Abstract
The response of the uterine epithelium to female sex steroid hormones provides an excellent model to study cell proliferation in vivo since both stimulation and inhibition of cell proliferation can be studied. Thus, when administered to ovariectomized adult mice 17β-estradiol (E2) stimulates a synchronized wave of DNA synthesis and cell division in the epithelial cells, while pretreatment with progesterone (P4) completely inhibits this E2-induced cell proliferation. Using a simple method to isolate the uterine epithelium with high purity, we have shown that E2 treatment induces a relocalization of cyclin D1 and, to a lesser extent, cdk4 from the cytoplasm into the nucleus and results in the orderly activation of cyclin E- and cyclin A-cdk2 kinases and hyperphosphorylation of pRb and p107. P4 pretreatment did not alter overall levels of cyclin D1, cdk4, or cdk6 nor their associated kinase activities but instead inhibited the E2-induced nuclear localization of cyclin D1 to below the control level and, to a lesser extent, nuclear cdk4 levels, with a consequent inhibition of pRb and p107 phosphorylation. In addition, it abrogated E2-induced cyclin E-cdk2 activation by dephosphorylation of cdk2, followed by inhibition of cyclin A expression and consequently of cyclin A-cdk2 kinase activity and further inhibition of phosphorylation of pRb and p107. P4 is used therapeutically to oppose the effect of E2 during hormone replacement therapy and in the treatment of uterine adenocarcinoma. This study showing a novel mechanism of cell cycle inhibition by P4 may provide the basis for the development of new antiestrogens.
Estrogen exposure is the major risk factor in the genesis of breast and endometrial cancers (29), with the majority of tumors initially dependent on estrogen for their proliferation before becoming hormone independent (4). Thus, treatment of these tumors at early stages with antiestrogens has proven therapeutic value. In the normal uterus, 17β-estradiol (E2) synthesized at every estrus or menstrual cycle causes the epithelial cells to undergo a wave of cell proliferation (20). In contrast, progesterone (P4) inhibits this estrogen-induced cell proliferation and stimulates epithelial differentiation in preparation for embryo implantation (21). Consequently, P4 is used therapeutically to inhibit the proliferation of estrogen-dependent endometrial cancers and to oppose estrogen action in postmenopausal women during hormone replacement therapy (9, 25).
The uterine cellular dynamics observed during the estrous cycle and early pregnancy can be faithfully reproduced in adult ovariectomized mice by administration of exogenous female sex steroid hormones. A single injection of E2 dramatically shortens G1 in the epithelial cells with the result that they undergo a synchronized wave of cell proliferation, with DNA synthesis in essentially all the cells commencing 6 to 9 h after hormone administration and peaking at 12 to 15 h, followed by a wave of cell division (22, 40, 41, 56). Cells thereafter enter into a second round of cell proliferation (40, 41). P4 completely inhibits the E2-induced DNA synthesis and cell proliferation and reduces the basal level of epithelial cell proliferation to zero (13, 36). In addition, both E2 and P4 inhibit apoptosis in the uterine epithelial cells (41, 54, 66). In contrast to its effects on epithelial cell proliferation, P4 treatment permits the uterine stromal cells to respond to E2 with a single round of cell proliferation that shows kinetics similar to those of, but with a lower amplitude than, that observed in the uterine epithelium following E2 treatment alone (38, 39). These hormonal actions are receptor mediated because estrogen receptor (ER) α nullizygous mice have hypoplastic uteri in response to E2 stimulation (34) while P4’s action on both epithelial and stromal cell proliferation can be completely blocked by the progesterone receptor (PR) antagonist RU 486 (11), and in ovariectomized PR-null mutant mice, the uterine epithelium is hyperplastic in response to E2 and P4 treatment (35).
Studies in which P4 was administered to ovariectomized mice at varying times after E2 treatment indicated that it acts within the first 3 h of G1 after E2 administration (13). P4 does not inhibit the binding of E2 to the ER (57, 58), nor in the mouse does it influence the uterine metabolism of E2 (8). Furthermore, P4 pretreatment does not block the E2-induced increase in protein and rRNA synthesis; the induction of early-response genes including c-fos, c-myc, and Ha-ras; the expression of cell-cycle-associated genes, such as that for ornithine decarboxylase; or epithelial cell hypertrophy (6, 7, 12, 51), although it does alter lipid metabolism (64). These data show that P4 inhibits epithelial hyperplasia without affecting hypertrophy and suggest that P4 does not interfere generally with E2-induced signaling but specifically inhibits some event(s) in the E2-induced cell proliferation signaling pathway.
The orderly progression through the cell cycle is regulated by the activation of specific cyclin-dependent kinases (cdk’s) that bind to their appropriate cyclin partners. These include the cyclin D-cdk4 and -cdk6 complexes acting in G1 and the cyclin E-cdk2 complex acting at the G1-to-S transition. Both kinase complexes hyperphosphorylate and inactivate members of the Rb family of proteins, with the result that the negative control over transcription factors such as the E2Fs is released. These transcription factors promote expression of genes required for S phase and the expression of S-phase cyclin A, which together with its partner, cdk2, is required for initiation and maintenance of S-phase progression (reviewed in references 26 and 59–62).
The cdk activities are regulated positively by their assembly with cyclins and their phosphorylation by cdk-activating kinases (CAKs) (47) and negatively by their association with cdk inhibitors (CKIs) (59, 62). Two families of CKIs play a major role in regulating cyclin-cdk activities. One is the Ink4 family, which specifically inhibits cyclin D-cdk4 and -cdk6 activities, while the other one, the Cip/kip family, binds to and inhibits a broad range of cyclin-cdk complexes (42, 62).
The basic mechanisms for cell cycle regulation appear to be universal and may also apply in E2-induced epithelial cell proliferation. The effects of E2 and P4 have been studied in breast cancer cells in culture. In these cases, E2 stimulates cyclin D1 expression, cdk4 and cdk6 activity, and cyclin E-cdk2 activity and induces hyperphosphorylation of pRb (48, 53, 55), while P4 alone can inhibit these events (49). However, little has been done to study hormonal regulation of epithelial cells in vivo. Following the hormonal treatment of ovariectomized mice, the mouse uterine epithelium can be isolated with a high degree of purity (up to 95%) in a state suitable for biochemical analysis (16). This, together with the E2-induced proliferation and the P4-induced inhibition of cell proliferation, means that both “on” and “off” switches of cell proliferation can be studied in uterine epithelial cells in vivo. Using this system, we have shown that E2 first stimulates the nuclear localization of cyclin D1 and cdk4 and phosphorylation of pRb and p107, followed by activation of cyclin E- and cyclin A-cdk2-dependent kinases and hyperphosphorylation of pRb and p107. In contrast, P4 prohibited cyclin D1 and to a lesser extent cdk4 translocation into the nucleus and abrogated E2-induced cyclin E-cdk2-associated kinase activation, followed by inhibition of cyclin A synthesis and its associated cdk2 kinase activity and dephosphorylation of pRb and p107. The identification of a novel mechanism of cyclin D1 regulation through controlling its access to nuclear substrates may have significant implication for studies of therapy designed to interfere with estrogen-induced carcinogenesis.
MATERIALS AND METHODS
Animals and treatments.
Female CD1 mice, obtained from Charles River (Wilmington, Mass.), were maintained on 12-h light–12-h dark cycles. Female mice containing a null mutation in the p27Kip1 gene (30) were derived from crosses of heterozygous founders with nullizygous male mice obtained from A. Koff (Memorial Sloan-Kettering Cancer Center, New York, N.Y.). Mice were ovariectomized at 10 to 12 weeks of age via a dorsal incision under tribromoethanol (2.5% Avertin) anesthesia. After resting for 2 to 3 weeks, mice were “primed” for 2 days by subcutaneous (s.c.) injection of 100 ng of 17β-estradiol in 0.1 ml of peanut oil 6 days prior to the experiment. In most experiments, groups of two to five mice were killed by cervical dislocation at different time points after one of the following treatments: (a) no treatment (control), (b) one s.c. injection of 50 ng of E2 in 0.05 ml of peanut oil (E2 treatment), (c) 4 days of s.c. injection of 1 mg of P4 in 0.1 ml of peanut oil (P4 treatment), or (d) 4 days of s.c. injections of 1 mg of P4 with one s.c. injection of 50 ng of E2 at the same time as the last P4 injection (P4E2 treatment). All experiments were repeated at least three times with similar results.
Hormones and antibodies.
17β-Estradiol and progesterone were purchased from Sigma. Rabbit polyclonal anti-p27 antibody was a generous gift from Andrew Koff (Memorial Sloan-Kettering Cancer Center). Polyclonal antibodies for cdk2 (sc-163), cdk4 (sc-260), cdk6 (sc-177), p27 (sc-528), p107 (sc-318), cyclin A (sc-597), and cyclin E (sc-481), with their competitive peptides when available, were obtained from Santa Cruz Biotechnology, Inc., Santa Cruz, Calif. Monoclonal antibodies for pRb (G3-245) were obtained from Pharmingen, San Diego, Calif.; those for cyclin D1 (DCS-6, DCS-11, and Ab-3) were obtained from Neomarkers, Fremont, Calif.; and that for proliferating cell nuclear antigen (PCNA) (PC10) was obtained from Boehringer Mannheim.
Preparation of epithelial cell extracts.
