Abstract
Veterinary pharmaceuticals may cause unexpected adverse effects on non-target aquatic species. While these pharmaceuticals were previously identified as priority compounds in ambient water, their ecological risks are relatively unknown. In this study, a series of chronic toxicity tests were conducted for these pharmaceuticals using algae, two cladocerans, and a fish. After a 21-d exposure to amoxicillin, enrofloxacin, and neomycin, no observed effect concentration (NOEC) for the reproduction of Daphnia magna was detected at 27.2, 3.3, and 0.15 mg/L, respectively. For the survival of juvenile Oryzias latipes following the 40-d exposure, NOEC was found at 21.8, 3.2, and 0.87 mg/L, respectively. Based on the results of the chronic toxicity tests and those reported in the literature, predicted no-effect concentrations (PNECs) were determined at 0.078, 4.9, and 3.0 µg/L for amoxicillin, enrofloxacin, and neomycin, respectively. Their hazard quotients (HQs) were less than 1 at their average levels of occurrence in ambient freshwater. However, HQs based on the maximum detected levels of amoxicillin and enrofloxacin were determined at 21.2 and 6.1, respectively, suggesting potential ecological risks. As the potential ecological risks of these veterinary pharmaceuticals at heavily contaminated sites cannot be ignored, hotspot delineation and its management are required.
Keywords: veterinary medicine, antibiotics, amoxicillin, enrofloxacin, neomycin, chronic toxicity, risk assessment, surface water
1. Introduction
Veterinary pharmaceuticals have been used for the treatment and/or prevention of diseases in both companions and livestock animals. After use, proportions of pharmaceuticals can be excreted from the body unchanged or as active metabolites [1]. In addition, veterinary pharmaceuticals can reach the environment via direct application in aquaculture or through the disposal of the unused [2,3]. Therefore, veterinary pharmaceuticals have been frequently reported in ambient water worldwide [4,5,6,7,8]. Given that pharmaceuticals are designed for specific therapeutic functions, these compounds may cause unexpected physiological effects on non-target species [6,9,10]. Hence, their potential consequences in aquatic environment have been of concern.
Antibiotics and antimicrobials are used to control pathogenic bacteria [4,11] and have been widely used in veterinary medicine to prevent diseases and promote the growth of livestock and fish [12]. In terms of the amount of use, these groups of veterinary pharmaceuticals occupy among the highest ranks in many countries; and hence have been detected in ambient environments at high concentrations, often at as high as µg/L levels [6,10,13]. According to prioritization studies in the United Kingdom (UK) and Korean environments, amoxicillin, enrofloxacin, and neomycin have been suggested as compounds with high hazard potential, mainly due to their higher possibility to reach the environment [2,14]. Because of their potential ecological risks, ecotoxicological assessments have been conducted for many veterinary antibiotics and antimicrobials, but they are often only limited to their acute toxicity [9].
Amoxicillin is a broad-spectrum β-lactam antibiotic that belongs to the penicillin family. Amoxicillin has been used for the treatment of certain gastrointestinal and systemic infections [15]. This compound has been detected at the ng/L level in various countries such as Ghana [16], Turkey [17], Italy [8,18], and Australia [7], with a maximum concentration of 1.65 µg/L. For amoxicillin, no observed effect concentrations (NOECs) were derived at 0.78 µg/L based on its chronic toxicity on blue-green algae [19]. Enrofloxacin is a fluoroquinolone antibiotic that inhibits the activity of bacterial DNA gyrase, which is essential for replication and transcription in prokaryotes [20]. Enrofloxacin has been frequently detected in surface waters worldwide, including Asia [21,22,23,24], Europe [25,26], North America [27], and Oceania [7]. However, ecotoxicity information for enrofloxacin is restricted to acute exposure, and chronic toxicity values are available only for algae and invertebrate species [12]. Neomycin is a water-soluble aminoglycoside that has been used for gastrointestinal infections and mastitis [28]. Nevertheless, both the occurrence and ecotoxicity of neomycin are not very well characterized.
In the present study, the ecological hazards of amoxicillin, enrofloxacin, and neomycin were evaluated using an algae Pseudokirchneriella subcapitata, two invertebrate species, Daphnia magna and Moina macrocopa, and a vertebrate Oryzias latipes, representing three trophic levels in freshwater ecosystems. Predicted no effect concentrations (PNECs) for these drugs were derived based on the toxicity information obtained in the present study and those reported in the literature. Potential ecological risks were estimated by comparing the surface water concentrations of these compounds reported in the literature and the PNECs. The results of this study will provide useful information on the potential ecological risks of these veterinary pharmaceuticals and, if necessary, help develop relevant risk management options in freshwater environments.
2. Materials and Methods
2.1. Test Chemicals
Reagent grade amoxicillin (CAS RN: 26787-78-0, purity ≥ 90%), enrofloxacin (CAS RN: 93106-60-6; purity ≥ 98.0%), and neomycin sulfate (CAS RN: 1405-10-3; 734 μg neomycin/mg) were purchased from Sigma Aldrich (St. Louis, MO, USA). The physicochemical characteristics of the pharmaceuticals are shown in Table S1. Test solutions of each compound were prepared immediately prior to the experiments. The test concentrations for each pharmaceutical that were employed for the acute test were determined by preliminary range-finding tests (data not shown). The concentration range for chronic exposure was determined based on the results of the acute toxicity tests, i.e., the highest exposure concentration of the chronic exposure was set at about one-half to a tenth of an acute EC50 of each pharmaceutical. The actual concentrations of the test solutions were measured following the method shown in the Supplementary Materials and Methods, and the average measured concentrations for each pharmaceutical are reported in Table S2.
2.2. Test Organisms and Maintenance
All test organisms were maintained at the Environmental Toxicology Laboratory of Seoul National University (Seoul, Korea). Pseudokirchneriella subcapitata was cultured in a temperature-controlled shaking chamber at 22 °C, with a shaking speed of 220 rpm [29] under continuous illumination at 4306 lx [30]. The two cladocerans, D. magna and M. macrocopa, were cultured in-house in M4 media-manufactured following OECD guideline 211 [31]. Daphnia magna was maintained at 21 ± 1 °C in 6-L glass jars, and M. macrocopa was maintained at 25 ± 1 °C in 3-L glass beakers. Daphnia magna and M. macrocopa were fed daily with algae. Japanese medaka (O. latipes) were cultured in a temperature controlled incubation room (25 ± 1 °C) under a photoperiod of 16: 8 h light:dark. The fish were fed Artemia nauplii (<24 h after hatching) twice daily. Water quality parameters such as pH, conductivity, temperature, and dissolved oxygen were routinely monitored.
2.3. Toxicity Tests
A 72-h growth inhibition test was carried out for P. subcapitata, following the OECD test guideline 201 [29] with a minor modification on the initial cell densities. Three replicates with a cell density of 1.0 × 105 cells/mL were exposed to various concentrations of amoxicillin (0, 1.6, 8.0, 40, 200, or 1000 mg/L), enrofloxacin (0, 1.1, 3.3, 10, or 30 mg/L), or neomycin (0, 0.2, 1.0, 5.0, 25, or 125 mg/L).
For D. magna and M. macrocopa, 48-h acute tests were performed following the OECD test guideline 202 [32]. Four replicates of five neonates (<24-h old) were exposed to a series of concentrations of amoxicillin (0, 12.3, 37.0, 111, 333, or 1000 mg/L), enrofloxacin (0, 12.5, 25, 50, 100, or 200 mg/L), or neomycin (0, 1.85, 5.55, 16.7, 50.0, or 150 mg/L). The number of immobile organisms was recorded after the 48-h exposure. During the acute tests, the test organisms were not fed.
The chronic 21-d D. magna and 7-d M. macrocopa tests were conducted following the OECD test guideline 211 [31] and Oh and Choi [33], respectively. Ten replicates with one neonate each (<24-h old) were exposed to various concentrations of amoxicillin (0, 3.70, 11.1, 33.3, 100, or 300 mg/L), enrofloxacin (0, 0.123, 0.370, 1.11, 3.33, or 10.0 mg/L for D. magna; 0, 0.247, 0.741, 2.22, 6.67, or 20.0 mg/L for M. macrocopa), or neomycin (0, 0.0617, 0.185, 0.556, 1.67, or 5.00 mg/L). The exposure medium was renewed at least three times per week. The mortality of the organisms and the number of living offspring were recorded daily. At the end of the test, the body length of each D. magna, from the top of the head capsule to the base of the shell spine, was measured using a stereomicroscope (Dongwon, Bucheon, Korea) as described by Olmstead and LeBlanc [34].
