Abstract
The atypical protein kinase C (PKC) isotypes (λ/ιPKC and ζPKC) have been shown to be critically involved in important cell functions such as proliferation and survival. Previous studies have demonstrated that the atypical PKCs are stimulated by tumor necrosis factor alpha (TNF-α) and are required for the activation of NF-κB by this cytokine through a mechanism that most probably involves the phosphorylation of IκB. The inability of these PKC isotypes to directly phosphorylate IκB led to the hypothesis that ζPKC may use a putative IκB kinase to functionally inactivate IκB. Recently several groups have molecularly characterized and cloned two IκB kinases (IKKα and IKKβ) which phosphorylate the residues in the IκB molecule that serve to target it for ubiquitination and degradation. In this study we have addressed the possibility that different PKCs may control NF-κB through the activation of the IKKs. We report here that αPKC as well as the atypical PKCs bind to the IKKs in vitro and in vivo. In addition, overexpression of ζPKC positively modulates IKKβ activity but not that of IKKα, whereas the transfection of a ζPKC dominant negative mutant severely impairs the activation of IKKβ but not IKKα in TNF-α-stimulated cells. We also show that cell stimulation with phorbol 12-myristate 13-acetate activates IKKβ, which is entirely dependent on the activity of αPKC but not that of the atypical isoforms. In contrast, the inhibition of αPKC does not affect the activation of IKKβ by TNF-α. Interestingly, recombinant active ζPKC and αPKC are able to stimulate in vitro the activity of IKKβ but not that of IKKα. In addition, evidence is presented here that recombinant ζPKC directly phosphorylates IKKβ in vitro, involving Ser177 and Ser181. Collectively, these results demonstrate a critical role for the PKC isoforms in the NF-κB pathway at the level of IKKβ activation and IκB degradation.
The transcription factor NF-κB plays a critical role in a number of cell functions, including key inflammatory and immune responses (2, 16). NF-κB is composed of dimers of different members of the Rel protein family (1, 2, 30). The most classical form of NF-κB is a heterodimer of p50 and p65 (RelA) (1, 2, 30) that is sequestered in the cytosol by IκB, which prevents its nuclear translocation and activity (30, 31). Upon cell stimulation by inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) or interleukin 1 (IL-1), IκBα is phosphorylated in residues 32 and 36, which trigger the ubiquitination and subsequent degradation of IκB through the proteasome pathway (31). These events release NF-κB which translocates to the nucleus, where it activates several genes (1, 2, 30, 31). The identification of the kinase responsible for the signal-induced phosphorylation of IκB has been the subject of intense research. Recently, several groups have succeeded in the identification and molecular cloning of two IκB kinase (IKK) activities (IKKα and IKKβ) that phosphorylate residues 32 and 36 of IκBα and whose activity is potently stimulated by TNF-α and IL-1 (9, 22, 25, 33, 34). The IKKs bind NF-κB-inducing kinase (NIK) (25, 33), a member of the mitogen-activated protein (MAP) kinase kinase kinase family that interacts with TNF receptor-associated factor 2 (20), linking IκB degradation and NF-κB activation to the TNF receptor complex. TNF-α and interleukin 1 are potent activators of protein kinase C ζ (ζPKC) in vivo (19, 23, 26). Interestingly, we and others have previously shown that the atypical PKC isoforms ζ and λ/ι play a critical role during NF-κB activation (4–6, 8, 10, 11, 19, 28). Thus, the blockade of the atypical PKCs with either microinjected pseudosubstrate peptide inhibitors (10), antisense oligonucleotides (10, 11), or the transfection of kinase-dead dominant negative mutants of ζPKC or λ/ιPKC (4–6, 8, 11, 19, 28) dramatically impairs NF-κB activation. However, the mechanisms whereby the atypical PKCs participate in this pathway have not yet been elucidated. Because ζPKC is unable to directly phosphorylate IκB (7), it is possible that the signals generated by the stimulation of the atypical PKCs could be mediated by the novel IKKs.
We report here that the atypical PKCs bind to the IKKs in vitro and in vivo. Importantly, overexpression of ζPKC positively modulates IKKβ activity but not that of IKKα whereas the transfection of a ζPKC dominant negative mutant severely impairs the activation of IKKβ but not that of IKKα in TNF-α-stimulated cells. In addition, recombinant active ζPKC dramatically stimulates in vitro IKKβ activity but not that of IKKα from unstimulated cells. Collectively these results demonstrate a critical role for the atypical PKCs in the NF-κB pathway through the regulation of IKKβ activity.
MATERIALS AND METHODS
Plasmids, cell culture, and transfections.
The hemagglutinin (HA)-tagged expression plasmids for ζPKC, λ/ιPKC, Raf, ζPKCCAT, ζPKCMUT, and λ/ιPKCMUT have previously been described (4, 8). The HA-αPKC was made by inserting an EcoRI-EcoRV fragment encompassing the full-length bovine αPKC into pCDNA3. The Flag-IKKβ and IKKα constructs were provided by D. Goeddel (Tularik, Inc.) and A. Israel, respectively. The Flag-IκBα and the p65 constructs were generously provided by D. Ballard (Vanderbilt University). The Flag-IKKα plasmid was made by inserting the EcoRI fragment containing the rat IKKα cDNA into pCDNA3-Flag. Flag-tagged constructs encompassing the kinase or the regulatory domains of IKKα or IKKβ were generated by PCR. The Flag-IKKβKD and Flag-IKKβAA (S177A S181A) constructs were obtained by site-directed mutagenesis (Stratagene). The glutathione S-transferase (GST)-IκBΔC and GST-IκBΔCA32/36 were transformed into Escherichia coli JM101, and expression of GST fusion proteins and their purification on glutathione-Sepharose were carried out according to the manufacturer’s procedures. Cultures of 293 cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, penicillin G (100 μg/ml), and streptomycin (100 μg/ml) (Flow). Subconfluent cells were transfected by the calcium phosphate method (Clontech, Inc.).
In vitro translation and immunoprecipitation.
