Abstract
Pex14p is a central component of the peroxisomal protein import machinery, which has been suggested to provide the point of convergence for PTS1- and PTS2-dependent protein import in yeast cells. Here we describe the identification of a human peroxisome-associated protein (HsPex14p) which shows significant similarity to the yeast Pex14p. HsPex14p is a carbonate-resistant peroxisomal membrane protein with its C terminus exposed to the cytosol. The N terminus of the protein is not accessible to exogenously added antibodies or protease and thus might protrude into the peroxisomal lumen. HsPex14p overexpression leads to the decoration of tubular structures and mislocalization of peroxisomal catalase to the cytosol. HsPex14p binds the cytosolic receptor for the peroxisomal targeting signal 1 (PTS1), a result consistent with a function as a membrane receptor in peroxisomal protein import. Homo-oligomerization of HsPex14p or interaction of the protein with the PTS2-receptor or HsPex13p was not observed. This distinguishes the human Pex14p from its counterpart in yeast cells and thus supports recent data suggesting that not all aspects of peroxisomal protein import are conserved between yeasts and humans. The role of HsPex14p in mammalian peroxisome biogenesis makes HsPEX14 a candidate PBD gene for being responsible for an unrecognized complementation group of human peroxisome biogenesis disorders.
Eukaryotic cells have developed elaborate mechanisms for recognizing newly synthesized organellar proteins and directing them to their proper destinations. Peroxisomal matrix proteins and at least a subset of the peroxisomal membrane proteins are synthesized on free ribosomes and are posttranslationally imported into the organelles (40). Newly synthesized peroxisomal matrix proteins are directed to the peroxisomal lumen by peroxisomal targeting signals (PTS), namely, the C-terminal PTS1 or the N-terminal PTS2 (30, 61, 62). A PTS for the posttranslational targeting of peroxisomal membrane proteins (mPTS) that is distinct from the PTS1 and PTS2 has been identified (16, 46). It has been reported that the targeting of peroxisomal matrix and membrane proteins is performed by distinct protein import machineries (18, 20, 29). Components of the transport machinery for peroxisomal membrane proteins have not yet been identified, while five proteins are known to be involved in the import of proteins into the peroxisomal matrix. The two PTS receptors, Pex5p and Pex7p, serve as specific recognition factors for the peroxisomal targeting signals PTS1 and PTS2, respectively (9, 45, 47, 56, 59, 66, 70, 71). The membrane-bound SH3-domain containing protein Pex13p is thought to function as a docking protein in PTS1-dependent protein import (17, 20, 22, 29, 59). Pex14p has been reported to provide binding sites at the peroxisomal surface for both the PTS1- and PTS2-specific receptors (1, 10, 35). Thus, Pex14p is a candidate for the predicted point of convergence of the PTS1- and PTS2-dependent protein import pathways (1). In addition, yeast ScPex14p has been reported to homo-oligomerize and to interact with ScPex13p (1). The fifth component of the peroxisomal import machinery for matrix proteins, Pex17p, functionally interacts with the peroxisomal Pex14p (32).
Identification of the components of the peroxisomal import machinery for matrix proteins was facilitated by the isolation of yeast pex mutants affected in peroxisomal protein import (21, 39, 59, 61, 67). However, significant contributions to our current understanding of peroxisome biogenesis in general and peroxisomal protein import in particular have been obtained from the analysis of peroxisome biogenesis disorders (PBD). These genetically heterogeneous, lethal diseases in humans are caused by mutations in proteins required for the biogenesis of peroxisomes (peroxins) (41). Eighteen yeast peroxins have been identified, but only ten human peroxins have yet been identified (22, 26, 28, 37, 60). Mutations in six of these human peroxins have been demonstrated to be the molecular cause for PBD (26, 37, 60). All 10 human peroxins known have yeast homologues which are peroxins as well. This observation supports the view that the basic mechanisms of peroxisome biogenesis are conserved from yeasts to humans. However, recent studies disclosed a remarkable difference between the mammalian and yeast systems. In yeasts, the PTS1 receptor Pex5p is dispensable for the peroxisomal import of PTS2 proteins. This observation led to the conclusion that the PTS1- and PTS2-dependent protein import pathways function independently prior to the binding of the import receptors to the membrane-bound Pex14p, the proposed point of convergence of these pathways in yeasts (1, 22). In contrast, the import of PTS2 proteins in humans strictly depends on the presence of the PTS1 receptor, opening the possibility of an earlier point of convergence of the PTS1- and PTS2-dependent protein import pathways in humans (3, 6, 7, 14, 25, 44, 52, 68).
Here we report the identification and characterization of HsPex14p, a peroxisomal membrane-bound human homologue of the yeast Pex14p. HsPex14p binds the cytosolic receptor for PTS1, suggesting that the protein is a component of the human import machinery for peroxisomal matrix proteins. This function makes HsPEX14 a candidate gene for a PBD. Interestingly, the binding capabilities of the human HsPex14p for other components of the peroxisomal protein import machinery seem to differ from those observed for its yeast counterpart. This observation supports the notion that the mechanism of protein import into peroxisomes might not be conserved in every detail between yeasts and humans.
MATERIALS AND METHODS
Cloning and sequencing.
BLAST searches of the database of expressed sequence tags (dbESTs) were performed as described previously (2). The IMAGE Consortium cDNA clone H16035 (ATCC 397396) was obtained from The American Type Culture Collection (Rockville, Md.). Both strands of the cDNA clone were sequenced with an ABI automated sequencer, and the HsPEX14 sequence extended from bp −5 (where 1 is the A of the initiation methionine) to the start of the poly(A) tail (bp 1903).
Northern blot analysis.
A multiple tissue Northern blot (Clontech, Palo Alto, Calif.) was probed with digoxigenin dUTP-labeled cDNA corresponding to the entire HsPEX14 open reading frame. Prehybridization and hybridization was performed according to the manufacturer’s protocol (Clontech). Detection of digoxigenin-labeled nucleotides was accomplished by using chemiluminescence according to the instructions of the manufacturer (Boehringer GmbH, Mannheim, Germany).
Generation of antibodies.
For the generation of antibodies against HsPex14p, a 402-bp fragment encoding amino acids 1 to 134 of HsPex14p was amplified by PCR with primers KU253 (see below) and KU254 (5′-GAAGGCCTCATGCGGCCGCGTCGACGAGGGGGAGCAGGTATTT-3′) and cloned into pET21b (Novagen, Abingdon, United Kingdom) by using the primer-derived BamHI and SalI sites. Escherichia coli BL21(DE3) was transformed with the plasmid, resulting in an IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible expression of His6-tagged HsPex14p. The soluble protein was purified by affinity chromatography on an Ni-nitrilotriacetic acid resin according to the instructions of the manufacturer (Quiagen, Hilden, Germany). Rabbit polyclonal antibodies to His6-tagged HsPex14p were produced by Eurogentec (Seraing, Belgium). Polyclonal sheep anti-human catalase antibodies were from The Binding Site (Heidelberg, Germany). The polyclonal antibodies against rat PMP69 (34) were kindly provided by Wilhelm Just (53).
Plasmid construction.
The HsPEX14 open reading frame was amplified from the human HsPEX14 cDNA by PCR with oligonucleotides KU 253 (5′-CGCGGATCC-GATATCTCATATGGCGTCCTCGGAGCAG-3′) and KU 255 (5′-GAAGGCCTG-CGGCCGCGTCGACCTAGTCCCGCTCACTCTC-3′).
The amplification product was inserted into EcoRV-NotI-restricted pBluescript SK(+) (Stratagene, Heidelberg, Germany), and the resulting plasmid was designated pSK-HsPEX14. For expression in human fibroblasts and in vitro synthesis of HsPex14p, the HindIII-NotI fragment of pSK-HsPEX14, encoding the entire HsPEX14 open reading frame, was subcloned into pcDNA3 (Invitrogen, Leek, The Netherlands), resulting in pcDNA3-HsPEX14. The plasmid expressing the myc epitope-tagged PMP69 was kindly provided by Stephen Gould (Johns Hopkins University, Baltimore, Md.). For complementation studies in Saccharomyces cerevisiae, the entire HsPEX14 open reading frame was subcloned into yeast pYADE4 plasmid (11) by using the EcoRV-SalI site of pSK-HsPEX14.
For the C-terminal tagging of HsPex14p with the myc epitope (23), HsPEX14 was amplified by PCR with pSK-HsPEX14 cDNA as template with primers KU253 (see above) and KU400 (5′-GAAGGCCTGCGGCCGCTCTAGACTA CAGGTCCTCCTCGCTGATCAGCTTCTGCTCGTCCCGCTCACTCTCGT TGC-3′). The amplified fragment was restricted with EcoRV-NotI and subcloned into Bluescript pSK(+) yielding pSK-HsPEX14myc. Taking advantage of the internal BamHI site and the primer-derived NotI sites, the 541-bp fragment of pcDNA3-HsPEX14 encoding the untagged C-terminal region of HsPex14p was exchanged with the corresponding tagged version of pSK-HsPEX14myc. The resulting plasmid encoding the C-terminal myc-tagged HsPex14p was designated pcDNA3-HsPEX14myc.
Plasmid pcDNA3-HsPEX13 was used for the in vitro synthesis of full-length HsPEX13. HsPEX13 was amplified by PCR with pcDNA3-IPMPGFP as a template, which was kindly provided by Stephen Gould. The oligonucleotides KU340 (5′-CGCAGAATTCGGATCCAGATGACAAGACCTGGACAA-3′) and KU343 (5′-CAGTCTAGACTGCAGTCAAAGATCTTGCTTTTCTCC-3′) were used for the amplification. The amplification product was subcloned into pcDNA3 by using the primer-derived BamHI and XbaI sites.
In vitro translation of proteins and immunoblot analysis.
Coupled in vitro transcription-translation reactions were performed with the TNT-coupled reticulocyte lysate system according to the manufacturer’s protocol (Promega, Madison, Wis.). The procedures for Western blot analysis were performed according to the standard protocols (31). Anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (Amersham, Braunschweig, Germany) or anti-sheep IgG-coupled horseradish peroxidase (Dianova, Heidelberg, Germany) were used as the second antibodies, and blots were developed by using the ECL system (Amersham).