Uteri were excised, trimmed of fat, and slit lengthwise. Uterine luminal epithelial cells were removed by the method of Fagg et al. (16). Briefly, two to six uterine horns were vortexed in round-bottomed 15-ml tissue culture tubes with five Teflon balls in 1 ml of vortexing buffer for 3 to 4 min, with intervals on ice. Homogenates were filtered through 205-μm-pore-size nylon mesh to remove residual tissues and balls, and the tissues were washed with 1 ml of washing buffer. The lysates were then sonicated and clarified by centrifugation. The vortexing buffer was either 10 mM Tris-HCl (pH 7.4)–0.1 M NaCl–1 mM NaF–0.1 mM Na3VO4–0.2 mM phenylmethylsulfonyl fluoride–10 μg of aprotinin per ml–10 μg of leupeptin per ml–10 μg of pepstatin A per ml or 10 mM HEPES-KOH (pH 7.5)–0.1 M NaCl–10 mM β-glycerophosphate–1 mM dithiothreitol (DTT)–1 mM NaF–0.1 mM Na3VO4–0.2 mM phenylmethylsulfonyl fluoride–10 μg of aprotinin per ml–10 μg of leupeptin per ml–10 μg of pepstatin A per ml. The former was combined with a washing buffer to constitute a final Nonidet P-40 (NP-40) immunoprecipitation (IP) buffer: 50 mM Tris-HCl (pH 7.4), 0.25 M NaCl, 5 mM EDTA, 0.5% (vol/vol) NP-40, 50 mM NaF, and proteinase inhibitors as described above. The latter was adjusted to a final Tween 20 IP buffer: 50 mM HEPES-KOH (pH 7.5), 0.15 M NaCl, 1 mM EDTA, 2.5 mM EGTA, 0.1% (vol/vol) Tween 20, 10% (vol/vol) glycerol, 10 mM β-glycerophosphate, and proteinase inhibitors.
Western blot analysis and IP.
Equal amounts of protein or cell number equivalents were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Immobilon P membranes (Millipore). The membranes were blocked in Tris-buffered saline with 0.1% (vol/vol) Tween 20 and 5% (wt/vol) nonfat dry milk for 3 h. They were then incubated for 1 to 2 h with a dilution of the specific antibody in blocking solution and subsequently incubated for 0.5 to 1 h with a 1:5,000 dilution of horseradish peroxidase-linked secondary antibody (Amersham). Immunodetection was achieved with an enhanced chemiluminescence system (ECL; Amersham). For all the Western blots, cell lysates from the BAC1.2F5 macrophage cell line either unstimulated or stimulated with colony-stimulating factor 1 (CSF-1) were used as the positive control, and for every antibody used, titrations were performed to ensure that detection of the specific protein was on the linear portion of the curve.
For IP, the lysates were incubated with 1 to 2 μg of antibody for 1 to 2 h on ice and then precipitated with 30 μl of 50% slurry-protein A beads (Zymed or Santa Cruz). The beads were washed three times with the IP buffer and subjected to kinase assays or boiled in SDS sample buffer for electrophoresis.
Kinase assay.
For histone H1 phosphorylation, the protein lysates were immunoprecipitated with anti-cyclin E, anti-cyclin A, or anti-cdk2 antibodies in NP-40 IP buffer. The beads were washed three times in IP buffer and twice with kinase buffer (20 mM Tris-HCl [pH 7.5], 7.5 mM MgCl2, 1 mM DTT). The reactions were performed in 50 μl of kinase mix (kinase buffer containing 30 μM ATP with 2 μg of histone H1 [Boehringer Mannheim] and 10 μCi of [γ-32P]ATP) for 30 min at 37°C. The reactions were stopped by addition of SDS sample buffer, and the mixtures were separated by SDS-PAGE. The gels were fixed and dried, followed by autoradiography. Specificity was confirmed by the loss of signal when IPs were performed in the presence of a competitive peptide.
The Rb kinase assay was performed as described by Matsushime et al. (43). The cells were lysed in Tween 20 IP buffer, and total cell extracts were precipitated with anti-cdk4 or anti-cdk6 antibodies. The beads were washed three times with IP buffer and twice with kinase buffer (50 mM HEPES-KOH [pH 7.5], 10 mM MgCl2, 1 mM DTT, 2.5 mM EGTA, 10 mM glycerophosphate, 0.1 mM Na3VO4, 1 mM NaF). The kinase reaction was started by the addition of 30 μl of Rb-kinase mix (kinase buffer with 20 μM ATP, 0.3 μg of glutathione S-transferase [GST]–Rb [769; Santa Cruz] or recombinant truncated Rb protein [p56Rb; amino acids 379 to 928; QED Bioscience, San Diego, Calif.], and 10 μCi of [γ-32P]ATP). The reaction mixtures were incubated for 30 min at 30°C, reactions were stopped with SDS sample buffer, and reaction mixtures were analyzed by SDS-PAGE followed by autoradiography.
Isolation of nuclear fraction from uterine epithelium.
Uterine epithelial cell lysates were extracted as described above except for the use of sucrose vortexing buffer: 10 mM HEPES (pH 7.5), 50 mM NaCl, 0.5 M sucrose, 1 mM EDTA, 0.25 mM EGTA, 1 mM DTT, 0.6 mM spermidine, and proteinase inhibitors. NP-40 was added to the homogenates to a final concentration of 0.7% after filtration. The lysates were vortexed vigorously for 10 s and then passed through 22-gauge needles. The nuclei were isolated by centrifugation at 800 × g for 10 min at 4°C and washed once with sucrose vortexing buffer plus 0.7% NP-40. The nuclei were resuspended in SDS sample buffer and prepared for Western blotting.
Immunohistochemistry.
Uteri were removed, fixed overnight in Bouin’s solution or peroxidate–lysine–2% paraformaldehyde–0.05% glutaraldehyde (PLPG), and processed for paraffin embedding. Cross sections (5-μm thickness) were stained for bromodeoxyuridine (BrdU) incorporation with the cell proliferation kit from Oncogene Science or processed by the following procedure. The sections were deparaffinized and subjected to microwaves in 0.01 M sodium citrate buffer (pH 6.0) for 10 to 30 min (for cdk4, cdk6, p27, and cyclin D1 staining). Nonspecific immunoglobulin binding was blocked by incubating sections in 10% normal goat serum for 20 min. The primary antibodies were added at appropriate dilutions. The sections were washed and incubated with biotin-conjugated secondary antibodies for 30 min, followed by incubation with avidin DH-biotinylated horseradish peroxidase H complex for 30 min (Vector Laboratories). Lastly, the sections were detected with the Metal Enhanced diaminobenzidine (DAB) substrate kit (Pierce) and counterstained with hematoxylin (Sigma), followed by Permount mounting (Sigma). Controls included incubation with normal serum corresponding to the antibody used and omission of the primary antibody. In all cases, these controls were consistently negative.
RESULTS
17β-Estradiol stimulated, while progesterone inhibited, uterine epithelial cell proliferation.
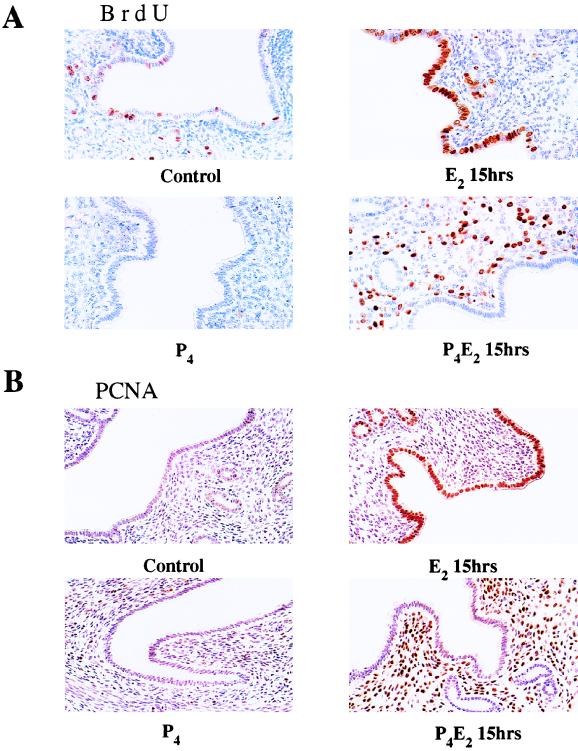
E2 treatment of ovariectomized adult mice results in uterine luminal and glandular epithelial cell proliferation. DNA synthesis commences about 6 h after E2 administration and peaks at 12 to 15 h with a consequent doubling of cell number at about 24 h (40, 41). P4 completely suppresses this E2-induced proliferation as well as the basal rate of epithelial cell proliferation but sensitizes the uterine stromal cells to respond to E2 with a wave of cell proliferation following a time course similar to those observed in the epithelium (13, 36–39). In the present studies, in order to confirm this cell cycle regulation and to determine the efficiency of the hormonal regimens used in adult CD1 mice, incorporation of BrdU as an index of DNA synthesis was measured by immunohistochemistry following a 2-h pulse in vivo. In untreated ovariectomized mice, there was a low basal rate of DNA synthesis in the luminal and glandular epithelium with 0 to 5% of the cells in S phase depending on the individual mouse (Fig. 1A). Consistent with previous results with [3H]thymidine labeling (40, 41), immunostaining for BrdU following 15 h of E2 treatment showed a dramatic increase in the number of BrdU-positive luminal and glandular epithelial cells (Fig. 1A). P4 pretreatment completely abolished this E2-induced BrdU incorporation as well as the basal rate of DNA synthesis in the uterine epithelium (Fig. 1A). For underlying stromal cells, E2 treatment alone had no significant effect on stromal cell proliferation, but P4 pretreatment induced about 30 to 40% of the underlying stromal cells to enter into DNA synthesis in response to E2 (Fig. 1A). These data confirmed the hormonally regulated switch in E2-induced uterine proliferation in response to P4 in this strain of mice.
FIG. 1.
