Abstract
What changes in cortical organization characterize global and localized variation between humans and chimpanzees remains a topic of considerable interest in evolutionary neuroscience. Here we examined regional variation in cortical thickness, gyrification and white matter in samples of human and chimpanzee brains. Both species were MRI scanned on the same platform using identical procedures. The images were processed and segmented by FSL and FreeSurfer and the relative changes in cortical thickness, gyrification and white matter across the entire cortex were compared between species. In general, relative to chimpanzees, humans had significantly greater gyrification and significantly thinner cortex, particularly in the frontal lobe. Human brains also had disproportionately higher white matter volumes in the frontal lobe, particularly in prefrontal regions. Collectively, the findings suggest that after the split from the common ancestor, white matter expansion and subsequently increasing gyrification occurred in the frontal lobe possibly due to increased selection for human cognitive and motor specializations.
Introduction
After adjustment for body size, the human brain is approximately 3.5 larger than that of our closet living relative, the chimpanzee (Rilling 2006; Schoenemann 2006; Sherwood et al. 2012). In addition to overall volumetric differences, it has also been reported that humans have a disproportionally more gyrified brain (Rilling and Insel 1999; Rogers et al. 2010; Zilles et al. 2013) and larger white compared to grey matter volumes (Rilling and Insel 1999; Rogers et al. 2010) than chimpanzees and other more distantly related primates. However, there is still considerable debate over whether changes in brain size and gyrification are uniform across the cortex or are disproportionally larger for some areas compared to others. Some have suggested that the temporal, parietal and frontal lobes, and specifically prefrontal cortex (PFC), have become disproportionally larger in humans compared to chimpanzees and other more distantly related primates (Semendeferi et al. 2001; Semendeferi et al. 1997; Semendeferi et al. 2002; McBride et al. 1999; Deacon 1997; Rilling and Seligman 2002; Holloway 2002; Brodmann 1912) and this change reflects increasing selection for human-specific cognitive, motor and linguistic functions in Homo after the split with the common ancestor with Pan (Reader and Laland 2002; Deacon 1997; De Felipe 2011). For example, Schoenemann et al. (2005) compared the relative volume of prefrontal cortex in 11 primate species and found that humans had disproportionally larger white but not grey volumes compared to all other primates, including chimpanzees. Similarly, Rilling and Insel (1999) measured gyrification from 10 equally spaced coronal slices along the anterior-posterior axes of brains of 11 primate species. Regression of gyrification on brain volume showed that humans had a disproportionately more gyrified prefrontal region, especially in the most anterior region.
One challenge in the study of comparative brain organization is that these investigations traditionally rely on common sulcal and other anatomical landmarks to define specific regions of interest. For highly conserved brain regions, such as the lobes, this is less problematic (Semendeferi and Damasio 2000; Semendeferi et al. 1997; Semendeferi et al. 2002) but for more specific regions within a lobe such as the PFC, this can be prohibitive due to individual and phylogenetic variation in local cortical folding and gyrification (Sherwood et al. 2003; Keller et al. 2009a; Sherwood et al. 2005b). Moreover, the procedure of manually outlining the region of interest can be very time consuming and rater subjective. A second limitation in extant comparative brain studies is that different dimensions of cortical organization are evaluated separately or independently of other potentially relevant neurological features. For instance, differences in cortical surface area, gyrification and white matter volume have been investigated separately without consideration of the potential interrelationship between these measures of neural organization (Sherwood et al. 2012; Schoenemann 2006). This latter point is particularly important because some have hypothesized that expansion in white matter volume or connectivity underlies both individual and phylogenetic variation in gyrification and cortical thickness (Van Essen 1997; Seldon 2005; Kevala et al. 2013).
As a means of further exploring brain evolution in primates, relative white matter distribution, gyrification and cortical thickness were compared in magnetic resonance images collected in humans and chimpanzees. The software program FreeSurfer was used for the analysis and this allowed comparison between human and chimpanzee white matter, gyrification and cortical thickness using two different strategies. First, FreeSurfer determines a vertex-wise correspondence between the folding pattern of an individual brain and a reference template based on high-dimensional non-linear registration in a spherical space. With the inter-subject correspondence projected back to the native space, all brains can be put in a common coordinate system and are allowed for convenient vertex-by-vertex comparison. Thereby, the use of vertex-based analyses within and between the chimpanzee and human brain scans facilities the comparison between these species in the measurement of local gyrification and cortical thickness across the entire cortex. Second, for individual brains, FreeSurfer can assign an anatomical label to each of the surface vertices based on the probabilistic information estimated from its neighboring folding pattern and a set of manually pre-labeled training sets. For example, using the so-called Desikan-Killany atlas, FreeSurfer can parcellate the cortex into 34 different cortical labels (Fischl et al. 2004b; Desikan et al. 2006) (See Figure 1a and 1b). Though the Desikan-Killany atlas was developed for the human brain, a case can be made for its application to the chimpanzee brain. Specifically, despite the difference in overall brain size and convolution, the general brain anatomy is similar between humans and chimpanzees and the Desikan-Killany parcellation was largely based on common and large-scale gyri and sulci shared between species. In addition, the non-linear registration procedure in FreeSurfer gives a greater effect to those consistent folding patterns than more variable patterns by the introduction of a folding pattern variance component in the registration force energy function (Dale et al. 1999b). Therefore, in this paper, we applied the Desikan-Killany atlas to the chimpanzee brain (Figure 1b). The preliminary analyses of the scans indicated that, despite the difference in overall brain size and convolution, the Desikan-Killany maps retained good spatial alignment when warped onto the chimpanzee brain (see Movie S1).
Figure 1a and b:
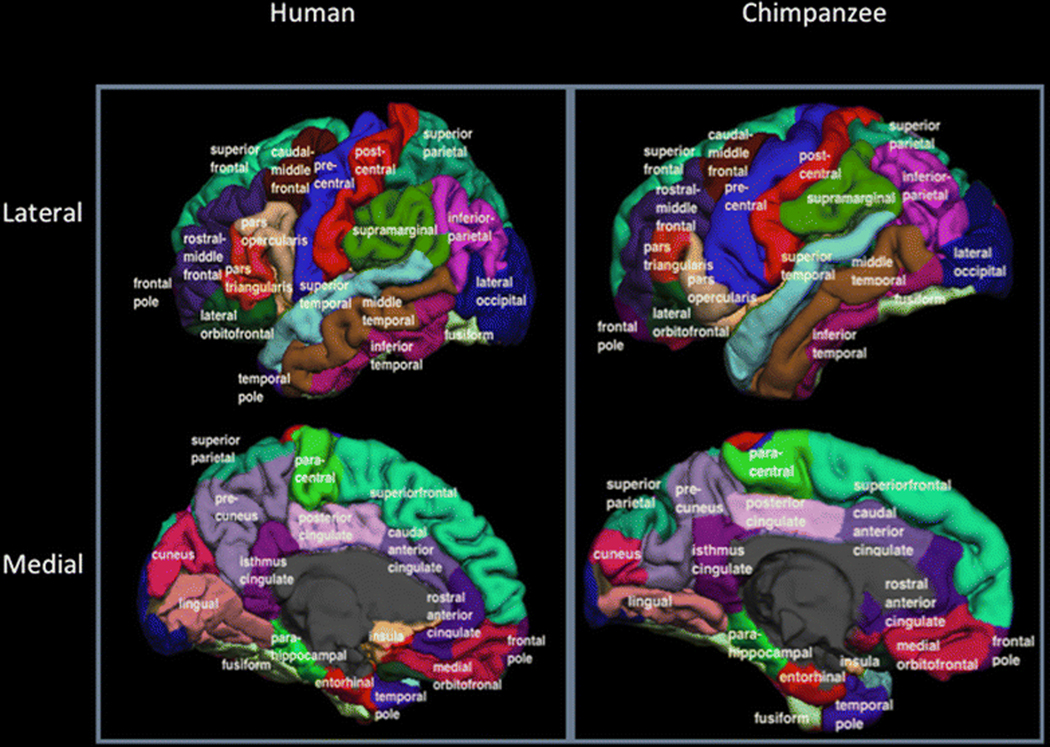
Cortical surface parcellation based on the Desikan-Killiany atlas in an example human and chimpanzee Brain. Gyri for each species are labelled.
By using this approach it was possible to quantify the relative cortical thickness, gyrification and underlying white matter for well defined regions of interest that are largely overlapping between humans and chimpanzees. For both strategies, two hypotheses were tested. First, Van Essen (1997) has proposed that localized variation in cortical folding is caused by increased tension formed by development of cortical connections between different regions. Thus, global and localized differences in gyrification would presumably reflect variation in white matter connectivity within and between species. In addition to gyrification, Seldon (2005) has proposed that increasing white matter during primate brain evolution resulted in decreasing global and localized variation in cortical thickness. In this model, as white matter volume expanded, cortical surface area also expanded which resulted in a thinning in cortical thickness. Based on the assumption and previous reports that humans have disproportionally larger white matter volume within prefrontal cortex, the theories proposed by Van Essen (1997) and by Seldon (2005) predict that humans have significantly thinner and more gyrified cortex compared to chimpanzees, particularly within prefrontal cortex.
Methods
Subjects
Twenty-seven adult humans (13 females, 14 males) and 29 chimpanzees (17 females, 12 males) were MRI scanned using the exact same scanning platform and protocol (see Keller et al. 2009b for description). The chimpanzees were all members of the colony housed at the Yerkes National Primate Research Center (YNPRC) of Emory University and ranged in age from 13 to 50 years. Institutional approval for the study of humans and animals in this study was approved prior to the onset of data collection.
