Abstract: Background
This review examines three bodies of literature related to herb–drug interactions: case reports, clinical studies, evaluations found in six drug interaction checking resources. The aim of the study is to examine the congruity of resources and to assess the degree to which case reports signal for further study. A qualitative review of case reports seeks to determine needs and perspectives of case report authors. Methods: Systematic search of Medline identified clinical studies and case reports of interacting herb–drug combinations. Interacting herb–drug pairs were searched in six drug interaction resources. Case reports were analyzed qualitatively for completeness and to identify underlying themes. Results: Ninety-nine case-report documents detailed 107 cases. Sixty-five clinical studies evaluated 93 mechanisms of interaction relevant to herbs reported in case studies, involving 30 different herbal products; 52.7% of these investigations offered evidence supporting reported reactions. Cohen’s kappa found no agreement between any interaction checker and case report corpus. Case reports often lacked full information. Need for further information, attitudes about herbs and herb use, and strategies to reduce risk from interaction were three primary themes in the case report corpus. Conclusions: Reliable herb–drug information is needed, including open and respectful discussion with patients.
Keywords: herb drug interaction, pharmacovigilance, phytovigilance, drug interaction
1. Introduction
Use of herbal supplements in the United States is a multi-billion-dollar industry with sales in 2019 reaching nearly ten billion dollars [1]. Several surveys have revealed that more than one-third of U.S. citizens report use of at least one herbal product [2,3,4], with many of the herb-using respondents specifying that the use of herbals demonstrates independence in self-management of their health [4,5]. Pharmacovigilance for drug–drug interactions is fraught with complexity, confounded by factors such as comorbid conditions, pharmacogenomic variations in response and metabolism, and the influence of polypharmacy [6,7]. Phytovigilance for potential herb–drug interactions is further complicated by the multiplicity of constituents in botanicals, confusion caused by use of shared common plant names, misidentification of species, mislabeling of products, contamination of botanicals, and combination of botanicals or use of multi-botanical products [8,9,10,11,12,13,14,15]. Where the botanical product is an extract, the extraction process may alter constituents, thus altering the nature or extent of interaction with pharmaceutical agents [10,14,16]. The full complement of constituents in whole plant parts may also impact pharmacodynamics and pharmacokinetics compared to extracts in what has been termed in cannabis research as “the entourage effect” [17]. For example, bioavailability of the anti-malarial compound artemisinin is reportedly 45 times greater when whole leaf of Artemisia annua is administered compared to administration of pure artemisinin alone [18]. Production of secondary metabolite constituents by a plant species is also variable, impacted by geography, genotype, plant part used, and seasonal variation. Harvest, preparation, and storage of crude plant may similarly impact constituent content [10,14,19].
Sources of information regarding herb–drug interactions are themselves potentially problematic. Results from in vitro studies may offer mechanistic insight but may not translate well into clinical practice [10,20,21,22]. Animal models may offer insight into the role of metabolites in interaction, but these metabolites may not always be applicable to humans and require further clinical confirmation [23]. Clinical trials are often performed in healthy homogenous adult populations, and sometimes test inappropriate plant parts or inappropriately prepared products [10,19]. Case reports in the literature often signal that further research is required and may offer pragmatic insight into the clinical nature of herb–drug interactions but may introduce reporting bias skewed toward risk rather than benefit, may overlook confounding factors, rarely establish causality, and seldom include perspectives outside the framework of the current medical culture [14]. Interaction checking databases and publications often cite results of clinical studies, in vitro findings, and case reports from the literature. These important clinical tools may reflect risks that lack clinical relevance and may fail to offer insight into combinations that offer benefit. A review published by Ng et al. [24] provides an excellent evaluation of sources of herb–drug interaction and adverse effect information.
As we continue our progress toward integrative patient-centered care, a thorough understanding of herb–drug interactions is necessary to maximize positive outcomes from beneficial interaction while minimizing risks from potentially harmful combinations. Cooperation between those who care for patients in the context of the dominant medical belief system, those who provide care through non-dominant systems, and input from the patients themselves is needed to gather the data to form valid conclusions about the risks and benefits of any individual medicinal product [14,25]. The United States FDA adverse event reporting system (FAERS) is publicly accessible, includes consumer reporting and allows for reporting of botanical precipitants in herb–drug interaction but does not separate herbs from mainstream pharmaceuticals [26]. The World Health Organization Vigibase™ [27] is a global repository of pharmacovigilance data but is not freely accessible to individual clinicians.
Ivan Stockley, an innovator in the field of pharmacovigilance for drug–drug interactions, stated that information regarding drug–drug interactions is sometimes “no more than speculative and theoretical scaremongering guesswork, hallowed by repeated quotation until they become virtually set in stone” [16] (p. 2). The overarching goal of this scoping review is to explore the current state of clinically relevant herb–drug interaction information. Specific aims include qualitative assessment of clinical case reports, evaluation of the degree to which the herbs involved in case reports appear to serve as a signal for investigation through clinical study, and comparative evaluation of the inter-source agreement of herb–drug interaction information between case reports and clinical studies, and between case reports and selected herb–drug interaction checking resources. A secondary aim is a qualitative assessment of the attitudes toward herbal medicines expressed by authors of herb–drug interaction case reports. Review of herb–drug interaction mechanisms and extensive evaluation of individual interactions are beyond the scope of this review; the reader is referred to recent reviews by Awortwe [21,28], Borse [29], Chen [22], Liu [30], and Rombola [31] for further information on these topics.
2. Materials and Methods
A systematic search of Medline was performed on 28 October 2020, for journal articles and editorials using the MeSH terms “Herb-Drug Interactions” and “Drug Interactions” AND “Herbal Medicines” for case reports and clinical human research studies published in English without date restriction. Exclusion criteria included review articles, ex vivo studies, animal studies, and articles without report of herb–drug interacting combinations (e.g., those reporting only adverse effects). Retrieval precision was improved through the addition of filters including “human”, “randomized controlled trials”, “letters”, “case reports”, and “editorials”. Case report documents were mined to identify additional cases.
Case reports were evaluated for completeness by at least two separate authors using the rating criteria seen in Table 1, which represents modification of a previously published reliability rating scale [32]. Discrepant ratings were resolved through discussion. A reliability index for each report was calculated by dividing the sum of scored points by the number of applicable points. Rechallenge with problematic substances is often deemed unethical and thus is often not performed. Rather than allowing an ethical decision to impact completeness scoring, where rechallenge was not performed, 0 points were added to the numerator and 1 point was removed from the denominator for that report in calculation of the report reliability index.
Table 1.
Case report completeness rating tool, adapted from reference [32].
| Measure | Scoring |
|---|---|
| Is reporting of relevant demographics (sex, age, relevant conditions) adequate? | Y = +1 N = 0 |
| Are concomitant diseases and other medications associated with adverse events included (including dosing)? | |
| Are concomitant medications and other herbals/supplements documented (including dosing)? | |
| Are interactors adequately described, including scientific name of botanicals? | |
| Have alternate explanations been excluded? | |
| Is chronology complete? | |
| Is chronology sequence reasonable? | |
| Is adverse reaction adequately described? | |
| Is interaction pharmacologically feasible? | |
| Does event cease upon stopping herb? | |
| Does event recur upon rechallenge? | Y = +1 N = 0 N/A = 0 and remove from denominator |
MaxQDA 2020 (Verbi software) [33] was used to facilitate qualitative analysis of case reports. Case reports were analyzed to identify themes regarding the authors’ perspectives and attitudes regarding herbal medications and the roles of herbal medicines in altering response to pharmaceuticals. Reactions in case reports were evaluated for severity of reaction as fatal, severe requiring hospitalization or treatment, minor requiring no treatment beyond herb or drug withholding, beneficial if the author clearly stated benefit, or none if no interaction was found. Herb–drug interacting pairs were labeled categorically based upon interaction that increased activity/caused abnormal toxicity of the target drug, lack of interaction present, or interaction decreasing activity/level of the target drug. Individual herbs from reports involving more than 5 combined botanicals were eliminated from the analysis, as this number reflects a common cut-point for polypharmacy beyond which adverse effects become more common [34,35]. For case reports involving 5 or fewer botanicals, each individual botanical was evaluated against the primary suspected target drug(s). For case reports using only the common name “ginseng”, the three most popular botanicals using this common name were each included for the inter-source analysis. Conflict in nature of interaction between multiple reports was resolved through exclusion of the score related to multi-herbal report, which resolved all conflicts.
Herbal products studied in clinical trials investigating actions of individual herbs were identified and quantified for comparison to those identified in case reports; trials exploring multiherbal formulations were excluded from the analysis. When studied herbs or constituents were involved in case reports, the pharmacokinetic pathways for drugs noted in the case reports were identified through Lexicomp [36] and/or the Pharm GKB database [37]; details are found in Table S1. Findings of pharmacokinetic studies were matched to the relevant case report drugs via reported pharmacokinetic pathways and assigned a categorical value for findings that would be predicted to increase drug effect, for those finding no likely interaction, and for findings that would likely decrease target drug effect. Pharmacodynamic studies were similarly labeled based upon additive/synergistic findings, no interaction, or antagonistic finding. Studies demonstrating benefit were so noted; details are presented in Table S2. Percent agreement/disagreement for case reports versus study findings was calculated by matching pharmacokinetic pathways and pharmacodynamic actions of target drug in case reports with the pathways/actions explored for the precipitating herb in clinical studies, counting matches that supported case reports, those offering no support, and those that contradicted the reported interactions, summing each group and dividing by the number of studies that provided data for each relevant pathway or action.
Herb–drug interacting pairs identified through case reports were searched for interaction in six drug interaction information sources including Integrative Pro [38], Lexicomp [36], Medscape [39], Memorial Sloan-Kettering Cancer Center [40], Natural Medicines Database [41], and Stokely’s Herbal Medicines Interactions [16]. Three of these sources were selected for their frequent use by clinical research team members and three were selected through their position when returned by Google™ search using the terms “herb drug interaction checker”; the latter to provide insight into information found in non-subscription databases. Each herb and target drug involved in the case reports involving fewer than 5 botanicals was investigated in each database and assigned a categorical value to indicate increased effect or concentration of target drug, lack of reported interaction, antagonistic interaction, lack of data regarding at least one of the interactors, or beneficial interaction. Where a database returned conflicting information (both increasing and decreasing effect for a pair), the interaction was scored specifically for the drug if the conflicting information was generic for the class or pharmacokinetic, otherwise the interaction was scored based upon strength of the supporting information presented. Warfarin was searched in databases as a proxy for the less commonly used vitamin K antagonists fluindione and phenprocoumin. Table S3 details the findings. Cohen’s kappa was calculated for each database compared to case reports as a measure of inter-source reliability using IBM SPSS statistical package [42].
3. Results
The initial literature search returned 1745 records. After exclusion of duplicates (n = 30) and articles published in languages other than English (n = 15), application of filters excluded an additional 1476 articles. Screening of titles and abstract led to exclusion of 104 articles, with 53 case reports and 67 clinical study documents remaining for full review. Of these, three case reports were unretrievable. Two case reports were excluded for off-target report of adverse effect rather than herb drug interaction. Mining of case reports led to identification of an additional 53 records; one was excluded as a duplicate and three for off-target reporting of adverse effect, leaving a total of 99 case reports or series covering 107 herb–drug interaction reports and 65 clinical studies involving 82 investigations of 30 different herbs or herb combinations for review. The PRISMA flow diagram is depicted in Figure 1.
Figure 1.
PRISMA diagram [43].