For in vitro translation studies, ζPKC, ζPKCCAT, λ/ιPKC, αPKC, or Raf were in vitro translated in rabbit reticulocyte lysates, either alone or together with Flag-IKKα, Flag-IKKβ, or their respective catalytic and regulatory domains, exactly as described in the manufacturer’s protocol (Promega), and the Flag-tagged proteins were immunoprecipitated with the monoclonal M2 anti-Flag antibody (Kodak) as described previously (8). Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography in an InstantImager (Packard). For coimmunoprecipitation experiments, subconfluent 293 cells plated on 10-cm-diameter dishes were transfected with 10 μg of expression plasmid. After transfection (36 h), cells were or were not stimulated with 20 ng of TNF-α (Promega) per ml or 5 μM phorbol 12-myristate 13-acetate (PMA) (Sigma) for different times. In some experiments, cells were incubated with 10 nM GF109203X (Calbiochem) for 10 min prior to the stimulation. Cells were then harvested and lysed in buffer A (40 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.1% Nonidet P-40, 6 mM EDTA, 6 mM EGTA, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM PNPP [para-nitrophenyl-phosphate], 300 μM Na3VO4, 1 mM benzamidine, 2 M PMSF [phenylmethylsulfonyl fluoride], aprotinin [10 μg/ml], leupeptin [1 μg/ml], pepstatin [1 μg/ml], 1 mM dithiothreitol [DTT]). The IKK proteins were precipitated with 3 μg of M2 monoclonal antibody to the Flag epitope (Kodak) and 10 μl of protein G-agarose and then immunoblotted with a polyclonal antiserum to the HA-tagged PKCs or to the endogenous PKCs (Santa Cruz Biotechnology, Inc.). The immunocomplexes were washed in a high-salt buffer (500 mM NaCl). Proteins were detected with ECL reagent (Amersham). In another set of experiments, cell extracts prepared as described above were immunoprecipitated with a polyclonal anti-MKP-1 (MAP kinase phosphatase 1) antibody (Santa Cruz Biotechnology, Inc.), and the extensively washed immunocomplexes were analyzed by immunoblotting with monoclonal anti-λ/ιPKC antibody (Transduction Laboratories). For the detection of endogenous IKK a polyclonal anti-IKKα antibody (H-744; Santa Cruz Biotechnology) was used.
IKK kinase assay.
Kinase activity was assayed in a solution consisting of 20 mM HEPES (pH 7.7), 10 mM β-glycerophosphate, 2 mM MgCl2, 2 mM MnCl2, 10 mM PNPP, 300 μM Na3VO4, 1 mM dithiothreitol, 10 μM ATP, 1 mM benzamidine, 2 M PMSF, aprotinin (10 μg/ml), leupeptin (1 μg/ml), pepstatin (1 μg/ml), and 2 μCi of [γ-32P]ATP at 30°C for 30 min. IκB substrate proteins were expressed and purified from E. coli. Flag-tagged IKK immune complexes were isolated as described above and washed in kinase buffer before the level of kinase activity was determined. The kinase reaction was stopped by the addition of 5× SDS-PAGE sample buffer, subjected to SDS-PAGE analysis, and visualized in an InstantImager. In some experiments, the immunoprecipitates of Flag-IKKs, either untreated or inactivated with FSBA [5′-(4-fluorosulfonylbenzoyl)adenine] as described previously (7), were or were not incubated with recombinant preparations of either αPKC (maximally activated by phosphatidylserine plus diacylglycerol according to the manufacturer’s instructions) or a permanently active mutant of ζPKC, both produced from baculovirus in Sf9 insect cells. The recombinant baculovirus αPKC was obtained from Panvera. The recombinant ζPKCCAT was prepared by using the Bac-to-Bac baculovirus expression system (Life Technologies).
Reporter assays.
For reporter gene assays, 293 cells were seeded into six-well plates. Cells were transfected the following day by the calcium phosphate precipitation method with 100 ng of κB-luciferase reporter gene plasmid and various amounts of each expression construct. The total amount of DNA transfected (5 μg) was kept constant by supplementation with the control vector pCDNA3. After 24 h, cells either were left untreated or were stimulated with TNF-α (20 ng/ml) for 6 h prior to harvest. Extracts were prepared, and the level of luciferase activity was determined as described previously (8).
RESULTS
Interaction of PKC isoforms with the IKKs in vitro.
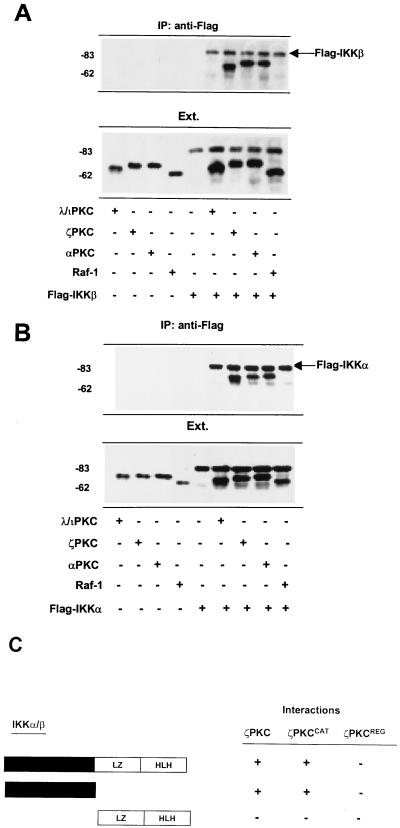
Binding assays were performed with in vitro-translated 35S-labeled Flag-tagged IKKβ or IKKα and HA-tagged λ/ιPKC, ζPKC, αPKC, or Raf-1. The immunoprecipitation of IKKβ with an anti-Flag antibody reveals that IKKβ associates in vitro with both atypical PKCs and αPKC but that it is unable to interact with Raf-1 (Fig. 1A). The same results were obtained when the interaction of IKKα with all these kinases was investigated (Fig. 1B). In order to map the regions in the IKKs and PKCs that mediate their interaction, in vitro-translated 35S-labeled IKKα or IKKβ, or fragments of these kinases encompassing either their catalytic domain or the regulatory region (leucine zipper plus the helix-loop-helix), were incubated with HA-tagged versions of either full-length ζPKC or two fragments corresponding to the catalytic domain and the regulatory region of this kinase. Experiments similar to those whose results are shown in Fig. 1A and B were carried out, and the results are shown in Fig. 1C. Interestingly, it seems that both catalytic domains are responsible for the interaction between IKK and PKC.