Transfections and indirect immunofluorescence microscopy.
Skin fibroblast cell lines were kindly provided by A. B. Moser and H. W. Moser (Kennedy Institute, Baltimore, Md.). We only used transformed derivates of the cell lines, which were kindly provided by Stephen Gould. Fibroblast cell lines from patients with peroxisomal disorders are referred to by their PBD number (58). The cells were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and penicillin-streptomycin as described earlier (69). The subcellular localization of HsPex14p was detected in normal skin fibroblasts (GM5756). Transfections were done on transformed cells by using Lipofectamine (Gibco-BRL, Eggenstein, Germany) according to the method of Yahraus et al. (69). Two days after transfection, the cells were fixed, permeabilized, and processed for indirect immunofluorescence as described earlier (58). Fixation of cells was done for 20 min at room temperature with 3% formaldehyde in phosphate-buffered saline (PBS). Fixed cells were incubated for 5 min in PBS containing 1% Triton X-100 to permeabilize all cellular membranes or with 25 μg of digitonin per ml to permeabilize the plasma membrane only. Double immunofluorescence studies were performed with polyclonal rabbit anti-HsPex14p antibodies in conjunction with a polyclonal sheep anti-human catalase antibody (The Binding Site) or monoclonal 9E10 antiserum (23) to detect the c-myc epitope fused to PMP69 or HsPex14p. The primary antibodies were either detected with fluorescein-conjugated donkey anti-rabbit IgG (Dianova), donkey rhodamine-labeled anti-sheep secondary antibodies (Dianova), or CY3-conjugated donkey anti-mouse antibodies (Dianova). The micrographs were taken with a Zeiss Axiophot microscope with a Kodak Ektachrome ASA 400 film.
Subcellular fractionation, extraction of peroxisomes, protease protection, and enzyme assays.
Homogenization and preparation of the postnuclear supernatant was performed as described earlier (14). For the preparation of organellar pellets, 1 ml of the postnuclear supernatants was loaded on 0.5 M sucrose cushions in homogenization buffer. Centrifugation was at 25,000 × g for 30 min (HFA22.50 rotor; 15,000 rpm). Extractions of sedimented organelles with low salt, high salt, and carbonate were essentially performed as described previously (19). For the protease protection experiment, sedimented organelles were treated with increasing amounts of proteinase K according to the method of Albertini et al. (1). Peroxisomal catalase and mitochondrial fumarase activities were assayed as described by Peters et al. (54) and Bergmeyer et al. (5), respectively.
Yeast two-hybrid methodology.
The EcoRV-NotI fragment of pSK-HsPEX14 was subcloned into the SmaI-NotI-digested yeast two-hybrid system plasmids pPC86 and pPC97 (12), resulting in fusion of the entire HsPex14p to the transcription activation or DNA binding domains of Gal4p, respectively. Construction of Gal4p-AD-ScPex5p has been described previously (20). The complete long and short forms of the HsPEX5 cDNA were fused to the activation domain of Gal4p in pPC86, resulting in pPC86-HsPEX5s and pPC86-HsPEX5l (13a). Cotransformation of two-hybrid vectors into strain HF7c (Clontech) or PCY2 (12) was performed according to the manufacturer’s protocol (Clontech). The β-galactosidase filter and liquid assay, as well as the test for HIS prototrophy, was performed as described previously (1).
Mammalian two-hybrid methodology.
We used the Mammalian Matchmaker Two-Hybrid assay kit (Clontech) to investigate a possible interaction between human peroxins. Two proteins were cloned behind the GAL4 DNA-binding domain of the pM vector or the VP16 transcription activation domain of the pVP16 vector. The EcoRV-SalI fragment of pSK-HsPEX14, encoding the entire HsPEX14 open reading frame, was cloned into the EcoRI (blunted with Klenow polymerase) and SalI sites of pVP16 or into the SmaI and SalI sites of pM, respectively. The pM and pV16 plasmids expressing the human short and long forms of HsPEX5, the HsPEX7, or the SH3 domain and the full-length form of HsPEX13 have been described (13a). For transfection, human skin fibroblasts (GM5756) were seeded onto 35-mm-diameter dishes. Cells were incubated in the presence of 5 μl of Lipofectamine with 0.7 μg of DNA of pM and pVP16 derivatives, as well as with 0.15 μg of the reporter plasmid pG5CAT (Clontech). Two days after transfection, cells were lysed in 0.5 ml of lysis buffer for 30 min at 4°C, and the supernatants were cleared at 15,000 × g for 15 min. Proteins were estimated by using the BCA protein assay reagent (Pierce, Rockford, Ill.). The amount of chloramphenicol acetyltransferase (CAT) in lysates was estimated with the CAT enzyme-linked immunoassay (ELISA) kit (Boehringer). In parallel, transfected cells were analyzed for the presence of the two-hybrid fusion proteins and for CAT expression by immunofluorescence with antibodies against the Gal4p binding domain (Santa Cruz Biotechnology, Heidelberg, Germany) and with antibodies against CAT (5′Prime→3′Prime, Boulder, Colo.).
Immunoprecipitations.
HspEX5l (pPEX51 [7, 8]), HsPEX13 (pcDNA3-HsPEX13), and HsPEX14 (pcDNA3-HsPEX14) were transcribed and translated in vitro for 1 h by using the TNT-coupled reticulocyte lysate system (Promega). All proteins were labeled with [35S]methionine (1,175 Ci/mmol) (NEN, Cologne, Germany). The translation was terminated by the addition of cycloheximide to a final concentration of 100 μg/ml. Equal amounts of either HsPex13p and HsPex14p or HsPex5p and HsPex14p translation reactions (10 μl) were mixed and incubated together for an additional hour at 30°C. The reaction mixture was diluted to 200 μl with binding buffer (20 mM HEPES, pH 7.3; 110 mM potassium acetate; 5 mM sodium acetate; 2 mM magnesium acetate; 1 mM EDTA; 0.1% Triton X-100; 0.5 μg of leupeptin and 0.5 μg of pepstatin per ml; 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated with 50 μl of anti-rabbit IgG Dynabeads (Dynal, Hamburg, Germany) and saturated with anti-HsPex14p antibodies for 2 h at 4°C in a rotary shaker. Immunoprecipitates were collected with a magnet. The precipitates were washed five times with 0.5 ml of binding buffer containing 0.1% Triton X-100 and 0.02% sodium dodecyl sulfate (SDS), resuspended in 20 μl of SDS sample buffer, and denatured for 5 min at 95°C. The samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (10% acrylamide gel). The gel was then treated with 0.5 M sodium salicylate for 30 min, dried, and subjected to fluorography.
For immunoprecipitation of HsPex14p from lysates of human fibroblasts (PBD005), cells were plated to 80% confluency on 60-mm Petri dishes. The transfection with plasmid pEB13.10 encoding an N-terminal myc-tagged HsPex7p (13a) and plasmids pPEX5s or pPEX5l (7), encoding the short and long forms of the human Pex5p, were done as described above. Two days after the transfections, the cells were washed with PBS followed by incubation with 0.8 ml of binding buffer (see above) containing 0.5% Triton X-100, 0.5 μg of leupeptin and 0.5 μg of pepstatin per ml, and 0.1 mM PMSF for 30 min on ice. The cell lysates were cleared in a microfuge at 15,000 × g for 15 min. Then, 450 μl of cell lysate was incubated with 50 μl of anti-rabbit IgG Dynabeads saturated with anti-HsPex14p antibodies. The precipitates were washed five times with 0.5 ml of binding buffer containing 0.5% Triton X-100 and 0.01% SDS, resuspended in 30 μl of nonreducing SDS sample buffer, and denatured for 5 min at 95°C. After separation of the Dynabeads, the samples were supplemented with 2 μl of β-mercaptoethanol and incubated again for 5 min at 95°C. The samples were separated by SDS-PAGE (10% acrylamide gel). Immunoblot blot analysis was performed by using polyclonal anti-HsPex5p antibodies (14) or monoclonal 9E10 antiserum (23) to detect N-terminal tagged myc-HsPex7p.
Ligand blot assay.
Plasmids pET9d-His-PEX5l, pET9d-His-PEX5s, and pET9d-His-PEX14 for the bacterial expression of HsPex5ps, HsPex5pl, and HsPex14p, respectively, were kindly provided by Wolfgang Schliebs (Ruhr-University Bochum, Bochum, Germany [57a]). Expression of these proteins in host cell E. coli BL21(DE3) and the purification of His-tagged HsPex14p was performed with Ni-nitriloacetic acid agarose according to the protocol of the manufacturer of the pET system (Novagen). Cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose. In order to renature the proteins, the membranes were incubated for 4 h in buffer A (50 mM Tris-HCl, pH 7.5; 100 mM potassium acetate, 150 mM NaCl; 1 mM dithiothreitol; 5 mM MgCl2; 1 mM EDTA; 0.3% Tween 20; 100 μM ZnCl2; 5% [wt/vol] nonfat milk; 100 mM methionine). After renaturation, the membranes were incubated for 14 h with purified HsPex14p in buffer A. HsPex14p-containing complexes on the membranes were visualized by immunoblot analysis with anti-HsPex14p antibodies.
RESULTS
Identification of HsPEX14 and detection of HsPex14p.
We used the BLAST algorithm to probe the dbESTs with the S. cerevisiae ScPex14p sequence and identified a human candidate HsPEX14 cDNA (GenBank number H16035) from a breast cell library. Sequence analysis revealed the presence of an open reading frame of 1,131 bp with the potential to encode a protein of 41 kDa (Fig. 1A). Alignment of the deduced amino acid sequence with S. cerevisiae ScPex14p and Hansenula polymorpha HpPex14p (1, 35) showed overall identities of 26 and 29%, and similarities of 43 and 45%, respectively (Fig. 1B). All three predicted proteins are of similar size and share two conserved coiled-coil regions with probabilities of 0.6 and 0.8 according to the pair-coil program algorithm (4). Based on these similarities, the identified human gene was designated HsPEX14.
FIG. 1.
