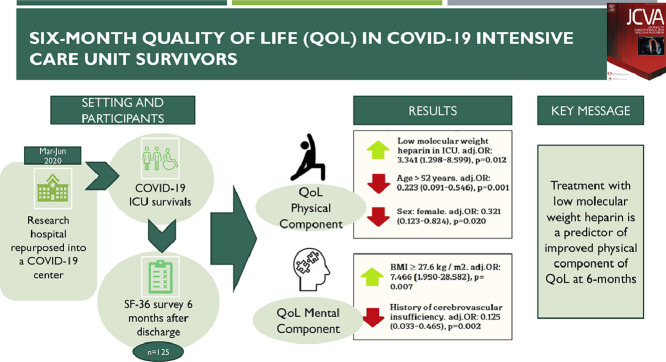
Abstract
Objectives
Because there is increasing evidence of serious deterioration in long-term quality of life (QoL) in coronavirus 2019 (COVID-19) intensive care unit (ICU) survivors, the authors identified predictors of poor quality of life in these patients.
Design
Prospective cohort study.
Setting
Research hospital repurposed into a COVID-19 center.
Participants
Consecutive patients admitted in COVID-19 ICUs between March and June 2020.
Interventions
An SF-36 questionnaire, which included physical and mental items, was used six months after patient's discharge.
Measurements and Main Results
A total of 403 patients were managed in the ICU, with a hospital mortality of 181 of 403 (44.9%), and 16 (4.0%) patients died within six months. Among the 125 questionnaire responders, only 32.0% and 52% had a normal quality of life in terms of the physical and mental component of health. Multivariate analysis identified low-molecular-weight heparin treatment in the ICU as the only modifiable factor associated with an increase in physical component of QoL odds ratio (OR) 3.341 (95% confidence interval 1.298-8.599), p = 0.012, and age ≥52 years OR 0.223 and female sex OR 0.321 were significantly associated with a decrease in the physical component. Medical history of cerebrovascular insufficiency was significantly associated with a decrease in mental component of QoL OR 0.125, and the only factor associated with an increase in the mental health component was body mass index ≥27.6 kg/m2 OR 7.466.
Conclusions
In COVID-19 ICU survivors the authors identified treatment with low- molecular-weight heparin as a predictor of improved physical component of QoL at 6 months.
Key Words: COVID-19, quality life, SARS-CoV-2, low molecular weight heparin, critical care, Intensive Care, mortality, quality of life
Graphical abstract
LONG-TERM consequences of coronavirus 2019 (COVID-19) still are seldom reported1, 2, 3, 4, 5, 6 and include fatigue and muscle weakness,3 , 6 dyspnea with minimal exertion,4 , 5 sleep disturbance, anxiety, and depression.1 Sonnweber et al. reported that 100 days after discharge a relevant percentage of COVID-19 patients had persisting symptoms and lung function impairment, along with pulmonary abnormalities.7 Therefore, emerging evidence suggests a persistent and serious deterioration in quality of life (QoL) of COVID-19 survivors and this represents a serious medical and social problem.
Long-term full recovery after ICU discharge has been an issue of great interest for intensive care specialists in the last decade; causes, diagnoses, and treatment of postintensive care syndrome (PICS) have been widely discussed.8 PICS describes a variety of health disorders occurring often among patients who survive critical illness and intensive care.9 It generally is accepted that patients with PICS require long-term medical and social rehabilitation.10 PICS patients showed a significantly worse QoL, along with higher risk of sudden death, compared with the general population.11 However, there still are few data regarding the features of PICS in COVID-19 survivors. Furthermore, predictors of QoL are poorly investigated.
In this study, the authors present a large case series of COVID-19 ICU survivors. The authors’ aim was to assess QoL of these patients at a six-month follow-up. Furthermore, the authors tried to identify modifiable risk factors and outcome predictors.
Methods
Study Design and Participants
Consecutive COVID-19 patients admitted into the ICUs of a single center between March and June 2020 were enrolled with approval of the Ethics Committee (No. 2/21/1 on 02.16.2021). All patients signed a written consent before answering the questionnaire. Exclusion criteria were cardiac arrest before or at the moment of ICU admission (n = 13), transferred to another hospital irrespective of the reason (n = 88), and preliminary diagnosis of COVID-19 not confirmed after additional examination (n = 66).