Cell proliferation in response to E2 and P4 treatment in the mouse uterus. Shown are the results of immunohistochemistry studies of transverse sections of uteri from ovariectomized mice given BrdU intraperitoneally 2 h before killing and 15 h after the following hormone treatments: control (no treatment), 50 ng of E2 (E2 15hrs), 1 mg of P4 for 4 days (P4), and 1 mg of P4 for 4 days and 50 ng of E2 on the fourth day (P4E2 15hrs). (A) Bouin’s fixed uteri immunostained for BrdU; (B) PLPG-fixed uteri immunostained for PCNA. In the immunohistochemistry studies, the figures shown are representative of five mice analyzed per group (magnification, ×400).
The expression of PCNA was also analyzed by immunostaining. PCNA is a component of DNA polymerase δ, which is required for entry into S phase, and its nuclear localization is used as a marker for DNA replication (27). Consistent with the BrdU labeling, strong PCNA nuclear staining involving over 90% of the cells was detected at 15 h after E2 administration. P4 pretreatment completely prevented this E2-induced PCNA nuclear localization in epithelial cells but greatly increased its nuclear localization in stromal cells (Fig. 1B). In both E2- and P4E2-treated uteri, a higher percentage of cells were positive for PCNA staining than for BrdU staining. This may be due to the fact that the nuclear accumulation of PCNA begins in G1 and is cumulative throughout S phase, while BrdU staining is a result of a short period of incorporation during S phase.
Progesterone inhibited 17β-estradiol-induced pRb and p107 hyperphosphorylation and differentially regulated their cellular localization.
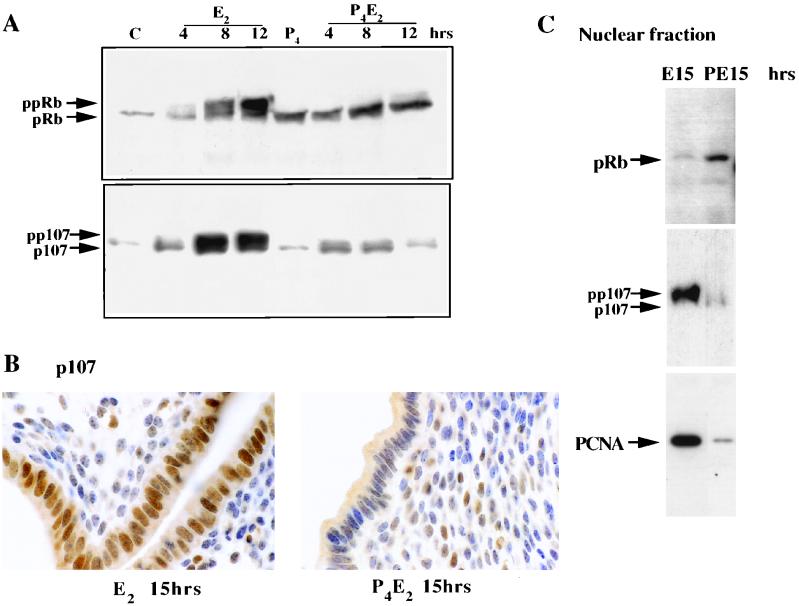
Central to the regulation of the G1-to-S-phase transition are the pocket proteins, pRb, p107, and p130. The hypophosphorylated forms of these proteins are potent inhibitors of E2F-mediated transcriptional transactivation and S-phase entry (60). To explore functional roles of pocket proteins in P4-mediated inhibition of DNA synthesis in uterine epithelial cells, we determined the phosphorylation status of these proteins in epithelial cells following sex steroid hormone treatment. The uterine epithelial cells were isolated as described in Materials and Methods. This method has been extensively characterized elsewhere (6, 12a, 16, 64) and gives a highly enriched (up to 95%) epithelial fraction with minimal contamination by stromal cells. The same cell number equivalents of protein were processed for Western blot analysis. E2 induced gradual hyperphosphorylation of pRb and p107 detectable within 4 h of treatment as was shown by a slower-migrating band on the SDS-PAGE gel, with intense phosphorylation being detected at 8 to 12 h after treatment, coincident with entry into S phase (Fig. 2A). Pretreatment with P4 abolished the hyperphosphorylation of pRb and p107 in response to E2 (Fig. 2A). The protein levels of pRb and p107 were also significantly increased at 8 to 12 h following E2 treatment, while their concentration remained low after P4 pretreatment, with the reduction being greatest for p107 levels. Analysis of p130 phosphorylation by Western blotting with anti-p130 antibodies (sc-317; Santa Cruz) failed to detect any specific changes in either the concentration or the phosphorylation state of this protein (data not shown).
FIG. 2.
Phosphorylation status and localization of pRb and p107 following E2 or P4E2 treatment. (A) Uterine epithelial lysates from the same numbers of cells were prepared from control (C) mice at the indicated times after E2 or P4E2 treatment and analyzed for pRb (top) and p107 (bottom) proteins by Western blotting. The hypophosphorylated (pRb) and hyperphosphorylated (ppRb) bands are indicated, as are p107 and its phosphorylated form (pp107). (B) Immunohistochemical localization of p107 following 15 h of E2 and P4E2 treatment. Note the strong nuclear localization following E2 treatment (magnification, ca. ×900). (C) Western blot showing nuclear localization of pRb preferentially in the epithelial nuclear fraction from P4E2 at 15 h (PE15) compared to the fraction from E2 at 15 h (E15) and that of p107 preferentially in the nuclear fraction from E2 at 15 h compared to the fraction from P4E2 at 15 h. PCNA immunodetection acts as a control for the E2 effect.
In order to determine the cellular localization of these pocket proteins, their distribution 15 h after treatment was determined by Western blotting and immunohistochemistry for p107 and, as our anti-pRb antibodies do not function in immunohistochemical applications, by Western blotting of a nuclear fraction for pRb. Following E2 treatment, hyperphosphorylated p107 was largely nucleus associated, but even given its lower concentration in the P4E2-treated epithelium, p107 appeared to be largely retained in the cytoplasm following this hormone treatment (Fig. 2B and C). In contrast, the hypophosphorylated form of pRb was nucleus associated following P4E2 treatment, while this form is significantly lower in concentration in the nuclei of E2-treated luminal epithelial cells (Fig. 2C). The absence of the hyperphosphorylated forms in these preparations is due to the well-documented leaching of these forms from the nuclei during isolation (44, 45) and is entirely consistent with the E2-induced phosphorylation status of pRb. In these experiments, PCNA acts as a control for the nuclear fraction, since it is found predominantly in the nucleus following E2 treatment.
Progesterone and 17β-estradiol differentially regulated the expression and localization of cyclins and CKIs.
Phosphorylation of pRb and p107 is known to be driven by cdk’s. We next characterized the changes in the cellular content of cyclin D1, cyclin E, and cyclin A proteins following E2 or P4E2 treatment.
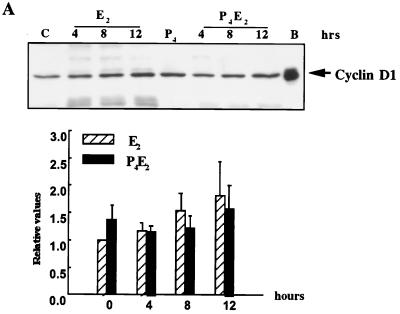
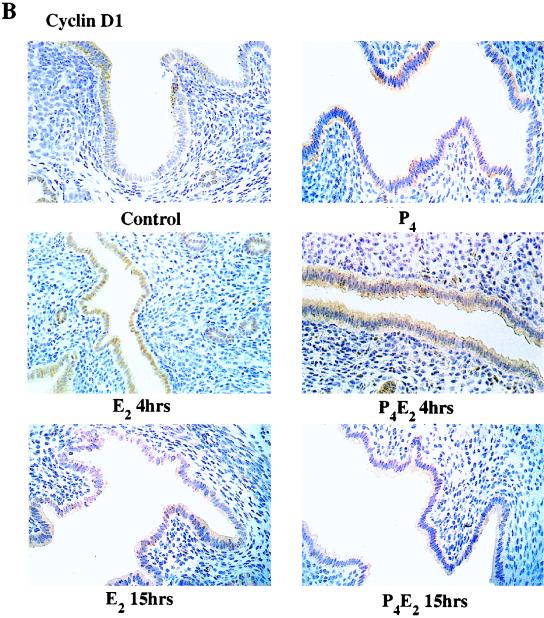
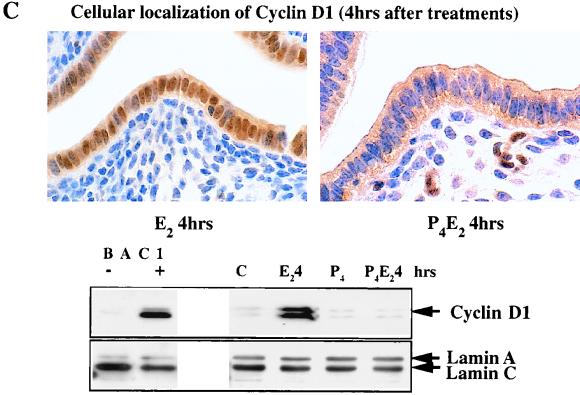
In these epithelial cells, of the three different cyclins D, cyclin D2 was not detected and D3 was found only at very low and unchanging concentrations, but cyclin D1 was readily detected. Over five independent experiments, there was no significant change in cyclin D1 protein concentration in total cell lysates of uterine epithelial cells following E2 administration over the first 8 h with only a very slight increase of ∼70% at 12 h, compared to untreated ovariectomized control mice (Fig. 3A). In the P4-treated uterine epithelium, the cyclin D1 concentration also does not change over this time course following E2 treatment (Fig. 3A). Given the importance of cyclin D1 in cell cycle regulation and the fairly small magnitude of its change together with the absence of effect by P4, we further explored its regulation by determining its subcellular localization by immunohistochemistry with anti-cyclin D1 antibodies following the different hormonal treatments (Fig. 3B). Cyclin D1 protein is predominantly, although not exclusively, cytoplasmic in the epithelium of untreated control mice (Fig. 3B). However, E2 induced a nuclear accumulation of cyclin D1 by 4 h after treatment (early G1 phase), and the signal in the cytoplasm was proportionately reduced (Fig. 3B and C). By 15 h (S phase), cyclin D1 had largely left the nucleus and appeared dispersed again in the cytoplasm (Fig. 3B). In a kinetic study with immunohistochemistry, cyclin D1 nuclear accumulation was apparent by 2 h and reached a plateau between 4 and 8 h after E2 treatment followed by a progressive loss until 15 h (data not shown). P4 completely inhibited this nuclear localization of cyclin D1 in early G1, and cyclin D1 proteins remained in the cytoplasm throughout the G1-S phase (Fig. 3B and C). Interestingly, E2 also induced cyclin D1 nuclear accumulation in stromal cells pretreated with P4 (Fig. 3C).
