Abstract
Oncoprotein 18/stathmin (Op18) is a recently identified phosphorylation-responsive regulator of the microtubule (MT) system. It was originally proposed that Op18 specifically regulates dynamic properties of MTs by associating with tubulin, but it has subsequently been proposed that Op18 acts simply by sequestering of tubulin heterodimers. We have dissected the mechanistic action of Op18 by generation of two distinct classes of mutants. One class has interruptions of the heptad repeats of a potential coiled-coil region of Op18, and the other involves substitution at all four phosphorylation sites with negatively charged Glu residues. Both types of mutation result in Op18 proteins with a limited decrease in tubulin complex formation. However, the MT-destabilizing activities of the coiled-coil mutants are more severely reduced in transfected leukemia cells than those of the Glu-substituted Op18 derivative, providing evidence for tubulin-directed regulatory activities distinct from tubulin complex formation. Analysis of Op18-mediated regulation of tubulin GTPase activity and taxol-promoted tubulin polymerization showed that while wild-type and Glu-substituted Op18 derivatives are active, the coiled-coil mutants are essentially inactive. This suggests that Op18-tubulin contact involves structural motifs that deliver a signal of regulatory importance to the MT system.
Microtubules (MTs) participate in a variety of cellular processes, including chromosome segregation during mitosis, cell motility, and intracellular vesicle transport. MTs are known to be ever-changing dynamic structures that switch abruptly between elongation and shortening. The switch from growth to shortening is called catastrophe, and the switch between shortening and growth is called rescue (for a review, see reference 8). Classically, regulation of MT dynamics has been ascribed to a class of nonmotor proteins collectively termed MT-associated proteins (MAPs). More recently, a family of MT motors has been shown to regulate MT dynamics both in vivo and in vitro (for a review, see reference 15). Besides these two classes of MT regulators, it has recently been shown that a cytosolic protein termed oncoprotein 18/stathmin (Op18) regulates MT dynamics both in vitro (2) and in intact cells (13, 21).
Several lines of evidence suggest that Op18 is an important phosphorylation-responsive regulator of the MT system in intact cells (for a review, see reference 18). Phosphorylation by either cell surface receptor or cell cycle-regulated kinase systems on four distinct Ser residues decreases the MT-directed activity of Op18 both in vitro and in intact cells (10, 17, 23). The kinase systems involved have been identified as members of the mitogen-activated protein kinase (MAPK), CaM kinase IV/Gr (CaMK IV/Gr), cyclic AMP-dependent kinase (PKA), and cyclin-dependent kinase (CDK) families (for a review, see reference 7).
Op18 has been identified as a factor that both forms complexes with tubulin heterodimers and destabilizes MTs by promoting catastrophes (2). However, the mechanism by which Op18-tubulin complex formation causes destabilization of MTs or promotion of catastrophes is still unresolved. Two recent reports have questioned the original proposal, namely, that Op18 has a specific catastrophe-promoting activity, and the authors propose that Op18 acts simply by sequestering the available pool of unpolymerized tubulin heterodimers. In one of these reports (16), the main arguments presented were based on analysis of the stoichiometric content of stable Op18-tubulin complexes (ratio 1:2) combined with determination of the stoichiometry required for Op18-mediated inhibition of MT assembly. In the other study (6), the authors failed to reproduce the original finding of specific promotion of catastrophes.
The two proposed mechanistic possibilities for Op18 action lead to different predictions. In simple sequestering, ability to bind tubulin is predicted to correlate with activity, and it is unlikely that Op18-tubulin contact leads to modulation of intrinsic tubulin properties, such as its GTPase activity. On the other hand, if Op18 acts as an authentic catastrophe-promoting factor, it can be predicted that Op18 binding to tubulin, either with free heterodimers or at MT ends, results in transmission of putative tubulin-directed regulatory signals and modulation of intrinsic tubulin activities. Evidence for the latter of these two possibilities of Op18 action requires identification of tubulin-directed regulatory activities of Op18 that can be dissociated from tubulin binding per se. In the present study we have searched for the mechanism responsible for Op18-mediated regulation of the MT system by comparing the overexpression phenotypes of specific Op18 mutants. Transfection studies, with a human leukemia cell line, showed that mutations of the potential coiled-coil motif of Op18 have only a limited effect on Op18-tubulin complex formation while causing a dramatic reduction of the MT destabilizing activity. The results of analysis of in vitro properties of wild-type (wt) and mutated Op18 derivatives, such as (i) tubulin-complex formation, (ii) the demonstrated modulation of tubulin GTPase activity, and (iii) inhibition of taxol-driven MT polymerization, were consistent with the phenotypes of the mutants observed in intact cells. Taken together, the results of the present study demonstrate tubulin-directed regulatory activities of Op18 and show that tubulin sequestering is neither the only nor the major mechanism by which Op18 regulates the MT system.
MATERIALS AND METHODS
DNA constructs and transfections.
DNA isolations and manipulations were performed by standard techniques. Construction of a mutant Op18 cDNA, where the codons for both Ser-25 and Ser-38 are replaced with those for Glu (Op18-S25,38E), has previously been described (20). Generation of mutants followed a general strategy where mutations were introduced into subfragments of the coding region by single or overlapping PCR (12) with wt or the Op18-S25,38E cDNA as a template. The subfragments were cloned along with the remaining wt or mutant cDNA fragments into pBluescript SK(+) to regenerate the entire coding sequence of Op18 in its native configuration. For construction of a mutant where the codons for Ser-16, Ser-25, and Ser-38 are replaced with codons for Glu (Op18-S16,25,38E), Op18-S25,38E was used as a template in an overlapping PCR with the T7 primer for pBluescript SK(+) and 5′-CTG GCC TTC GGC ACG CT-3′, 5′-AGC GTG CCG AAG GCC AG-3′, and 5′-CTT TGG ATA TTT AGG AAG GGG-3′ (the introduced mutations are underlined). Construction of a mutant where the codon for Ser-63 is replaced with a codon for Glu (Op18-S63E) was performed with Op18-wt template and the primer 5′-TTA GAA GCT GCA GAA GAA AGA CGC AAG GAA CAT GAA GCT G-3′ and T3 primer for pBluescript SK(+). To generate the Op18-S16,25,38,63E (tetraE) mutant containing Ser-to-Glu substitutions at positions 16, 25, 38, and 63, a 765-bp PstI-to-BamHI fragment containing the S63E mutation was combined with the Op18-S16,25,38E cDNA. Construction of the Op18-cc m1 mutant, where the codons for Leu-47, Ile-50, and Leu-54 are replaced with Ala codons, was performed with Op18-wt as a template, the T7 primer for pBluescript SK(+), and 5′-GTC TTT CTT CTG CAG CTT CTG CTT TCT TCT GAG CTT CCT CCG CGG AAA GAT-3′. In the case of the Op18-cc m2 mutant, where the codons for Leu-47 and Ile-50 are replaced with those for Lys and Leu-54 is replaced with the codon for Glu, the T7 primer for pBluescript SK(+) and 5′-GTC TTT CTT CTG CAG CTT CTT CTT TCT TCT GCT TTT CCT CCT TGG AAA GAT-3′ were used. The coding sequences of PCR-generated fragments were confirmed by nucleotide sequence analysis with an ABI PRISM dye terminator cycle sequencing kit from Perkin-Elmer. Where indicated, an 8-amino-acid C-terminal Flag epitope was introduced as previously described (22). Op18 cDNA derivatives described above were excised from pBluescript SK(+) as either (i) NcoI-to- BamHI fragments that were cloned into pET3d (Novagen) for expression in Escherichia coli or (ii) HindIII-to-BamHI fragments that were cloned into pMEP4 (Invitrogen [11]) for expression in mammalian cells. The conditions used for transfection studies and the pMEP4 shuttle vector system have previously been described (22). In brief, pMEP4 contains the Epstein-Barr virus origin of replication and the EBNA-1 gene to allow high-copy-number episomal replication, and the hph gene, which confers hygromycin B resistance in mammalian cells (11). Conditional expression of various Op18 derivatives was achieved by employing the hMTIIa promoter, which can be suppressed by low concentrations of EDTA (50 μM) and induced by Cd2+ (0.03 to 0.2 μM) (22).