The primary endpoint of the study was QoL six months after hospital discharge. The authors estimated QoL through the SF-36 questionnaire, which includes items about physical and mental health.
Criteria for ICU admission are specified in the Supplement (Supplemental Table 1). Treatment in the ICU is detailed in the Supplement (Supplemental Table 2). Prescription of low-molecular-weight heparin (LMWH) or unfractionated heparin was made at the discretion of the attending physician. Collected data (Supplemental Table 3) consisted of demographic characteristics (age, sex, body mass index [BMI]), comorbidities according to Charlson comorbidity index12 and detailed in the Supplement, PaO2/FIO2 levels and laboratory parameters at time of ICU admission, patient's condition at admission (national early warning score13) and Sequential Organ Failure Assessment (SOFA) score. Also, days on ventilator, days in the ICU, and hospital stay were considered. Computed tomography was evaluated by the same team of radiologists using the same criteria—each of the five lung lobes was scored visually for the degree of lung involvement, using a pointscale: no involvement; 1%-to-25% involvement; 26%-to-49% involvement; 50%-to- 75% involvement; and 76%-to-100% involvement.
The six-month follow-up period started at hospital discharge. Experienced staff made up to three attempts to contact the patients through the phone. If the patient agreed to fill in the survey (available in the Supplement), the login and password to the unique record of the questionnaire were sent to the participant. Moreover, the authors attached the consent form to the email, asking kindly to fill it in and send it back.
The analysis of QoL was carried out through SF-36 Health Status Survey. SF-36 refers to nonspecific questionnaires designed to measure health and QoL at the individual level in clinical practice and is used widely in the United States and European countries. It is composed of 11 questions and 36 items that cover eight domains of health (Supplemental material). Health domains (multiple-item subscales) evaluate physical function (limitations in physical activities because of health problems), social functioning (limitations in social activities because of physical or emotional problems), role limitations due to physical problems, role limitations due to emotional problems, mental health, bodily pain, vitality (energy and fatigue), and general health perception (psychologic distress and well-being). The overall score on each SF-36 subscale ranges from 0 to 100, and a higher score indicates a better QoL. Scores for the different subscales were converted and pooled using a scoring key (Z-scores) and then two total measurements were calculated by combining the subscale scores—physical and mental components of QoL.14 A reference level of 50 points was adopted for physical and mental health components, as calculated for the United States population15 and validated in Australia, France, and Italy. SF-36 uses norm-based scoring algorithms for all eight subscales (T-score transformation with mean, 50 ± 10 [SD]). Subsequently, patients were divided into two groups based on physical and mental components as follows: (1) fewer than 50 points (decreased QoL), and (2) 50 or more points (normal QoL).
Statistical Analysis
The Shapiro-Wilk test was used to assess the normality of data. Descriptive statistics were used to describe the sample; continuous variables were described using mean and standard deviation (SD). For nonnormal distributions, such as medians and interquartile ranges (IQR), categorical variables were described using frequency and percentages.
Group differences (normal versus reduced QoL) were explored using Mann-Whitney U test for continuous variables (with the values of the statistics of the criterion U and Z). Categorical variables were analyzed using Fisher's exact test. The Spearman rank correlation coefficient was used as a hypothesis test to study the relationship between variables. The risk of outcome in univariate analysis was assessed using the odds ratio (OR) and its 95% confidence interval (CI) for each predictor studied. In multivariate analysis, adjusted OR was calculated using binary logistic regression in order to take into account the influence of confounders (eg, age, gender, comorbidity, and ongoing therapy). Predictors were included in the regression model based on the forward stepwise (Wald) method. To assess the quality of various quantitative indicators as predictors of QoL, the authors used the receiver operating characteristic analysis with the assessment of Area Under the Curve (AUC) parameter and its 95% CI. The cut-off value was chosen in order to achieve the optimal sensitivity/specificity ratio according to the results of the receiver operating characteristic analysis (Youden's J statistic). All analyses were carried out using SPSS Software version 25 and MedCalc Statistical Software version 19.5.6. The significance level was set at 0.05.