FIG. 3.
Levels and localization of cyclin D1 in mouse uterus influenced by E2 and P4. (A) Western blotting of cyclin D1 with equivalent amounts of protein from total epithelial cell lysates with the DCS-6 mouse monoclonal anti-cyclin D1 antibody. The histogram indicates densitometric determination of cyclin D1 expression for four independent determinations (means ± standard errors of the means) (lane B, BAC1.2F5 cell lysates; lane C, untreated control epithelial cell lysates). (B) Localization of cyclin D1 determined by immunohistochemistry of transverse sections of uteri from ovariectomized mice after the following hormone treatments: control (no treatment), 50 ng of E2 (E2 4hrs), 50 ng of E2 (E2 15hrs); 1 mg of P4 for 4 days (P4), 1 mg of P4 for 4 days and 50 ng of E2 on the fourth day (P4E2 4hrs), and the treatment designated P4E2 15hrs. (magnification, ×400). (C) Immunohistochemistry of cyclin D1 in transverse sections of mouse uteri after the treatments designated E2 4hrs and P4E2 4hrs (magnification, ca. ×800). The lower portion shows concentrations of cyclin D1 in the nuclear fraction of the epithelial cells determined by Western blotting with the Ab-3 rabbit polyclonal anti-cyclin D1 antibody. It should be noted that this polyclonal antibody recognizes the upper D1 band with greater sensitivity than that of the mouse monoclonal antibody used in panel A. As loading controls, the expression levels of lamins A and C (constitutively expressed) are shown. (BAC1 −, BAC1.2F5 cells without CSF-1 stimulation; BAC1 +, BAC1.2F5 cells 6 h after CSF-1 stimulation).
The nuclear translocation of cyclin D1 at 4 h following E2 treatment was also demonstrated by Western blotting with a nuclear fraction isolated from uterine epithelial cells (Fig. 3C). The cyclin D1 level in the nuclear fraction was significantly increased 4 h following E2 treatment, while pretreatment with P4 completely inhibited this nuclear accumulation (Fig. 3C), consistent with the immunohistochemistry also shown in Fig. 3C. The expression of nuclear lamins A and C was the same under all hormonal treatments and acted as a loading control in these Western blotting experiments (Fig. 3C). In BAC1.2F5, a macrophage cell line (46), from which the cyclin D1 cDNA was originally isolated (43), CSF-1 dramatically stimulates cyclin D1 protein expression in total cell lysates. These lysates from unstimulated and CSF-1-stimulated BAC1.2F5 cells were used as a positive control for the cyclin D1 antibodies and our ability to detect cell-cycle-associated changes in cyclin D1 concentration (Fig. 3A and C) as well as for other cyclins and cdk’s (data not shown).
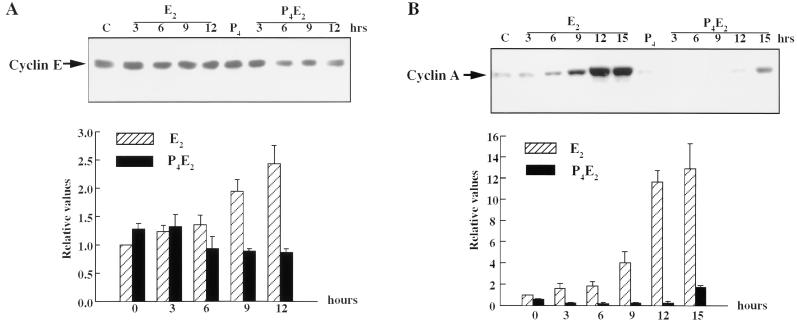
Cyclin E protein accumulation in the total cell lysates of uterine epithelial cells was gradually induced by E2 (Fig. 4A). It was significantly increased twofold at 9 h after treatment, and this increase continued until 12 h (Fig. 4A). Cyclin E levels remained relatively constant, perhaps even showing a small decline, throughout G1-S phase following P4E2 administration (Fig. 4A).
FIG. 4.
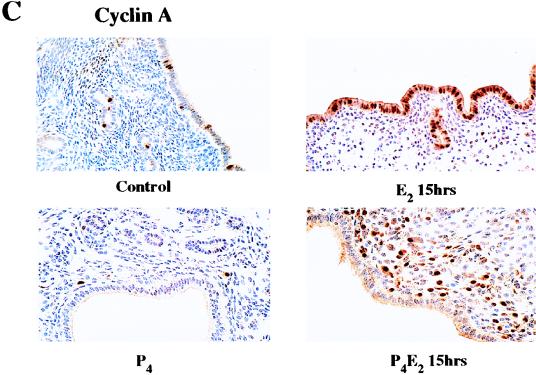
E2 and P4 effects on the levels of cyclin E and cyclin A in mouse uterine epithelial cells. (A and B) Uterine epithelial cell extracts, prepared at the indicated times, were analyzed for cyclin E (A) and cyclin A (B) protein expression by Western blotting. Lanes C, epithelial cell lysates from untreated control mice. Relative values are shown for three to five determinations per time point in the bar figures underneath (means ± standard errors of the means). (C) Immunohistochemistry with anti-cyclin A antibodies of uterine transverse sections from ovariectomized mice after the hormone treatments, as described for Fig. 1 (magnification, ×400).
In contrast to these small effects on cyclin D1 and E concentrations, cyclin A protein accumulation in the epithelial cells was induced fourfold at 9 h after E2 treatment, followed by an acute increase to 13-fold at 12 to 15 h. P4 completely abrogated this E2 induction of cyclin A such that it was barely detected in the uterine epithelium (Fig. 4B). This result was confirmed by immunohistochemical staining of transverse sections of uteri with anti-cyclin A antibodies (Fig. 4C). In untreated control uteri, cyclin A protein levels in the cytoplasm of the epithelial cells remained proportional to the low level of basal cell proliferation. Fifteen hours following E2 treatment, cyclin A protein accumulated at a high concentration in the nucleus of almost all epithelial cells and was also significantly increased in the cytoplasm of these cells (Fig. 4C). P4 treatment resulted in very little cyclin A expression being detected, with the protein being retained in the cytoplasm of the epithelial cells. Interestingly, P4 pretreatment caused the accumulation of cyclin A in the nuclei of a proportion of the underlying stromal cells following E2 treatment, suggesting a similar involvement of cyclin A in the proliferation of these cells (Fig. 4C). The correlation of the Western blot results and immunohistochemical results of cyclin A expression confirmed the high purity of the uterine epithelial cell isolation.
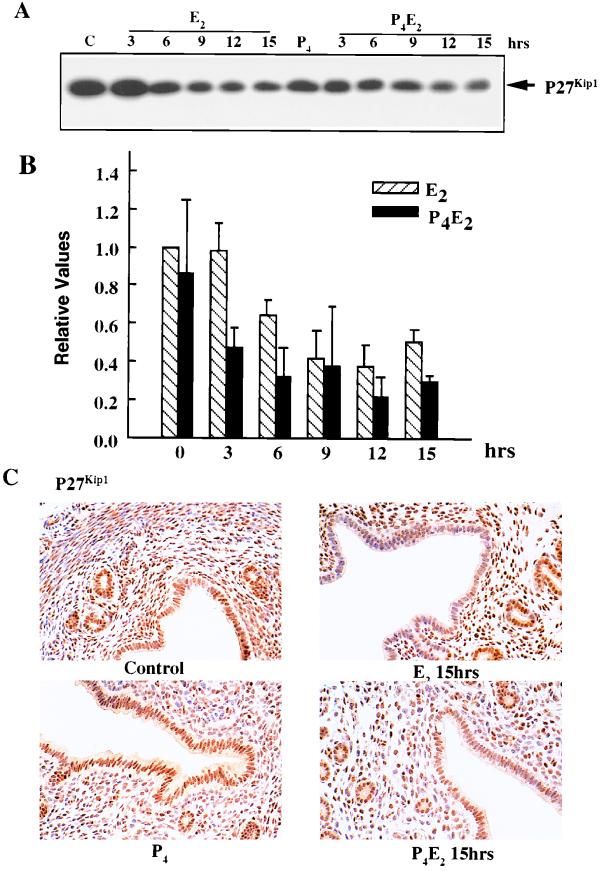
The cyclin-cdk complexes are subject to inhibition by the binding of various CKIs. The expression levels of p21Cip/Waf1, p57Kip2, and p15Ink4b, if present, are below the level of detection with currently available antibodies. The expression of p16Ink4a, p18Ink4c, and p19Ink4d is also minimal and shows little change under different hormone treatments (data not shown). However, p27Kip1 is expressed at high levels in uterine epithelial cells (Fig. 5A), suggesting that p27Kip1 could be the major CKI involved in cell cycle control in the uterus. Western blot analysis showed that E2 treatment resulted in a reduction of about 50% of the p27Kip1 level in uterine epithelial cells in both E2 and P4E2 treatments (Fig. 5A and B). Similar results could be shown by immunohistochemistry with a rather greater reduction in signal following P4E2 treatment (Fig. 5C). Thus, p27Kip1 levels seem to be hormonally regulated, either directly or indirectly, but the reduced level is not correlated with S-phase progression (see below).