A fish early life stage (ELS) toxicity test was initiated with fertilized eggs (<24 h of spawning) and carried out until 30 d post-hatching (dph) following the OECD guideline 210 [35]. The hatching rate, survival, and growth were measured for exposed or hatched fish. Four replicates (15 newly fertilized eggs per replicate) were exposed to a series of concentrations of amoxicillin (0, 1.23, 3.70, 11.1, 33.3, or 100 mg/L), enrofloxacin (0, 0.005, 0.05, 0.5, 5, or 50 mg/L), and neomycin (0, 0.01, 0.1, 1.0, 10, or 100 mg/L) until 30 dph. At 30 dph, five fish per treatment group were randomly selected and their body length and weight were measured. Fish were anesthetized in ice-cold water following the guidelines of the Seoul National University Institutional Animal Care and Use Committee.
2.4. Hazard Quotient Calculation
The hazard quotient (HQ) of each tested pharmaceutical was calculated by dividing the measured environmental concentrations (MECs) reported in the literature by the PNEC derived for each pharmaceutical. Among the available MECs from each location, the maximum values of MECmean and MECmax were chosen and compared with PNEC for each tested pharmaceutical which was determined following the European Commission [36]. For the calculation of MECmean, concentrations below the LOQ were not included. For the PNEC derivation, toxicity data based on ecologically relevant toxicity endpoints (e.g., mortality, immobilization, reproduction, or growth inhibition) were considered, and the most sensitive ecotoxicological data obtained in the present study and those reported by others were employed. If the HQ value is less than 1, the ecological impact is considered negligible.
2.5. Statistical Analysis
The median effective concentration (EC50) of the algae was determined using REGTOX ver. 7.0.3 (GNU General Public License, Boston, MA, USA). For the cladocerans, the EC50 and associated confidence intervals were calculated by probit analysis using Toxstat® (Ver. 3.5; West, Cheyenne, WY, USA). Fisher’s exact test was used to calculate NOEC for the chronic survival of cladocerans. To analyze the reproduction and growth data of cladocerans and all the data of fish, one-way analysis of variance (ANOVA) and Dunnett’s T post-hoc test were performed using SPSS 20.0 for Windows (SPSS, Chicago, IL, USA). Before conducting the ANOVA, normality of data and homogeneity of variance were confirmed. When necessary, a non-parametric Kruskal–Wallis test was performed. The population growth rate (PGR) for the cladocerans was calculated according to Lotka [37].
3. Results and Discussion
3.1. Acute and Chronic Toxicity of the Tested Pharmaceuticals
3.1.1. Amoxicillin
All the ecotoxicity data obtained from the current study are summarized in Table 1, along with those reported from previous studies. For algae, the 72-h growth EC50 was determined at 213.14 mg/L in the present study. This value was lower than that of González-Pleiter et al. [38] (>1500 mg/L); however, it was much higher than that reported for another algae species (cyanobacteria): For Synechococcus leopoliensis, the 96-h EC50 was reported at 2.22 µg/L [19]. Compared to green algae, cyanobacteria are generally more sensitive to most antibiotics such as aminoglycosides, macrolides, quinolones, sulfonamides, and tetracyclines [39,40,41]. The difference in sensitivity among algae species might be due to the fact that many antibiotics inhibit protein synthesis of prokaryotic cyanobacteria through binding to ribosome subunits; however, chloroplast division in eukaryotic green algae may not be affected [39].
Table 1.
Ecotoxicity of tested pharmaceuticals on aquatic organisms obtained from the present study and from the literature.
| Pharmaceuticals/ Taxonomic Group |
Species | Test Duration /Endpoint |
Concentration (mg/L) | Reference |
|---|---|---|---|---|
| Amoxicillin | ||||
| Bacteria | Vibrio fischeri | 5 min, IC50 | 1320.0 | Park and Choi [12] |
| Bacteria | Vibrio fischeri | 15 min, IC50 | 3597.0 | Park and Choi [12] |
| Algae | Microcystis aeruginosa | 7 d, EC50 | 0.0037 | Lützhøft et al. [40] |
| Algae | Microcystis aeruginosa | 7 d, EC50 | 0.00803 | Liu et al. [1] |
| Algae | Pseudokirchneriella subcapitata | 7 d, NOEC | 250 | Lützhøft et al. [40] |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC10 | 4.75 | This study |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC50 | 213.14 | This study |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC50 | >1500 | González-Pleiter et al. [38] |
| Algae | Rhodomonas salina | 7 d, EC50 | 3108 | Lützhøft et al. [40] |
| Algae | Synechococcus leopoliensis | 96 h, EC50 | 0.00222 | Andreozzi et al. [19] |
| Algae | Synechococcus leopoliensis | 96 h, NOEC | 0.00078 | Andreozzi et al. [19] |
| Algae | Synechococcus leopoliensis | 96 h, LOEC | 0.00156 | Andreozzi et al. [19] |
| Aquatic plant | Lemna gibba | 7 d, EC10 | >1 | Brain et al. [54] |
| Invertebrate | Daphnia Magna | 48 h, EC50 | >1000 | Park and Choi [12] |
| Invertebrate | Daphnia Magna | 48 h, EC50 | >1000 | This study |
| Invertebrate | Daphnia Magna | 21 d, survival NOEC | >266 | This study |
| Invertebrate | Daphnia Magna | 21 d, reproduction NOEC | 27.2 | This study |
| Invertebrate | Daphnia Magna | 21 d, growth NOEC | 27.2 | This study |
| Invertebrate | Moina macrocopa | 48 h, EC50 | >1000 | Park and Choi [12] |
| Invertebrate | Moina macrocopa | 48 h, EC50 | >1000 | This study |
| Invertebrate | Moina macrocopa | 7 d, survival NOEC | >266 | This study |
| Invertebrate | Moina macrocopa | 7 d, reproduction NOEC | 2.05 | This study |
| Fish | Danio rerio | 48 h, EC50 premature hatching | 132.4 | Oliveira et al. [42] |
| Fish | Danio rerio | 96 h, LC50 embryo, adult | >100 | Oliveira et al. [42] |
| Fish | Oryzias latipes | 96 h, LC50 | >1000 | Park and Choi [12] |
| Fish | Oryzias latipes | Hatchability NOEC | 1.37 | This study |
| Fish | Oryzias latipes | Time-to-hatch NOEC | >38.9 | This study |
| Fish | Oryzias latipes | 40 d, juvenile survival NOEC | 21.8 | This study |
| Fish | Oryzias latipes | 40 d, juvenile growth NOEC | 21.8 | This study |
| Fish | Tilapia nilotica | 96 h, LC50 | 0.03572 | Yasser and Nabila [43] |
| Enrofloxacin | ||||
| Bacteria | Vibrio fischeri | 5 min, IC50 | 272.25 | Oh [48] |
| Bacteria | Vibrio fischeri | 15 min, IC50 | 306.35 | Oh [48] |
| Bacteria | Vibrio fischeri | 5 min, IC50 | 425.0 | Park and Choi [12] |
| Bacteria | Vibrio fischeri | 15 min, IC50 | 326.8 | Park and Choi [12] |
| Bacteria | Vibrio fischeri | 5 min, EC50 | >8.4 | Hernandoet al. [55] |
| Bacteria | Vibrio fischeri | 15 min, EC50 | >8.4 | Hernando et al. [55] |
| Bacteria | Vibrio fischeri | 30 min, EC50 | >8.4 | Hernando et al. [55] |
| Algae | Anabaena flos-aquae | 72 h, EC50 | 0.173 | Ebert et al. [20] |
| Algae | Chlorella sp. | 72 h, EC50 | 111 | Andrieu et al. [21] |
