Abstract
Background
The association of asthma with the risk for mortality among coronavirus disease 2019 (COVID-19) patients is not clear.
Objective
To investigate the association between asthma and the risk for mortality among COVID-19 patients.
Methods
We performed systematic searches through electronic databases including PubMed, EMBASE, and Web of Science to identify potential articles reporting adjusted effect estimates on the association of asthma with fatal COVID-19. A random-effects model was conducted to estimate pooled effects. Sensitivity analysis, subgroup analysis, meta-regression, Begg's test and Egger's test were also performed.
Results
Based on 62 studies with 2,457,205 cases reporting adjusted effect estimates, COVID-19 patients with asthma had a significantly reduced risk for mortality compared with those without it (15 cohort studies: 829,670 patients, pooled hazard ratio [HR] = 0.88, 95% confidence interval [CI], 0.82-0.95, I2 = 65.9%, P < .001; 34 cohort studies: 1,008,015 patients, pooled odds ratio [OR] = 0.88, 95% CI, 0.82-0.94, I2 = 39.4%, P = .011; and 11 cross-sectional studies: 1,134,738 patients, pooled OR = 0.87, 95% CI, 0.78-0.97, I2 = 41.1%, P = .075). Subgroup analysis based on types of adjusted factors indicated that COVID-19 patients with asthma had a significantly reduced risk for mortality among studies adjusting for demographic, clinical, and epidemiologic variables (pooled OR = 0.87, 95% CI, 0.83-0.92, I2 = 36.3%, P = .013; pooled HR = 0.90, 95% CI, 0.83-0.97, I2 = 69.2%, P < .001), but not among studies adjusting only for demographic variables (pooled OR = 0.88, 95% CI, 0.70-1.12, I2 = 40.5%, P = .097; pooled HR = 0.82, 95% CI, 0.64-1.06, I2 = 0%, P = .495). Sensitivity analysis proved that our results were stable and robust. Both Begg's test and Egger's test indicated that potential publication bias did not exist.
Conclusions
Our data based on adjusted effect estimates indicated that asthma was significantly related to a reduced risk for COVID-19 mortality.
Key words: Asthma, COVID-19, Mortality, Meta-analysis, Adjusted effect estimate
Abbreviations used: COVID-19, Coronavirus disease 2019
What is already known about this topic? The pooled prevalence of asthma in COVID-19 patients has been reported to be similar to that in the general population. However, the association of asthma with the risk for COVID-19 mortality is less evident.
What does this article add to our knowledge? Asthma was significantly related to a reduced risk for COVID-19 mortality.
How does this study impact current management guidelines? Asthma was an independent protective factor for the mortality of COVID-19 patients. Routine interventions and treatment for asthma patients infected with severe acute respiratory syndrome coronavirus 2 should be continued.
Introduction
A recent systematic review by Liu et al1 suggested that coronavirus disease 2019 (COVID-19) patients with asthma had a lower risk for death compared with those without it, based on crude effects from six studies. Another systematic review by Shi et al,2 based on 12 eligible articles reporting adjusted effects, also indicated that asthma was associated with a significantly reduced risk for COVID-19 mortality. These two studies are interesting. However, these systematic reviews do not explore sources of heterogeneity; also, more recent primary studies with larger sample sizes have been published. Therefore, an updated meta-analysis based on risk factor-adjusted effects was performed to verify the relationship between asthma and COVID-19 mortality, considering that several factors (sex, age, and underlying comorbidities) significantly affected the clinical outcomes of COVID-19 patients.3, 4, 5, 6, 7
Methods
This meta-analysis was conducted in line with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.8 Systematic searches were carried out in Web of Science, PubMed and EMBASE to identify potential articles as of June 25, 2021. Search terms used were “2019-nCoV” or “SARS-CoV-2” or “COVID-19” or “coronavirus disease 2019” or “severe acute respiratory syndrome coronavirus 2” and “asthma.” Full queries for each bibliographic database are available in Table E1 (in this article's Online Repository at www.jaci-inpractice.org). We included primary studies comparing COVID-19 patients with asthma versus those without asthma regarding mortality, and in which adjusted effect estimates on the association between asthma and COVID-19 mortality were reported. In the case of a study resulting in more than one publication, only the one with more complete data was included. Duplicated publications, reviews, errata, protocols, comments, case reports, and studies with incomplete data were excluded.
Two independent authors (H. Hou and Y. Li) screened articles and abstracted essential information from all included eligible studies. Any disagreement was resolved by discussion. An assessment of study quality using National Institutes of Health Study Quality Assessment tools was performed by two independent reviewers (Y. Wang and H.Yang). For all included articles, study quality was judged as good, fair, or poor (see Table E2 in this article's Online Repository at www.jaci-inpractice.org). Basic information, including the first author, study period, prevalence of asthma, county or region, number of cases, age (means and SDs or medians with interquartile ranges), percentage of males, study design, adjusted risk factors, and adjusted effects, was extracted from each eligible article.
We conducted statistical analyses using STATA (version 12.1, StataCorp LP, College Station, Tex) and R (version 3.6.3, The R Foundation,Vienna, Austria). The pooled effect (pooled odds ratio [OR] and/or hazard ratio [HR]) and its 95% confidence interval (CI) were estimated by a random-effects model. Moreover, we presented separate results for the pooled OR and pooled HR. Heterogeneity across studies was assessed by Higgins I 2 statistic and chi squared–based Q test. Publication bias was investigated by Begg's test and Egger's test. Sensitivity analysis was conducted to evaluate the stability of our results by omitting each eligible study one at a time. Meta-regression and subgroup analyses were performed to investigate potential sources of heterogeneity (such as age, sex, region, data collection period (number of months since the first COVID-19 case), hospitalization status, and the types of adjusted factors). Two-tailed P less than .05 was considered statistically significant.
Results
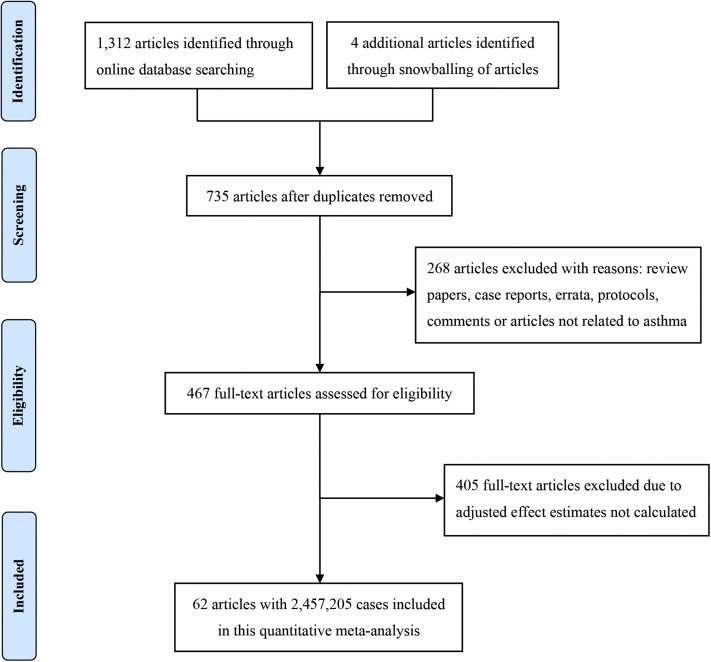
A total of 62 studies9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 with 2,457,205 patients were included. Basic characteristics of the included studies are presented in Table I . A flowchart of the study search and selection is shown in Figure 1 . Sample sizes across the included studies ranged from 132 to 654,858. There were 30 studies conducted in North America (21 in the United States, eight in Mexico, and one in Canada), 15 in Europe (eight in the United Kingdom, two in Spain, and one each in Ireland, Italy, France, Belgium, and Sweden), 11 in Asia (six in Korea and one each in China, Turkey, Iran, Kuwait, and Saudi Arabia), and six in other regions (three in Brazil, one in Nigeria, one in Libya, and one from an international center). There were 39 retrospective cohort studies, 11 cross-sectional studies, nine prospective cohort studies, two case-control studies, and one case series.
Table I.
Main characteristics of studies included in this meta-analysis
| First author | Study period | Country | Prevalence of asthma, n (%) | Patients, n | Population | Male (%) | Age, y | Study design | Adjusted-effect (95% confidence interval) | Confounders |
|---|---|---|---|---|---|---|---|---|---|---|
| Shah9 | March 2 to May 6, 2020 | United States | 68 (13.0) | 522 | All hospitalized patients with confirmed COVID-19 | 41.8 | 63 (50-72) | Case-control study | OR: 0.74 (0.33-1.64) | Age, BMI, sex, race, all baseline comorbidities |
| Arshad10 | March 10 to May 2, 2020 | United States | 251 (9.9) | 2541 | All hospitalized adult patients with confirmed COVID-19 | 51.1 | 63.7 ± 16.5 | Retrospective cohort study | HR: 0.916 (0.632-1.327) | Hydroxychloroquine alone, azithromycin alone, hydroxychloroquine plus azithromycin, age, sex, race, BMI, lung comorbidity, immunodeficiency comorbidity, cardiovascular comorbidity, CKD, COPD, HTN, cancer comorbidity, DM, percent O2 saturation <95, admitted to ICU, ventilator, given steroid, given tocilizumab |
| Mato11 | February 17 to April 30, 2020 | International center | 12 (6.1) | 198 | All patients diagnosed with confirmed COVID-19 | 63 | 63 (35-92) | Retrospective cohort study | HR: 2.5 (1.1-5.8) | Age, CIRS score, DM, chronic renal disease |
| Poblador-Plou12 | March 4 to May 17, 2020 | Spain | NR | 4412 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 41.2 | 67.7 ± 20.7 | Retrospective cohort study | OR: 0.45 (0.18-1.11) OR: 0.68 (0.40-1.17) |
Age |
| van Gerwen13 | March 1 to May 13, 2020 | United States | 430 (11.6) | 3703 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 55.3 | 56.8 ± 18.2 | Retrospective cohort study | OR: 0.89 (0.64-1.25) | Age group, sex, race, BMI, smoking status, comorbidities (HTN, CAD, AF, CHF, PVD, CVA/TIA, dementia, DM, hypothyroidism, CKD, malignancy, COPD, and prior VTE) |
| Hernandez-Galdamez14 | Up to June 27, 2020 | Mexico | 5854 (2.77) | 211,003 | Laboratory-confirmed COVID-19 cases | 54.71 | 45.7 ± 16.3 | Cross-sectional study | OR: 0.82 (0.74-0.90) | Age, sex, CKD, immunosuppression, DM, COPD, HTN, CVD, obesity and smoking |
| Hernandez-Vasquez15 | Up to May 18, 2020 | Mexico | 1590 (3.1) | 51,053 | Patients with confirmed COVID-19 | 57.6 | 46.6 ± 15.8 | Cross-sectional study | OR: 1.02 (0.84-1.23) | Age, gender, smoking |