FIG. 5.
Levels and localization of p27Kip1 in the mouse uterus influenced by E2 and P4. (A) Western blotting of uterine epithelial cell lysates at different time points following E2 and P4E2 treatments with anti-p27 antibodies. Lane C, epithelial cell lysates from untreated control mice. (B) Densitometric quantitation of p27Kip1 concentrations; three to five independent determinations per time point (means ± standard errors of the means). (C) Immunohistochemical determination of p27Kip1 distribution in the uterine epithelium (magnification, ×400).
Progesterone suppresses 17β-estradiol-induced cyclin E-cdk2 and cyclin A-cdk2 kinase activities.
E2-induced cyclin E-associated activity gradually increased in the total cell lysates of the epithelial cells, with elevated levels being detected at 4 h and a peak at 12 h, while P4 abolished this induction (Fig. 6A). E2 also induced cyclin A-associated kinase activity significantly in the epithelial cells, with levels being dramatically elevated at 12 h, while P4 decreased this activity to below the control level (Fig. 6B). We observed that E2 induction of cyclin E-associated kinase activity started as early as mid-G1, while E2 stimulated cyclin A-associated kinase activity acutely at the border of G1 and S phases. This is correlated with phosphorylation of pRb and p107 (Fig. 2).
FIG. 6.
Suppression of E2-induced cyclin E- and cyclin A-cdk2-associated kinase activities by P4. Cyclin E (A)-, cyclin A (B)-, or cdk2 (C [top])-associated kinase activities were assessed with histone H1 as a substrate. The total cell lysates were analyzed for cdk2 protein by Western blotting (C [bottom]). Thr 160-P indicates the threonine-160-phosphorylated active form of cdk2, and Thr 160 indicates the nonphosphorylated form. Also shown are cyclin E- and cyclin A-cdk2 kinase activities in extracts from uterine epithelial cells of p27Kip1 nullizygous mice (p27KO) 12 h after E2 or P4E2 treatment (D). Details are as described for panels A to C. Abbreviations: NRS, normal rabbit immunoglobulin G; WT, wild type; C, control; HH1, histone H1; WB, Western blot.
Direct measurements of cdk2-associated kinase activities confirmed the above findings. In uterine epithelial cells, E2 gradually stimulated cdk2 kinase activity, with a peak at 12 h, while P4 completely inhibited this induction and reduced it to below the control level (Fig. 6C). The regulation of cdk2 kinase activity was demonstrated by Western blot analysis of cdk2 protein expression from the same cell lysates in which the total cell lysates from E2-induced proliferating epithelial cells exhibited a faster-migrating form of cdk2 (Fig. 6C), corresponding to the Thr-160-phosphorylated, active form of cdk2 (47). In untreated control mice, there was a very low level of the active form of cdk2. E2 acutely stimulated this active form so that it was as abundant as the inactive slow-migrating form of cdk2 at 12 h. In contrast, the inactive form of cdk2 was the major form detected in P4-induced cell-cycle-arrested cells over the 12-h course of E2 stimulation (Fig. 6C). The active form of cdk2 was significantly reduced to even below the control level following P4 treatment (Fig. 6C). These results suggest that P4 exerts its inhibition of cell proliferation by inhibiting E2-induced cdk2 activity through the dephosphorylation of cdk2.
Role of p27Kip1 in regulating cdk2 kinase activity.
To ascertain the role of p27Kip1 in the uterine epithelial cells, cyclin E- and cyclin A-cdk2 kinase activities were compared in extracts isolated following the different hormonal treatments of mice having a null mutation in the p27Kip1 gene (19, 30, 50). In a similar fashion as in wild-type littermate mice, in p27Kip1−/− mice P4 treatment resulted in a significant inhibition of these kinase activities compared to the activity in extracts from mice treated solely with E2 (Fig. 6D). This suggests that p27Kip1 is not regulating this inhibition. However, of interest is the consistent observation that the specific activity of the kinases is considerably enhanced in the p27Kip1−/− extracts compared to those from wild-type littermates even though the cyclin E, cyclin A, and cdk2 protein levels were not enhanced (Fig. 6D). This indicates that p27Kip1 does play a role in determining the overall level of activity of these kinases.
Progesterone and 17β-estradiol differentially regulated cdk4 and cdk6 cellular distribution.
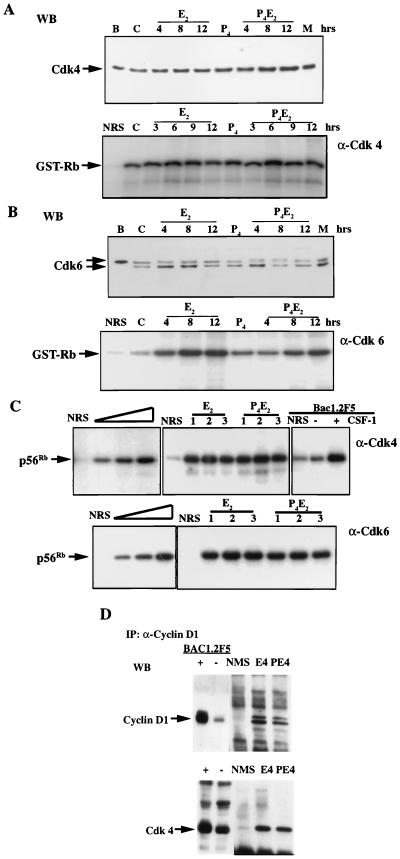
Over several independent experiments, we could not detect any changes in cdk4 protein concentration in total cell lysates of the epithelial cells over a 12-h course after either E2 or P4E2 administration (Fig. 7A). The cdk4-associated kinase activity was also indistinguishable between E2 and P4E2 treatment at 4 h (Fig. 7C) and over the entire 12-h course of treatment (Fig. 7A). cdk6 protein levels in total cell lysates of the epithelial cells remained approximately constant following the different hormone treatments (Fig. 7B). cdk6 concentrations did not change following P4E2 treatment. We noticed that there were consistently two bands on cdk6 Western blots. The lysates from BAC1.2F5 cells showed only one slower-migrating band while MEF (passage 5) cell lysates showed two bands comigrating with those from the uterine epithelial cell lysates (Fig. 7B). We do not know whether these two bands represent differently modified cdk6 proteins, nor do we know their functional difference. cdk6-associated kinase activities from the same lysates were increased gradually over a 12-h course following E2 or P4E2 administration (Fig. 7B). However, no significant difference was observed in activities at the same time points between any of the different hormone treatments (Fig. 7B). cdk4 and cdk6 proteins are stably expressed in excess in most cell types. Their regulation is believed to be imposed through the regulation of their partners, the D-type cyclins (47). Our data suggest that this may also be the case in uterine epithelial cells. However, it seems contradictory that P4 can abolish pRb and p107 hyperphosphorylation but does not inhibit the kinases, cdk4 and cdk6, which are thought to be responsible for Rb family phosphorylation. In order to confirm that the condition we used for kinase assay is in the linear range, we performed titration experiments to ensure that the amount of the lysates we used could detect differences in activities (Fig. 7C). cdk4 kinase assays of BAC1.2F5 cells with or without CSF-1 stimulation were used as positive controls, and in our hands, as reported before (15), they showed dramatic stimulation of cdk4 activity in response to CSF-1 (Fig. 7C). However, in more than five independent experiments performed under conditions that detected the kinase over a linear range as shown by titration (Fig. 7C) and that were positively controlled by BAC1.2F5 lysates, no significant differences could be detected between the E2 and P4E2 extracts, three of which are shown in Fig. 7C. Thus, we are confident that, had there been a difference in activity, we would have detected it. Furthermore, we could detect a similar amount of cdk4 associated with cyclin D1 by IP of lysates with anti-cyclin D1 antibodies followed by detection of cdk4 by Western blotting (Fig. 7D), consistent with the equivalence in cdk4 kinase activities between these two treatment groups.
FIG. 7.
Concentrations and kinase activities of cdk4 and cdk6 following E2 or P4E2 treatment. (A) cdk4 levels and associated kinase activities remain unchanged following different hormonal treatments as determined by Western blotting (WB) (top) and IP kinase assay with GST-Rb (769) as substrate (bottom). (B) cdk6 levels and associated kinase activities are comparable between E2 and P4E2 treatments as determined by Western blotting (WB) (top) and IP kinase assay with GST-Rb (769) as substrate (bottom). (Abbreviations: B, BAC1.2F5; M, MEF; C, control; NRS, normal rabbit immunoglobulin G). (C) (upper panel) Left, titration of kinase activity with increasing concentrations of uterine epithelial cell lysate. Middle, three independent experiments (1 to 3) with cdk4 activities 4 h after E2 and P4E2 treatment with recombinant truncated Rb (p56Rb) as substrate. Total epithelial cell lysates with protein concentrations in the middle of the linear portion of the titration were used in the experiments. Right, BAC1.2F5 cells either unstimulated or stimulated with CSF-1 for 9 h were used as positive controls. (Lower panel) As described for the upper panel, except that cdk6 kinase activities were determined. (D) Coimmunoprecipitation of cdk4 with cyclin D1, at 4 h following E2 and P4E2 treatments. NMS, normal mouse immunoglobulin G; WB, Western blot.