FIG. 1.
In vitro interaction of PKC with IKK. 35S-labeled Flag-tagged IKKβ (A) or IKKα (B) and HA-tagged λ/ιPKC, ζPKC, αPKC, or Raf-1 were incubated either alone or in combination as described in Materials and Methods. IKKβ and IKKα were immunoprecipitated (IP) with an anti-Flag antibody, and the immunoprecipitates were fractionated by SDS-PAGE, followed by autoradiography in an InstantImager. An aliquot (one-tenth of the amount of labeled protein used for the in vitro binding reaction) was loaded in parallel (Ext.). Essentially identical results were obtained in two other independent experiments. (C) Summary of results of three independent experiments in which 35S-labeled Flag-tagged versions of either full-length IKKβ or two fragments of this kinase encompassing the catalytic (black box) or the regulatory domain (leucine zipper [LZ] plus the helix-loop-helix [HLH]) were incubated with either full-length ζPKC or its catalytic (ζPKCCAT) and regulatory (ζPKCREG) regions, after which IKKβ was immunoprecipitated as described above, and the level of association of the ζPKC constructs was determined by SDS-PAGE and autoradiography. The numbers at left of panels indicate positions of molecular mass markers in kilodaltons.
Interaction of the atypical PKCs and of αPKC with the IKKs in vivo.
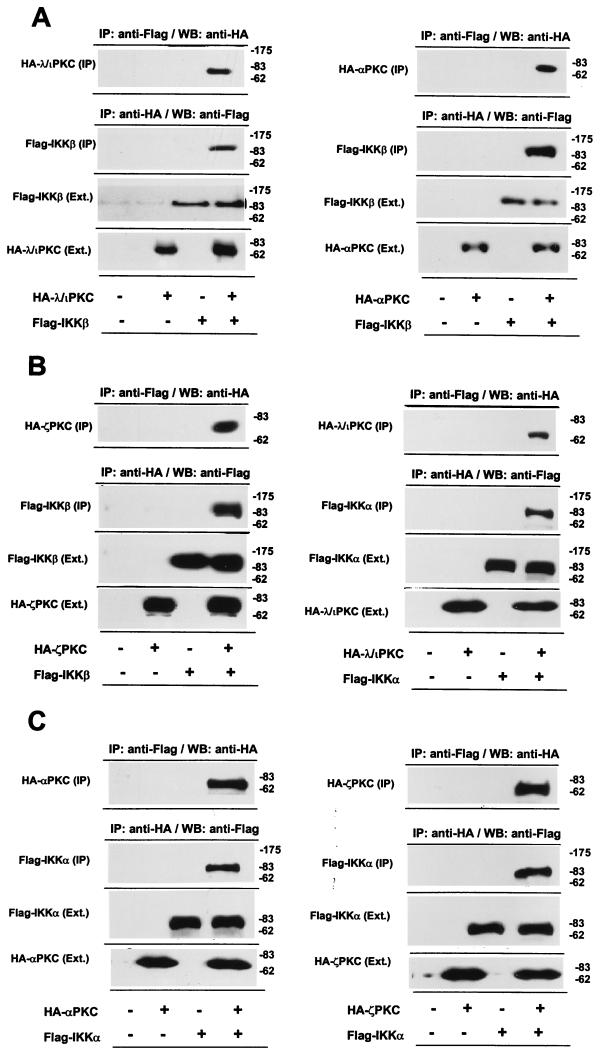
To determine whether the IKKs bind to the atypical PKCs in vivo, 293 cells were transfected with HA-tagged λ/ιPKC, ζPKC, or αPKC along with Flag-tagged IKKβ or IKKα. Cell lysates were immunoprecipitated with an anti-HA antibody, and the immunoprecipitates were resolved by SDS-PAGE and analyzed by immunoblotting with an anti-Flag antibody. An immunoreactive band corresponding to Flag-IKKβ was detected only in immunoprecipitates from cells transfected with HA-λ/ιPKC (Fig. 2A, left panel), HA-αPKC (Fig. 2A, right panel), or HA-ζPKC (Fig. 2B, left panel). Similar data were obtained when cell lysates were immunoprecipitated with an anti-Flag antibody and immunoblotted with the anti-HA antibody (Fig. 2A, left and right panels, and Fig. 2B, left panel). Also, similar results were obtained when the interaction of λ/ιPKC, ζPKC, or αPKC with IKKα was investigated (Fig. 2B, right panel, and both panels of Fig. 2C). In marked contrast, when this experiment was performed with HA-Raf and Flag-IKKβ or Flag-IKKα, no association was detected (data not shown).
FIG. 2.
λ/ιPKC and αPKC interact with IKKβ in vivo. Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected with 10 μg of either pCDNA3 or expression vectors for either HA-λ/ιPKC (A, left panel; B, right panel), HA-αPKC (A, right panel; C, left panel), HA-ζPKC (B, left panel; C, right panel), Flag-IKKβ (A, both panels; B, left panel), or Flag-IKKα (B, right panel; C, both panels) and enough empty vector to give 20 μg of total DNA. Parallel cultures were transfected with 10 μg of Flag-IKKβ or Flag-IKKα plus 10 μg of either HA-λ/ιPKC, HA-αPKC, or HA-ζPKC. After transfection (36 h), cell extracts were immunoprecipitated with an anti-Flag antibody or an anti-HA antibody. Immunoprecipitates were extensively washed in high-salt buffer (500 mM NaCl), fractionated by SDS-PAGE, and analyzed by immunoblotting with anti-HA or anti-Flag antibodies (IP). An aliquot (one-tenth of the amount of extract [Ext.] used for the immunoprecipitation) was loaded in parallel gels and analyzed by immunoblotting with the corresponding antibodies. Essentially identical results were obtained in two other independent experiments. The numbers at right of panels indicate positions of molecular mass markers in kilodaltons. WB, Western blot.
Taken together the data suggest that the atypical PKCs, as well as αPKC, can interact with the IKKs when ectopically expressed in 293 cells.