| Algae | Chlamydomonas mexicana | 96 h, EC50 | 10.76 | Xiong et al. [41] |
| Algae | Chlorella vulgaris | 96 h, EC50 | 12.2 | Xiong et al. [46] |
| Algae | Desmodesmus subspicatus | 72 h, EC50 | 5.568 | Ebert et al. [20] |
| Algae | Microcystis aeruginosa | 5 d, EC50 | 0.049 | Robinson et al. [44] |
| Algae | Micractinium resseri | 96 h, EC50 | 12.03 | Xiong et al. [46] |
| Algae | Ourococcus mutipsorus | 96 h, EC50 | 14.98 | Xiong et al. [46] |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC50 | 3.1 | Robinson et al. [44] |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC10 | 0.83 | This study |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC50 | 3.33 | This study |
| Algae | Scenedesmus obliquus | 24 h, EC50 | 88.39 | Qin et al. [45] |
| Algae | Scenedesmus obliquus | 48 h, EC50 | 63.86 | Qin et al. [45] |
| Algae | Scenedesmus obliquus | 72 h, EC50 | 45.1 | Qin et al. [45] |
| Algae | Scenedesmus obliquus | 96 h, EC50 | 59.16 | Qin et al. [45] |
| Algae | Scenedesmus obliquus | 96 h, EC50 | 9.86 | Xiong et al. [46] |
| Aquatic plant | Lemna minor | 7 d, EC50 | 0.114 | Robinson et al. [44] |
| Aquatic plant | Lemna minor | 7 d, EC50 | 0.107 | Ebert et al. [20] |
| Aquatic plant | Myriophyllum spicatum | 14 d, EC50 | >44.3 | Ebert et al. [20] |
| Invertebrate | Daphnia curvirostris | 48 h, EC50 | 4.33 | Dalla Bona et al. [49] |
| Invertebrate | Daphnia magna | 24 h, EC50 | 26.75 | Oh [48] |
| Invertebrate | Daphnia magna | 48 h, EC50 | 15.7 | Oh [48] |
| Invertebrate | Daphnia magna | 24 h, EC50 | 131.7 | Park and Choi [12] |
| Invertebrate | Daphnia magna | 48 h, EC50 | 56.7 | Park and Choi [12] |
| Invertebrate | Daphnia magna | 48 h, EC50 (pH 7.4) | 45.8 | Kim et al. [47] |
| Invertebrate | Daphnia magna | 48 h, EC50 | 16.34 | Dalla Bona et al. [49] |
| Invertebrate | Daphnia magna | 48 h, EC50 | 20.1 | This study |
| Invertebrate | Daphnia magna | 21 d, survival, NOEC | 5 | Park and Choi [12] |
| Invertebrate | Daphnia magna | 21 d, reproduction, NOEC | 5 | Park and Choi [12] |
| Invertebrate | Daphnia magna | 21 d, survival, NOEC | 3.33 | This study |
| Invertebrate | Daphnia magna | 21 d, reproduction, NOEC | 3.33 | This study |
| Invertebrate | Daphnia magna | 21 d, growth NOEC | 0.12 | This study |
| Invertebrate | Gammarus pulex | 48 h, EC50 (pH7.0) | 42.1 | Sun et al. [50] |
| Invertebrate | Gammarus pulex | 96 h, EC50 (pH7.0) | 15.6 | Sun et al. [50] |
| Invertebrate | Moina macrocopa | 24 h, EC50 | 285.7 | Park and Choi [12] |
| Invertebrate | Moina macrocopa | 48 h, EC50 | >200 | Park and Choi [12] |
| Invertebrate | Moina macrocopa | 48 h, EC50 | 69 | Andrieu et al. [21] |
| Invertebrate | Moina macrocopa | 48 h, EC50 | 85.2 | This study |
| Invertebrate | Moina macrocopa | 7 d, survival, NOEC | 2.47 | This study |
| Invertebrate | Moina macrocopa | 7 d, reproduction, NOEC | >2.47 | This study |
| Invertebrate | Physella acuta | 48 h, EC50 (pH 7.0) | 133 | Sun et al. [50] |
| Invertebrate | Physella acuta | 96 h, EC50 (pH 7.0) | 122 | Sun et al. [50] |
| Fish | Oryzias latipes | 96 h, EC50 | >100 | Park and Choi [12] |
| Fish | Oryzias latipes | 48 h, EC50 | >100 | Park and Choi [12] |
| Fish | Oryzias latipes | Hatchability, NOEC | >11 | This study |
| Fish | Oryzias latipes | Time-to-hatch, NOEC | >11 | This study |
| Fish | Oryzias latipes | 40 d, juvenile survival | 3.2 | This study |
| Fish | Oryzias latipes | 40 d, juvenile growth | >3.2 | This study |
| Neomycin | ||||
| Bacteria | Vibrio fischeri | 5 min, IC50 | >1000 | Park and Choi [12] |
| Algae | Anacystis nidulans | 6 h, NOEC | 0.2 | Whitton [52] |
| Algae | Microcystis aeruginosa | 24 h, NOEC | 0.1 | Vance [51] |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC10 | 4.28 | This study |
| Algae | Pseudokirchneriella subcapitata | 72 h, EC50 | 4.60 | This study |
| Aquatic plant | Lemna gibba | 7 d, EC10 | >1.0 | Brain et al. [54] |
| Invertebrate | Daphnia magna | 48 h, EC50 | 42.1 | Park and Choi [12] |
| Invertebrate | Daphnia magna | 48 h, EC50 | 56.0 | This study |
| Invertebrate | Daphnia magna | 21 d, NOEC | 0.03 | Park and Choi [12] |
| Invertebrate | Daphnia magna | 21 d, survival NOEC | 1.5 | This study |
| Invertebrate | Daphnia magna | 21 d, reproduction NOEC | 0.15 | This study |
| Invertebrate | Daphnia magna | 21 d, growth NOEC | 0.15 | This study |
| Invertebrate | Moina macrocopa | 48 h, EC50 | 34.1 | Park and Choi [12] |
| Invertebrate | Moina macrocopa | 48 h, EC50 | 22.9 | This study |
| Invertebrate | Moina macrocopa | 7 d, NOEC | 0.5 | Park and Choi [12] |
| Invertebrate | Moina macrocopa | 7 d, survival NOEC | >5.3 | This study |
| Invertebrate | Moina macrocopa | 7 d, reproduction NOEC | >5.3 | This study |
| Mollusks | Crassostrea gigas | 48 h, EC50 | >800 | US EPA, ECOTOX [53] |
| Fish | Anguilla japonica | LC50 | 2829 | US EPA, ECOTOX [53] |
| Fish | Oryzias latipes | 96 h, LC50 | 80.8 | Park and Choi [12] |
| Fish | Oryzias latipes | Hatchability NOEC | 11 | This study |
| Fish | Oryzias latipes | Time-to-hatch NOEC | >100 | This study |
| Fish | Oryzias latipes | 40 d, juvenile survival NOEC | 0.87 | This study |
| Fish | Oryzias latipes | 40 d, juvenile growth NOEC | 11 | This study |
EC50, median effective concentration; IC50, median inhibitory concentration; NOEC, no observed effect concentration; LOEC, lowest observed effect concentration.
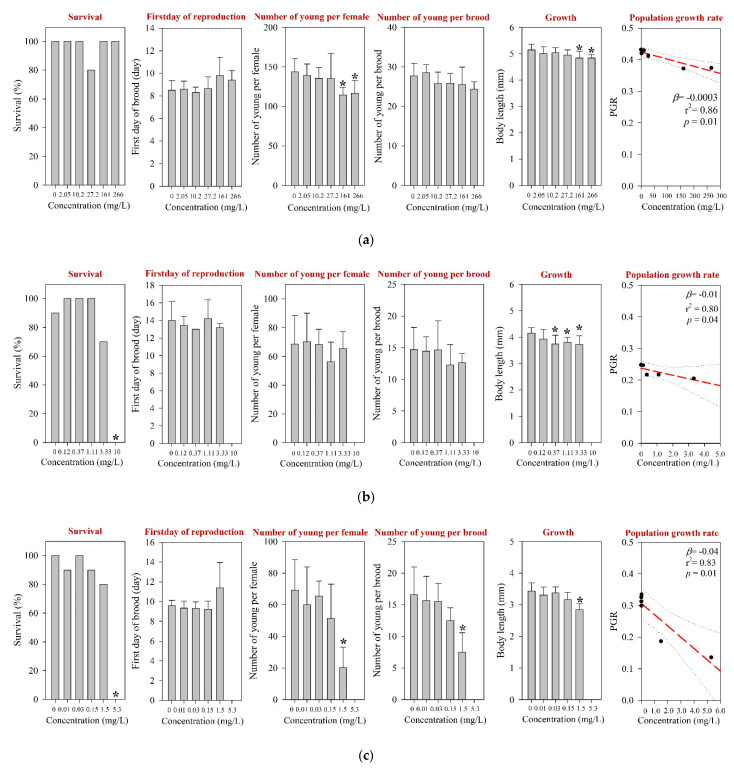
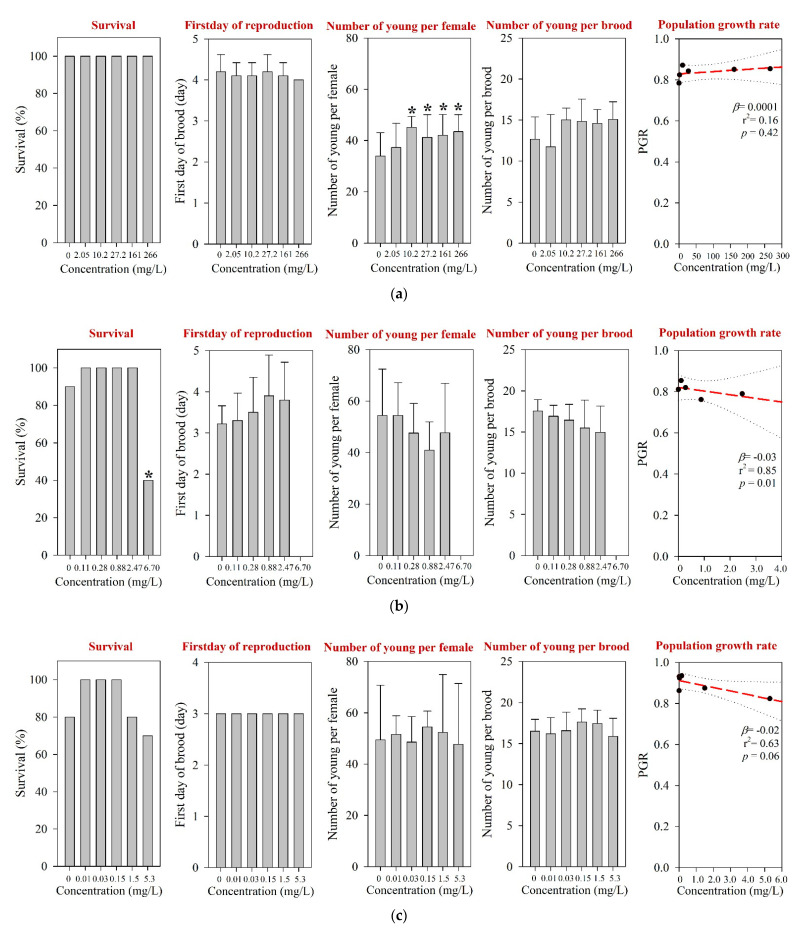
For D. magna and M. macrocopa, the 48-h EC50s values were determined at >1000 mg/L, which was the maximum experimental concentration (Table 1 and Table S3). These values were comparable to those reported in the literature [12]. The results of the 21-d chronic D. magna exposure for the tested pharmaceuticals are shown in Figure 1. The reproduction NOEC of amoxicillin in the 21-d chronic D. magna test was determined at 27.2 mg/L (Table 1 and Figure 1a). However, survival and other reproductive-related endpoints, e.g., the first day of reproduction and number of young per brood, were not affected at concentrations up to 266 mg/L. In addition, the population growth rate (PGR) showed a significant decreasing trend (Figure 1a). The results of the chronic M. macrocopa exposure for tested pharmaceuticals are depicted in Figure 2. The M. macrocopa reproduction NOEC for amoxicillin was determined at 2.05 mg/L, but the change was in a positive direction (Table 1 and Figure 2a). This positive or increasing trend of M. macrocopa reproduction by amoxicillin exposure should not be considered beneficial, because, the extent of change was small, and in D. magna, we found the opposite direction of the reproduction effect (Figure 1a). For amoxicillin, no chronic toxicity value for cladocerans is available in the literature; therefore, the present data could not be compared.