3.1. Case Report Analysis
Hypericum perforatum (St. John’s wort) was the most common herb implicated as a single precipitant (n = 17), followed by Vaccinium spp. (cranberry n = 8), Panax ginseng (n = 5), Ginkgo biloba (n = 4), ginseng species not otherwise specified (NOS), Lycium barbarum (Goji), Curcuma longa (source of turmeric), and Cathus edulus (khat) (n = 3 each). Not surprisingly the most common drugs implicated are often those with narrow therapeutic indices including as a single agent warfarin (n = 30), and as classes of drugs serotoninergic (n = 14), calcineurin inhibitors (n = 13), chemotherapy and antiretroviral therapy (n = 8 each). Five reported fatalities occurred, two associated with a combination of cranberry and warfarin, one ginkgo-related bleed in a patient taking ibuprofen, a ginkgo-related seizure fatality in a patient taking phenytoin and divalproex, and a Mitragyna speciosa-related (kratom) case of toxic quetiapine levels. Table 2 summarizes the interactions of the most commonly involved herbs and/or interactions with fatal outcomes; further information regarding the document group titled “case reports” are found in Table S4. It is interesting to note that kratom is implicated in 58 herb–drug interaction reports and 42 fatalities in the FAERS database, while cranberry is associated with only 9 reports, none of them fatal [26].
Table 2.
Herbs most commonly involved and/or associated with fatal outcomes.
| Latin Name, Common Name | Drug(s) or Class | N Reports | Severity (N) | Reaction |
|---|---|---|---|---|
| Curcuma longa, turmeric | Fluindione | 1 | Minor | Increased INR |
| Tacrolimus | 1 | Severe | Increased tacrolimus level | |
| contaminated with microcystin | Paclitaxel | 1 | Severe | Hepatotoxicity |
| Equisetum arvense, horsetail | Antiretrovirals | 2 | Minor | Increased viral load |
| Gingko biloba, ginkgo | Antiretrovirals | 1 | Severe | Treatment failure |
| Ibuprofen | 1 | Fatal | Intracerebral bleed | |
| Trazodone | 1 | Severe | Altered mental status | |
| Aescinate | 1 | Severe | Acute kidney injury | |
| Ginkgo biloba in multiherbal | Divalproate, phenytoin | 1 | Fatal | Seizures |
| Efavirenz | 1 | Mild | Increased viral load | |
| G biloba and H perforatum | Buspirone, fluoxetine | 1 | Mild | Serotonin syndrome |
| Hypericum perforatum, St. John’s wort | Serotoninergics | 6 | Mild (4) Severe (2) |
Serotonin syndrome |
| Calcineurin inhibitors | 6 | Mild (3) Severe (3) |
Decreased drug levels | |
| Clozapine | 1 | Mild | Schizophrenia | |
| Sertraline | 1 | Severe | Mania | |
| Oral contraceptive | 1 | Severe | Pregnancy | |
| H. perforatum in multiherbal | Cyclosporin | 1 | Mild | Decreased drug level |
| Lycium barbarum, goji | Warfarin | 3 | Mild (2) Severe |
Increased INR Bleeding |
| Mitragyna speciosa, kratom | Quetiapine | 1 | Fatal | Increased drug level, neuroleptic malignant syndrome |
| Panax ginseng, ginseng | Phenelzine | 1 | Mild | Mania |
| Imatinib | 1 | Severe | Hepatotoxicity | |
| Raltegravir | 1 | Severe | Increased drug level | |
| contaminated with germanium | Furosemide | 1 | Severe | Treatment failure |
| P ginseng in multiherbal | Warfarin | 1 | Severe | Intracerebral bleeding |
| Vaccinium spp, cranberry | Warfarin | 8 | Mild (6) Fatal (2) |
6 Increased INR, 2 GI and pericardial bleeding |
These discrepant findings highlight a challenge in pharmacovigilance: consistency collection of data [12,14,44,45]. Several tools are available to aid in the objective rating of the probability that an adverse drug event or drug interaction occurred, including the Naranjo Adverse Drug Reaction Probability Scale [46] and the Horn Drug Interaction Probability Scale (DIPS) [47]. In application of these tools, a point is scored for each criterion that is associated with an increased likelihood that an observed reaction is due to the product in question. A rating tool was used by the case reporter to evaluate the probability for 28 cases in the case report document set, with 5 reports as possible (DIPS n = 2, Naranjo n = 3), 24 as probable (Naranjo n = 14, DIPS n = 8, other = 2, two reporters used two scales each), while one reporter claimed use of the Naranjo scale but did not disclose the result.
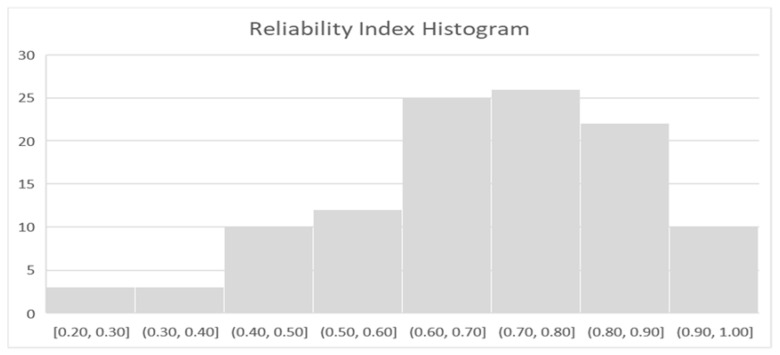
In an earlier study, Fugh-Berman and Ernst [32] applied a 10-point reliability rating score to evaluate the reliability of herb drug reports in the literature. To evaluate completeness of each of the 107 case reports in this study in a standardized manner, a “reliability index” based upon the Fugh-Berman scoring was calculated for each report. In addition to the original criteria, a point was added for pharmacologic feasibility, a provision for the full scientific name of botanicals as a minimum for “adequate description of interactors” was added, and the score was not reduced when rechallenge was omitted. Figure 2 is a histogram representing the reliability index calculated for each of the 107 cases, with a maximum attainable score of 1.
Figure 2.
Histogram of calculated report reliability indices.
3.1.1. Reliability Index Results
The reliability index scores are a measure of report completeness that ranged from 0.2 to 1, with median 0.8 and mode 0.8. The point most often lacking in the reliability index score was full evaluation of alternative explanations, with 46 (43.0%) reports rated as lacking in this domain. The next most frequently lacking points included identification of comorbidities and other medications/herbals associated with adverse effects (n = 40, 37.4%), identification of concomitant medications and herbs (n = 37, 34.6%), adequate description of interactors (n = 35, 32.7%), and information regarding remission upon de-challenge (n = 31, 29.0%). In the 15 cases that involved rechallenge, 3 (20%) did not lead to re-emergence of the reaction. Inadequacy of event description (n = 4, 3.7%), inadequate description of patient demographics (n = 9, 8.4%) incompleteness of chronology (n = 8, 7.5%), lack of reason in chronology (n = 5, 4.7%), and lack of pharmacologic feasibility (n = 13, 12.1%) were deemed least problematic.
3.1.2. Qualitative Analysis of Case Reports
Three themes emerged from qualitative analysis of the case report document set: attitudes toward herbs, herb use, and patient autonomy, approaches to risk mitigation, and calls for information and education.
On one end of the attitude about herbs spectrum, four documents offered positive evidence for an herbal supplement, and one offered a balanced input comparing risk from herbs and conventional drugs, thus 5 documents (5.1% of all case reports) were rated with a non-judgmental/positive attitude toward herbs. Only two reports—including the earliest record found—hinted that the interaction may be applied for benefit. On the other end of the attitude spectrum, thirteen documents (13.1%) made statements using language that was dismissive of herbs or posited that all risk was from the herb, without acknowledgment of the risk-related role of narrow therapeutic index drugs. Additionally, fourteen reports cited irrelevant herb–drug interactions to support their case. Seven reports discussed patient decision to use herbs in a disrespectful fashion.
Twenty documents (20.2%) emphasize the importance of asking the patient about herb and vitamin supplements. Several risk mitigation strategies are discussed: four authors offer strategies to reduce risk from a medication for patients who desire the herbal product(s), 10 authors suggest increased therapeutic drug monitoring, 12 authors suggest avoidance of herbal medicine, two authors call for greater regulation of herbal sales, and one author suggests monitoring of herbal consumption for patients on high-risk medications.
Information was a theme in 32 documents. Fifteen authors identified the need for education, with eight prioritizing patient education, three prioritizing provider education, and four recommending increased education for both. General lack of information was cited by six authors as problematic. Three authors suggested a need for improved labeling, two called for improved pharmacovigilance reporting, two for better electronic resources, and one emphasized the need to identify the composition of products involved in reports.
3.2. Case Report and Study Comparison
Thirty different herbal products were investigated in clinical studies, including six multiherbal formulations against 24 different potential interaction mechanisms. Of the tested mechanisms, 93 were relevant to interactions found in the case report document set with 91 related to pharmacokinetic interactions and two to pharmacodynamic interaction specific to an herb–drug pair reported in at least one case report.
The herbs with most frequent study excluding multiherb formulations can be seen in Table 3, which compares the number of studies and case reports to the two lists (each from a different source) of top selling herbal products in 2019 published in HerbalGram, the journal of the American Botanical Council [1]. Of the three herbs associated with fatal case reports, ginkgo was most frequently studied, cranberry was investigated once, kratom was not found in the clinical trial document set. A summary of clinical study materials and methods including source of botanical is found in Table S5.
Table 3.
Frequency of herb study compared to frequency of case report and rank on two top selling herb lists. NOS, species not otherwise specified, multiherb, identified only as a component in a case involving multiple herbs.
| Clinical Studies | N Studies | N Case Reports | Top Selling Herb Lists Ranks |
|---|---|---|---|
| Hypericum perforatum St. John’s wort | 17 | 17 | 0, 0 |
| Ginkgo biloba, ginkgo | 11 | 7 | 17, 21 |
| Panax ginseng, ginseng | 6 | 4 plus 3 NOS | 30, 30 |
| Allium sativum, garlic | 3 | 1 (multiherb) | 8, 15 |
| Hydrastis canadensis, goldenseal | 3 | 0 | 0, 0 |
| Piper methysticum, kava-kava | 3 | 2 (multiherb) | 0, 28 |
| Silybum marianum, milk thistle | 3 | 0 | 23, 10 |
| Actea racemosa, black cohosh | 2 | 0 | 15, 0 |
| Curcuma longa, turmeric | 2 | 3 | 4, 3 |
| Crataegus spp, hawthorne | 2 | 0 | 0, 38 |
The number of herbs shared between the set of herbs in case reports (n = 61) and those studied (n = 30) was fourteen, a union representing 46.6% of studied herbs. Thirteen studied herbs are listed as best sellers; nine of these were involved in a case report.
In the analysis of agreement for the relevant studies, 52.7% of pharmacokinetic findings supported the case reports, 37.4% offered no support, and 16.5% found interaction in that opposed the nature of the case report. Pharmacodynamic studies (n = 2) offered 100% support for the relevant case-reported interactions.
3.3. Case Report and Interaction Checker Comparison
Cohen’s kappa for inter-source reliability between each interaction checker and case reports are presented in Table 4. For fatal case reports, all interaction checkers agreed that ginkgo and ibuprofen have additive risk for bleed, four and three of six agree that gingko could reduce efficacy of phenytoin and divalproex, respectively, two of six agree that kratom could produce additive effects with quetiapine, and all agree that cranberry may have additive effects with warfarin.
Table 4.
Cohen’s kappa and 95% confidence intervals (CI) comparing checkers to case reports.
4. Discussion
It is not the intent of this study to malign those who have taken the time to share their experience nor to dismiss the work that they have done to improve our knowledge of herb–drug interactions. The purpose of this study is exploration of three bodies of information to identify limitations for their clinical application and to initiate conversations that expand our understanding of this phenomenon. One must always respect the evaluation of those with first-hand knowledge of the case. Contextual information is lacking when performing a review of case reports.
Many herbs belong in a subset of the category “drugs”. Pharmaceuticals themselves often originate from natural sources. As drugs, there is no doubt that herbs may interact with other drugs or nutrients. Herbs are fortunately available to all for use. Unfortunately, this availability adds a further layer of complexity to phytovigilance: prevalence of use for any herb is difficult to discern.
