Abstract
Shrimp, as a high-protein animal food commodity, are one of the fastest growing food producing sectors in the world. It has emerged as a highly traded seafood product, currently exceeding 8 MT of high value. However, disease outbreaks, which are considered as the primary cause of production loss in shrimp farming, have moved to the forefront in recent years and brought socio-economic and environmental unsustainability to the shrimp aquaculture industry. Acute hepatopancreatic necrosis disease (AHPND), caused by Vibrio spp., is a relatively new farmed penaeid shrimp bacterial disease. The shrimp production in AHPND affected regions has dropped to ~60%, and the disease has caused a global loss of USD 43 billion to the shrimp farming industry. The conventional approaches, such as antibiotics and disinfectants, often applied for the mitigation or cure of AHPND, have had limited success. Additionally, their usage has been associated with alteration of host gut microbiota and immunity and development of antibiotic resistance in bacterial pathogens. For example, the Mexico AHPND-causing V. parahaemolyticus strain (13-306D/4 and 13-511/A1) were reported to carry tetB gene coding for tetracycline resistance gene, and V. campbellii from China was found to carry multiple antibiotic resistance genes. As a consequence, there is an urgent need to thoroughly understand the virulence mechanism of AHPND-causing Vibrio spp. and develop novel management strategies to control AHPND in shrimp aquaculture, that will be crucially important to ensure food security in the future and offer economic stability to farmers. In this review, the most important findings of AHPND are highlighted, discussed and put in perspective, and some directions for future research are presented.
Keywords: shrimp aquaculture, AHPND, V. parahaemolyticus, virulence mechanism, management strategies
1. Introduction
Crustaceans, usually treated as a subphylum, form a large group of arthropods—mainly aquatic invertebrates—which represent a group of animals important to aquaculture. Crustaceans are considered as economic relevant aquaculture products with high worldwide demand [1,2]. The total crustacean aquaculture production in 2017, from over 30 different species, was 8.4 MT valued at USD 61.06 billion, with an average annual growth rate of 9.92% per year since 2000 [3]. The marine shrimp currently dominate crustacean aquaculture at 5.51 MT or 65.3% of total crustaceans (valued at USD 34.2 billion), followed by freshwater crustacean (2.53 MT or 29.9% total crustacean) and valued at USD 24.3 billion. Although shrimp represents only 6% of the global aquaculture production, they contribute to around 16% of the production value of traded seafood products.
Shrimp production mainly consists of three species, i.e., Litopenaeus vannamei, Penaeus monodon and Macrobrachium rosenbergii. Countries in East and Southeast Asia and Latin America account by far for the major share shrimp production for, but a large proportion of consumption takes place in the developed countries. Among crustaceans, the white leg shrimp (L. vannamei) was reported to have the highest unit value at USD 26.7 billion [4]. The giant tiger shrimp P. monodon makes up ~15% of total shrimp production, its production reached 0.74 million tonnes, worth USD 5.59 billion [3]. The giant river prawn (M. rosenbergii) makes up the rest of the farmed shrimp volumes. M. rosenbergii is native to Asia and production reached 0.23 million tons globally, with a value of more than USD 1.90 billion [5,6]. As surveyed in GOAL 2019, the shrimp market is expected to grow further with an annual growth rate of 5.4% between 2017–2021. This will result in a global farmed shrimp harvest of 5.03 million tonnes (approx. 5.4 million tonnes including M. rosenbergii) [7,8].
Moreover, as the global human population continues to expand at a high rate and is expected to reach over 9 billion by 2030, shrimp aquaculture can provide global food and nutritional security to people in both developed and developing countries and support the livelihood and jobs of the global population [3,9]. However, due to the global demand increase, the pressure for intensification and expansion of shrimp aquaculture systems has rendered most aquaculture business fragile. In the aquaculture industry, economic losses from disease outbreak have been estimated by the FAO to be over of USD 9 billion per year, which is approximately 15% of the value of world farmed fish and shellfish production. In particular, bacterial diseases have brought socio-economic and environmental unsustainability to the shrimp aquaculture industry during the last decades. Vibriosis, an important bacterial disease, caused by opportunistic Vibrio spp. continues as the most serious threat to shrimp farmers in the region [10,11,12,13]. V. harveyi, V. alginolyticus, V. anguillarum, V. splendidus, V. salmonicida, V. vulnificus and V. parahaemolyticus strains have been found as main causative organisms of vibriosis [14,15,16]. However, apart from “classical” vibriosis, some Vibrio spp. are also responsible for causing acute hepatopancreatic necrosis disease (AHPND), originally known as early mortality syndrome (EMS) [17,18,19]. The AHPND in shrimp aquaculture has escalated since late 2013, when the industry collapsed in South-Asian countries. AHPND, having a devastating impact on the shrimp aquaculture industry, develops quickly, starting approximately 8 days post stocking and severe mortalities (up to 100%) occur within 20–30 days [20,21]. Hence, in this review at first an overview of the current knowledge on acute hepatopancreatic necrosis disease (AHPND) is given, including the disease associated gross signs and histopathology changes. Later, the current status on management/mitigation solutions for acute hepatopancreatic necrosis disease (AHPND) with respect to shrimp aquaculture are summarized.