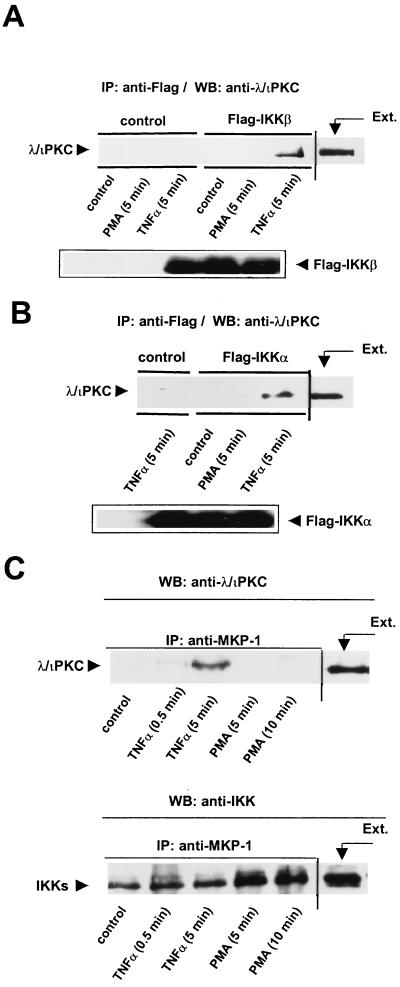
TNF-α-dependent interaction of endogenous λ/ιPKC with the IKKs and the signalosome.
To further analyze these interactions, 293 cells transfected with either Flag-IKKβ or Flag-IKKα were or were not stimulated with TNF-α or PMA. Cell lysates were immunoprecipitated with a monoclonal anti-Flag antibody, and the immunoprecipitates were resolved by SDS-PAGE and analyzed with a polyclonal anti-λ/ιPKC antibody. Interestingly, the addition of TNF-α but not that of PMA promotes the interaction of endogenous λ/ιPKC with IKKβ (Fig. 3A) and IKKα (Fig. 3B). Similar results were obtained when the immunoprecipitates were analyzed with a ζPKC polyclonal antibody that also cross-reacts with λ/ιPKC (data not shown). The lack of a reliable antibody with an absolute specificity for ζPKC precludes the definitive identification of this atypical PKC isoform in the IKK complex. However, the evidence presented in Fig. 1 and 2, in conjunction with the functional data shown below, strongly indicates that most probably native ζPKC, like λ/ιPKC, will associate with the IKKs in TNF-α-activated cells. Of note, αPKC is the only other PKC isotype detectable in 293 cells (14a). When the IKK immunoprecipitates were analyzed by immunoblotting with an antibody selective for αPKC, no association of endogenous αPKC with IKKα or IKKβ was observed in unstimulated cells or in PMA- or TNF-α-treated cells (data not shown).
FIG. 3.
Interaction of endogenous λ/ιPKC with IKKβ and with the signalosome. (A and B) Subconfluent cultures of 293 cells in 100-mm-diameter plates transfected with 10 μg of either Flag-IKKβ (A) or Flag-IKKα (B) were stimulated with 20 ng of TNF-α per ml or PMA (5 μM) for 5 min. Afterward, cell extracts (200 μg) were immunoprecipitated (IP) with a monoclonal anti-Flag antibody, and the immunoprecipitates were analyzed by immunoblotting (WB) with a polyclonal anti-λ/ιPKC antibody. The immunoprecipitates were analyzed in parallel gels by immunoblotting with an anti-Flag antibody. The extract (Ext.) lane contained 20 μg of cell protein. Essentially identical results were obtained in two other independent experiments. (C) Subconfluent cultures of 293 cells in 100-mm-diameter plates were stimulated with 20 ng of TNF-α per ml or PMA (5 μM) for different times. Afterward, cell extracts (200 μg) were immunoprecipitated (IP) with a polyclonal anti-MKP-1 antibody, and immunoprecipitates were analyzed by immunoblotting (WB) with a monoclonal anti-λ/ιPKC antibody. The immunoprecipitates were analyzed in parallel gels by immunoblotting with an anti-IKK antibody. The extract (Ext.) lane contained 20 μg of cell protein. Essentially identical results were obtained in two other independent experiments.
Recent evidence indicates that the IKKs are part of a large complex termed the signalosome that can be immunoprecipitated with an antibody raised against MKP-1 (22). Therefore, it was of interest to determine if the atypical PKCs could be recruited to the signalosome upon cell stimulation. To address this possibility, 293 cells were stimulated either with PMA or TNF-α for different times and cell lysates were immunoprecipitated with a polyclonal anti-MKP-1 antibody and analyzed by immunoblotting with a monoclonal anti-λ/ιPKC antibody. The upper panel of Fig. 3C shows that the stimulation with TNF-α but not that with PMA promotes the recruitment of λ/ιPKC to the signalosome complex. Analysis with an anti-IKK antibody reveals that the anti-MKP-1 immunoprecipitates contained similar amounts of IKK (Fig. 3C, lower panel). However, no association of αPKC with the signalosome complex was detected in these experiments (data not shown). This observation and the lack of any association of endogenous αPKC with the transfected IKKβ or IKKα suggest that αPKC, in contrast to the atypical isoforms, does not stably associate with the IKKs in vivo unless it is overexpressed in cotransfection experiments.
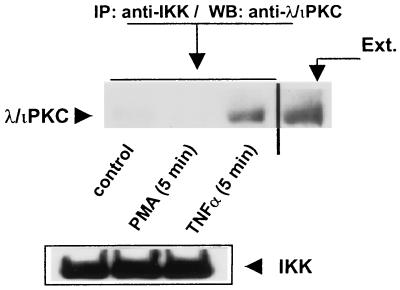
To further establish the interaction of the atypical PKCs with IKK under physiological conditions, 293 cells either were left untreated or were stimulated with TNF-α or PMA for 5 min, after which the native IKK complex was immunoprecipitated with an anti-IKKα antibody that also cross-reacts with IKKβ, and the association of the atypical PKCs was analyzed with an anti-λ/ιPKC antibody. Interestingly, treatment with TNF-α but not that with PMA provokes a reproducible interaction of native λ/ιPKC with native IKK (Fig. 4). Similar results were obtained when the immunoprecipitates were analyzed with a ζPKC polyclonal antibody that also cross-reacts with λ/ιPKC (data not shown). Again, when the immunoprecipitates were analyzed with an anti-αPKC antibody, no association of this PKC with the IKK complex was observed (data not shown).
FIG. 4.