| Almazeedi16 | February 24 to April 20, 2020 | Kuwait | 43 (3.9) | 1096 | All patients with confirmed COVID-19 | 81 | 41 (25-75) | Retrospective cohort study | OR: 4.92 (1.03-23.44) | Age, obesity, DM, HTN, chronic renal disease, smoker, qSOFA score, elevated procalcitonin, and elevated CRP |
| Perez-Guzman17 | February 25 to May 1, 2020 | United Kingdom | 56 (9.1) | 614 | Patients admitted for COVID-19 | 62.21 | 69 ± 25 | Retrospective cohort study | OR: 0.42 (0.19-0.91) | Age |
| Tartof18 | February 13 to May 23, 2020 | United States | 1273 (18.4) | 6916 | Members diagnosed with COVID-19 | 44.98 | 49.1 ± 16.6 | Retrospective cohort study | Risk ratio: 0.81 (0.54-1.21) | BMI, age, sex, race and ethnicity, smoking, metastatic tumor/cancer, MI, other immune condition, organ transplant, CHF, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, HTN, DM status, and time |
| Parra-Bracamonte19 | January 13 to June 13, 2020 | Mexico | 4028 (2.8) | 142,690 | All cases positive for COVID-19 | 56 | 45 (34.0-57.0) | Cross-sectional study | OR: 0.949 (0.832-1.082) | Age, sex, smoking habits, hospitalization, and comorbidity traits |
| Fox20 | March 1 to April 24, 2020 | United States | 27 (7.6) | 355 | All hospitalized adult patients with confirmed COVID-19 | 49 | 66.21 ± 14.21 | Retrospective cohort study | OR: 0.714 (0.076-6.670) | Age, BMI, sex, ethnicity, COPD, heart failure, HTN, CAD, AF, and CKD |
| Yehia21 | February 19 to June 25, 2020 | United States | 628 (5.6) | 11,210 | All hospitalized adult patients with confirmed COVID-19 | 49.8 | 61 (46-74) | Retrospective cohort study | HR: 0.91 (0.74-1.12) | Race, age, sex, insurance, Agency for Healthcare Research and Quality Elixhauser Comorbidity Index scores, neighborhood deprivation index scores, cancer, CKD, COPD, CHF, CAD, DM, and obesity |
| Emami22 | February 20 to March 1, 2020 | Iran | 25 (2.0) | 1239 | All hospitalized patients with confirmed COVID-19 | 55.9 | 51.48 ± 19.54 | Retrospective cohort study | HR: 1.04 (0.53-2.02) | Age, DM, CVD, chronic liver disease, CKD, cancer, human immunodeficiency virus, smoking, and immunodeficiency disease |
| Trabulus23 | March 15 to June 1, 2020 | Turkey | 20 (6.0) | 336 | All hospitalized adult patients with confirmed COVID-19 | 57.1 | 55.0 ± 16.0 | Retrospective cohort study | OR: 3.087 (0.382-24.965) | Age |
| Santos24 | February 20 to June 2, 2020 | Brazil | 488 (5.7) | 80,102 | All hospitalized patients with confirmed COVID-19 | 57.3 | NR | Retrospective cohort study | HR: 0.71 (0.61-0.81) | ICU, DM, neurological, kidney disease, cardiopathy, race, and pneumopathy |
| Ioannou25 | February 28 to June 22, 2020 | United States | 745 (7.4) | 10,131 | Patients with confirmed COVID-19 | 91 | 63.6 ± 16.2 | Retrospective cohort study | HR: 0.80 (0.60-1.05) | All sociodemographic characteristics, comorbid conditions, and symptoms |
| HR: 0.85 (0.65-0.11) | Age | |||||||||
| Gutierrez26 | Through September 16, 2020 | Mexico | 17,026 (2.6) | 654,858 | Adult (age ≥20 y) patients with confirmed COVID-19 | 52.21 | 46.1 (45.8-46.3) | Cross-sectional study | OR: 0.90 (0.84-0.96) OR: 0.96 (0.75-1.24) OR: 1.07 (0.86-1.34) OR: 0.44 (0.29-0.68) |
Sex, age, indigenous speaker, obese, smoking, COPD, chronic renal disease, CVD, ministry of health, social security, private health provider, and quintiles of share poverty |
| Clift27 | January 24 to April 30, 2020 | United Kingdom | 825,422 (13.57) | 10,776 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 55.33 | 69.6 ± 17.9 | Prospective cohort study | HR: 0.84 (0.73-0.97) HR: 1.03 (0.91-1.17) |
Age, BMI, Townsend score (linear), ethnic group, domicile (residential care, homeless, neither), and range of conditions and treatments |
| Kim28 | March 1 to May 12, 2020 | United States | 903 (8.3) | 10,861 | All hospitalized adult patients with confirmed COVID-19 | 59.6 | 65 (54-77) | Prospective cohort study | OR: 0.81 (0.67-0.98) | Age, sex, race and ethnicity, presence of comorbidities, smoking status, hospital type, and BMI groups |
| Tang29 | March 1 to June 16, 2020 | United States | 54 (7.2) | 752 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 39.9 | 72.1 ± 11.9 | Retrospective cohort study | HR: 0.64 (0.30-1.40) | Age, sex, race, and facility |
| Ken-Dror30 | March to April, 2020 | United Kingdom | 42 (12.8) | 429 | All hospitalized adult patients with confirmed COVID-19 | 56.4 | 70 ± 18 | Prospective cohort study | OR: 3.22 (1.16-8.92) | Age, CRP, respiratory rate, diastolic blood pressure, dementia, Akaike information criterion, area under the curve, and sensitivity/specificity |
| Choi31 | NR | Korea | 96 (2.3) | 4057 | Hospitalized patients with mild to critical COVID-19 nationwide | 42.5 | NR | Prospective cohort study | HR: 2.20 (1.02-4.76) | Age, sex, obesity, systolic blood pressure, diastolic blood pressure, heart rate, temperature, DM, HTN, heart failure, chronic heart disease, COPD, CKD, cancer, chronic liver disease, rheumatic or autoimmune disease, and dementia |
| Nyabera32 | February 1 to April 30, 2020 | United States | 18 (6.2) | 290 | Older adult inpatients (≥65 y) with laboratory-confirmed COVID- 19 infection | 51.7 | 77.6 ± 8.3 | Retrospective cohort study | OR: 0.66 (0.24-1.83) | BMI, age, CAD, COPD, DM, end-stage renal disease, and HTN |
| Lee33 | January 20 to May 27, 2020 | Korea | 686 (9.4) | 7272 | Adult COVID-19 patients | 40.3 | NR | Retrospective cohort study | OR: 1.06 (0.71-1.59) | Age, sex, and CCI |
| Murillo-Zamora34 | March 4 to August 15, 2020 | Mexico | NR | 66,123 | All hospitalized adult patients with confirmed COVID-19 | 60.7 | NR | Retrospective cohort study | HR: 0.92 (0.85-0.99) | Sex, age, clinically diagnosed pneumonia at hospital admission, tobacco use, obesity, COPD, type 2 DM arterial HTN, immunosuppression, and CKD |
| Ling35 | January 27 to August 7, 2020 | United Kingdom | 52 (11.7) | 444 | All hospitalized adult patients with confirmed COVID-19 | 55.1 | 74 (63-83) | Cross-sectional study | OR: 0.31 (0.13-0.71) | Age, sex, obesity, ethnicity, and presence of DM (types 1 and 2 combined) |
| Izurieta36 | April 1 to May 8, 2020 | United States | 962,666 (3.8) | 27,961 | All elderly patients (ages ≥65 y) with confirmed COVID-19 | 48.8 | 75 (70-85) | Retrospective cohort study | OR: 0.93 (0.85-1.03) | Sex, age, area deprivation index national rank, circulation rate, population density, vaccination, presence of medical conditions, frailty conditions, immune compromised status, and race |
| Lundon37 | March 28 to April 26, 2020 | United States | 403 (4.5) | 8928 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 46.2 | 58.0 ± 18.8 | Cross-sectional study | OR: 0.68 (0.51-0.91) | Age, sex, race and ethnicity, New York City borough, English as preferred language, smoking status, COPD, HTN, obesity, DM, CKD, human immunodeficiency virus, and cancer |
| Schwartz38 | January 21 to September 30, 2020 | Canada | 2655 (4.7) | 56,606 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 48.4 | NR | Cross-sectional study | OR: 0.85 (0.66-1.09) | Sex (male vs female), age (<30 y, 30-44 y, 60-4 y, or ≥75 y, compared with 45-59 y), comorbidities (COPD, renal disease, cardiac disease, DM, immune compromised or cancer, obesity, or other comorbidities, compared with no comorbidities), working or residing in long-term care home (yes vs no), and symptoms (fever and/or cough, other symptoms, or missing symptoms compared with asymptomatic) |
| Martos-Benítez39 | January 1 to May 13, 2020 | Mexico | NR | 38,324 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 58.3 | 46.9 ± 15.7 | Retrospective cohort study | OR: 0.86 (0.64-1.16) | Age, sex, smoking habit, time from symptoms onset to medical contact, COPD, high blood pressure, CVD, DM, obesity, CKD, and other comorbidities |
| Oh40 | January 1 to June 4, 2020 | Korea | NR | 7780 | Adult (age ≥20 y) patients with confirmed COVID-19 | NR | NR | Retrospective cohort study | OR: 1.03 (0.76-1.41) | COPD, interstitial lung disease, lung cancer, lung disease d/t external agent, obstructive sleep apnea, tuberculosis of lung, age, income level, sex, residence, underlying disability, CCI, HTN, DM, peripheral vascular disease, renal disease, rheumatic disease, dementia, peptic ulcer paraplegia, hemiplegia or paraplegia, moderate or severe liver disease, mild liver disease, cerebrovascular disease, CHF, MI, malignancy, metastatic solid tumor, and acquired immunodeficiency syndrome/human immunodeficiency virus |
| Park41 | February 15 to April 24, 2020 | Korea | NR | 2269 | Patients hospitalized with COVID-19 | 35.9 | 55.5 ± 20.2 | Retrospective cohort study | OR: 2.13 (0.74-6.13) | Age, male, respiratory rate, fever, altered consciousness, hemoptysis, sore throat, malaise, COPD, CKD, malignancy, chronic neurological disorder, and preexisting cardiovascular risk factor/CVD |
| Ahlstrom42 | March 6 to May 27, 2020 | Sweden | 261 (2.6) | 1981 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 74 | 61 (52-69) | Case-control study | HR: 1.52 (1.04-2.22) | Simplified acute physiology score 3, age, sex, ischemic heart disease, nonischemic heart disease, HTN, type 1 DM, type 2 DM, stroke, chronic renal disease, COPD, immunosuppressed, and cancer |
| Lopez Zuniga43 | February 4 to April 30, 2020 | Spain | NR | 318 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 58.5 | 64.9 ± 14.1 | Prospective cohort study | HR: 2.235 (0.554-9.02) | Age, sex, HTN, COPD, immunosuppression, chronic heart disease, AF, obesity, tumor, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, needed high oxygen volume, DM, qSOFA, hydroxychloroquine azithromycin, lopinavir/ritonavir, interferon, corticosteroids, tocilizumab, vitamin D supplementation, and anticoagulation therapy |
| Mollalo44 | January 22 to November 22,2020 | United States | NR | NR | All individuals with laboratory-confirmed infection by SARS-CoV-2 | NR | NR | Cross-sectional study | OR: 4.584 (2.583-8.137) OR: 0.818 (0.461-1.452) |