2. Acute Hepatopancreatic Necrosis Disease (AHPND)—An Overview
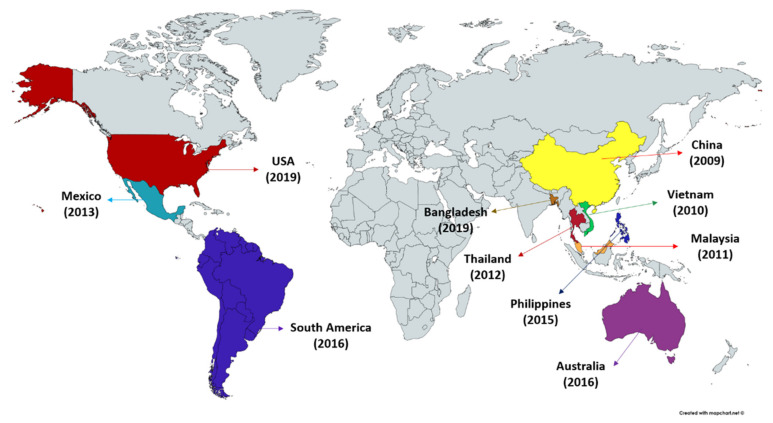
Acute hepatopancreatic necrosis disease (AHPND), a relatively new farmed penaeid shrimp bacterial disease originally known as early mortality syndrome (EMS) has been causing havoc in the shrimp industry. Since the AHPND outbreak first appeared in China in 2009, it has spread to Vietnam (2010), Malaysia (2011), Thailand (2012), Mexico (2013), Philippines (2015) and South America (2016) (Figure 1) [17,18,19,22]. The shrimp production in AHPND affected regions has dropped temporarily to ~60% and has resulted in collective losses exceeding an estimated USD 43 billion across Asia (China, Malaysia, Thailand, Vietnam) and in Mexico [23,24,25,26,27]. AHPND affects multiple species of shrimp including commercial species, P. monodon, L. vannamei and M. rosenbergii and crustacean model Artemia franciscana [24,28,29]. Interestingly, the brine shrimp (A. franciscana), an aquatic invertebrate characteristically small, highly osmotolerant and branchiopod crustacean that can be reared under gnotobiotic conditions (allowing full control over the host-associated microbial communities), serve as exceptional model organism to study the host-pathogen interactions in commercially important shrimps and other crustacean species [30,31]. Moreover, the early life stages of shrimp, in general, are more susceptible to AHPND infection. AHPND is characterized by severe atrophy of the shrimp hepatopancreas accompanied by unique histopathological changes at the acute stage of disease [18]. Furthermore, as disease progress massive sloughing of hepatopancreatic or digestive tract epithelial cells in the absence of any accompanying pathogen can be observed within approximately first 30 days of shrimp post-larvae stocking [21,32]. In fact, the AHPND-causing bacteria were reported to mainly target the digestive gland (hepatopancreas) and damage the hepatopancreatic R (resorptive), B (blister), F (fibrillar) and E (embryonic) cells, resulting in dysfunction and massive mortalities of shrimp [19,33,34]. The shrimp affected with AHPND exhibits lethargy, anorexia, slow growth, empty digestive tract and a pale to white hepatopancreas. However, these reported clinical signs for AHPND are common for some other diseases. For instance, the gross signs induced by chemical factor, e.g., nitrite and ammonia or secondary bacterial (traditional vibriosis) and viral (white spot syndrome virus, yellow head virus, etc.) infections could also lead to AHPND pathology [28]. Hence, identification of bacterial virulence factor and AHPND-specific cellular changes coupled with gross clinical signs are considered to be helpful for confirmatory diagnosis of AHPND in shrimp.
Figure 1.
Occurrence of acute hepatopancreatic necrosis disease (AHPND) in shrimp.
2.1. Gross Signs and Histopathology of AHPND
The AHPND-affected shrimps are lethargic and display erratic swimming behaviour. The external appearance of shrimps is slightly changed with expanded chromatophores across cuticles. Moreover, based on bacterial density and histological appearance, the natural AHPND-affected shrimp are divided into three phases: (a) initial, (b) acute and (c) terminal phase [35,36].
2.1.1. Initial Phase
The shrimp exhibits signs of damage in the hepatopancreas and in the gut there is partial or total absence of food. Moreover, the changes in the digestive tract and hepatopancreas are visualized better by dissecting and removing epithelial membrane (Figure 2b). The hepatopancreatic tubular epithelial cells are modified and elongated (display drops like appearance) towards the lumen (Figure 3a) causing cellular desquamation. Moreover, as AHPND progresses, the size of hepatopancreatic R and B cells are further reduced (Figure 3b).
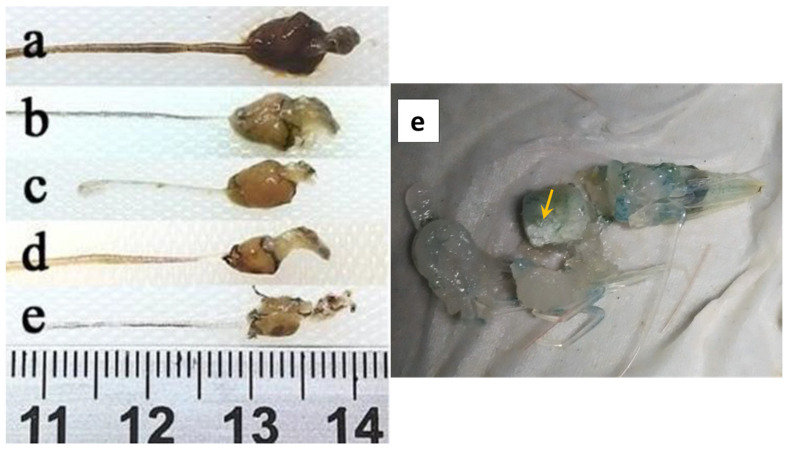
Figure 2.
Macroscopic observation of L. vannamei digestive tract affected by acute hepatopancreatic necrosis disease (AHPND). (a) Healthy shrimp; (b) initial phase; (c,d) acute phase; (e) terminal phase. Yellow arrowhead demonstrates completely damaged fibrous appearance hepatopancreas [36].