During planning discussions for this project, different attitudes regarding herb–drug interactions among team members from different disciplines of medicine became apparent, which signaled a need to include a thematic investigation. The Council for International Organizations of Medical Science includes beneficial interaction in their definition of pharmacovigilance “signal” regarding drug–drug interactions [48]. In conventional medicine, interaction between pharmaceuticals is occasionally used for benefit. Examples include the use of ritonavir to “boost” other protease inhibitors via CYP 3A4 inhibition [49] and the use of probenecid to prevent cidofovir-related renal toxicity through inhibition of renal anion transporters [50]. That piperine in black pepper inhibits the p-glycoprotein transporter should not automatically be judged in a negative light; it may enhance bioavailability/effectiveness of chemotherapeutic agents and other botanical constituents via inhibition of p-glycoprotein [51,52].
Given that pharmacovigilance systems target safety more than therapeutics it is not surprising that the case reports—and findings in FAERS—are biased toward reporting harm. Two case reports hinted at the possibility of benefit from interaction, one interaction checker reported two interactions as beneficial (albeit in opposition to case report findings), and two clinical studies reported benefit from interaction. Perhaps the ability of botanical constituents to decrease pharmaceutical drug clearance could be harnessed to reduce drug costs through reduction in dose requirements, lower the burden of drug excretion into the water supply, or enhance bioavailability of nutrients [53,54]. Further exploration could conceivably improve patient quality of life through reduction in side effects [53]. While the possibility of occasional benefit from interactions exists, far too often inappropriate combinations of drugs from any source can lead to serious harm or even death.
Complementary and Alternative Medicine (CAM) can be beneficial and aid in the treatment of many diseases. When it comes to cancer treatments, roughly half of the patients receiving treatment use CAM [55]. Patients decide to use alternative medicine alongside chemotherapy for many reasons including strengthening of the immune system, improving well-being, and relieving symptoms of either the disease or disease treatment, such as nausea, insomnia, and pain [56]. Publications evidencing positive and negative effects of the use of herbs with chemotherapy abound. To expand patient options and respect patient autonomy, physicians should be encouraged to open discussion with patients, seeking input from experts in integrative medicine when needed so that we may all participate actively in informed shared decision-making [57].
Patient autonomy is one of the four principles stated by Beauchamp and Childress in 1985 to define the morals that guide medical ethics [58]. Patient choices regarding healthcare often do not align with the tenets of the current medical paradigm. Four documents contained strong language to support autonomy and respectful discussion with patients such as “we hope to inspire the discussion about the safety of any herbal formulas in combination with cytotoxic therapies and encourage physicians to seek faithful conversations dealing with the use of CAM in cancer patients” [59] (p. 3) and “it is our professional duty to increase our understanding of alternative medicine and to provide unbiased information without sounding judgmental or indifferent” [60] (p. 1653). Seven documents contained language with the opposite tone including “the fact that this patient was a registered nurse also did not prevent her from combining medications, although it is arguable that her judgement may have been subtly affected by the TBI” (traumatic brain injury) [61] (p. 363) and “the patient was informed that discontinuation of SJW (St. John’s wort) would be next if her symptoms fail to resolve” [62] (p. 683).
Eight case report documents and three clinical study documents state that herbal medicines are used due to the fallacious belief that “natural means safe”. An example found in the case report corpus states that it is a “common belief that natural plant products never do any harm to the body and that only pharmaceutical products manufactured by humans may have harmful side effects [63] (p. 219). Only one document offered a citation for this commonly stated conception about herb-user beliefs. The cited source document reported survey results where respondents reported “natural” and “safe” as separate reasons for use and concluded that this meant that users believe that “natural means safe” [64]. One must question the validity of this conclusion, especially considering the higher levels of education among herb-users in the United States [64,65,66]. This oft-cited statement may belong in the realm of Ivan Stokely’s misconceptions that become hallowed fact through the argument of repetition. Further study is needed to assess the validity of this statement.
Cohen’s kappa is a descriptive statistic that corrects for agreement due to chance when defining inter-rater reliability. Perfect congruence of rating returns a value of “1”, lack of agreement is reflected by scores close to zero, and disagreement by negative scores. [67]. None of the kappa scores reveal agreement for interaction checkers compared to case reports. This does not mean that the checkers are invalid, but rather reflects the complexity of the issue and the need for more information. The lack of agreement may equally be due to idiosyncratic reactions, contaminants, misidentification of herb. or lack of causality in case reports.
Herbs chosen for clinical studies seem to reflect the inter-related commonality of use and reports of herb–drug interaction. An agreement of more than 50% is laudable, given the potential number of confounding factors that may be present. Further study to compare reports to FAERS or use of data mining techniques as employed in pharmacovigilance [44,45,68] may yield greater insight into these relationships.
The case reports found in the literature were lacking in completeness, perhaps in part due to word count limitations associated with report venue or in part due to availability of new information gained since publication of the case [45], which impacted the score for consideration of alternate explanations. Lack of identification by scientific name is problematic, but even inclusion of the appropriate label does not guarantee that the herb was correctly identified, appropriately harvested, stored, prepared, and was contaminant-free [14].
One of the major limitations to this study is in the use of only one database to identify case reports and clinical studies, though the mining of case reports for additional cases returned a significantly higher number of cases than did additional database searches as seen in the excellent 2018 review by Awortwe et al. [21]. The risk of bias toward a particular herb is conceivably increased through reference mining. The robustness of this study would be improved through inclusion of official pharmacovigilance data, for example from FAERS, despite that none of the case report authors reported using this as a source to evaluate prior reports of interaction. The convenience sample of 3 interaction checkers may not reflect resources that are commonly used by clinicians, but likely reflect information used by those outside the clinical realm. Fifteen case report authors identified sources of information used to evaluate the reported case interaction. Most commonly Medline®/Pubmed® [69] was used by 13 (86.6%) followed by Natural Medicines Database [41] and Embase® [70] used by three (20%), IBM Micromedex® [71] by two authors, and UptoDate (powered by Lexicomp® [36]), Toxline® [69], and Drug Interaction Facts [72] one each.
5. Conclusions
The identification of information as a recurring qualitative theme signals the need for improved phytovigilance that includes exploration of potential harms and benefits from combined use of herbal medications with pharmaceuticals. As seen in Table S1, pharmaceutical drugs induce and inhibit enzymes, indicating the need to explore the role of pharmaceutics in altering response to botanic drugs. Application of data mining could expand our understanding vastly but would be critically impacted when scientific name is omitted [14,45] and would still lack needed contextual insight.
Most importantly, we need to ask, listen, and learn with respect and without bias. The author of a cranberry and warfarin interaction report said it well: “I thought I knew pretty much everything there was to know about warfarin. I had a team to help me but was not willing to ask…if you are lucky, you will learn a new nugget of knowledge or a new skill, and if you are very lucky, your attitude will improve as well.” [73] (p. 1).
Acknowledgments
The authors are indebted to Gayle Hamman, DO for her insight and thoughtful review of this document.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/medicines8080044/s1, Table S1: Pharmacokinetic Pathways for Drugs Implicated in Herb–Drug Interactions Where Herb Was Clinically Studied; Table S2: Findings of Clinical Studies; Table S3: Comparison of Case Reports to Herb–Drug Interaction Checkers; Table S4: Case Report Summaries; Table S5: Materials and Methods of Clinical Studies. (References [74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220] are referred in supplementary materials).
Author Contributions
Individual author contributions include: conceptualization, M.B.B., D.H., and P.H.; methodology, M.B.B.; software, M.B.B.; validation, M.B.B., M.H.; formal analysis, M.B.B.; investigation, M.B.B., M.H., M.K., T.M., F.M., J.V.S.-P., L.R., V.S.; resources, M.B.B.; data curation, M.B.B.; writing—original draft preparation, M.B.B., D.H., M.K., J.V.S.-P.; writing—review and editing, all authors; visualization, M.B.B.; supervision, M.B.B.; project administration, M.B.B.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith T., May G., Eckl V., Morton Reynolds C. US Sales of Herbal Supplements Increase by 8.6% in 2019. [(accessed on 20 December 2020)];HerbalGram. 2020 127:54–69. Available online: http://cms.herbalgram.org/herbalgram/issue127/hg127-mktrpt-2019.html. [Google Scholar]
- 2.Martin K.J., Jordan T.R., Vassar A.D., White D.B. Herbal and Nonherbal Alternative Medicine Use in Northwest Ohio. Ann. Pharmacother. 2002;36:1862–1869. doi: 10.1345/aph.1A215. [DOI] [PubMed] [Google Scholar]
- 3.Clarke T.C., Black L.I., Stussman B.J., Barnes P.M., Nahin R.L. Trends in the Use of Complementary Health Approaches among Adults: United States, 2002–2012 National Health Statistics Reports No. 79. National Center for Health Statistics; Hyattsville, MD, USA: 2015. [PMC free article] [PubMed] [Google Scholar]
- 4.Rashrash M., Schommer J.C., Brown L.M. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J. Patient Exp. 2017;4:108–113. doi: 10.1177/2374373517706612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low Dog T. The use of botanicals during pregnancy and lactation. Altern. Ther. Health Med. 2009;15:54–58. [PubMed] [Google Scholar]
- 6.Percha B., Altman R.B. Informatics confronts drug–drug interactions. Trends Pharmacol. Sci. 2013;34:178–184. doi: 10.1016/j.tips.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Q., Li Y. Interaction between Warfarin and the Herbal Product Shengmai-Yin: A Case Report of Intracerebral Hematoma. Yonsei Med. J. 2010;51:793–796. doi: 10.3349/ymj.2010.51.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker B., Greene J., Evanson J., Chidsey G., Stone W. Ginseng-induced diuretic resistance. JAMA. 1996;276:606–607. doi: 10.1001/jama.1996.03540080028021. [DOI] [PubMed] [Google Scholar]
- 9.Costa M.L., Rodrigues J.A., Azevedo J., Vasconcelos V., Eiras E., Campos M.G. Hepatotoxicity induced by paclitaxel interaction with turmeric in association with a microcystin from a contaminated dietary supplement. Toxicon. 2018;150:207–211. doi: 10.1016/j.toxicon.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Fasinu P.S., Bouic P.J., Rosenkranz B. An Overview of the Evidence and Mechanisms of Herb–Drug Interactions. Front. Pharmacol. 2012;3:69. doi: 10.3389/fphar.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupiec T., Raj V. Fatal Seizures Due to Potential Herb-Drug Interactions with Ginkgo biloba. J. Anal. Toxicol. 2005;29:755–758. doi: 10.1093/jat/29.7.755. [DOI] [PubMed] [Google Scholar]
- 12.Lee A., Chui P.T., Aun C.S.T., Gin T., Lau A.S.C. Possible interaction between sevoflurane and Aloe vera. Ann. Pharmacother. 2004;38:1651–1654. doi: 10.1345/aph.1E098. [DOI] [PubMed] [Google Scholar]
- 13.Mergenhagen K.A., Sherman O. Elevated International Normalized Ratio after concurrent ingestion of cranberry sauce and warfarin. Am. J. Health Pharm. 2008;65:2113–2116. doi: 10.2146/ajhp080135. [DOI] [PubMed] [Google Scholar]
- 14.Shaw D., Graeme L., Pierre D., Elizabeth W., Kelvin C. Pharmacovigilance of herbal medicine. J. Ethnopharmacol. 2012;140:513–518. doi: 10.1016/j.jep.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Shetti S., Kumar C.D., Sriwastava N.K., Sharma I.P. Pharmacovigilance of herbal medicines: Current state and future directions. Pharmacogn. Mag. 2011;7:69–73. doi: 10.4103/0973-1296.75905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson E., Driver S., Baxter K., editors. Stockley’s Herbal Medicines Interactions. Pharmaceutical Press; London, UK: 2009. [Google Scholar]
- 17.Russo E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weathers P.J., Arsenault P., Covello P.S., McMickle A., Teoh K.H., Reed D. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem. Rev. 2010;10:173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobieraj D.M., Freyer C.W. Probable Hypoglycemic Adverse Drug Reaction Associated with Prickly Pear Cactus, Glipizide, and Metformin in a Patient with Type 2 Diabetes Mellitus. Ann. Pharmacother. 2010;44:1334–1337. doi: 10.1345/aph.1P148. [DOI] [PubMed] [Google Scholar]
- 20.Al-Jenoobi F.I., Al-Thukair A.A., Alam M.A., Abbas F.A., Al-Mohizea A.M., Alkharfy K.M., Al-Suwayeh S.A. Effect of Curcuma longa on CYP2D6- and CYP3A4-mediated metabolism of dextromethorphan in human liver microsomes and healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 2014;40:61–66. doi: 10.1007/s13318-014-0180-2. [DOI] [PubMed] [Google Scholar]
- 21.Awortwe C., Makiwane M., Reuter H., Muller C., Louw J., Rosenkranz B. Critical evaluation of causality assessment of herb-drug interactions in patients. Br. J. Clin. Pharmacol. 2018;84:679–693. doi: 10.1111/bcp.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X.-W., Sneed K.B., Pan S.-Y., Cao C., Kanwar J.R., Chew H., Zhou S.-F. Herb-Drug Interactions and Mechanistic and Clinical Considerations. Curr. Drug Metab. 2012;13:640–651. doi: 10.2174/1389200211209050640. [DOI] [PubMed] [Google Scholar]
- 23.Aronson J.K. Toward standardized reporting of drug interactions: The READI checklist for anecdotal reports. Expert Rev. Clin. Pharmacol. 2015;8:399–409. doi: 10.1586/17512433.2015.1049598. [DOI] [PubMed] [Google Scholar]
- 24.Ng J.Y., Munford V., Thakar H. Web-based online resources about adverse interactions or side effects associated with complementary and alternative medicine: A systematic review, summarization and quality assessment. BMC Med. Inform. Decis. Mak. 2020;20:1–20. doi: 10.1186/s12911-020-01298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Essential Medicines and Health Products. 2015. [(accessed on 7 January 2021)]. The WHO Programme for International Drug Monitoring. WHO Website. Available online: who.int/medicines/areas/quality_safety/safety_efficacy/National_PV_Centres_Map/en/ [Google Scholar]
- 26.United States Food and Drug Administration FDA Adverse Event Reporting System (FAERS) Public Dashboard. [(accessed on 28 October 2020)];2021 Available online: https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis.
