | 0.007 | 4 | 23 | 0.11 (0.03–0.43) | 0.0014 | 1.2×10−5 |
CI: confidence interval. OR: odds ratio; sPTB: Spontaneous preterm birth; TB: Term birth.
The “low” category as the reference group in each genotype strata.
Adjusted for genotyping batch, maternal genetic ancestry, age at delivery, marital status, parity, social support from the baby’s father and newborn sex.
The interaction effect was analyzed in the total sample by adding lifetime stress, rs35331017 (under the additive genetic model) and their interaction term into the regression model, with adjustment of the same covariates as mentioned above.
Figure 2. Joint associations between rs35331017 in the PTPRD gene and maternal perceived stress on sPTB in African-American mothers from the BBC.
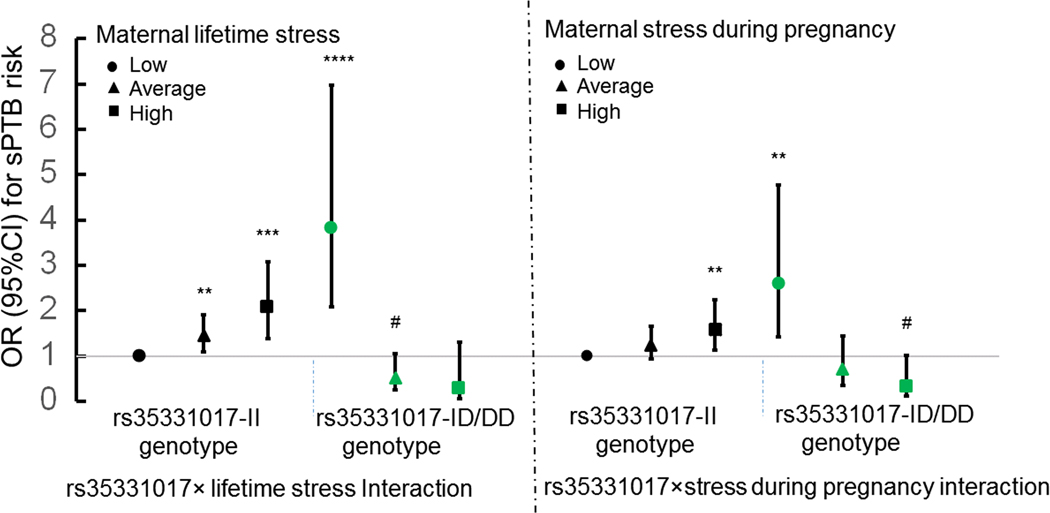
Y axis reflects the odds ratio (OR) and 95% confidence interval (CI) of sPTB risk for each subgroup stratified by the genotype of rs35331017 and maternal lifetime stress (Fig. 2A) or stress during pregnancy (Fig. 2B), with low-stress mothers carrying the rs35331017-II genotype as the reference group. This analysis was conducted based on multiple logistic regression models adjusted for genotyping batch, maternal genetic ancestry, age at delivery, marital status, parity, social support from the baby’s father and newborn sex. # 0.05<P < 0.10; ** P < 0.01; *** P < 0.001, **** P < 0.0001.
We further performed sensitivity analyses to assess the robustness of the maternal rs35331017× lifetime stress interaction on PTB subtypes. As presented in Supplementary Table 1, the effect size and direction of the rs35331017×maternal lifetime stress interaction was comparable for early sPTB (<33 weeks; PG×E =0.0005) and late sPTB (33–36 weeks; PG×E =1.3×10−6). Lastly, we stratified our interaction analyses by newborn sex, which revealed that the rs35331017 × maternal lifetime stress interaction on risk of sPTB tended to be stronger among females (PG×E =1.8×10−6) than males (PG×E =0.046) (Supplementary Table 2).
Replication studies and meta analyses
The replication sample includes 337 AA mothers and 738 Caucasian mothers from the GPN study, with their population characteristics shown in Supplementary Table 3. Among the 337 AA mothers, a marginally significant rs35331017 × maternal stress interaction was observed on sPTB (PG×E = 0.088), with the association in the same direction as in the BBC. Interestingly, we found that there was a rs35331017 × maternal stress interaction for sPTB risk in 738 Caucasian mothers (PG×E = 0.023, Table 3 & Supplementary Fig 2), as well as in the combined cohort of AA and Caucasian mothers (P =0.009) from the GPN study (which included adjustment for maternal ancestry). With the meta analyses combining the discovery and replication samples, we identified an even stronger rs35331017 × maternal stress interaction on risk of sPTB (P =4.5×10−10).
Table 3.