Interaction of endogenous λ/ιPKC with endogenous IKK. Subconfluent cultures of 293 cells in 100-mm-diameter plates were stimulated with 20 ng of TNF-α per ml or PMA (5 μM) for 5 min. Afterward, cell extracts (200 μg) were immunoprecipitated (IP) with a polyclonal anti-IKK antibody, and immunoprecipitates were analyzed by immunoblotting (WB) with a monoclonal anti-λ/ιPKC antibody. The immunoprecipitates were analyzed in parallel gels by immunoblotting with an anti-IKK antibody. The extract (Ext.) lane contained 20 μg of cell protein. Essentially identical results were obtained in two other independent experiments.
Role for the PKCs in the activation of IKKβ and IKKα in response to TNF-α and PMA.
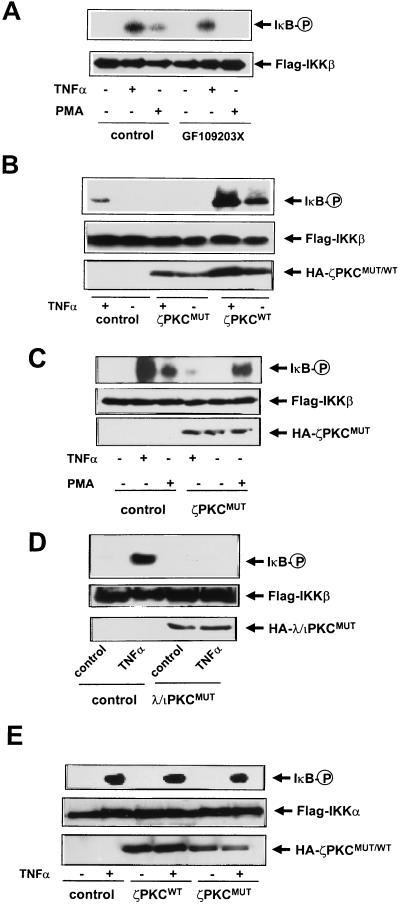
Collectively the above findings suggest that PKCs may be critically involved in the regulation of IKK activity in vivo. To begin analyzing this possibility, 293 cells were transfected with Flag-tagged IKKβ, and 36 h post-transfection they either were left untreated or were stimulated with TNF-α or PMA. Afterward, cell extracts were immunoprecipitated with an anti-Flag antibody, and the ability of IKKβ to phosphorylate a GST-IκB construct containing the first 250 amino acids of IκBα (25) was determined. Cell stimulation with TNF-α or PMA activates the ability of IKKβ to phosphorylate GST-IκB (Fig. 5A) but not a mutant in which Ser32 and Ser36 were replaced by Ala (data not shown). The PMA effect is most likely accounted for by the activation of αPKC. Consistent with this notion, the incubation with GF109203X completely abrogated the activation of IKKβ by PMA but not that by TNF-α (Fig. 5A). This strongly suggests that αPKC mediates the activation of IKKβ by PMA but not that by TNF-α, which is entirely consistent with previous observations demonstrating that the PMA-sensitive PKC isoforms are not involved in the activation of NF-κB by TNF-α but are responsible for the PMA effects (6, 10, 11, and references therein). In order to determine whether the atypical PKCs could be involved in the activation of IKKβ by TNF-α, 293 cells were transfected with Flag-IKKβ along with either a plasmid control or expression vectors for wild-type or dominant negative ζPKC. Thirty-six hours posttransfection, cells were stimulated with either TNF-α or PMA for 7 min and the level of activity of IKKβ was determined as described above. Interestingly, Fig. 5B shows that the simple overexpression of wild-type ζPKC was sufficient to stimulate IKKβ and synergistically increase its activation by TNF-α (Fig. 5B).
FIG. 5.
Role for αPKC and ζPKC in the activation of IKKβ by PMA and TNF-α. (A) Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected with Flag-IKKβ (10 μg), and 36 h posttransfection, cells either were left untreated or were incubated with GF109203X (10 nM) 15 min prior to stimulation with TNF-α (20 ng/ml) or PMA (5 μM) for 7 min. Afterward, Flag-IKKβ was immunoprecipitated, and the level of its activity was determined by using recombinant GST-IκB as the substrate as described in Materials and Methods. (B) Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected with Flag-IKKβ (10 μg) along with 10 μg of empty plasmid or expression vectors for HA-tagged versions of wild-type (ζPKCWT) or dominant negative (ζPKCMUT) ζPKC. Thirty-six hours posttransfection, cells either were left untreated or were stimulated with TNF-α (20 ng/ml) for 7 min. Afterward, Flag-IKKβ was immunoprecipitated, and the level of its activity was determined as described above. (C) Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected with Flag-IKKβ (10 μg) along with 10 μg of empty plasmid or an expression vector for the HA-tagged dominant negative ζPKC (ζPKCMUT). Thirty-six hours posttransfection, cells either were left untreated or were stimulated with TNF-α (20 ng/ml) or PMA (5 μM) for 7 min. Afterward, Flag-IKKβ was immunoprecipitated, and the level of its activity was determined as described above. (D) Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected with Flag-IKKβ (10 μg) along with 10 μg of empty plasmid or expression vectors for HA-tagged dominant negative (λ/ιPKCMUT) λ/ιPKC. Thirty-six hours posttransfection, cells either were left untreated or were stimulated with TNF-α (20 ng/ml) for 7 min. Afterward, Flag-IKKβ was immunoprecipitated, and the level of its activity was determined as described above. (E) Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected with Flag-IKKα (10 μg), and 36 h posttransfection, cells either were left untreated or were stimulated with TNF-α (20 ng/ml) for 7 min. Afterward, Flag-IKKα was immunoprecipitated, and the level of its activity was determined as described above. The expression levels of the different constructs were determined by using the corresponding antitag antibodies. Essentially identical results were obtained in two other independent experiments. P, phosphorylated protein.