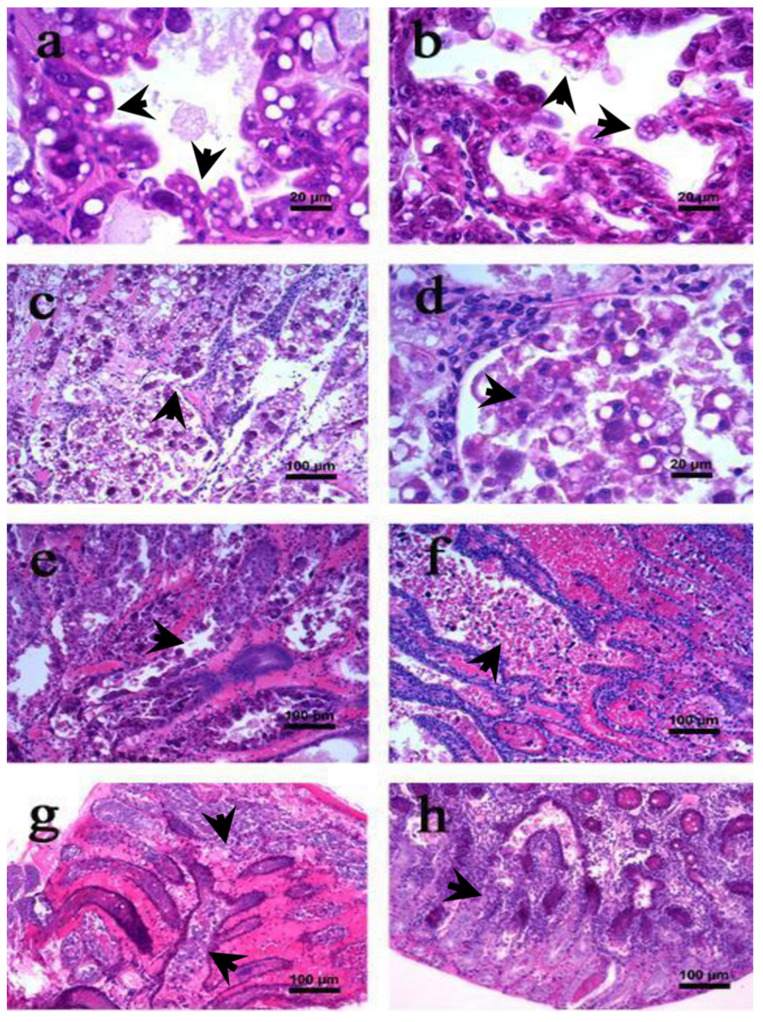
Figure 3.
Haematoxylin and eosin (H & E) stained section of hepatopancreas of L. vannamei with lesions associated with acute hepatopancreatic necrosis disease (AHPND). (a,b) Initial phase; (c–f) acute phase; (g,h) terminal phase [36]. The arrowhead in figures (a–h) represent the cellular changes associated different AHPND phases in affected shrimp.
2.1.2. Acute Phase
The AHPND-affected shrimp exhibit signs of anorexia and lethargy with empty digestive tract and loss of tissue pigmentation (Figure 2c). The hepatopancreas becomes atrophied and whitish in appearance (Figure 2d). During the first hour of infection, the hepatopancreatic tissue are friable with an aqueous consistency. However, as disease progress the tissue develops a hard consistency, becoming difficult to disintegrate. Microscopically, massive shedding or sloughing of hepatopancreatic tubular epithelial cells are observed. In addition, the tubular epithelial cells are severely necrotized and have massive accumulation of haemocytes and dead cells in the lumen, a pathognomonic lesion reported for AHPND (Figure 3c,d) [17,18,35,37].
Furthermore, at the first hour post-exposure, mitosis is interrupted in hepatopancreatic E cells and the presence of cytoplasmic vacuoles is observed in the B and R cells (Figure 3e). However, as the disease progresses the vacuoles disappear from the hepatopancreatic cells. Interestingly, during the acute phase, no bacterial cells are observed in the AHPND-affected tissue, which suggests that AHPND-causing bacteria secreted binary toxins, i.e., PirAVP and PirBVP might be responsible for mediating AHPND in shrimp at later stage of infection. The PirAVP and PirBVP toxins are found to bind and induce significant damage to the hepatopancreatic tubular epithelial cells, a phenomenon not observed in any other organ or tissue of AHPND-affected shrimp [33,35,38]. At the end of acute phase, the tubular epithelium is severely necrotized, in some instances it is completely absent, and significant high amount of haemocytic infiltration are observed in the intratubular tissues (Figure 3f).
2.1.3. Terminal Phase
Similar to acute phase, the shrimps at terminal phase of AHPND are anorexic, lethargic and have a completely empty stomach. The chromatophores are significantly expanded and hepatopancreas are atrophied and whitish in coloration (Figure 2e). Furthermore, when the hepatopancreas is squashed, it gives a fibrous appearance. Ultrastructure details showed the presence of black streaks, indicating focal melanisation in the hepatopancreatic tubular cells. In addition, the intratubular connective tissue, filled with a large amount of haemocytic cells, are surrounded by haemocytic capsules as a response of bacterial load and necrotic tissue (Figure 3g). During the terminal phase, the damage of tissue is mostly done by PirAVP and PirBVP toxins. However, bacterial proliferation at the site of damage is caused by secondary bacterial infections, possibly by a vibriosis (Figure 3h).
2.2. Causative Agent of AHPND
The AHPND is caused by specific strain of bacteria, e.g., V. parahaemolyticus, V. punensis, V. harveyi, V. owensii, V. campbelli and Shewanella sp. that contains pVA1 plasmid (63–70 kb) encoding the binary PirAVP and PirBVP toxins, homologous to the Photorhabdus luminescens insect-related (Pir) toxins PirA/PirB (Table 1) [18,39,40,41,42,43]. The PirAVP and PirBVP are the primary virulence factor of AHPND-causing bacteria that mediates AHPND aetiology and mortality in shrimp [20,39,44]
Table 1.