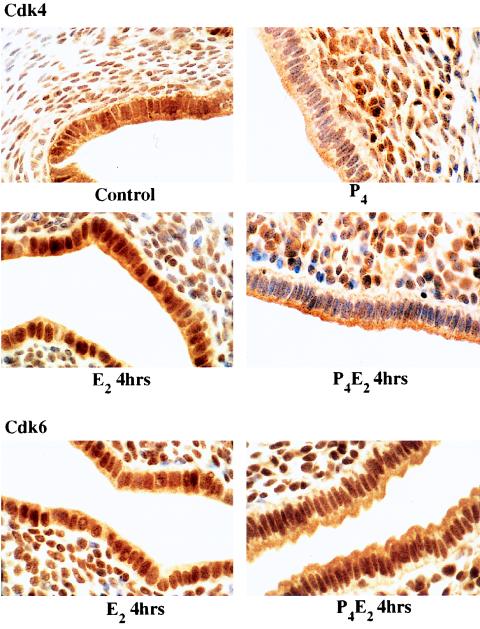
P4 did not inhibit the overall activation of cdk4 or cdk6 but did inhibit cyclin D1 nuclear translocation. Thus, P4 might influence the substrate accessibility of cyclin D1-cdk4 or -cdk6 complexes, or E2 signaling through cyclin D1 is cdk4 or cdk6 independent. Therefore, we studied the distribution of cdk4 and cdk6 following the various hormonal treatments. The cdk6 level remained high in both the nucleus and the cytoplasm of uterine epithelial cells under both E2 and P4E2 treatments over a 15-h course (data not shown) as illustrated for the 4-h point (Fig. 8). cdk4 was present at a high level and was distributed in both the nucleus and the cytoplasm in control uterine epithelial cells (Fig. 8). The nuclear association of cdk4 was increased following E2 treatment, with a maximal level being observed at 4 h (Fig. 8). cdk4 was also present in a proportion of underlying stromal cells. Treatment with P4 alone severely reduced cdk4 accumulation in the nucleus of epithelial cells, while the cdk4 level remained high in the cytoplasm (Fig. 8). P4 pretreatment also prevented cdk4 mobilization into the nucleus following E2 treatment (Fig. 8). Therefore, E2 and P4 differentially regulate cdk4, but not cdk6, distribution. The redistribution of cdk4 to the nucleus and the prevention of this accumulation by P4 parallel that of cyclin D1 and are correlated with the phosphorylation of the nuclear substrates pRb and p107.
FIG. 8.
Immunostaining of uterine transverse sections for cdk4 (0 and 4 h) and cdk6 (4 h) after E2 and P4E2 treatments. Magnification, ×1,000.
DISCUSSION
17β-Estradiol synthesized during every estrous cycle acts through the ER to selectively stimulate uterine luminal and glandular epithelial cell proliferation. Progesterone inhibits this E2-induced epithelial cell proliferation while preparing the underlying stromal cells to respond to E2 with a single wave of cell proliferation. P4 causes the epithelial cells to differentiate in preparation for implantation which is initiated at day 4.5 of pregnancy by a nidatory surge of E2 acting upon the epithelium and causing it to become receptive to blastocyst attachment. The correct interplay of these hormones is essential for pregnancy, and any inhibition of their actions terminates this process. These hormones are also involved in tumorigenesis, with estrogen being the primary risk factor for adenocarcinoma of the breast and endometrium. It is important, therefore, to determine the mechanism of action of sex steroid hormones in regulating epithelial cell proliferation in vivo. Physiological concentrations of E2 and P4 given exogenously to ovariectomized mice can recapitulate the cell cycle kinetics of the estrous cycle and early pregnancy. Taking advantage of the fact that uterine epithelial cells can be isolated with high purity for biochemical studies, we have exploited the E2 and P4 regulation of uterine epithelial cell proliferation in ovariectomized mice in this study.
Estrogen stimulates and progesterone blocks cyclin D1-cdk4 nuclear accumulation.
The cyclin D-, E-, and A-dependent kinases are central to the regulation of cell proliferation. They act to phosphorylate pRb, thereby alleviating transcriptional repression of important cell cycle genes and allowing cell cycle progression. Of these cdks, the D cyclins in association with cdk4 and cdk6 are thought to act first, early in G1. E2 shortens the G1 phase of the cell cycle, while P4 is inhibitory only if administered within the first 3 h of G1 (13). Thus, cyclin D1 would appear to be a likely target for regulation by these steroid hormones. Regulation of cyclin D1-cdk4 and cyclin D1-cdk6 complexes by growth factors has been shown at several different levels. These include (i) increased transcription (43) of the cyclin D1 mRNA, (ii) stabilization of cyclin D1 protein (15), (iii) association with cdk’s, (iv) increased phosphorylation of an associated cdk through CAKs or inhibition of the cyclin-dependent phosphatases (47), and (v) association with CKIs (26, 62). In this report, we demonstrate another mechanism whereby regulation of nuclear accumulation of cyclin D1 provides access to its nuclear substrates. Thus, E2 does not stimulate overall cellular cyclin D1-cdk4 or cyclin D1-cdk6 activities but instead causes a significant redistribution of cyclin D1 and, to a lesser extent, cdk4 to the nucleus. This process begins within 2 h of E2 administration at the peak of uterine ER occupancy (41), is maximal within 3 to 5 h coincident with significantly enhanced phosphorylation of pRb and p107, and returns to basal levels by 15 h when DNA synthesis is maximal. These data are consistent with those for cultured cycling fibroblasts that showed cyclin D1 entering the nucleus during G1 and disappearing during S phase (3). This nuclear association of cyclin D1 may also activate other cyclin D1-dependent functions, such as the recently reported ligand-independent transcriptional activation of the ER by cyclin D1 association (52, 68).
P4 does not inhibit cyclin D1-cdk4 or -cdk6 activities in total cell lysates even while it completely suppresses cell proliferation. A similar lack of regulation of cdk4 and cdk6 kinase activity has been reported during fibrillar collagen inhibition of arterial smooth muscle proliferation (31) and during anchorage-induced cell proliferation in fibroblasts (17). However, the access of cyclin D1-cdk4 and cyclin D1-cdk6 to nuclear substrates or targets following E2 treatment is inhibited by P4 pretreatment, which completely blocks the E2-induced nuclear accumulation of cyclin D1 and, to a lesser extent, cdk4, although not cdk6. This exclusion results in the observed absence of phosphorylation of pRb and p107. Interestingly, p107 concentration is increased following E2 treatment, and in the former case, the p107 is largely nucleus localized. In contrast, the hypophosphorylated form of pRb is found at a higher concentration in the nucleus following P4E2 treatment, and this hypophosphorylated form may act as a transcriptional repressor of cell cycle gene expression. These data are consistent with the P4 inhibition of both the E2-stimulated epithelial cell proliferation and the complete suppression of the basal sex steroid hormone-independent rate of cell proliferation by P4. This nuclear translocation of cyclin D1 is the earliest E2-induced event that P4 has been shown to inhibit and strongly suggests that it is central to the regulation of cell proliferation by these steroid hormones.
The mechanism of cyclin D1 and cdk4 translocation into the nucleus by E2 or the inhibition of this translocation by P4 is currently unknown. It could be due to either inhibition of translocation or stimulation of nuclear export. Coimmunoprecipitation experiments have shown that cyclin D1 forms complexes with cdk4 in the presence or absence of P4 following E2 treatment. This suggests that they cotranslocate as a complex following E2 treatment. Since cyclin D1 and cdk4 lack obvious nuclear localization sequences, it is likely that other cellular proteins may provide this function. A dominant-negative mutant of cyclin D1 (T156A) can still bind to cdk4, but the complex is not imported into the nucleus (14), suggesting that a conformational state is recognized by such a carrier protein. p50Cdc37/Hsp90, acting as a protein kinase chaperon, can bind to and stabilize cdk4 (65). However, the cytoplasmic property of this chaperon probably excludes it from being a nuclear transporter, but it could be involved in retention of cyclin D1-cdk4 complexes in the cytoplasm by P4. The Cip/kip inhibitors p21Cip/Waf1, p27Kip1, and p57Kip2 can also target cyclin D1-cdk4 to the nucleus (32). However, we were unable to detect p21Cip/Waf1 or p57Kip2 in the uterine epithelial lysates. In contrast, p27Kip1 could readily be detected. However, both epithelial cell proliferation (67) and nuclear translocation of cyclin D1 occur in p27Kip1−/− ovariectomized mice following E2 treatment (unpublished data), and the inhibition of these events by P4 also occurred in these null mutant mice (67). These data are similar to those found in MEFs null for p21Cip1 and p27Kip1 where cyclin D1 was still nucleus associated (5). Thus, these inhibitors are unlikely to be involved in the regulation of cyclin D1 translocation, although the presence of another adapter and/or inhibitor whose identity is unknown cannot be ruled out. Overall, these data suggest that transport of an active cyclin D1-cdk4 complex could be targeted to the nucleus in response to E2 via novel nuclear transporters.
A caveat to the role of cyclin D1 as a central regulator of uterine cell cycle in response to E2 is the findings obtained from cyclin D1 null mutant mice (18, 63). Although the status of uterine cell proliferation has not been assessed in these mice, they are fertile, suggesting that the hormonally induced uterine cell proliferation is unaffected by the absence of cyclin D1. In vitro studies have shown that cyclin D1 is essential for fibroblast proliferation because inhibition of cyclin D1 by microinjection of anti-cyclin D1 antibody or cyclin D1 antisense plasmids into fibroblasts results in inhibition of DNA synthesis (3). However, both in vivo and in vitro fibroblast proliferation is also apparently normal in these cyclin D1 null mutant mice (63). These results, although clearly indicating that the function of cyclin D1 is not required in these cells in vivo, may suggest some regulative development to compensate for the absence of cyclin D1, perhaps through the expression of cyclin D2 or D3, which could interact with cdk4 once this molecule has become preferentially associated with the nucleus after E2 treatment.