Figure 1.
Results of the 21-d chronic D. magna test for (a) amoxicillin, (b) enrofloxacin, and (c) neomycin. The results are shown as mean ± standard deviation (n = 10). The Asterisk (*) denotes a significant difference in the observation endpoint from that of the control (p < 0.05). Monotonous trend was assumed for statistical analysis of the growth of D. magna following exposure to enrofloxacin (b). Nominal concentration was used for enrofloxacin (b). β, slope; r2, coefficient of determination; p, probability value.
Figure 2.
Results of the 7-d chronic M. macrocopa test for (a) amoxicillin, (b) enrofloxacin, and (c) neomycin. The results are shown as mean ± standard deviation (n = 10). Asterisk (*) denotes a significant difference in the observation endpoint from that of the control (p < 0.05). Monotonous trend was assumed as the number of young per female of M. macrocopa exposed to amoxicillin (a). β, slope; r2, coefficient of determination; p, probability value.
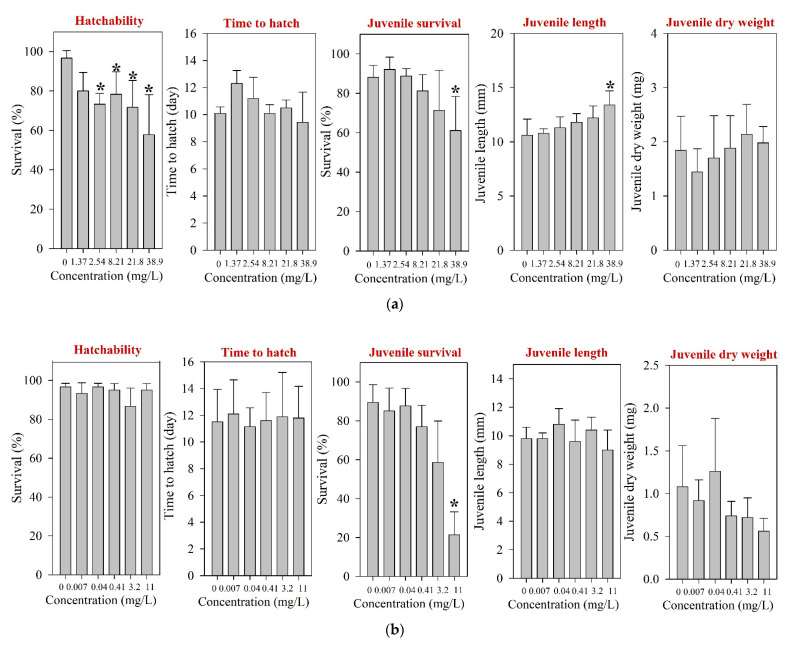
For fish, only acute toxicity information is available to date [12,42,43]. After 96 h of exposure, LC50s were reported at >100 and 1000 mg/L in Danio rerio and O. latipes, respectively [12,42]. However, for Tilapia nilotica, LC50 was determined at 0.0357 mg/L [43], suggesting notable variation of sensitivity by fish species. The results of ELS O. latipes exposure obtained in the present study for tested pharmaceuticals are shown in Figure 3. After 30 d of ELS exposure of O. latipes, we observed the hatchability NOEC at 1.37 mg/L (Figure 3a), and survival LOEC at 38.9 mg/L. Our result shows that the acute to chronic ratio of amoxicillin for O. latipes is very high (1000 vs. 1.37 mg/L).
Figure 3.
Results of the early life stage test of O. latipes for (a) amoxicillin, (b) enrofloxacin, and (c) neomycin. The results are shown as mean ± standard deviation (n = 4). Asterisk (*) denotes a significant difference in the observation endpoint from that of the control (p < 0.05). Monotonous trend was assumed for statistical analysis of hatchability of O. latipes exposed to amoxicillin (a).
3.1.2. Enrofloxacin
For P. subcapitata, the 72-h growth EC50 of enrofloxacin was determined at 3.33 mg/L (Table 1). This result corresponded well with previous reports which were made on the same species [20,44]. The EC50s values reported for other algal species such as Chlomydomonas Mexicana, Chlorella vulgaris, Scenedesmus obliquus, Micractinium resseri, and Ourococcus mutipsorus are slightly higher, despite the longer exposure duration [45,46]. The lowest EC50 reported for algae species was 0.049 mg/L, which was observed from freshwater cyanobacteria, Microcystis aeruginosa, following a 5-d exposure [44].
For D. magna and M. macrocopa, the 48-h EC50s were determined at 20.1 mg/L and 85.2 mg/L, respectively (Table 1 and Table S3). The 48-h EC50s from both cladoceran species obtained from the present study are comparable to those reported elsewhere, e.g., for D. magna ranging between 15.7 and 56.7 mg/L [12,47,48] and for M. macrocopa at 69 mg/L [21]. The EC50s values reported for other invertebrates, including D. curvirostris, Gammarus pulex, and Physella acuta, ranged between 4.33 and 133 mg/L [49,50]. Following a 21-d exposure of D. magna, survival and growth NOECs were determined at 3.33 mg/L and 0.12 mg/L, respectively (Figure 1b). The body length was significantly reduced at 0.37 mg/L, but reproduction was not affected at concentrations of up to 3.33 mg/L, which was the highest concentration without significant lethal effects. The survival NOEC of M. macrocopa was determined at 2.47 mg/L, which was similar to that of D. magna (Table 1 and Figure 2b). Due to the low survival rate, the PGRs in both D. magna and M. macrocopa were significantly reduced in a concentration-dependent manner (Figure 1b and Figure 2b).
Following the fish ELS exposure, survival NOEC was determined at 3.2 mg/L (Table 1), and significant juvenile mortality was observed at 11 mg/L (Figure 3b). However, the hatchability and time-to-hatch of O. latipes were not influenced by the exposure at up to 11 mg/L. For fish, ecotoxicity information for enrofloxacin is very limited to date; only one report on acute toxicity is available [12].
3.1.3. Neomycin
For neomycin, the 72-h growth EC50 for P. subcapitata was determined at 4.60 mg/L (Table 1). This observation is quite different from the reports made on other algae, e.g., Anacystis nidulans (6-h NOEC of 0.2 mg/L) and Microcystis aeruginosa (24-h NOEC of 0.1 mg/L) [51,52]. Different experimental species and conditions, for example, different cell densities, light intensities, and endpoints were employed in these studies, and hence direct comparison with that of the present study may not be appropriate.