The main effects of maternal rs35331017, maternal stress and their interaction effects on spontaneous PTB in the mothers from the Boston Birth Cohort and from the GPN study
| Variable | BBC discovery an=1,484b | GPN Replication cAfrican-American Mothers n=337 | GPN Replication cCaucasian Mothers n=738 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| rs35331017 | 3.51 | 1.98–6.22 | 1.73×10−5 | 45.6 | 0.43–482.0 | 0.108 | 4.35 | 1.18–16.5 | 0.030 |
| Perceived stress | 1.44 | 1.19–1.75 | 1.75×10−4 | 1.76 | 1.14–2.71 | 0.010 | 1.78 | 1.35–2.56 | 0.0005 |
| rs35331017×stress interaction d | 0.14 | 0.07–0.28 | 4.7×10−8 | 0.12 | 0.01–1.37 | 0.088 | 0.46 | 0.20–0.86 | 0.023 |
BBC: Boston Birth Cohort; CI: confidence interval; GPN: Genomic and Proteomic Network for Preterm Birth Research; PTB: preterm birth; OR: odds ratio.
The analysis was conducted using the logistic regression model, adjusted for genotyping batch, maternal genetic ancestry, age at delivery, marital status, parity, social support from the baby’s father and newborn sex in the BBC discovery sample.
n=1484 after removing 6 mothers with missing genotypic data on rs35331017.
The analysis was conducted using the logistic regression model, adjusted for genetic ancestry, maternal age at enrollment, parity, marital status and newborn sex in the GPN replication sample.
Here P value for the interaction effect was estimated using an interaction term of maternal stress and the 35331017 genotype (under an additive genetic model).
Discussion
This is the first study to investigate maternal genome-wide gene × maternal perceived stress interactions on the risk of sPTB in African Americans, a high-risk population for PTB. Specifically, we have identified a genome-wide significant interaction between maternal PTPRD genetic variants and maternal lifetime stress in AA women from the BBC, and further replicated this interaction in AA and Caucasian women from the GPN study. These findings, if further validated in other large populations, underscore the importance of considering maternal genetic factors and G×E interactions when assessing socio-environmental determinants of sPTB.
Our study may contribute new insight into the relationship between maternal stress and sPTB. To our knowledge, previous studies evaluating associations between maternal perceived stress and risk of sPTB are inconclusive. Some studies have reported maternal perceived stress as a risk factor for sPTB,(8, 11, 14) while others demonstrated a negative association between them.(9, 10, 13) Findings from the current study may help to explain such inconsistent findings. Our data indicated that the associations between maternal lifetime stress and risk of PTB may vary in women with different genetic backgrounds. Among mothers carrying the rs35331017-II genotype, those reporting high lifetime stress were at about 2-fold higher risk of sPTB than those reporting low lifetime stress. However, this association was reversed among mothers carrying the DD or ID genotype at rs35331017. A similar pattern, but with a relatively modest effect size, was observed for the interaction between rs35331017 genotypes and maternal stress during pregnancy on risk of sPTB. Furthermore, we observed that, among all the genotyped mothers, high lifetime stress was more prevalent in mothers of sPTB than in mothers of TB, while maternal stress during pregnancy was only marginally different between these two groups. One possible explanation is that lifetime stress is better able to capture cumulative stress that may affect maternal preconception health as well as health during pregnancy, which is consistent with a life course health framework.
Although the underlying mechanism linking maternal perceived stress with risk of sPTB is not yet clear, several biological pathways have been implicated. First, activation of the hypothalamic-pituitary-adrenal axis, a major endocrine pathway, is activated in response to stress. Maternal stress triggers norepinephrine and cortisol release, which results in activation of placental corticotrophin-releasing hormone (CRH) gene expression. CRH gradually increases as the pregnancy progresses, and serves as a “placental clock” determining the timing of parturition.(31) It has been hypothesized that in mothers experiencing high levels of stress, increased CRH levels may alter the timing of parturition and ultimately promote PTB.(32, 33) Another biological pathway implicated in the pathophysiology of sPTB is inflammation/infection. Higher stress levels are associated with higher circulating levels of inflammatory markers (such as C-reactive protein, interleukin (IL) 1 beta and IL-6), and lower levels of anti-inflammatory cytokines such as IL-10, (34, 35) leading to the T-helper 1 skewing and increased sPTB risk.
The 35331017 × stress interaction was further replicated in Caucasian women from the GPN study, suggesting a shared effect across different ethnic populations. It is largely unknown how the identified maternal rs35331017 × maternal perceived stress interaction may affect sPTB risk. SNP rs35331017 is a T-nucleotide insertion/deletion polymorphism located in the intronic region of the gene coding protein-tyrosine phosphatase receptor Type D (PTPRD). Protein PTPRD, which is highly expressed in the human brain, plays a potential role in psychopathology because it can bidirectionally induce pre- and post-synaptic differentiation of neurons by mediating interaction with IL1 receptor accessory protein and IL1 receptor accessory protein like 1. (36) Previous studies have linked the PTPRD gene with multiple traits, such as substance addiction, (37, 38) and gestational diabetes,(39) which show some degree of association with PTB. For example, It is likely that substance addiction during pregnancy, which may directly or indirectly target placental transport systems between maternal and fetal circulation, may lead to accumulated levels of serotonin and norepinephrine in the intravillous space.(40) Increased levels of these neurotransmitters may be associated with an increased risk of sPTB via altering CRH levels, a shared pathway underlying maternal stress - PTB associations, which may help to explain the interaction effect identified in this study. It would be interesting to further investigate whether the identified rs35331017 × maternal stress interactions on sPTB risk are mediated by CRH levels.