Characterization of the human HsPEX14 cDNA. (A) Nucleotide sequence and deduced amino acid sequence of human HsPEX14 cDNA. Boxed amino acids indicate the leucine zipper of the predicted coiled-coil regions. (B) Amino acid alignment of the human, S. cerevisiae, and H. polymorpha Pex14p. Sequence alignment was performed by using Lasergene (DNASTAR, London, United Kingdom). Amino acids identical or similar in at least two proteins are highlighted with black. Similarity rules were as follows: G = A = S; A = V; V = I = L = M; I = L = M = F = Y = W; K = R = H; D = E = Q = N; and S = T = Q = N.
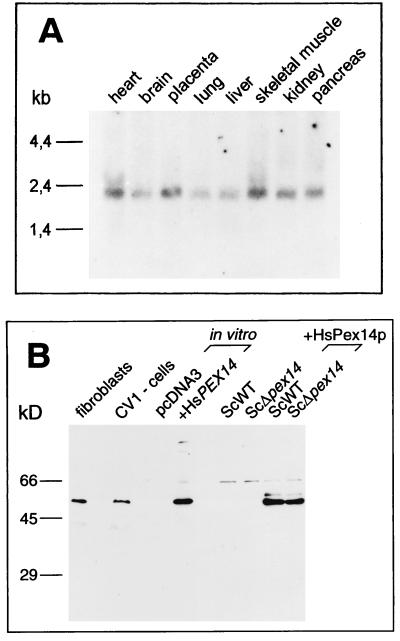
The expression of HsPex14p in different tissues was examined by Northern blot analysis (Fig. 2A). The mRNA for the gene is expressed in all of the tissues examined, thus exhibiting an expression pattern expected for a protein required for peroxisome biogenesis (8). The size of the mature HsPEX14 transcript of 2.1 kb corresponds well to the size of the isolated cDNA. To further analyze whether the cDNA contained the full-length open reading frame, we synthesized the product of the HsPEX14 cDNA in a coupled in vitro transcription-translation reaction and compared its migration mobility on SDS-PAGE to the endogenous HsPex14p of mammalian cells. With polyclonal antibodies raised against HsPex14p, immunoblot analysis showed that the in vitro translation product of the isolated HsPEX14 cDNA was indistinguishable in its mobility from the endogenous HsPex14p of human fibroblasts and monkey CV1 cells (Fig. 2B). 5′ Rapid amplification of cDNA ends experiments did not reveal cDNAs longer than the identified EST clones (data not shown). These observations suggested that the isolated cDNA contained the complete open reading frame of the HsPEX14 gene. The low pI of 4.9 might explain why HsPex14p migrated with a molecular mass of 55 kDa instead of its calculated mass of 41 kDa.
FIG. 2.
HsPEX14 expression and immunological detection of the HsPEX14 gene product. (A) A multiple-tissue Northern blot loaded with 2 μg of mRNA per lane was probed with digoxigenin dUTP-labeled cDNA corresponding to the entire HsPEX14 open reading frame. A 2.1-kb HsPEX14 transcript is present in all tissues. (B) Immunological detection of the HsPEX14 gene product. The endogenous mammalian HsPex14p was identified in protein extracts of normal human fibroblasts (lane fibroblasts) and monkey kidney CV1 cells (lane CV1-cells). The HsPEX14 cDNA gene product synthesized in a coupled in vitro transcription-translation reaction (lane +HsPEX14) showed the same relative molecular mass as the endogenous mammalian HsPex14p. The in vitro transcription-translation of the empty vector (lane pcDNA3) served as a negative control. HsPex14p of the same size also was detected in homogenates of S. cerevisiae wild-type and Δpex14 null mutant strains expressing the HsPEX14 cDNA under the control of the yeast alcohol dehydrogenase promoter from a high-copy-number plasmid (lanes ScWT+HsPex14p and ScΔpex14+HsPex14p). Detection of HsPex14p was done by immunoblot analysis with polyclonal antibodies against the protein. The antibodies against the HsPex14p did not recognize the yeast orthologue (lane ScWT).
To test whether the HsPex14p can functionally replace the yeast orthologue, we expressed HsPex1p in S. cerevisiae pex14Δ cells (Fig. 2B). Deficiency in ScPex14p results in a characteristic defect in peroxisome biogenesis, including an inability of the yeast to grow on oleic acid as single carbon source (1, 10). The growth defect on oleic acid medium could not be rescued by the expression of the human HsPex14p, indicating that the proteins are not functionally interchangeable (data not shown).
Association of HsPex14p with peroxisomes.
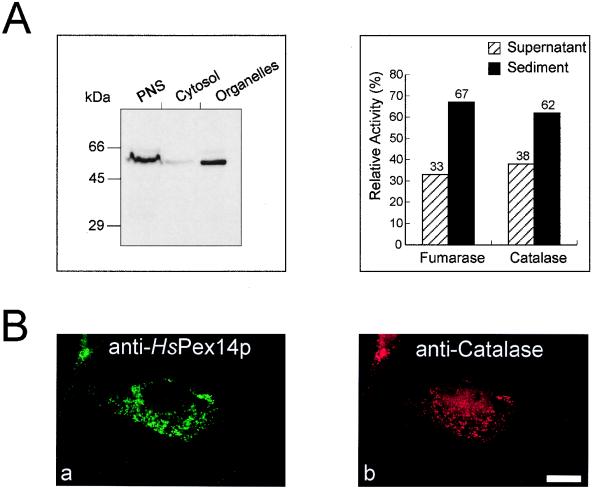
The subcellular localization of HsPex14p was determined by indirect double immunofluorescence microscopy and subcellular fractionation studies. Immunoblot analysis of fractions obtained by differential centrifugation of homogenates from human fibroblasts revealed that HsPex14p is almost exclusively present in the organellar pellet (Fig. 3A). These observations were corroborated by the localization of the protein by double immunofluorescence microscopy. The congruent punctate fluorescence pattern observed for HsPex14p and peroxisomal catalase revealed that both proteins share the same subcellular localization, indicating that HsPex14p is peroxisomal (Fig. 3Ba and b). Occasionally, an additional, very faint staining of tubular structures was observed upon detection of endogenous HsPex14p (see below).
FIG. 3.
Peroxisomal localization of HsPex14p. (A) Immunological and enzymatic detection of HsPex14p and organellar marker enzymes in cell fractions that were obtained by differential centrifugation of a homogenate from human skin fibroblasts (GM5756). HsPex14p was almost exclusively localized in the organellar pellet. The enzyme activities of the peroxisomal marker catalase and the mitochondrial marker fumarase in fractions served as internal controls for the integrity of the isolated organelles. Equal portions were loaded per lane. (B) Double immunofluorescence localization of the endogenous HsPex14p (a) and catalase (b) in human fibroblasts. Triton X-100 permeabilized human skin fibroblasts (GM5756) were processed for indirect double immunofluorescence with polyclonal rabbit anti-HsPex14p antibodies and polyclonal sheep antibodies against human peroxisomal catalase. Secondary antibodies were fluorescein-labeled donkey anti-rabbit antibodies (a) and rhodamine-labeled donkey anti-sheep (b). Bar, 28 μm.
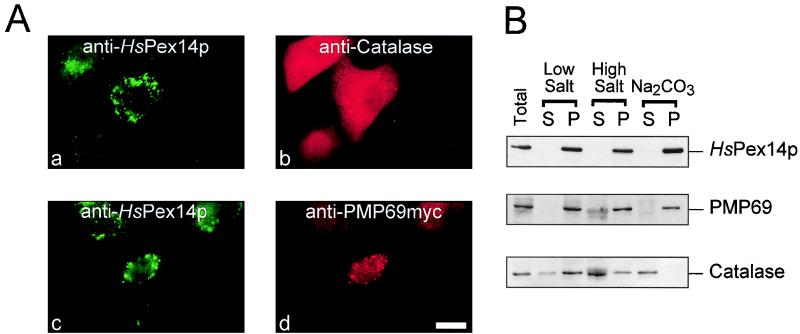
PBD cell lines that are affected in the import of peroxisomal matrix proteins maintain correct targeting and insertion of all or at least some peroxisomal membrane proteins, leading to the presence of numerous matrix-deficient peroxisomal membrane ghosts in these cells (57) (Fig. 4Ac and d). Immunofluorescence localization of HsPex14p and of the peroxisomal matrix marker catalase in an HsPEX1-defective PBD cell line (complementation group 1; PBD009) revealed a diffuse staining pattern for catalase, a finding consistent with the mislocalization of this protein to the cytosol (Fig. 4Ab). Additional staining of the same cells for HsPex14p by immunofluorescence microscopy revealed a punctate pattern, suggesting that HsPex14p is associated with peroxisomal membrane ghosts (Fig. 4Aa). Double immunofluorescence microscopic localization of HsPex14p and the peroxisomal membrane marker PMP69 (27, 33, 34) revealed colocalization of both proteins in PBD cells (Fig. 4Ac and d). This result indicated that HsPex14p is targeted to peroxisomes independent of the matrix protein import pathway, an observation expected for a peroxisomal membrane protein. A fibroblast organellar fraction was subjected to extraction by low-salt and high-salt conditions and carbonate at pH 11 (Fig. 4B). None of the extractions led to the release of HsPex14p from the membranes. In this respect, HsPex14p behaved like the integral membrane protein PMP69, while the peroxisomal matrix protein catalase was at least partially extracted by all means.
FIG. 4.
Subperoxisomal localization of HsPex14p. (A) HsPex14p is targeted to peroxisomal membrane ghosts. HsPEX1-deficient CG1 cells of patient PBD009 were transfected with pcDNA3-PMP69myc and processed for indirect double immunofluorescence after Triton X-100 permeabilization by using anti-HsPex14p (a and c), anti-human catalase (b), and monoclonal anti-myc (d) antibodies. The diffuse labeling pattern for catalase reflects the inability of these cells to import peroxisomal matrix proteins. In contrast, HsPex14p shared the same subcellular distribution as the peroxisomal membrane protein PMP69, a finding suggestive of both proteins residing in peroxisomal membrane ghosts. Secondary antibodies were fluorescein-labeled donkey anti-rabbit antibodies (a and c), rhodamine-labeled donkey anti-sheep antibodies (b), or Cy3-labeled donkey anti-mouse antibodies (d). Bar, 29 μm. (B) HsPex14p resists extraction with carbonate. Isolated organelles from human skin fibroblasts were subjected to extraction with low- and high-salt concentrations and to carbonate extraction as indicated. Extracted membranes were sedimented, and equal portions of all fractions were analyzed by immunoblot analysis for the presence of HsPex14p, as well as for the peroxisomal matrix marker catalase and the membrane marker PMP69.