Bacterial species reported to mediate AHPND in crustacean species.
| Bacterial Species | Host Range | Geographical Distribution | References |
|---|---|---|---|
| Vibrio parahaemolyticus | P. monodon, L. vannamei | Worldwide | [17,18,33,45,46,47,48,49] |
| V. parahaemolyticus | Artemia franciscana | Laboratory condition | [16,24,29,32,39,44] |
| V. parahaemolyticus | Macrobrachium rosenbergii | Laboratory condition | [24,44] |
| V. punensis | L. vannamei | South America | [42] |
| V. harveyi | L. vannamei | China, Malaysia, Vietnam | [42,50,51] |
| V. owensii | L. vannamei | China | [41] |
| V. campbelli | L. vannamei | China | [40] |
| Shewanella sp. | L. vannamei | Thailand | [43,52] |
The Photorhabdus insect-related (Pir) toxins are first identified in Photorhabdus luminescens, a bacterium that maintains a symbiotic relationship with entomopathogenic nematodes of the family Heterorhabditidae [53,54,55]. In moths and mosquitoes, the binary PirAB toxins, encoded by PirA and PirB genes, are necessary for oral toxicity [56,57]. In fact, during infection, the pathology of oral toxicity can be visualized in the midgut epithelium of moth Plutella xylostella larvae, resulting in swelling and shedding of the (apical) epithelial cells [56]. In shrimp aquaculture, the virulent AHPND-causing bacteria containing pVA1 plasmid that encodes PirABVP toxin genes, homologous to the insecticidal PirA/PirB toxins genes, are absent in all non-AHPND bacterial species [33,58].
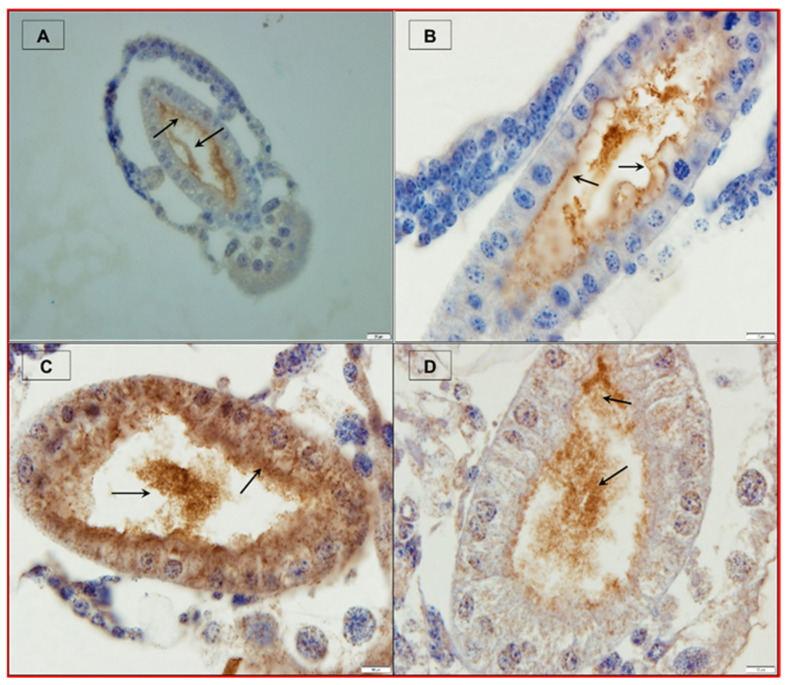
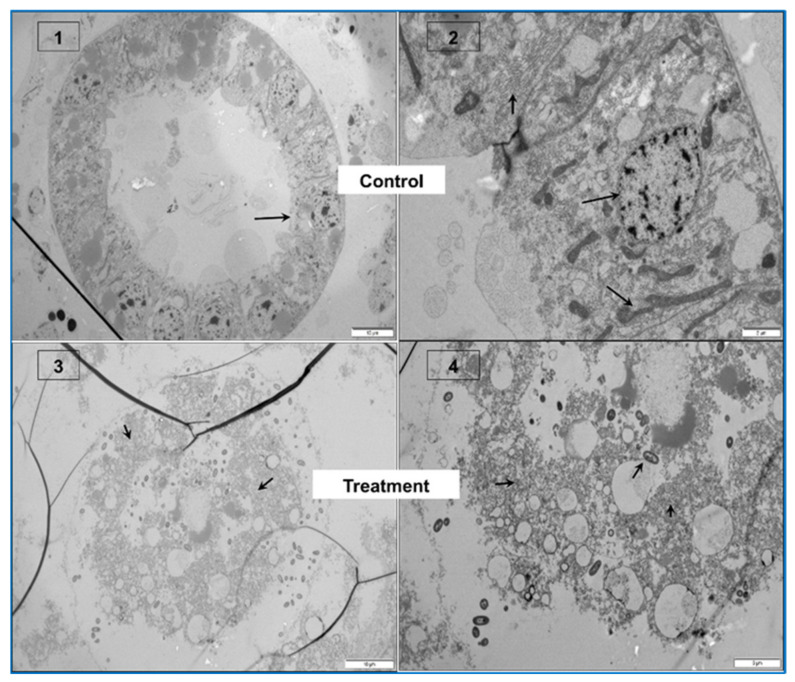
Among the PirABVP binary toxin, PirAVP binds with specific ligands on the cell membrane and receptors (e.g., monosaccharides like N-acetylgalactosamine (GalNAC) and oligosaccharides) and facilitates target-specific recognition. While PirBVP toxin, containing N-terminal domain (PirBN) and C-terminal domain (PirBC), induces cell death in host via pore formation and is involved in protein–protein and protein–ligand interactions [28,59,60,61]. The PirABVP toxins have been reported to bind with hepatopancreatic epithelial tissue, possibly via recognition and binding to certain ligands on the cell membrane and receptor that leads to oligomerization and pore formation and subsequent cell death [34,37,59]. Interestingly, Kumar et al. (2019) reported that PirABVP toxins induced focal to extensive necrosis and damage epithelial enterocytes in the midgut and hindgut regions resulting in nuclear pyknosis, cell vacuolisation, mitochondrial and rough endoplasmic reticulum (RER) damage in different degree in gnotobiotic A. franciscana. In fact, as the disease proceeded, the epithelium was severely damaged, and the remaining cellular components were further detached into the lumen and showed signs of degeneration such as pyknotic nuclei and lysed cellular membranes, which leads to the subsequent death of challenged larvae (Figure 4). Furthermore, the study showed that PirABVP toxin affected the digestive process and A. franciscana larvae were unable to digest the supplied food [39].