Results
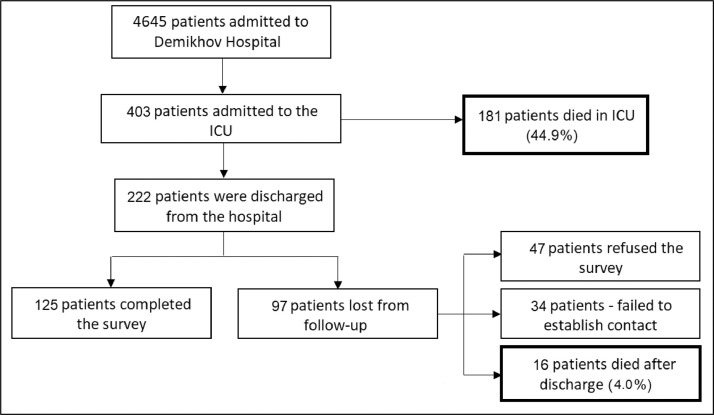
Out of 4,565 patients admitted to the hospital during the first pandemic wave, 403 were transferred to the ICU. Mean age of ICU patients was 62 ± 15.3 (range 21-97 y) and 231 of 403 (57.3%) were men. In-hospital mortality rate of ICU patients was 44.9% (181/403 patients died). Further 16 of 403 patients (4.0%) died after hospital discharge (Fig 1 ).
Fig 1.
Flow chart of the study. ICU, intensive care unit.
Out of the 125 survivors who signed the informed consent (74 men, 59.2%), 40 (32.0%) had a normal QoL in terms of physical health component, with differences between gender as follows: 32/74 (43.2%) men versus eight/51 (15.7%) women (p = 0.002); 65 patients (52.0%) had a normal QoL in terms of mental health component, with no differences between gender: 38/74 (51.4%) men versus 27/51 (52.9%) women (p = 0.9).
Overall median SF-36 physical QoL level was 43.7 (IQR: 31.7-52.7), with significant differences between men 48.3 (IQR: 36.6-53.7) and women 34.8 (IQR: 28.5-47.2), p < 0.001. No differences in mental SF-36 QoL values were found in terms of gender; men had 50.3 (IQR: 42.4-55.2), and women had 52.7 (IQR: 39.9-56.6), p = 0.7.
Comorbidities, patient characteristics, prescribed therapy, and their association with six-month QoL are showed in Table 1 and Table 2 . Patients were comparable in terms of lung involvement at computed tomography and hospital stay before transfer to the ICU (Table 2); frequency of antibiotic prescription, PaO2/FIO2 levels, and laboratory parameters at the time of admission to the ICU did not differ significantly in patients with normal and decreased QoL (Supplemental Table 3).
Table 1.
Frequency of Comorbid Conditions in Patients With Normal/Reduced Physical and Mental Components
| Comorbid Condition | Patients With a Normal Physical Component n = 40 |
Patients With a Reduced Physical Component n = 85 |
p Value | Patients With a Normal Mental Component n = 65 |
Patients With a Reduced Mental Component n = 60 |
p Value |
|---|---|---|---|---|---|---|
| Chronic obstructive pulmonary disease | 3 (7.5%) | 12 (14.1%) | 0.4 | 4 (6.2%) | 11 (18.3%) | 0.053 |
| Cerebrovascular insufficiency | 8 (20.0%) | 30 (35.3%) | 0.1 | 13 (20.0%) | 25 (41.7%) | 0.01 |
| Peripheral arterial disease | 22 (55.0%) | 60 (70.6%) | 0.1 | 43 (66.2%) | 39 (65.0%) | 0.9 |
| Diabetes | 6 (15.0%) | 20 (23.5%) | 0.3 | 12 (18.5%) | 14 (23.3%) | 0.5 |
| Arterial hypertension | 18 (45.0%) | 53 (62.4%) | 0.8 | 37 (56.9%) | 34 (56.7%) | 0.9 |
| Chronic kidney disease | 5 (12.5%) | 19 (22.4%) | 0.2 | 12 (18.5%) | 12 (20.0%) | 0.9 |
| Myocardial infarction | 4 (10.0%) | 19 (22.4%) | 0.1 | 10 (15.4%) | 14 (23.3%) | 0.4 |
| Congestive heart failure | 1 (2.5%) | 4 (4.7%) | 0.9 | 2 (3.1%) | 3 (5.0%) | 0.7 |
| Liver failure | 0 (0.0%) | 3 (3.5%) | 0.6 | 1 (1.5%) | 2 (3.3%) | 0.6 |
| Diabetic organ damage | 6 (15.0%) | 16 (18.8%) | 0.8 | 10 (15.4%) | 12 (20.0%) | 0.6 |
| Peptic ulcer | 2 (5.0%) | 5 (5.9%) | 0.9 | 5 (7.7%) | 2 (3.3%) | 0.4 |
| Hemiplegia | 0 (0.0%) | 4 (4.7%) | 0.3 | 0 (0.0%) | 4 (6.7%) | 0.051 |
| Malignant neoplasms | 1 (2.5%) | 7 (8.2%) | 0.4 | 2 (3.1%) | 3 (5.0%) | 0.7 |
| Charlson comorbidity index | 1 (IQR: 0-3) | 2 (IQR: 1-5) | 0.019 | 2 (IQR: 1-4) | 2 (IQR: 1-5) | 0.5 |
Abbreviations: IQR, interquartile range.