The implication of G×E interactions in sPTB could be far-reaching. In addition to further elucidating the “missing heritability” of PTB, the discovery of significant G×E interactions creates the opportunity for translational application. For example, our findings, if further confirmed, could prove valuable for the prediction and prevention of sPTB, since rs35331017 genotypes and maternal perceived lifetime stress can be measured well before pregnancy and maternal stress is, to an extent, a modifiable factor. Our findings also indicate that effective prevention strategies targeting sPTB may differ for women with different genetic backgrounds. Specifically, women carrying the rs35331017-II genotype are at particular risk for stress-related sPTB, and they may therefore benefit from prioritizing stress reduction before and during pregnancy to decrease the risk of sPTB. Conversely, women carrying the rs35331017 DD or ID genotype may demonstrate more resilience in the face of acute and chronic stress, and for these women, emphasis on avoiding and mitigating other risk factors may be more effective in preventing PTB. However, these strategies require further investigation.
Several limitations should be acknowledged. First, perceived stress levels during a mother’s lifetime and during pregnancy were self-reported by the mothers, which may lead to recall bias. We believe that this bias is largely random and does not significantly affect our G×E findings, since mothers were not aware of their genotypes at the time of report, and the findings were successfully replicated in an independent cohort. Second, other stress-related variables, such as depressive symptoms and adverse life events, are less frequent and thus were not analyzed in the current study due to limited statistical power. Third, the current study may have a limited power to test G×E for SNPs with low minor allele frequencies (i.e., MAF < 2 %) and/or with relatively modest interaction effects with maternal stress. Fourth, the results of the study remain purely associative, with unknown mechanisms, and warrant future investigation. The identified variant, rs35331017, is an insertion/deletion polymorphism in an intronic region, whose function is not known. Finally, the BBC was specifically designed to study PTB in a predominantly urban, low-income minority population. Caution is warranted when generalizing our findings to other populations.
In conclusion, this is the first study to demonstrate a significant genome-wide maternal gene × stress interaction on sPTB risk in a high-risk AA population in Boston, MA. Our study highlights the importance of considering genetic factors and G×E interactions when investigating socio-environmental determinants of sPTB. Our findings, if further confirmed, may provide new insight into individual susceptibility to stress-induced sPTB; help advance prediction and prevention of sPTB; and stimulate future studies on the functionality of the identified target gene and SNPs, and validations of this finding in other ethnic groups.
Supplementary Material
Impact.
This was the first preterm study to demonstrate a significant genome-wide gene-stress interaction in African Americans, specifically, PTPRD gene variants can interact with maternal perceived stress to affect risk of spontaneous preterm birth.
The PTPRD × maternal stress interaction was demonstrated in African Americans and replicated in both African Americans and Caucasians from the GPN study.
Our findings highlight the importance of considering genetic susceptibility in assessing the role of maternal stress on spontaneous preterm birth.
Acknowledgements
We thank all of the study participants in the BBC for supporting this study. We are also grateful for the dedication and hard work of the field team at the Department of Pediatrics, Boston University School of Medicine, and for the support of the obstetric nursing staff at Boston Medical Center. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to the Johns Hopkins University, contract numbers HSN268200782096C and HHSN268201200008I. The GWAS data cleaning was performed by Dr. Laurie and her team at Washington University following the GENEVA protocol. The authors thank Linda Rosen of the Boston University Clinical Data Warehouse for assistance in obtaining relevant clinical information; the Clinical Data Warehouse service is supported by Boston University Clinical and Translational Institute and the National Institutes of Health Clinical and Translational Science Award (grant U54-TR001012).
Funding Support
This work is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; grant numbers include R03HD096136, R21HD085556, 2R01HD041702, R01HD086013 and R01HD098232) and the Johns Hopkins Population Center (NICHD R24HD042854). The Boston Birth Cohort (the parent study) is also supported in part by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) grants (R40MC27443 and UJ2MC31074). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by NIH, HRSA, HHS or the U.S. Government.
Footnotes
Patient consent
Each participant provided written informed consent prior to participation. The study protocol was approved by the Institutional Review Boards of Boston University Medical Center, and of the Johns Hopkins Bloomberg School of Public Health.