Figure 4.
PirABVP toxin binds the digestive tract and induces sloughing of epithelial cells in brine shrimp larvae. (A–D) Immunohistochemistry analysis of brine shrimp larvae challenged with PirABVP toxin. (A,B): PirABVP toxin binds to epithelial cells and induces shedding or sloughing of enterocytes in midgut and hindgut digestive tract. (C,D): Necrosis and damage of epithelial cells and intestinal lumen filled with moderately electron dense cells. (1–4) Transmission electron microscopy (TEM) analysis of control and treatment group brine shrimp larvae. (1,2): The digestive tract epithelial enterocytes appeared normal with an intact mitochondrion, nucleus, rough endoplasmic reticulum (RER) and intercellular junctions. (3,4): PirABVP toxin challenge produce focal to extensive necrosis and damages epithelial cells in midgut and hindgut region. The arrowhead in figures represent the cellular changes associated with AHPND in affected brine shrimp [39].
Interestingly, it is important to mention that in some publications it has been proposed that PirBVP toxin alone can induce cell damage and mortality in the host, e.g., mosquito larvae (Aedes aegypti and Aedes albopictus) and shrimp larvae (L. vannamei) [62,63]. The simultaneous occurrence of sloughing and presence of PirBVP toxin in the hepatopancreas provides evidence that PirBVP toxin is enough to cause AHPND infection in shrimp [62]. However, PirAVP and PirBVP toxin mixture was reported to form complex and through receptor binding, oligomerization and pore formation, exhibits a higher toxic effect on experimental animals [19,28,38,53]. Although, PirAVP and PirBVP toxins are directly responsible for shrimp mortality during AHPND [33,59], several other pathogenic extracellular proteins (ECP) are identified in V. parahaemolyticus strains like hemin; enterobactin; vibrioferrin; type I, II and VI secretion system protein; chemotaxis protein (60 kDa); flagellin (40 kDa); metalloproteases (PrtV protein, 62 kDa; VppC protein, 90 kDa and VPM protein, 90 kDa); and serine proteases (VPP1, 43 kDa; VpSP37, 37 kDa and PrtA, 71 kda), which might contribute in toxicity of AHPND-causing bacteria [63,64,65,66,67]. For example, the AHPND pathology induced by 1 µg of crude protein (60% ammonium sulfate) precipitated from AHPND-causing V. parahaemolyticus broth culture, is equivalent to AHPND caused by 10 µg each of pure PirAVP and PirBVP toxins [28,37]. This indicates that ~10 times more recombinant PirAVP and PirBVP toxin is required to achieve the same results in shrimp larvae caused by crude total protein obtained from V. parahaemolyticus AHPND strain. Hence, the AHPND-causing V. parahaemolyticus extracellular proteins apparently contain some other toxins or proteins apart from PirAVP and PirBVP, which aggravate the toxic effect of PirABVP toxins [39].
Since, AHPND-causing PirABVP toxins are released extracellularly, the presence of toxins in the aquatic environment, apart from mediating AHPND and mortality in shrimp (e.g., 20 µg toxin/g shrimp were reported to induce AHPND) [37], may modulate vibriosis, caused by non-AHPND Vibrio species. Vibriosis caused by the opportunistic Vibrio sp. has negative impacts on fish, crustaceans and molluscs [10,11,12,13]. V. harveyi, V. alginolyticus, V. anguillarum, V. splendidus, V. salmonicida, V. vulnificus and non-AHPND V. parahaemolyticus have been found as main causative organisms of Vibriosis [14]. Interestingly, Tran et al. (2020) demonstrated that the presence of PirABVP toxins modulates the virulence of non-AHPND Vibrio spp. in both in vivo and in vitro conditions. The PirABVP toxin interacts synergistically with V. harveyi and V. alginolyticus and aggravating vibriosis in a gnotobiotic A. franciscana model. However, supplementation of PirABVP toxin has significant antagonistic interaction on in vivo virulence of V. campbellii, V. parahaemolyticus, V. proteolyticus and V. anguillarum strain in the same model [16]. One of the factors that might interfere with virulence of Vibrio spp. is the digestive physiology of the host animal [68,69]. The increased virulence might be a result of digestive tract epithelium damage that possibly gives a suitable site of bacterial attachment and further helps in the entry of the pathogen [70]. Kumar et al. (2019) reported that PirABVP toxins bind with epithelial cells in the midgut and hindgut regions of A. franciscana larvae and induce necrosis and damage the cellular structure [32]. Hence, when the Artemia larvae were exposed to PirABVP toxin and Vibrio spp. together, the toxin-induced damage of epithelial cells in the digestive tract of larvae might be giving a portal of entry for pathogens, resulting in a significant synergistic increase in vivo virulence of Vibrio species. Moreover, the cellular and humoral components of immune system present in the digestive tract play important role in preventing the potential binding and invasion of intestinal layer by an incoming pathogen [71]. As reported in sea bass larvae (Dicentrarchus labrax), the gut epithelial enterocytes containing lysosomes mediate intracellular elimination of pathogenic V. anguillarum cells [72,73]. The binding of PirABVP toxin with epithelial cells of the digestive tract, might have induced immunological response in brine shrimp larvae [34,74], which subsequently prevents the attachment and entry of pathogenic bacteria and decreases the in vivo virulence of Vibrio species (and hence the antagonistic effect of PirAB toxin on vibriosis caused by certain Vibrio species/strains). Therefore, it appears that PirABVP toxins will not always aggravate vibriosis. Damage of epithelial cells might lead to synergistic effects while an immunological response might result in antagonism, all strains are dependent.