Table 2.
Descriptive Statistics: Patient Characteristics and Prescribed Therapy in Patients With Normal/Reduced Physical and Mental Components
| Parameter | Patients With a Normal Physical Component n = 40 |
Patients With a Reduced Physical Component n = 85 |
p Value | Patients With a Normal Mental Component n = 65 |
Patients With a Reduced Mental Component n = 60 |
p Value |
|---|---|---|---|---|---|---|
| Medical and demographic parameters | ||||||
| Sex, female | 8 (20.0%) | 43 (50.6%) | 0.002 | 27 (41.5%) | 24 (40.0%) | 0.9 |
| Age, y | 50.0 (IQR: 40.3-58.0) | 58.0 (IQR: 51.0-65.5) | <0.001 | 54.0 (IQR: 48.5-62.0) | 57.0 (IQR: 48.0-66.0) | 0.2 |
| Body mass index, kg/m2 | 28.1 (IQR: 25.1-31.8) | 28.0 (IQR: 25.2-36.8) | 0.8 | 29.4 (IQR: 27.0-37.6) | 26.0 (IQR: 24.5-32.5) | 0.013 |
| Mechanical ventilation | 2 (5.0%) | 5 (5.9%) | 0.9 | 3 (4.6%) | 4 (6.7%) | 0.7 |
| Characteristics of the severity of the condition on admission | ||||||
| SOFA, score | 1.0 (IQR: 1.0-2.0) | 1.0 (IQR: 1.0-2.0) | 0.9 | 1.0 (IQR: 1.0-2.0) | 1.5 (IQR: 1.0-2.0) | 0.6 |
| NEWS, score | 7.0 (IQR: 5.0-8.0) | 7.0 (IQR: 5.0-8.0) | 0.7 | 7.0 (IQR: 5.0-9.0) | 7 (IQR: 5.0-8.0) | 0.4 |
| CT, no involvement | 3 (7.5%) | 11 (12.9%) | 0.5 | 5 (7.7%) | 9 (15.0%) | 0.3 |
| CT, 1-25% involvement | 3 (7.5%) | 5 (5.9%) | 0.7 | 6 (9.2%) | 2 (3.3%) | 0.3 |
| CT, 26-49% involvement | 11 (27.5%) | 20 (23.5%) | 0.7 | 18 (27.7%) | 13 (21.7%) | 0.5 |
| CT, 50-75% involvement | 19 (47.5%) | 40 (47.1%) | 0.9 | 30 (46.2%) | 29 (48.3%) | 0.9 |
| CT, 76-100% involvement | 4 (10.0%) | 9 (10.6%) | 0.9 | 6 (9.2%) | 7 (11.7%) | 0.8 |
| Length of stay, d | ||||||
| Intensive care unit stay | 4.5 (IQR: 3.0-7.0) | 5.0 (IQR: 3.0-7.0) | 0.9 | 5.0 (IQR: 3.0-7.0) | 4.0 (IQR: 3.0-7.0) | 0.7 |
| Hospital stay | 16.0 (IQR: 12.3-18.0) | 16.0 (IQR: 12.0-22.5) | 0.7 | 16.0 (IQR: 13.0-19.5) | 17.0 (IQR: 12.0-22.8) | 0.7 |
| Prescribed therapy | ||||||
| Enoxaparin | 29 (72.5%) | 44 (51.8%) | 0.03 | 42 (64.6%) | 31 (51.7%) | 0.2 |
| Unfractionated Heparin | 9 (22.5%) | 27 (31.8%) | 0.4 | 20 (30.8%) | 16 (26.7%) | 0.7 |
| Tocilizumab | 17 (42.5%) | 31 (36.5%) | 0.6 | 26 (40.0%) | 22 (36.7%) | 0.7 |
| Hydroxychloroquine | 28 (70.0%) | 56 (65.9%) | 0.7 | 50 (76.9%) | 34 (56.7%) | 0.022 |
| Antiviral therapy | 14 (35.0%) | 27 (31.8%) | 0.8 | 23 (35.4%) | 18 (30.0%) | 0.6 |
Abbreviations: CT, computed tomography; IQR, interquartile range; NEWS, National Early Warning Score; SOFA, Sequential Organ Failure Assessment.