The 48-h EC50s for D. magna and M. macrocopa were determined at 56.0 mg/L and 22.9 mg/L, respectively (Table 1 and Table S3); which were comparable with a previous report [12]. For D. magna, chronic survival and reproduction NOECs were determined at 1.5 mg/L and 0.15 mg/L, respectively (Table 1). Neomycin exposure decreased reproduction performance, including the number of young per female and the number of young per brood of D. magna (Figure 1c), and PGR. Neomycin exposure led to the steepest decline of the PGR slope for D. magna among the three pharmaceuticals tested in this study. Based on the M. macrocopa chronic toxicity test, however, no significant changes in both survival and reproduction were observed at all experimental concentrations up to 5.3 mg/L neomycin (Figure 2c), which was above the NOEC reported previously [12]. The PGR of M. macrocopa showed a slightly decreasing pattern, with marginal statistical significance (p = 0.06).
Following the fish ELS exposure, hatching was significantly affected at 127 mg/L; the hatchability of O. latipes at 127 mg/L neomycin was 6.7% (Figure 3c). The survival of juvenile fish was significantly impaired at 11 mg/L neomycin. However, the growth of O. latipes, i.e., juvenile length and dry weight, was not altered by the neomycin exposure. Previously, a couple of studies have reported toxicity values of neomycin on aquatic vertebrates, and they were much higher than the survival NOEC (40-d juvenile survival, 0.87 mg/L) of the juvenile fish observed in the present study: A 96-h LC50 of 80.8 mg/L was reported for O. latipes and an LC50 of 2928 mg/L (without specification of the exposure period) was reported for Anguilla japonica [12,53].
3.1.4. Acute to Chronic Ratio
Acute to chronic ratio (ACR) of two cladoceran species which was calculated by dividing the 48-h acute EC50 by the chronic NOEC for D. magna or M. macrocopa, ranged from 34.5 to >487.8 (Table S3). These ACRs are generally within the ranges reported for other pharmaceuticals. In a previous study [56], the mean ACR of aquatic invertebrate for pharmaceuticals was reported at 314 (n = 27; range: 1–3108 and median: 17.6). The ACR is useful in ecological risk assessment because a reliable ACR would allow the use of acute toxicity data to estimate chronic effect concentrations [56,57].
3.2. Levels of Environmental Occurrence
The tested pharmaceuticals were reported in the aquatic environments worldwide, and these occurrence data are summarized in Table 2. The literature information shows that both amoxicillin and enrofloxacin have been frequently detected in the aquatic environment worldwide, while neomycin has seldom been reported (Table 2). The maximum values of MECmean reported for amoxicillin, enrofloxacin, and neomycin, in the literature were 0.068 µg/L, 0.087 µg/L, and 1.18 µg/L, respectively (Table 2). It should be noted however that the maximum MECmean of neomycin was derived from only two countries, India and Korea [58,59,60]. More information is warranted on the environmental occurrences of neomycin in other geographical areas, and this should be a subject of future research. The maximum reported concentrations (MECmax) ranged between 1 and 2 µg/L for amoxicillin and neomycin, but enrofloxacin was reported at up to 30 µg/L in the Isakavagu-Nakkavagu rivers of India [13].
Table 2.
Concentrations of amoxicillin, enrofloxacin, and neomycin reported in surface waters worldwide.
| Pharmaceuticals /Location |
Number of Detect (Total n) |
LOQ (µg/L) | Concentration (µg/L) | Reference | ||
|---|---|---|---|---|---|---|
| Mean | Min. | Max. | ||||
| Amoxicillin | ||||||
| Africa | ||||||
| Ghana | ||||||
| Kumasi region (Rivers) |
–(39) | - | - | <LOQ | 0.0027 | Azanu et al. [16] |
| Asia | ||||||
| India | ||||||
| Yamuna River | 4 (7) | - | 0.18 | - | - | Velpandian et al. [60] |
| Korea | ||||||
| Four Major River water a | 0 (40) | 0.00442 | <LOQ | <LOQ | <LOQ | NIER [58] |
| Turkey | ||||||
| Buyukcekmece Lake | 2 (5) | 0.0015 | 0.00291 b | <LOQ | 0.00400 | Aydin and Talinli [17] |
| Karasu River | 5 (5) | 0.0015 | 0.0214 b | 0.00389 | 0.0639 | Aydin and Talinli [17] |
| Tahtakopru River | 4 (5) | 0.0015 | 0.00635 b | <LOQ | 0.0142 | Aydin and Talinli [17] |
| Hamza River | 4 (5) | 0.0015 | 0.0123 b | <LOQ | 0.0573 | Aydin and Talinli [17] |
| Ahlat River | 5 (5) | 0.0015 | 0.0406 b | 0.00640 | 1.654 | Aydin and Talinli [17] |
| Beylikcayi River | 5 (5) | 0.0015 | 0.0138 b | 0.00280 | 0.0336 | Aydin and Talinli [17] |
| Europe | ||||||
| France | ||||||
| Seine River | - | 0.0392 | 0.068 | - | - | Dinh et al. [61] |
| Italy | ||||||
| River Po and Arno | 0 (8) | <0.001 | <LOQ | <LOQ | <LOQ | Calamari et al. [18] |
| River Arno (Castelfranco) |
4 (4) | <0.00208 | 0.00557 | 0.00357 | 0.00991 | Zuccato et al. [8] |
| River Arno (Limite sull’Arno) |
- | <0.00208 | 0.00377 | - | - | Zuccato et al. [8] |
| River Arno (Pisa) | - | <0.00208 | 0.00991 | - | - | Zuccato et al. [8] |
| River Po (Monticelli PV) |
- | <0.00208 | <0.00208 | - | - | Zuccato et al. [8] |
| Oceania | ||||||
| South-East Queensland, drinking water | 0 (20) | 0.020 | <LOQ | <LOQ | <LOQ | Watkinson et al. [7] |
| South-East Queensland, environmental water | 29 (98) | 0.020 | <LOQ | <LOQ | 0.2 | Watkinson et al. [7] |
| Enrofloxacin | ||||||
| Asia | ||||||
| China | ||||||
| Chentaizi drainage River | 3 (4) | 0.0001 | 0.0044 | ND | 0.0112 | Gao et al. [22] |
| Dagu drainage River | 1 (6) | 0.0001 | 0.0002 | ND | 0.0012 | Gao et al. [22] |
| Duliujian River | 2 (2) | 0.0001 | 0.0041 | 0.002 | 0.0062 | Gao et al. [22] |
| Guangzhou –Tap water | –(10) | 0.00028 | 0.002 b | ND | 0.0083 | Yiruhan et al. [62] |
| Haihe River | 4 (9) | 0.0001 | 0.0004 | ND | 0.001 | Gao et al. [22] |
| Haihe River, tributary | 2 (6) | 0.0001 | 0.0012 | ND | 0.0051 | Gao et al. [22] |
| Huangpu River | 2 (38) | 0.01134 | <LOQ | ND | <LOQ | Jiang et al. [23] |
| Huangpu River | 5 (13) | - | 0.0028 | ND | 0.0146 | Chen and Zhou [63] |
| Nansha River | 12 (12) | 0.001 | 0.00867 | 0.003 | 0.02 | Shao et al. [64] |
| Qiantang River, Hangzhou | 2 (2) | 0.027 | 0.0146 | 0.0105 | 0.0187 | Tong et al. [65] |
| River discharging to Laizhou Bay | 13 (23) | 0.005 | 0.0106 | ND | 0.0246 | Zhang et al. [66] |
| River in Shandong province | 12 (25) | 0.00133 | 0.00274 | 0.0002 | 0.0522 | Hanna et al. [67] |
| Shahu county, Jianghan | 19 (20) | 0.00145 d | 0.02457 | 0.00017 | 0.136 | Yao et al. [68] |
| Tai Lake | 6 (101) | - | 0.00508 | - | 0.183 | Song et al. [24] |
| Yangtz estuary | 4 (28) | 0.00168 | - | ND | 0.00477 | Yan et al. [69] |
| India | ||||||
| Isakavagu-Nakkavagu Rivers | 4 (5) | 0.01 | 0.064 b | ND | 30 | Fick et al. [13] |