NR |
| Lohia45 | March 10 to June 30, 2020 | United States | 134 (7.2) | 1871 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 51.6 | 64.1 ± 16 | Retrospective cohort study | OR: 0.98 (0.61-1.58) | Age, sex, race, BMI, and comorbidities including HTN, CAD, DM, CKD, ESRD on dialysis, CHF, any cancer, any liver disease, hyperlipidemia, and history of stroke |
| Cedano46 | March 3 to April 22, 2020 | United States | 7 (5) | 132 | All adult patients admitted to ICU with severe COVID-19 infection | 59 | 63 (53-71) | Retrospective cohort study | OR: 2.13 (0.10-45.4) | Age, male sex, arterial HTN, DM, COPD, CAD, systolic heart failure, diastolic heart failure, CKD, end-stage kidney disease, BMI, and mechanical ventilation |
| Girardin47 | March 2 to May 24, 2020 | United States | 493 (11.7) | 4446 | All hospitalized patients with confirmed COVID-19 | 58.1 | 62 ± 18 | Case series | HR: 0.83 (0.67-1.04) | Age, ethnic minority, male sex, low income, smoking, obesity, COPD, sleep apnea, HTN, DM, peripheral artery disease, CAD, autoimmune disease, and cancer |
| Cao48 | March to September 2020 | United States | 72 (21.0) | 343 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 56 | 60.7 ± 15.9 | Prospective cohort study | OR: 0.72 (0.31-1.57) | Age, race (Black or not Black), sex, COPD, and obesity |
| Ho49 | March 7 to June 7, 2020 | United States | 468 (4.4) | 10,523 | All adult patients with laboratory-confirmed diagnosis of COVID-19 | 54.2 | 58.4 ± 18.8 | Retrospective cohort study | OR: 0.64 (0.53-0.77) | Age, sex, BMI, race, COVID-19 disease severity, CCI, COPD, CRP (>150), interleukin-6 (>80), ferritin (>2000), D-dimer (>2.0 μg/L), use of anticoagulation, use of corticosteroids, and smoking (current and former) |
| Guan50 | December 2019 to May 6th, 2020 | China | 830 (2.1) | 39,420 | All hospitalized patients with confirmed COVID-19 | 49.9 | 55.7 | Retrospective cohort study | OR: 0.84 (0.48-1.48) | Presence of any other systemic comorbidities, female sex, and age |
| Bloom51 | January 17 to August 17, 2020 | United Kingdom | 7859 (10.4) | 75,463 | All hospitalized patients with confirmed COVID-19 | 55.4 | NR | Prospective cohort study | HR: 1.17 (0.73-1.86) HR: 0.99 (0.61-1.58) HR: 0.94 (0.62-1.43) HR: 1.02 (0.67-1.54) HR: 1.96 (1.25-3.08) HR: 0.97 (0.89-1.05) HR: 0.86 (0.80-0.92) HR: 1.13 (1.01-1.28) HR: 0.97 (0.89-1.06) |
Age, sex, ethnicity, deprivation, obesity, smoking, chronic cardiac disease, CKD, and malignancy |
| Osibogun52 | February 27 to July 6, 2020 | Nigeria | 45 (2.1) | 2184 | All hospitalized patients with confirmed COVID-19 | 65.8 | 43 (33-55) | Retrospective cohort study | OR: 1.52 (0.41-5.57) | Age and sex |
| de Souza53 | February 26 to August 10, 2020 | Brazil | 4566 (7.15) | 44,128 | All hospitalized patients with confirmed COVID-19 | 54.2 | NR | Retrospective cohort study | HR: 0.79 (0.73-0.85) | Male sex, age, fever, cough, dyspnea, respiratory distress, blood oxygen saturation <95%, diarrhea, other symptom, cardiac disease, liver disease, immunodepression, DM, neuropathy, pneumopathy, kidney disease, other comorbidity, flu vaccine, ICU admission, invasive mechanical ventilation, and noninvasive ventilation |
| Mulhem54 | March 13 to April 29, 2020 | United States | 429 (13.3) | 3219 | All hospitalized patients with confirmed COVID-19 | 49 | 65.2 (52.6-77.2) | Retrospective cohort study | OR: 1.14 (0.84-1.55) | Gender, age, race, current smoking and comorbidities |
| Topless55 | March 16 to August 24, 2020 | United Kingdom | 40,898 (8.6) | 2118 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | NR | NR | Retrospective cohort study | OR: 1.11 (0.80-1.53) | Current age, sex, ethnicity, Townsend deprivation index, BMI, and smoking status |
| Bennett56 | March 2 to September 14, 2020 | Ireland | 467 (2.4) | 19,789 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 43.6 | NR | Retrospective cohort study | OR: 0.82 (0.50-1.35) | Age (linear, quadratic, and cubic), chronic heart disease, chronic neurological disease, chronic respiratory disease, and CKD |
| Chronic liver disease, immunodeficiency, DM, BMI ≥40, cancer, other comorbidity, unknown comorbidity, community health office, residential care facility, and route of transmission | ||||||||||
| OR: 0.82 (0.53-1.26) | Age | |||||||||
| Lieberman-Cribbin57 | February 29 to April 24, 2020 | United States | 272 (4.4) | 6250 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | NR | NR | Cross-sectional study | OR: 0.94 (0.66-1.34) | Age, sex, and race |
| Calmes58 | March 18 to April 17, 2020 | Belgium | 57 (9.6) | 596 | All hospitalized adult patients with confirmed COVID-19 | 49.3 | 58.8 ± 18.9 | Retrospective cohort study | OR: 0.74 (0.24-2.3) | Age, sex, cardiopathy, immunosuppressive disease, and COPD |
| OR: 0.59 (0.20-1.8) | Age and sex | |||||||||
| Choi59 | Up to May 15, 2020 | Korea | 218 (2.9) | 7590 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 40.8 | NR | Retrospective cohort study | OR: 1.317 (0.708–2.451) | Age, sex, and underlying diseases |
| Kim60 | February to May 2020 | Korea | 70 (3.2) | 2200 | All hospitalized adult patients with confirmed COVID-19 | 35.7 | 56.7 ± 19.0 | Cross-sectional study | OR: 1.762 (0.813-3.822) | Age and sex |
| OR: 1.656 (0.624-4.395) | Age, sex, BMI, smoking history, underlying comorbidity (COPD, DM, HTN, heart failure, other heart disease, CKD, chronic liver disease, cancer, autoimmune disease, dementia, and other psychological disorder), and medication (antiretroviral, hydroxychloroquine, systemic steroid, and azithromycin) | |||||||||
| Alwafi61 | March 15 to August 15, 2020 | Saudi Arabia | 28 (4.0) | 706 | All hospitalized patients with confirmed COVID-19 | 68.5 | 48.0 ± 15.6 | Cross-sectional study | OR: 0.80 (0.07-8.82) | Age, sex, and comorbidities |
| Vera-Zertuche62 | February 24 to April 26, 2020 | Mexico | 542 (3.5) | 15,529 | All individuals with laboratory-confirmed infection by SARS-CoV-2 | 57.8 | 46.6 ± 15.5 | Retrospective cohort study | OR: 0.63 (0.24-1.70) | Sex, age, and time from symptom onset to care, social lag index, aging index, afro-descendant/100 inhabitants, indigenous language-speaking/100 inhabitants, affiliation to health services/100 inhabitants, members per household, hospitals/10,000 inhabitants, and hospital beds/10,000 inhabitants |
| Elhadi63 | May 29 to December 30, 2020 | Libya | 51 (11) | 465 | All adult COVID-19 patients admitted to ICUs | 51.6 | 69 (56.5-75) | Prospective cohort study | HR: 0.66 (0.40–1.10) | Age, BMI, comorbidities, laboratory findings during admission, qSOFA score, type of intubation during admission, developed sepsis/septic shock at during ICU admission, inotropes/vasopressor, antibiotic, and major complications or events |
| Cummins64 | February 1 to June 30, 2020 | United Kingdom | 244 (13.7) | 1781 | All adult (age ≥16 y) patients with laboratory-confirmed diagnosis of COVID-19 | 55.2 | NR | Retrospective cohort study | OR: 1.03 (0.70-1.50) | Age, sex, ethnicity, top 30% most deprived areas, obese, smoker (current), AF, cancer, chronic heart disease, CKD, COPD, dementia, depression, type 1 DM, type 2 DM, epilepsy, heart failure, HTN, learning disability, severe mental illness, peripheral arterial disease, and stroke |
| Castro65 | By 14 December 2020 | Brazil | 14,567 (2.8) | 522,167 | All hospitalized patients with confirmed COVID-19 | 56.0 | 61 (47-73) | Retrospective cohort study | OR: 0.81 (0.77-0.86) | Age, sex, ethno-racial self-classification, region, ICU, obesity, DM, chronic liver disease, chronic neurological disease, chronic lung disease, immunodeficiency, and CKD |
| HR: 0.88 (0.84-0.92) | ||||||||||
| Beltramo66 | March 1 to April 30, 2020 | France | 3273 (3.7) | 89,530 | All hospitalized patients with confirmed COVID-19 | 53.1 | 65 ± 20 | Retrospective cohort study | OR: 0.82 (0.71-0.94) | Lung cancer, COPD, pulmonary sarcoidosis, ILD, emphysema, sleep apnea, chronic respiratory failure, and pulmonary HTN |
| Robles-Pérez67 | March to December 2020 | Mexico | 2403 (3.2) | 75,595 | All Social Security workers with confirmed COVID-19 | 42.4 | NR | Retrospective cohort study | OR: 0.96 (0.51-1.79) | Age, sex, and presence of comorbidities |
| De Rosa68 | February 27 to June 15, 2020 | Italy | 23 (1.5) | 1538 | Hospitalized adult patients with confirmed COVID-19 | 58 | 74 (61-83) | Retrospective cohort study | OR: 1.45 (0.44-4.78) | Age, sex, smoking, DM, HTN, CVD, COPD, immunodepression, P/F, lymphocytopenia, LDH, eGFR, D-dimer, and CRP |
| Marciniak69 | January 17, 2020 to February 15, 2021 | United Kingdom | NR | 73,832 | Hospitalized adult patients with confirmed COVID-19 | NR | NR | Prospective cohort study | OR: 0.90 (0.85-0.96) | Age, sex, and comorbidities |
| Kelly70 | March 2 to October 31, 2020 | United States | 1487 (5.4) | 27,640 | All veterans with confirmed COVID-19 | 88.6 | 57.2 ± 16.6 | Retrospective cohort study | OR: 0.88 (0.65-1.19) | Age, sex, race, ethnicity, marital status, clinical factors, health care facility, and month of COVID-19 diagnosis |
AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CCI, Charlson comorbidity index; CHF, congestive heart failure; CIRS, cumulative illness rating scale score; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease comorbidity; CRP, C-reactive protein; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, hazard ratio; HTN, hypertension; ICU, intensive care unit; IQR, interquartile range; LDH, lactate dehydrogenase; MI, myocardial infarction; NR, not reported; OR, odds ratio; P/F, arterial oxygen tension/inspired oxygen fraction; qSOFA, quick sequential organ failure assessment.
Values of age are presented as means ± SDs or medians (IQRs).
Figure 1.
Flowchart of study search and selection.