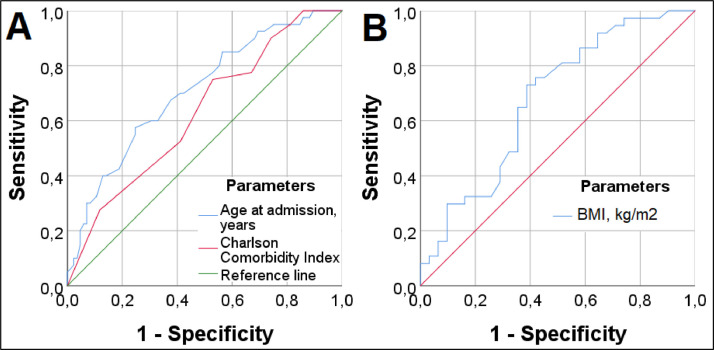
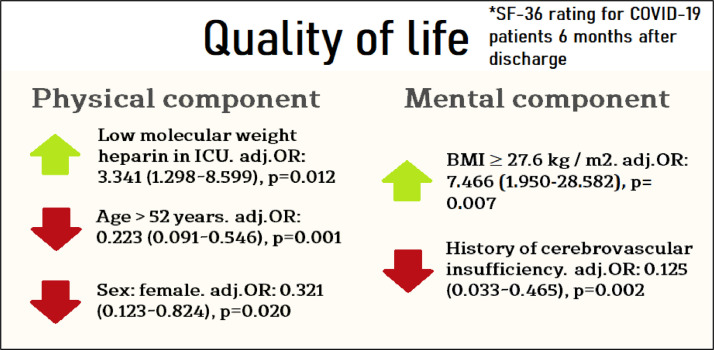
Univariate predictors of low physical component of health were female sex, old age, Charlson morbidity index, and absence of treatment with enoxaparin. Age (AUC: 0.707, 95% CI 0.611-0.803, p < 0.001) had the best results (in terms of predicting value) among quantitative predictors of patients' physical health. The optimal cut-off point was 52 years (OR: 0.266, 95% CI 0.118-0.596; sensitivity-57.5%, specificity-75.3%); patients older than 52 years had a 3.8-fold lower chance of a normal physical component of QoL six months after COVID-19 than the younger ones (Fig 2 , A). The Charlson comorbidity index was a weak predictor of the physical component of health (AUC: 0.628, 95% CI 0.526-0.730, p = 0.021) (Fig 2, A). A multivariate analysis showed that treatment with LMWH in the ICU (adjusted OR: 3.341 [95% CI 1.298-8.599], p = 0.012), but not with unfractionated heparin, was the only significant factor associated with increased odds of a normal QoL in terms of the physical health component. Age ≥52 years (adjusted OR: 0.223 [95% CI 0.091-0.546], p = 0.001) and female sex (adjusted OR: 0.321 (95% CI 0.123-0.824), p = 0.020) were significantly associated with a decrease in the physical health component (Fig 3 ). To understand how LMWH could increase the percentage of patients with normal physical QoL, the authors analyzed the relationship between LMWH treatment and development of thrombotic complications (p = 0.9), Acute Respiratory Distress Syndrome (ARDS) (p = 0.1), and stroke (p = 0.3). The authors found a significant association (p = 0.047) only when assessing the composite outcome ARDS and/or stroke (36.1% v 45.9%).
Fig 2.
Receiver operating characteristic analysis: quantitative predictors of the physical (A) and mental (B) components of QoL. BMI, body mass index; QoL; quality of life.
Fig 3.
Predictors of physical and mental components of COVID-19 survivors quality of life at a multivariate analysis. BMI, body mass index; COVID-19, coronavirus 2019; ICU, intensive care unit; OR, odds ratio.