Analysis of MT polymerization status, SDS-PAGE, and Western blotting.
Preparation of total cellular proteins and separation of proteins by 10 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) have been described (22). The cellular MT polymer content was determined by extracting soluble tubulin in an MT-stabilizing buffer followed by quantification of tubulin in the particulate and soluble fractions as described (21, 24)). Affinity purified anti-Op18 specific for the C-terminal region (anti-Op18:34-149) was prepared and used for Western blot analysis as described (4). 125I-labeled protein A or the ECL detection system (Amersham) was used to reveal bound antibodies, as indicated. PhosphorImager analysis of radioactive bands was used for quantification.
Immunofluorescence and flow cytometric analysis.
Cells were extracted with MT-stabilizing buffer (see above) containing 0.05% saponin–10 μg of RNase per ml. Cells were fixed in 4% paraformaldehyde–0.5% glutaraldehyde for 15 min followed by quenching with NaBH4 and thereafter stained with anti-α-tubulin (clone B-5-1-2; Sigma). Bound antibodies were revealed by fluorescein-conjugated rabbit anti-mouse immunoglobulin, and DNA was stained with 1 μg of propidium iodide per ml. Cells were mounted with 1 mg of p-phenylenediamine/ml in phosphate-buffered saline with 80% glycerol and analyzed by epifluorescence. MT fluorescence was also analyzed by flow cytometry as described (25).
Cross-linking of Op18-tubulin complexes by using crude cell extracts.
Cross-linking of Op18-tubulin complexes in crude cell extracts was performed as follows. Cells were lysed in PEM buffer (80 mM piperazine-N,N′-bis[2-ethanesulfonic acid], 1 mM EGTA, 4 mM Mg2+ [pH 6.8]) containing Triton X-100 (0.5%), β-glycerophosphate (10 mM), leupeptin (20 μM), Pefabloc (1 mM), and benzamide (1 mM). The cell extract was clarified by centrifugation and thereafter passed through a desalting column (P-6 Micro Bio-Spin; Bio-Rad) equilibrated with PEM buffer. The protein concentration of the extracts was adjusted to 1 mg/ml, and the extracts were incubated with GTP (1 mM)–bovine tubulin (10 μM) (Cytoskeleton, Denver, Colo.) in PEM buffer (pH 6.8). After 30 min on ice, 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (12 mM) (EDC; Sigma) was added for 15 min before quenching with 2-mercaptoethanol–glycine (final concentrations, 5% and 0.1 M, respectively). Samples were precipitated with 66% acetone, and cross-linked Op18-tubulin complexes were analyzed by SDS-PAGE.
Analyses of tubulin interaction with bead-bound Op18.
Wt and mutated derivatives of purified E. coli-derived Op18 were prepared, and protein mass was determined by analysis of amino acid composition, as described (4). An Op18 protein derivative, with a carboxy-terminal fusion of the 8-amino-acid Flag epitope (Op18-F), was used to coat beads covalently coupled with the anti-Flag M2 monoclonal antibody (Kodak). M2 beads were incubated with Op18 in PEM buffer at 37°C for at least 1 h and extensively washed in PEM buffer at the indicated pH, and 10 μl of beads was used per assay point. The binding capacity of the M2-coupled beads was approximately 0.2 to 0.3 mg of Op18 per ml of beads. The dissociation of bead-bound Op18-F was not influenced by the pH range used in the present study and was below 10% during a 15-min course at 37°C. To allow rapid separation of tubulin bound to Op18-coated beads, the bead suspension was applied into the cap of a 1.5-ml Eppendorf tube containing 0.4 ml of PEM (pH 6.8)–40% sucrose and a top layer of 0.2 ml of PEM (pH 6.8). The caps were closed with care, to keep the bead suspension hanging in the cap, and the samples were centrifuged at the indicated time points (for 1 min at 21,000 × g). Sedimented beads were boiled in SDS sample buffer, and eluted material was separated on a 10 to 20% gradient by SDS-PAGE. Tubulin and Op18 contents were quantitated by Coomassie blue staining of protein bands followed by scanning with a Personal Densitometer (Molecular Dynamics). Bovine tubulin and a standard recombinant Op18 preparation, in which the protein mass had been determined by amino acid analysis, were used as internal standards. The errors between independent determinations were routinely less than 5%.
Assays of GTP exchange/hydrolysis and taxol-mediated assembly of MTs.
GTP exchange of tubulin was analyzed by incubating tubulin (5 μM) loaded with cold GTP in a PEM buffer containing 1 mM adenyl-5′-yl imidodiphosphate (AMP-PNP, to inhibit non-tubulin-mediated GTPase activity) and [γ-32P]GTP (100 μM; 2 × 106 dpm per 25 μl of reaction mixture). To inhibit GTP hydrolysis, all incubations were performed on ice. After 40 min, unbound [γ-32P]GTP was removed by applying 25 μl of the reaction mixture onto a desalting column (P-30 Micro Bio-Spin; Bio-Rad). In the absence of tubulin but in the presence of bovine serum albumin or Op18 (15 μM), these columns retained more than 99.99% of all [γ-32P]GTP. The GTP exchange was calculated by quantifying the radioactivity of the samples before and after separation on a desalting column. Determination of tubulin-mediated GTPase activities was performed under the same buffer conditions as for analysis of GTP exchange activity. In the most sensitive protocol, tubulin (5 μM) was preloaded together with [γ-32P]GTP (100 μM; 2 × 106 dpm per 25 μl of reaction mixture) on ice for 30 min in the presence or absence of Op18. Tubulin associated with [γ-32P]GTP was recovered by employing a desalting column as described above, and GTPase activity was followed at 37°C. Analysis with SDS-PAGE confirmed that both tubulin and Op18 were quantitatively recovered in the run-through, and control experiments showed that Op18 neither bound nor hydrolyzed [γ-32P]GTP. Where indicated, a less-sensitive but simpler protocol, in which tubulin GTPase activity was monitored by incubating tubulin (5 μM) with [γ-32P]GTP (100 μM; 2 × 106 dpm per 25 μl of reaction mixture) at 37°C, was used.