MRI Scanning Procedure
Detailed methods of the scanning parameters for each species have been described elsewhere (Keller et al. 2009b). Briefly, T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) MR images were obtained using a Siemens 3 tesla Trio MR system (TR = 2300 ms, TE = 4.4 ms, TI = 1100 ms, Flip angle = 8, FOV = 200mm X 200 mm). Chimpanzee images were acquired at Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia. Human images were acquired at the Magnetic Resonance and Image Analysis Research Centre at the University of Liverpool (Liverpool, UK). Acquisition parameters were made to be as similar as possible for humans and chimpanzees (see Table 1 from Keller et al, 2009). The data matrix size was 320X320 for both species but the slice thickness was 0.6 mm for the chimpanzees and 1 mm for humans, in the y plane. Reducing the voxel resolution to 1.0 mm in the y direction in humans resulted in an acquisition time of 12 min, which contrasted to the 36 min (y = 0.6 mm) chimpanzee scan. However, the in-plane voxel resolution was 0.6 X 0.6 mm for both humans and chimpanzees, resulting in a very similar between-tissue contrast. All humans provided written consent to participate in this study, which had local Research Ethics Committee (REC) approval. For the chimpanzee scans, subjects were first immobilized by ketamine injection (10 mg/kg) and subsequently anesthetized with propofol (40–60 mg/kg/hr) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility and remained anesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time, ~2 h). Subjects were placed in the scanner in a supine position with their head fitted inside the human-head coil.
Table 1.
Average Neocortical, White Matter and Cerebral Hemisphere Volume in 27 Humans and 29 Chimpanzees
| Cerebral cortex | White Matter | Total Brain | |
|---|---|---|---|
| Human | |||
| Left | 252.2 | 252.8 | |
| Right | 251.5 | 2524 | |
| Total | 503.6 | 505.2 | 1199.6 |
| Chimpanzee | |||
| Right | 50.9 | 67.4 | |
| Left | 50.7 | 67.4 | |
| Total | 101.6 | 134.8 | 295.5 |
Values are in cc3
Image Analysis
The analyses of differences in the variation in cortical thickness and gyrification and the distribution of white matter between the human and chimpanzee brain were performed using FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and FreeSurfer (https://surfer.nmr.mgh.harvard.edu/) software. In particular, within FSL (i) the BET function was used to automatically strip away the skull, (ii) the FAST function was used to correct for intensity variations due to magnetic susceptibility artefacts and radio frequency field inhomogeneities (i.e. bias field correction) and (iii) the FLIRT function was used to normalise the isolated brain to the MNI152 template brain using a 7 degree of freedom transformation (i.e. 3 translations, 3 rotations and 1 uniform scaling) which preserved the shape of individual brains. Next each brain was segmented using FreeSurfer. The fact that the brains are already isolated, bias field corrected and size normalized greatly assists with respect to segmenting the chimpanzee brain in FreeSurfer. FSL also has the specific benefit, as mentioned above, of enabling the individual brains to be spatially normalised with preserved brain shape, and the values of this transformation matrix and the scaling factor were saved for later use.
The following FreeSurfer pipeline was employed: (i) the bias field correction was repeated to assist in the classification of the voxel data into different tissue types and to locate the boundaries between different brain matters, (ii) the white matter is labelled, split into two cerebral hemispheres and the cerebellum and brain stem are removed, (iii) a triangular mesh is fitted to cover the outer voxels of the connected white matter component for each cerebral hemisphere and deformed with respect to the intensity gradients between the white and grey matter to produce a final mesh with sub-voxel resolution, (iv) the resulting surface mesh representing the boundary between the cerebral cortex and underlying white matter is expanded along the direction of the intensity gradients between grey matter and CSF until it coincides with the pial surface and topologically corrected (Dale et al. 1999a, b), (v) at each surface location (i.e., vertex), the detailed anatomical information (e.g., folding patterns) was modelled quantitatively by a set of curvature-based descriptors (e.g., the spatial relationship of a vertex to neighbouring vertices). These descriptors later served as natural anatomical landmarks to compute the inter-subject correspondence using a high-dimensinal non-linear registration on the sphere space between an individual brain and a reference template through a matching process. As such, the vertex-wise correspondence is determined so as to put all brains in a common coordinate system and allows convenient vertex-by-vertex comparison in corresponding folds and regions in native space (Fischl et al. 1999).
In this study, the primary focus for the surface based analysis was cortical thickness (CT) and local gyrification index (GI). CT at each surface location (or vertex) is defined as the average of the closest distance in either direction between the white and the pial surfaces (Fischl and Dale 2000). GI was computed in the following four step process, (i) the morphology closing was applied to fill the sulcus of brain volume, (ii) a triangle mesh is then fit to the sulcus filled volume to generate an outer smoothed surface that tightly wrapping the brain pial surface, (iii) at each sample vertex of the smoothed outer surface, a circular Region of Interest (ROI) was defined as the intersection of the outer surface and a sphere (with radius as 25mm) centred on the vertex. The corresponding region on the pial surface was found by searching for the closest points to the vertices on the perimeter of ROI on the pial surface and linking them via the geodesic path, iv) the GI at each vertex of the smoothed outer surface was computed as the area ratio between the two corresponding regions and was propagated to the cortical pial surface based on the contribution of the surface of each pial surface vertex to the computation of smoothed surface GI weighted by relative distance. More details can be found in Schaer et al. (2008). In order to increase the signal-to-noise ratio of the vertex-based comparison, a Gaussian smoothing with full-width half-maximum of 15 mm was applied to the computed values of CT and GI.
As mentioned above, FreeSurfer is able to automatically assign a neuroanatomical label to each vertex of cortical surface based on the probabilistic information estimated by incorporating geometric information (e.g., curvature) derived from brain cortical surface and the neuroanatomical convention represented in certain pre-trained atlases. For instance, the so-called Desikan-Killiany atlas, was trained from a reference cohort of 40 brains that were manually parcellated into 34 regions per hemisphere (Fischl et al. 2004a; Fischl et al. 2002). By virtue of the above atlas-based labelling technique, individual brains can be parcellated into 68 regions (two hemispheres X 34 maps) and average values of CT and GI may be reported for each brain for all these regions. In order to allow comparison of the relative regional variation of CT and GI between humans and chimpanzees, values were standardized to an average value of unity within the left and right hemispheres for individuals within each species. This resulted in values that reflected the relative increase or decrease within each species, after adjustment for within individual and species variation for each brain trait. A second analysis concerned the distribution of white matter. Based on the abovementioned atlas labelling, each white matter voxel in the volume was given the label of its closest cortical vertex. The regional white matter volume was obtained by summing the voxels belonging to each ROI. Subsequently, regional white matter volume at each ROI was expressed as a percentage of total white matter volume. We also calculated asymmetries in cortical thickness (CT-AQ), gyrification (GI-AQ) and white matter (WM-AQ) for each Desikan region. This was accomplished using the formula [AQ=(R – L)/ ((R + L) * 5)] where R and L represented the right and left hemisphere CT, GI and WM values for each subject and region.
Data Analysis
For the ROI based comparisons, analyses of variance (ANOVA) or t-tests were performed to compare relative CT, GI and white matter volume between species with alpha was set to p < 0.05. For the vertex-based analyses, voxel-by-voxel t-tests were performed with alpha set to p < 0.01, uncorrected for multiple comparisons. Population-level asymmetries for each region within each species were tested using one sample t-tests with alpha set to p < 0.01. The raw data used in these analyses can be found in Supplementary Table 1.
Results
Descriptive Data on Total Neocortical, White Matter and Cerebral Hemisphere Volume
The volume of the neocortex, white matter and total cerebral hemisphere volume are presented in Table 1. The total volume of the human brain was 4.06 times larger than the chimpanzee brain. Moreover the human brain had 4.95 times more cerebral cortex than the chimpanzee brain and 3.75 times more white matter such that the ratio between these quantities is 1 for the human brain and 0.75 for the chimpanzee brain.
Vertex Based Analysis
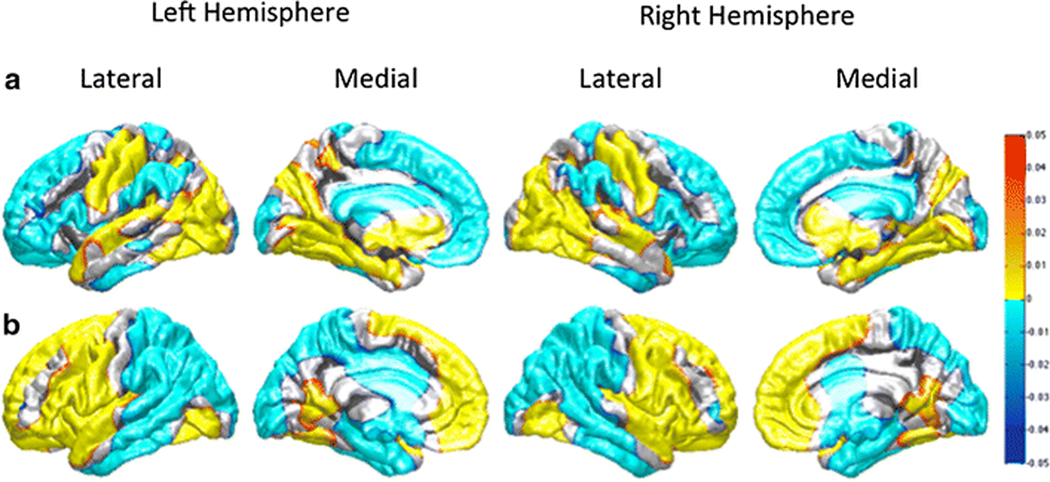
Inter-species variation in the relative distribution of CT and GI between human and chimpanzees was initially investigated using the vertex-based approach (see Figure 2a and 2b). Figure 2a shows that compared to chimpanzees, humans have a relatively thinner cortex in the lateral and medial premotor and prefrontal but not primary motor cortex within the frontal lobe. Further, humans have thinner cortex within the inferior parietal region, supramarginal gyrus, and the middle and anterior regions of the inferior temporal cortex. In contrast, humans have thicker cortex in primary motor and somatosensory cortex, primary auditory cortex within the temporal lobe and throughout most of the lateral and medial occipital lobe. The comparative results on GI revealed largely opposite results to those found for cortical thickness (Figure 2b). With the exception of a small region in the dorso-lateral prefrontal cortex, humans had more gyrified frontal lobes than chimpanzees. Humans also had more gyrified cortex in the anterior regions of the temporal lobe including the parahippocampal and entorhinal cortex.