- 27.Uppsala Monitoring Center Vigibase. [(accessed on 3 March 2021)];2012 Available online: https://www.who-umc.org/vigibase/vigibase/
- 28.Awortwe C., Bruckmueller H., Cascorbi I. Interaction of herbal products with prescribed medications: A systematic review and meta-analysis. Pharmacol. Res. 2019;141:397–408. doi: 10.1016/j.phrs.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Borse S.P., Singh D.P., Nivsarkar M. Understanding the relevance of herb–drug interaction studies with special focus on interplays: A prerequisite for integrative medicine. Porto Biomed. J. 2019;4:e15. doi: 10.1016/j.pbj.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D., Zhang L., Duan L.X., Wu J.J., Hu M., Liu Z.Q., Wang C.Y. Potential of herb-drug / herb interactions between substrates and inhibitors of UGTs derived from herbal medicines. Pharmacol. Res. 2019;150:104510. doi: 10.1016/j.phrs.2019.104510. [DOI] [PubMed] [Google Scholar]
- 31.Rombolà L., Scuteri D., Marilisa S., Watanabe C., Morrone L.A., Bagetta G., Corasaniti M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life. 2020;10:106. doi: 10.3390/life10070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fugh-Berman A., Ernst E. Herb-drug interactions: Review and assessment of report reliability. Br. J. Clin. Pharmacol. 2001;52:587–595. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VERBI Software . MAXQDA 2020. VERBI Software; Berlin, Germany: 2019. [Google Scholar]
- 34.Gnjidic D., Hilmer S., Blyth F.M., Naganathan V., Waite L., Seibel M., McLachlan A., Cumming R., Handelsman D.J., Le Couteur D. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J. Clin. Epidemiol. 2012;65:989–995. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Turner J.P., Jamsen K.M., Shakib S., Singhal N., Prowse R., Bell J.S. Polypharmacy cut-points in older people with cancer: How many medications are too many? Support. Care Cancer. 2016;24:1831–1840. doi: 10.1007/s00520-015-2970-8. [DOI] [PubMed] [Google Scholar]
- 36.Lexicomp Online Database. Lexicomp Inc.; Hudson, OH, USA: 2021. [(accessed on 15 May 2021)]. Available online: http://online.lexi.com. [Google Scholar]
- 37.Whirl-Carrillo M., McDonagh E.M., Hebert J.M., Gong L., Sangkuhl K., Thorn C.F., Altman R.B., Klein T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012;92:414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.IntegrativePro Drug-Nutrient Interaction Checker. Integrative Therapeutics, LLC; Green Bay, WI, USA: 2021. [(accessed on 15 May 2021)]. Available online: https://www.integrativepro.com/drug-nutrient-interaction-checker. [Google Scholar]
- 39.Medscape Drug Interaction Checker. [(accessed on 15 May 2021)];2021 Available online: https://reference.medscape.com/drug-interactionchecker.
- 40.Memorial Sloan Kettering Cancer Center Search About Herbs. [(accessed on 15 May 2021)];2021 Available online: https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs/search.
- 41.Natural Medicines Online Database. Therapeutic Research Center; San Francisco, CA, USA: 2021. [(accessed on 15 May 2021)]. Available online: https://naturalmedicines.therapeuticresearch.com. [Google Scholar]
- 42.IBM Corp . IBM SPSS Statistics for Windows. IBM Corp; Armonk, NY, USA: 2020. Version 27.0. [Google Scholar]
- 43.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben Abacha A., Chowdhury F.M., Karanasiou A., Mrabet Y., Lavelli A., Zweigenbaum P. Text mining for pharmacovigilance: Using machine learning for drug name recognition and drug–drug interaction extraction and classification. J. Biomed. Inform. 2015;58:122–132. doi: 10.1016/j.jbi.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Thompson P., Daikou S., Ueno K., Batista-Navarro R., Tsujii J., Ananiadou S. Annotation and detection of drug effects in text for pharmacovigilance. J. Cheminform. 2018;10:1–33. doi: 10.1186/s13321-018-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I., Roberts E.A., Janecek E., Domecq C., Greenblatt D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 47.Horn J.R., Hansten P.D., Chan L.-N. Proposal for a New Tool to Evaluate Drug Interaction Cases. Ann. Pharmacother. 2007;41:674–680. doi: 10.1345/aph.1H423. [DOI] [PubMed] [Google Scholar]
- 48.Council for International Organizations of Medical Sciences Cumulative Pharmacovigilance Glossary. [Internet Site] [(accessed on 15 June 2001)];2021 Available online: https://cioms.ch/wp-content/uploads/2021/03/CIOMS-Cumulative-Glossary_v1.1_3Jun2021.pdf.
- 49.Hill A., Van Der Lugt J., Sawyer W., Boffito M. How much ritonavir is needed to boost protease inhibitors? Systematic review of 17 dose-ranging pharmacokinetic trials. AIDS. 2009;23:2237–2245. doi: 10.1097/QAD.0b013e328332c3a5. [DOI] [PubMed] [Google Scholar]
- 50.Alcamo A.M., Wolf M.S., Alessi L.J., Chong H.J., Green M., Williams J.V., Simon D.W. Successful Use of Cidofovir in an Immunocompetent Child With Severe Adenoviral Sepsis. Pediatrics. 2020;145:e20191632. doi: 10.1542/peds.2019-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan M., Maryam A., Mehmood T., Zhang Y., Ma T. Enhancing Activity of Anticancer Drugs in Multidrug Resistant Tumors by Modulating P-Glycoprotein through Dietary Nutraceuticals. Asian Pac. J. Cancer Prev. 2015;16:6831–6839. doi: 10.7314/APJCP.2015.16.16.6831. [DOI] [PubMed] [Google Scholar]
- 52.Meghwal M., Goswami T.K. Piper nigrum and Piperine: An Update. Phytother. Res. 2013;27:1121–1130. doi: 10.1002/ptr.4972. [DOI] [PubMed] [Google Scholar]
- 53.Jiang W., Wang X., Xu X., Kong L. Effect of Schisandra sphenanthera extract on the concentration of tacrolimus in the blood of liver transplant patients. Int. J. Clin. Pharmacol. Ther. 2010;48:224–229. doi: 10.5414/CPP48224. [DOI] [PubMed] [Google Scholar]
- 54.Souirti Z., Loukili M., Soudy I.D., Rtibi K., Özel A., Limas-Nzouzi N., El Ouezzani S., Eto B. Hibiscus sabdariffaincreases hydroxocobalamin oral bioavailability and clinical efficacy in vitamin B12deficiency with neurological symptoms. Fundam. Clin. Pharmacol. 2016;30:568–576. doi: 10.1111/fcp.12220. [DOI] [PubMed] [Google Scholar]
- 55.Berretta M., della Pepa C., Tralongo P., Fulvi A., Martellotta F., Lleshi A., Nasti G., Fisichella R., Romano C., De Divitiis C., et al. Use of Complementary and Alternative Medicine (CAM) in cancer patients: An Italian multicenter survey. Oncotarget. 2017;8:24401–24414. doi: 10.18632/oncotarget.14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knecht K., Kinder D., Stockert A. Biologically-Based Complementary and Alternative Medicine (CAM) Use in Cancer Patients: The Good, the Bad, the Misunderstood. Front. Nutr. 2020;6:196. doi: 10.3389/fnut.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung K.S., Gubili J., Mao J.J. Herb-Drug Interactions in Cancer Care. Oncology. 2018;32:516–520. [PubMed] [Google Scholar]
- 58.Beauchamp T.L., Childress J.F. Principles of Biomedical Ethics. 7th ed. Oxford University Press; New York, NY, USA: 2019. [Google Scholar]
- 59.Melchardt T., Magnes T., Weiss L., Grundbichler M., Strasser M., Hufnagl C., Moik M., Greil R., Egle A. Liver toxicity during temozolomide chemotherapy caused by Chinese herbs. BMC Complement. Altern. Med. 2014;14:115. doi: 10.1186/1472-6882-14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spinella M., Eaton L.A. Hypomania induced by herbal and pharmaceutical psychotropic medicines following mild traumatic brain injury. Brain Inj. 2002;16:359–367. doi: 10.1080/02699050110103319. [DOI] [PubMed] [Google Scholar]
- 61.Lee N., Fermo J. Warfarin and royal jelly interaction. Pharmacotherapy. 2006;26:583–586. doi: 10.1592/phco.26.4.583. [DOI] [PubMed] [Google Scholar]
- 62.Khalid Z., Osuagwu F.C., Shah B., Roy N., Dillon J.E., Bradley R. Celery root extract as an inducer of mania induction in a patient on venlafaxine and St John’s Wort. Postgrad. Med. 2016;128:682–683. doi: 10.1080/00325481.2016.1218263. [DOI] [PubMed] [Google Scholar]
- 63.Al Faraj S. Antagonism of the anticoagulant effect of warfarin caused by the use of Commiphora molmol as a herbal medication: A case report. Ann. Trop. Med. Parasitol. 2005;99:219–220. doi: 10.1179/136485905X17434. [DOI] [PubMed] [Google Scholar]
- 64.Ernst E., Pittler M. Herbal medicine. Med. Clin. North Am. 2002;86:149–161. doi: 10.1016/S0025-7125(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 65.Bruno J.J., Ellis J. Herbal Use among US Elderly: 2002 National Health Interview Survey. Ann. Pharmacother. 2005;39:643–648. doi: 10.1345/aph.1E460. [DOI] [PubMed] [Google Scholar]
- 66.Kelly J.P., Kaufman D.W., Kelley K., Rosenberg L., Anderson T.E., Mitchell A.A. Recent Trends in Use of Herbal and Other Natural Products. Arch. Intern. Med. 2005;165:281–286. doi: 10.1001/archinte.165.3.281. [DOI] [PubMed] [Google Scholar]
- 67.McHugh M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ventola C.L. Big data and pharmacovigilance: Data mining for adverse drug events and interactions. Pharm. Ther. 2018;43:340–351. [PMC free article] [PubMed] [Google Scholar]
- 69.National Center for Biotechnology Information (NCBI) National Library of Medicine (US), National Center for Biotechnology Information, Bethesda, MD, US. [(accessed on 18 May 2021)]; Available online: https://www.ncbi.nlm.nih.gov/
- 70.Elsevier Embase®. [(accessed on 18 May 2021)]; Available online: https://www.elsevier.com/solutions/embase-biomedical-research.