| Korea | ||||||
| 4 Major Rivers a | 5 (40) | 0.010 | 0.0608 c | <LOQ | 0.188 | NIER [70] |
| 4 Major Rivers a | 1 (40) | 0.0829 | 0.0870 c | <LOQ | 0.0870 | NIER [58] |
| 4 Major Rivers a | 8 (80) | 0.00316 | 0.0156 c | <LOQ | 0.0300 | NIER [59] |
| 4 Major Rivers a | 0 (80) | 0.0407 | <LOQ c | <LOQ | <LOQ | NIER [71] |
| 4 Major Rivers a | 0 (80) | 0.009 | <LOQ c | <LOQ | <LOQ | NIER [72] |
| 4 Major Rivers a | 1 (80) | 0.008 | 0.011 c | <LOQ | <LOQ | NIER [73] |
| Macao | ||||||
| Macao -Tap water | –(12) | 0.00028 | 0.0040 b | 0.0028 | 0.0052 | Yiruhan et al. [62] |
| Vietnam | ||||||
| Freshwater near Mekong delta | 42 (154) | 0.001 | 0.012 b | < LOQ | 0.059 | Nguyen DangGiang et al. [74] |
| Panguasius catfish pond | –(19) | 0.02 | 0.05 | 0.68 | Andrieu et al. [21] | |
| Europe | ||||||
| France | ||||||
| Seine River | 0 (44) | 0.01 | - | - | < 0.01 | Tamtam et al. [26] |
| Seine River | - | 0.011 | <LOQ | <LOQ | <LOQ | Dinh et al. [61] |
| Portugal | ||||||
| Mondego River | 8 (22) | 0.025 | - | <LOQ | 0.1025 | Pena et al. [25] |
| Spain | ||||||
| Castellon and Valencia provinces | 18 (18) | 0.009 | - | - | 0.070 | Gracia-Lor et al. [75] |
| North America | ||||||
| United States | ||||||
| 139 Streams | 0 (115) | 0.02 d | ND b | - | ND | Kolpin et al. [5] |
| 23 Streams in Iowa, high-flow | 0 (23) | 0.01 d | ND | - | ND | Kolpin et al. [27] |
| 23 streams in Iowa, normal-flow | 0 (23) | 0.01 d | ND | - | ND | Kolpin et al. [27] |
| 23 streams in Iowa, low-flow | 1 (30) | 0.01 d | - | - | 0.01 | Kolpin et al. [27] |
| Oceania | ||||||
| Australia | ||||||
| South-East Queensland, drinking water | 0 (20) | 0.001 | ND b | <LOQ | <LOQ | Watkinson et al. [7] |
| South-East Queensland, environmental water | 43 (97) | 0.001 | ND b | - | 0.30 | Watkinson et al. [7] |
| Neomycin | ||||||
| Asia | ||||||
| India | ||||||
| Yamuna River | 3 (7) | - | 1.18 | - | - | Velpandian et al. [60] |
| Korea | ||||||
| 4 Major Rivers a | 1 (40) | 0.00008 | 0.94 c | <LOQ | 0.94 | NIER [58] |
| 4 Major Rivers a | 0 (80) | 0.001 | <LOQ | <LOQ | <LOQ | NIER [59] |
ND, not detected; LOQ, limit of quantification; -, not available. a Four major rivers in Korea include the Han River, Geum River, Youngsan River, and Nakdong River. b Median concentration. c Concentration below LOQ were not included in the calculation of mean values. d Limit of detection.
3.3. PNEC of Each Pharmaceutical
Based on the acute and chronic ecotoxicity information obtained in the present study and in the literature (Table 1), the most sensitive toxicity value that was identified for each compound was 0.00078 mg/L for amoxicillin [19], 0.049 mg/L for enrofloxacin [44], and 0.03 mg/L for neomycin [12]. Because the chronic toxicity data from three representative trophic levels—that is, algae, daphnids, and fish—were available, an uncertainty factor of 10 was used for each of three veterinary pharmaceuticals for the derivation of PNECs [36]. The PNECs that were determined for the tested pharmaceuticals are shown in Table 3, and these are 0.078 µg/L, 4.9 µg/L, and 3.0 µg/L for amoxicillin, enrofloxacin, and neomycin, respectively (Table 3). With an uncertainty factor of 10, the derived PNECs are expected to provide reasonable measures to estimate potential risks of these pharmaceuticals in ambient water. If necessary, however, the PNECs for the tested pharmaceuticals can be further refined with more chronic ecotoxicological data for diverse taxa, and by employing species sensitivity distribution approach.
Table 3.
Hazard quotients derived for amoxicillin, enrofloxacin, and neomycin.
| Pharmaceuticals | MECmean (µg/L) | MECmax (µg/L) | Lowest NOEC (mg/L) | AF | PNEC (µg/L) | HQ Based on MECmean |
HQ Based on MECmax |
|---|---|---|---|---|---|---|---|
| Amoxicillin | 0.068 | 1.654 | 0.00078 b | 10 | 0.078 | 0.87 | 21.2 |
| Enrofloxacin | 0.087 | 30 | 0.049 c | 10 | 4.9 | 0.018 | 6.1 |
| Neomycin | 1.18 | 1.18 a | 0.03 d | 10 | 3.0 | 0.39 | 0.39 |
3.4. Ecological Risks
The HQs derived for the MECmean of amoxicillin and enrofloxacin were less than one, suggesting negligible risks (Table 3), suggesting negligible ecological risks in the aquatic environment in general. However, at MECmax, the HQs for amoxicillin and enrofloxacin were 21.2 and 6.1, respectively. This finding implies that both amoxicillin and enrofloxacin can cause potential ecological risks in hotspot areas, e.g., near the sources. Potential risks of both pharmaceuticals especially at the sites with MECmax indicate that efforts for identification of hotspots and development of appropriate risk management may be required for these pharmaceuticals. For neomycin, negligible risks were expected with an HQ of 0.39. However, considering the fact that the occurrence information for neomycin was very restricted, further surveillance is recommended before its ecological risk can be characterized with greater confidence.
4. Conclusions
In conclusion, amoxicillin and enrofloxacin were identified as pharmaceuticals of potential ecological concerns in certain hotspot areas. Further efforts are required to identify their sources of contamination, and to investigate the ecological consequences of both pharmaceuticals. For neomycin, environmental monitoring in ambient water should be followed before its ecological risk can be properly characterized.
Acknowledgments
This study was supported by the National Institute of Environmental Research (NIER) of Korea and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1F1A1074971).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxics9080196/s1. Table S1: Physicochemical characteristics of tested veterinary pharmaceuticals, Table S2: Nominal and measured concentrations of amoxicillin, enrofloxacin, and neomycin exposure, Table S3: Toxicity value obtained from acute and chronic test of D. magna and M. macrocopa after acute or chronic exposure to tested pharmaceuticals.
Author Contributions
Conceptualization, S.L. (Sangwoo Lee) and K.C.; software, S.L. (Sangwoo Lee); validation, Y.K.; formal analysis, S.L. (Sangwoo Lee), C.K., X.L, S.L.; resources, P.K. and W.-K.K.; data curation, S.L. (Sangwoo Lee), C.K., and W.-K.K.; writing—original draft preparation, S.L. (Sangwoo Lee); writing—review and editing, K.C.; visualization, S.L. (Sangwoo Lee), C.K., X.L., S.L. (Saeram Lee); supervision, K.C.; project administration, K.C.; funding acquisition, K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Institute of Environmental Research (NIER), funded by the Ministry of Environment (ME) of Korea (Risk Assessment of Pharmaceuticals in the Environment (IV)).