2.3. Vibrio parahaemolyticus as a Causative Agent of AHPND
V. parahaemolyticus is the predominant species causing AHPND in shrimp [18,45]. V. parahaemolyticus is heterogenous Gram-negative, non-spore forming and comma-shaped bacteria with a polar flagellum or with several flagella. This pathogen is part of the autochthonous microflora of estuarine and coastal environments, as well as fish, bivalves and crustaceans in tropical to temperate zones all over the world [75,76]. Apart from fish and shellfish species (including shrimp and molluscs), this bacterium has been isolated from water, sediment, plankton, and marine mammals [77,78]. Moreover, the level of V. parahaemolyticus in the environment and in various fish and shellfish species may vary depending on environmental and geographical factors. V. parahaemolyticus can thrive in high sodium chloride concentration, ranging from 0.5 to 10% with optimal levels between 1 to 3%, and can grow in moderate temperature (5 to 37 °C) [79].
In shrimp aquaculture, V. parahaemolyticus is an important aquatic pathogen and several strains are capable of causing acute hepatopancreatic necrosis disease (AHPND) and other important disease resulting in significant economic losses [38,44,80]. The V. parahaemolyticus strains implicated in AHPND are unique in carrying a pVA1 plasmid (70 kb) harbouring the virulence genes, PirAVP and PirBVP encoding the binary PirAVP/PirBVP toxins [17]. The pVA1 plasmid are reported to contain 45 open reading frames (ORF) with known functions. These include, five putative transposases, one putative ORF with homology to toxin-antitoxin gene pndA associated with post-segregational killing (PSK) system, operon that encodes proteins (~30% homology) to PirAVP and PirBVP toxins, a cluster of conjugative transfer genes and two plasmid mobilization genes. The PirAB operon has both upstream and downstream transposases, suggesting that the operon can be acquired by lateral gene transfer. The PSK system ensures that only progeny containing the plasmid survive, since the stable PSK mRNA in a plasmid-negative strain will be translated into bactericidal pndA toxin [17].
Since, AHPND-causing pVA1 plasmid reported to contain two plasmid mobilization genes and a group of transfer genes for conjugation [17], the plasmid has been reported to mobilize to other Vibrio strains (V. punensis, V. harveyi, V. owensii, V. campbelli) and even non-Vibrio spp. (Shewanella sp.) [41,43,50,52]. These processes explain the huge possibility of conversion from non-pathogenic to pathogenic AHPND strain that positively enhance the spread of AHPND [42]. In addition, the 70-kb AHPND plasmid present in V. parahaemolyticus strains are not clonal, but genetically diverse, suggesting that the virulent plasmid has been acquired from several genotypes of V. parahaemolyticus by lateral gene transfer [81]. Recently, it has been shown that V. parahaemolyticus harbouring intact pVA1 plasmid and PirABVP genes (tested positive by PCR), did not produce AHPND-causing PirABVP toxins. In addition, the AHPND positive strains failed to exhibit characteristic AHPND histopathological lesions and mortality in shrimps [82]. Hence, the virulence of AHPND-causing V. parahaemolyticus is reported to depend on the production of secreted proteins, PirABVP toxins, and not on the copy number of PirAVP/PirBVP gene [83].
It is also noteworthy to mention that all human pathogenic V. parahaemolyticus strains produce thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH), as the main virulence factors [79]. The V. parahaemolyticus strains possess two sets of type III secretion systems (TTSS); TTSS1 in all strains whereas TTSS2 (α and β, containing tdh and trh genes) only in human pathogenic strains [84]. Moreover, the AHPND-causing V. parahaemolyticus strains studied so far are reported to lack the genes for tdh, trh and TTSS2 [50,63]. For example, Chonsin et al. (2016) investigated the conventional virulence factors of human pathogenic V. parahaemolyticus strains and AHPND-causing V. parahaemolyticus strains. The results showed that none of the AHPND-causing strains possess tdh, trh or TTSS2-related genes of human pathogenic strains [81]. There are two identified types of V. parahaemolyticus AHPND bacteria reported based on geographical variations [85]. The V. parahaemolyticus AHPND-causing strains from Mexico and Central USA are reported to contain a 4243 bp Tn3-like transposon insert at ORF4, that is not present in the Asian isolates (isolated from China, Vietnam and Thailand) [33]. Moreover, the transposon-like insert is unrelated with AHPND aetiology and shows no difference in the virulence between two AHPND isolates, even if it is found on the virulent plasmid [18]. Recently, González-Gómez et al. (2020) analysed nine AHPND isolates of V. parahaemolyticus from Mexico harbouring pVA1 sequences along with 38 previously reported pVA1- harbouring V. parahaemolyticus. The AHPND strain nucleotide sequences were clustered into three phylogenetic clades (Latin American, Malaysian, and Cosmopolitan) through pangenomic and phylogenetic analysis. The results highlight that among Latin American and Asian AHPND strains, the main structural difference is the absence of Tn3 transposon in the Asian strains. In addition, some deletion in the PirAB region were also found in two of the Latin American strains. Interestingly, the study demonstrates that diagnosis of AHPND through PirAB toxin gene detection may be inadequate due to structural variability of these genes, as noticed in different isolates [86].