- 71.IBM Micromedex Truven Health Analytics/IBM Watson Health. [(accessed on 18 May 2021)];2020 Available online: https://www.micromedexsolutions.com.
- 72.Tatro D., editor. Drug Interaction Facts: The Authority on Drug Interactions. Wolters Kluwer Health; Philadelphia, PA, USA: 2015. [Google Scholar]
- 73.Walsh K.M. Getting to Yes. J. Am. Geriatr. Soc. 2005;53:1072. doi: 10.1111/j.1532-5415.2005.53326.x. [DOI] [PubMed] [Google Scholar]
- 74.Mazaleuskaya L.L., Theken K., Gong L., Thorn C.F., Fitzgerald G.A., Altman R., Klein T.E. PharmGKB summary: Ibuprofen pathways. Pharm. Genom. 2015;25:96–106. doi: 10.1097/FPC.0000000000000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Khan I.A., Shah A. In Vivo Effects of Goldenseal, Kava Kava, Black Cohosh, and Valerian on Human Cytochrome P450 1A2, 2D6, 2E1, and 3A4 Phenotypes. Clin. Pharmacol. Ther. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gurley B.J., Barone G.W., Williams D.K., Carrier J., Breen P., Yates C.R., Song P.-F., Hubbard M.A., Tong Y., Cheboyina S. Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab. Dispos. 2006;34:69–74. doi: 10.1124/dmd.105.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdul M.I.M., Jiang X., Williams K.M., Day R., Roufogalis B., Liauw W.S., Xu H., McLachlan A.J. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br. J. Pharmacol. 2008;154:1691–1700. doi: 10.1038/bjp.2008.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 79.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y. Clinical assessment of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John’s wort, garlic oil, Panax ginseng, and Ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Q., Ye Z., Ruan Z., Zeng S. Investigation on modulation of human P-gp by multiple doses of Radix Astragali extract granules using fexofenadine as a phenotyping probe. J. Ethnopharmacol. 2013;146:744–749. doi: 10.1016/j.jep.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 81.Fan L., Zhang W., Guo D., Tan Z.-R., Xu P., Li Q., Liu Y.-Z., Zhang L., He T.-Y., Hu D.-L., et al. The Effect of Herbal Medicine Baicalin on Pharmacokinetics of Rosuvastatin, Substrate of Organic Anion-transporting Polypeptide 1B1. Clin. Pharmacol. Ther. 2008;83:471–476. doi: 10.1038/sj.clpt.6100318. [DOI] [PubMed] [Google Scholar]
- 82.Hu M., Mak V.W.L., Yin O.Q.P., Chu T.T.W., Tomlinson B. Effects of Grapefruit Juice and SLCO1B1 388A>G Polymorphism on the Pharmacokinetics of Pitavastatin. Drug Metab. Pharmacokinet. 2013;28:104–108. doi: 10.2133/dmpk.DMPK-12-RG-067. [DOI] [PubMed] [Google Scholar]
- 83.Nicandro J.P., Tsourounis C., Frassetto L., Guglielmo B.J. In vivo effect of I’m-Yunity on hepatic cytochrome P450 3A4. J. Herb. Pharmacother. 2007;7:39–56. doi: 10.1080/J157v07n01_04. [DOI] [PubMed] [Google Scholar]
- 84.Tankanow R., Tamer H.R., Streetman D.S., Smith S.G., Welton J.L., Annesley T., Aaronson K.D., Bleske B.E. Interaction Study between Digoxin and a Preparation of Hawthorn (Crataegus oxyacantha) J. Clin. Pharmacol. 2003;43:637–642. doi: 10.1177/0091270003253417. [DOI] [PubMed] [Google Scholar]
- 85.Walker A.F., Marakis G., Simpson E., Hope J.L., Robinson P.A., Hassanein M., Simpson H.C. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: A randomized controlled trial. Br. J. Gen. Pract. 2006;56:437–443. [PMC free article] [PubMed] [Google Scholar]
- 86.Ikehata M., Ohnishi N., Egami S., Kishi H., Shin Y., Takara K., Tsuchishita Y., Tokuda N., Hori S., Yatani Y., et al. Effects of Turmeric Extract on the Pharmacokinetics of Nifedipine After a Single Oral Administration in Healthy Volunteers. J. Diet. Suppl. 2008;5:401–410. doi: 10.1080/19390210802519713. [DOI] [PubMed] [Google Scholar]
- 87.Kim T.-E., Ha N., Kim Y., Kim H., Lee J.W., Jeon J.-Y., Kim M.-G. Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers. Drug Des. Dev. Ther. 2017;11:1409–1416. doi: 10.2147/DDDT.S130050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moltó J., Valle M., Miranda C., Cedeño S., Negredo E., Clotet B. Herb-Drug Interaction between Echinacea purpurea and Etravirine in HIV-Infected Patients. Antimicrob. Agents Chemother. 2012;56:5328–5331. doi: 10.1128/AAC.01205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salman S.A.B., Amrah S., Wahab M.S.A., Ismail Z., Ismail R., Yuen K.H., Gan S.H., Msc S.A.B.S., Mpharm R.I. Modification of propranolol’s bioavailability by Eurycoma longifolia water-based extract. J. Clin. Pharm. Ther. 2010;35:691–696. doi: 10.1111/j.1365-2710.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- 90.Xiao C.-Q., Chen R., Lin J., Wang G., Chen Y., Tan Z.-R., Zhou H.-H. Effect of genistein on the activities of cytochrome P450 3A and P-glycoprotein in Chinese healthy participants. Xenobiotica. 2011;42:173–178. doi: 10.3109/00498254.2011.615954. [DOI] [PubMed] [Google Scholar]
- 91.Aruna D., Naidu M.U.R. Pharmacodynamic interaction studies of Ginkgo biloba with cilostazol and clopidogrel in healthy human subjects. Br. J. Clin. Pharmacol. 2007;63:333–338. doi: 10.1111/j.1365-2125.2006.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dai L.-L., Fan L., Wu H.-Z., Tan Z.-R., Chen Y., Peng X.-D., Shen M.-X., Yang G.-P., Zhou H.-H. Assessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and Ginkgo biloba extracts in healthy subjects. Xenobiotica. 2013;43:862–867. doi: 10.3109/00498254.2013.773385. [DOI] [PubMed] [Google Scholar]
- 93.Fan L., Tao G.-Y., Wang G., Chen Y., Zhang W., He Y.-J., Li Q., Lei H.-P., Jiang F., Hu D.-L., et al. Effects of Ginkgo biloba Extract Ingestion on the Pharmacokinetics of Talinolol in Healthy Chinese Volunteers. Ann. Pharmacother. 2009;43:944–949. doi: 10.1345/aph.1L656. [DOI] [PubMed] [Google Scholar]
- 94.Guo C.-X., Pei Q., Yin J.-Y., Peng X., Zhou B.-T., Zhao Y.-C., Wu L.-X., Meng X.-G., Wang G., Li Q., et al. Effects of Ginkgo biloba extracts on pharmacokinetics and efficacy of atorvastatin based on plasma indices. Xenobiotica. 2012;42:784–790. doi: 10.3109/00498254.2012.661100. [DOI] [PubMed] [Google Scholar]
- 95.Jiang X., Blair E.Y.L., McLachlan A.J. Investigation of the Effects of Herbal Medicines on Warfarin Response in Healthy Subjects: A Population Pharmacokinetic-Pharmacodynamic Modeling Approach. J. Clin. Pharmacol. 2006;46:1370–1378. doi: 10.1177/0091270006292124. [DOI] [PubMed] [Google Scholar]
- 96.Kim B.-H., Kim K.-P., Lim K.S., Kim J.-R., Yoon S.H., Cho J.-Y., Lee Y.-O., Lee K.-H., Jang I.-J., Shin S.-G., et al. Influence of Ginkgo biloba extract on the pharmacodynamic effects and pharmacokinetic properties of ticlopidine: An open-label, randomized, two-period, two-treatment, two-sequence, single-dose crossover study in healthy Korean male volunteers. Clin. Ther. 2010;32:380–390. doi: 10.1016/j.clinthera.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 97.Mohutsky M.A., Anderson G.D., Miller J.W., Elmer G.W. Ginkgo biloba: Evaluation of CYP2C9 Drug Interactions In Vitro and In Vivo. Am. J. Ther. 2006;13:24–31. doi: 10.1097/01.mjt.0000143695.68285.31. [DOI] [PubMed] [Google Scholar]
- 98.Zadoyan G., Rokitta D., Klement S., Dienel A., Hoerr R., Gramatté T., Fuhr U. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: A cocktail interaction study in healthy volunteers. Eur. J. Clin. Pharmacol. 2012;68:553–560. doi: 10.1007/s00228-011-1174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Breemen R.B., Chen L., Tonsing-Carter A., Banuvar S., Barengolts E., Viana M., Chen S.-N., Pauli G.F., Bolton J.L. Pharmacokinetic Interactions of a Hop Dietary Supplement with Drug Metabolism in Perimenopausal and Postmenopausal Women. J. Agric. Food Chem. 2020;68:5212–5220. doi: 10.1021/acs.jafc.0c01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gurley B.J., Swain A., Barone G.W., Williams D.K., Breen P., Yates C.R., Stuart L.B., Hubbard M.A., Tong Y., Cheboyina S., et al. Effect of Goldenseal (Hydrastis canadensis) and Kava Kava (Piper methysticum) Supplementation on Digoxin Pharmacokinetics in Humans. Drug Metab. Dispos. 2006;35:240–245. doi: 10.1124/dmd.106.012708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gurley B.J., Swain A., Hubbard M.A., Hartsfield F., Thaden J., Williams D.K., Gentry W.B., Tong Y. Supplementation With Goldenseal (Hydrastis canadensis), but not Kava Kava (Piper methysticum), Inhibits Human CYP3A Activity In Vivo. Clin. Pharmacol. Ther. 2008;83:61–69. doi: 10.1038/sj.clpt.6100222. [DOI] [PubMed] [Google Scholar]
- 102.Andrén L., Andreasson A., Eggertsen R. Interaction between a commercially available St. John’s wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur. J. Clin. Pharmacol. 2007;63:913–916. doi: 10.1007/s00228-007-0345-x. [DOI] [PubMed] [Google Scholar]
- 103.Bell E.C., Ravis W.R., Chan H.M., Lin Y.-J. Lack of Pharmacokinetic Interaction Between St. John’s Wort and Prednisone. Ann. Pharmacother. 2007;41:1819–1824. doi: 10.1345/aph.1K316. [DOI] [PubMed] [Google Scholar]
- 104.Bell E.C., Ravis W.R., Lloyd K.B., Stokes T.J. Effects of St. John’s Wort Supplementation on Ibuprofen Pharmacokinetics. Ann. Pharmacother. 2007;41:229–234. doi: 10.1345/aph.1H602. [DOI] [PubMed] [Google Scholar]
- 105.Nieminen T.H., Hagelberg N.M., Saari T.I., Neuvonen M., Laine K., Neuvonen P., Olkkola K.T. St John’s wort greatly reduces the concentrations of oral oxycodone. Eur. J. Pain. 2010;14:854–859. doi: 10.1016/j.ejpain.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 106.Fan L., Zhou G., Guo D., Liu Y.-L., Chen W.-Q., Liu Z., Tan Z.-R., Sheng D., Zhou H.-H., Zhang W. The Pregnane X Receptor Agonist St Johnʼs Wort Has No Effects on the Pharmacokinetics and Pharmacodynamics of Repaglinide. Clin. Pharmacokinet. 2011;50:605–611. doi: 10.2165/11587310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 107.Goey A.K.L., Meijerman I., Rosing H., Marchetti S., Mergui-Roelvink M., Keessen M., Burgers J.A., Beijnen J.H., Schellens J.H.M. The Effect of St John’s Wort on the Pharmacokinetics of Docetaxel. Clin. Pharmacokinet. 2013;53:103–110. doi: 10.1007/s40262-013-0102-5. [DOI] [PubMed] [Google Scholar]