Importantly, the expression of a dominant negative mutant of ζPKC severely impaired the activation of IKKβ by TNF-α (Fig. 5B and C) but not that by PMA (Fig. 5C). Similar results were obtained when cells were transfected with a dominant negative mutant of λ/ιPKC (Fig. 5D). Next, 293 cells were transfected with Flag-IKKα along with either a plasmid control or expression vectors for wild-type or dominant negative ζPKC. Thirty-six hours posttransfection, cells were stimulated with TNF-α for 7 min and the level of activity of IKKα was determined as described above. Of note, Fig. 5E shows that the overexpression of wild-type ζPKC produced little or no effect on IKKα activity or on its activation by TNF-α. Likewise, the expression of a dominant negative mutant of ζPKC does not significantly affect the activation of IKKα by TNF-α (Fig. 5E). Collectively these results indicate that the atypical PKCs are critically involved in the activation by TNF-α of IKKβ but not that of IKKα, whereas αPKC is responsible for the activation of IKKβ by PMA.
Stimulation of IKKβ in vitro by recombinant ζPKC and αPKC.
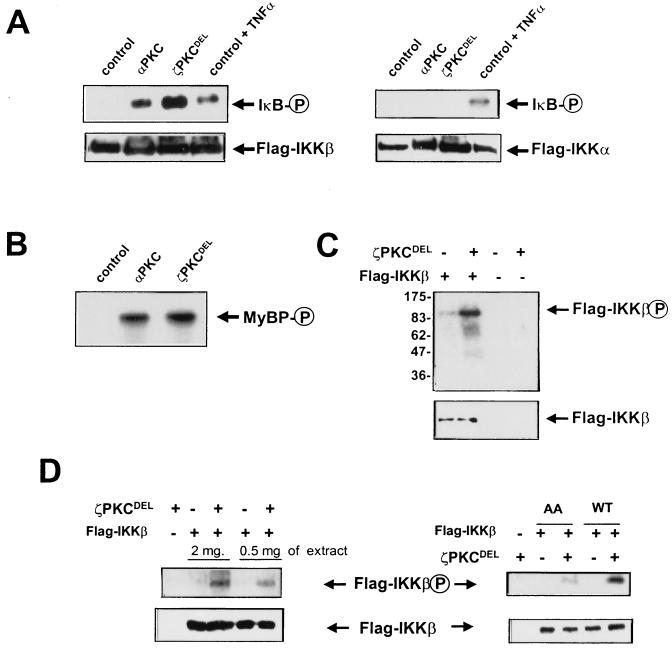
To further explore the activation of the IKKs by these PKC isotypes, we carried out an in vitro coupled assay in which immunoprecipitated IKKβ or IKKα from untreated cells was incubated in vitro with recombinant preparations of αPKC or a permanently active mutant of ζPKC, both produced from baculovirus in insect cells. Figures 6A and B show that the presence of catalytically active recombinant ζPKC dramatically reactivates IKKβ but not IKKα in vitro to an extent comparable to that produced by cell stimulation with TNF-α. Likewise, maximally activated αPKC was able to activate IKKβ but not IKKα in vitro (Fig. 6A). Control incubations demonstrate that the different PKC isoforms were unable to phosphorylate GST-IκB by themselves in the absence of IKKβ (data not shown). In addition, the results shown in Fig. 6C demonstrate that recombinant active ζPKC directly phosphorylates immunopurified IKKβ. To further establish the direct phosphorylation of IKKβ by ζPKC, immunoprecipitates of IKKβ were treated with FSBA to inactivate its kinase activity as well as that of any hypothetical contaminant associated kinase. Afterward, the level of phosphorylation of the inactivated IKKβ was determined. The results shown in Fig. 6D demonstrate the capability of recombinant active ζPKC to phosphorylate inactivated IKKβ (left panel). Interestingly, the mutation of serines 177 and 181 to alanine substantially inhibits IKKβ phosphorylation by recombinant ζPKC (Fig. 6D, right panel).
FIG. 6.
Recombinant active ζPKC and αPKC stimulate IKKβ but not IKKα in vitro. (A) Immunoprecipitates of Flag-IKKβ (left panel) or Flag-IKKα (right panel) expressed in untreated 293 cells were or were not incubated with recombinant preparations of either αPKC (maximally activated by phosphatidylserine plus diacylglycerol) or a permanently active mutant of ζPKC (ζPKCDEL), both produced from baculovirus in insect cells. Reactions were carried out at 30°C for 30 min in the presence of GST-IκB, after which the level of IκB phosphorylation was determined as described above. As a positive control, the activities of both IKKs from TNF-α-activated cells (20 ng/ml; 7 min) were included. (B) The levels of activity of the PKC recombinant preparations used in these experiments were assayed with myelin basic protein (MyBP) as a control. (C) Immunoprecipitates of Flag-IKKβ expressed in untreated 293 cells were or were not incubated with recombinant ζPKC at 30°C for 30 min in the absence of GST-IκB, after which the level of direct phosphorylation of IKKβ was determined. The numbers at left indicate positions of molecular mass markers in kilodaltons. (D, left panel) Immunoprecipitates of wild-type IKKβ from either 0.5 or 2 mg of protein extracts were inactivated by treatment with FSBA, as described in Materials and Methods. Afterward, they were or were not incubated with recombinant ζPKC as described above, and the level of phosphorylation of kinase-inactive IKKβ was determined. (D, right panel) Immunoprecipitates of either wild-type (WT) or activation loop mutant (AA) IKKβ from 1 mg of protein extracts were inactivated by treatment with FSBA, as described above, after which they were or were not incubated with recombinant ζPKC and the level of phosphorylation of kinase-inactive IKKβ was determined. The reaction mixtures in every experiment were analyzed in parallel gels by immunoblotting with an anti-Flag antibody. Essentially identical results were obtained in three other experiments. P, phosphorylated protein.
Role for the atypical PKCs in the TNF-α-induced degradation of IκB and activation of a κB-dependent promoter.