Disclosure statement: The authors declare no completing interests
Category of study: Population study.
References
- 1.Liu L, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 388, 3027–3035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn JE, Kinney M. Preterm birth: now the leading cause of child death worldwide. Sci Transl Med. 6, 263ed221 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Pritchard MA, et al. Autism in Toddlers Born Very Preterm. Pediatrics. 137, e20151949 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Wang G, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA : the journal of the American Medical Association. 311, 587–596 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ Births: Final Data for 2015. Natl Vital Stat Rep. 66, 1 (2017). [PubMed] [Google Scholar]
- 6.Grobman WA, et al. Racial/Ethnic Disparities in Measures of Self-reported Psychosocial States and Traits during Pregnancy. Am J Perinatol. 33, 1426–1432 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadhwa PD, Entringer S, Buss C, Lu MC The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 38, 351–384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub H, Adams M, Kim JJ, Silver RK Antenatal depressive symptoms increase the likelihood of preterm birth. Am J Obstet Gynecol. 207, 329 e321–324 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 191, 691–699 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Kramer MS, et al. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 169, 1319–1326 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Dole N, et al. Maternal stress and preterm birth. Am J Epidemiol. 157, 14–24 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kitai T, et al. A comparison of maternal and neonatal outcomes of pregnancy with mental disorders: results of an analysis using propensity score-based weighting. Arch Gynecol Obstet. 290, 883–889 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Krabbendam L, et al. The impact of maternal stress on pregnancy outcome in a well-educated Caucasian population. Paediatr Perinat Epidemiol. 19, 421–425 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Yonkers KA, et al. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry. 71, 897–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce TW 2019The Orchid and the Dandelion: Why Some Children Struggle and How All Can Thrive. (Knopf, New York, 2019) [Google Scholar]
- 16.Massey SH, et al. Does MAOA increase susceptibility to prenatal stress in young children? Neurotoxicol Teratol. 61, 82–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green CG, et al. Prenatal maternal depression and child serotonin transporter linked polymorphic region (5-HTTLPR) and dopamine receptor D4 (DRD4) genotype predict negative emotionality from 3 to 36 months. Dev Psychopathol. 29, 901–917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mparmpakas D, et al. Differential expression of placental glucocorticoid receptors and growth arrest-specific transcript 5 in term and preterm pregnancies: evidence for involvement of maternal stress. Obstet Gynecol Int. 2014, 239278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, et al. Variants in the fetal genome near pro-inflammatory cytokine genes on 2q13 associate with gestational duration. Nat Commun. 10, 3927 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, et al. Genetic Associations with Gestational Duration and Spontaneous Preterm Birth. N Engl J Med. 377, 1156–1167 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 287, 195–202 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Hong X, et al. Genome-wide approach identifies a novel gene-maternal pre-pregnancy BMI interaction on preterm birth. Nat Commun. 8, 15608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 311, 587–596 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaneau O, Marchini J, Zagury JF A linear complexity phasing method for thousands of genomes. Nat Methods. 9, 179–181 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 44, 955–959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 38, 904–909 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogarten SM, et al. GWASTools: an R/Bioconductor package for quality control and analysis of genome-wide association studies. Bioinformatics. 28, 3329–3331 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruim RJ, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 26, 2336–2337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, et al. A genome-wide association study of early spontaneous preterm delivery. Genet Epidemiol. 39, 217–226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrekoussis T, et al. The role of stress in female reproduction and pregnancy: an update. Ann N Y Acad Sci. 1205, 69–75 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 180, S257–263 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Hobel CJ, Dunkel-Schetter C, Roesch S. Maternal stress as a signal to the fetus. Prenat Neonat Med. 3, 116–120 (1998). [Google Scholar]
- 34.Coussons-Read ME, Okun ML, Nettles CD Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 21, 343–350 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Christian LM, Franco A, Glaser R, Iams JD Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 23, 750–754 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagata A, et al. Mechanisms of splicing-dependent trans-synaptic adhesion by PTPdelta-IL1RAPL1/IL-1RAcP for synaptic differentiation. Nat Commun. 6, 6926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drgon T, et al. “Replicated” genome wide association for dependence on illegal substances: genomic regions identified by overlapping clusters of nominally positive SNPs. Am J Med Genet B Neuropsychiatr Genet. 156, 125–138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhl GR, et al. Cocaine reward is reduced by decreased expression of receptor-type protein tyrosine phosphatase D (PTPRD) and by a novel PTPRD antagonist. Proc Natl Acad Sci U S A. 115, 11597–11602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T, et al. Genetic variants in PTPRD and risk of gestational diabetes mellitus. Oncotarget. 7, 76101–76107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baer RJ, et al. Risk of preterm birth among women using drugs during pregnancy with elevated alpha-fetoprotein. J Perinatol. 37, 220–225 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.