Univariate predictors of reduced mental health were cerebrovascular insufficiency, low BMI (AUC: 0.676, 95% CI 0.545-0.806, p = 0.013) (Fig 2, B), and treatment with hydroxychloroquine. A multivariate analysis showed that the only predictor associated with increased odds of a normal QoL in terms of the mental health component was a BMI ≥27.6 kg/m2 (adjusted OR: 7.466 [95% CI 1.950-28.582], p = 0.007), and cerebrovascular insufficiency at baseline (adjusted OR: 0.125 [95% CI 0.033-0.465], p = 0.002) was the only factor associated with a decreased mental health component QoL (Fig 3). The negative effect of hydroxychloroquine treatment was not confirmed by the multivariate analysis, probably because patients receiving hydroxychloroquine had significantly less frequent cerebrovascular insufficiency according to medical records (19/84, 22.6%) compared with patients who did not receive this treatment (19/41, 46.3%), p = 0.012. To better understand the counterintuitive finding on BMI ≥27.6 kg/m2 being associated with normal QoL in terms of the mental health component, the authors explored mortality in obese and nonobese patients (49.0% v 45.9%, p = 0.7) and ARDS in obese and nonobese patients (41.8% v 26.3%, p = 0.016).
When ventilated patients were excluded from the regression analysis, independent predictors did not change, with OR values and CI insignificantly changing. For the physical component, the modified predictors were age ≥52 years (adjusted OR: 0.286 [95% CI 0.117-0.703], p = 0.006), female sex (adjusted OR: 0.271 [95% CI 0.101-0.725], p = 0.009), and LMWH (adjusted OR: 3.343 [95% CI 1.282-8.718], p = 0.014). For the mental component the modified predictors were BMI ≥27.6 kg/m2 (adjusted OR: 3.897 [95% CI 1.253-12.122], p = 0.019) and history of cerebrovascular insufficiency (adjusted OR: 0.175 [95% CI 0.053-0.584], p = 0.005). Explorative correlation analysis revealed a significant positive weak relationship of the degree of lung involvement at computed tomography and BMI (r = 0.2, p = 0.002), length of stay in the ICU (r = 0.2, p < 0.001), and the C-reactive protein level at the time of the ICU admission (r = 0.2, p = 0.001).
Discussion
The majority (68%) of COVID-19 survivors had serious problems in terms of the physical component of health during the six-month follow-up according to SF-36 assessment. The authors identified LMWH treatment in the ICU as a strong and modifiable predictor of a better physical component of the QoL. Furthermore, 48% of patients reported serious problems in terms of the mental health component.
The authors’ findings were worse than those reported in patients who outlasted septic shock. Hammond et al. reported 15%-to-30% of patients with moderate-to-severe problems in QoL six months after discharge.16
In the authors’ study, six months after discharge approximately half of the patients reported problems in the mental health component of QoL. These features represent a serious challenge for patients, their families, and for the social system. Similarly, Prescott et al. recently reported a relatively high prevalence of mental health problems in patients with sepsis 90 days after discharge, including anxiety (32% of survivors), depression (29%), or posttraumatic stress disorder (44%).17
In this study, sex and age appeared to be the most significant predictors of QoL in terms of the physical component health—patients older than 52 had 4.5 times lower chances of a normal QoL than younger patients (adjusted OR: 0.223 [95% CI 0.091-0.546], p = 0.001) and female sex was reported with three times decreased odds (adjusted OR: 0.321 [95% CI 0.123-0.824], p = 0.020). The negative impact of advanced age on short-term clinical outcomes is intuitive and was demonstrated in previous studies,18 , 19 including a recent study on six-months' QoL that was performed with the SF-36 scale in patients with COVID-19.20 The authors set the age cutoff at >52 years, which was significantly lower than the one usually found in the literature (>65),21 , 22 and this can be attributed to the specific Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus characteristics and to the impact of environmental factors in different countries.23 The surprising findings of female sex being a predictor of poor outcome in terms of the physical component of QoL might be, at least in part, a consequence of the drawbacks of the SF-36 scale itself. Some aspects related to physical activity are a priori easier to perform for men than for women, and this could lead to an underestimation of the physical component of QoL in women. Furthermore, previous studies that identified female sex as a predictor of positive outcomes24 , 25 considered short-term outcomes and not six-months' follow-up. Further studies are needed to clarify and confirm this important issue.