- 108.Hennessy M., Kelleher D., Spiers P., Barry M., Kavanagh P., Back D., Mulcahy F., Feely J. St John’s Wort increases expression of P-glycoprotein: Implications for drug interactions. Br. J. Clin. Pharmacol. 2002;53:75–82. doi: 10.1046/j.0306-5251.2001.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang X., Williams K.M., Liauw W.S., Ammit A., Roufogalis B., Duke C.C., Day R., McLachlan A.J. Effect of St John’s wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2004;57:592–599. doi: 10.1111/j.1365-2125.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loughren M.J., Kharasch E.D., Kelton-Rehkopf M.C., Syrjala K.L., Shen D.D. Influence of St. John’s Wort on Intravenous Fentanyl Pharmacokinetics, Pharmacodynamics, and Clinical Effects. Anesthesiology. 2020;132:491–503. doi: 10.1097/ALN.0000000000003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Markert C., Kastner I.M., Hellwig R., Kalafut P., Schweizer Y., Hoffmann M.M., Burhenne J., Weiss J., Mikus G., Haefeli W.E. The Effect of Induction of CYP3A4 by St John’s Wort on Ambrisentan Plasma Pharmacokinetics in Volunteers of knownCYP2C19Genotype. Basic Clin. Pharmacol. Toxicol. 2014;116:423–428. doi: 10.1111/bcpt.12332. [DOI] [PubMed] [Google Scholar]
- 112.Mueller S.C., Majcher-Peszynska J., Uehleke B., Klammt S., Mundkowski R.G., Miekisch W., Sievers H., Bauer S., Frank B., Kundt G., et al. The extent of induction of CYP3A by St. John’s wort varies among products and is linked to hyperforin dose. Eur. J. Clin. Pharmacol. 2006;62:29–36. doi: 10.1007/s00228-005-0061-3. [DOI] [PubMed] [Google Scholar]
- 113.Mueller S.C., Majcher-Peszynska J., Mundkowski R.G., Uehleke B., Klammt S., Sievers H., Lehnfeld R., Frank B., Thurow K., Kundt G., et al. No clinically relevant CYP3A induction after St. John’s wort with low hyperforin content in healthy volunteers. Eur. J. Clin. Pharmacol. 2008;65:81–87. doi: 10.1007/s00228-008-0554-y. [DOI] [PubMed] [Google Scholar]
- 114.Murphy P.A., Kern S.E., Stanczyk F.Z., Westhoff C.L. Interaction of St. John’s Wort with oral contraceptives: Effects on the pharmacokinetics of norethindrone and ethinyl estradiol, ovarian activity and breakthrough bleeding. Contracept. 2005;71:402–408. doi: 10.1016/j.contraception.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 115.Piscitelli S.C., Burstein A.H., Chaitt D., Alfaro R.M., Falloon J. Indinavir concentrations and St John’s wort. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 116.Portolés A., Terleira A., Calvo A., Martínez M.I., Resplandy G. Effects of Hypericum perforatum on Ivabradine Pharmacokinetics in Healthy Volunteers: An Open-Label, Pharmacokinetic Interaction Clinical Trial. J. Clin. Pharmacol. 2006;46:1188–1194. doi: 10.1177/0091270006291623. [DOI] [PubMed] [Google Scholar]
- 117.Xie R., Tan L.H., Polasek E.C., Hong C., Teillol-Foo M., Gordi T., Sharma A., Ms D.J.N., Arakawa T., Knuth D.W., et al. CYP3A and P-Glycoprotein Activity Induction With St. John’s Wort in Healthy Volunteers From 6 Ethnic Populations. J. Clin. Pharmacol. 2005;45:352–356. doi: 10.1177/0091270004273320. [DOI] [PubMed] [Google Scholar]
- 118.Xu H., Williams K.M., Liauw W.S., Murray M., Day R., McLachlan A.J. Effects of St John’s wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide. Br. J. Pharmacol. 2008;153:1579–1586. doi: 10.1038/sj.bjp.0707685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heger-Mahn D., Pabst G., Dienel A., Schlafke S., Klipping C. No interacting influence of lavender oil preparation silexan on oral contraception using an ethinyl estradiol/levonorgestrel combination. Drugs R&D. 2014;14:265–272. doi: 10.1007/s40268-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen Y., Ouyang D.-S., Kang Z., Yang G.-P., Tan Z.-R., Zhou G., Yan J. Effect of a traditional Chinese medicine Liu Wei Di Huang Wan on the activities of CYP2C19, CYP2D6 and CYP3A4 in healthy volunteers. Xenobiotica. 2011;42:596–602. doi: 10.3109/00498254.2011.644596. [DOI] [PubMed] [Google Scholar]
- 121.Fan L., Wang G., Wang L.-S., Chen Y., Zhang W., Huang Y.-F., Huang R.-X., Hu D.-L., Wang D., Zhou H.-H. Herbal medicine Yin Zhi Huang induces CYP3A4-mediated sulfoxidation and CYP2C19-dependent hydroxylation of omeprazole. Acta Pharmacol. Sin. 2007;28:1685–1692. doi: 10.1111/j.1745-7254.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 122.Kim H., Bae S.K., Park S.-J., Shim E.-J., Kim H.-S., Shon J.-H., Liu K.-H., Shin J.-G. Effects of woohwangcheongsimwon suspension on the pharmacokinetics of bupropion and its active metabolite, 4-hydroxybupropion, in healthy subjects. Br. J. Clin. Pharmacol. 2010;70:126–131. doi: 10.1111/j.1365-2125.2010.03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakao M., Muramoto Y., Hisadome M., Yamano N., Shoji M., Fukushima Y., Saruwatari J., Nakagawa K. The effect of Shoseiryuto, a traditional Japanese medicine, on cytochrome P450s, N-acetyltransferase 2 and xanthine oxidase, in extensive or intermediate metabolizers of CYP2D6. Eur. J. Clin. Pharmacol. 2007;63:345–353. doi: 10.1007/s00228-006-0253-5. [DOI] [PubMed] [Google Scholar]
- 124.Park S.-I., Park J.-Y., Park M.-J., Yim S.-V., Kim B.-H. Effects of Ojeok-san on the Pharmacokinetics of Celecoxib at Steady-state in Healthy Volunteers. Basic Clin. Pharmacol. Toxicol. 2018;123:51–57. doi: 10.1111/bcpt.12971. [DOI] [PubMed] [Google Scholar]
- 125.Saruwatari J., Takaishi C., Yoshida K., Takashima A., Fujimura Y., Umemoto Y., Abe T., Kitamado M., Shimomasuda M., Muramoto Y., et al. A herbal-drug interaction study of keishi-bukuryo-gan, a traditional herbal preparation used for menopausal symptoms, in healthy female volunteers. J. Pharm. Pharmacol. 2012;64:670–676. doi: 10.1111/j.2042-7158.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- 126.Wang P., Sun H., Yang L., Li L.-Y., Hao J., Ruff D., Guo Z.-X. Absence of an effect of T89 on the steady-state pharmacokinetics and pharmacodynamics of warfarin in healthy volunteers. J. Clin. Pharmacol. 2014;54:234–239. doi: 10.1002/jcph.209. [DOI] [PubMed] [Google Scholar]
- 127.Lee Y.H., Lee B.K., Choi Y.J., Yoon I.K., Chang B.C., Gwak H.S. Interaction between warfarin and Korean red ginseng in patients with cardiac valve replacement. Int. J. Cardiol. 2010;145:275–276. doi: 10.1016/j.ijcard.2009.09.553. [DOI] [PubMed] [Google Scholar]
- 128.Malati C.Y., Robertson S.M., Hunt J.D., Chairez C., Alfaro R.M., Kovacs J.A., Penzak S.R. Influence of Panax ginseng on Cytochrome P450 (CYP)3A and P-glycoprotein (P-gp) Activity in Healthy Participants. J. Clin. Pharmacol. 2012;52:932–939. doi: 10.1177/0091270011407194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fatima N., Pingali U., Muralidhar N. Study of pharmacodynamic interaction of Phyllanthus emblica extract with clopidogrel and ecosprin in patients with type II diabetes mellitus. Phytomedicine. 2014;21:579–585. doi: 10.1016/j.phymed.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 130.Bedada S.K., Neerati P. Resveratrol Pretreatment Affects CYP2E1 Activity of Chlorzoxazone in Healthy Human Volunteers. Phytotherapy Res. 2016;30:463–468. doi: 10.1002/ptr.5549. [DOI] [PubMed] [Google Scholar]
- 131.Fuhr U., Beckmann-Knopp S., Jetter A., Lück H., Mengs U. The Effect of Silymarin on Oral Nifedipine Pharmacokinetics. Planta Medica. 2007;73:1429–1435. doi: 10.1055/s-2007-990256. [DOI] [PubMed] [Google Scholar]
- 132.Kawaguchi-Suzuki M., Frye R.F., Zhu H.-J., Brinda B.J., Chavin K.D., Bernstein H.J., Markowitz J.S. The Effects of Milk Thistle (Silybum marianum) on Human Cytochrome P450 Activity. Drug Metab. Dispos. 2014;42:1611–1616. doi: 10.1124/dmd.114.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tocaciu S., Oliver L.J., Lowenthal R.M., Peterson G.M., Patel R., Shastri M., McGuinness G., Olesen I., Fitton J.H. The Effect of Undaria pinnatifida Fucoidan on the Pharmacokinetics of Letrozole and Tamoxifen in Patients With Breast Cancer. Integr. Cancer Ther. 2018;17:99–105. doi: 10.1177/1534735416684014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thronicke A., Steele M.L., Grah C., Matthes B., Schad F. Clinical safety of combined therapy of immune checkpoint inhibitors and Viscum album L. therapy in patients with advanced or metastatic cancer. BMC Complement. Altern. Med. 2017;17:534. doi: 10.1186/s12906-017-2045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Açikgöz S.K., Açıkgöz E. Gastrointestinal bleeding secondary to interaction of Artemisia absinthium with warfarin. Drug Metab. Drug Interact. 2013;28:187–189. doi: 10.1515/dmdi-2013-0021. [DOI] [PubMed] [Google Scholar]
- 136.Almeida J., Grimsley E. Coma from the Health Food Store: Interaction between Kava and Alprazolam. Ann. Intern. Med. 1996;125:940–941. doi: 10.7326/0003-4819-125-11-199612010-00023. [DOI] [PubMed] [Google Scholar]
- 137.Alscher D.M., Klotz U. Drug interaction of herbal tea containing St. John’s wort with cyclosporine. Transpl. Int. 2003;16:543–544. doi: 10.1111/j.1432-2277.2003.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 138.Aslam M., Stockley I. Interaction between curry ingredient (karela) and drug (chlorpropamide) Lancet. 1979;313:607. doi: 10.1016/S0140-6736(79)91028-6. [DOI] [PubMed] [Google Scholar]