Topology of HsPex14p.
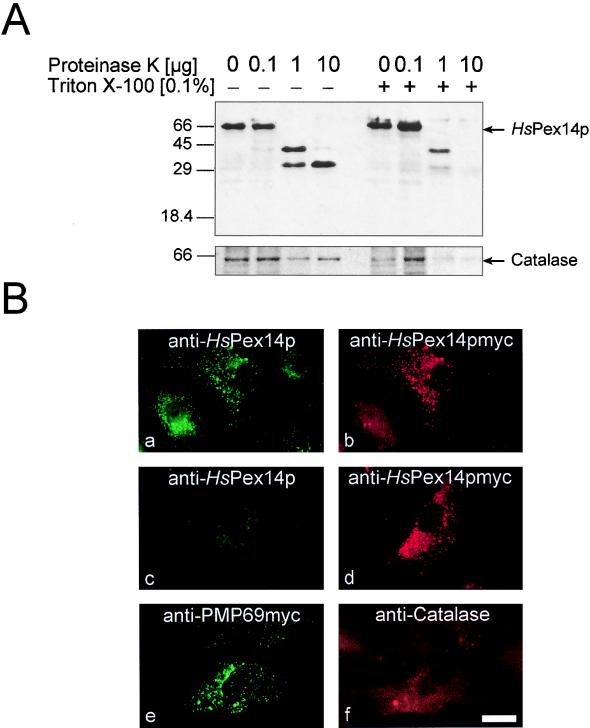
In order to determine the topology of HsPex14p, isolated organelles were incubated with proteinase K in the presence or absence of detergent. Figure 5A shows that HsPex14p was extremely sensitive to protease even in the absence of detergent, indicating that the protein is at least partially exposed to the cytosol. In the absence of detergent a protease-resistant 30-kDa degradation product was observed that was rapidly degraded after detergent was added. This result might indicate that the 30-kDa fragment is protected from the proteolytic digestion by compartmentation or protein association. For a more detailed analysis, we studied the topology of a C-terminally myc-tagged HsPex14p by immunofluorescence microscopy. Human fibroblasts expressing the tagged HsPex14p were fixed, incubated with detergent to permeabilize the cellular membranes, and processed for immunofluorescence microscopy. Double immunofluorescence microscopy localization of the myc-tagged HsPex14p and PMP69 revealed a congruent fluorescence pattern, indicating that the tagged HsPex14p is targeted to peroxisomes (data not shown). Upon permeabilization with Triton X-100, a congruent fluorescence pattern was observed for the double immunofluorescence localization of HsPex14p with polyclonal antibodies against the N-terminal 1 to 134 amino acids of the protein in combination with monoclonal antibodies against the C-terminal myc tag (Fig. 5Ba and b). The same cell population was also processed for indirect immunofluorescence microscopy after permeabilization with digitonin instead of Triton X-100. Under these conditions, only the plasma membrane is permeabilized and intraperoxisomal antigens are inaccessible to exogenous antibodies. This is shown for the intraperoxisomal catalase in Fig. 5Bf, which is not recognized under these conditions, in contrast to the integral membrane protein PMP69 (Fig. 5Be). Double immunofluorescence microscopy localization of mycHsPex14p in digitonin-permeabilized cells was performed with the antibodies against the myc epitope and the antibodies against the N-terminal amino acids 1 to 134 of HsPex14p. A punctate pattern was observed for the localization of the C-terminal myc epitope (Fig. 5Bd), while the N-terminal region was not detected under these conditions (Fig. 5Bc). This observation suggests that the C terminus of HsPex14p is exposed to the cytosol, while the N terminus of the protein is not accessible to the antibodies.
FIG. 5.
Membrane topology of HsPex14p. (A) Protease protection analysis of isolated organelles. HsPex14p is accessible to exogenously added protease. A 30-kDa degradation product is protected in the absence of detergent but is rapidly degraded upon permeabilization of the membrane with Triton X-100. Equal amounts of an organellar pellet from human skin fibroblasts were incubated in the absence or presence of detergent with increasing amounts of proteinase K as indicated. Detection of HsPex14p in fractions was performed with polyclonal antibodies against amino acids 1 to 134 of the protein. The intraperoxisomal catalase was stable in the absence of detergent, serving as an internal control for the integrity of the peroxisomes. (B) HsPex14p is a peroxisomal membrane protein with its C terminus exposed to the cytosol. Human skin fibroblasts (GM5756) transfected with pcDNA3-HsPEX14myc were processed for indirect double immunofluorescence microscopy by permeabilization of fixed cells with either 1% Triton X-100 (a and b) or 25 μg of digitonin per ml (c to f). Corresponding sets of cells were incubated with the following antibody combinations: a to d, anti-HsPex14p (1–134)/anti-myc; e and f, anti-PMP69myc and anti-catalase. The congruent fluorescence pattern in panels a and b indicates that the myc-tagged HsPex14p is targeted to peroxisomes. When the peroxisomal membrane remains intact, neither the intraperoxisomal catalase (f) nor the N terminus of HsPex14p (c) is detected, while the C-terminal myc tag (d) or the membrane protein PMP69 (e) is recognized under these conditions. Bar, 44 μm.
Effect of HsPex14p overexpression on peroxisome biogenesis.
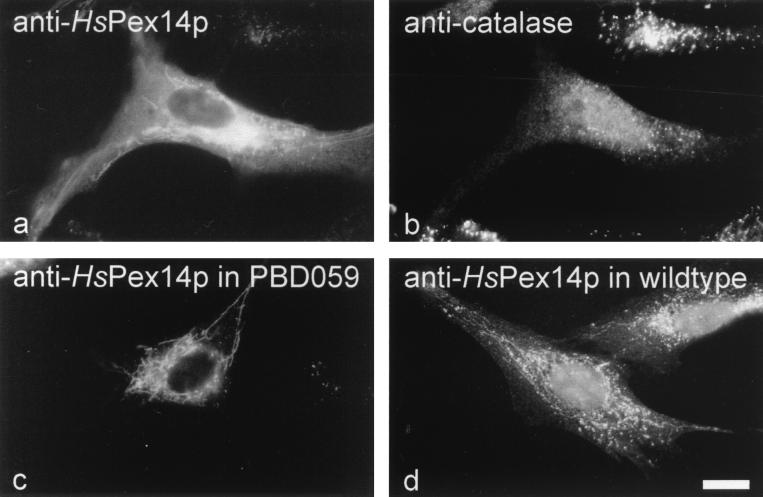
We studied the effect of HsPEX14 overexpression in the GM5756 cell line (normal) and in a cell line from a PBD patient (complementation group 8; PBD059). Immunofluorescence localization of overexpressed HsPex14p revealed an overall fluorescence accompanied by a labeling of a tubular system of unknown origin (Fig. 6a and c). Remarkably, HsPEX14 overexpression led to a partial mislocalization of peroxisomal catalase to the cytosol evident in the immunofluorescence microscopic image as fading of the peroxisomal punctate pattern and an increase of the cytosolic staining (Fig. 6b). Double immunofluorescence microscopy localization suggested that the HsPex14p-containing tubules do not contain catalase (Fig. 6a and b). Upon HsPex14p overexpression, the HsPex14p-containing tubular structures were also observed in the GM5756 cell line (Fig. 6; panel a) and in cell lines from PBD patients (Fig. 6c). Often they were more striking in PBD cell lines than in GM5756 cells. Interestingly, in addition to the predominant localization to “normal” peroxisomes, a faint labeling of an HsPex14p-containing tubular system was occasionally observed in nontransfected normal GM5756 cells (Fig. 6d).
FIG. 6.
Effects of HsPEX14 overexpression in normal fibroblast (GM5756) and PBD patient cell lines. (a and b) Immunofluorescence microscopy localization of HsPex14p (a) and peroxisomal catalase (b) in GM5756 fibroblasts overexpressing HsPex14p from pcDNA-HsPEX14. Overexpression led to a partial mislocalization of catalase, as judged by the disappearance of the prominent punctate pattern and the increase in cytosolic background labeling (b). Note the bright punctate labeling for catalase in the adjacent control cell which does not overproduce the HsPex14p. In addition to the congruent punctate, staining for HsPex14p revealed HsPex14p-containing tubules of unknown origin. (c) Immunofluorescence localization of overexpressed HsPex14p in a PBD patient cell line of complementation group 8 (PBD059) expressing pcDNA-HsPEX14. Staining for HsPex14p revealed a bright labeling of tubular structures. (d) Immunofluorescence localization of endogenous HsPex14p of nontransfected normal GM5756 fibroblasts. Note the faint labeling of tubular structures in addition to the bright punctate staining for peroxisomes. Bar, 20 μm.
HsPEX14 is not responsible for one of the known complementation groups of PBD.
We tested the ability of HsPEX14 cDNA to restore the peroxisome biogenesis defect in fibroblasts representing 10 complementation groups (CG1 to CG4 and CG6 to CG11) of the PBD (26, 49). As judged by mislocalization of PTS1-containing proteins to the cytosol, the expression of HsPex14p did not rescue the peroxisomal protein import defect in any of the PBD fibroblasts (data not shown). Consequently, the HsPEX14 gene is not responsible for the defects in any of the known PBD complementation groups. However, the yeast orthologue ScPex14p is essential for peroxisome biogenesis. Deficiency in ScPex14p (1, 10) or HpPex14p (35) abolishes peroxisomal protein import and leads to the absence of morphologically normal peroxisomes, a phenotype also typical for most PBD (41). In analogy, it can be expected that mutations abolishing the function of HsPex14p will lead to a PBD. However, we also have to consider the possibility that HsPex14p is not essential for human peroxisome biogenesis, for instance, due to redundancy in this pathway in humans. In this respect, it is interesting to note that two isoforms of Pex11p have been discovered in mammals, Trypanosoma brucei and Candida boidinii (42, 48, 53), while only one Pex11p orthologue exists in S. cerevisiae (19, 43).