To separate free phosphate from GTP, a previously described protocol was used (1). In brief, aliquots were removed at the times indicated, adjusted to contain 0.1% SDS, and heated for 2 min at 80°C. Aliquots (0.6 μl) were spotted onto polyethyleneimine cellulose plates (Merck), and the free phosphate was separated from GTP by ascending chromatography in 0.75 M potassium hydrogen phosphate buffer. PhosphorImager analysis of radioactive spots was used for quantification, and phosphate release was calculated as a percentage of the total amount of radioactivity of each sample.
In vitro assembly of tubulin (5 μM) in the presence of various amounts of Op18 was performed in assembly buffer (25 μl of PEM containing 1 mM GTP and 4 μg of taxol/ml) as previously described (17).
RESULTS
Tubulin binding of Op18 wt and mutated derivatives in crude cell extracts.
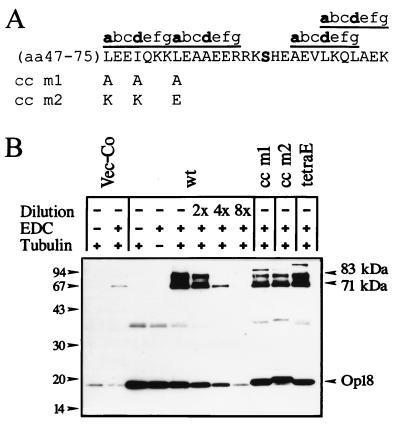
The amino acid sequence of Op18 contains a potential coiled-coil region with three heptad repeats, denoted abcdefg, where positions a and d are occupied by hydrophobic amino acids (reference 9, and see Fig. 1A). In a search for structural motifs of functional importance for MT regulation by Op18, two mutants were constructed by replacement of three hydrophobic residues with either Ala (Op18-cc m1) or charged amino acids (Op18-cc m2), as outlined in Fig. 1A. These mutants were subsequently expressed in the K562 leukemia cell line, using a previously described inducible expression system (22). For comparison, we also expressed Op18-wt and an Op18 mutant with replacement of Ser residues at all four in vivo phosphorylation sites with negatively charged Glu residues (Op18-tetraE). As the first step in characterizing transfected cells, tubulin interactions with wt and mutated Op18 derivatives in crude lysates of transfected cells were analyzed. For this purpose, we have developed an assay that involves cross-linking of cellular Op18 to bovine tubulin added to the cell extract. It is shown in Fig. 1B that, compared to the endogenous gene product expressed in vector control transfected cells, all transfected Op18 derivatives were expressed at higher levels. It is also shown that in all cases, addition of both tubulin and the zero-length cross-linker EDC to extracts prepared from cells expressing the indicated Op18 derivatives generates two major Op18-containing complexes that migrate at 71 and 83 kDa. In vector control transfected cells, however, only faint bands were observed. The 71- and 83-kDa complexes migrate at the same position as the Op18-tubulin complexes observed after cross-linking of purified Op18 and tubulin proteins (17). Moreover, the intensities of the 71- and 83-kDa bands are dramatically increased by overexpression of Op18, and these bands are observed only after addition of bovine tubulin, i.e., their appearance is both Op18 and tubulin dependent. Comparison of the intensities of Op18-tubulin complexes suggests that the three Op18 mutants analyzed have only slightly reduced tubulin binding activities as compared to the wt proteins (they showed about twofold-decreased complex formation as judged from dilution of the Op18-wt extract). Thus, the result indicates that neither disruption of the potential coiled-coil region nor substitution at phosphorylation sites with Glu has major effects on tubulin binding of Op18 in a crude cell extract.
FIG. 1.
Cross-linking of tubulin to wt and mutated Op18 in crude cell extracts of transfected K562 cells. (A) The locations of mutations in Op18-cc m1 (L47A, I50A, and L54A) and Op18-cc m2 (L47K, I50K, and L54E) in the putative coiled-coil region are depicted. The Ser-63 phosphorylation site is indicated in bold. (B) K562 cells were transfected with pMEP4 (Vec-Co), pMEP-Op18-wt (wt), pMEP-Op18-cc m1 (cc m1), pMEP-Op18-cc m2 (cc m2), or pMEP-Op18-S16,25,38,63E (tetraE), and cell lines were selected as described in Materials and Methods. Cells were treated with Cd2+ (0.1 μM) for 7 h to induce expression from the hMTIIa promoter and subsequently lysed. In each case, crude cell extract was mixed with bovine tubulin and subjected to the EDC cross-linking as described in Materials and Methods. After separation with SDS-PAGE, complexes were revealed by rabbit anti-Op18:33-149. For semi-quantification of complex formation, the sample of cells expressing Op18-wt was diluted twofold (2×), fourfold (4×), and eightfold (8×) as indicated. The positions of the 71- and 83-kDa Op18-tubulin complexes are indicated. Data are representative for two experiments.
In addition to the 71- and 83-kDa Op18-tubulin complexes shown in Fig. 1B, other anti-Op18 reactive proteins are also evident. For example, some minor bands of unknown identity migrate above 83 kDa. The induced expression of Op18-wt and Op18 mutated derivatives also results in a band migrating at about 38 kDa, which is hardly affected by cross-linking or addition of tubulin. The 38-kDa band appears to represent a minor cellular fraction of dimeric Op18, since the molecular weight is about twice that of Op18 and its migration reflects the slight size variation of specific Op18 derivatives. The significance of the 38-kDa Op18 species and the minor bands migrating above 83 kDa is as yet unclear.
MT-regulating activity of wt and mutated Op18 derivatives in intact cells.
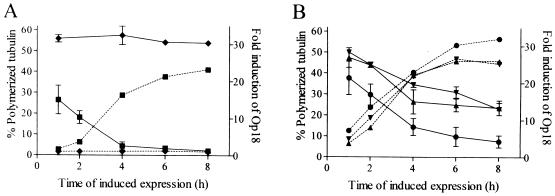
As outlined above, mutations of the potential coiled-coil motif have a marginal effect on Op18-tubulin complex formation similar to that of substitution at all four phosphorylation sites with Glu. To determine the effects of these mutations on the MT-regulating activity of Op18 in transfected cells, the level of induced ectopic expression and the amount of polymerized MTs were monitored for 8 h (Fig. 2). The results showed rapid induction of expression, and after 6 h comparable levels of all tested Op18 derivatives were observed (the levels were about 25-fold greater than that of endogenous Op18). In agreement with previous studies, induced expression of Op18-wt results in rapid destabilization of cellular MTs, and after 8 h less than 5% of the original MT content remains intact as judged from an extraction assay (Fig. 2A). In contrast, Op18-cc m1 and Op18-cc m2 are both severely impaired in their MT destabilizing activity, and about 45% of all the original MT content remains intact (Fig. 2B). It is also shown that Op18-tetraE is clearly more active than the coiled-coil mutants (about 15% of the original MT content remains intact after 8 h [Fig. 2B]).
FIG. 2.