Figure 2:
Vertex-wise Comparison significance maps. (a) Vertex-wise thickness comparison significance map. The hot color regions (yellow) show where the cortex is relatively thicker in humans than chimpanzees. The cool color (cyan) shows where the cortex is relatively thicker in chimpanzees than humans. b) Vertex-wise gyrification comparison significance map. The hot color regions (yellow) show where gyrification is relatively greater in humans than chimpanzees. The cool color (cyan) shows where gyrification is relatively greater in chimpanzees than humans.
Desikan Based Analysis
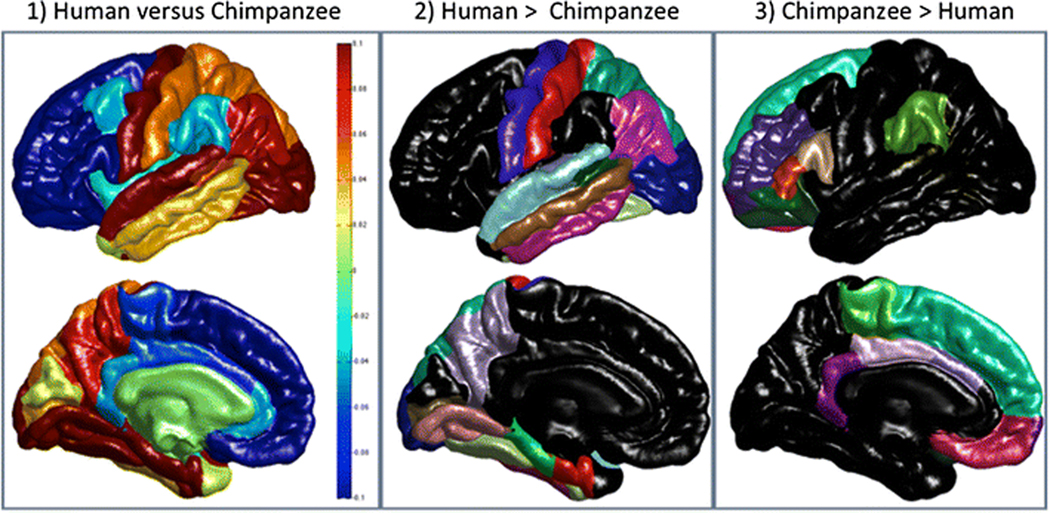
Multiple analysis of variance (MANOVA) was used for these analyses with each region-of-interest serving as dependent measure while sex and species served as between group factors. For CT, the MANOVA revealed a significant main effect for species F (34, 19) = 36.98, p < 0.001. No other main effects or interactions were found. The subsequent univariate F-tests for each region are shown in Table 1. Significant differences were found in 29 of the 34 regions (see Figure 3). Humans had, relatively speaking, significantly thinner cortex in 13 regions and 9 of these were within the frontal lobe (see Figure 3). Humans had significant thicker cortex in 16 regions, 15 within the temporal, parietal and temporal regions and 1 within the frontal lobe (see Figure 3).
Figure 3:
Cortical Thickness Comparisons: Lateral and medial views of maps of the difference in cortical thickness between the two species are shown in the first column accompanied by a vertical scale such that yellow to red colours indicate that the cortex is relatively thicker in humans than chimpanzees (scaled values > 0) and light to dark blue colours (scaled values < 0) indicate that the cortex is relatively thicker in chimpanzees than humans. The second column shows those regions of the Deskian-Killany atlas where the cortex is found be significantly thicker in humans and the third column shows regions where chimpanzees have significantly thicker cortex.
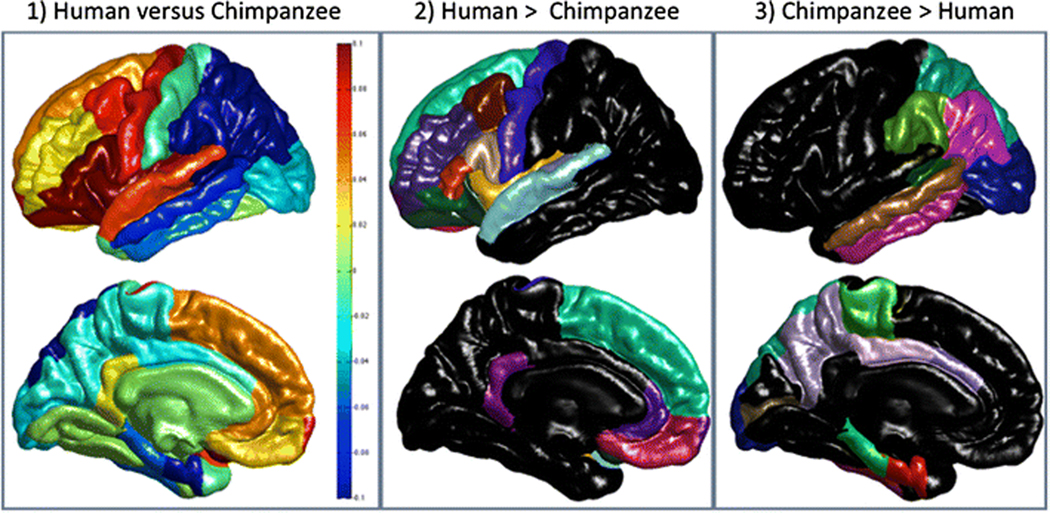
For GI, the MANOVA similarly revealed a significant main effect for species F (34, 19) = 38.73, p < 0.001. No other main effects or interactions were found. The subsequent univariate F-tests for each region are shown in Table 2. Like the CT results, significant species differences were found for a majority of the regions (29 of 34 possible regions) (see Figure 4). The human brain was, relatively speaking, significantly more gyrified in 15 regions, 11 of which were located in the frontal lobe, 2 in the temporal lobe and 2 in the cingulate (see Figure 4). Humans had significantly less gyrification in 14 regions, one of which is in the frontal lobe (see Figure 4).
Table 2.
Mean (+/− s.e.) Adjusted Grey Matter Thickness For Humans and Chimpanzees
| Region | Human | Chimpanzee | F | DIFF | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| Caudal Middle Frontal | 1.076 | (.018) | 1.112 | (.125) | 2.17 | H=C |
| Lateral Orbital Frontal | 1.140 | (.035) | 1.402 | (.067) | 327.03 | H<C |
| Medial Orbital Frontal | 0.971 | (.046) | 1.240 | (.112) | 133.78 | H<C |
| Para-central | 0.990 | (.024) | 1.053 | (.081) | 15.05 | H<C |
| Pars opercularis | 1.105 | (.023) | 1.230 | (.056) | 116.32 | H<C |
| Pars orbitalis | 1.134 | (.033) | 1.315 | (.086) | 104.86 | H<C |
| Pars triangularis | 1.081 | (.021) | 1.226 | (.071) | 102.86 | H<C |
| Pre Central | 1.044 | (.036) | 0.916 | (.117) | 29.70 | H>C |
| Rostral Middle Frontal | 1.021 | (.023) | 1.148 | (.044) | 180.79 | H<C |
| Superior Frontal | 1.155 | (.027) | 1.390 | (.055) | 397.41 | H<C |
| Frontal Pole | 1.080 | (.050) | 1.293 | (.143) | 53.51 | H<C |
| Insula | 1.254 | (.035) | 1.277 | (.072) | 2.15 | H=C |
| Temporal | ||||||
| Bank STS | 1.113 | (.029) | 1.054 | (.054) | 24.78 | H>C |
| Entorhinal | 1.346 | (.065) | 1.192 | (.143) | 26.26 | H>C |
| Fusiform | 1.104 | (.025) | 0.937 | (.070) | 138.91 | H>C |
| Inferior Temporal | 1.144 | (.030) | 1.109 | (.079) | 4.51 | H>C |
| Middle Temporal | 1.197 | (.030) | 1.164 | (.055) | 7.45 | H>C |
| Parahippocampal | 1.067 | (.049) | 0.919 | (.104) | 45.30 | H>C |
| Superior Temporal | 1.198 | (.031) | 1.092 | (.052) | 84.30 | H>C |
| Temporal Pole | 1.484 | (.072) | 1.470 | (.143) | 0.20 | H=C |
| Transverse Temporal | 1.052 | (.040) | 0.932 | (.066) | 66.80 | H>C |
| Parietal | ||||||
| Inferior Parietal | 1.050 | (.022) | 0.963 | (.060) | 49.80 | H>C |
| Post Central | 0.908 | (.030) | 0.859 | (.028) | 39.93 | H>C |
| Precuneus | 1.000 | (.030) | 0.917 | (.089) | 21.20 | H>C |
| Superior Parietal | 0.940 | (.020) | 0.890 | (.057) | 18.54 | H>C |
| Supramarginal | 1.083 | (.027) | 1.120 | (.046) | 13.28 | H<C |
| Occipital | ||||||
| Cuneus | 0.825 | (.033) | 0.808 | (.087) | 0.90 | H=C |
| Lateral Occipital | 0.934 | (.021) | 0.831 | (.060) | 71.46 | H>C |
| Lingual | 0.870 | (.033) | 0.749 | (.064) | 76.01 | H>C |
| Peri-calcarine | 0.761 | (.047) | 0.721 | (.073) | 5.66 | H>C |
| Cingulate | ||||||
| Caudal Anterior Cingulate | 0.979 | (.051) | 1.052 | (.111) | 9.51 | H<C |
| Rostral Anterior Cingulate | 1.003 | (.050) | 1.041 | (.177) | 1.20 | H=C |
| Posterior Cingulate | 0.987 | (.033) | 1.049 | (.074) | 15.78 | H<C |
| Isthmus Cingulate | 0.993 | (.042) | 1.036 | (.065) | 8.55 | H<C |
Red indicates human values significantly less than chimpanzees; Blue values indicate human values significantly higher than chimpanzee values. All univariate F-values evaluated at p < .05 with 1 and 52 degrees of freedom.