HsPex14p binds the PTS1 receptor in the yeast and human two-hybrid systems.
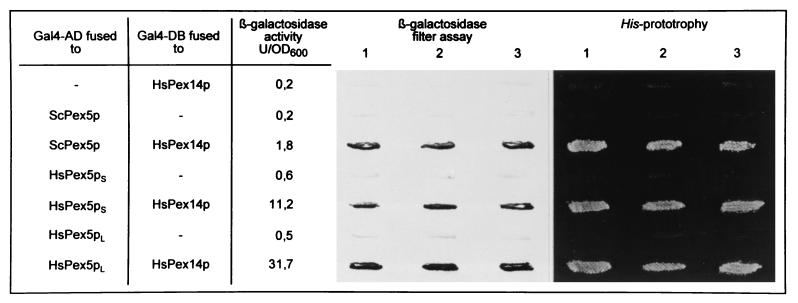
We used the yeast and human two-hybrid systems to detect homologous and heterologous in vivo protein-protein interactions (13, 24) between HsPex14p and different yeast and human peroxins. These included the PTS1 receptor from S. cerevisiae (ScPex5p) (9, 66) and both the long and the short forms of the human PTS1 receptor, HsPex5pl and HsPex5ps (7, 8, 14, 25, 68). The two forms of the human PTS1 receptor might derive by alternative mRNA splicing and differ by a 37-amino-acid insertion in the longer form (8, 14, 25, 44, 68). Other proteins included in the study were the PTS2 receptors ScPex7p (56, 71) and HsPex7p (8, 50, 55) and the putative docking protein for PTS1-dependent protein import HsPex13p (17, 20, 29). The yeast peroxins included in this study have been reported to be binding partners of the yeast Pex14p (1, 10). Fusion constructs were prepared by cloning the human PEX genes into plasmids encoding either the VP16 or the Gal4p-transcription activation domain or else the Gal4p-DNA binding domain. In the yeast two-hybrid system, physical interaction of HsPex14p with these peroxins was expected to result in the activation of lacZ and HIS3 transcription in reporter strains PCY2 and HF7c, respectively, as indicated by β-galactosidase expression and the His prototrophy of transformants (Fig. 7). In the mammalian two-hybrid system, the interaction of components results in the expression of CAT, which can be monitored by immunofluorescence microscopy with antibodies against the protein (Fig. 8a). Quantification of CAT expression was performed by ELISA (Table 1).
FIG. 7.
HsPex14p interacts with the PTS1 receptor Pex5p. Analysis of HsPex14p interaction with either S. cerevisiae ScPex5p or human HsPex5ps and HsPex5pl in a yeast two-hybrid system by means of HIS3 and lacZ transcription activation. Yeast strains PCY2 and HF7c were transformed with plasmids expressing peroxins fused to either the Gal4p-DNA binding domain (Gal4p-DB) or the Gal4p-transcription activation domain (Gal4p-AD) as indicated. The amount of β-galactosidase activity in PCY2 double transformants expressing the indicated combinations of Gal4p-peroxin fusion proteins is given on the left. The β-galactosidase activity shown is the average of duplicate measurements for two independent transformants harboring each set of plasmids. The color intensity of these strains after the β-galactosidase filter assay is shown in the middle panel. The His-prototropy assay for HF7c double transformants harboring the indicated plasmid combination is shown on the right. HsPex5ps and Pex5pl, short and long forms of the human PTS1 receptor, respectively.
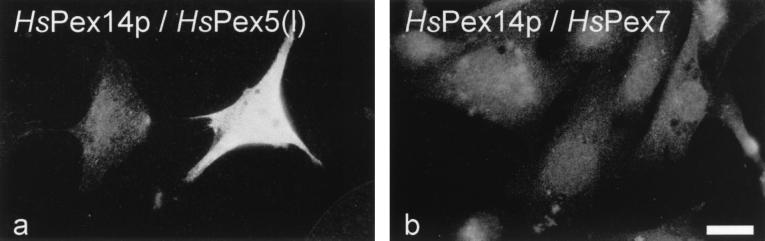
FIG. 8.
Analysis of protein-protein interactions of HsPex14p with HsPex5p (a) or HsPex7p (b) in the mammalian two-hybrid system. Immunofluorescence microscopy detection of CAT expression in cells containing the CAT plasmid in conjunction with the plasmid expressing HsPex14p fused to the activation domain of VP16 and either the short form of HsPex5p (a) or HsPex7p (b) each fused to the DNA-binding domain of Gal4p. Coexpression of the HsPex5pl fusion construct did result in a bright fluorescence, indicating the expression of CAT and thus interaction of HsPex14p with HsPex5pl (a). The nonfluorescent cell on the left is shown for comparison. Coexpression of the HsPex7p fusion construct did not lead to fluorescence above the background level (b), suggesting that HsPex14p and HsPex7p do not interact in the mammalian two-hybrid system. Bar, 20 μm.
TABLE 1.
Quantitative analysis of protein-protein interactions of Pex14p with peroxins in the mammalian two-hybrid systema
| No. of test | Fusions to the Gal4p-DNA binding domain (pM vector) | Fusions to the VP16-activation domain (pVP16) | CAT expression (ng/mg of protein) |
|---|---|---|---|
| 1 | HsPex5pl | HsPex14p | 3.28 |
| 2 | HsPex5ps | HsPex14p | 2.6 |
| 3 | HsPex7p | HsPex14p | ND |
| 4 | HsPex13p | HsPex14p | ND |
| 5 | HsPex13p (SH3) | HsPex14p | ND |
| 5 | HsPex14p | HsPex14p | ND |
| 6 | HsPex5pl | pVP16 | 0.22 |
| 7 | HsPex5ps | pVP16 | 0.1 |
| 8 | HsPex7p | pVP16 | ND |
| 9 | HsPex13p | pVP16 | ND |
| 10 | HsPex14p | pVP16 | ND |
| 11 | pM | HsPex14p | ND |
CAT expression was detected in cells containing pG5CAT in conjunction with the plasmid expressing HsPex14p fused to the VP16 activation domain and fusions of the Gal4p-DNA binding domain either with the short and long forms of HsPex5p, HsPex7p, the SH3 domain of HsPex13p, and the full-length HsPex13p or with HsPex14p itself. A significant CAT expression was only observed for the coexpression of the HsPex14p fusion constructs together with the fusion constructs containing either the long or the short form of HsPex5p. This result indicates a two-hybrid interaction of HsPex14p with both forms of the PTS1 receptor HsPex5p. Other combination of peroxin fusions with Gal4p domains or expression of constructs in combination with nonfused Gal4p domains did not result in a significant expression of CAT. ND, not detected.
Yeast cells coexpressing HsPex14p and either the long or the short form of HsPex5p fused to the corresponding Gal4p domains both were His prototrophic and expressed β-galactosidase (Fig. 7). The corresponding combinations expressed in the mammalian two-hybrid system resulted in the expression of CAT, as judged by the immunofluorescence microscopic detection of the protein (Fig. 8a) and by quantitative analysis of CAT expression (Table 1). These results demonstrate that HsPex14p is capable of binding both the long and the short forms of HsPex5p in vivo. Interestingly, HsPex14p was also found to interact with the yeast PTS1 receptor (ScPex5p; Fig. 7). However, coexpression of HsPEX14-GAL4AD (yeast system) or HsPEX14-VP16AD (human system) with HsPEX13-GAL4DB, HsPEX7-GAL4DB, HsPEX14-GAL4DB, or HsPEX13 (SH3)-GAL4DB, which encodes the cytosolic SH3-domain of HsPex13p, did not result in lacZ or HIS3 transcription activation (data not shown) or in CAT expression (Fig. 8b; Table 1). Transfection of Pex7p-defective PBD074 (CG11) cells with HsPEX7-GAL4DB results in a complementation of the peroxisome biogenesis defect of these cells, suggesting that the Gal4pDB-Pex7p is still functional (data not shown). The results of the quantification of the two-hybrid interactions paralleled the staining intensities obtained with the filter assay or immunofluorescence microscopy (Fig. 7, Table 1). While HsPex14p strongly interacts with the long and the short forms of the human PTS1 receptor, the binding to the yeast counterpart was rather weak.
The controls included in the two-hybrid experiments show that coexpression of either of the fusion proteins, together with the respective binding or activation domains alone, did not significantly support transcription activation of the reporter genes. Peroxins which did not interact with HsPex14p in the two-hybrid system showed β-galactosidase and CAT levels in the range of the controls.
In vivo and in vitro binding of HsPex14p and HsPex5p.
The two-hybrid data on HsPex14p interaction with HsPex5pl and HsPex5ps and its lack of interaction with HsPex7p and HsPex13p were corroborated by in vitro and in vivo coimmunoprecipitation studies, as well as by ligand blot analysis.