Alterations in MT polymerization status in response to overexpressed wt and mutated Op18. K562 cells were transfected with (A) pMEP4 (⧫) or Op18-wt (■) and with (B) Op18-cc m1 (▴), Op18-cc m2 (▾), or Op18-tetraE (●) as described in the legend for Fig. 1. Transfected cell lines were induced with 0.1 μM Cd2+ for the indicated times, and the fraction of polymerized tubulin was determined by the extraction protocol described in Materials and Methods. The mean of two independent determinations is shown. In parallel the induced levels of ectopic Op18 were determined by quantitative Western blot analysis (dashed lines), and data are presented as fold induction over that by endogenous Op18. Data are representative for two independent transfection experiments.
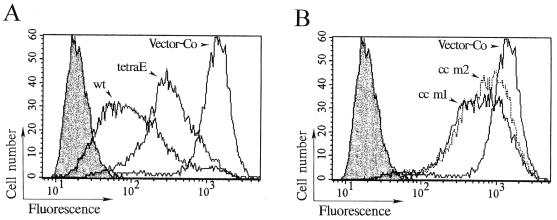
To extend the present analyses, MTs were studied by immunofluorescence in transfected cells. To release unpolymerized tubulin, cells were extracted with an MT-stabilizing buffer prior to fixation. MT-specific immunofluorescence was thereafter analyzed by flow cytometry, which allows both quantification of fluorescence intensity and evaluation of heterogeneity within the cell population. The results presented in Fig. 3 show that induced expression of Op18-wt results in 94% reduction of the median fluorescence intensity as compared with that of vector control transfected cells (note the log scale). The Op18-tetraE mutant shows reduced activity as compared with Op18-wt (Fig. 3A) but is still more active than either of the Op18-cc m1 and Op18-cc m2 derivatives (Fig. 3B). The histograms of cells expressing either cc m1 or cc m2 Op18 derivatives show a partial overlap in fluorescence intensity with vector control transfected cells (Fig. 3B). Thus, quantification of MTs of transfected cells by immunofluorescence combined with flow cytometry is consistent with the biochemical determination of polymerized tubulin shown in Fig. 2.
FIG. 3.
Flow cytometric analysis of MTs in cells overexpressing wt and mutated Op18. Transfected K562 cells were induced with 0.1 μM Cd2+ for 5.5 h, extracted with MT-stabilizing buffer, fixed, and stained with anti-α-tubulin. Open graphs depict α-tubulin-specific fluorescence of cells transfected with the indicated pMEP4-Op18 constructs, and shaded graphs show control staining in the absence of anti-α-tubulin but in the presence of fluorescein-conjugated rabbit anti-mouse immunoglobulin. To facilitate comparison, the histograms depicting control staining and cells expressing vector control (pMEP4) are shown in both panel A and B. The median fluorescence signals were as follows: control staining, 19; vector control (Vector-Co), 1,275; Op18-wt, 95; Op18-cc m1, 567; Op18-cc m2, 692; and Op18-tetraE, 311.
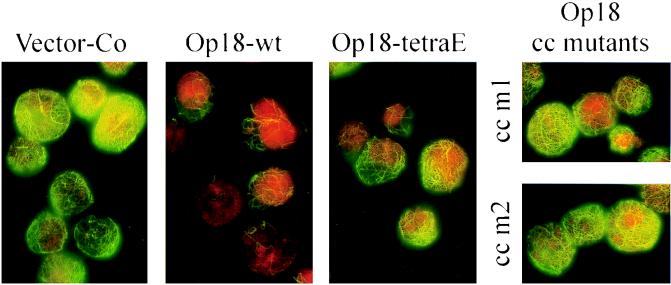
The morphology of the interphase network of MTs, in cells induced to express the indicated Op18 derivatives, is shown in Fig. 4. The distribution of MT-specific immunofluorescence, as analyzed by flow cytometry, suggested that overexpression of Op18-wt results in a mixed population of cells with low but varying amounts of MTs. This agrees with analysis by epifluorescence shown in Fig. 4. The few “Op18-resistant” MTs that remain intact often contain kinks. Cells overexpressing Op18-tetraE gave a similar array but generally with a significantly higher density of MTs, which agrees with flow cytometric analysis shown above. The results obtained with the cc m1 and cc m2 mutant Op18 derivatives were also found to be in agreement with flow cytometric analysis. Thus, compared to Op18-tetraE expressing cells, the average densities of MTs were clearly higher, and a significant proportion of all cells appeared similar to vector control transfected cells. Besides a somewhat lower general density of MTs, detailed examination failed to reveal any morphological differences between the MT networks displayed in cells expressing coiled-coil mutants of Op18 and vector control. Taken together, the results presented above reveal that the cc-m1 and cc-m2 mutants of Op18 are clearly less active in their MT-destabilizing activity than Op18-tetraE. Since the tetraE and coiled-coil mutants of Op18 have comparable tubulin-binding activities in crude cell extracts, this result indicates that Op18, in addition to tubulin binding, has other tubulin-directed activities in intact cells.
FIG. 4.
Immunodetection of MTs in cells induced to overexpress Op18. K562 cells, transfected with the indicated pMEP4-based constructs, were induced with 0.1 μM Cd2+ for 5.5 h and double stained with anti-α-tubulin (green) and propidium iodide (DNA staining; red). Representative interphase cells analyzed by epifluorescence (1,000-fold original magnification) are shown.
Tubulin-directed in vitro activities of wt and mutated Op18 derivatives.
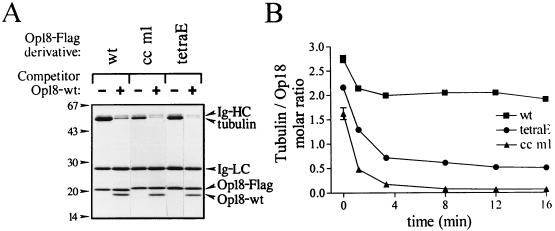
To analyze Op18-tubulin complex formation in more detail, we used an assay designed to allow rapid isolation of Op18-tubulin complexes. For these experiments we fused Op18 with an 8-amino-acid C-terminal Flag epitope tag. E. coli-expressed and purified Flag-tagged derivatives of Op18-wt, Op18-cc m1, and Op18-tetraE were bound to agarose beads coupled with the M2 antibody specific to the Flag epitope (M2 beads). When these beads were incubated with 10 μM tubulin and then rapidly pelleted through a sucrose cushion, tubulin bound to the Op18-F/M2 beads was detected in the pellet (Fig. 5A). More than 90% of all binding was competed by addition of excess soluble Op18-wt, which demonstrates the specificity of this assay system. Note that competitor Op18 copellets with Op18-F/M2 beads at a molar ratio close to 1:1. Unflagged Op18 binds less to M2 beads alone (data not shown), indicating the existence of specific Op18-Op18 interactions. Most importantly, although Op18-wt-F/M2 pelleted somewhat more tubulin than the mutated derivatives, the data show only a limited difference in tubulin-binding capacity. This is in line with analyses of Op18-tubulin association in a crude cell lysate by cross-linking as shown above (Fig. 1).
FIG. 5.