Furthermore, treatment with LMWH in the ICU was an independent and modifiable predictor of improvement in the physical component of QoL six months after hospital discharge. This was in line with existing evidence that severe SARS-Cov-2 pneumonia is a consequence of systemic inflammatory response and microvascular pulmonary thrombosis. Therefore, anticoagulant therapy is confirmed to be an essential and mandatory component of COVID-19 treatment.26, 27, 28 Surprisingly, the authors failed to confirm a similar beneficial effect for unfractionated heparin. This might be explained by the pleiotropic pharmacologic effects of LMWH29 or by the use of a relatively low-dose unfractionated heparin. The authors’ findings were in agreement with Sholzberg et al., who recently found a better survival associated with high-dose heparin in moderately ill patients with COVID-19.30 Furthermore, even if the authors used a multivariate model to minimize the effect of confounders, they cannot exclude that the small sample size and the severity of the patient's condition at admission (SOFA, National Early Warning Score (NEWS) scores, as well as gender, age, and comorbidity), confounded the findings (eg, prescription of unfractionated heparin to the most severe patients). This also could be explained by a selection bias induced by the clinician who might be more tempted to use LMWH in patients who do well compared with others who might need an invasive procedure for example, due to their worse clinical status. All other medications, such as tocilizumab, hydroxychloroquine, and antivirals (lopinavir–ritonavir and oseltamivir), showed no benefits in terms of the physical component of QoL in the authors’ cohort.
In contrast with reported evidence linking obesity with poor mental QoL outcomes in COVID-19 survivors, the authors’ data suggested that a BMI ≥27.6 kg/m2 might be a predictor of good outcome in the mental health component of QoL (adjusted OR 7.466 [95% CI 1.950-28.582], p = 0.007).31 Although counterintuitive, the authors’ findings were not in contrast with previous literature on the relationship between the mental health component of QoL and BMI. Chen et al.32 found that obesity in COVID-19 patients was an independent predictor of a decline in the physical health component, but not the mental one. Hańczewski et al.33 found that after laparoscopic appendectomy the mental health component of QoL was 72.0 versus 79.5 in patients with BMI ≤25 versus >25. Unsurprisingly, the authors confirmed that cerebrovascular insufficiency was significantly associated with a decrease in the mental health component of QoL.34 Steffens et al. already described an association between depressive symptoms and cerebrovascular disease in the elderly general population.35
Limitations
This study had strengths and limitations. It was a single-center study, with several patients lost to follow-up; and, therefore, its external validity is limited. The sickest patients, many with serious comorbidities, would have succumbed given the relatively high mortality rate of the study cohort. Therefore, even though comorbidities did not surface as independent negative predictors, they certainly cannot be ignored. The authors also acknowledge that they did not register this (nonrandomized) study in an international registry. Moreover, the authors failed to collect and include in the model several in-hospital clinically relevant data and treatments. Finally, the authors did not investigate if young patients were able to return to their “before-COVID-19” job. The authors were not able to describe the causes of death after hospital discharge and did not assess all possible domains of QoL (eg, memory loss). At the same time, the studied population was relatively large and only a few midterm outcome studies including the QoL questionnaire have been performed so far in COVID-19 survivors. Moreover, decrease in QoL in COVID-19 ICU survivors is a topic of great interest because it represents a social and medical problem, and modifiable risk factors and predictors are not known so far. Therefore, the authors also underline the originality of their findings.
Conclusion
Only 32% of Russian ICU survivors did not report serious problems with the physical health component six months after discharge, and 52% had no problems related to the mental health component. LMWH treatment in the ICU was the only modifiable predictor in the physical health component in critically ill patients with COVID-19 six months after discharge. Age older than 52 and female sex were independent risk factors for worse physical outcomes. None of the investigated drugs had an impact on the six-month mental health outcomes. BMI ≤27.6 kg/m2 and cerebrovascular impairment at admission were independent risk factors for adverse outcomes in terms of the mental health component of QoL.
Acknowledgments
Conflict of Interest
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2021.08.036.