Figure 4:
Local Gyrification Comparisons: Lateral and medial views of maps of the difference in local gyrification between the two species are shown in the first column accompanied by a vertical scale such that yellow to red colours indicate that the cortex is relatively more gyrified in humans than chimpanzees (scaled values > 0) and light to dark blue colours (scaled values < 0) indicate that the cortex is relatively more gyrified in chimpanzees than humans. The second column shows those regions of the Desian-Killany atlas (same colour coding and labelling as in Figure 3) where human are significantly more gyrified and the third column shows regions where chimpanzees have comparatively greater gyrification.
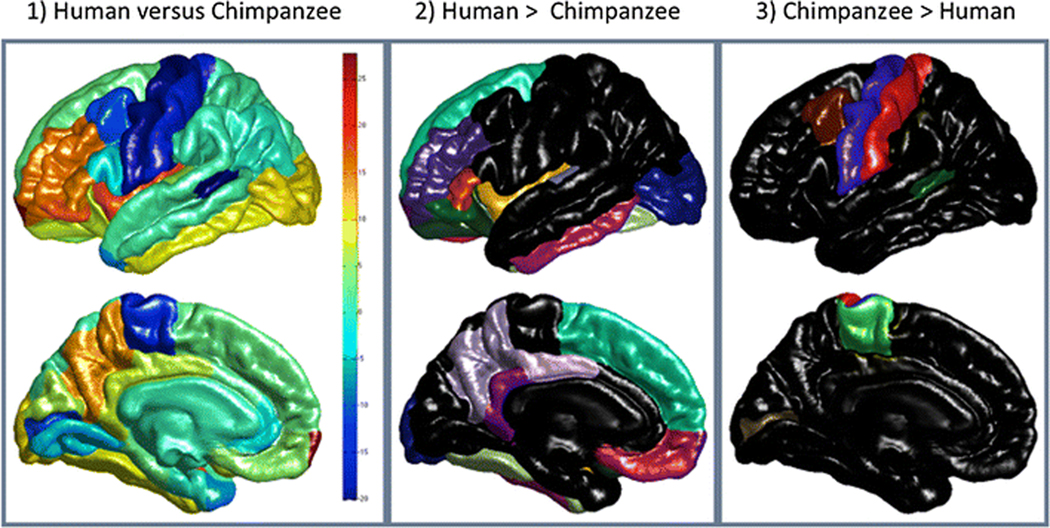
In the final ROI based analysis, the relative distribution of white matter was compared between humans and chimpanzees and the findings are presented in Figure 5 and in Table 3. As was the case for CT and GI, the MANOVA revealed a significant main effect for species F (34, 19) = 33.75, p <0. 001, but no main effect for sex nor was there a significant interaction between species and sex. The univariate F-tests revealed significant differences between humans and chimpanzees for 23 regions with humans having, relatively speaking, a significantly higher proportion of white matter for 17 regions, 8 of which are in the frontal lobe, and with humans having a lower proportion in 6 regions, 3 of which were in the frontal lobe.
Figure 5:
White Matter Volume Comparisons: Lateral and medial views of maps of the difference in white matter volume between the two species are shown in the first column accompanied by a vertical scale such that yellow to red colours indicate where white matter volume is greater in humans than chimpanzees (scaled values > 0) and light to dark blue colours (scaled values < 0) indicate white matter volume is relatively greater in chimpanzees. The second column shows those regions of the Desikan-Killany atlas (same colour coding and labelling as in Figure 3) where humans have significantly higher percentages of white matter volume and the third column shows regions where chimpanzees have comparatively more white matter volume.
Table 3.
Mean (+/− s.e.) Adjusted GI Scores for Humans and Chimpanzees
| Region | Human | Chimp | F | DIFF | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| Caudal Middle Frontal | 1.062 | (.038) | 0.989 | (.034) | 56.69 | H>C |
| Lateral Orbital Frontal | 0.893 | (.027) | 0.772 | (.032) | 227.88 | H>C |
| Medial Orbital Frontal | 0.679 | (.020) | 0.646 | (.023) | 33.03 | H>C |
| Para-central | 0.776 | (.023) | 0.796 | (.034) | 6.56 | H<C |
| Pars opercularis | 1.460 | (.050) | 1.133 | (.049) | 604.56 | H>C |
| Pars orbitalis | 1.004 | (.052) | 0.875 | (.035) | 121.78 | H>C |
| Pars triangularis | 1.279 | (.052) | 0.989 | (.037) | 587.95 | H>C |
| Pre Central | 1.160 | (.028) | 1.080 | (.022) | 143.97 | H>C |
| Rostral Middle Frontal | 0.927 | (.034) | 0.902 | (.025) | 10.14 | H>C |
| Superior Frontal | 0.734 | (.014) | 0.690 | (.018) | 99.295 | H>C |
| Frontal Pole | 0.677 | (.019) | 0.595 | (.027) | 166.3 | H>C |
| Insula | 1.549 | (.064) | 1.291 | (.057) | 251.25 | H>C |
| Temporal | ||||||
| Bank STS | 1.145 | (.046) | 1.264 | (.053) | 79.25 | H<C |
| Entorhinal | 0.819 | (.029) | 0.912 | (.052) | 67.97 | H<C |
| Fusiform | 0.846 | (.028) | 0.842 | (.029) | 0.21 | H=C |
| Inferior Temporal | 0.849 | (.025) | 0.901 | (.030) | 49.35 | H<C |
| Middle Temporal | 1.071 | (.030) | 1.140 | (.037) | 57.5 | H<C |
| Parahippocampal | 0.889 | (.037) | 0.943 | (.060) | 16.22 | H<C |
| Superior Temporal | 1.384 | (.042) | 1.324 | (.034) | 35.96 | H>C |
| Temporal Pole | 0.806 | (.032) | 0.814 | −0.047 | 0.53 | H=C |
| Transverse Temporal | 1.606 | (.056) | 1.527 | −0.047 | 32.61 | H>C |
| Parietal | ||||||
| Inferior Parietal | 0.849 | (.025) | 0.901 | (.030) | 49.35 | H<C |
| Post Central | 1.182 | (.025) | 1.193 | (.030) | 2.29 | H=C |
| Precuneus | 0.973 | (.033) | 0.994 | (.046) | 4.06 | H<C |
| Superior Parietal | 0.978 | −0.027 | 1.129 | (.044) | 230.38 | H<C |
| Supramarginal | 1.198 | (.028) | 1.294 | (.039) | 111.65 | H<C |
| Occipital | ||||||
| Cuneus | 0.988 | (.036) | 1.007 | (.055) | 2.35 | H=C |
| Lateral Occipital | 0.845 | −0.027 | 0.875 | −0.037 | 11.7 | H<C |
| Lingual | 0.944 | (.039) | 0.94 | −0.037 | 0.1 | H=C |
| Peri-calcarine | 0.965 | (.031) | 0.996 | (.044) | 9.13 | H<C |
| Cingulate | ||||||
| Caudal Anterior Cingulate | 0.643 | (.021) | 0.658 | (.023) | 5.97 | H<C |
| Rostral Anterior Cingulate | 0.682 | (.021) | 0.636 | (.023) | 61.36 | H>C |
| Posterior Cingulate | 0.734 | (.026) | 0.761 | −0.027 | 13.71 | H<C |
| Isthmus Cingulate | 0.931 | (.042) | 0.909 | (.051) | 3.08 | H>C |
Red indicates human values significantly less than chimpanzees; Blue values indicate human values significantly higher than chimpanzee values. All univariate F-values evaluated at p < .05 with 1 and 52 degrees of freedom.
Asymmetries
MANOVA analyses comparing the species revealed significant main effects for the CT_AQ F (34, 19) = 2.85, p < 0.008, GI_AQ F (34, 19) = 3.59, p < 0.002 and WM_AQ F (34, 19) = 4.72, p < 0.001 measures. Mean AQ scores in CT, GI and WM for each species and region as well as corresponding univariate F-values are shown in Tables 5 to 7. For all three measures, both chimpanzees and humans showed population-level asymmetries. For CT, human showed significant rightward or leftward asymmetries in 16 regions compared to 9 in the chimpanzees. For GI and WM, humans showed significant leftward or rightward asymmetries in 12 and 17 regions compared to 14 and 20 regions in chimpanzees, respectively. Further, for each measure, there was some overlap in directional asymmetries (or lack there of) between humans and chimpanzees and these are indicated in Table 5 to 7.