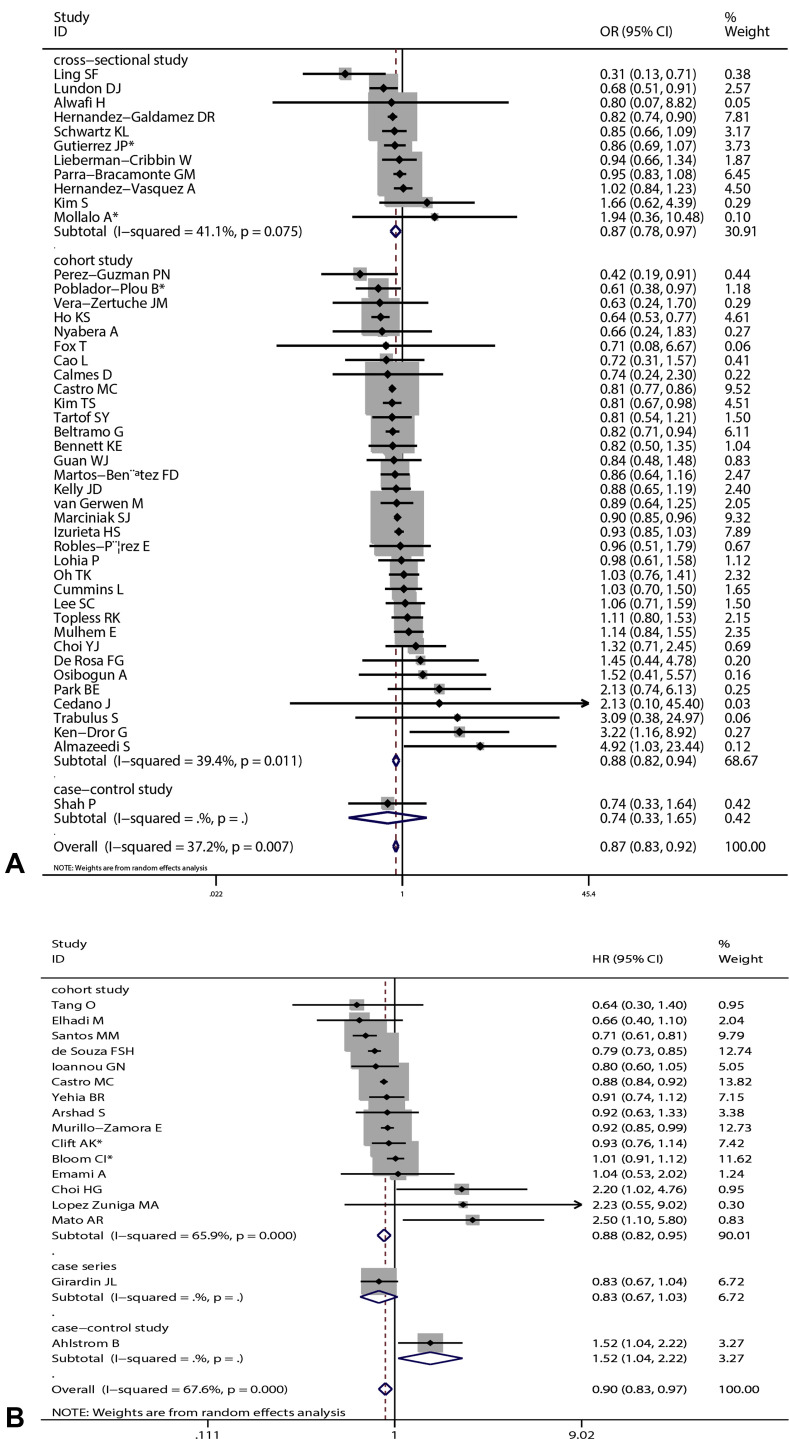
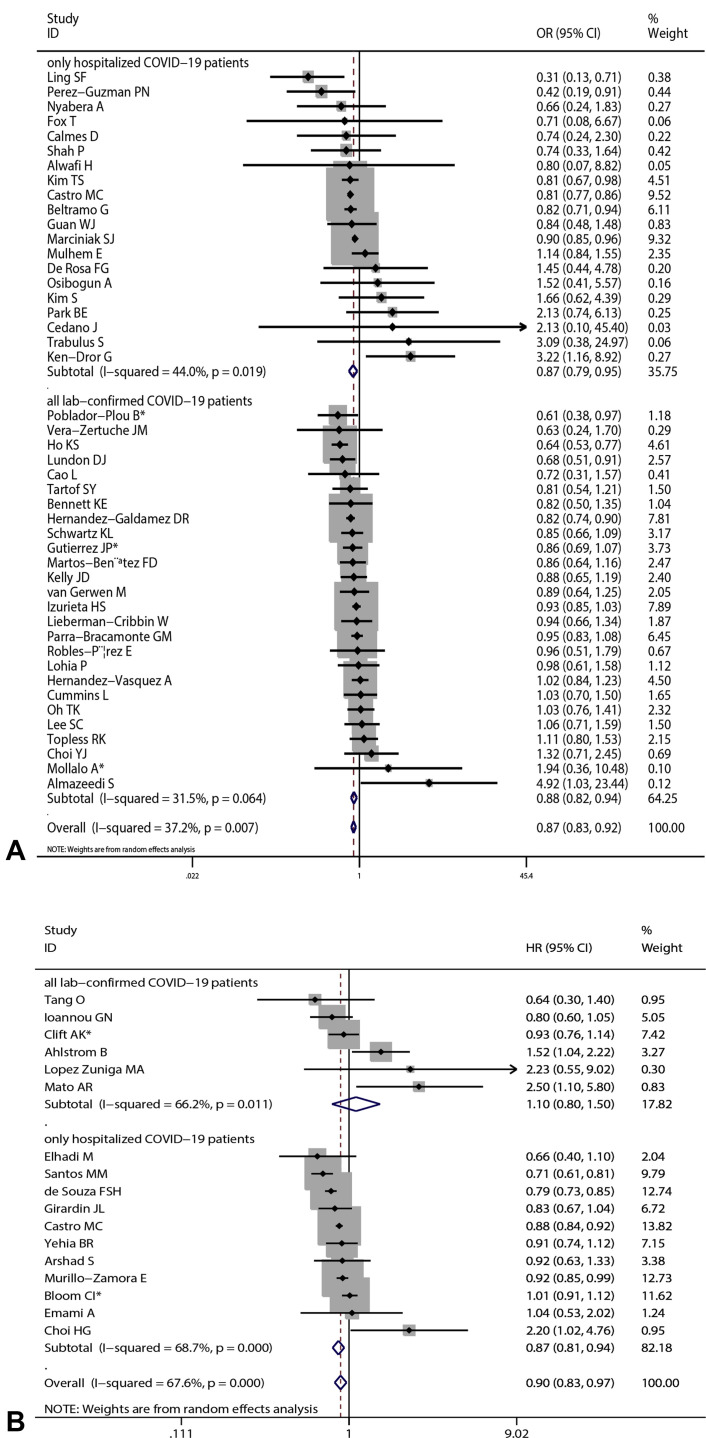
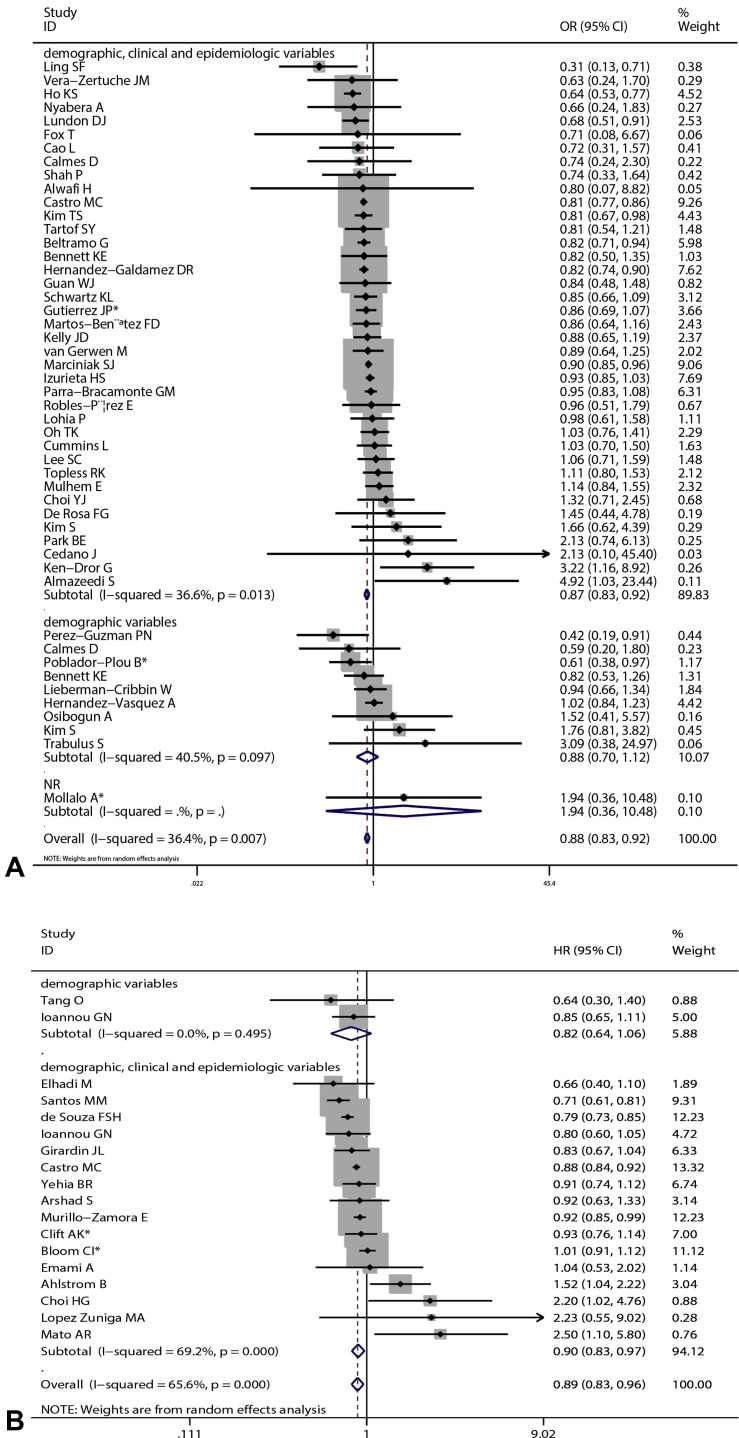
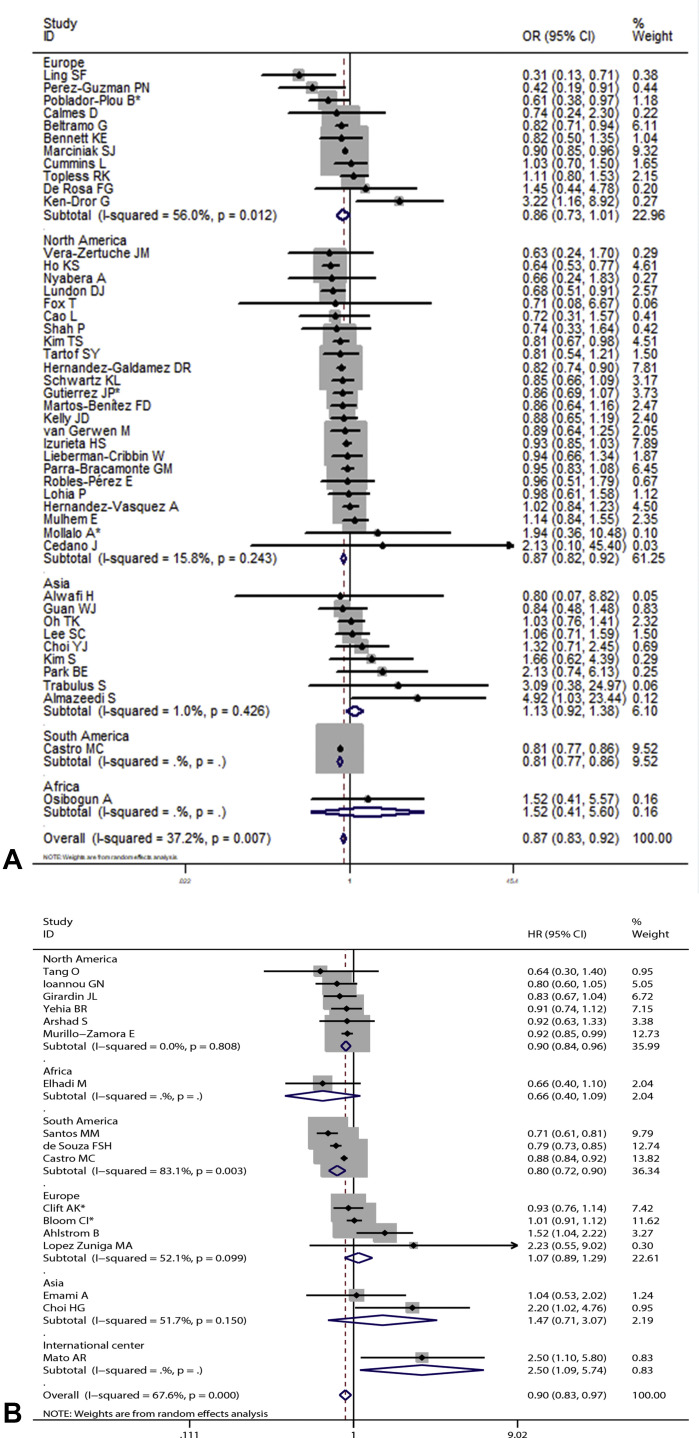
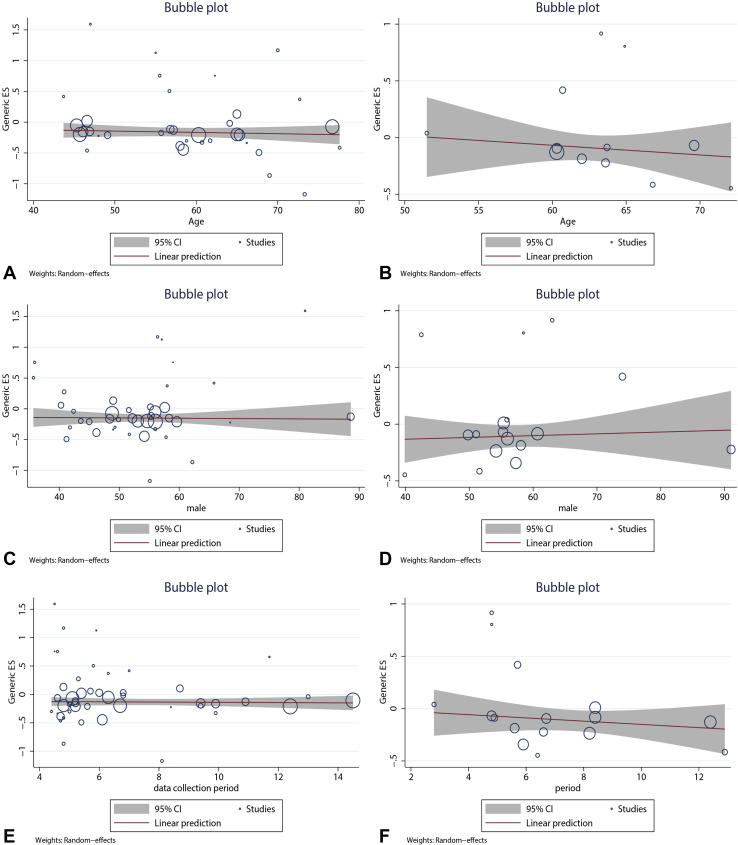
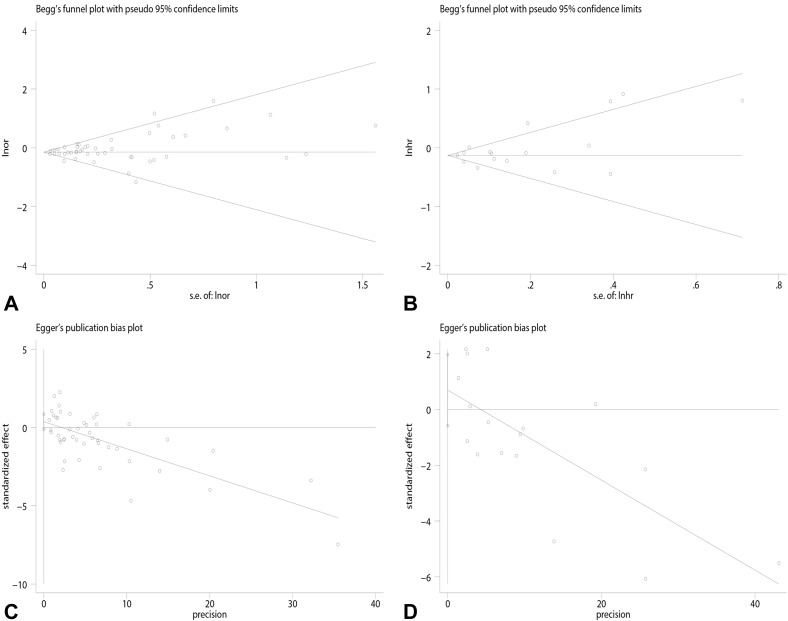
Overall results based on adjusted effect estimates demonstrated that COVID-19 patients with asthma had a significantly reduced risk for mortality compared with those without it (15 cohort studies: 829,670 patients, pooled HR = 0.88, 95% CI, 0.82-0.95, I 2 = 65.9%, P < .001; 34 cohort studies: 1,008,015 patients, pooled OR = 0.88, 95% CI, 0.82-0.94, I 2 = 39.4%, P = .011; 11 cross-sectional studies: 1,134,738 patients, pooled OR = 0.87, 95% CI, 0.78-0.97, I 2 = 41.1%, P = .075) (Figure 2 ). The results of subgroup analysis based on hospitalization status showed that asthma was associated with a significantly reduced risk for mortality in COVID-19 patients when we restricted the analysis to studies that included only hospitalized patients (20 studies: 751,644 patients, pooled OR = 0.87, 95% CI, 0.79-0.95, I 2 = 44.0%, P = .019; 11 studies: 811,941 patients, pooled HR = 0.87, 95% CI, 0.81-0.94, I 2 = 68.7%, P < .001) (Figure 3 ). The significant association was observed in studies reporting ORs but not in those reporting HRs in subgroups that included all laboratory-confirmed patients (36 studies: 1,391,631 patients, pooled OR = 0.88, 95% CI, 0.82-0.94, I 2 = 31.5%, P = .064; six studies: 24,156 patients, pooled HR = 1.10, 95% CI, 0.80-1.50, I 2 = 66.2%, P = .011) (Figure 3). The inconsistency of results may be a result of the difference in the number of studies in each subgroup; subgroups with fewer studies tended to conclude more often that asthma was not associated with mortality in COVID-19 patients. Subgroup analysis based on types of adjusted factors indicated that COVID-19 patients with asthma had a significantly reduced risk for mortality among studies adjusting for demographic, clinical, and epidemiologic variables (39 studies: 2,078,426 patients, pooled OR = 0.87, 95% CI, 0.83-0.92, I 2 = 36.3%, P = .013; 16 studies: 835,345 patients, pooled HR = 0.90, 95% CI, 0.83-0.97, I 2 = 69.2%, P < .001) (Figure 4 ), but not among studies adjusting only for demographic variables (nine studies: 97,434 patients, pooled OR = 0.88, 95% CI, 0.70-1.12, I 2 = 40.5%, P = .097; two studies: 10,883 patients, pooled HR = 0.82, 95% CI, 0.64-1.06, I 2 = 0%, P = .495) (Figure 4). Further subgroup analysis by region revealed that COVID-19 patients with asthma had a significantly reduced risk for mortality compared with patients without asthma among North American patients (24 studies: 1,355,172 patients, pooled OR = 0.87, 95% CI, 0.82-0.92, I 2 = 15.8%, P = .243; six studies: 95,203 patients, pooled HR = 0.90, 95% CI, 0.84-0.96, I 2 = 0%, P = .808 (Figure 5 ) and South American patients (3 studies: 646,397 patients, pooled HR = 0.80, 95% CI, 0.72-0.90, I 2 = 83.1%, P = .003) (Figure 5), but not among Asian patients (9 studies: 68,669 patients, pooled OR = 1.13, 95% CI, 0.92-1.38, I 2 = 1.0%, P = .426; 2 studies: 5296 patients, pooled HR = 1.47, 95% CI, 0.71-3.07, I 2 = 51.7%, P = .150) (Figure 5) or European patients (11 studies: 195,083 patients, pooled OR = 0.86, 95% CI, 0.73-1.01, I 2 = 56.0%, P = .012; 4 studies: 88,538 patients, pooled HR = 1.07, 95% CI, 0.89-1.29, I 2 = 52.1%, P = .099) (Figure 5). Age (OR: tau2 = 0.010, t = –0.62, P = .542; HR: tau2 = 0.008, t = –0.63, P = .540) (Figure 6 , A and B), sex (OR: tau2 = 0.007, t = –0.14, P = .889; HR: tau2 = 0.016, t = 0.33, P = .743) (Figure 6, C and D), and data collection periods (OR: tau2 = 0.007, t = –0.28, P = .777; HR: tau2 = 0.017, t = –0.82, P = .428) (Figure 6, E and F) could not explain potential sources of heterogeneity by meta-regression. We did not observe potential publication bias in Begg's test (OR: P = .394; HR: P = .343) (Figure 7 , A and B) or Egger's test (OR: P = .142, HR: P = .265) (Figure 7, C and D). Sensitivity analysis proved that our results were stable.
Figure 2.
Forest plots indicating that coronavirus disease 2019 (COVID-19) patients with asthma had a significantly reduced risk for mortality compared with those without it. Arrow indicates that the 95% confidence interval (CI) for effect size in the study was equal to or greater than the x-axis value. Sizes of the shaded area reflect the study-specific statistical weights. (A) Pooled odds ratio (OR). (B) Pooled hazard ratio (HR). ∗Combined effects based on subgroups.
Figure 3.
Subgroup analysis by hospitalization status: (A) pooled odds ratio (OR) and (B) pooled hazard ratio (HR). ∗Combined effects based on subgroups. CI, confidence interval.
Figure 4.
Subgroup analysis by type of adjusted factors: (A) pooled odds ratio (OR) and (B) pooled hazard ratio (HR). ∗Combined effects based on subgroups. CI, confidence interval.
Figure 5.
Subgroup analysis based on region: (A) pooled odds ratio (OR) and (B) pooled hazard ratio (HR). ∗Combined effects based on subgroups. CI, confidence interval.
Figure 6.
Meta-regression for age to evaluate association between asthma and mortality of COVID-19 patients: (A) pooled odds ratio [OR]; (B) pooled hazard ratio (HR) and sex; (C) pooled OR; (D) pooled HR and data collection period; (E) pooled OR; and (F) pooled HR. CI, confidence interval.
Figure 7.
Publication bias was evaluated by Begg's test: (A) pooled odds ratio (OR); (B) pooled hazard ratio (HR) and Egger's test; (C) pooled OR; (D) pooled HR.
Discussion
This meta-analysis on the basis of adjusted effects estimates found that COVID-19 patients with asthma had a significantly reduced risk for mortality compared with those without asthma, which suggests that asthma might be an independent protective factor for developing fatal outcomes among COVID-19 patients. Meta-regression and subgroup analyses showed that none of these factors (such as age, sex, region, hospitalization status, data collection period, and the types of adjusted factors) could explain the potential sources of heterogeneity. Although the detailed mechanisms underlying the association between asthma and the reduced risk for COVID-19 mortality are unclear, several possibilities exist: (1) COVID-19 patients with asthma may receive more medical care in clinical practice; (2) the use of inhaled corticosteroids, allergen immunotherapy, and biological agents might be beneficial through suppressing viral replication and alleviating inflammation71; and (3) type 2 immune response in patients with asthma might counteract the severe acute respiratory syndrome coronavirus 2 infection-induced inflammatory process.72 Further studies should focus on underlying mechanisms of preexisting asthma reducing the risk for fatal COVID-19. The association between having asthma and lower COVID-19 mortality may also have resulted from study bias, including selection bias (eg, a lack of representativeness), information bias (asthma underreporting or overreporting), and confounding bias (eg, asthma may have been relatively underrepresented among patients with other comorbidities that predispose more often to COVID-19 mortality, such as diabetes, obesity, or smoking, because asthma is common among younger patients).
A strength of this study was the large number of included studies (62 eligible articles) with 2,457,205 cases reporting adjusted effect estimates, which consider the influences of confounding factors such as age, sex, and underlying diseases on the association between asthma and mortality among COVID-19 patients. However, several limitations should be acknowledged. First, most included studies were from North America, and one should be cautious when extrapolating the findings to other regions. Second, most of the eligible studies were designed retrospectively. Thus, further well-designed prospective studies with large sample sizes are warranted to verify the findings. Third, the pooled effects were estimated based on risk factor-adjusted effects, but the adjusted risk factors were not fully consistent across the included studies. We performed subgroup analysis according to the types of adjusted factors, which yielded inconsistent results. These results may have been because the number of studies adjusting only for demographic variables was significantly smaller than the number of studies adjusting for demographic, clinical, and epidemiologic variables, which warrants further studies based on more primary studies and larger sample sizes. Fourth, we did not investigate the effects of medication on the association between asthma and COVID-19 mortality, which should be addressed in the future when sufficient data are available. Fifth, there was heterogeneity across studies, which was why we performed meta-regression and further subgroup analyses but did not identify potential sources of heterogeneity. In addition, excluding articles that were not written in English might be a source of publication bias. However, publication bias was not detected by Begg's test or Egger's test. Our data indicate that asthma is related to a significantly reduced risk for COVID-19 mortality. Thus, routine interventions and treatment for asthma patients infected with severe acute respiratory syndrome coronavirus 2 should be continued. We hope the updated data will contribute to more accurate elaboration and substantiation of findings from the study of Liu et al.1
Acknowledgments
The authors thank Ying Wang, Li Shi, Wenwei Xiao, Xuan Liang, Jian Wu, and Peihua Zhang (all from the Department of Epidemiology, School of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data, and valuable suggestions for data analysis. H. Yang and Y. Wang designed the study. H. Hou and Y. Li performed literature search. H. Hou and H. Yang performed data extraction. H. Hou and J. Xu performed statistical analyses. H. Yang, H. Hou, and Y. Wang wrote and reviewed the manuscript. All authors approved the final version of the manuscript.
Footnotes
This work was supported by National Natural Science Foundation of China Grant No. 81973105, Key Scientific Research Project of Henan Institution of Higher Education Grant No. 21A330008, and Joint Construction Project of Henan Medical Science and Technology Research Plan Grant No. LHGJ20190679. The funders have no role in the data collection, data analysis, preparation of manuscript, and decision in submission.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository.
Table E1.
Search strategies
| Database | Search strategies |
|---|---|
| PubMed | (“coronavirus disease 2019” OR “COVID-19” OR “SARS-CoV-2” OR “2019-nCoV” OR “novel coronavirus”) AND (“mortality” OR “fatality” OR “death” OR “non-survivor” OR “deceased”) AND (“asthma”) |
| EMBASE | (‘coronavirus disease 2019’ OR ‘covid-19’ OR ‘sars-cov-2’ OR ‘2019-ncov’ OR ‘novel coronavirus’) AND (‘asthma’) AND (‘mortality’ OR ‘fatality’ OR ‘death’ OR ‘non-survivor’ OR ‘deceased’) |
| Web of Science | TS = ((“coronavirus disease 2019” OR “covid-19” OR “sars-cov-2” OR “2019-ncov” OR “novel coronavirus”) AND (“asthma”) AND (“mortality” OR “fatality” OR “death” OR “non-survivor” OR “deceased”)) |
Table E2.
Assessment of risk for bias in each study with National Institutes of Health Study Quality Assessment tools in prognosis meta-analysis
| First author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort and cross-sectional studies | |||||||||||||||
| Arshad SE1 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Mato ARE2 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Poblador-Plou BE3 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| van Gerwen ME4 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Hernandez-Galdamez DRE5 | Yes | Yes | No | Yes | No | Yes | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Hernandez-Vasquez AE6 | Yes | Yes | NR | Yes | No | Yes | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Almazeedi SE7 | Yes | Yes | Yes | Yes | No | Yes | NA | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Perez-Guzman PNE8 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Tartof SYE9 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Parra-Bracamonte GME10 | Yes | Yes | NR | Yes | No | NR | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Fox TE11 | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Yehia BRE12 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Emami AE13 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Trabulus S E14 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Santos MME15 | Yes | Yes | NR | Yes | No | NR | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Ioannou GNE16 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Gutierrez JPE17 | Yes | Yes | NR | Yes | No | Yes | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Clift AKE18 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Kim TSE19 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Tang OE20 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Ken-Dror GE21 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Choi HGE22 | Yes | Yes | NR | Yes | No | Yes | NR | No | Yes | NR | Yes | NR | NR | Yes | i |
| Nyabera AE23 | Yes | Yes | NR | Yes | No | Yes | NR | No | NR | NR | NR | NR | Yes | Yes | i |
| Lee SCE24 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | ii |
| Murillo-Zamora EE25 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Ling SFE26 | Yes | Yes | NR | Yes | No | Yes | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Izurieta HSE27 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Lundon DJE28 | Yes | Yes | Yes | Yes | No | NR | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Schwartz KLE29 | Yes | Yes | NR | Yes | No | NR | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Martos-Benítez FDE30 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Oh TKE31 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Park BEE32 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | NR | Yes | i |
| Lopez Zuniga MAE33 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Mollalo AE34 | Yes | Yes | Yes | Yes | No | NR | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Lohia PE35 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Cedano JE36 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Cao LE37 | Yes | Yes | NR | Yes | No | Yes | NR | No | Yes | NR | Yes | NR | NR | Yes | i |
| Ho KSE38 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Guan WJE39 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Bloom CIE40 | Yes | Yes | NR | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | ii |
| Osibogun AE41 | Yes | Yes | NR | Yes | No | Yes | Yes | No | No | NR | Yes | NR | Yes | Yes | i |
| de Souza FSHE42 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Mulhem EE43 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Topless RKE44 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Bennett KEE45 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Lieberman-Cribbin WE46 | Yes | Yes | NR | Yes | Yes | NR | NA | No | No | NR | Yes | NR | NA | Yes | i |
| Calmes DE47 | Yes | Yes | NR | Yes | No | NR | NR | No | Yes | No | Yes | NR | Yes | Yes | i |
| Choi YJE48 | Yes | Yes | NR | Yes | No | Yes | NR | Yes | Yes | NR | Yes | NR | Yes | Yes | i |
| Kim SE49 | Yes | Yes | Yes | Yes | No | Yes | NA | No | Yes | NR | Yes | NR | NA | Yes | i |
| Alwafi HE50 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Vera-Zertuche JME51 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | Yes | Yes | NR | Yes | Yes | i |
| Elhadi ME52 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Cummins LE53 | Yes | Yes | Yes | Yes | No | Yes | NR | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Castro MCE54 | Yes | Yes | Yes | NR | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Beltramo GE55 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Robles-Pérez EE56 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | NR | NR | Yes | Yes | i |
| De Rosa FGE57 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Marciniak SJE58 | Yes | Yes | NR | Yes | No | Yes | NR | No | Yes | NR | Yes | NR | NR | Yes | i |
| Kelly JDE59 | Yes | Yes | NR | Yes | No | Yes | Yes | No | Yes | NR | Yes | NR | Yes | Yes | i |
| Case-control studies | |||||||||||||||
| Shah PE60 | Yes | Yes | No | Yes | Yes | Yes | No | No | No | Yes | NR | Yes | i | ||
| Ahlstrom BE61 | Yes | Yes | NR | Yes | Yes | Yes | NR | NR | Yes | Yes | NR | Yes | i | ||
| Case series studies | |||||||||||||||
| Girardin JLE62 | Yes | Yes | NR | NR | Yes | NR | NR | Yes | Yes | i |
NA, not applicable; NR, not reported.
For cohort and cross-sectional studies, quality was rated as 0 for poor (0-4 of 14 questions), i for fair (5-10 of 14 questions), or ii for good (11-14 of 14 questions): (1) Was the research question or objective in this paper clearly stated? (2) Was the study population clearly specified and defined? (3) Was the participation rate of eligible persons at least 50%? (4) Were all of the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? (5) Was a sample size justification, power description, or variance and effect estimates provided? (6) For the analyses in this paper, were the exposure(s) of interest measured before the outcome(s) being measured? (7) Was the time frame sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? (8) For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (eg, categories of exposure, or exposure measured as continuous variable)? (9) Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? (10) Was the exposure(s) assessed more than once over time? (11) Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? (12) Were the outcome assessors blinded to the exposure status of participants? (13) Was loss to follow-up after baseline 20% or less? (14) Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? For case-control studies, quality was rated as 0 for poor (0-3 of 12 questions), i for fair (4-8 of 12 questions), or ii for good (9-12 of 12 questions): (1) Was the research question or objective in this paper clearly stated and appropriate? (2) Was the study population clearly specified and defined? (3) Did the authors include a sample size justification? (4) Were controls selected or recruited from the same or similar population that gave rise to the cases (including the same time frame)? (5) Were the definitions, inclusion and exclusion criteria, algorithms, or processes used to identify or select cases and controls valid, reliable, and implemented consistently across all study participants? (6) Were the cases clearly defined and differentiated from controls? (7) If less than 100% of eligible cases and/or controls were selected for the study, were the cases and/or controls randomly selected from those eligible? (8) Was there use of concurrent controls? (9). Were the investigators able to confirm that the exposure or risk occurred before the development of the condition or event that defined a participant as a case? (10) Were the measures of exposure or risk clearly defined, valid, reliable, and implemented consistently (including the same time period) across all study participants? (11) Were the assessors of exposure or risk blinded to the case or control status of participants? (12) Were key potential confounding variables measured and adjusted statistically in the analyses? If matching was used, did the investigators account for matching during study analysis? For case series studies, quality was rated as 0 for poor (0-2 of nine questions), i for fair (3-6 of nine questions), or ii for good (7-9 of nine questions): (1) Was the study question or objective clearly stated? (2) Was the study population clearly and fully described, including a case definition? (3) Were the cases consecutive? (4) Were the subjects comparable? (5) Was the intervention clearly described? (6). Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? (7) Was the length of follow-up adequate? (8) Were the statistical methods well-described? (9) Were the results well-described?
References
- 1.Liu S., Cao Y., Du T., Zhi Y. Prevalence of comorbid asthma and related outcomes in COVID-19: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2021;9:693–701. doi: 10.1016/j.jaip.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L., Xu J., Xiao W., Wang Y., Jin Y., Chen S., et al. Asthma in patients with coronavirus disease 2019: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2021;126:524–534. doi: 10.1016/j.anai.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang X., Li S., Yu H., Wang P., Zhang Y., Chen Z., et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang X., Shi L., Wang Y., Xiao W., Duan G., Yang H., et al. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J Infect. 2020;81:e44–e47. doi: 10.1016/j.jinf.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X., Xu J., Xiao W., Shi L., Yang H. The association of diabetes with COVID-19 disease severity: evidence from adjusted effect estimates. Hormones (Athens) 2021;20:409–414. doi: 10.1007/s42000-020-00259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J., Xiao W., Liang X., Zhang P., Shi L., Wang Y., et al. The association of cerebrovascular disease with adverse outcomes in COVID-19 patients: a meta-analysis based on adjusted effect estimates. J Stroke Cerebrovasc Dis. 2020;29:105283. doi: 10.1016/j.jstrokecerebrovasdis.2020.105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., Xu J., Liang X., Shi L., Wang Y. Chronic liver disease independently associated with COVID-19 severity: evidence based on adjusted effect estimates. Hepatol Int. 2021;15:217–222. doi: 10.1007/s12072-020-10133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 9.Shah P., Owens J., Franklin J., Mehta A., Heymann W., Sewell W., et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52:354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M., et al. Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int J Environ Res Public Health. 2020;17:5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gerwen M., Alsen M., Little C., Barlow J., Genden E., Naymagon L., et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93:907–915. doi: 10.1002/jmv.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández-Galdamez D.R., González-Block M., Romo-Dueñas D.K., Lima-Morales R., Hernández-Vicente I.A., Lumbreras-Guzmán M., et al. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51:683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández-Vásquez A., Azañedo D., Vargas-Fernández R., Bendezu-Quispe G. Association of comorbidities with pneumonia and death among COVID-19 patients in Mexico: a nationwide cross-sectional study. J Prev Med Public Health. 2020;53:211–219. doi: 10.3961/jpmph.20.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Guzman P.N., Daunt A., Mukherjee S., Crook P., Forlano R., Kont M.D., et al. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. https://doi.org/10.1093/cid/ciaa1091 Clin Infect Dis. Published online August 7, 2020. [DOI] [PMC free article] [PubMed]
- 18.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parra-Bracamonte G.M., Lopez-Villalobos N., Parra-Bracamonte F.E. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020;52:93–98.e2. doi: 10.1016/j.annepidem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox T., Ruddiman K., Lo K.B., Peterson E., DeJoy R., 3rd, Salacup G., et al. The relationship between diabetes and clinical outcomes in COVID-19: a single-center retrospective analysis. Acta Diabetol. 2021;58:33–38. doi: 10.1007/s00592-020-01592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yehia B.R., Winegar A., Fogel R., Fakih M., Ottenbacher A., Jesser C., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3:e2018039. doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emami A., Javanmardi F., Akbari A., Kojuri J., Bakhtiari H., Rezaei T., et al. Survival rate in hypertensive patients with COVID-19. Clin Exp Hypertens. 2021;43:77–80. doi: 10.1080/10641963.2020.1812624. [DOI] [PubMed] [Google Scholar]