Characterization of in vitro tubulin binding to wt and mutated Op18. (A) Tubulin (10 μM in PEM, pH 6.8) was mixed with the indicated Flag-epitope-tagged Op18 derivative coupled to M2 beads (Op18-F/M2 beads). To control for nonspecific binding, competitor Op18-wt (50 μM), which does not bind to M2 beads, was added where indicated (+). After a 10-min incubation at 37°C, bead-bound material was pelleted through a sucrose cushion and separated by SDS-PAGE. Proteins were detected by Coomassie blue staining, and the positions of the immunoglobulin (Ig-) heavy (HC) and light (LC) chain derived from the M2 antibody, as well as tubulin, Op18-F (Op18-Flag), and competitor Op18, are indicated. (B) Tubulin sufficient for saturated binding (20 μM in PEM, pH 6.8) was allowed to bind for 15 min at 37°C to Op18-wt-F (■), Op18-cc m1-F (▴), or Op18-tetraE-F (●) coupled to M2 beads. Beads were thereafter either pelleted through a sucrose cushion (t = 0) or diluted 40-fold, and dissociation of tubulin was monitored at 37°C by pelleting of beads at the indicated time points. The molar ratio of tubulin associated with Op18 was determined as described in Materials and Methods, and the contribution of nonspecific binding (about 8%) was subtracted from the presented data. Data are representative for at least two independent experiments.
Binding of tubulin to Op18-F/M2 beads coated with either of the three Op18-Flag derivatives tested was rapid (saturation within 5 min). Using the present assay system, we were unable to document potential differences between Op18-wt and mutated derivatives in association kinetics (data not shown). However, on the level of dissociation rates, a clear-cut difference between wt and mutated Op18 was evident. As shown in Fig. 5B (t = 0), incubation of 20 μM tubulin with Op18-wt/M2 beads, which saturates tubulin binding to the beads (data not shown), results in association of close to 3 moles of tubulin per mole of Op18. A 40-fold dilution results in a rapid dissociation of about one-third of the associated tubulin, while the remaining two-thirds was tightly bound to Op18 during the period analyzed. It is also shown that the total tubulin-binding capacity of Op18-cc m1 is about 75% of that of Op18-tetraE at equilibrium conditions (i.e., before dilution). Importantly, dilution reveals an enhanced dissociation of tubulin bound to either of the mutated derivatives as compared to that bound to Op18-wt. For Op18-cc m1, essentially all tubulin is released after 8 min, while the dissociation curve of tubulin bound to Op18-tetraE appears biphasic with a small fraction of the initially bound tubulin present in a stable complex. Taken together, the data in Fig. 5 show that under equilibrium conditions both types of Op18 mutants form complexes with significant amounts of tubulin but that there is a dramatic effect of each type of mutation on the level of the stability of the complex. Interestingly, under equilibrium conditions the molar ratio of pelleted material indicates that Op18-wt binds about three tubulin heterodimers. Upon dilution, however, about one-third of all tubulin dissociates rapidly but the remaining complex of Op18-wt associated with close to two tubulin heterodimers appears stable. The estimated composition of this stable complex is in accordance with the previously reported stoichiometry (ratio, 1:2) of a tight Op18-tubulin complex which resisted gel filtration and analytical ultracentrifugation (6, 16).
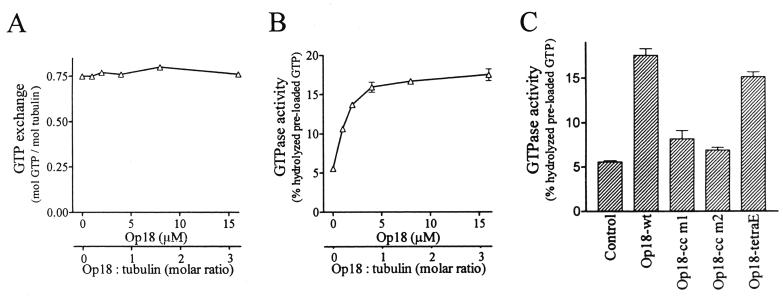
To analyze the functional consequences of Op18 association to tubulin and the potential effects of mutations, we studied modulation of tubulin GTP exchange and GTPase activity. For these experiments we used E. coli-expressed and purified Op18 in its native form, without the Flag epitope tag. As shown in Fig. 6A, Op18 does not have any detectable effect on [γ-32P]GTP loading to tubulin. However, Op18 induced a threefold increase in the low basal GTPase activity of tubulin preloaded with [γ-32P]GTP (Fig. 6B) and a plateau level of activity was obtained at about 4 μM. Note that the experiments were conducted with 5 μM tubulin, so the observed GTP hydrolysis was independent of polymerization. Most interestingly, as shown in Fig. 6C, at a concentration of 16 μM the coiled-coil mutants of Op18 were almost inactive while the Op18-tetraE mutant was essentially as active as Op18-wt.
FIG. 6.
Tubulin-directed activities of wt and mutated Op18. (A) Tubulin (5 μM) was incubated with a graded concentration of Op18-wt on ice for 30 min in the presence of [γ-32P]GTP. GTP exchange was calculated by determination of tubulin-associated [γ-32P]GTP as described in Materials and Methods. (B) [γ-32P]GTP was allowed to bind to tubulin in the presence of graded concentrations of Op18 as described for panel A. Unbound [γ-32P]GTP was thereafter removed on a desalting column, and [γ-32P]GTP-loaded tubulin and Op18 were incubated at 37°C. The mean of duplicate determinations of hydrolyzed GTP, after 40 min of incubation, is shown. (C) Modulation of tubulin GTPase activity by 16 μM concentration of the indicated Op18 derivative was determined as described for panel B. Data are representative for at least two independent experiments.
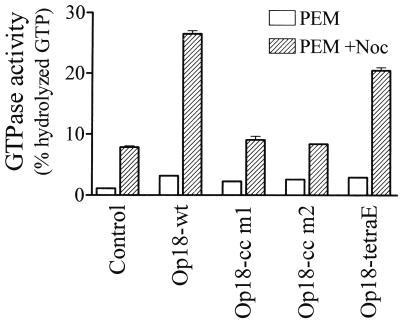
During our search for tubulin-directed activities, we also analyzed potential Op18-mediated modulation of nocodazole-induced tubulin GTPase activity. As shown in Fig. 7, nocodazole alone increased tubulin GTPase activity by eightfold, as contrasted with a threefold increase in the presence of Op18-wt alone. Most interestingly, in the presence of both nocodazole and Op18, a synergistic response is evident, with a 26-fold increase in tubulin GTPase activity. In agreement with data presented above, the coiled-coil mutants of Op18 were essentially inactive, while Op18-tetraE was almost as active as Op18-wt. Thus, Op18 stimulates the basal GTPase activity of tubulin and shows a synergism with nocodazole. Most importantly, in contrast to the tubulin binding activity, these tubulin-directed activities of Op18 are extremely sensitive to mutation of the potential coiled-coil region.
FIG. 7.
Modulation of nocodazole-stimulated tubulin GTPase activity by wt and mutated Op18. Tubulin (5 μM) was incubated at 37°C in the presence of [γ-32P]GTP in the absence (open bars) or presence (striped bars) of nocodazole (33 μM). The indicated Op18 derivative (16 μM) was also included in the reaction mixtures. The means of duplicate determinations of hydrolyzed GTP, after 120 min of incubation, are shown. Data are representative for at least two independent experiments.