- 139.Barbenel D.M., Yusufi B., Bench C.J., O’Shea D. Mania in a patient receiving testosterone replacement post-orchidectomy taking St John’s wort and sertraline. J. Psychopharmacol. 2000;14:84–86. doi: 10.1177/026988110001400113. [DOI] [PubMed] [Google Scholar]
- 140.Barone G.W., Gurley B.J., Ketel B.L., Lightfoot M.L., Abul-Ezz S.R. Drug Interaction between St. John’s Wort and Cyclosporine. Ann. Pharmacother. 2000;34:1013–1016. doi: 10.1345/aph.10088. [DOI] [PubMed] [Google Scholar]
- 141.Bilgi N., Bell K., Ananthakrishnan A.N., Atallah E. Imatinib and Panax ginseng: A Potential Interaction Resulting in Liver Toxicity. Ann. Pharmacother. 2010;44:926–928. doi: 10.1345/aph.1M715. [DOI] [PubMed] [Google Scholar]
- 142.Bolley R., Zülke C., Kammerl M., Fischereder M., Krämer B.K. Tacrolimus-induced nephrotoxicity unmasked by induction of the CYP3A4 system with ST JOHN’S wort. Transplantation. 2002;73:1009. doi: 10.1097/00007890-200203270-00035. [DOI] [PubMed] [Google Scholar]
- 143.Bossaer J.B., Odle B.L. Probable Etoposide Interaction with Echinacea. J. Diet. Suppl. 2012;9:90–95. doi: 10.3109/19390211.2012.682643. [DOI] [PubMed] [Google Scholar]
- 144.Breidenbach T., Hoffmann M.W., Becke T., Schlitt H., Klempnauer J. Drug interaction of St John’s wort with ciclosporin. Lancet. 2000;355:1912. doi: 10.1016/S0140-6736(05)73359-6. [DOI] [PubMed] [Google Scholar]
- 145.Buckley M.S., Goff A.D., Knapp W.E. Fish Oil Interaction with Warfarin. Ann. Pharmacother. 2004;38:50–53. doi: 10.1345/aph.1D007. [DOI] [PubMed] [Google Scholar]
- 146.Bamgbade O. The perioperative implications of khat use. Eur. J. Anaesthesiol. 2008;25:170–172. doi: 10.1017/S026502150700258X. [DOI] [PubMed] [Google Scholar]
- 147.Campos M., Machado J., Costa M., Lino S., Correia F., Maltez F. Case Report: Severe Hematological, Muscle and Liver Toxicity Caused by Drugs and Artichoke Infusion Interaction in an Elderly Polymedicated Patient. Curr. Drug Saf. 2018;13:44–50. doi: 10.2174/1574886312666170912163746. [DOI] [PubMed] [Google Scholar]
- 148.Cappuzzo K.A. Herbal Product Use in a Patient with Polypharmacy. Consult. Pharm. 2006;21:911–915. doi: 10.4140/TCP.n.2006.911. [DOI] [PubMed] [Google Scholar]
- 149.Carbajal R., Yisfalem A., Pradhan N., Baumstein D., Chaudhari A. Case Report: Boldo (Peumus boldus) and Tacrolimus Interaction in a Renal Transplant Patient. Transplant. Proc. 2014;46:2400–2402. doi: 10.1016/j.transproceed.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 150.Carr M., Klotz J., Bergeron M. Coumadin resistance and the vitamin supplement “noni”. Am. J. Hematol. 2004;77:103. doi: 10.1002/ajh.20062. [DOI] [PubMed] [Google Scholar]
- 151.Carrasco M.C., Vallejo J.R., Pardo-de-Santayana M., Peral D., Martín M.Á., Altimiras J. Interactions of Valeriana officinalis L. and Passiflora incarnata L. in a patient treated with lorazepam. Phytother. Res. 2009;23:1795–1796. doi: 10.1002/ptr.2847. [DOI] [PubMed] [Google Scholar]
- 152.Carter J., Yeh R.F., Braunschweig I., Barta S. Unreported use of an herbal supplement resulting in decreased clearance of intravenous busulfan in a patient undergoing auto-SCT. Bone Marrow Transplant. 2014;49:313–314. doi: 10.1038/bmt.2013.169. [DOI] [PubMed] [Google Scholar]
- 153.Cattaneo D., Fusi M., Gervasoni C. No effects of Hypericum-containing complex on dolutegravir plasma trough concentrations: A case report. Eur. J. Clin. Pharmacol. 2019;75:1467–1468. doi: 10.1007/s00228-019-02714-0. [DOI] [PubMed] [Google Scholar]
- 154.Chan J.C.M., Ng M.-H., Wong R.S.M., Tomlinson B. A case of simvastatin-induced myopathy with SLCO1B1 genetic predisposition and co-ingestion of linagliptin and Stevia rebaudiana. J. Clin. Pharm. Ther. 2019;44:381–383. doi: 10.1111/jcpt.12805. [DOI] [PubMed] [Google Scholar]
- 155.Chang Y.-Y., Liu J.-S., Lai S.-L., Wu H.-S., Lan M.-Y. Cerebellar Hemorrhage Provoked by Combined Use of Nattokinase and Aspirin in a Patient with Cerebral Microbleeds. Intern. Med. 2008;47:467–469. doi: 10.2169/internalmedicine.47.0620. [DOI] [PubMed] [Google Scholar]
- 156.Constable S., Ham A., Pirmohamed M. Herbal medicines and acute medical emergency admissions to hospital. Br. J. Clin. Pharmacol. 2007;63:247–248. doi: 10.1111/j.1365-2125.2006.02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cordova E., Morganti L., Rodriguez C. Possible Drug–Herb Interaction between Herbal Supplement Containing Horsetail (Equisetum arvense) and Antiretroviral Drugs. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2017;16:11–13. doi: 10.1177/2325957416680295. [DOI] [PubMed] [Google Scholar]
- 158.Damato A., Larocca M., Rondini E., Menga M., Pinto C., Versari A. Severe Rhabdomyolysis during Treatment with Trabectedin in Combination with a Herbal Drug in a Patient with Metastatic Synovial Sarcoma: A Case Report. Case Rep. Oncol. 2017;10:258–264. doi: 10.1159/000464440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dannawi M. Possible serotonin syndrome after combination of buspirone and St John’s Wort. J. Psychopharmacol. 2002;16:401. doi: 10.1177/026988110201600420. [DOI] [PubMed] [Google Scholar]
- 160.Daveluy A., Géniaux H., Thibaud L., Mallaret M., Miremont-Salamé G., Haramburu F. Probable Interaction Between an Oral Vitamin K Antagonist and Turmeric (Curcuma longa) Therapie. 2014;69:519–520. doi: 10.2515/therapie/2014062. [DOI] [PubMed] [Google Scholar]
- 161.Epstein R.J., Leung T.W.T., Cheung P.S.Y. Panmucositis and chemosensitisation associated with betel quid chewing during dose-dense adjuvant breast cancer chemotherapy. Cancer Chemother. Pharmacol. 2006;58:835–837. doi: 10.1007/s00280-006-0218-5. [DOI] [PubMed] [Google Scholar]
- 162.Galera R.M.L., Pascuet E.R., Mur J.I.E., Montoro-Ronsano J.B., Juarez-Gimenez J.C. Interaction between cat’s claw and protease inhibitors atazanavir, ritonavir and saquinavir. Eur. J. Clin. Pharmacol. 2008;64:1235–1236. doi: 10.1007/s00228-008-0551-1. [DOI] [PubMed] [Google Scholar]
- 163.Galluzzi S., Zanetti O., Binetti G., Trabucchi M., Frisoni G.B. Coma in a patient with Alzheimer’s disease taking low dose trazodone and Ginkgo biloba. J. Neurol. Neurosurg. Psychiatry. 2000;68:679–680. doi: 10.1136/jnnp.68.5.679a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hamann G.L., Campbell J.D., George C.M. Warfarin-Cranberry Juice Interaction. Ann. Pharmacother. 2011;45:420. doi: 10.1345/aph.1P451. [DOI] [PubMed] [Google Scholar]
- 165.Gordon R.Y., Becker D.J., Rader D.J. Reduced Efficacy of Rosuvastatin by St. John’s Wort. Am. J. Med. 2009;122:e1–e2. doi: 10.1016/j.amjmed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 166.Griffiths A., Beddall A., Pegler S. Fatal haemopericardium and gastrointestinal haemorrhage due to possible interaction of cranberry juice with warfarin. J. R. Soc. Promot. Health. 2008;128:324–326. doi: 10.1177/1466424008096615. [DOI] [PubMed] [Google Scholar]
- 167.Hanselin M. INR elevation with maitake extract in combination with warfarin. Ann. Pharmacother. 2010;44:223–224. doi: 10.1345/aph.1M510. [DOI] [PubMed] [Google Scholar]
- 168.Hou Q., Han W., Fu X. Pharmacokinetic interaction between tacrolimus and berberine in a child with idiopathic nephrotic syndrome. Eur. J. Clin. Pharmacol. 2013;69:1861–1862. doi: 10.1007/s00228-013-1537-1. [DOI] [PubMed] [Google Scholar]
- 169.Hughes R.L. Fatal combination of mitragynine and quetiapine—A case report with discussion of a potential herb-drug interaction. Forensic Sci. Med. Pathol. 2018;15:110–113. doi: 10.1007/s12024-018-0049-9. [DOI] [PubMed] [Google Scholar]
- 170.Hurren K.M., Lewis C.L. Probable interaction between warfarin and bee pollen. Am. J. Health Pharm. 2010;67:2034–2037. doi: 10.2146/ajhp090489. [DOI] [PubMed] [Google Scholar]
- 171.Hwang S.-W., Han H.-S., Lim K.Y., Han J.-Y. Drug Interaction Between Complementary Herbal Medicines and Gefitinib. J. Thorac. Oncol. 2008;3:942–943. doi: 10.1097/JTO.0b013e3181803f1e. [DOI] [PubMed] [Google Scholar]
- 172.Iida R., Otsuka Y., Matsumoto K., Kuriyama S., Hosoya T. Pseudoaldosteronism due to the concurrent use of two herbal medicines containing glycyrrhizin: Interaction of glycyrrhizin with angiotensin-converting enzyme inhibitor. Clin. Exp. Nephrol. 2006;10:131–135. doi: 10.1007/s10157-006-0415-x. [DOI] [PubMed] [Google Scholar]
- 173.Janetzky K., Morreale A.P. Drug Experience Warfarin, and ginseng. Am. J. Healh Pharm. 1997;54:692–693. doi: 10.1093/ajhp/54.6.692. [DOI] [PubMed] [Google Scholar]
- 174.Ji H., Zhang G., Yue F., Zhou X. Adverse event due to a likely interaction between sodium aescinate and Ginkgo biloba extract: A case report. J. Clin. Pharm. Ther. 2017;42:237–238. doi: 10.1111/jcpt.12500. [DOI] [PubMed] [Google Scholar]
- 175.Jones B.D., Runikis A.M. Interaction of Ginseng with Phenelzine. J. Clin. Psychopharmacol. 1987;7:201–202. doi: 10.1097/00004714-198706000-00030. [DOI] [PubMed] [Google Scholar]
- 176.Kang Y.-C., Chen M.-H., Lai S.-L. Potentially Unsafe Herb-drug Interactions Between a Commercial Product of Noni Juice and Phenytoin- A Case Report. Acta Neurol. Taiwanica. 2015;24:43–46. [PubMed] [Google Scholar]
- 177.Karhova M., Trelchel U., Malagb M., Fnllmg A., Gerken G., Broelsch C.E. Interaction of Hypericum perforatum (St. John’s wort) with cycbsporin A metabolism in a patient after liver transplantation. J. Hepatol. 2000;33:853. doi: 10.1016/s0168-8278(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 178.Cambria-Kiely J.A. Effect of Soy Milk on Warfarin Efficacy. Ann. Pharmacother. 2002;36:1893–1896. doi: 10.1345/aph.1C160. [DOI] [PubMed] [Google Scholar]