Table 5.
Summary of Differences Between Humans and Chimpanzees in Cortical Thickness, Gyrification and White Matter For Each Region
| Region | |||||
|---|---|---|---|---|---|
| Frontal (12) | Temporal (9) | Region (5) | Occipital (4) | Cingulate (4) | |
| Thickness | |||||
| H > C | 1 | 8 | 4 | 3 | 0 |
| H < C | 9 | 0 | 1 | 0 | 3 |
| H = C | 2 | 1 | 0 | 1 | 1 |
| Gyrification | |||||
| H > C | 11 | 2 | 0 | 0 | 2 |
| H < C | 1 | 5 | 4 | 2 | 2 |
| H = C | 0 | 2 | 1 | 2 | 0 |
| White Matter | |||||
| H > C | 8 | 5 | 1 | 1 | 2 |
| H < C | 3 | 1 | 1 | 1 | 0 |
| H = C | 1 | 3 | 3 | 2 | 2 |
H > C = humans greater than chimpanzees, H < C = chimpanzees greater than humans, H = C = no significant difference between chimpanzees and humans. Numbers in parentheses indicated the number of Desikan regions that were quantified within each of the 5 cortical regions of interest.
Table 7.
Mean (+/− s.e) Gyrification AQ Scores For Humans and Chimpanzees
| Region | Human | Chimpanzee | F | DIFF | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| Caudal Middle Frontal | +.002 | (.011) | +.008 | (.010) | 0.17 | No |
| Lateral Orbital Frontal | −.036 | (.009) | +.025 | (.009) | 24.18 | Yes |
| Medial Orbital Frontal | +.008 | (.008) | −.033 | (.008) | 12.53 | Yes |
| Para-central | +.011 | (.008) | +.003 | (.008) | 0.56 | No |
| Pars opercularis | (.000 | (.012) | +.014 | (.012) | 0.73 | No |
| Pars orbitalis | −.025 | (.014) | +.018 | (.013) | 5.01 | Yes |
| Pars triangularis | +.001 | (.013) | +.009 | (.013) | 0.15 | No |
| Pre Central | −.012 | (.006) | −.009 | (.006) | 0.13 | No |
| Rostral Middle Frontal | +.006 | (.007) | +.009 | (.007) | 0.11 | No |
| Superior Frontal | +.028 | (.007) | +.023 | (.007) | 0.22 | No |
| Frontal Pole | +.030 | (.010) | +.015 | (.010) | 1.12 | No |
| Insula | −.046 | (.011) | −.028 | (.011) | 1.34 | No |
| Temporal | ||||||
| Bank STS | +.001 | (.010) | −.032 | (.010) | 4.83 | Yes |
| Entorhinal | +.007 | (.012) | −.005 | (.011) | 0.55 | No |
| Fusiform | −.011 | (.008) | −.004 | (.008) | 0.29 | No |
| Inferior Temporal | −.031 | (.009) | −.039 | (.009) | 0.49 | No |
| Middle Temporal | −.040 | (.008) | −.046 | (.008) | 0.27 | No |
| Parahippocampal | +.006 | (.013) | +.002 | (.012) | 0.06 | No |
| Superior Temporal | −.017 | (.008) | −.011 | (.007) | 0.34 | No |
| Temporal Pole | −.018 | (.012) | −.014 | (.012) | 0.07 | No |
| Transverse Temporal | +.002 | (.010) | −.021 | (.010) | 2.56 | No |
| Parietal | ||||||
| Inferior Parietal | +.001 | (.009) | −.012 | (.008) | 1.25 | No |
| Post Central | −.007 | (.006) | −.003 | (.005) | 0.23 | No |
| Precuneus | +.040 | (.009) | +.038 | (.009) | 0.01 | No |
| Superior Parietal | −.006 | (.007) | −.001 | (.007) | 0.23 | No |
| Supramarginal | −.001 | (.007) | −.003 | (.007) | 0.05 | No |
| Occipital | ||||||
| Cuneus | +.033 | (.012) | +.032 | (.011) | 0.01 | No |
| Lateral Occipital | −.009 | (.008) | −.021 | (.008) | 1.08 | No |
| Lingual | +.017 | (.010) | +.039 | (.010) | 2.24 | No |
| Peri-calcarine | +.021 | (.011) | +.050 | (.011) | 3.47 | No |
| Cingulate | ||||||
| Caudal Anterior Cingulate | +.029 | (.006) | +.014 | (.006) | 3.38 | No |
| Rostral Anterior Cingulate | +.028 | (.007) | +.026 | (.007) | 0.08 | No |
| Posterior Cingulate | +.021 | (.009) | −.019 | (.009) | 9.21 | Yes |
| Isthmus Cingulate | +.022 | (.014) | +.026 | (.014) | 0.04 | No |
Red indicates significant leftward asymmetries; Blue values indicate significant rightward asymmetries. All univariate F-values evaluated at p < .05 with 1 and 52 degrees of freedom. One sample t-tests were used on the AQ scores to determine population-level asymmetries within each species with alpha set to p < .05.
Discussion
The results of this study indicate that there are significant differences between humans and chimpanzees in terms of cortical thickness, gyrification and white matter (see Table 4 for summary). Specifically, humans have thinner cortex more gyrified brains and increased white matter, particularly within regions of the frontal lobe including prefrontal and premotor but not the precentral gyrus. These results are generally consistent with the suggestion that expansion in white matter during primate evolution in the human line after the split from the common ancestor was a potentially important factor in the development of human specific cognitive and linguistic specializations. Our findings further indicate that both humans and chimpanzees show region specific population-level asymmetries in CT, GI and WM with some between species differences.
TABLE 4.
Mean (+/− s.e.) Percentage White Matter for Humans and Chimpanzees
| Region | Human | Chimp | F | DIFF | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| Caudal Middle Frontal | 3.424 | (.414) | 4.247 | (.542) | 40.26 | H<C |
| Lateral Orbital Frontal | 3.082 | (.222) | 2.675 | (.599) | 11.04 | H>C |
| Medial Orbital Frontal | 1.908 | (.272) | 1.748 | (.301) | 4.31 | H>C |
| Para-central | 2.036 | (.211) | 2.601 | (.329) | 57.53 | H<C |
| Pars opercularis | 1.598 | (.194) | 1.686 | (.315) | 1.54 | H=C |
| Pars orbitalis | 0.493 | (.056) | 0.353 | (.129) | 26.88 | H>C |
| Pars triangularis | 1.631 | (.184) | 1.214 | (.313) | 36.18 | H>C |
| Pre Central | 6.507 | (.545) | 9.777 | (.981) | 232.79 | H<C |
| Rostral Middle Frontal | 6.397 | (.695) | 4.697 | (.730) | 79.35 | H>C |
| Superior Frontal | 8.461 | (.547) | 8.048 | (.737) | 5.6 | H>C |
| Frontal Pole | 0.161 | (.063) | 0.091 | (.043) | 24.02 | H>C |
| Insula | 6.859 | (.446) | 4.827 | (.875) | 117.17 | H>C |
| Temporal | ||||||
| Bank STS | 1.354 | (.215) | 2.014 | (.250) | 111.43 | H<C |
| Entorhinal | 0.247 | (.051) | 0.247 | (.094) | 0.01 | H=C |
| Fusiform | 2.719 | (.231) | 2.299 | (.412) | 21.74 | H>C |
| Inferior Temporal | 2.416 | (.253) | 2.014 | (.282) | 31.33 | H>C |
| Middle Temporal | 2.340 | (.248) | 2.291 | (.337) | 0.37 | H>C |
| Parahippocampal | 0.712 | (.100) | 0.673 | (.269) | 0.5 | H>C |
| Superior Temporal | 3.176 | (.257) | 3.106 | (.371) | 0.67 | H=C |
| Temporal Pole | 0.186 | (.052) | 0.215 | (.105) | 1.6 | H=C |
| Transverse Temporal | 0.324 | (.052) | 0.294 | (.040) | 6.21 | H>C |
| Parietal | ||||||
| Inferior Parietal | 4.937 | (.506) | 4.943 | −0.677 | 0.01 | H=C |
| Post Central | 3.139 | (.327) | 4.335 | (.893) | 42.93 | H<C |
| Precuneus | 4.477 | (.349) | 3.395 | (.523) | 81.55 | H>C |
| Superior Parietal | 5.453 | (.467) | 5.334 | (.619) | 0.65 | H=C |
| Supramarginal | 4.219 | (.424) | 4.209 | (.355) | 0.01 | H=C |
| Occipital | ||||||
| Cuneus | 0.873 | (.101) | 0.783 | (.316) | 1.97 | H=C |
| Lateral Occipital | 3.707 | (.462) | 3.033 | −0.822 | 14.03 | H>C |
| Lingual | 2.051 | (.320) | 2.232 | (.472) | 2.77 | H=C |
| Peri-calcarine | 1.207 | (.234) | 1.492 | (.407) | 10.11 | H<C |
| Cingulate | ||||||
| Caudal Anterior Cingulate | 1.861 | (.200) | 1.798 | (.222) | 1.23 | H=C |
| Rostral Anterior Cingulate | 1.296 | (.190) | 1.354 | (.310) | 0.68 | H=C |
| Posterior Cingulate | 2.702 | (.201) | 2.389 | −0.276 | 23.28 | H>C |
| Isthmus Cingulate | 1.917 | (.196) | 1.647 | −0.243 | 20.76 | H>C |
Red indicates human values significantly less than chimpanzees; Blue values indicate human values significantly higher than chimpanzee values. All univariate F-values evaluated at p < .05 with 1 and 52 degrees of freedom.