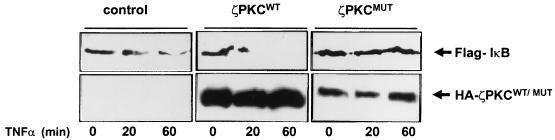
Consistent with the physiological implications of all these findings are the results of the following experiment. We transfected 293 cells with a Flag-tagged version of IκBα and an expression vector for p65 to stabilize the ectopic IκBα molecule, according to the protocol described by Chu et al. (5a), and either a control plasmid or expression vectors for wild-type or dominant negative ζPKC. Afterward, cells were stimulated with TNF-α in the presence of cycloheximide, and the ectopically expressed IκB was detected in cell extracts by immunoblot analysis with the anti-Flag antibody. Figure 7 shows that stimulation with TNF-α triggers the degradation of IκBα, consistent with previously reported data (9, 22, 25, 33, 34). The overexpression of wild-type ζPKC synergistically increases the ability of TNF-α to induce the degradation of IκBα (Fig. 7). More importantly, the expression of the dominant negative ζPKC construct completely abrogates the degradation of IκBα in response to TNF-α (Fig. 7). Similar results were obtained when cells were transfected with a dominant negative mutant of λ/ιPKC (data not shown). These results demonstrate that the ability of the atypical PKCs to bind and regulate the IKK activity is critical to the control of IκB degradation in TNF-α-activated cells.
FIG. 7.
Role for ζPKC in the induced degradation of IκB. Subconfluent cultures of 293 cells were transfected with 5 μg of expression plasmid for Flag-tagged IκBα along with 5 μg of an expression vector for p65 with 10 μg of either control vector or expression plasmids for HA-tagged versions of wild-type (ζPKCWT) or dominant negative (ζPKCMUT) ζPKC. Thirty-six hours posttransfection cells were incubated with cycloheximide (50 μg/ml) for 1 h in the presence of TNF-α (20 ng/ml) for different times. Afterward, cell extracts were analyzed by immunoblotting with anti-Flag and anti-HA antibodies. Essentially identical results were obtained in three other experiments.
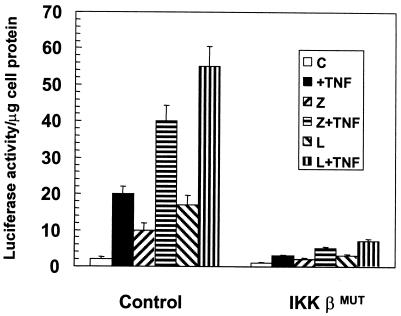
In addition, 293 cells were transfected with a κB-dependent luciferase reporter plasmid along with either a control or an expression vector for a kinase-inactive dominant negative mutant of IKKβ (either with or without expression plasmids for wild-type ζPKC or λ/ιPKC). Cells were stimulated with TNF-α for 6 h, and the level of luciferase activity was determined in cell extracts. The results shown in Fig. 8 demonstrate that the simple overexpression of ζPKC or λ/ιPKC is sufficient to activate a κB-dependent transcription in keeping with previously reported results (8) and to synergize with TNF-α. Interestingly, the transfection of a dominant negative mutant of IKKβ severely impairs not only the TNF-α effects but also those of both atypical PKCs.
FIG. 8.
IKKβ is required for NF-κB activation by the atypical PKCs. Subconfluent cultures of 293 cells were transfected with 100 ng of the κB-luciferase reporter gene plasmid and 2 μg of each kinase construct. The amount of total DNA transfected (5 μg) was kept constant by supplementation with the control vector PCDNA3. After 24 h, cells either were left untreated or were stimulated with TNF-α (20 ng/ml) for 6 h prior to harvest. Extracts were prepared, and the level of luciferase activity was determined as described in Materials and Methods. Results are means ± standard deviations from three independent experiments with incubations in duplicate. C, control; Z, ζPKC; L, λ/ιPKC.
DISCUSSION
The identification and molecular cloning of the IKKs constitute a great advance in the understanding of NF-κB activation (9, 22, 25, 33, 34). However, the mechanisms whereby these kinases are regulated are not yet completely understood (13, 29, 32). We show here that the atypical PKCs and αPKC seem to be important intermediaries in the activation of IKKβ by TNF-α and PMA, respectively. These findings would be consistent with the reported role played by the atypical PKCs in NF-κB activation in TNF-α-stimulated cells (4–6, 10, 11, 19) and establish the mechanism whereby the PKC signaling cascades regulate this important transcription factor. We have shown previously that ζPKC was unable to directly phosphorylate IκB in vitro but that it associated with a putative IκB kinase activity in immunoprecipitates (7). The findings reported in this study suggest that the IκB kinase activity detected in the ζPKC immunoprecipitates in previous work (7) could be accounted for at least in part by IKKβ. However, reconstitution experiments, in which recombinant ζPKC was incubated with cell extracts, demonstrated the association of an IκB kinase activity that in in-gel kinase assays gave a molecular mass of about 50kDa, which is very different from that of the IKKs. This 50-kDa protein has now been identified as casein kinase 2 (24b), which selectively phosphorylates the C terminus of IκB (21). These phosphorylation sites are not involved in the induced degradation of IκB but rather in the control of its stability (21). The inability of the IKKs to renature in the in-gel kinase assays (8a) explains why they remained undetected in previous studies (7).
The atypical PKCs can also stimulate the MAP/extracellular signal-regulated kinase (ERK) kinase (MEK)–ERK signaling pathway through a still-to-be-defined Raf-independent mechanism (4, 27). This pathway is also relevant for the activation of the κB-dependent transcription, since the overexpression of a dominant negative ERK mutant severely impairs the κB-dependent promoter activity stimulated by the overexpression of a ζPKC active mutant or the presence of TNF-α (3, 4). However, that mechanism does not involve the actual translocation of NF-κB to the nucleus (4) but could be mediated through the action of ERK on the transactivation domain of p65 (3, 4a). This, together with the evidence presented here that the atypical PKCs directly regulate IKKβ in vitro and in vivo, strongly suggests that the atypical PKCs may control the NF-κB pathway at two levels, which would ensure the maximal efficiency in the activation of NF-κB-regulated genes.