Institutional Review Board Statement
The study was conducted according to the guidelines of Seoul National University Institutional Animal Care and Use Committee.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu Y., Gao B., Yue Q., Guan Y., Wang Y., Huang L. Influences of two antibiotic contaminants on the production, release and toxicity of microcystins. Ecotoxicol. Environ. Saf. 2012;77:79–87. doi: 10.1016/j.ecoenv.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y., Jung J., Kim M., Park J., Boxall A.B.A., Choi K. Prioritizing veterinary pharmaceuticals for aquatic environment in Korea. Environ. Toxicol. Pharmacol. 2008;26:167–176. doi: 10.1016/j.etap.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Li W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014;187:193–201. doi: 10.1016/j.envpol.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Danner M.C., Robertson A., Behrends V., Reiss J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019;664:793–804. doi: 10.1016/j.scitotenv.2019.01.406. [DOI] [PubMed] [Google Scholar]
- 5.Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B., Buxton H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 6.Osorio V., Larrañaga A., Aceña J., Pérez S., Barceló D. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian rivers. Sci. Total Environ. 2016;540:267–277. doi: 10.1016/j.scitotenv.2015.06.143. [DOI] [PubMed] [Google Scholar]
- 7.Watkinson A.J., Murby E.J., Kolpin D.W., Costanzo S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009;407:2711–2723. doi: 10.1016/j.scitotenv.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Zuccato E., Castiglioni S., Bagnati R., Melis M., Fanelli R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010;179:1042–1048. doi: 10.1016/j.jhazmat.2010.03.110. [DOI] [PubMed] [Google Scholar]
- 9.Boxall A.B., Rudd M.A., Brooks B.W., Caldwell D.J., Choi K., Hickmann S., Innes E., Ostapyk K., Staveley J.P., Verslycke T., et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012;120:1221–1229. doi: 10.1289/ehp.1104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebele A.J., Abou-Elwafa Abdallah M., Harrad S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017;3:1–16. doi: 10.1016/j.emcon.2016.12.004. [DOI] [Google Scholar]
- 11.Yılmaz Ç., Özcengiz G. Antibiotics: Pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Biochem. Pharmacol. 2017;133:43–62. doi: 10.1016/j.bcp.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Park S., Choi K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology. 2008;17:526–538. doi: 10.1007/s10646-008-0209-x. [DOI] [PubMed] [Google Scholar]
- 13.Fick J., Söderström H., Lindberg R.H., Phan C., Tysklind M., Larsson D.G.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009;28:2522–2527. doi: 10.1897/09-073.1. [DOI] [PubMed] [Google Scholar]
- 14.Boxall A.B.A., Kolpin D.W., Halling-Sørensen B., Tolls J. Are veterinary medicines causing environmental risks? Environ. Sci. Technol. 2003;37:286A–294A. doi: 10.1021/es032519b. [DOI] [PubMed] [Google Scholar]
- 15.Putra E.K., Pranowo R., Sunarso J., Indraswati N., Ismadji S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009;43:2419–2430. doi: 10.1016/j.watres.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Azanu D., Styrishave B., Darko G., Weisser J.J., Abaidoo R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018;622–623:293–305. doi: 10.1016/j.scitotenv.2017.11.287. [DOI] [PubMed] [Google Scholar]
- 17.Aydin E., Talinli I. Analysis, occurrence and fate of commonly used pharmaceuticals and hormones in the Buyukcekmece Watershed, Turkey. Chemosphere. 2013;90:2004–2012. doi: 10.1016/j.chemosphere.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 18.Calamari D., Zuccato E., Castiglioni S., Bagnati R., Fanelli R. Strategic survey of therapeutic drugs in the rivers Po and Lambro in Northern Italy. Environ. Sci. Technol. 2003;37:1241–1248. doi: 10.1021/es020158e. [DOI] [Google Scholar]
- 19.Andreozzi R., Caprio V., Ciniglia C., de Champdoré M., Lo Giudice R., Marotta R., Zuccato E. Antibiotics in the environment: Occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 2004;38:6832–6838. doi: 10.1021/es049509a. [DOI] [PubMed] [Google Scholar]
- 20.Ebert I., Bachmann J., Kühnen U., Küster A., Kussatz C., Maletzki D., Schlüter C. Toxicity of the fluoroquinolone antibiotics enrofloxacin and ciprofloxacin to photoautotrophic aquatic organisms. Environ. Toxicol. Chem. 2011;30:2786–2792. doi: 10.1002/etc.678. [DOI] [PubMed] [Google Scholar]
- 21.Andrieu M., Rico A., Phu T.M., Huong D.T.T., Phuong N.T., Van den Brink P.J. Ecological risk assessment of the antibiotic enrofloxacin applied to Pangasius catfish farms in the Mekong Delta, Vietnam. Chemosphere. 2015;119:407–414. doi: 10.1016/j.chemosphere.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 22.Gao L., Shi Y., Li W., Liu J., Cai Y. Occurrence, distribution and bioaccumulation of antibiotics in the Haihe River in China. J. Environ. Monit. 2012;14:1248–1255. doi: 10.1039/c2em10916f. [DOI] [PubMed] [Google Scholar]
- 23.Jiang L., Hu X., Yin D., Zhang H., Yu Z. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere. 2011;82:822–828. doi: 10.1016/j.chemosphere.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Song C., Zhang C., Fan L., Qiu L., Wu W., Meng S., Hu G., Kamira B., Chen J. Occurrence of antibiotics and their impacts to primary productivity in fishponds around Tai Lake, China. Chemosphere. 2016;161:127–135. doi: 10.1016/j.chemosphere.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Pena A., Chmielova D., Lino C.M., Solich P. Determination of fluoroquinolone antibiotics in surface waters from Mondego River by high performance liquid chromatography using a monolithic column. J. Sep. Sci. 2007;30:2924–2928. doi: 10.1002/jssc.200700363. [DOI] [PubMed] [Google Scholar]
- 26.Tamtam F., Mercier F., Le Bot B., Eurin J., Tuc Dinh Q., Clément M., Chevreuil M. Occurrence and fate of antibiotics in the seine river in various hydrological conditions. Sci. Total Environ. 2008;393:84–95. doi: 10.1016/j.scitotenv.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Kolpin D.W., Skopec M., Meyer M.T., Furlong E.T., Zaugg S.D. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci. Total Environ. 2004;328:119–130. doi: 10.1016/j.scitotenv.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Keswani N., Choudhary S., Kishore N. Interaction of weakly bound antibiotics neomycin and lincomycin with bovine and human serum albumin: Biophysical approach. J. Biochem. 2010;148:71–84. doi: 10.1093/jb/mvq035. [DOI] [PubMed] [Google Scholar]
- 29.Organization for Economic Co-Operation and Development . OECD Guideline for Testing of Chemicals, Test NO. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Publishing; Paris, France: 2011. Section 2: Effects on biotic systems. [Google Scholar]
- 30.US Environmental Protection Agency . Ecological Effects Test Guidelines. OPPTS 850.5400. Algal Toxicity, Tiers I and II. United States Environmental Protection Agency; Washington, DC, USA: 1996. EPA 712-C96-164. [Google Scholar]
- 31.Organization for Economic Co-Operation and Development . OECD Guideline for Testing of Chemicals, Test NO. 211: Daphnia magna Reproduction Test. OECD Publishing; Paris, France: 2008. Section 2: Effects on biotic systems. [Google Scholar]
- 32.Organization for Economic Co-Operation and Development . OECD Guideline for Testing of Chemicals, Test NO. 202: Daphnia sp. Acute Immobilization Test. OECD Publishing; Paris, France: 2004. Section 2: Effects on biotic systems. [Google Scholar]
- 33.Oh S., Choi K. Optimal conditions for three brood chronic toxicity test method using a freshwater macroinvertebrate Moina macrocopa. Environ. Monit. Assess. 2012;184:3687–3695. doi: 10.1007/s10661-011-2216-2. [DOI] [PubMed] [Google Scholar]
- 34.Olmstead A.W., LeBlanc G.A. Effects of endocrine-active chemicals on the development of sex characteristics of Daphnia magna. Environ. Toxicol. Chem. 2000;19:2107–2113. doi: 10.1002/etc.5620190821. [DOI] [Google Scholar]
- 35.Organization for Economic Co-Operation and Development . OECD Guideline for Testing of Chemicals, Test NO. 210: Fish, Early-Life Stage Toxicity Test. OECD Publishing; Paris, France: 2013. Section 2: Effects on biotic systems. [Google Scholar]
- 36.European Commission . Technical Guidance Document in Support of Commission Directive 93/67 EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) Joint Research Centre; Ispra, Italy: 2003. No 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European Parliament and of the Council concerning the Placing of Biocidal Products on the Market. [Google Scholar]
- 37.Lotka A.J. A natural population norm I. [(accessed on 15 July 2021)];J. Wash. Acad. Sci. 1913 3:241–248. Available online: https://www.jstor.org/stable/24521071. [Google Scholar]
- 38.González-Pleiter M., Gonzalo S., Rodea-Palomares I., Leganés F., Rosal R., Boltes K., Marco E., Fernández-Piñas F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013;47:2050–2064. doi: 10.1016/j.watres.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Halling-Sørensen B. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere. 2000;40:731–739. doi: 10.1016/S0045-6535(99)00445-2. [DOI] [PubMed] [Google Scholar]
- 40.Lützhøft H.H., Halling-Sørensen B., Jørgensen S.E. Algal toxicity of antibacterial agents applied in danish fish farming. Arch. Environ. Contam. Toxicol. 1999;36:1–6. doi: 10.1007/s002449900435. [DOI] [PubMed] [Google Scholar]