With specific reference to white matter, Schoenemann et al. (2005) reported that humans had a disproportionately larger prefrontal region volume compared to apes and monkeys and the results of the present study appear to support these findings; however, the differences between humans and chimpanzees in white matter reported here differ from those of Schoenemann et al. (2005) in several important ways. First, differences in the relative proportion of white matter between humans and chimpanzees reported here were much more region specific than those reported by Schoenemann et al. (2005). Specifically, humans had relatively larger white matter percentages in the superior, lateral and orbital regions within the prefrontal cortex but the caudal regions did not differ. The method of defining the prefrontal cortex used by Schoenemann et al. (2005) was not as precise as the methods employed here and this likely explains these discrepancies. Notably, Schoenemann et al (2005) defined all the tissue anterior to the genu of the corpus callosum as prefrontal cortex without parcellation into either dorsalventral or medial-lateral divisions, as was the case in this study.
Second, the magnitude of human-chimpanzee differences in percentage white matter was relatively small compared to those reported by Schoenemann et al. (2005). These authors reported that white matter volume in humans was 41% larger than would be predicted for a primate of their brain size. In particular, Schoenemannn et al. (2005) used regression analyses to allometrically scale prefrontal white matter volume that included data from 11 primate species and as noted by Sherwood et al. (2005a), the predicted residual values can be influenced by the taxonomic composition of the sample. In a re-analysis of the data reported by Schoenemann et al. (2005), Sherwood and colleagues (2005a) restricted the taxonomic composition to the great ape clad (humans, chimpanzees, bonobos, gorillas, and orangutans) and found that human white matter prefrontal cortex was only 12% larger (as opposed to the original 41% value). If in the present study data on the precentral and paracentral regions are excluded and the average difference in white matter percentage between humans and chimpanzees is calculated, the value is 9.22%, a value similar to that reported by Sherwood et al. Further to this point, the range of percentage differences in white matter within the frontal lobe regions ranged from 27.78% (frontal pole) to −10.73% (caudal middle frontal lobe, see Table 3). Thus, the maximum percentage difference in white matter never reached the value reported by Schoenemann et al. (2005). This suggests that phylogenetic variation in white matter volume, particularly within the frontal lobe, is much more nuanced and perhaps reflects regional variation in connectivity associated with specific motor or cognitive skills. Further, estimates of allometric scaling in brain regions across primate species appear to be influenced by the range taxonomic groups represented in the sample and this needs to be considered when making inferences about the magnitude of differences between species. In sum, the find on white matter are largely consistent with previous comparative studies that have focused on comparisons between great apes and monkeys (Semendeferi and Damasio 2000; Semendeferi et al. 1997; Semendeferi et al. 2002; Schoenemann et al. 2005; Sherwood et al. 2005b).
As expected the present study confirms that humans have more gyrified brains than chimpanzees particularly within frontal lobe regions (Armstrong et al. 1993; Kochunov et al. 2010; Zilles et al. 1989; Zilles et al. 2013). In many ways, the differences found between humans and chimpanzees mimic those found for percentage white matter. In principal, this finding is consistent with the hypothesis of Van Essen that increasing white matter connectivity may be related to increasing gyrification both within and between species. Rilling and Insel (1999) reported that human prefrontal cortex was disproportionately more gyrified compared to other primates and there is support for this claim based on the results reported here. In particular, it is clear from Table 2 that the percentage difference in GI scores are much higher in frontal lobe regions compared to the temporal, parietal, occipital and cingulate regions. Moreover, the largest difference in GI scores was for the frontal pole, the cortical region most proximal to the region quantified by Rillling and Insel (1999). However, within frontal lobe (and indeed the entire brain) the largest difference in human and chimpanzee gyrification is in the pars opercularis and pars triangularis, regions that overlap with the cytoarchitectonic definition of Broca’s area in the left and right cerebral hemispheres (Schenker et al. 2010a; Amunts et al. 1999; Uylings et al. 2006; Amunts and Zilles 2006).
The difference in GI between humans and chimpanzees within Broca’s area was not restricted to the Desikan gyral analysis but was also evident in the vertex-based analysis (see Figures 5a & 5b); therefore these results do not seem to be an artifact of the sulci used to define the regions of interest. The differences in GI values between humans and chimpanzees in these two regions are largely consistent with anatomical descriptions of the sulcal folding patterns in these two species. Though the sulci used to define the pars opercularis are similar between humans and chimpanzees, several sulci used to define the pars triangularis, such as the horizontal ramus, which defines the anterior and inferior border of this region, are absent in nearly all chimpanzee post-mortem brains (Keller et al. 2012; Keller et al. 2007; Keller et al. 2009b). Furthermore, within human brains, some possess a dimple within the inferior frontal gyrus, which also appears absent in the chimpanzee brain. More generally, this finding is consistent with other results suggesting that there has been considerable expansion of Broca’s area in humans compared to chimpanzees and that this likely reflects human specializations for motor and cognitive functions associated with language and potentially other abilities such as praxic functions associated with tool-use and tool-making (Schenker et al. 2010a; Sherwood et al. 2012; Stout and Chaminade 2012). Also, it is worth noting that asymmetries in the pars opercularis and pars triangularis were largely consistent between humans and chimpanzees (see Tables 5 to 7); thus, the expansion of Broca’s area does not appear to have been selected for more in one hemisphere compared to the other (but see Schenker et al. 2010b).
Finally, the present study has revealed significant differences in cortical thickness between human and chimpanzees. Though both species tend to show thinner cortex in primary motor and sensory regions, humans had thinner cortex particularly within the frontal lobe. Combining these findings with those for white matter, the results are consistent with the hypothesis proposed by Seldon (2005) that increased white matter is associated with decreased cortical thickness.
A word of caution regarding cortical thickness is warranted in the context of the results reported here based on measures derived from MRI in comparison to studies in human and nonhuman brain quantified from post-mortem material. Cortical thickness, when measured from fixed post-mortem brain tissue, is considered highly conserved among primates, and indeed, mammalian brains (Changizi 2001). Further, the range of regional variation in cortical thickness is much smaller in post-mortem brains (2 – 4 mm) compared to in vivo measurements (2 to 7 mm) (Fischl and Dale 2000; Rabinowicz et al. 1999). The MRI measures reflect the sensitivity of the imaging sequence to distinguish between the pial surface and the white and grey matter boundary beneath the cortical surface. This is a less reliable measure of cortical thickness than can be obtained from stained post-mortem material. Therefore, the data reported here should not be interpreted as absolute measures of cortical thickness but rather as approximations of true cortical thickness.
With respect to the findings on asymmetry, both humans and chimpanzees showed region specific population-level asymmetries for all three measures though directional biases were not entirely consistent across regions within species. Thus, for example, humans showed a significant rightward asymmetry in the bank of the STS in cortical thickness but no significant biases in this same region for gyrification and white matter. In contrast, chimpanzees showed a significant leftward asymmetry in the bank of the STS for white matter but no significant biases in cortical thickness or gyrification. Some have suggested that asymmetries in cortical folding in regions within the STS distinguish humans from chimpanzees (LeRoy et al. 2015) but these findings would suggest that such differences may not similarly express themselves across different levels in cortical organization. Many of the directional asymmetries were consistent between species, though both absolute and directional differences were found for some regions. We considered absolute differences in asymmetry to be instances in which both species showed a significant population-level bias in the direction but the absolute of the AQ score was lesser in one species than the other. There were very few instances of absolute differences in asymmetry for CT, GI and WM. In contrast, directional asymmetries were those instances in which either both humans and chimpanzees showed a population-level but in opposite directions (i.e., the sign of the mean AQ scores were the opposite) or one species showed a significant population-level bias and the other did not. For CT, GI and WM, differences in directional asymmetry were found for 15, 6, and 13 regions, respectively.
We believe that some caution should be exercised in interpreting the findings on asymmetry for two reasons. First, compared to other studies on neuroanatomical asymmetries in humans and chimpanzees (Hopkins and Nir 2010; Bogart et al. 2012; Gomez-Robles et al. 2013; Hopkins 2013), the sample size is relatively small and the potential for Type I error is potentially too high. Second, the chimpanzee sample was fairly heterogeneous in terms of their individual hand preferences whereas the human sample was presumably, largely right-handed (though this was not tested). Assuming that handedness might influence some of the measures of asymmetry, without explicitly controlling for this factor, this could lead to spurious results.
One obvious limitation of this study is the sulcal landmarks used to define the 34 Desikan regions applied to the human and chimpanzee brains. The original Desikan maps were derived from a set of human reference brains and it must be acknowledged that though the same landmarks delineate most of the gyral maps, not every sulcus used to define the human regions of interest applies to the chimpanzee brain. For instance, as noted above, within the inferior frontal gyrus, some of the sulci used to define the pars triangularis are not present in the chimpanzee brain. This may conveniently explain the difference in GI scores found between humans and chimpanzees within this region. This is less of an issue for the cortical thickness and GI measure because the vertex-based, landmark free analyses largely confirmed the findings using the Desikan maps (see above). Notwithstanding the above caveats, the development of a sulcus-based cortical parcellation of the chimpanzee brain would be useful for determining exactly those folds and gyri that distinguish the species, once individual variation within the species is taken into account.