Importance of the potential coiled-coil motif for Op18-mediated inhibition of MT polymerization.
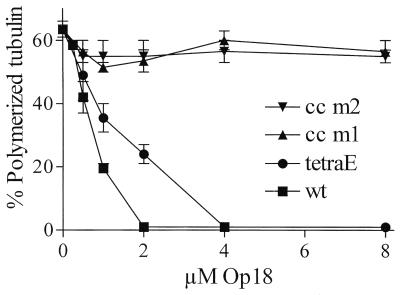
Op18-mediated inhibition of taxol-driven in vitro MT assembly is likely to reflect inhibition of polymerization rather than destabilization of MTs. As shown in Fig. 8, in the presence of taxol and 5 μM tubulin, both Op18-wt and Op18-tetraE are potent inhibitors of MT polymerization. Most interestingly, however, the Op18-cc m1 and Op18-cc m2 proteins are essentially inactive in this assay system, which is in line with the analysis of GTPase activity described above. Given that coiled-coil mutants associate with tubulin almost as efficiently as Op18-tetraE at equilibrium conditions (Fig. 1 and 5), the result indicates that Op18 does not simply inhibit polymerization by association to tubulin. It follows that a distinct tubulin-directed regulatory activity of Op18 may be required for its antipolymerizing activity, as implied by the data obtained from intact cells shown in a previous section.
FIG. 8.
Op18-mediated inhibition of taxol-driven MT polymerization. Tubulin was incubated with taxol (5 μM) in the presence of graded concentrations of the indicated Op18 derivatives for 30 min at 37°C. Polymerized tubulin was sedimented by centrifugation and quantitated by using the bicinchoninic acid protein assay. Incubation of tubulin with taxol on ice resulted in sedimentation of less than 2 to 3% of all tubulin protein. The means of duplicate determinations are shown. Data are representative for at least two independent experiments.
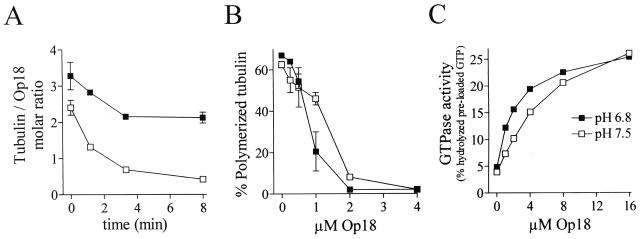
To further address the relative importance of Op18 binding characteristics to tubulin for the potency of Op18 activity, we took advantage of the recently shown pH dependence of Op18-tubulin binding. As determined by plasmon resonance measurement, Op18 binds tubulin optimally at pH 6.5 while binding activity at pH 7.5 is low (6). As shown in Fig. 9A, the pH dependence of Op18-tubulin binding is not found to be dramatic when analyzed under equilibrium conditions in the presence of 20 μM tubulin. However, upon dilution a pH-dependent effect on Op18-tubulin interaction is readily observed. Thus, while a stable Op18-tubulin complex (ratio, about 1:2) is observed after the initial rapid dissociation phase (about 3 min) at pH 6.8, increased pH results in an unstable complex with dissociation characteristics that appear to be intermediate between those of the Op18-cc m1 and Op18-tetraE mutants (as analyzed at pH 6.8; see Fig. 5). Interestingly, on the level of inhibition of tubulin polymerization, the Op18 dose responses at pH 6.8 and pH 7.5 show less than twofold differences (Fig. 9B). This suggests that Op18 in an unstable complex with tubulin is essentially as potent an inhibitor of tubulin polymerization as Op18 in a stable tubulin complex. Moreover, on the level of Op18-mediated stimulation of tubulin GTPase activity, the dose response was shifted only about twofold and the peak activities were similar at pH 6.8 and 7.5. This is in line with the phenotype of the Op18-tetraE mutant, which is inefficient in forming a stable complex with tubulin (Fig. 5) but is still a potent inhibitor of tubulin polymerization (Fig. 8) and stimulator of tubulin GTPase activity (Fig. 6). Thus, several lines of evidence indicate that tight tubulin binding is not required for Op18 activity, which in turn supports the importance of tubulin-directed regulatory activities for Op18-mediated regulation of the MT system.
FIG. 9.
pH dependent modulation of Op18-tubulin interactions and tubulin-directed activities. (A) Tubulin (20 μM) was allowed to bind for 15 min at 37°C to Op18-wt-F-coupled M2 beads in PEM buffer at pH 6.8 (■) or pH 7.5 (□). Tubulin binding at t = 0 and tubulin dissociation were determined at each pH as described for Fig. 5. (B) Op18-mediated inhibition of taxol-driven MT polymerization in PEM buffer at pH 6.8 (■) and pH 7.5 (□) was determined as described for Fig. 8. (C) Op18-stimulated tubulin GTPase activity at pH 6.8 (■) and pH 7.5 (□) was determined as described for Fig. 6.
DISCUSSION
In the present study we have addressed the mechanism responsible for Op18-mediated regulation of the MT system by comparing the phenotypes of distinct classes of Op18 mutants. In terms of in vitro modulation of MT dynamics, Op18 has been identified as a factor that both forms complexes with tubulin and destabilizes MTs by promoting catastrophes (2). However, two subsequent in vitro studies brought into question the proposed catastrophe-promoting activity of Op18, and the authors argued that Op18 regulates MT polymerization simply by forming a stable complex with tubulin and thereby sequestering the available pool of tubulin, rather than directly regulating the dynamic properties of MTs (6, 16).
The approach used here to dissect the mechanistic action of Op18 involved the generation of two classes of mutant Op18. One class has interruptions of the potential coiled-coil region of Op18, and the other involves substitution at all four phosphorylation sites of negatively charged Glu residues to create a “pseudophosphorylated” Op18 derivative. Functional consequences of these mutations were evaluated both in intact cells and by in vitro assays. Both types of mutation resulted in Op18 derivatives with decreased, but comparable, levels of tubulin association, as evaluated by cross-linking studies both in crude cell extracts and by interaction with M2 beads coated with purified Op18 proteins (Fig. 1 and 5). Thus, these two classes of mutants provide a system to differentiate between effects of tubulin association and other potential Op18-mediated activities. That such potential activities exist was first indicated by the observed in vivo overexpression phenotype of the Op18 mutants. The class with a disrupted coiled-coil motif was strongly reduced in its ability to destabilize MTs, as compared to both the Op18-wt and Op18-tetraE derivative. Moreover, in vitro analysis of tubulin-directed activities demonstrated that while the Op18-tetraE mutant was almost as active as Op18-wt, the coiled-coil mutants were essentially devoid of tubulin-directed activities. Hence, these experiments indicate that Op18-tubulin association is not sufficient for a functional consequence.