- 179.Krüth P., Brosi E., Fux R., Mörike K., Gleiter C.H. Ginger-Associated Overanticoagulation by Phenprocoumon. Ann. Pharmacother. 2004;38:257–260. doi: 10.1345/aph.1D225. [DOI] [PubMed] [Google Scholar]
- 180.Lam A.Y., Elmer G.W., Mohutsky M.A. Possible interaction between warfarin and Lycium barbarum L. Ann. Pharmacother. 2001;35:1199–1201. doi: 10.1345/aph.1Z442. [DOI] [PubMed] [Google Scholar]
- 181.Lambert J.-P., Cormier J. Potential Interaction between Warfarin and Boldo-Fenugreek. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001;21:509–512. doi: 10.1592/phco.21.5.509.34492. [DOI] [PubMed] [Google Scholar]
- 182.Lantz M.S., Buchalter E., Giambanco V. St. John’s wort and antidepressant drug interactions in the elderly. J. Geriatr. Psychiatry Neurol. 1999;12:7–10. doi: 10.1177/089198879901200103. [DOI] [PubMed] [Google Scholar]
- 183.Leung H., Hung A., Hui A., Chan T. Warfarin overdose due to the possible effects of Lycium barbarum L. Food Chem. Toxicol. 2008;46:1860–1862. doi: 10.1016/j.fct.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 184.Mai I., Krüger H., Budde K., Johne A., Brockmöller J., Neumayer H.-H., Roots I. Hazardous pharmacokinetic interaction of Saint John’s wort (Hypericum perforatum) with the immunosuppressant cyclosporin. Int. J. Clin. Pharmacol. Ther. 2000;38:500–502. doi: 10.5414/CPP38500. [DOI] [PubMed] [Google Scholar]
- 185.Mateo-Carrasco H., Gálvez-Contreras M.C., Fernández-Ginés F.D., Nguyen T.V. Elevated liver enzymes resulting from an interaction between Raltegravir and Panax ginseng: A case report and brief review. Drug Metab. Drug Interact. 2012;27:171–175. doi: 10.1515/dmdi-2012-0019. [DOI] [PubMed] [Google Scholar]
- 186.McGovern E., McDonnell T. Herbal Medicine—Sets the Heart Racing! Ir. Med. J. 2009;1:517–538. [PubMed] [Google Scholar]
- 187.McCrae S. Elevated serum digoxin levels in a patient taking digoxin and Siberian ginseng. CMAJ. 1996;155:293–295. [PMC free article] [PubMed] [Google Scholar]
- 188.Meisel C. Fatal intracerebral mass bleeding associated with Ginkgo biloba and ibuprofen. Atherosclerosis. 2003;167:367. doi: 10.1016/S0021-9150(03)00015-7. [DOI] [PubMed] [Google Scholar]
- 189.Mendoza C.E., Ferreira A.C., De Marchena E. Warfarin and herbal products interaction causing prosthetic aortic valve thrombosis presenting as acute myocardial infarction. J. Hear. Valve Dis. 2004;13:22–24. [PubMed] [Google Scholar]
- 190.Myers A.P., Watson T.A., Strock S.B. Drug Reaction with Eosinophilia and Systemic Symptoms Syndrome Probably Induced by a Lamotrigine-Ginseng Drug Interaction. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015;35:e9–e12. doi: 10.1002/phar.1550. [DOI] [PubMed] [Google Scholar]
- 191.Naccarato M., Yoong D., Gough K. A Potential Drug–Herbal Interaction between Ginkgo biloba and Efavirenz. J. Int. Assoc. Physicians AIDS Care. 2012;11:98–100. doi: 10.1177/1545109711435364. [DOI] [PubMed] [Google Scholar]
- 192.Nayeri A., Wu S., Adams E., Tanner C., Meshman J., Saini I., Reid W. Acute Calcineurin Inhibitor Nephrotoxicity Secondary to Turmeric Intake: A Case Report. Transplant. Proc. 2017;49:198–200. doi: 10.1016/j.transproceed.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 193.Nebel A., Schneider B.J., Baker R.K., Kroll D.J. Potential metabolic interaction between St. John’s wort and theophylline. Ann. Pharmacother. 1999;33:502. doi: 10.1345/aph.18252. [DOI] [PubMed] [Google Scholar]
- 194.Nowack R., Nowak B. Herbal teas interfere with cyclosporin levels in renal transplant patients. Nephrol. Dial. Transplant. 2005;20:2554–2556. doi: 10.1093/ndt/gfi003. [DOI] [PubMed] [Google Scholar]
- 195.Orr A., Parker R. Red clover causing symptoms suggestive of methotrexate toxicity in a patient on high-dose methotrexate. Menopause Int. Integr. J. Postreprod. Health. 2013;19:133–134. doi: 10.1177/1754045313502473. [DOI] [PubMed] [Google Scholar]
- 196.Paeng C.H., Sprague M., Jackevicius C.A. Interaction between Warfarin and Cranberry Juice. Clin. Ther. 2007;29:1730–1735. doi: 10.1016/j.clinthera.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 197.Page R.L., Lawrence J.D. Potentiation of Warfarin by Dong Quai. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1999;19:870–876. doi: 10.1592/phco.19.10.870.31558. [DOI] [PubMed] [Google Scholar]
- 198.Jacquin-Porretaz C., Nardin C., Blanc D., Aubin F., Gérard B., Drobacheff-Thiebaut C., Jacoulet P., Westeel V. Cutaneous Toxicity Induced by Hibiscus Tea in a Patient Treated with Erlotinib. J. Thorac. Oncol. 2017;12:e47–e48. doi: 10.1016/j.jtho.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 199.Prasad K., Tharangani P., Samaranayake C. Recurrent relapses of depression in a patient established on sertraline after taking herbal medicinal mixtures—A herb–drug interaction? J. Psychopharmacol. 2008;23:216–219. doi: 10.1177/0269881108089808. [DOI] [PubMed] [Google Scholar]
- 200.Rindone J.P., Murphy T.W. Warfarin-cranberry juice 9nteraction resulting in profound hypoprothrombinemia and bleeding. Am. J. Ther. 2005;13:283–284. doi: 10.1097/01.mjt.0000178908.32892.2f. [DOI] [PubMed] [Google Scholar]
- 201.Rivera C.A., Ferro C.L., Bursua A.J., Gerber B.S. Probable Interaction Between Lycium barbarum (Goji) and Warfarin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012;32:e50–e53. doi: 10.1002/j.1875-9114.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 202.Rosado M.F. Thrombosis of a Prosthetic Aortic Valve Disclosing a Hazardous Interaction between Warfarin and a Commercial Ginseng Product. Cardiol. 2003;99:111. doi: 10.1159/000069720. [DOI] [PubMed] [Google Scholar]
- 203.Rozenfeld V., Crain J.L., Callahan A.K. Possible augmentation of warfarin effect by glucosamine-chondroitin. Am. J. Health Pharm. 2004;61:306–307. doi: 10.1093/ajhp/61.3.306. [DOI] [PubMed] [Google Scholar]
- 204.Rubin D., McGovern B., Kopelman R.I. Back to Basics. Am. J. Med. 2006;119:482–483. doi: 10.1016/j.amjmed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 205.Ruschitzka F., Meier P.J., Turina M., Lüscher T.F., Noll G. Acute heart transplant rejection due to Saint John’s wort. Lancet. 2000;355:548–549. doi: 10.1016/S0140-6736(99)05467-7. [DOI] [PubMed] [Google Scholar]
- 206.Schwarz U.I., Büschel B., Kirch W. Unwanted pregnancy on self-medication with St John’s wort despite hormonal contraception. Br. J. Clin. Pharmacol. 2003;55:112–113. doi: 10.1046/j.1365-2125.2003.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Segal R. Warfarin interaction with Matricaria chamomilla. Can. Med. Assoc. J. 2006;174:1281–1282. doi: 10.1503/cmaj.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Strippoli S., Lorusso V., Albano A., Guida M. Herbal-drug interaction induced rhabdomyolysis in a liposarcoma patient receiving trabectedin. BMC Complement. Altern. Med. 2013;13:199. doi: 10.1186/1472-6882-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Suvarna R., Pirmohamed M., Henderson L. Possible interaction between warfarin and cranberry juice. BMJ. 2003;327:1454. doi: 10.1136/bmj.327.7429.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tam L.S., Chan T.Y.K., Leung W.K., Critchley J.A.J.H. Warfarin interactions with Chinese traditional medicines: Danshen and methyl salicylate medicated oil. Aust. N. Z. J. Med. 1995;25:258. doi: 10.1111/j.1445-5994.1995.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 211.Tamura S., Warabi Y., Matsubara S. Severe liver dysfunction possibly caused by the combination of interferon beta-1b therapy and melilot (sweet clover) supplement. J. Clin. Pharm. Ther. 2012;37:724–725. doi: 10.1111/j.1365-2710.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- 212.Taylor J., Wilt V. Probably antagonism of warfarin by green tea. Ann. Pharmacother. 1999;33:426–428. doi: 10.1345/aph.18238. [DOI] [PubMed] [Google Scholar]
- 213.van den Bout C.J., Bosch M.E., Burger D.M., Koopmans P.P., van der Ven A.J. Toxic lopinavir concentrations in an HIV-1 infected patient taking herbal medications. AIDS. 2008;22:1243–1244. doi: 10.1097/QAD.0b013e32830261f4. [DOI] [PubMed] [Google Scholar]
- 214.Van Strater A.C., Bogers J.P. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int. Clin. Psychopharmacol. 2012;27:121–124. doi: 10.1097/YIC.0b013e32834e8afd. [DOI] [PubMed] [Google Scholar]
- 215.Welch J., Forster K. Probably elevation in international normalized ratio from cranberry juice. J. Pharm. Technol. 2007;23:104–107. doi: 10.1177/875512250702300207. [DOI] [Google Scholar]
- 216.Werba J.P., Misaka S., Giroli M.G., Shimomura K., Amato M., Simonelli N., Vigo L., Tremoli E. Update of green tea interactions with cardiovascular drugs and putative mechanisms. J. Food Drug Anal. 2018;26:S72–S77. doi: 10.1016/j.jfda.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wiegman D.-J., Brinkman K., Franssen E.J.F. Interaction of Ginkgo biloba with efavirenz. AIDS. 2009;23:1184–1185. doi: 10.1097/QAD.0b013e32832c412b. [DOI] [PubMed] [Google Scholar]
- 218.Wong A.L.N., Chan T.Y.K. Interaction between warfarin and the herbal product quilinggao. Ann. Pharmacother. 2003;37:836–838. doi: 10.1345/aph.1C503. [DOI] [PubMed] [Google Scholar]
- 219.Yu C.M., Chan J., Sanderson J.E. Chinese herbs and warfarin potentiation by ’Danshen’. J. Intern. Med. 1997;241:337–339. doi: 10.1046/j.1365-2796.1997.134137000.x. [DOI] [PubMed] [Google Scholar]
- 220.Kim H.-S., Kim G.-Y., Yeo C.-W., Oh M., Ghim J.-L., Shon J.-H., Kim E.-Y., Kim D.H., Shin J.-G. The effect of Ginkgo biloba extracts on the pharmacokinetics and pharmacodynamics of cilostazol and its active metabolites in healthy Korean subjects. Br. J. Clin. Pharmacol. 2014;77:821–830. doi: 10.1111/bcp.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable.