Abstract
Background & aims
COVID-19 is a severe viral infection of the respiratory tract and has become a worldwide pandemic. Months after the initial infection several people report persistent limitations in daily life. Previous studies have identified body composition as a predictor of clinical progression in cases of COVID-19. However, body impedance measurements were limited to baseline and not repeated in serial measurements. In this study we analyzed the impact of a moderate oxygen-dependent COVID-19 infection on body composition during hospitalization.
Methods
We enrolled 12 consecutive patients hospitalized due to an oxygen-dependent SARS-CoV-2 infection. Body impedance analysis was performed within 24 h of admission and repeated on day 3 ± 1 as well as on the day of discharge. Endpoints were any significant changes in body composition.
Results
Median age of enrolled patients was 70.6 years with a BMI of 30.8 kg/m2. Patients were hospitalized for 14 days. Median oxygen demand was 3 l/min, 2 patients required mechanical ventilation. Body water and fat remained unchanged during the study period. We observed a significant decrease of phase angle (−0.6, p < 0.01) and body cell mass (−2.3%, p < 0.01) with an increase in extracellular mass on day 3. Values returned to baseline along recovery.
Conclusion
We found a significant reduction in body cell mass and phase angle during the active infection with slow regression towards hospital discharge. Future studies are needed to clarify if nutrition and training programs during and after COVID-19 might limit these changes and have a positive impact on clinical course and rehabilitation.
Keywords: COVID-19, Body impedance analysis, Phase angle, Rehabilitation
1. Introduction
Coronavirus disease 2019 (COVID-19) is a potentially severe viral infection of the respiratory tract, which caused a worldwide pandemic in the last 18 months. Until today, we have recorded over 170 million infections and almost 4 million deaths with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Primary prevention by vaccinations is rapidly expanding with over 2 billion at least partially vaccinated people in June 2021 [1]. However, medical therapy to successfully treat COVID-19 is limited and current strategies for hospitalized patients are mainly based on a prophylaxis of thrombosis by heparin as well as dexamethasone for patients requiring oxygen. Post infection several patients report persistent limitations in daily activities with rehospitalization rates of 10–20% [2]. Immobility due to the infection itself as well as quarantine requirements challenge the organism and lead to changes in body physiology. As previous studies have already identified phase angle as a risk predictor for the clinical course, we aimed to investigate if COVID-19 is associated with a significant change in body composition during hospitalization in serial measurements [[3], [4], [5]]. As a future perspective, we hypothesize, that the identification of significant longitudinal changes in body composition might become a relevant factor in COVID-19 therapy as early counteractive measurements could improve clinical course and reduce long-persisting symptoms.
2. Methods
2.1. Patients
In this pilot study, we investigated the effect of COVID-19 on body composition for hospitalized patients between February 17th and April 26th 2021. We enrolled 12 consecutive patients with a PCR-confirmed oxygen-dependent SARS-CoV-2 infection (10 out of 12 patients with B.1.1.7 variant, 2 undetermined). Two patients required intensive care unit (ICU) treatment including mechanical ventilation and were excluded from follow-up body impedance analysis (BIA) measurements due to missing data. Baseline values (excluding BIA measurements and maximum oxygen demand) for these two patients remained in the analysis to reflect the full consecutive cohort. In compliance with the Declaration of Helsinki and German data protection laws, all patients in this analysis provided informed consent and the study was approved by the local ethics committee (LMU Munich, Germany).
2.2. Study definition and endpoints
All patients admitted to hospital due to an oxygen-dependent SARS-CoV-2 infection were eligible to participate in this trial. Patients suitable for outpatient treatment as well as those directly admitted to ICU were excluded. Study endpoints were any significant changes in body composition – i.e. body water, body fat, phase angle, body cell mass (BCM) or extracellular mass (ECM). Corrected body fat relies on a calculation that was designed to determine values of body fat which are less dependent on short-term alterations in body water (Data Input, Germany).
2.3. Data collection
Baseline data including laboratory values were collected as part of the routine diagnostic and treatment protocol. Body impedance analysis was performed according to the manufacturer's instructions with a Nutribox body impedance analyzer (Data Input, Germany) within 24 h of admission to our COVID-19 ward and repeated on day 3 ± 1 as well as on the day of discharge. The Nutribox measurement is based on the bioelectrical impedance vector analysis. Three parameters determine body composition: resistance (R) as a measure of body water, reactance (Xc) as a measure for body cell mass, i.e. cell membranes, and finally the corresponding phase angle (PhA) as a result of electrical phase shift of alternating current. These parameters allow the best possible calculation of all values of body composition.
2.4. Statistical analysis
Statistical analysis was performed using Prism 9 software (GraphPad, USA). Normally distributed continuous variables were reported as median and interquartile range (IQR). T-test was used for group comparisons of normally distributed variables. All tests were 2-tailed and paired. P values < 0.05 were considered as statistically significant. In this pilot study we included all patients matching the inclusion criteria. No prior sample size calculation was performed.
3. Results
3.1. Baseline characteristics and clinical course
We enrolled 12 consecutive patients admitted to our COVID-19 ward between February 17th and April 26th 2021. Median age was 71 years and two third of our patients were male. Body mass index was elevated with 30.8 mg/m2 which represents a relevant risk factor for a symptomatic COVID-19 infections in preceding studies [6,7]. SARS-CoV-2 α variant – also known as variant of concern B.1.1.7 - was identified in 10 out of 12 cases (2 were undetermined) with an average viral load of 60 million copies per ml. Median duration of symptoms before admission was 4.5 days. Half of our patients had a past medical history of hypertension, one quarter to one third had coronary artery disease, chronic kidney disease or diabetes mellitus (Table 1 ).
Table 1.
Baseline characteristics.
| n = 12 | |
|---|---|
| Clinical parameters | |
| Age (years) | 70.6 (49.5; 72.9) |
| Male | 66.7% (8) |
| Body mass index (kg/m2) | 30.8 (26.1; 33.5) |
| Symptoms before admission (days) | 4.5 (2.3; 6.0) |
| Duration of hospitalization (days) | 13.5 (8.3; 27.8) |
| ICU treatment | 16.7% (2) |
| Maximum oxygen demand (l/min) | 3 (2; 4) |
| Average viral load (copies/ml) | ~60∗106 (B.1.1.7 Sars-CoV2 variant) |
| Medical history | |
| Coronary artery disease | 33.3% (4) |
| Hypertension | 50% (6) |
| Pulmonary disease | 8.3% (1) |
| Chronic kidney disease | 25% (3) |
| Dialysis | 16.7% (2) |
| Liver disease | 8.3% (1) |
| History of thromboembolism (DVT, PE) | 16.7% (2) |
| Diabetes mellitus | 25% (3) |
| Laboratory values (max.) | |
| CRP (mg/dl) | 11.5 (6.4; 14.9) |
| IL6 (pg/ml) | 92.0 (34.5; 133.0) |
| PCT (ng/ml) | 0.2 (0.1; 0.6) |
| Ferritin (ng/ml) | 1940 (1000; 2310) |
| Creatinine (mg/dl) | 1.0 (0.9; 1.4) |
| BUN (mg/dl) | 42.5 (40.0; 61.3) |
| Albumin (g/dl) | 4.1 (3.8; 4.3) |
All values are depicted as median and interquartile range (IQR). ICU, intensive care unit. DVT, deep vein thrombosis. PE, pulmonary embolism. CRP, C-reactive protein. IL6, interleukin 6. PCT, procalcitonin. BUN, blood urea nitrogen.
Patients were hospitalized for about two weeks until discharge. The oxygen demand was low to moderate with maximum rates of 3 l/min. According to the guideline recommendation all patients received high dose steroid therapy (6 mg dexamethasone for 10 days or until discharge, whichever is shorter) and prophylaxis of thrombosis if anticoagulant therapy was not indicated otherwise. 10 patients received antibiotic therapy due to bacterial superinfection. 2 out of 12 patients required mechanical ventilation and were transferred to ICU. Laboratory values showed moderately elevated infection markers as expected (Table 1).
3.2. Body water and body fat remain stable during hospitalization
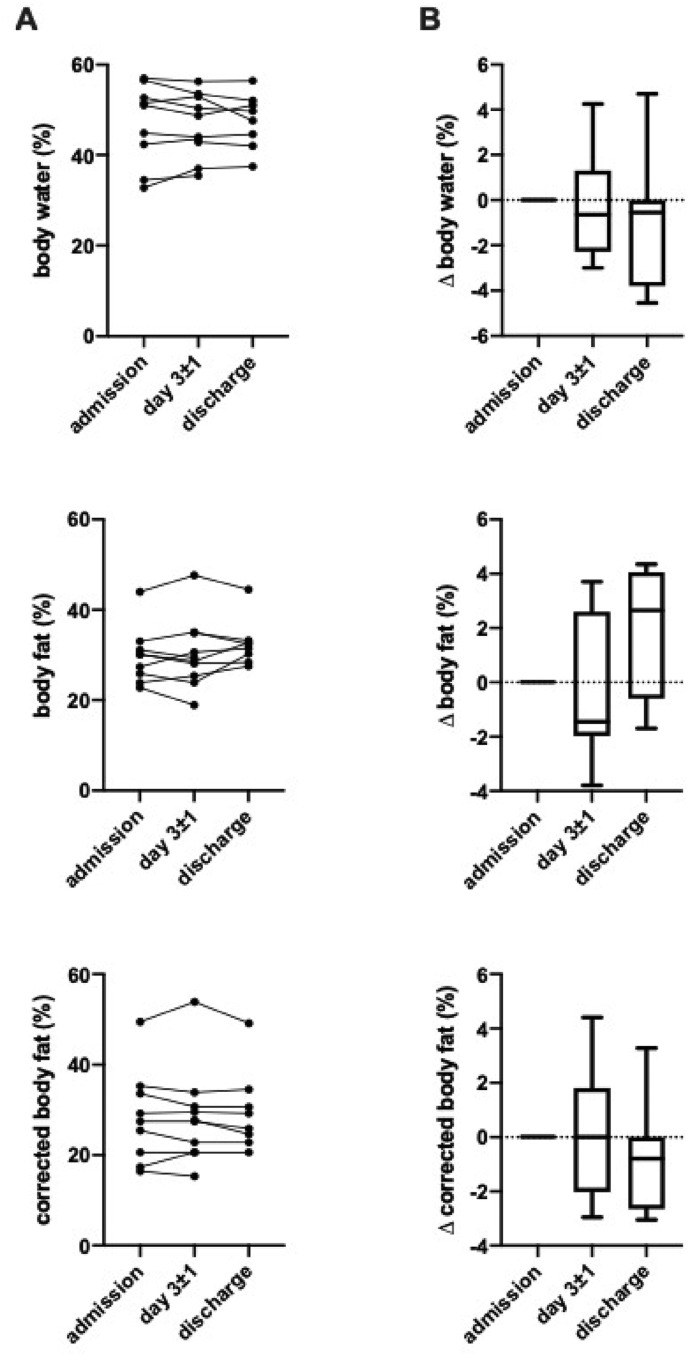
Total body water was average to low with a median value of 51.0% (IQR 38,5%; 54.6%) and did not change during the course of hospitalization. Baseline body fat was slightly elevated in COVID-19 patients with 30.1% (IQR 24.9%; 32.1%). Again, we observed no significant changes during the course of infection (Fig. 1 ).
Fig. 1.
Body water and fat during hospitalization. Measures of body water, body fat and corrected body fat for indicated time points presented (A) in absolute numbers and (B) absolute change after admission. All differences between time points are non-significant (n = 10).
3.3. COVID-19 is associated with significant changes in extracellular and body cell mass
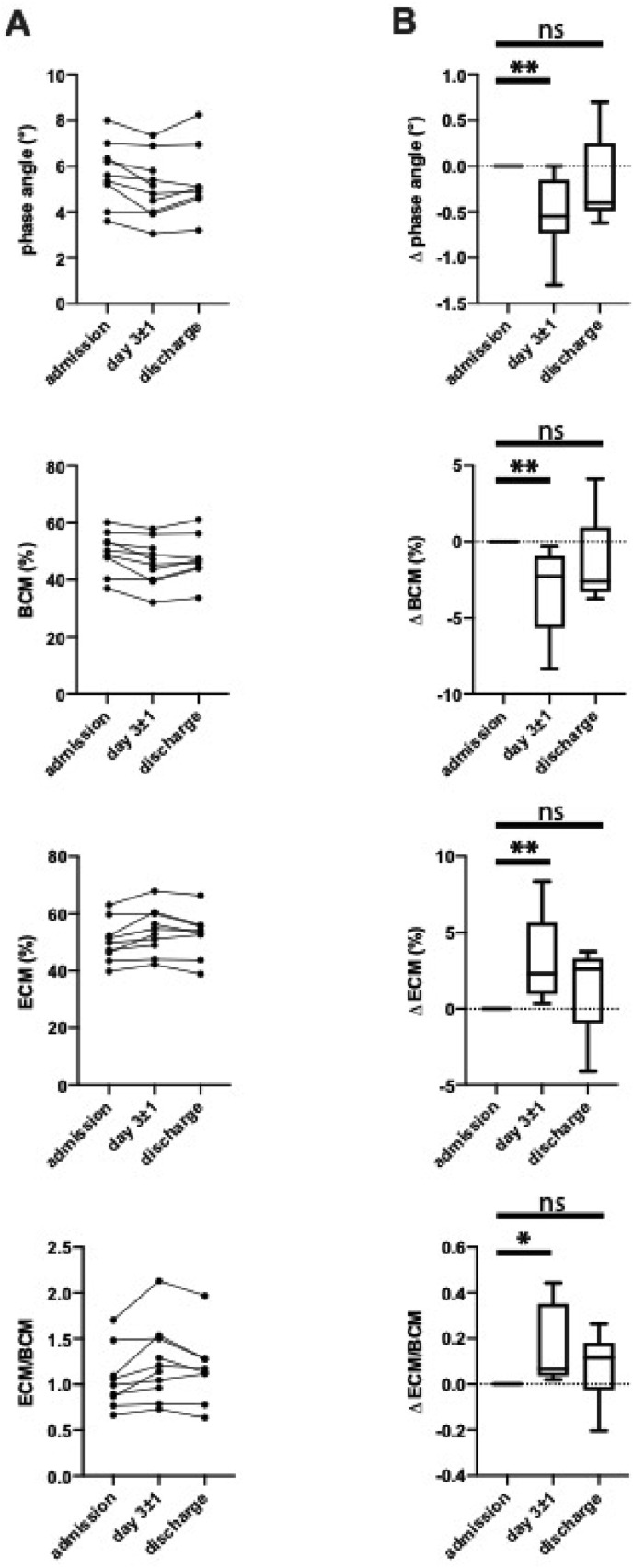
Phase-sensitive modern BIA devices determine phase angle (PhA), extracellular mass (ECM) and body cell mass (BCM) as measures of physical fitness and healthy body composition. While PhA–as the ratio of electrical resistance (intra- and extracellular body water) to reactance (cell membranes) – is the direct technical correlate of the measurement process and relatively independent of the hydration status, BCM reflects metabolic active cells, i.e. muscle tissue. Vice versa, ECM reflects metabolic inactive tissue such as connective tissue and bones. Therefore, a higher PhA and an ECM/BCM ratio lower than 1 is associated with a good physical condition.
In patients hospitalized for COVID-19 we found baseline values of 50.2% (IQR 44.1; 55.1%) for BCM and 49.8% (IQR 44.9%; 56.0%) for ECM, resulting in an ECM/BCM ratio of 0.99 (IQR 0.82; 1.29). Interestingly, there was a significant loss of 2.3% in BCM with a corresponding relative increase in ECM on day 3 of hospitalization. PhA was 5.6 on admission and decreased by 0.6. This trend was reversed during the following process of recovery and reached normal values at hospital discharge, approximately 2 weeks after admission (Fig. 2).
Fig. 2.
Alterations in body composition. Depicted are measures for phase angle as well as body cell mass (BCM) and extracellular mass (ECM) for indicated time points (A) in absolute numbers and (B) absolute change after admission (∗) p < 0.05, (∗∗) p < 0.01, (∗∗∗) p < 0.001, (ns) non-significant (n = 10).
4. Discussion
In this study we analyzed body composition during hospitalization in patients with moderate oxygen-dependent COVID-19. We identified a significant decrease in BCM and PhA during the early phase of active infection with a slow subsequent regeneration to baseline parameters towards hospital discharge (Fig. 2). Of note, body water and body fat were not affected during this short-term observation (Fig. 1). In accordance with proceeding studies baseline BMI and body fat were slightly increased in COVID-19 patients [6,7].
COVID-19 is known to cause a rapid progression of clinical symptoms, even in patients without significant comorbidities. Moreover, the process of regeneration is slow, and several patients report persistent symptoms weeks and months after the infection. Previous studies on body composition in the intensive care unit setting highlighted the difficulty in short-term longitudinal BIA measurements due to its dependence on the patient's hydration status and therefore used the PhA as the most robust parameter and mortality risk predictor [8,9]. PhA is known to decrease with age and is greater in men then in women [10]. Although modern phase-sensitive impedance vector-based BIA devices, as used in this study, were designed to at least partially overcome this limitation, we cannot exclude an influence of the intracellular hydration status on our results. Yet, we did not observe any significant changes in body water during our observational period in impedance vector-based calculations (Fig. 1 ).
We are aware of the fact that this pilot study is limited by its small population size and therefore our conclusions need to be validated in larger upcoming trials. As we included all patients admitted to our non-ICU COVID-19 ward in the study period consecutively, no prior sample size calculation was performed. The rationale of our study was to statistically evaluate a subjective perception during routine diagnostics, that COVID-19 leads to significant changes in body composition. Of note, similar trends have been described for other cohorts, including patients with sepsis. However, COVID-19 is a largely unknown and challenging disease which demands special attention and independent analysis as this might by a key factor for optimized treatment strategies in the future.
5. Conclusion
Our present study shows analogies to the clinical course of COVID-19 as BCM, i.e. metabolic active muscle tissue and the intracellular hydration status, was altered during the early active phase of the disease and only regained slowly towards hospital discharge. Beyond our observations in the hospital setting, these effects may also apply to milder COVID-19 infections which are usually treated in the outpatient setting, where additional quarantine requirements limit the rehabilitation process.
We suggested to monitor changes in body composition in these patients and start early structured physical training and nutrition programs to allow best possible rehabilitation. The effects of these programs should be systematically analyzed in larger following studies to counter severe courses of COVID-19, especially in patients with long-persisting symptoms.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Antonia Kellnar: Methodology, validation and writing – original draft. John M. Hoppe: Investigation, validation and data curation. Stefan Brunner: Conceptualization and supervision. Christopher Stremmel: Conceptualization, methodology, formal analysis, writing – reviewing and editing.
Declaration of competing interest
None of the authors report a conflict of interest related to the study.
References
- 1.WHO. World Health Organisation - COVID-19 https://covid19.who.int2021
- 2.Bowles K.H., McDonald M., Barron Y., Kennedy E., O'Connor M., Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med. 2021;174:316–325. doi: 10.7326/M20-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Giorno R., Quarenghi M., Stefanelli K., Capelli S., Giagulli A., Quarleri L. Nutritional risk screening and body composition in COVID-19 patients hospitalized in an internal medicine ward. Int J Gen Med. 2020;13:1643–1651. doi: 10.2147/IJGM.S286484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moonen H., van Zanten F.J.L., Driessen L., de Smet V., Slingerland-Boot R., Mensink M. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: the BIAC-19 study. Clin Nutr. 2021;40:2328–2336. doi: 10.1016/j.clnu.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornejo-Pareja I., Vegas-Aguilar I.M., Garcia-Almeida J.M., Bellido-Guerrero D., Talluri A., Lukaski H. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin Nutr. 2021 Feb 17 doi: 10.1016/j.clnu.2021.02.017. S0261-5614(21)00091-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 Years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe Acute respiratory Syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thibault R., Makhlouf A.M., Mulliez A., Cristina Gonzalez M., Kekstas G., Kozjek N.R. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: the international prospective observational study Phase Angle Project. Intensive Care Med. 2016;42:1445–1453. doi: 10.1007/s00134-016-4468-3. [DOI] [PubMed] [Google Scholar]
- 9.Looijaard W., Molinger J., Weijs P.J.M. Measuring and monitoring lean body mass in critical illness. Curr Opin Crit Care. 2018;24:241–247. doi: 10.1097/MCC.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbosa-Silva M.C., Barros A.J., Wang J., Heymsfield S.B., Pierson R.N., Jr. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]