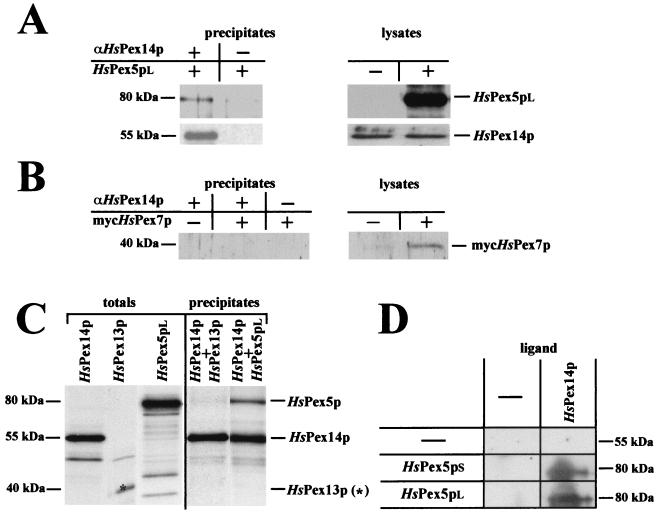
An N-terminally myc-tagged form of HsPex7p was expressed in HsPex7p-defective cell line PBD074 (CG11). As the expression resulted in a functional complementation of the mutant phenotype of this cell line (data not shown), the tagging apparently did not interfere with the function of HsPex7p in peroxisome biogenesis. Thus, the results obtained with this fusion protein can be expected to closely mirror the wild-type situation. For the immunoprecipitation of HsPex14p, cell lysates were prepared from HsPex5p-defective PBD005 (CG2) cell lines, expressing HsPex5pl, HsPex5ps, or mycHsPex7p. Nontransfected cells served as controls for the expression. Endogenous HsPex14p was precipitated from the cell lysates with anti-HsPex14p antibodies and precipitates were analyzed for the presence of HsPex5p or mycHsPex7p with anti-HsPex5p or anti-myc antibodies (Fig. 9A and B). HsPex5pl was found to coprecipitate with HsPex14p (Fig. 9A). The minute amount of HsPex5pl present in the precipitate in the absence of anti-HsPex14p antibodies indicates that a small amount of HsPex5p unspecifically binds to the Dynabeads. However, a significantly higher amount of HsPex5pl is precipitated with anti-HsPex14p-coated beads, indicating an interaction of HsPex14p and HsPex5pl. The same result was obtained for the short form of HsPex5p (data not shown). In contrast, myc HsPex7p was not detected in the precipitates (Fig. 9B), thus supporting the observed lack of interaction of both proteins in the yeast and mammalian two-hybrid systems (Fig. 8 and Table 1).
FIG. 9.
In vivo and in vitro studies on the interaction between HsPex14p and HsPex5p, HsPex7p and HsPex13p. (A and B) Immunoprecipitation of HsPex14p from cell lysates of HsPex5pl (A) or mycHsPex7p (B) transfected HsPex5p-defective PBD005 (CG2) cells. Immunoprecipitates and lysates (4 μg of protein) were subjected to SDS-PAGE and immunoblot analysis. Immunoprecipitation was performed with anti-HsPex14p antibodies. The amount of precipitates loaded on the gel equals 10 times the amount of lysate loaded. (C) In vitro synthesized [35S]HsPex14p was incubated with lysates containing either [35S]HsPex5pl or [35S]HsPex13p. [35S]HsPex14p was immunoprecipitated with anti-HsPex14p antibodies and precipitates, as well as the original lysates, were subjected to SDS-PAGE and autoradiography. The amount of precipitates loaded on the gel corresponds to two portions of lysate loaded. (D) Ligand blot analysis of the HsPex14p interaction with HsPex5pl and HsPex5ps. Bacterial lysates (20 μg protein) containing HsPex5l, HsPex5ps, or no recombinant protein were subjected to SDS-PAGE and transferred to nitrocellulose. Individual membranes were incubated with buffer containing either purified His6-tagged HsPex14p (1 μg) or no recombinant protein. HsPex14p-containing complexes were visualized by immunoblotting with anti-HsPex14p antibodies.
Furthermore, binding of HsPex5pl and HsPex13p to HsPex14p was analyzed by the combination of in vitro transcription-translation and coimmunoprecipitation, as well as by ligand blotting. In vitro synthesized 35S-labeled HsPex14p was incubated with lysates containing either [35S]HsPex5pl or [35S]HsPex13p and, subsequently, the HsPex14p was immunoprecipitated with anti-HsPex14p antibodies. As shown in Fig. 9C, [35S]HsPex5pl was recovered in the immunoprecipitates but no [35S]HsPex13p coprecipitated with [35S]HsPex14p. Even after a longer exposure, the [35S]HsPex13p could not be detected in the precipitate. For the ligand blots, bacterial lysates containing either HsPex5ps, HsPex5pl, or the SH3 domain of HsPex13p (amino acids 231 to 364) were separated by SDS-PAGE, immobilized on nitrocellulose and analyzed for their interaction with recombinant, purified HsPex14p. We found that HsPex14p bound to the long and the short forms of HsPex5p (Fig. 9D), but we did not observe an interaction of HsPex14p with the SH3 domain of HsPex13p (data not shown).
DISCUSSION
Evolutionary and functional relationship of human HsPex14p and yeast ScPex14p.
Several lines of evidence suggest that HsPex14p represents the human orthologue of yeast Pex14p. First, HsPex14p bears a striking 43 to 45% sequence similarity and a 26 to 29% sequence identity to the Pex14p of yeasts. This similarity is distributed over the entire length of the proteins, and it is in the range of known yeast and mammalian peroxin orthologues. Second, both the yeast Pex14p and the human Pex14p are localized to the peroxisomal membrane, although the membrane topology of the two proteins might be different (see below). Third, both yeast and human types of Pex14p interact with the PTS1 receptor Pex5p (Fig. 7 to 9). This observation supports the notion that both proteins are involved in peroxisomal protein import and that they provide a binding site for the PTS1 receptor at the peroxisomal membrane. Taken together, the similarities between the yeast and the human forms of HsPex14p strongly suggest that these proteins not only are descendents from a common gene but that they also perform a similar function in peroxisome biogenesis and thus are true orthologues.
Subcellular localization and topology of HsPex14p.
HsPex14p is predominantly associated with peroxisomes (Fig. 3 and 4). Interestingly, immunofluorescence microscopic localization of endogenous HsPex14p revealed an additional faint labeling of tubular structures (Fig. 6). The labeling was more pronounced upon overexpression of the protein, and it is also visible in PBD cells (Fig. 6). Whether the labeling is due to a partial mislocalization of HsPex14p or whether these tubular structures represent a matrix-deficient peroxisomal compartment has not yet been determined.
HsPex14p was entirely resistant to extraction by carbonate or high salt concentrations, suggesting that the protein is tightly associated with the peroxisomal membrane (Fig. 3 and 4). In line with this observation, HsPex14p is efficiently targeted to peroxisomal membrane ghosts in fibroblasts of PBD patients (Fig. 4c). In PBD fibroblasts, matrix proteins are typically mislocalized to the cytosol, whereas membrane proteins are still correctly targeted to their destination (57). One putative transmembrane segment (amino acids 108 to 127) is predicted in the primary sequence of HsPex14p by (38). Studies on the topology of the HsPex14p were performed with the endogenous protein, as well as with a C-terminal myc-tagged HsPex14p. Remarkably, the C-terminal myc tag of the protein was detected under conditions in which the N terminus of HsPex14p was not accessible to exogenously added antibodies against the first 134 amino acids of the protein (Fig. 5). This observation suggests that the N terminus of HsPex14p protrudes into the peroxisomal lumen. In line with this assumption, a degradation product of HsPex14p, which is protected against exogenously added protease (Fig. 5A), is also recognized by antibodies against the N-terminal region of the protein. However, considering the position of the predicted transmembrane segment (amino acids 108 to 127), the expected size of the protected fragment is much smaller than the observed 31 kDa. Expression of amino acids 1 to 134 fused to His6 in E. coli results in a protein of only 23 kDa (data not shown). If it turns out that the protection of the 30-kDa fragment is indeed caused by compartmentalization, it will be a challenge to determine the molecular cause for the 7-kDa difference between the expected and the observed sizes. In this respect, it is interesting to note that the endogenous HsPex14p is detected as a 55-kDa protein; this is bigger than the 41-kDa size predicted from its amino acid sequence and thus opens the possibility of an as-yet-unidentified posttranslational modification of the human protein.
Based on carbonate extractability and protease sensitivity, the ScPex14p was found to be a peripheral membrane protein residing on the cytoplasmic face of the peroxisomal membrane (1). However, a second group showed that the protein was partially carbonate resistant (10), a property also attributed to the H. polymorpha orthologue (35). These observations in yeast cells should be reevaluated in light of our observations which suggest that HsPex14p might traverse the peroxisomal membrane. However, our data do not rigorously rule out the possibility that HsPex14p is also a peripheral membrane protein. In support of this assumption, homo- and heterologously overexpressed HsPex14p is at least partially soluble, a property not typically observed for integral membrane proteins. As a possible explanation for the inaccessibility of the N terminus of HsPex14p to antibodies and protease, we also have to consider that the protection of this region could also be due to interaction with other proteins. In this respect, it is interesting to note that in vitro binding experiments showed that the N terminus of HsPex14p directly interacts with the human PTS1 receptor HsPex5p (36, 57a). It has been reported that the human PTS1 receptor cycles between the cytosol and peroxisomes, thus predicting specific receptor docking sites at the peroxisomal membrane (15). If the N terminus of HsPex14p faces the cytosol, HsPex14p could provide such a docking site for the PTS1 receptor as has been described for its yeast counterpart (1, 10). To address this possibility, we attempted to inhibit peroxisomal protein import by microinjection of antiserum against the N-terminal region of HsPex14p into the cytosol of GM5756 fibroblasts. We monitored the subcellular localization of endogenous catalase, as well as the localization of a green-fluorescent protein (GFP) fused to a PTS1 by immunofluorescence and fluorescence microscopy, respectively, over a period of several days. The GFP-PTS1 fusion protein was expressed from a coinjected or sequentially injected plasmid. No difference between microinjected cells and control cells was observed (data not shown). This result is in agreement with the observed inaccessability of HsPex14p for antibodies against the N-terminal region in digitonin-permeabilized cells (Fig. 5Bc).
Giving special attention to the carbonate resistance of the human HsPex14p, as well as to the inaccessibility of the N terminus from the cytosolic side, we at least have to consider that the N terminus of HsPex14p might face the peroxisomal lumen. In conjunction with the observed interaction of this region with the human PTS1 receptor (36, 57a), this would suggest that the predominantly cytosolic human PTS1 receptor might be localized in the peroxisomal lumen at some stage of the protein import process. This would be in support of the “extended shuttle model of peroxisomal protein import” (14, 22, 65), which suggests that the import receptors might bind their cargo proteins in the cytosol and target them across the peroxisomal membrane. In the peroxisomal lumen, the cargo might be released from the receptors, which subsequently shuttle back to the cytosol.
Different binding properties of yeast and human Pex14p.