- 23.Trabulus S., Karaca C., Balkan, Dincer M.T., Murt A., Ozcan S.G., et al. Kidney function on admission predicts in-hospital mortality in COVID-19. PLoS One. 2020;15:e0238680. doi: 10.1371/journal.pone.0238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos M.M., Lucena E.E.S., Lima K.C., Brito A.A.C., Bay M.B., Bonfada D. Survival and predictors of deaths of patients hospitalised due to COVID-19 from a retrospective and multicentre cohort study in Brazil. Epidemiol Infect. 2020;148:e198. doi: 10.1017/S0950268820002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannou G.N., Locke E., Green P., Berry K., O'Hare A.M., Shah J.A., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3:e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez J.P., Bertozzi S.M. Non-communicable diseases and inequalities increase risk of death among COVID-19 patients in Mexico. PLoS One. 2020;15:e0240394. doi: 10.1371/journal.pone.0240394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim T.S., Roslin M., Wang J.J., Kane J., Hirsch J.S., Kim E.J. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity (Silver Spring) 2021;29:279–284. doi: 10.1002/oby.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang O., Bigelow B.F., Sheikh F., Peters M., Zenilman J.M., Bennett R., et al. Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: results of universal testing of 1970 residents. J Am Med Dir Assoc. 2020;21:1767–1773.e1. doi: 10.1016/j.jamda.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ken-Dror G., Wade C., Sharma S., Law J., Russo C., Sharma A., et al. COVID-19 outcomes in UK centre within highest health and wealth band: a prospective cohort study. BMJ Open. 2020;10:e042090. doi: 10.1136/bmjopen-2020-042090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi H.G., Wee J.H., Kim S.Y., Kim J.H., Il Kim H., Park J.Y., et al. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean Disease Control and Prevention. Allergy. 2021;76:921–924. doi: 10.1111/all.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyabera A., Lakhdar S., Li M., Trandafirescu T., Ouedraogo Tall S. The association between BMI and inpatient mortality outcomes in older adults with COVID-19. Cureus. 2020;12:e11183. doi: 10.7759/cureus.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.C., Son K.J., Han C.H., Jung J.Y., Park S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19) Sci Rep. 2020;10:21805. doi: 10.1038/s41598-020-77791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murillo-Zamora E., Hernandez-Suarez C.M. Survival in adult inpatients with COVID-19. Public Health. 2021;190:1–3. doi: 10.1016/j.puhe.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling S.F., Broad E., Murphy R., Pappachan J.M., Pardesi-Newton S., Kong M.F., et al. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020;12:3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izurieta H.S., Graham D.J., Jiao Y., Hu M., Lu Y., Wu Y., et al. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. J Infect Dis. 2021;223:945–956. doi: 10.1093/infdis/jiaa767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundon D.J., Mohamed N., Lantz A., Goltz H.H., Kelly B.D., Tewari A.K. Social determinants predict outcomes in data from a multi-ethnic cohort of 20,899 patients investigated for COVID-19. Front Public Health. 2020;8:571364. doi: 10.3389/fpubh.2020.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz K.L., Achonu C., Buchan S.A., Brown K.A., Lee B., Whelan M., et al. Epidemiology, clinical characteristics, household transmission, and lethality of severe acute respiratory syndrome coronavirus-2 infection among healthcare workers in Ontario, Canada. PLoS One. 2020;15:e0244477. doi: 10.1371/journal.pone.0244477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martos-Benítez F.D., Soler-Morejón C.D., García-Del Barco D. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med. 2021;6:1507–1517. doi: 10.1007/s11739-020-02597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh T.K., Song I.A. Impact of coronavirus disease-2019 on chronic respiratory disease in South Korea: an NHIS COVID-19 database cohort study. BMC Pulm Med. 2021;21:12. doi: 10.1186/s12890-020-01387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park B.E., Lee J.H., Park H.K., Kim H.N., Jang S.Y., Bae M.H., et al. Impact of cardiovascular risk factors and cardiovascular diseases on outcomes in patients hospitalized with COVID-19 in Daegu metropolitan city. J Korean Med Sci. 2021;36:e15. doi: 10.3346/jkms.2021.36.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahlström B., Frithiof R., Hultström M., Larsson I.M., Strandberg G., Lipcsey M. The Swedish covid-19 intensive care cohort: Risk factors of ICU admission and ICU mortality. Acta Anaesthesiol Scand. 2021;65:525–533. doi: 10.1111/aas.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López Zúñiga M., Moreno-Moral A., Ocaña-Granados A., Padilla-Moreno F.A., Castillo-Fernández A.M., Guillamón-Fernández D., et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021;16:e0243964. doi: 10.1371/journal.pone.0243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mollalo A., Rivera K.M., Vahabi N. Spatial statistical analysis of pre-existing mortalities of 20 diseases with COVID-19 mortalities in the continental United States. Sustain Cities Soc. 2021;67:102738. doi: 10.1016/j.scs.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lohia P., Sreeram K., Nguyen P., Choudhary A., Khicher S., Yarandi H., et al. Preexisting respiratory diseases and clinical outcomes in COVID-19: a multihospital cohort study on predominantly African American population. Respir Res. 2021;22:37. doi: 10.1186/s12931-021-01647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cedano J., Fabian Corona E., Gonzalez-Lara M., Santana M., Younes I., Ayad S., et al. Characteristics and outcomes of patients with COVID-19 in an intensive care unit of a community hospital; retrospective cohort study. J Community Hosp Intern Med Perspect. 2021;11:27–32. doi: 10.1080/20009666.2020.1830516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girardin J.L., Seixas A., Ramos Cejudo J., Osorio R.S., Avirappattu G., Reid M., et al. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron Respir Dis. 2021;18 doi: 10.1177/1479973120986806. 1479973120986806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao L., Lee S., Krings J.G., Rauseo A.M., Reynolds D., Presti R., et al. Asthma in patients with suspected and diagnosed coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126:535–541.e2. doi: 10.1016/j.anai.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho K.S., Howell D., Rogers L., Narasimhan B., Verma H., Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. 2021;127:42–48. doi: 10.1016/j.anai.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan W.J., Liang W.H., Shi Y., Gan L.X., Wang H.B., He J.X., et al. Chronic respiratory diseases and the outcomes of COVID-19: a nationwide retrospective cohort study of 39,420 cases. J Allergy Clin Immunol Pract. 2021;9:2645–2655.e14. doi: 10.1016/j.jaip.2021.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloom C.I., Drake T.M., Docherty A.B., Lipworth B.J., Johnston S.L., Nguyen-Van-Tam J.S., et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:699–711. doi: 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osibogun A., Balogun M., Abayomi A., Idris J., Kuyinu Y., Odukoya O., et al. Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. PLoS One. 2021;16:e0248281. doi: 10.1371/journal.pone.0248281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Souza F.S.H., Hojo-Souza N.S., Batista B.D.O., da Silva C.M., Guidoni D.L. On the analysis of mortality risk factors for hospitalized COVID-19 patients: a data-driven study using the major Brazilian database. PLoS One. 2021;16:e0248580. doi: 10.1371/journal.pone.0248580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulhem E., Oleszkowicz A., Lick D. 3219 hospitalised patients with COVID-19 in Southeast Michigan: a retrospective case cohort study. BMJ Open. 2021;11:e042042. doi: 10.1136/bmjopen-2020-042042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Topless R.K., Phipps-Green A., Leask M., Dalbeth N., Stamp L.K., Robinson P.C., et al. Gout, rheumatoid arthritis, and the risk of death related to coronavirus disease 2019: an analysis of the UK Biobank. ACR Open Rheumatol. 2021;3:333–340. doi: 10.1002/acr2.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bennett K.E., Mullooly M., O'Loughlin M., Fitzgerald M., O'Donnell J., O'Connor L., et al. Underlying conditions and risk of hospitalisation, ICU admission and mortality among those with COVID-19 in Ireland: a national surveillance study. Lancet Reg Health Eur. 2021;5:100097. doi: 10.1016/j.lanepe.2021.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lieberman-Cribbin W., Rapp J., Alpert N., Tuminello S., Taioli E. The impact of asthma on mortality in patients with COVID-19. Chest. 2020;158:2290–2291. doi: 10.1016/j.chest.2020.05.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calmes D., Graff S., Maes N., Frix A.N., Thys M., Bonhomme O., et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9:160–169. doi: 10.1016/j.jaip.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi Y.J., Park J.Y., Lee H.S., Suh J., Song J.Y., Byun M.K., et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. 2021;57:2002226. doi: 10.1183/13993003.02226-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S., Jung C.G., Lee J.Y., Kim G., Choi S.W., Jin H.J., et al. Characterization of asthma and risk factors for delayed SARS-CoV-2 clearance in adult COVID-19 inpatients in Daegu. Allergy. 2021;76:918–921. doi: 10.1111/all.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alwafi H., Naser A.Y., Qanash S., Brinji A.S., Ghazawi M.A., Alotaibi B., et al. Predictors of length of hospital stay, mortality, and outcomes among hospitalised COVID-19 patients in Saudi Arabia: a cross-sectional study. J Multidiscip Healthc. 2021;14:839–852. doi: 10.2147/JMDH.S304788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vera-Zertuche J.M., Mancilla-Galindo J., Tlalpa-Prisco M., Aguilar-Alonso P., Aguirre-García M.M., Segura-Badilla O., et al. Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: a national observational study. Epidemiol Infect. 2021;149:e109. doi: 10.1017/S0950268821001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elhadi M., Alsoufi A., Abusalama A., Alkaseek A., Abdeewi S., Yahya M., et al. Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: a prospective multi-center cohort study. PLoS One. 2021;16:e0251085. doi: 10.1371/journal.pone.0251085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cummins L., Ebyarimpa I., Cheetham N., Tzortziou Brown V., Brennan K., Panovska-Griffiths J. Factors associated with COVID-19 related hospitalisation, critical care admission and mortality using linked primary and secondary care data. Influenza Other Respir Viruses. 2021;15:577–588. doi: 10.1111/irv.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro M.C., Gurzenda S., Macário E.M., França G.V.A. Characteristics, outcomes and risk factors for mortality of 522 167 patients hospitalised with COVID-19 in Brazil: a retrospective cohort study. BMJ Open. 2021;11:e049089. doi: 10.1136/bmjopen-2021-049089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beltramo G., Cottenet J., Mariet A.S., Georges M., Piroth L., Tubert-Bitter P., et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. https://doi.org/10.1183/13993003.04474-2020 Eur Respir J. Published online May 20, 2021. [DOI] [PMC free article] [PubMed]
- 67.Robles-Pérez E., González-Díaz B., Miranda-García M., Borja-Aburto V.H. Infection and death by COVID-19 in a cohort of healthcare workers in Mexico. Scand J Work Environ Health. 2021;47:349–355. doi: 10.5271/sjweh.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Rosa F.G., Palazzo A., Rosso T., Shbaklo N., Mussa M., Boglione L., et al. Risk factors for mortality in COVID-19 hospitalized patients in Piedmont, Italy: results from the multicenter, regional, CORACLE registry. J Clin Med. 2021;10:1951. doi: 10.3390/jcm10091951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marciniak S.J., Farrell J., Rostron A., Smith I., Openshaw P.J.M., Baillie J.K., et al. COVID-19 pneumothorax in the United Kingdom: a prospective observational study using the ISARIC WHO clinical characterisation protocol. Eur Respir J. 2021;58:2100929. doi: 10.1183/13993003.00929-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly J.D., Bravata D.M., Bent S., Wray C.M., Leonard S.J., Boscardin W.J., et al. Association of social and behavioral risk factors with mortality among US veterans with COVID-19. JAMA Netw Open. 2021;4:e2113031. doi: 10.1001/jamanetworkopen.2021.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamaya M., Nishimura H., Deng X., Sugawara M., Watanabe O., Nomura K., et al. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59:78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Cano-Del Pozo M., et al. Baseline Chronic Comorbidity and Mortality in Laboratory-Confirmed COVID-19 Cases: Results from the PRECOVID Study in Spain. Int J Environ Res Public Health. 2020;17:5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerwen M., Alsen M., Little C., Barlow J., Genden E., Naymagon L., et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93:907–915. doi: 10.1002/jmv.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Galdamez D.R., González-Block M., Romo-Dueñas D.K., Lima-Morales R., Hernández-Vicente I.A., Lumbreras-Guzmán M., et al. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51:683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Vásquez A., Azañedo D., Vargas-Fernández R., Bendezu-Quispe G. Association of comorbidities with pneumonia and death among COVID-19 patients in Mexico: a nationwide cross-sectional study. J Prev Med Public Health. 2020;53:211–219. doi: 10.3961/jpmph.20.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almazeedi S., Al-Youha S., Jamal M.H., Al-Haddad M., Al-Muhaini A., Al-Ghimlas F., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Guzman P.N., Daunt A., Mukherjee S., Crook P., Forlano R., Kont M.D., et al. Clinical characteristics and predictors of outcomes of hospitalized patients with COVID-19 in a multi-ethnic London NHS Trust: a retrospective cohort study. Clin Infect Dis. Published online August. 2020;7 doi: 10.1093/cid/ciaa1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., et al. obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Bracamonte G.M., Lopez-Villalobos N., Parra-Bracamonte F.E. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020;52:93–98.e2. doi: 10.1016/j.annepidem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T., Ruddiman K., Lo K.B., Peterson E., DeJoy R., 3rd, Salacup G., et al. The relationship between diabetes and clinical outcomes in COVID-19: a single-center retrospective analysis. Acta Diabetol. 2021;58:33–38. doi: 10.1007/s00592-020-01592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia B.R., Winegar A., Fogel R., Fakih M., Ottenbacher A., Jesser C., et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A., Javanmardi F., Akbari A., Kojuri J., Bakhtiari H., Rezaei T., et al. Survival rate in hypertensive patients with COVID-19. Clin Exp Hypertens. 2021;43:77–80. doi: 10.1080/10641963.2020.1812624. [DOI] [PubMed] [Google Scholar]