Finally, though not the focus of this study, another interesting finding was the lack of significant sex differences in cortical thickness. Previous studies in humans and chimpanzees have reported small but nonetheless significant sex differences in cortical thickness (Im et al. 2006; Luders et al. 2006). These previous studies had larger sample sizes than the present study, which may have been underpowered in terms of being able to detect statistically significant sex differences.
In conclusion, the results reported here show that after the split from the common ancestor approximately 5–6 mya, the human brain became increasingly gyrified and the cortex became relatively thinner, particularly within but not exclusively within prefrontal cortex. Comparative studies in other species should remind us though that having a large brain is not a necessary condition for having a more gyrified brain (Zilles et al. 2013). For instance, the recent paper by Kazu and colleagues (2014) nicely demonstrates that when species form wide taxonomic groups are matched on brain size, significant differences can be found, suggesting that here are likely other important neuronal characteristics influencing gyrification including neuron number and density within cortical regions. Finally, though there was a corresponding expansion in white matter in humans compared to chimpanzees, the extent was not as great as has been reported by some (Deacon 1997). Rather, the findings presented here suggest that increases in white matter in prefrontal regions were relatively modest in humans and were not uniform across the different regions.
Supplementary Material
Table 6.
Mean (+/− s.e) Grey Matter Thickness AQ Scores For Humans and Chimpanzees
| Region | Human | Chimpanzee | F | DIFF | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| Caudal Middle Frontal | −.003 | (.012) | −.018 | (.012) | 0.87 | No |
| Lateral Orbital Frontal | −.022 | (.009) | −.048 | (.009) | 3.92 | No |
| Medial Orbital Frontal | −.035 | (.014) | +.016 | (.014) | 6.79 | Yes |
| Para-central | −.005 | (.011) | +.031 | (.010) | 5.91 | Yes |
| Pars opercularis | +.005 | (.011) | −.014 | (.014) | 1.46 | No |
| Pars orbitalis | +.017 | (.019) | −.043 | (.018) | 3.74 | No |
| Pars triangularis | +.023 | (.017) | +.026 | (.016) | 0.01 | No |
| Pre Central | −.013 | (.011) | −.002 | (.010) | 0.53 | No |
| Rostral Middle Frontal | −.014 | (.010) | −.014 | (.010) | 0.01 | No |
| Superior Frontal | −.020 | (.006) | −.010 | (.006) | 1.32 | No |
| Frontal Pole | −.052 | (.036) | +.015 | (.035) | 1.82 | No |
| Insula | −.002 | (.009) | −.028 | (.009) | 3.90 | No |
| Temporal | ||||||
| Bank STS | +.045 | (.018) | −.016 | (.017) | 5.97 | Yes |
| Entorhinal | +.049 | (.037) | +.012 | (.036) | 0.51 | No |
| Fusiform | +.010 | (.012) | −.005 | (.012) | 0.75 | No |
| Inferior Temporal | +.013 | (.014) | −.012 | (.013) | 1.58 | No |
| Middle Temporal | +.016 | (.010) | −.017 | (.009) | 6.16 | Yes |
| Parahippocampal | −.013 | (018) | +.046 | (.017) | 5.65 | Yes |
| Superior Temporal | +.001 | (.008) | +.010 | (.008) | 0.60 | No |
| Temporal Pole | +.054 | (.020) | +.047 | (.019) | 0.54 | No |
| Transverse Temporal | −.012 | (.020) | +.077 | (.019) | 10.55 | Yes |
| Parietal | ||||||
| Inferior Parietal | +.024 | (.008) | −.006 | (.008) | 6.71 | Yes |
| Post Central | −.007 | (007) | −.001 | (.007) | 0.43 | No |
| Precuneus | −.009 | (.011) | .024 | (.010) | 5.34 | Yes |
| Superior Parietal | −.008 | (.009) | −.006 | (.009) | 0.02 | No |
| Supramarginal | +.013 | (.008) | −.007 | (.008) | 3.07 | No |
| Occipital | ||||||
| Cuneus | −.021 | (.013) | −.008 | (.013) | 0.46 | No |
| Lateral Occipital | +.035 | (.008) | +.009 | (.008) | 4.96 | Yes |
| Lingual | +.018 | (.019) | +.023 | (.008) | 0.18 | No |
| Peri-calcarine | −.008 | (016) | −.006 | (.015) | 0.01 | No |
| Cingulate | ||||||
| Caudal Anterior Cingulate | −.057 | (.018) | −.094 | (.018) | 2.11 | No |
| Rostral Anterior Cingulate | −.028 | (.021) | +.009 | (.020) | 1.56 | No |
| Posterior Cingulate | −.032 | (.013) | (.000 | (.013) | 2.96 | No |
| Isthmus Cingulate | −.041 | (015) | +.046 | (.015) | 17.12 | Yes |
Red indicates significant leftward asymmetries; Blue values indicate significant rightward asymmetries. All univariate F-values evaluated at p < .05 with 1 and 52 degrees of freedom. One sample t-tests were used on the AQ scores to determine population-level asymmetries within each species with alpha set to p < .05.
Table 8.
Mean (+/− s.e) White Matter AQ Scores For Humans and Chimpanzees
| Region | Human | Chimpanzee | F | DIFF | ||
|---|---|---|---|---|---|---|
| Frontal | ||||||
| Caudal Middle Frontal | −.143 | (.031) | −.167 | (.030) | 0.32 | No |
| Lateral Orbital Frontal | +.026 | (.025) | +.226 | (.024) | 32.84 | Yes |
| Medial Orbital Frontal | −.076 | (.033) | −.194 | (.032) | 6.73 | Yes |
| Para-central | +.205 | (.020) | +.210 | (.019) | 0.03 | No |
| Pars opercularis | −.081 | (.035) | −.136 | (.034) | 1.27 | No |
| Pars orbitalis | +.216 | (.059) | +.370 | (.013) | 3.55 | No |
| Pars triangularis | +.071 | (.053) | −.022 | (.026) | 1.58 | No |
| Pre Central | +.015 | (.014) | −.012 | (.014) | 1.86 | No |
| Rostral Middle Frontal | +.040 | (.027) | +.070 | (.026) | 0.65 | No |
| Superior Frontal | −.032 | (.016) | +.019 | (.016) | 5.07 | Yes |
| Frontal Pole | +.375 | (.049) | +.354 | (.047) | 0.1 | No |
| Insula | +.011 | (.021) | −.020 | (.020) | 1.07 | No |
| Temporal | ||||||
| Bank STS | −.028 | (.044) | −.095 | (.042) | 1.21 | No |
| Entorhinal | −.182 | (.069) | −.089 | (.067) | 0.94 | No |
| Fusiform | −.022 | (.019) | −.020 | (.019) | 0.01 | No |
| Inferior Temporal | −.055 | (.022) | −.039 | (.022) | 0.28 | No |
| Middle Temporal | +.098 | (.008) | +.167 | (.026) | 3.31 | No |
| Parahippocampal | +.028 | (.049) | −.025 | (.048) | 0.59 | No |
| Superior Temporal | −.104 | (.015) | +.012 | (.014) | 32.45 | Yes |
| Temporal Pole | −.070 | (.062) | −.011 | (.060) | 0.46 | No |
| Transverse Temporal | −.445 | (.030) | −.41 | −.029 | 2.42 | No |
| Parietal | ||||||
| Inferior Parietal | +.174 | (.019) | +.078 | (.018) | 13.08 | Yes |
| Post Central | +.028 | (.021) | −.069 | (.021) | 10.76 | Yes |
| Precuneus | +.065 | (.022) | +.043 | (.021) | 0.51 | No |
| Superior Parietal | −.043 | (.015) | −.053 | (.015) | 0.22 | No |
| Supramarginal | +.027 | (.016) | +.058 | (.016) | 1.83 | No |
| Occipital | ||||||
| Cuneus | −.021 | (.047) | −.154 | (.046) | 4.09 | Yes |
| Lateral Occipital | −.021 | (.020) | −.05 | (.019) | 1.05 | No |
| Lingual | .000 | (.023) | .007 | (.022) | 0.42 | No |
| Peri-calcarine | +.030 | (.045) | −.035 | (.044) | 1.09 | No |
| Cingulate | ||||||
| Caudal Anterior Cingulate | +.067 | (.031) | −.004 | (.030) | 2.63 | No |
| Rostral Anterior Cingulate | −.209 | (.030) | −.245 | (.031) | 0.67 | No |
| Posterior Cingulate | −.015 | (.025) | −.052 | (.025) | 1.10 | No |
| Isthmus Cingulate | −.123 | −.027 | −.090 | (.026) | 0.78 | No |
Red indicates significant leftward asymmetries; Blue values indicate significant rightward asymmetries. All univariate F-values evaluated at p < .05 with 1 and 52 degrees of freedom. One sample t-tests were used on the AQ scores to determine population-level asymmetries within each species with alpha set to p < .05.