3. Control and Management of Acute Hepatopancreatic Necrosis Disease (AHPND)—Current Status
The prophylaxis measures to control AHPND mainly focus on pond management (aeration, feeding, etc.) and disinfections before shrimp post-larvae stocking [87]. However, these approaches are not capable to stop the epidemiological situation once AHPND has emerged in a pond or in neighbouring ponds and hence more effective therapeutic measures are urgently needed to control AHPND in shrimps. The conventional approach applied so far in the mitigation or cure of V. parahaemolyticus AHPND strains, such as interrupting feeding or application of antibiotics and disinfectants has had limited success [28]. In addition, due to development of multiple resistance, their usage in the food producing sector is under severe scientific and public scrutiny [88]. For example, the AHPND-causing V. parahaemolyticus strain from Mexico (13-511/A1 and 13-306D/4) were reported to carry tetB gene coding for tetracycline resistance gene [89], and V. campbellii from China was found to carry multiple antibiotic resistance genes [19], hence the application of traditional methods like antibiotics may be ineffective to control the AHPND in shrimp farming system, especially in the long term.
Most of the therapeutic and control measures developed mainly targets AHPND-causing V. parahaemolyticus. However, the presence of AHPND-causing pVA1 plasmid (63–70 kb) encoding the binary toxins named PirAVP and PirBVP in non-Vibrio parahaemolyticus and even on non-Vibrio species has generated concerns since the management measures used to control a particular AHPND causing bacterial strain may not be useful and can generate unwanted economic pressure to farmers. Therefore, the management measures adopted, based on presence or absence of PirABVP toxins in the shrimp and aquaculture system, can be more suitable to control and eradicate AHPND from shrimp culture system.
Shrimp, lacking an adaptive immune system, rely on their cellular and humoral components of innate immune responses to combat the invading pathogens, due to which the development of therapeutic agents that enhances the adaptive immune response, e.g., vaccines against infectious disease in shrimp aquaculture had very limited success [28,85]. Therefore, methods that can boost the host innate immune response and enhance disease resistance against diseases have drawn much interest in recent years [2,90]. The disease caused by bacterial pathogens in shrimp farming systems are generally controlled by using appropriate management strategies, including supplementation of immunostimulants, prebiotics, probiotics or phages, maintaining optimum water quality, stocking density, post-larvae quality, aeration and feed quality and quantity [91,92]. However, since the outbreak of AHPND in China in 2009, the research has mainly focused on epidemiological studies including characterization of AHPND aetiological agents and associated pathological changes from various geographical locations. Hence, there is an urgent need to develop promising new methods that can become a potential tool to protect the shrimp against AHPND-causing V. parahaemolyticus. Moreover, some studies have reported management strategies to control the disease and possibly prevent AHPND outbreak in shrimp aquaculture. Details of potential therapeutic or control agents are summarized below.
3.1. Probiotics
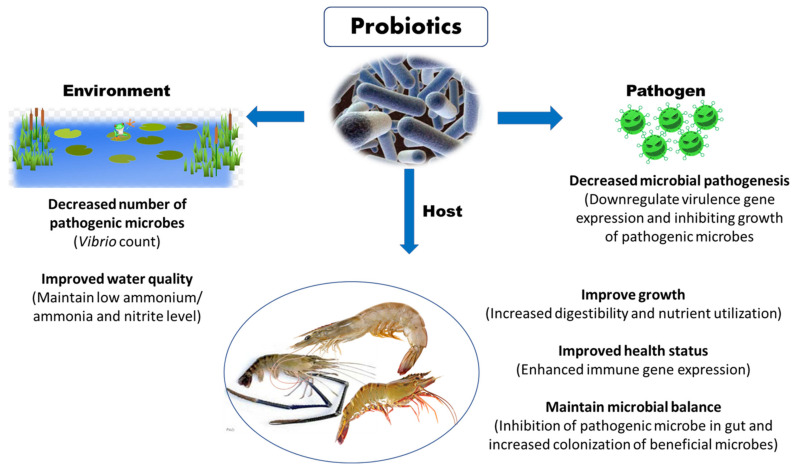
Probiotics have emerged as promising alternatives for improving disease resistance in farmed shrimp against AHPND. The probiotics microbes potentially secrete a wide range of extracellular substances and antimicrobial peptides, which improve feed digestion and absorption, boost shrimp health and immunity, promote shrimp growth and reproduction, and enhance survival upon exposure to pathogenic microorganism (Figure 5) [93]. Moreover, the beneficial effect of probiotic microorganism is generally influenced by several factors related to rearing conditions under larger scale, survival ability until reaching the gastrointestinal tract of the host, method of administration, dosage, probiotic strain and shrimp species [2]. Therefore, before application, attention must be paid in course of selecting an appropriate probiotic strain, since unsuitable strain can negatively impact the colonization, nutrient metabolism and assimilation, growth response, immunomodulation and resistance against pathogenic microorganisms.
Figure 5.
Potential beneficial role of probiotics in shrimp aquaculture.