- Trabulus S., Karaca C., Balkan I.I., Dincer M.T., Murt A., Ozcan S.G., et al. Kidney function on admission predicts in-hospital mortality in COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M.M., Lucena E.E.S., Lima K.C., Brito A.A.C., Bay M.B., Bonfada D. Survival and predictors of deaths of patients hospitalised due to COVID-19 from a retrospective and multicentre cohort study in Brazil. Epidemiol Infect. 2020;148:e198. doi: 10.1017/S0950268820002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou G.N., Locke E., Green P., Berry K., O'Hare A.M., Shah J.A., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J.P., Bertozzi S.M. Non-communicable diseases and inequalities increase risk of death among COVID-19 patients in Mexico. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.S., Roslin M., Wang J.J., Kane J., Hirsch J.S., Kim E.J. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity (Silver Spring) 2021;29:279–284. doi: 10.1002/oby.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang O., Bigelow B.F., Sheikh F., Peters M., Zenilman J.M., Bennett R., et al. Outcomes of nursing home COVID-19 patients by initial symptoms and comorbidity: results of universal testing of 1970 residents. J Am Med Dir Assoc. 2020;21:1767–1773.e1. doi: 10.1016/j.jamda.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ken-Dror G., Wade C., Sharma S., Law J., Russo C., Sharma A., et al. COVID-19 outcomes in UK centre within highest health and wealth band: a prospective cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.G., Wee J.H., Kim S.Y., Kim J.H., Il Kim H., Park J.Y., et al. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean Disease Control and Prevention. Allergy. 2021;76:921–924. doi: 10.1111/all.14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyabera A., Lakhdar S., Li M., Trandafirescu T., Ouedraogo Tall S. The association between BMI and inpatient mortality outcomes in older adults with COVID-19. Cureus. 2020;12:e11183. doi: 10.7759/cureus.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.C., Son K.J., Han C.H., Jung J.Y., Park S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19) Sci Rep. 2020;10:21805. doi: 10.1038/s41598-020-77791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Zamora E., Hernandez-Suarez C.M. Survival in adult inpatients with COVID-19. Public Health. 2021;190:1–3. doi: 10.1016/j.puhe.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S.F., Broad E., Murphy R., Pappachan J.M., Pardesi-Newton S., Kong M.F., et al. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020;12:3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izurieta H.S., Graham D.J., Jiao Y., Hu M., Lu Y., Wu Y., et al. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. J Infect Dis. 2021;223:945–956. doi: 10.1093/infdis/jiaa767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundon D.J., Mohamed N., Lantz A., Goltz H.H., Kelly B.D., Tewari A.K. Social determinants predict outcomes in data from a multi-ethnic cohort of 20,899 patients investigated for COVID-19. Front Public Health. 2020;8:571364. doi: 10.3389/fpubh.2020.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K.L., Achonu C., Buchan S.A., Brown K.A., Lee B., Whelan M., et al. Epidemiology, clinical characteristics, household transmission, and lethality of severe acute respiratory syndrome coronavirus-2 infection among healthcare workers in Ontario, Canada. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martos-Benítez F.D., Soler-Morejón C.D., García-Del Barco D. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med. 2021;6:1507–1517. doi: 10.1007/s11739-020-02597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh T.K., Song I.A. Impact of coronavirus disease-2019 on chronic respiratory disease in South Korea: an NHIS COVID-19 database cohort study. BMC Pulm Med. 2021;21:12. doi: 10.1186/s12890-020-01387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.E., Lee J.H., Park H.K., Kim H.N., Jang S.Y., Bae M.H., et al. Impact of cardiovascular risk factors and cardiovascular diseases on outcomes in patients hospitalized with COVID-19 in Daegu metropolitan city. J Korean Med Sci. 2021;36:e15. doi: 10.3346/jkms.2021.36.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Zúñiga M., Moreno-Moral A., Ocaña-Granados A., Padilla-Moreno F.A., Castillo-Fernández A.M., Guillamón-Fernández D., et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021;16 doi: 10.1371/journal.pone.0243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollalo A., Rivera K.M., Vahabi N. Spatial statistical analysis of pre-existing mortalities of 20 diseases with COVID-19 mortalities in the continental United States. Sustain Cities Soc. 2021;67:102738. doi: 10.1016/j.scs.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohia P., Sreeram K., Nguyen P., Choudhary A., Khicher S., Yarandi H., et al. Preexisting respiratory diseases and clinical outcomes in COVID-19: a multihospital cohort study on predominantly African American population. Respir Res. 2021;22:37. doi: 10.1186/s12931-021-01647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedano J., Fabian Corona E., Gonzalez-Lara M., Santana M., Younes I., Ayad S., et al. Characteristics and outcomes of patients with COVID-19 in an intensive care unit of a community hospital; retrospective cohort study. J Community Hosp Intern Med Perspect. 2021;11:27–32. doi: 10.1080/20009666.2020.1830516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Lee S., Krings J.G., Rauseo A.M., Reynolds D., Presti R., et al. Asthma in patients with suspected and diagnosed coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021;126:535–541.e2. doi: 10.1016/j.anai.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K.S., Howell D., Rogers L., Narasimhan B., Verma H., Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. 2021;127:42–48. doi: 10.1016/j.anai.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Shi Y., Gan L.X., Wang H.B., He J.X., et al. Chronic respiratory diseases and the outcomes of COVID-19: a nationwide retrospective cohort study of 39,420 cases. J Allergy Clin Immunol Pract. 2021 doi: 10.1016/j.jaip.2021.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom C.I., Drake T.M., Docherty A.B., Lipworth B.J., Johnston S.L., Nguyen-Van-Tam J.S., et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:699–711. doi: 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osibogun A., Balogun M., Abayomi A., Idris J., Kuyinu Y., Odukoya O., et al. Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza F.S.H., Hojo-Souza N.S., Batista B.D.O., da Silva C.M., Guidoni D.L. On the analysis of mortality risk factors for hospitalized COVID-19 patients: A data-driven study using the major Brazilian database. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhem E., Oleszkowicz A., Lick D. 3219 hospitalised patients with COVID-19 in Southeast Michigan: a retrospective case cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topless R.K., Phipps-Green A., Leask M., Dalbeth N., Stamp L.K., Robinson P.C., et al. Gout, Rheumatoid Arthritis, and the Risk of Death Related to Coronavirus Disease 2019: An Analysis of the UK Biobank. ACR Open Rheumatol. 2021;3:333–340. doi: 10.1002/acr2.11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K.E., Mullooly M., O'Loughlin M., Fitzgerald M., O'Donnell J., O'Connor L., et al. Underlying conditions and risk of hospitalisation, ICU admission and mortality among those with COVID-19 in Ireland: A national surveillance study. Lancet Reg Health Eur. 2021;5:100097. doi: 10.1016/j.lanepe.2021.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Cribbin W., Rapp J., Alpert N., Tuminello S., Taioli E. The impact of asthma on mortality in patients with COVID-19. Chest. 2020;158:2290–2291. doi: 10.1016/j.chest.2020.05.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmes D., Graff S., Maes N., Frix A.N., Thys M., Bonhomme O., et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021;9:160–169. doi: 10.1016/j.jaip.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Park J.Y., Lee H.S., Suh J., Song J.Y., Byun M.K., et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. 2021;57:2002226. doi: 10.1183/13993003.02226-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jung C.G., Lee J.Y., Kim G., Choi S.W., Jin H.J., et al. Characterization of asthma and risk factors for delayed SARS-CoV-2 clearance in adult COVID-19 inpatients in Daegu. Allergy. 2021;76:918–921. doi: 10.1111/all.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwafi H., Naser A.Y., Qanash S., Brinji A.S., Ghazawi M.A., Alotaibi B., et al. Predictors of length of hospital stay, mortality, and outcomes among hospitalised COVID-19 patients in Saudi Arabia: a cross-sectional study. J Multidiscip Healthc. 2021;14:839–852. doi: 10.2147/JMDH.S304788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Zertuche J.M., Mancilla-Galindo J., Tlalpa-Prisco M., Aguilar-Alonso P., Aguirre-García M.M., Segura-Badilla O., et al. Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: a national observational study. Epidemiol Infect. 2021;149:e109. doi: 10.1017/S0950268821001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhadi M., Alsoufi A., Abusalama A., Alkaseek A., Abdeewi S., Yahya M., et al. Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: A prospective multi-center cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins L., Ebyarimpa I., Cheetham N., Tzortziou Brown V., Brennan K., Panovska-Griffiths J. Factors associated with COVID-19 related hospitalisation, critical care admission and mortality using linked primary and secondary care data. Influenza Other Respir Viruses. 2021;15:577–588. doi: 10.1111/irv.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M.C., Gurzenda S., Macário E.M., França G.V.A. Characteristics, outcomes and risk factors for mortality of 522 167 patients hospitalised with COVID-19 in Brazil: a retrospective cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo G., Cottenet J., Mariet A.S., Georges M., Piroth L., Tubert-Bitter P., et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. Eur Respir J. 2021 doi: 10.1183/13993003.04474-2020. Published online May 20, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Pérez E., González-Díaz B., Miranda-García M., Borja-Aburto V.H. Infection and death by COVID-19 in a cohort of healthcare workers in Mexico. Scand J Work Environ Health. 2021;47:349–355. doi: 10.5271/sjweh.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa F.G., Palazzo A., Rosso T., Shbaklo N., Mussa M., Boglione L., et al. Risk factors for mortality in COVID-19 hospitalized patients in Piedmont, Italy: results from the multicenter, regional, CORACLE registry. J Clin Med. 2021;10:1951. doi: 10.3390/jcm10091951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak S.J., Farrell J., Rostron A., Smith I., Openshaw P.J.M., Baillie J.K., et al. COVID-19 pneumothorax in the United Kingdom: a prospective observational study using the ISARIC WHO clinical characterisation protocol. Eur Respir J Published online September. 2021;16 doi: 10.1183/13993003.00929-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.D., Bravata D.M., Bent S., Wray C.M., Leonard S.J., Boscardin W.J., et al. Association of social and behavioral risk factors with mortality among US veterans with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Owens J., Franklin J., Mehta A., Heymann W., Sewell W., et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52:354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlström B., Frithiof R., Hultström M., Larsson I.M., Strandberg G., Lipcsey M. The swedish covid-19 intensive care cohort: Risk factors of ICU admission and ICU mortality. Acta Anaesthesiol Scand. 2021;65:525–533. doi: 10.1111/aas.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin J.L., Seixas A., Ramos Cejudo J., Osorio R.S., Avirappattu G., Reid M., et al. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron Respir Dis. 2021;18 doi: 10.1177/1479973120986806. 1479973120986806. [DOI] [PMC free article] [PubMed] [Google Scholar]