It has recently been suggested that Op18 interacts with two tubulin heterodimers to form a tight ternary complex. The analyses were performed by using a standard tubulin buffer (PEM buffer [pH 6.8]), and it was proposed that formation of this tight ternary complex was the sole mechanism behind the observed MT regulatory properties of Op18 (6, 16). The present study confirms that a stable ternary Op18-tubulin complex is formed at pH 6.8, but our results show that a stable complex is not essential for Op18 function. Firstly, the Op18-tetraE derivative, which does not form a stable ternary complex, was almost as potent as Op18-wt in activating tubulin GTPase activity and inhibiting tubulin polymerization (Fig. 5 to 7). Secondly, a stable ternary complex is not formed at pH 7.5 but the potency of Op18 at this pH as evaluated by inhibition of tubulin polymerization and analysis of tubulin GTPase activity is not significantly altered (Fig. 9). Finally, since the cytosolic pH is around 7.2 (3), the physiological significance of the tight ternary complex observed at pH 6.8 is unclear.
By using an assay designed to allow rapid isolation of Op18-tubulin complexes, the present study demonstrates that Op18 forms complexes with more than two tubulin dimers under equilibrium conditions. Thus, it was shown that a complex corresponding to one Op18 and about three tubulin heterodimers can be isolated but that this complex is unstable. Within 3 min after dilution about one-third of the bound tubulin dissociates, which leaves a complex that corresponds to a stable ternary complex (Fig. 5 and 9). Given the rapid dissociation, it seems possible that Op18 may associate to even more than three tubulin heterodimers, since an unknown amount of tubulin associated during equilibrium may be released during the 30 to 60 s it takes to pellet the Op18-F/M2 beads through a sucrose cushion. This complexity of Op18-tubulin interactions indicates that Op18 has three or more distinct binding sites for tubulin. Mapping of these binding sites and identification of their importance for tubulin-directed regulatory activities are likely to be essential for future understanding of the mechanism of Op18 function.
Op18 is a protein that shows large cell-type-specific variations in abundance. For example, in some acute leukemia cells Op18 constitutes about 0.5% of all cytosolic proteins, while primary lymphocytes contain 100-fold less Op18. Most nontransformed cells that proliferate in tissue culture (e.g., primary fibroblast and lymphoblastoid cell lines) contain intermediate levels of Op18, ranging from 0.02 to 0.08% of all cellular proteins (4). In the overexpression experiments presented here, ectopic Op18 expression in K562 cells increased Op18 levels about 25-fold (from 0.1 to 2.5% of all cytosolic proteins). It should be noted that at these levels of overexpression, both wt Op18 and the coiled-coil mutants of Op18 still allow formation of the mitotic spindle and subsequent cell division, which is due to multisite phosphorylation of the expressed protein during mitosis (references 17 and 21 and data not shown). At these high Op18 levels, it seems likely that Op18 has the potential to sequester a major part of all cellular tubulin. This possibility probably underlies the observation that ectopic expression of coiled-coil Op18 mutants, which were essentially inactive in functional assays performed in vitro, still caused a limited reduction of cellular MT content in intact cells. However, in cells expressing Op18-wt, a marked decrease in cellular MT content is also evident at lower expression levels, which are observed early after induction. Thus, ectopic Op18-wt, but not the coiled-coil mutants, shows a potent MT-regulatory activity at expression levels that are in the range of those observed in some acute leukemia cells (i.e., about 0.5% of all cytosolic proteins) (4). The high, but variable, Op18 levels in normal and malignant cells make it likely that tubulin sequestering at least in some cases is of physiological importance. However, the observed differences in the potency with which coiled-coil and pseudophosphorylated Op18 mutants destabilize cellular MTs indicate that tubulin sequestering is not the only, nor major, mechanism by which Op18 regulates the MT system. This interpretation is in line with the results of in vitro experiments indicating dissociation of Op18-tubulin complex stability and polymerization inhibitory activity, via both pH and the Op18-tetraE mutant, which suggest that the stability of Op18-tubulin interactions is of minor regulatory importance.
During our search for Op18 activities that could modulate MT dynamics, we found that Op18 increases the basal tubulin GTPase activity (Fig. 6). Moreover, we also found that Op18 acts in synergy with nocodazole in stimulating tubulin GTPase activity (Fig. 7). The latter finding suggests that Op18 stimulates this activity by a mechanism that is distinct from nocodazole and other MT poisons that act through binding to the colchicine site of tubulin (5). The Op18-tetraE derivative was almost as efficient as Op18-wt in GTPase-inducing activity, while mutations of the coiled-coil motif essentially abolished the activity. Analysis of in vitro tubulin binding of Op18-cc m1 and Op18-tetraE, under equilibrium conditions, did not reveal any dramatic decrease as compared to that of Op18-wt. However, the stability of the tubulin complex was severely decreased for both types of mutants, the decrease being most dramatic for the Op18-cc m1 derivative (Fig. 5). As argued above, in vitro experiments suggest that manipulation of the stability of the Op18-tubulin complex, via either pH or Glu substitutions at phosphorylation sites, has only minor effects on Op18 activity. However, the possibility that the observed difference in tubulin binding stability accounts for or exacerbates some of the differences between the two types of mutants used in this study cannot be excluded. Nevertheless, since the coiled-coil mutants were clearly able to interact with tubulin (Fig. 1 and 5), these results demonstrate that tubulin interactions per se are not sufficient for tubulin-directed activities elicited by Op18. This conclusion is consistent with the observed importance of the potential coiled-coil motif for Op18-mediated inhibition of taxol-driven MT polymerization (Fig. 8). Thus, although Op18-tubulin contact is most likely a prerequisite for all tubulin-directed activities, the present study indicates that Op18 contains structural motifs that by contact with tubulin deliver a signal of regulatory importance to the MT system.
The present study reveals that a tight Op18-tubulin complex is not required for two distinct in vitro activities of Op18, namely, inhibition of tubulin polymerization and stimulation of tubulin GTPase activity. This is in line with a very recent study of the dynamic properties of individual MTs aimed at dissecting tubulin-sequestering and MT-catastrophe-promoting activities of Op18 (14). The results showed that under conditions where stable Op18-tubulin complexes are generated (i.e., in PEM buffer at pH 6.8), tubulin sequestering is the predominant activity of Op18. Most interestingly, under conditions designed to decrease the stability of Op18-tubulin complexes (i.e., in PEM buffer at pH 7.5 or after truncation of the C-terminal end of Op18), tubulin sequestering was not observed but the catastrophe-promoting activity of Op18 was retained. Hence, these data show that the stability of the Op18-tubulin complex is not important for Op18 activity per se but may determine the specific functional consequence of the interaction.