Yeast Pex14p has been reported to interact with the PTS1- and the PTS2 receptors, as well as with Pex13p, and the protein is also supposed to homo-oligomerize (1, 10). In vivo and in vitro binding studies revealed that HsPex14p interacts with the PTS1 receptor HsPex5p (Fig. 7 to 9, Table 1). Our studies did not show a homo-oligomerization of the protein or an interaction of HsPex14p with the SH3 domain of HsPex13p. Remarkably, we observed no interactions of HsPex14p with the PTS2 receptor HsPex7p in any of the two-hybrid systems applied or in the coimmunoprecipitation analyses (Fig. 7 to 9). Since not every protein-protein interaction that takes place in the living cell may show up in two-hybrid or coimmunoprecipitation studies, these negative results have to be interpreted with caution. However, the possibility that human HsPex14p might bind the PTS1 but not the PTS2 receptor would add to recent data suggesting that the yeast and human mechanisms of peroxisomal protein import might not be conserved in every detail. Yeast cells efficiently import proteins of the PTS2 variety into peroxisomes in the absence of the PTS1 receptor (47, 51, 63, 64, 66). Consequently, in yeast cells the PTS2 receptor functions independently of the PTS1 receptor and thus requires its own docking site at the peroxisomal membrane. Pex14p has been proposed to provide the peroxisomal docking site for Pex7p in yeast (1). In contrast to yeast cells, the PTS1 receptor HsPex5p in human cells is also needed for the peroxisomal import of PTS2 proteins (3, 6, 52). The HsPex5p dependency of the import of PTS2 proteins might suggest that both pathways converge at the level of HsPex5p. In fact, in line with this assumption, HsPex5p is supposed to interact with the human HsPex7p (13a), which opens the possibility that HsPex7p is targeted to the peroxisomal membrane via HsPex5p. In this case, the human HsPex7p might not need its own docking site at the peroxisomal membrane, which would explain the observed lack of interaction between HsPex14p and HsPex7p (Fig. 7 to 9, Table 1).
ACKNOWLEDGMENTS
We are grateful to Stephen Gould, Wilhelm Just, and Wolfgang Schliebs for kindly providing antibodies and plasmids. We are indebted to Ulrike Freimann, Uta Ricken, and Sigrid Wüthrich for technical assistance. We thank Peter Rehling and Michael Schwierskott for reading of the manuscript.
G. K. Will was supported by a fellowship from the Graduiertenkolleg der Ruhr-Universität Bochum. G. Dodt was supported by a Lise Meitner fellowship from NRW. This work was supported by grants from the Deutsche Forschungsgemeinschaft (Er178/2-1, Ku329/17-3, and SFB480) and by the Fond der Chemischen Industrie.
ADDENDUM IN PROOF
While this paper was in review, Fransen et al. (Proc. Natl. Acad. Sci. USA 95:8087–8092, 1998) reported that HsPex14p interacts with Pex5p and Pex13p (SH3) and is directly required for peroxisomal protein import.
REFERENCES
- 1.Albertini M, Rehling P, Erdmann R, Girzalsky W, Kiel J A K W, Veenhuis M, Kunau W-H. Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell. 1997;89:83–92. doi: 10.1016/s0092-8674(00)80185-3. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Baes M, Gressens P, Baumgart E, Carmeliet P, Casteels M, Fransen M, Evrard P, Fahimi D, Declercq P E, Collen D, van Veldhoven P P, Mannaerts G P. A mouse model for Zellweger syndrome. Nat Genet. 1997;17:49–57. doi: 10.1038/ng0997-49. [DOI] [PubMed] [Google Scholar]
- 4.Berger B, Wilson D B, Wolf E, Tonchev T, Milla M, Kim P S. Predicting coils coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmeyer H U, Gawehn K, Grassel M. Fumarase of pig heart. In: Bergmeyer H U, editor. Methods of enzymatic analysis I. Weinheim, Germany: Verlag Chemie; 1974. pp. 479–480. [Google Scholar]
- 6.Braverman N, Dodt G, Gould S J, Valle D. Disorders of peroxisome biogenesis. Hum Mol Genet. 1995;4:1791–1798. doi: 10.1093/hmg/4.suppl_1.1791. [DOI] [PubMed] [Google Scholar]
- 7.Braverman N, Dodt G, Gould S J, Valle D. Isoform of Pex5p, the human PTS1 receptor, is required for the import of PTS2 proteins into peroxisomes. Hum Mol Gen. 1998;7:1195–1205. doi: 10.1093/hmg/7.8.1195. [DOI] [PubMed] [Google Scholar]
- 8.Braverman N, Steel G, Obie C, Moser A, Moser H, Gould S J, Valle D. Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet. 1997;15:369–376. doi: 10.1038/ng0497-369. [DOI] [PubMed] [Google Scholar]
- 9.Brocard C, Kragler F, Simon M M, Schuster T, Hartig A. The tetraticopeptide repeat-domain of the Pas10 protein of Saccharomyces cerevisiae is essential for binding the peroxisomal targeting signal SKL. Biochem Biophys Res Commun. 1994;204:1016–1022. doi: 10.1006/bbrc.1994.2564. [DOI] [PubMed] [Google Scholar]
- 10.Brocard C, Lametschwandtner G, Koudelka R, Hartig A. Pex14p is a member of the protein linkage map of Pex5p. EMBO J. 1997;16:5491–5500. doi: 10.1093/emboj/16.18.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunelli J P, Pall M L. A series of yeast shuttle vectors for expression of cDNAs and other DNA sequences. Yeast. 1993;9:1299–1308. doi: 10.1002/yea.320091203. [DOI] [PubMed] [Google Scholar]
- 12.Chevray P M, Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci USA. 1992;89:5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien C T, Bartel P L, Sternglanz R. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci USA. 1991;88:9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Dodt, G., D. Warren, T. Yahraus, M. Soukupova, P. Rehling, and S. J. Gould. Submitted for publication.
- 14.Dodt G, Braverman N, Wong C, Moser A, Moser H W, Watkins P, Valle D, Gould S J. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat Genet. 1995;9:115–125. doi: 10.1038/ng0295-115. [DOI] [PubMed] [Google Scholar]
- 15.Dodt G, Gould S J. Multiple PEX genes are required for proper subcellular distribution and stability of Pex5p, the PTS1 receptor: evidence that PTS1 protein import is mediated by a cycling receptor. J Cell Biol. 1996;135:1763–1774. doi: 10.1083/jcb.135.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer J M, McNew J A, Goodman M. The sorting sequence of the peroxisomal integral membrane protein PMP47 is contained within a short hydrophilic loop. J Cell Biol. 1996;133:269–280. doi: 10.1083/jcb.133.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elgersma Y, Kwast L, Klein A, Voorn-Brouwer T, van den Berg M, Metzig B, America T, Tabak H F, Distel B. The SH3 domain of the Saccharomyces cerevisiae peroxisomal membrane protein Pex13p functions as a docking site for Pex5p, a mobile receptor for the import of PTS1 containing proteins. J Cell Biol. 1996;135:97–109. doi: 10.1083/jcb.135.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elgersma Y, Kwast L, van den Berg M, Snyder W B, Distel B, Subramani S, Tabak H F. Overexpression of Pex15p, a phosphorylated peroxisomal integral membrane protein required for peroxisome assembly in S. cerevisiae, causes proliferation of the endoplasmic reticulum membrane. EMBO J. 1997;16:7326–7341. doi: 10.1093/emboj/16.24.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdmann R, Blobel G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J Cell Biol. 1995;128:509–523. doi: 10.1083/jcb.128.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdmann R, Blobel G. Identification of a peroxisomal membrane receptor for the C-terminal tripeptide signal recognition factor. J Cell Biol. 1996;135:111–121. doi: 10.1083/jcb.135.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdmann R, Kunau W-H. A genetic approach to the biogenesis of peroxisomes in the yeast Saccharomyces cerevisiae. Cell Biochem Funct. 1992;10:167–174. doi: 10.1002/cbf.290100306. [DOI] [PubMed] [Google Scholar]
- 22.Erdmann R, Veenhuis M, Kunau W-H. Peroxisomes: organelles at the crossroads. Trends Cell Biol. 1997;7:400–407. doi: 10.1016/S0962-8924(97)01126-4. [DOI] [PubMed] [Google Scholar]
- 23.Evans G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields S, Song O K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 25.Fransen M, Brees C, Baumgart E, Vanhooren J C T, Baes M, Mannaerts G, van Veldhoven P P. Identification and characterization of the putative human peroxisomal C-terminal targeting signal import receptor. J Biol Chem. 1995;270:7731–7736. doi: 10.1074/jbc.270.13.7731. [DOI] [PubMed] [Google Scholar]
- 26.Fujiki Y. Molecular defects in genetic diseases of peroxisomes. Biochim Biophys Acta. 1997;1361:235–250. doi: 10.1016/s0925-4439(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 27.Gärtner J, Moser H, Valle D. Mutations in the 70K peroxisomal membrane protein gene in Zellweger syndrome. Nat Genet. 1992;1:16–23. doi: 10.1038/ng0492-16. [DOI] [PubMed] [Google Scholar]
- 28.Götte K, Girzalsky W, Linkert M, Baumgart E, Kammerer S, Kunau W H, Erdmann R. Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol Cell Biol. 1998;18:616–628. doi: 10.1128/mcb.18.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould S J, Kalish J E, Morrell J E, Bjorkman J, Urquhart A J, Crane D I. An SH3 protein in the peroxisome membrane is a docking factor for the PTS1 receptor. J Cell Biol. 1996;135:85–95. doi: 10.1083/jcb.135.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould S J, Keller G A, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989;108:1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harlow E, Lane D. Antibodies—a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 32.Huhse B, Rehling P, Albertini M, Blank L, Meller K, Kunau W H. Pex17p of Saccharomyces cerevisiae is a novel peroxin and component of the peroxisomal protein translocation machinery. J Cell Biol. 1998;140:49–60. doi: 10.1083/jcb.140.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imanaka T, Shiina Y, Takano T, Hashimoto T, Osumi T. Insertion of the 70-kDa peroxisomal membrane protein into peroxisomal membranes in vivo and in vitro. J Biol Chem. 1996;271:3706–3713. doi: 10.1074/jbc.271.7.3706. [DOI] [PubMed] [Google Scholar]
- 34.Kamijo K, Taketani S, Yokota S, Osumi T, Hashimoto T. The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J Biol Chem. 1990;265:4534–4540. [PubMed] [Google Scholar]
- 35.Komori M, Rasmussen S W, Kiel J A, Baerends R J, Cregg J M, van der Klei I J, Veenhuis M. The Hansenula polymorpha PEX14 gene encodes a novel peroxisomal membrane protein essential for peroxisome biogenesis. EMBO J. 1997;16:44–53. doi: 10.1093/emboj/16.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunau W H. Peroxisome: biogenesis, function and disease. CREST Research Conference, Fukuoka, Japan. 1998. Dissection of the peroxisomal translocation apparatus of Saccharomyces cerevisiae; p. I-2. [Google Scholar]
- 37.Kunau W H. Peroxisome biogenesis: from yeast to man. Curr Opin Microbiol. 1998;1:232–237. doi: 10.1016/s1369-5274(98)80016-7. [DOI] [PubMed] [Google Scholar]
- 38.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 39.Lazarow P B. Genetic approaches to study peroxisome biogenesis. Trends Cell Biol. 1993;157:105–132. doi: 10.1016/0962-8924(93)90079-g. [DOI] [PubMed] [Google Scholar]
- 40.Lazarow P B, Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- 41.Lazarow P B, Moser H W. Disorders in peroxisome biogenesis. In: Scriver C R, Beaudet A L, Sly W S, Valle D, editors. The metabolic bases of inherited diseases. 7th ed. New York, N.Y: McGraw-Hill, Inc.; 1995. pp. 2287–2324. [Google Scholar]
- 42.Lorenz P, Maier A G, Baumgart E, Erdmann R, Clayton C. Elongation and clustering of glycosomes in Trypanosoma brucei overexpressing the glycosomal Pex11p. EMBO J. 1998;17:3542–3555. doi: 10.1093/emboj/17.13.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall P A, Krimkevich Y I, Lark R H, Deyer J M, Veenhuis M, Goodman J M. PMP27 promotes peroxisome proliferation. J Cell Biol. 1995;129:345–355. doi: 10.1083/jcb.129.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marynen P, Fransen M, Raeymaekers P, Mannaerts G P, van Veldhoven P P. The gene for the peroxisomal targeting signal import receptor (PXR1) is located on human chromosome 12p13, flanked by TPI1 and D12S1089. Genomics. 1995;30:366–368. doi: 10.1006/geno.1995.0032. [DOI] [PubMed] [Google Scholar]
- 45.Marzioch M, Erdmann R, Veenhuis M, Kunau W-H. PAS7 encodes a novel yeast member of the WD-40 protein family essential for import of 3-oxoacyl-CoA thiolase, a PTS2-containing protein, into peroxisomes. EMBO J. 1994;13:4908–4918. doi: 10.1002/j.1460-2075.1994.tb06818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCammon M T, McNew J A, Willy P J, Goodman J M. An internal region of the peroxisomal membrane protein PMP47 is essential for sorting to peroxisomes. J Cell Biol. 1994;124:915–925. doi: 10.1083/jcb.124.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCollum D, Monosov E, Subramani S. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells: the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal and is a member of the TPR protein family. J Cell Biol. 1993;121:761–774. doi: 10.1083/jcb.121.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno M, Lark R, Campbell K L, Goodman J M. The peroxisomal membrane proteins of Candida boidinii: gene isolation and expression. Yeast. 1994;10:1447–1457. doi: 10.1002/yea.320101108. [DOI] [PubMed] [Google Scholar]
- 49.Moser A B, Rasmussen M, Naidu S, Watkins P A, McGuinness M, Hajra A K, Chen G, Raymond G, Liu A, Gordon D, Garnaas K, Walton D S, Skjeldal O H, Guggenheim M A, Jackson L G, Elias E R, Moser H W. Phenotype of patients with peroxisomal disorders subdivided into sixteen complementation groups. J Pediatr. 1995;127:13–22. doi: 10.1016/s0022-3476(95)70250-4. [DOI] [PubMed] [Google Scholar]
- 50.Motley A M, Hettema E H, Hogenhout E M, Brites P, ten Asbroek A L, Wijburg F A, Baas F, Heijmans H S, Tabak H F, Wanders R J, Distel B. Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat Genet. 1997;15:377–380. doi: 10.1038/ng0497-377. [DOI] [PubMed] [Google Scholar]
- 51.Nuttley W M, Szilard R K, Smith J J, Veenhuis M, Rachubinski R A. The PAH2 gene is required for peroxisome assembly in the methylotrophic yeast Hansenula polymorpha and encodes a member of the tetratricopeptide repeat family of proteins. Gene. 1995;160:33–39. doi: 10.1016/0378-1119(95)00230-4. [DOI] [PubMed] [Google Scholar]
- 52.Otera H, Okumoto K, Tateishi K, Ikoma Y, Matsuda E, Nishimura M, Tsukamoto T, Osumi T, Ohashi K, Higuchi O, Fujiki Y. Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: studies with PEX5-defective CHO cell mutants. Mol Cell Biol. 1998;18:388–399. doi: 10.1128/mcb.18.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passreiter M, Anton M, Lay D, Frank R, Harter C, Wieland F T, Gorgas K, Just W W. Peroxisome biogenesis: involvement of ARF and coatomer. J Cell Biol. 1998;141:373–383. doi: 10.1083/jcb.141.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters T J, Muller M, De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972;136:1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purdue P E, Zhang J W, Skoneczny M, Lazarow P B. Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat Genet. 1997;15:381–384. doi: 10.1038/ng0497-381. [DOI] [PubMed] [Google Scholar]
- 56.Rehling P, Marzioch M, Niesen F, Wittke E, Veenhuis M, Kunau W-H. The import receptor for the peroxisomal targeting signal 2 PTS2 in Saccharomyces cerevisiae is encoded by the PAS7 gene. EMBO J. 1996a;15:2901–2913. [PMC free article] [PubMed] [Google Scholar]
- 57.Santos M J, Imanaka T, Shio H, Small G M, Lazarow P B. Peroxisomal membrane ghosts in Zellweger syndrome—aberrant organelle assembly. Science. 1988;239:1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- 57a.Schliebs, W., J. Saidowsky, B. Agianian, G. Dodt, F. W. Herberg, and W. H. Kunau. Recombinant human PTS1-receptor PEX5: structural basis for interaction of PEX5 and Pex14. J. Biol. Chem., in press. [DOI] [PubMed]
- 58.Slawecki M L, Dodt G, Steinberg S, Moser A B, Moser H, Gould S J. Identification of three distinct peroxisomal protein import defects in patients with peroxisome biogenesis disorders. J Cell Sci. 1995;108:1817–1829. doi: 10.1242/jcs.108.5.1817. [DOI] [PubMed] [Google Scholar]
- 59.Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiol Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- 60.Subramani S. PEX genes on the rise. Nat Genet. 1997;15:331–333. doi: 10.1038/ng0497-331. [DOI] [PubMed] [Google Scholar]
- 61.Subramani S. Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol. 1993;9:445–478. doi: 10.1146/annurev.cb.09.110193.002305. [DOI] [PubMed] [Google Scholar]
- 62.Swinkels B W, Gould S J, Bodnar A G, Rachubinski R A, Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991;10:3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szilard K R, Titorenko V I, Veenhuis M, Rachubinski M. Pay32p of the yeast Yarrowia lipolytica is an intraperoxisomal component of the matrix protein translocation machinery. J Cell Biol. 1995;131:1453–1469. doi: 10.1083/jcb.131.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Klei I J, Hilbrands R E, Swaving G J, Waterham H R, Vrieling E G, Titorenko V I, Cregg J M, Harder W, Veenhuis M. The Hansenula polymorpha PER3 gene is essential for the import of PTS1 proteins into the peroxisomal matrix. J Biol Chem. 1995;270:17229–17236. doi: 10.1074/jbc.270.29.17229. [DOI] [PubMed] [Google Scholar]
- 65.van der Klei I J, Veenhuis M. Peroxisome biogenesis in the yeast Hansenula polymorpha: a structural and functional analysis. Ann NY Acad Sci. 1996;804:47–59. doi: 10.1111/j.1749-6632.1996.tb18607.x. [DOI] [PubMed] [Google Scholar]
- 66.van der Leij I, Franse M M, Elgersma Y, Distel B, Tabak H F. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1993;90:11782–11786. doi: 10.1073/pnas.90.24.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waterham H R, Cregg J M. Peroxisome biogenesis. Bioessays. 1997;19:57–66. doi: 10.1002/bies.950190110. [DOI] [PubMed] [Google Scholar]
- 68.Wiemer E A, Nuttley W M, Bertolaet B L, Li X, Francke U, Wheelock M J, Anné U K, Johnson K R, Subramani S. Human peroxisomal targeting signal-1 receptor restores peroxisomal protein import in cells from patients with fatal peroxisomal disorders. J Cell Biol. 1995;130:51–65. doi: 10.1083/jcb.130.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yahraus T, Braverman N, Dodt G, Kalish J E, Morrell J C, Moser H W, Valle D, Gould S J. The peroxisome biogenesis disorder group 4 gene, PXAAA1, encodes a cytoplasmic ATPase required for stability of the PTS1 receptor. EMBO J. 1996;15:2914–2923. [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J W, Lazarow P B. PEB1 (PAS7) in Saccharomyces cerevisiae encodes a hydrophilic, intra-peroxisomal protein that is a member of the WD repeat family and is essential for the import of thiolase into peroxisomes. J Cell Biol. 1995;129:65–80. doi: 10.1083/jcb.129.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J W, Lazarow P B. PEB1(PAS7) is a intraperoxisomal receptor for the NH2-terminal, type 2, peroxisomal targeting sequence of thiolase: Peb1p itself is targeted to peroxisomes by an NH2-terminal peptide. J Cell Biol. 1996;132:325–334. doi: 10.1083/jcb.132.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]