- 41.van der Grinten E., Pikkemaat M.G., van den Brandhof E.J., Stroomberg G.J., Kraak M.H. Comparing the sensitivity of algal, cyanobacterial and bacterial bioassays to different groups of antibiotics. Chemosphere. 2010;80:1–6. doi: 10.1016/j.chemosphere.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira R., McDonough S., Ladewig J.C.L., Soares A.M.V.M., Nogueira A.J.A., Domingues I. Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio) Environ. Toxicol. Pharmacol. 2013;36:903–912. doi: 10.1016/j.etap.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 43.Yasser E.N., El-Dahdouh N. Toxicity of amoxicillin and erythromycin to fish and mosquitoes. [(accessed on 15 July 2021)];Ecotoxicol. Environ. Contam. 2015 10:13–21. doi: 10.5132/eec.2015.01.03. Available online: http://hdl.handle.net/20.500.12358/25903. [DOI] [Google Scholar]
- 44.Robinson A.A., Belden J.B., Lydy M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. 2005;24:423–430. doi: 10.1897/04-210R.1. [DOI] [PubMed] [Google Scholar]
- 45.Qin H.W., Chen L.F., Lu N., Zhao Y.H., Yuan X. Toxic effects of enrofloxacin on Scenedesmus obliquus. Front. Environ. Sci. Eng. 2012;6:107–116. doi: 10.1007/s11783-011-0327-1. [DOI] [Google Scholar]
- 46.Xiong J.Q., Kurade M.B., Jeon B.H. Ecotoxicological effects of enrofloxacin and its removal by monoculture of microalgal species and their consortium. Environ. Pollut. 2017;226:486–493. doi: 10.1016/j.envpol.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 47.Kim J., Park J., Kim P.G., Lee C., Choi K., Choi K. Implication of global environmental changes on chemical toxicity-effect of water temperature, pH, and ultraviolet B irradiation on acute toxicity of several pharmaceuticals in Daphnia magna. Ecotoxicology. 2010;19:662–669. doi: 10.1007/s10646-009-0440-0. [DOI] [PubMed] [Google Scholar]
- 48.Oh S. Master’s Thesis. Seoul National University; Seoul, Korea: 2004. Aquatic Toxicities of Major Veterinary Antibiotics and Anthelmintics on Microbes and Macroinvertebrates. [Google Scholar]
- 49.Dalla Bona M., Di Leva V., De Liguoro M. The sensitivity of Daphnia magna and Daphnia curvirostris to 10 veterinary antibacterials and to some of their binary mixtures. Chemosphere. 2014;115:67–74. doi: 10.1016/j.chemosphere.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Sun M., Duker R.Q., Gillissen F., Van den Brink P.J., Focks A., Rico A. Influence of pH on the toxicity of ionisable pharmaceuticals and personal care products to freshwater invertebrates. Ecotoxicol. Environ. Saf. 2020;191:110172. doi: 10.1016/j.ecoenv.2020.110172. [DOI] [PubMed] [Google Scholar]
- 51.Vance B.D. Sensitivity of Microcystis aeruginosa and other blue-green algae and associated bacteria to selected antibiotics. J. Phycol. 1966;2:125–128. doi: 10.1111/j.1529-8817.1966.tb04607.x. [DOI] [PubMed] [Google Scholar]
- 52.Whitton B.A. Effect of light on toxicity of various substances to Anacystis nidulans. Plant Cell Physiol. 1968;9:23–26. doi: 10.1093/oxfordjournals.pcp.a079326. [DOI] [Google Scholar]
- 53.US Environmental Protection Agency, 2020 ECOTOX User Guide. ECOTOXicology Database System. Version 4.0. [(accessed on 17 March 2020)]; Available online: http://www.epa.gov/ecotox/
- 54.Brain R.A., Johnson D.J., Richards S.M., Sanderson H., Sibley P.K., Solomon K.R. Effects of 25 pharmaceutical compounds to Lemna gibba using a seven-day static-renewal test. Environ. Toxicol. Chem. 2004;23:371–382. doi: 10.1897/02-576. [DOI] [PubMed] [Google Scholar]
- 55.Hernando M.D., De Vettori S., Martínez Bueno M.J., Fernández-Alba A.R. Toxicity evaluation with Vibrio fischeri test of organic chemicals used in aquaculture. Chemosphere. 2007;68:724–730. doi: 10.1016/j.chemosphere.2006.12.097. [DOI] [PubMed] [Google Scholar]
- 56.Crane M., Watts C., Boucard T. Chronic aquatic environmental risks from exposure to human pharmaceuticals. Sci. Total Environ. 2006;367:23–41. doi: 10.1016/j.scitotenv.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Ji K., Kim S., Han S., Seo J., Lee S., Park Y., Choi K., Kho Y.L., Kim P.G., Park J., et al. Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: Are the current environmental concentrations safe? Ecotoxicology. 2012;21:2031–2050. doi: 10.1007/s10646-012-0956-6. [DOI] [PubMed] [Google Scholar]
- 58.National Institute of Environmental Research . Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products in Environment (II) Ministry of the Environment; Sejong, Korea: 2007. [(accessed on 15 July 2021)]. Available online: https://dl.nanet.go.kr/law/SearchDetailView.do?cn=NONB1200931362. [Google Scholar]
- 59.National Institute of Environmental Research . Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products Residues (I) Ministry of the Environment; Sejong, Korea: 2008. [(accessed on 15 July 2021)]. Available online: https://dl.nanet.go.kr/law/SearchDetailView.do?cn=NONB1200931438. [Google Scholar]
- 60.Velpandian T., Halder N., Nath M., Das U., Moksha L., Gowtham L., Batta S.P. Un-segregated waste disposal: An alarming threat of antimicrobials in surface and ground water sources in Delhi. Environ. Sci. Pollut. Res. Int. 2018;25:29518–29528. doi: 10.1007/s11356-018-2927-9. [DOI] [PubMed] [Google Scholar]
- 61.Dinh Q.T., Alliot F., Moreau-Guigon E., Eurin J., Chevreuil M., Labadie P. Measurement of trace levels of antibiotics in river water using on-line enrichment and triple-quadrupole LC-MS/MS. Talanta. 2011;85:1238–1245. doi: 10.1016/j.talanta.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q.J., Mo C.H., Li Y.W., Gao P., Tai Y.P., Zhang Y., Ruan Z.L., Xu J.W. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao. Environ. Pollut. 2010;158:2350–2358. doi: 10.1016/j.envpol.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Chen K., Zhou J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere. 2014;95:604–612. doi: 10.1016/j.chemosphere.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 64.Shao B., Chen D., Zhang J., Wu Y., Sun C. Determination of 76 pharmaceutical drugs by liquid chromatography-tandem mass spectrometry in slaughterhouse wastewater. J. Chromatogr. A. 2009;1216:8312–8318. doi: 10.1016/j.chroma.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 65.Tong C., Zhuo X., Guo Y. Occurrence and risk assessment of four typical fluoroquinolone antibiotics in raw and treated sewage and in receiving waters in Hangzhou, China. J. Agric. Food Chem. 2011;59:7303–7309. doi: 10.1021/jf2013937. [DOI] [PubMed] [Google Scholar]
- 66.Zhang R., Zhang G., Zheng Q., Tang J., Chen Y., Xu W., Zou Y., Chen X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012;80:208–215. doi: 10.1016/j.ecoenv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Hanna N., Sun P., Sun Q., Li X., Yang X., Ji X., Zou H., Ottoson J., Nilsson L.E., Berglund B., et al. Presence of antibiotic residues in various environmental compartments of Shandong Province in Eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018;114:131–142. doi: 10.1016/j.envint.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Yao L., Wang Y., Tong L., Deng Y., Li Y., Gan Y., Guo W., Dong C., Duan Y., Zhao K. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, Central China. Ecotoxicol. Environ. Saf. 2017;135:236–242. doi: 10.1016/j.ecoenv.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Yan C., Yang Y., Zhou J., Liu M., Nie M., Shi H., Gu L. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013;175:22–29. doi: 10.1016/j.envpol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 70.National Institute of Environmental Research . Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products in Environment (I) Ministry of the Environment; Sejong, Korea: 2006. [(accessed on 15 July 2021)]. Available online: http://dl.nanet.go.kr/law/SearchDetailView.do?cn=NONB1200931436. [Google Scholar]
- 71.National Institute of Environmental Research . Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products Residues (II) Ministry of the Environment; Sejong, Korea: 2009. [(accessed on 15 July 2021)]. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201300007870. [Google Scholar]
- 72.National Institute of Environmental Research . Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products Residues (III) Ministry of the Environment; Sejong, Korea: 2010. [(accessed on 15 July 2021)]. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201300007428. [Google Scholar]
- 73.National Institute of Environmental Research . Development of Analytical Method and Study of Exposure of Pharmaceuticals and Personal Care Products Residues (IV) Ministry of the Environment; Sejong, Korea: 2011. [(accessed on 15 July 2021)]. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201300007704. [Google Scholar]
- 74.Nguyen Dang Giang C., Sebesvari Z., Renaud F., Rosendahl I., Hoang Minh Q., Amelung W. Occurrence and dissipation of the antibiotics sulfamethoxazole, sulfadiazine, trimethoprim, and enrofloxacin in the Mekong Delta, Vietnam. PLoS ONE. 2015;10:e0131855. doi: 10.1371/journal.pone.0131855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gracia-Lor E., Sancho J.V., Hernández F. Multi-class determination of around 50 pharmaceuticals, including 26 antibiotics, in environmental and wastewater samples by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2011;1218:2264–2275. doi: 10.1016/j.chroma.2011.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.