Acknowledgement
This research was supported by NIH grants MH-92923, NS-42867, NS-73134 and HD-60563 to WDH and National Center for Research Resources P51RR165 to YNPRC, which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132). American Psychological Association and Institute of Medicine guidelines for the treatment of chimpanzees in research were followed during all aspects of this study. NR is at The University of Edinburgh Clinical Research Imaging Centre (CRIC: http://www.cric.ed.ac.uk) which is part of the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) collaboration (http://www.sinapse.ac.uk/) that is funded by the Scottish Funding Council, Scottish Executive Chief Scientist Office, and the six collaborator Universities. NR is a member of the Centre for Cognitive Ageing and Cognitive Epidemiology (http://www.ccace.ed.ac.uk) part of the cross council Lifelong Health and Wellbeing Initiative, G0700704/84698). XL is supported by the T.J. Crow Psychosis Trust Fund. Inquiries regarding this paper may be sent to: William D. Hopkins, Neuroscience Institute, Georgia State University, P.O. Box 5030, Atlanta, Georgia 30302-5030. whopkins4@gsu.edu or whopkin@emory.edu
References
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: Cytoarchitecture and intersubject variability. The Journal of Comparative Neurology 412 (2):319–341 [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K (2006) A multimodal analysis of structure and function in Broca’s area. In: Grodzinsky Y, Amunts K (eds) Broca’s Region. Oxford University Press, Oxford, [Google Scholar]
- Armstrong E, Zilles K, Schleicher A (1993) Cortical folding and the evolution of the human brain Journal of Human Evolution 20:341–348 [Google Scholar]
- Bogart SL, Mangin JF, Schapiro SJ, Reamer L, Bennett AJ, Pierre PJ, Hopkins WD (2012) Cortical sulci asymmetries in chimpanzees and macaques: A new look at an old idea. Neuroimage 61:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K (1912) Neue Ergebnisse über die vergleichende histologische localisation der grosshirnrinde mit besonderer berücksichtigung des stirnhirns. Anatomischer Anzeiger Supplement 41:157–216 [Google Scholar]
- Changizi MA (2001) Principles underlying mammalian neocortical scaling. Biological Cybernetics 84:207–215 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999a) Cortical surface-based analysis: I. Segementation and surface reconstruction. NeuroImage 9 (2):179–194 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999b) Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. NeuroImage 9 (2):195–207 [DOI] [PubMed] [Google Scholar]
- De Felipe J (2011) The evolution of the brain, the human nature of cortical curcuits and intellectual creatibity. Fronters in Neuroanatomy 5 (29):1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW (1997) The Symbolic Species: The coevolution of language and the brain. W. W. Norton and Company, New York [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRi scans into gyral based regions of interest. NeuroImage 31:968–980 [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences 97 (20):11050–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dietrich M, Haselgrove C, Kouwe Avd, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33 (3):341–355 [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999) High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping 8 (4):272–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D (2004a) Auomatically parcellating the human cerebral cortex. Cerebral Cortex 14 (1):11–22 [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kuwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004b) Automatically parcellating the human cerebral cortex. Cerebral Cortex 14:11–22 [DOI] [PubMed] [Google Scholar]
- Gomez-Robles A, Hopkins WD, Sherwood CC (2013) Increased morphological asymmetry, evolvability, and plasticity in human brain evolution. Proceedings of the Royal Society B: Biological Sciences 280 (1761):20130575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway RL (2002) Brief Communication: How much larger Is the relative volume of area 10 of the prefrontal cortex in humans? American Journal of Physical Anthropology 118 (389–401) [DOI] [PubMed] [Google Scholar]
- Hopkins WD (2013) Behavioral and brain asymmetries in chimpanzees: A case for continuity. Annals of the New York Academy of Sciences 1288:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Nir T (2010) Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): The effect of handedness and comparison within findings in humans. Behavioural Brain Research 208 (2):436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Shin YW, Kim IY, Kwon JS, Kim SI (2006) Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage 31:31–38 [DOI] [PubMed] [Google Scholar]
- Kazu RS, Maldonado J, Mota B, Manger PR, Herculano-Houzel S (2014) Cellular scaling rules for the brain of Artiodactyla include a highly folded cortex with few neurons. Frontiers in Neuroanatomy 12 (8):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Crow TJ, Foundas AL, Amunts K, Roberts N (2009a) Broca’s area: Nomenclature, anatomy, typology and asymmetry Brain and Language 109:29–48 [DOI] [PubMed] [Google Scholar]
- Keller SS, Deppe M, Herbin M, Gilissen E (2012) Variabilty and asymmetry of the suclal contours defining Broca’s area homologue in the chimpanzee brain. Journal of Comparative Neurology 520:1165–1180 [DOI] [PubMed] [Google Scholar]
- Keller SS, Highley JR, Garcia-Finana M, Sluming V, Rezaie R, Roberts N (2007) Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. Journal of Anatomy 211:534–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Hopkins WD (2009b) A comparative magnetic resonance imaging study of the anatomy, variability and asymmetry of Broca’s area in the human and chimpanzee brain Journal of Neuroscience 29:14607–14616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevala I, Lewitus E, Huttner WB (2013) The secondary loss of gyrencephaly as an example of evolutionary phenotypical reversal. Frontiers in Anatomy 7 (16):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov PV, Glahn DC, Fox PT, Lancaster JL, Saleem KS, Shelledy W, Zilles K, Thompson PM, Coulon O, Mangin JF, Blangero J, Rogers J (2010) Genetics of primary cerebral gyrification: Heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. NeuroImage 53 (3):1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy F, Cai Q, Bogart SL, Dubois J, Coulon O, Monzalvo K, Fischer C, Glasel H, Van der Haegen L, Benezit A, Lin CP, Kennedy DN, Ihara AS, Hertz-Pannier L, Moutard ML, Poupon C, Brysbaert M, Roberts N, Hopkins WD, Mangin JF, Dehaene-Lambertz G (2015) New human-specific brain landmark: The depth asymmetry of superior temporal sulcus. Proceedings of the National Academcy of Sciences [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, DeLuca H, Jancke L, Toga AW (2006) Gender effects on cortial thickness and the influence of scaling Human Brain Mapping 27:314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride T, Arnold SE, Gur RC (1999) A comparative volumetric analysis of the prefrontal cortex in human and baboon MRI. Brain, Behavior and Evolution 54:159–166 [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean DE, McFDonald-Comber J, Courten-Myers GM (1999) Gender differences in the human cerebral cortex: More neurons in males, more processes in females. Journal of Child Neurology 14:98–107 [DOI] [PubMed] [Google Scholar]
- Reader SM, Laland KN (2002) Social intelligence, innnovation, and enhanced brain size in primates. Proceedings of the National Academy of Sciences 99 (7):4436–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK (2006) Human and non-human primate brains: are they allometrically scaled versions of the same design?. Evolutionary Anthropology 15 (2):65–77 [Google Scholar]
- Rilling JK, Insel TR (1999) The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution 37:191–223. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Seligman RA (2002) A quantitative morphometric comparative analysis of the primate temporal lobe. Journal of Human Evolution 42:505–533 [DOI] [PubMed] [Google Scholar]
- Rogers J, Kochunov PV, Zilles K, Shelledy W, Lancaster JL, Thompson P, Duggirala R, Blangero J, Fox PT, Glahn DC (2010) On the genetic architecture of cortical folding and brain volume in primates. NeuroImage 53:1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra M, Tamarit L, Lazeyras F, Eliez S, Thiran J (2008) A surface based approach to quantify local cortical gyrification. IEEE Transactions on Medical Imaging 27 (2):161–170 [DOI] [PubMed] [Google Scholar]
- Schenker NM, Hopkins WD, Spocter MA, Garrison A, Stimpson CD, Erwin JM, Hof PR, Sherwood CC (2010a) Broca’s area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry and comparison to humans. Cerebral Cortex 20:730–742. doi:doi: 10.1.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker NM, Hopkins WD, Spocter MA, Garrison AR, Stimpson CD, Erwin JM, Hof PR, Sherwood CC (2010b) Broca’s area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry, and comparison to humans. Cerebral Cortex 20:730–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann PT (2006) Evolution of size and functional areas of the human brain. Annual Review of Anthropology 35:379–406 [Google Scholar]
- Schoenemann PT, Sheehan MJ, Glotzer LD (2005) Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nature Neuroscience 8 (2):242–252. [DOI] [PubMed] [Google Scholar]
- Seldon SH (2005) Does brain white matter growth expand the cortex like a balloon? Hypothesis and consequences. Laterality 10 (1):81–95 [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW (2001) Prefrontal cortex in humans and apes: A comparative study of area 10 American Journal of Physical Anthropology 114:224–241 [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H (2000) The brain and its main anatomical subdivisions in living hominids using magnetic resonance imaging. Journal of Human Evolution 38:317–332 [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW (1997) The evolution of the frontal lobes: a volumetric analysis based on three dimensional reconstructions of the magnetic resonance scans of human and ape brains Journal of Human Evolution 32:375–388 [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker NM, Damasio H (2002) Humans and great apes share a large frontal cortex. Nature Neuroscience 5 (3):272–276 [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Baurernfeind AL, Bianchi S, Raghanti MA, Hof PR (2012) Human brain evolution writ large and small. In: Hofman MA, Falk D (eds) Progress in Brain Research. Elsevier, [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR (2003) Variability of Broca’s area homologue in great apes: Implication for language evolution. The Anatomical Record 217A:276–285 [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Holloway RL, Semendeferi K, Hof PR (2005a) Is prefrontal white matter enlargement a human evolutionary specialization?. Nature Neuroscience 8 (5):537–538 [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Holloway RL, Semendeferi K, Hof PR (2005b) Is prefrontal white matter enlargement a human evolutionary specialization? Nature Neuroscience 8 (5):537–538 [DOI] [PubMed] [Google Scholar]
- Stout D, Chaminade T (2012) Stone tools, language and the brain in human evolution. Philosophical Transactions of the Royal Society B: Biological Sciences 367:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings H, Jacobsen A, Zilles K, Amunts K (2006) Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex 42 (4):652–658 [DOI] [PubMed] [Google Scholar]
- Van Essen DC (1997) A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385:313–318 [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H (1989) Gyrification in the cerebral cortex of primates. Brain, Behavior and Evolution 34:143–150 [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends in Neurosciences 36 (5):275–284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.