P4 inhibits E2-induced cdk2 phosphorylation and kinase activity.
Following the cyclin D1-cdk4 translocation, E2 induces, in an orderly sequence, a small but significant elevation in cyclin E protein concentration, increased cyclin E-cdk2 activity, pRb and p107 phosphorylation, elevated cyclin A protein expression, and dramatically elevated cyclin A-cdk2 activity that results in hyperphosphorylation of the pRb family proteins. This presumably releases transcriptional repression of the E2F family of transcription factors, resulting in the activation of genes that encode proteins required for DNA synthesis. Of note here is that the hypophosphorylated form of pRb remains high in the nuclei of P4-treated epithelial cells, suggesting a tight down-regulation of these cell cycle genes. In a recent study, E2 treatment of rats was reported to have no effect on cdk2 activity (2) in total uterine cell lysates. However, epithelial cells represent only ∼5% of total uterine cells, and it is only these epithelial cells that undergo proliferation directly in response to E2 (40). Thus, it is very likely that the residual, nonproliferating 95% of uterine cells obscured the detection of E2-induced cdk2 activity in these experiments. Our results are consistent with those reported for E2 stimulation of the MCF7 and T47D human mammary carcinoma cell lines, where estrogen stimulates cyclin E-cdk2 activation and hyperphosphorylation of pRb within 6 h of treatment (53, 55).
P4 inhibits cyclin E-cdk2 activity and cyclin A protein expression and consequently cyclin A-cdk2 activity and thus suppresses the phosphorylation of pRb and p107 throughout G1. P4 also inhibits arterial smooth muscle cell proliferation acting through the PR, and this is correlated with a decrease in cyclin A and cyclin E mRNA levels in these cells (33). P4 does not significantly reduce cyclin E or cdk2 protein concentration in uterine epithelial cells but instead acts through inhibition of cdk2 phosphorylation. The phosphorylation of cdk2 on Thr-160 and dephosphorylation on Tyr-15 and Thr-14 are required for its catalytic activity. The former is catalyzed by CAK, consisting of cdk7 and its regulatory partner, cyclin H, while the latter is regulated by the tyrosine phosphatase cdc25A (47). Given the fact that CAK activity is expressed throughout the cell cycle and is not rate limiting in fibroblasts (47), it is unlikely to be the target for P4 action. However, cdc25A could be a target for P4 in a manner analogous to that seen during transforming growth factor β inhibition of cell proliferation (28).
The activity of cdk2 is also regulated by its association with inhibitors, the CKIs. We were unable to detect in uterine epithelial cells significant expression of most of the known CKIs except p27Kip1. However, the E2-induced down-regulation of p27Kip1 in luminal epithelial cells is not reversed by P4 pretreatment. P4 also down-regulates the E2-induced increase in cdk2 activity in p27Kip1 nullizygous mice. Interestingly, in this case, the specific activity of the cdk2 and the cyclin E- and cyclin A-associated kinase activities are proportionately increased such that the inhibited level is equal to the stimulated level in mice wild type for the p27Kip1 gene. This suggests that p27Kip1 does have an effect upon the overall level of kinase activity in uterine cells consistent with its association in equal amounts with cyclin D-cdk4 and cyclin E-cdk2 complexes in both E2- and P4-treated mice (data not shown). However, in the absence of p27Kip1 the cells compensate for the higher activities and respond to the proportional reduction in kinase activity induced by P4. This study of uterine cell proliferation regulation by E2 and P4 in the uteri of p27Kip1 null mutant mice demonstrates that P4 can still inhibit E2-induced cell proliferation in uterine epithelial cells even in the absence of p27Kip1 (23), suggesting that p27Kip1 is not a nonredundant regulator of P4 action in these cells. These data also suggest that it is possible that a novel CKI is present in uterine epithelial cells.
Recent evidence with heterotypic tissue recombinants exploiting ERKO and PRKO mice suggests that, in both the mammary gland and the uterus in vivo, the actions of sex steroid hormones on cell proliferation are exerted though the stromal cells, causing them to send a paracrine signal to the epithelial cells (10). Given these data, it is feasible that P4 blocks release or synthesis of this stromal paracrine factor(s) or interferes with E2-ER interactions. However, P4 does not down-regulate ER levels or prevent metabolism of E2 in the mouse uterus, nor does it inhibit E2 in binding to the ER (8, 58). Furthermore, in the uterine epithelium, P4 does not inhibit the E2-induced hypertrophy (6) or the E2-induced expression of immediate-early genes (12) and some later cell cycle genes, such as that for ornithine decarboxylase (7), suggesting that P4 does not inhibit the early responses induced by E2 and that the expression of these genes is correlated with cell growth and survival rather than with cell proliferation. Instead, P4 selectively targets the cell-cycle-regulatory machinery, suggesting that it, perhaps through the synthesis of a paracrine factor, acts to inhibit this pathway by inhibiting cyclin D1 nuclear association and cdk2 phosphorylation specifically within the epithelial cells.
Implications for sex steroid hormone involvement in tumorigenesis.
Cell proliferation in subclones of the mammary tumor cell lines MCF7 and T47D is regulated by direct actions of sex steroid hormones acting through their receptors within the epithelial cells (1, 23, 49, 53). In these cell lines, E2 stimulates the expression of cyclin D1 together with their corresponding cdk4 and cdk6 kinase activities (1, 53). In the progesterone-responsive T47D subclone, P4, although initially stimulating cell proliferation, after 24 h of exposure inhibits it, and this is correlated with the inhibition of cyclin D, E, and A expression; increased CKI expression; and a consequent reduction in their associated kinase activity (23, 49). Although the inhibition of cdk2 activity by P4 is similar to the results presented in this report, the effects on cyclin D1-cdk4 and cyclin D1-cdk6 protein expression and activities and induction of CKIs are different. Furthermore, the data contrast with the actions of these steroid hormones in vivo since, in contrast to normal mammary epithelial cells (24), this cell line proliferates readily in the absence of any steroid hormone (49). Furthermore, while P4 does not inhibit epithelial cell proliferation in the mammary gland (24), it does so completely without any prior stimulation of cell proliferation in the uterus (13, 36). These data, together with the results with the heterotypic tissue recombinants described above (10), suggest that acquisition of epithelial cell responsiveness to direct cell cycle regulation by these steroid hormones either is a result of selection during tissue culture or, of greater interest, may be an important step in the transition of normal epithelial cells to the neoplastic state.
P4 is used therapeutically to oppose the effect of E2 on cell proliferation both in hormone replacement therapy and in the treatment of uterine adenocarcinoma. Since P4 is a well-defined cell cycle inhibitor in vivo, the present insights into its mode of action in the normal uterus indicating a novel regulation of cyclin D1 cellular localization might provide better opportunities for intervention strategies for opposing the effects of E2 in tumor growth and in the understanding of the transition of tumors to hormone independence.
ACKNOWLEDGMENTS
We thank L. Zhu, P. E. Cohen, A. Iavarone, and A. Koff for helpful discussion and A. Koff for kindly providing us with heterozygous breeding pairs of the p27Kip1 null mutant mice.
REFERENCES
- 1.Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker M G, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17β-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G1-arrested human breast cancer cells. Oncogene. 1996;12:2315–2324. [PubMed] [Google Scholar]
- 2.Altucci L, Addeo R, Cicatiello L, Germano D, Pacilio C, Battista T, Cancemi M, Petrizzi V B, Bresciani F, Weisz A. Estrogen induces early and timed activation of cyclin-dependent kinases 4, 5, and 6 and increases cyclin messenger ribonucleic acid expression in rat uterus. Endocrinology. 1997;138:978–984. doi: 10.1210/endo.138.3.5002. [DOI] [PubMed] [Google Scholar]
- 3.Baldin V, Lukas J, Marcote M, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 4.Castles C G, Fuqua S A W. Alterations within the estrogen receptor in breast cancer. In: Pasqualini J R, Katzenellenbogen B S, editors. Hormonal-dependent cancer. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 81–105. [Google Scholar]
- 5.Cheng M, Diehl J A, Fero M, Olivier P, Randel E, Roberts J M, Sherr C J, Roussel M F. Fourteenth Annual Meeting on Oncogenes. (Abstract.) 1998. Cdk inhibitors p27Kip1 and p21Cip1 are required for assembly of active cyclin D-dependent kinases in mouse fibroblasts; p. 39. [Google Scholar]
- 6.Cheng S V Y, MacDonald B S, Clark B F, Pollard J W. Cell growth and cell proliferation may be dissociated in the mouse uterine luminal epithelium treated with female sex steroids. Exp Cell Res. 1985;160:459–470. doi: 10.1016/0014-4827(85)90193-4. [DOI] [PubMed] [Google Scholar]
- 7.Cheng S V Y, Pollard J W. C-rasH and ornithine decarboxylase are induced by oestradiol-17β in the mouse uterine luminal epithelium independently of the proliferative status of the cell. FEBS Lett. 1986;196:309–314. doi: 10.1016/0014-5793(86)80269-1. [DOI] [PubMed] [Google Scholar]
- 8.Clark B F. Absence of oestradiol-17 beta dehydrogenase from the progesterone-dominated mouse uterus. J Endocrinol. 1980;85:155–159. doi: 10.1677/joe.0.0850155. [DOI] [PubMed] [Google Scholar]
- 9.Cohen C J, Bruckner H W, Deppe G. Multidrug treatment of advanced and recurrent endometrial carcinoma: a gynecologic oncology group study. Obstet Gynecol. 1984;63:719–726. [PubMed] [Google Scholar]
- 10.Cooke P S, Buchanan D L, Kurita T, Lubahn D B, Cunha G R. Stromal-epithelial cell communication in the female reproductive tract. In: Bazer F W, editor. Endocrinology of pregnancy. Totowa, N. J: Humana Press; 1998. pp. 491–506. [Google Scholar]
- 11.Cullingford T E, Pollard J W. RU 486 completely inhibits the action of progesterone on cell proliferation in the mouse uterus. J Reprod Fertil. 1988;83:909–914. doi: 10.1530/jrf.0.0830909. [DOI] [PubMed] [Google Scholar]
- 12.Cullingford T E, Pollard J W. Growth factors as mediators of sex-steroid hormone action in the uterus during the pre-implantation period. In: Khan S A, Stancel G M, editors. Protooncogenes and growth factors in steroid hormone-induced growth differentiation. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 13–30. [Google Scholar]
- 12a.Cullingford, T. E., and J. W. Pollard. Unpublished observations.