NIK is another kinase that binds to both IKKα and IKKβ (25, 33). It has recently been demonstrated that NIK activates and phosphorylates IKKα in cotransfection experiments but that it is unable to phosphorylate IKKβ (17, 25). The atypical PKCs also bind to both IKKs but in contrast to NIK activate only IKKβ and have no effect on IKKα. Thus, it seems that there are specific kinase pathways upstream of the different IKKs to control IκB phosphorylation and NF-κB activation. In this regard, MEK kinase 1 (MEKK1) has also been shown to selectively activate IKKβ and to have no effect on IKKα (24). However, in contrast with the atypical PKCs or NIK, MEKK1 appears to be unable to stably interact with the IKKs (24). Recent studies demonstrate that Ser176 in the activation loop of IKKα is the target of NIK (17) and together with Ser180 is essential for IKKα kinase activity (22). In the case of IKKβ, the mutation of serines 177 and 181 to alanine does not block its enzymatic activity (22); however, its activity is greatly increased when both residues are mutated to glutamic acid (22). This indicates that either or both serines may be important for the activation of IKKβ by upstream kinases. Actually, Lee et al. (15) demonstrate that a peptide comprising the activation loop of IKKβ is phosphorylated by MEKK1 on residues corresponding to serines 177 and 181. We show here the direct phosphorylation of IKKβ by recombinant ζPKC and the important contribution of those two residues to that phosphorylation. Although serines 177 and 181 do not conform strictly to the PKC consensus site, serine 181 is followed at position +1 by a hydrophobic amino acid which has been shown to be present in all bona fide PKC phosphorylation sites (24a). Another intriguing matter arising from this and other studies (15, 17, 22) is the fact that IKKα and IKKβ are selective for different upstream kinases, despite the fact that the sequence around the phosphorylated residues is highly conserved. This may suggest that other structural determinants in the IKK upstream kinases may be responsible for that specificity. Further studies will be required to answer this question.
A kinase-inactive mutant of IKKα blocks the activation by NIK of a κB-dependent reporter gene, reinforcing the notion that NIK is upstream of IKKα in the NF-κB pathway (25). The overexpression of MEKK1 is sufficient to activate a κB-dependent reporter gene (15), although the ability of a dominant negative MEKK1 to block NF-κB activation by TNF-α is still a matter for discussion (18). The observation that the atypical PKCs are critically involved in the regulation of NF-κB (4–6, 10, 11, 19) and IKKβ activity, in conjunction with the fact that they are potently activated by TNF-α (19, 23), strongly suggests that these PKCs are among the important players in the NF-κB pathway at the level of IKKβ activation. Recent data indicate that receptor-interacting protein is a critical molecule in the activation of NF-κB (12, 14). How the atypical PKCs are connected to receptor-interacting protein in the NF-κB signaling cascade is a matter of ongoing research in our laboratory.
ACKNOWLEDGMENTS
This work was supported by grants SAF96-0216 from CICYT, PM96-0002-C02 from DGICYT, and BIO4-CT97-2071 from the European Union and by funds from Glaxo Wellcome Spain and has benefited from an institutional grant from Fundación Ramón Areces to the CBM.
We are indebted to Esther Garcia, Carmen Ibañez, and Beatriz Ranera for technical assistance and to Gonzalo Paris and Isabel Perez for their help and enthusiasm. We thank Dave Goeddel for a critical reading of the manuscript and for helpful comments during this work.
REFERENCES
- 1.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Berghe W V, Plaisance S, Boone E, De Bosscher K, Schmitz M L, Fiers W, Haegeman G. p38 and the extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 4.Berra E, Diaz-Meco M T, Lozano J, Frutos S, Municio M M, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C ζ. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Berra, E., et al. Unpublished data.
- 5.Bjorkoy G, Overvatn A, Díaz-Meco M T, Moscat J, Johansen T. Evidence for a bifurcation of the mitogenic signaling pathway activated by ras and phosphatidylcholine-hydrolyzing. J Biol Chem. 1995;270:21299–21306. doi: 10.1074/jbc.270.36.21299. [DOI] [PubMed] [Google Scholar]
- 5a.Chu Z-L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Meco M T, Berra E, Municio M M, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera M T, Alcamí J, Payá C V, Arenzana-Seisdedos F, Virelizier J-L, Moscat J. A dominant negative protein kinase C ζ subspecies blocks NF-κB activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz-Meco M T, Lozano J, Municio M M, Berra E, Frutos S, Sanz L, Moscat J. ζPKC induces phosphorylation and inactivation of IκB-α in vitro. EMBO J. 1994;13:2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Meco M T, Municio M M, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ι and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/mcb.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Diaz-Meco, M. T., et al. Unpublished data.
- 9.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez I, Sanz L, Arenzana-Seisdedos F, Diaz-Meco M T, Virelizier J-L, Moscat J. Inhibition of protein kinase Cζ subspecies blocks the activation of an NF-κB-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993;13:1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folgueira L, McElhinny J A, Bren G D, MacMorran W S, Diaz-Meco M T, Moscat J, Paya C V. Protein kinase C-ζ mediates NF-κB activation in human immunodeficiency virus-infected monocytes. J Virol. 1996;70:223–231. doi: 10.1128/jvi.70.1.223-231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu H, Huang J, Shu H-B, Baichwal V, Goeddel D V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 13.Israël A. IκB kinase all zipped up. Nature. 1997;388:519–521. doi: 10.1038/41433. [DOI] [PubMed] [Google Scholar]
- 14.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 14a.Lallena, M.-J., et al. Unpublished data.
- 15.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 16.Lenardo M, Baltimore D. NF-κB: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- 17.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKKα by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 19.Lozano J, Berra E, Municio M M, Diaz-Meco M T, Dominguez I, Sanz L, Moscat J. Protein kinase C ζ isoform is critical for κB-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 20.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 21.McElhinny J A, Trushin S A, Bren G D, Chester N, Paya C V. Casein kinase II phosphorylates IκBα at S-283, S-289, S-293, and T-291 and is required for its degradation. Mol Cell Biol. 1996;16:899–906. doi: 10.1128/mcb.16.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J W, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 23.Müller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKCζ is a molecular switch in signal transduction of TNFα, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Nishikawa K, Toker A, Johannes F-J, Songyang Z, Cantley L C. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 24b.Paya, C. V., et al. Unpublished data.
- 25.Réginer C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 26.Rzymkiewicz D M, Tetsuka T, Daphna-Iken D, Srivastava S, Morrison A R. Interleukin-1β activates protein kinase Cζ in renal mesangial cells. J Biol Chem. 1996;271:17241–17246. doi: 10.1074/jbc.271.29.17241. [DOI] [PubMed] [Google Scholar]
- 27.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sontag E, Sontag J M, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase Cζ signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stancovski I, Baltimore D. NF-κB activation: the IκB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 30.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 31.Verma I M, Stevenson J K, Schwarz E M, VanAntwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 32.Verma I M, Stevenson J K. IκB kinase: beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 34.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]