Appendix. Supplementary materials
References
- 1.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C, Ye L, Xia R, et al. Chest CT and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020;17:1231–1237. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 – an observational prospective multi-center trial. Eur Respir J. 2021;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau AF, Prescott HC, Brett SJ, et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021;25:108. doi: 10.1186/s13054-021-03535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needham DM, Davidson J, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 10.Hirshberg EL, Wilson EL, Beesley SJ, et al. Impact of critical illness on resource utilization: A comparison of use in the year before and after ICU admission. Crit Care Med. 2019;47:1497–1504. doi: 10.1097/CCM.0000000000003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbertson BH, Roughton S, Vale L, et al. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6. doi: 10.1186/cc8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Physicians. National early warning score (NEWS): Standardising the assessment of acute-illness severity in the NHS. Available at: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2. Accessed August 8, 2021.
- 14.Liliane L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4 doi: 10.1177/2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware JE., Jr SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hammond NE, Finfer SR, Taylor C, et al. Health-related quality of life in survivors of septic shock: 6-month follow-up from the ADRENAL trial. Intensive Care Med. 2020;46:1696–1706. doi: 10.1007/s00134-020-06169-1. [DOI] [PubMed] [Google Scholar]
- 17.Prescott HC, Angus DC. Enhancing recovery from sepsis. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hägg S, Jylhävä J, Wang Y, et al. Age, frailty, and comorbidity as prognostic factors for short-term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21:1555–1559. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin M, Huang L, Zheng D, et al. Health-related quality of life of COVID-19 survivors at 6 months after hospital discharge: A cohort study [e-pub ahead of print] Research Square. 2021 doi: 10.21203/rs.3.rs-176489/v1. [DOI] [Google Scholar]
- 21.Jacobs LG, Gourna Paleoudis E, Aschner JL, et al. Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendren NS, de Lemos JA, Grodin JL, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: Results from the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 23.Landoni G, Maimeri N, Zangrillo A, et al. Why are Asian countries outperforming the Western world in controlling COVID-19 pandemic? Pathog Glob Health. 2021;115:70–72. doi: 10.1080/20477724.2020.1850982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kragholm K, Andersen MP, Gerds TA, et al. Association between male sex and outcomes of Coronavirus Disease 2019 (Covid-19)–a Danish nationwide, register-based study [e-pub ahead of print] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa924. Accessed September 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long L, Zeng X, Zhang X, et al. Short-term outcomes of COVID-19 and risk factors for progression. Eur Respir J. 2020;55 doi: 10.1183/13993003.00990-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Samkari H, Karp Leaf RS, Rosovsky RP, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nardelli P, Landoni G. COVID-19-related thromboinflammatory status: MicroCLOTS and Beyond (Editorial) General Reanimatology. 2020;16:14–15. [Google Scholar]
- 28.Sivaloganathan H, Ladikou EE, Chevassut T. COVID-19 mortality in patients on anticoagulants and antiplatelet agents. Br J Haematol. 2020;190:e192–e195. doi: 10.1111/bjh.16968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bal Dit Sollier C, Dillinger JG, Drouet L. Anticoagulant activity and pleiotropic effects of heparin. J Med Vasc. 2020;45:147–157. doi: 10.1016/j.jdmv.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Sholzberg M, Tang GH, Rahhal H, et al. Heparin for moderately ill patients with Covid-19 [e-pub ahead of print] medRxiv. 2021 doi: 10.1101/2021.07.08.21259351. Accessed September 14, 2021. [DOI] [Google Scholar]
- 31.Lofrano-Prado MC, do Prado WL, Botero WL, et al. The same storm but not the same boat: Effects of COVID-19 stay-at-home order on mental health in individuals with overweight. Clin Obes. 2021;11:e12425. doi: 10.1111/cob.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen KY, Li T, Gong FH, Zhang JS, et al. Predictors of health-related quality of life and influencing factors for COVID-19 patients, a follow-up at one month. Front Psychiatry. 2020;11:668. doi: 10.3389/fpsyt.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hańczewski M, Marciniak R. Effect of BMI on the quality of life in patients after appendectomy depending on surgical modality. Pol Przegl Chir. 2013;85:58–64. doi: 10.2478/pjs-2013-0011. [DOI] [PubMed] [Google Scholar]
- 34.Chandra A, Stone CR, Ding Y, et al. The cerebral circulation and cerebrovascular disease II: Pathogenesis of cerebrovascular disease. Brain Circ. 2017;3:57–65. doi: 10.4103/bc.bc_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffens DC, Helms MJ, Burke GL, et al. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30:2159–2166. doi: 10.1161/01.str.30.10.2159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.