- 13.Das R M, Martin L. Progesterone inhibition of mouse uterine epithelial proliferation. J Endocrinol. 1973;59:205–206. doi: 10.1677/joe.0.0590205. [DOI] [PubMed] [Google Scholar]
- 14.Diehl J A, Sherr C J. A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol Cell Biol. 1997;17:7362–7374. doi: 10.1128/mcb.17.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehl J A, Zindy F, Sherr C J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasone pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 16.Fagg B, Martin L, Rogers L A, Clark B F, Quarmby V E. A simple method for preparing pure samples of uterine epithelial cells. J Reprod Fertil. 1979;57:335–345. doi: 10.1530/jrf.0.0570335. [DOI] [PubMed] [Google Scholar]
- 17.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 18.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 19.Fero M L, Rivkin M, Tasch M, Porter P, Carow C E, Firpo E, Polyak K, Tsai L-H, Broudy V, Perlmutter R M, Kaushansky K, Roberts J M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 20.Finn C A, Martin L. The role of the oestrogen secreted before oestrus in the preparation of the uterus for implantation in the mouse. J Endocrinol. 1970;47:431–438. doi: 10.1677/joe.0.0470431. [DOI] [PubMed] [Google Scholar]
- 21.Finn C A, Porter D G. Handbooks in reproductive biology. 1. The uterus. London, United Kingdom: Elek Science; 1975. [Google Scholar]
- 22.Galand P, de Maertelaer V. Models of oestrogen action: a cell kineticist’s view. Epithelial Cell Biol. 1992;1:177–188. [PubMed] [Google Scholar]
- 23.Groshong S D, Owen G I, Grimison B, Schauer I E, Todd M C, Langan T A, Sclafani R A, Lange C A, Horwitz K B. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27Kip1. Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 24.Haslam S Z. The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones. Endocrinology. 1989;125:2766–2772. doi: 10.1210/endo-125-5-2766. [DOI] [PubMed] [Google Scholar]
- 25.Henderson B E, Ross R K, Pike M C. Hormonal chemoprevention of cancer in women. Science. 1993;259:633–638. doi: 10.1126/science.8381558. [DOI] [PubMed] [Google Scholar]
- 26.Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 27.Hyde-Dunn J, Jones G E. Visualization of cell replication using antibody to proliferating cell nuclear antigen. In: Pollard J W, Walker J M, editors. Basic cell culture protocols. Totowa, N.J: Humana Press; 1997. pp. 341–349. [DOI] [PubMed] [Google Scholar]
- 28.Iavarone A, Massagué J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-β in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- 29.Key T J A, Pike M C. The role of oestrogen and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1997;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 30.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 31.Koyama H, Raines E W, Bornfeldt J M, Roberts J M, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 32.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 33.Lee W-S, Harder J A, Yoshizumi M, Lee M-E, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med. 1997;3:1005–1008. doi: 10.1038/nm0997-1005. [DOI] [PubMed] [Google Scholar]
- 34.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lydon J P, DeMayo F J, Funk C, Mani S K, Hughes A R, Montgomery C A, Shyamala G, Conneely O M, O’Malley B W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 36.Martin L, Das R M, Finn C A. The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol. 1973;57:549–554. doi: 10.1677/joe.0.0570549. [DOI] [PubMed] [Google Scholar]
- 37.Martin L, Finn C A. Interactions of oestradiol and progestins in the mouse uterus. J Endocrinol. 1970;48:109–115. [PubMed] [Google Scholar]
- 38.Martin L, Finn C A. Oestrogen-gestagen interactions on mitosis in target tissues. In: Hubinont P O, et al., editors. Basic actions of sex steroids on target organs. S. Basel, Switzerland: Karger; 1971. pp. 172–188. [Google Scholar]
- 39.Martin L, Finn C A, Trinder G. DNA synthesis in the endometrium of progesterone-treated mice. J Endocrinol. 1973;56:303–307. doi: 10.1677/joe.0.0560303. [DOI] [PubMed] [Google Scholar]
- 40.Martin L, Finn C A, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 41.Martin L, Pollard J W, Fagg B. Oestriol, oestradiol-17β and the proliferation and death of uterine cells. J Endocrinol. 1976;69:103–115. doi: 10.1677/joe.0.0690103. [DOI] [PubMed] [Google Scholar]
- 42.Massagué J, Polyak K. Mammalian antiproliferative signals and their targets. Curr Opin Genet Dev. 1995;5:91–96. doi: 10.1016/s0959-437x(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 43.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 44.Mittnacht S, Lees J A, Desai D, Harlow E, Morgan D O, Weinberg R A. Distinct sub-populations of the retinoblastoma protein show a distinct pattern of phosphorylation. EMBO J. 1994;13:118–127. doi: 10.1002/j.1460-2075.1994.tb06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittnacht S, Weinberg R A. G1/S phosphorylation of the retinoblastoma protein is associated with an altered affinity for the nuclear compartment. Cell. 1991;65:381–393. doi: 10.1016/0092-8674(91)90456-9. [DOI] [PubMed] [Google Scholar]
- 46.Morgan C, Pollard J W, Stanley E R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC-1.2F5. J Cell Physiol. 1987;130:420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- 47.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 48.Musgrove E A, Lee C S L, Buckley M F, Sutherland R L. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci USA. 1994;91:8022–8026. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musgrove E A, Swarbrick A, Lee C S L, Cornish A L, Sutherland R L. Mechanisms of cyclin-dependent kinase inactivation by progestins. Mol Cell Biol. 1998;18:1812–1825. doi: 10.1128/mcb.18.4.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakayama K-I, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 51.Nephew K P, Peters G A, Khan S A. Cellular localization of estradiol-induced c-fos messenger ribonucleic acid in the rat uterus: c-fos expression and uterine cell proliferation do not correlate strictly. Endocrinology. 1995;136:3007–3015. doi: 10.1210/endo.136.7.7789326. [DOI] [PubMed] [Google Scholar]
- 52.Neuman E, Ladha M H, Lin N, Upton T M, Miller S J, DiRenzo J, Pestell R G, Hinds P W, Dowdy S F, Brown M, Ewen M E. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Planas-Silva M, Weinberg R A. Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollard J W, Pacy J, Cheng S V Y, Jordan E G. Estrogens and cell death in the mouse uterine luminal epithelium. Cell Tissue Res. 1987;249:533–540. doi: 10.1007/BF00217324. [DOI] [PubMed] [Google Scholar]
- 55.Prall O W J, Sarcevic B, Musgrove E A, Watts C K W, Sutherland R L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibition association. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 56.Quarmby V E, Korach K S. The influence of 17β-estradiol on patterns of cell division in the uterus. Endocrinology. 1984;114:694–702. doi: 10.1210/endo-114-3-694. [DOI] [PubMed] [Google Scholar]
- 57.Quarmby V E, Martin L. Effects of progesterone on uptake and metabolism of 17β-estradiol by mouse uterine luminal epithelium. Mol Cell Endocrinol. 1982;27:317–330. doi: 10.1016/0303-7207(82)90097-1. [DOI] [PubMed] [Google Scholar]
- 58.Quarmby V E, Martin L. Qualitative effects of progesterone on estrogen binding in mouse uterine luminal epithelium. Mol Cell Endocrinol. 1982;27:331–342. doi: 10.1016/0303-7207(82)90098-3. [DOI] [PubMed] [Google Scholar]
- 59.Ravitz M J, Wenner C E. Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-β. Adv Cancer Res. 1997;71:165–207. doi: 10.1016/s0065-230x(08)60099-8. [DOI] [PubMed] [Google Scholar]
- 60.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 61.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 62.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 63.Sicinski L P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 64.Stacey K, Beasley B, Wilce P A, Martin L. Effects of female sex hormones on lipid metabolism in the uterine epithelium of the mouse. Int J Biochem. 1991;23:371–376. doi: 10.1016/0020-711x(91)90121-3. [DOI] [PubMed] [Google Scholar]
- 65.Stepanova L, Leng X, Parker S B, Harper J W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 66.Terada N, Yamamoto R, Takada T, Miyake T, Terakawa N, Wakimoto H, Taniguchi H, Li W, Kitamura Y, Matsumoto K. Inhibitory effect of progesterone on cell death of mouse uterine epithelium. J Steroid Biochem. 1989;33:1091–1096. doi: 10.1016/0022-4731(89)90414-7. [DOI] [PubMed] [Google Scholar]
- 67.Tong W, Kiyokawa H, Soos T J, Park M S, Soares V C, Manova K, Pollard J W, Koff A. The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa→luteal transition. Cell Growth Differ. 1998;9:787–794. [PubMed] [Google Scholar]
- 68.Zwijsen R M L, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R J A M. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]