Two recent reports by us have investigated the physiological role of Op18 phosphorylation for regulation of the MT system (10, 23). It was shown that stoichiometric phosphorylation of endogenous Op18, by induced ectopic expression of CaM kinase IV/Gr or PKA, is associated with a rapid 20 to 50% increase in the cellular MT content. The direct involvement of Op18 phosphorylation during this process was indicated by the result of conditional coexpression of the cognate kinases and a series of kinase target site-deficient mutants of Op18. With PKA, which phosphorylates Op18 on both Ser-16 and Ser-63, the activity of overexpressed Op18 was completely suppressed. These results showed that phosphorylation of these two sites is sufficient to completely switch off the activity of overexpressed Op18 in intact cells. This is in contrast to the modest reduction of Op18 activity caused by substitution at all four kinase target sites with negatively charged Glu residues in the tetraE, pseudophosphorylation, mutant observed in the present study. Horwitz et al. (13) reported a more dramatic difference between a glutathione S-transferase–Op18-wt and a pseudophosphorylation derivative in which negatively charged Asp residues replaced the Ser residues. There are several possible explanations for the differences between the two studies. Firstly, the native protein was employed instead of a fusion protein in which the major part (27 kDa) is the fusion partner; secondly, Glu was used instead of Asp; and thirdly, we employed a conditional expression system that results in homogeneous and high expression levels, which allows biochemical or flow cytometric quantification of MT content, while their result was based on manual inspections of microinjected single cells. Nevertheless, despite the reported differences in the severity of phenotypes, our finding that Glu substitution at phosphorylation sites does have minor effects on the in vitro activities of Op18 is clearly in line with the previous study (13).
Coiled-coil structures are frequently occurring motifs involved in protein-protein interactions on many levels of cell regulation (19). Future structural studies are clearly required to establish if the heptad repeats noted in Op18 (9) form an authentic coiled-coil structure. The present mutant analysis shows that this region of Op18 is not essential for tubulin complex formation, although it has an important tubulin-directed functional role both in intact cells and in vitro.
ACKNOWLEDGMENTS
We thank Victoria Shingler for helpful discussions and critical reading of the manuscript. We thank Susann Haraldsson and Anna Falk for valuable help during the construction of mutated cDNA derivatives.
This work was supported by the Swedish Natural Science Research Council, the Foundation for Medical Research at the University of Umeå, and the Swedish Society for Medical Research.
REFERENCES
- 1.Austin S, Dixon R. The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J. 1992;11:2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belmont L D, Mitchison T J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 3.Boron W F. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. doi: 10.1146/annurev.ph.48.030186.002113. [DOI] [PubMed] [Google Scholar]
- 4.Brattsand G, Roos G, Marklund U, Ueda H, Landberg G, Nanberg E, Sideras P, Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993;7:569–579. [PubMed] [Google Scholar]
- 5.Correia J J. Effects of antimitotic agents on tubulin-nucleotide interactions. Pharmacol Ther. 1991;52:127–147. doi: 10.1016/0163-7258(91)90004-6. [DOI] [PubMed] [Google Scholar]
- 6.Curmi P A, Andersen S S, Lachkar S, Gavet O, Karsenti E, Knossow M, Sobel A. The stathmin/tubulin interaction in vitro. J Biol Chem. 1997;272:25029–25036. doi: 10.1074/jbc.272.40.25029. [DOI] [PubMed] [Google Scholar]
- 7.Deacon, H. W., T. J. Mitchison, and M. Gullberg. Op18/stathmin. In T. Kreis and R. Vale, Guidebook to the cytoskeletal and motor proteins, in press. Oxford University Press, Oxford, United Kingdom.
- 8.Desai A, Mitchison T J. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Doye V, Soubrier F, Bauw G, Boutterin M C, Beretta L, Koppel J, Vandekerckhove J, Sobel A. A single cDNA encodes two isoforms of stathmin, a developmentally regulated neuron-enriched phosphoprotein. J Biol Chem. 1989;264:12134–12137. [PubMed] [Google Scholar]
- 10.Gradin H M, Larsson N, Marklund U, Gullberg M. Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol. 1998;140:131–141. doi: 10.1083/jcb.140.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groger R K, Morrow D M, Tykocinski M L. Directional antisense and sense cDNA cloning using Epstein-Barr virus episomal expression vectors. Gene. 1989;81:285–294. doi: 10.1016/0378-1119(89)90189-3. [DOI] [PubMed] [Google Scholar]
- 12.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz S B, Shen H J, He L F, Dittmar P, Neef R, Chen J H, Schubart U K. The microtubule-destabilizing activity of metablastin (p19) is controlled by phosphorylation. J Biol Chem. 1997;272:8129–8132. doi: 10.1074/jbc.272.13.8129. [DOI] [PubMed] [Google Scholar]
- 14.Howell B, Larsson N, Gullberg M, Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol Biol Cell. 1999;10:105–118. doi: 10.1091/mbc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi H C. Microtubule dynamics in living cells. Curr Opin Cell Biol. 1998;10:35–44. doi: 10.1016/s0955-0674(98)80084-7. [DOI] [PubMed] [Google Scholar]
- 16.Jourdain L, Curmi P, Sobel A, Pantaloni D, Carlier M F. Stathmin: a tubulin-sequestering protein which forms a ternary T2S complex with two tubulin molecules. Biochemistry. 1997;36:10817–10821. doi: 10.1021/bi971491b. [DOI] [PubMed] [Google Scholar]
- 17.Larsson N, Marklund U, Melander Gradin H, Brattsand G, Gullberg M. Control of microtubule dynamics by oncoprotein 18: dissection of the regulatory role of multisite phosphorylation during mitosis. Mol Cell Biol. 1997;17:5530–5539. doi: 10.1128/mcb.17.9.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawler S. Microtubule dynamics—if you need a shrink try stathmin/Op18. Curr Biol. 1998;8:R212–R214. doi: 10.1016/s0960-9822(98)70128-9. [DOI] [PubMed] [Google Scholar]
- 19.Lupas A. Predicting coiled-coil regions in proteins. Curr Opin Struct Biol. 1997;7:388–393. doi: 10.1016/s0959-440x(97)80056-5. [DOI] [PubMed] [Google Scholar]
- 20.Marklund U, Larsson N, Brattsand G, Osterman O, Chatila T A, Gullberg M. Serine 16 of oncoprotein 18 is a major cytosolic target for the Ca2+/calmodulin-dependent kinase-Gr. Eur J Biochem. 1994;225:53–60. doi: 10.1111/j.1432-1033.1994.00053.x. [DOI] [PubMed] [Google Scholar]
- 21.Marklund U, Larsson N, Melander Gradin H, Brattsand G, Gullberg M. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J. 1996;15:5290–5298. [PMC free article] [PubMed] [Google Scholar]
- 22.Marklund U, Osterman O, Melander H, Bergh A, Gullberg M. The phenotype of a “Cdc2 kinase target site-deficient” mutant of oncoprotein 18 reveals a role of this protein in cell cycle control. J Biol Chem. 1994;269:30626–30635. [PubMed] [Google Scholar]
- 23.Melander Gradin H, Marklund U, Larsson N, Chatila T A, Gullberg M. Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol Cell Biol. 1997;17:3459–3467. doi: 10.1128/mcb.17.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minotti A M, Barlow S B, Cabral F. Resistance to antimitotic drugs in Chinese hamster ovary cells correlates with changes in the level of polymerized tubulin. J Biol Chem. 1991;266:3987–3994. [PubMed] [Google Scholar]
- 25.Roos G, Brattsand G, Landberg G, Marklund U, Gullberg M. Expression of oncoprotein 18 in human leukemias and lymphomas. Leukemia. 1993;7:1538–1546. [PubMed] [Google Scholar]