Maintaining a biological balance among bacteria and algae in aquaculture ponds and gastrointestinal tract of shrimp is one of the ways to reduce the effect of AHPND in shrimp [94]. Probiotics can participate in establishing a balance of gastrointestinal microbial flora, improving the digestive functions and immune system and increase the survival of L. vannamei against the pathogenic V. parahaemolyticus AHPND strain [95,96]. Since the AHPND-causing bacteria were reported to infect and damage hepatopancreas, subsequent studies to investigate the effect of probiotics mediated balanced gastrointestinal microbial flora on AHPND bacteria and hepatopancreas morphology add further understanding on probiotics mechanism of action. Moreover, Kewcharoen and Srisapoome (2019) reported that supplementation of Bacillus subtilis AQAHBS001 strain through the feed resulted in proliferation and colonization of this strain in gastrointestinal tract of shrimp. Additionally, the shrimp postlarvae exhibited enhanced growth performance and immune gene expression and increased disease resistance against V. parahaemolyticus AHPND strain [97]. In another study, Chomwong et al. (2018) found that two lactic acid bacteria (LAB), Lactobacillus plantarum SGLAB01 and Lactococcus lactis SGLAB02 strain activate the proPO system, by significantly increasing haemolymph phenoloxidase (PO) activity, improving the survival of L. vannamei upon challenge with AHPND-causing V. parahaemolyticus [98].
Moreover, several probiotic strains are reported to possess antimicrobial abilities against Vibrio species, especially V. parahaemolyticus, V. harveyi and V. alginolyticus [99]. The probiotic bacteria were reported to produce a wide range of extracellular substances such as trypsin, lipase, amylase and antimicrobial substances (e.g., bacteriocins and hydrogen peroxide), against a variety of bacterial pathogenic factors [100,101]. For instance, Bacillus, Lactobacillus, Rhodopseudomonas and Pseudoalteromonas probiotic strains are reported to inhibit the activity of pathogenic AHPND-causing bacteria by producing inhibitory compounds, one of the mechanisms of action of probiotics [95,96,99]. A total of 19 lactic acid bacteria (LAB), isolated from L. vannamei, were characterized based on morphological, biochemical, sequencing techniques and analysed for their ability to inhibit the AHPND-causing V. parahaemolyticus strain. The results showed that 3 among 19 isolated LAB strains have the highest antagonizing ability against AHPND V. parahaemolyticus strain in vitro, generating inhibition zones ranging from 18 to 20 mm in diameter. In addition, the shrimps fed with LAB supplemented diets displayed significantly higher survival (approximately 80%) upon AHPND V. parahaemolyticus challenge [102]. Recently, Wang et al. (2020) demonstrated that the natural product amicoumacins A purified from the cell-free supernatant of Bacillus subtilis BSXE-1601 strain harboured acillibactin, fengycin, surfactin, bacilysin and subtilosin A, which are responsible for both in vitro and in vivo anti-Vibrio activity against AHPND-causing V. parahaemolyticus strain in L. vannamei [103].
3.2. Phage Therapy
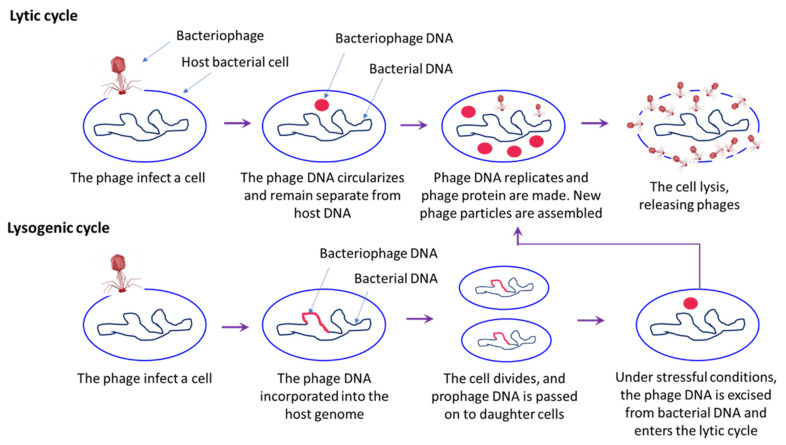
Bacteriophages are viruses, discovered for the first time over 100 years ago in bacterial host by Twort et al. (1915), with dsRNA, ssRNA, dsDNA and ssDNA genome, that can infect prokaryotic organism [104,105]. The bacteriophages are abundant in nature and have been found in both terrestrial and aquatic environment (non-polluted waters, 2 × 108 bacteriophage/mL), and in association with plants and animals [106]. Phages have been proposed as potential management strategy to control infectious disease in both human and animals [107]. The life cycle of bacteriophages includes either a lytic stage (bacteriolytic) or a lysogenic stage (Figure 6). Since, the emergence of bacterial antibiotic resistance problem in animals and humans, the use of phages as a therapeutic agent (shows an effective bacteriolytic activity) is advantageous as it is natural and relatively inexpensive, without serious or irreversible side effects reported to date [107,108,109]. In shrimp aquaculture, the use of phage therapy is well-documented; work is still going on to develop a commercial phage product for shrimp aquaculture. Bacteriophages used in shrimp bacterial pathogens may belong to the family Siphoviridae or Myoviridae [110,111]. In general, the family Siphoviridae member bacteriophages are reported to be lytic phages [112]. For instance, Yang et al. (2020) found that lytic bacteriophages, namely vB_VpS_BA3 and vB_VpS_CA8 (belong to the Siphoviridae family), isolated from sewage were capable of killing the multidrug resistant V. parahaemolyticus and hence its use was suggested as a potential biological control agent [113].
Figure 6.
A schematic overview of the bacteriophage life cycle, including lytic and lysogenic cycle. In lytic cycle, bacteriophages infect the host and release of viral genome into bacterial cells. Once a phage infects a bacterium, it shuts down the defence mechanism and takes over its cellular machinery to synthesis new phage particles. The number of phage particles synthesized eventually reaches a point where they rapture the bacterial cells resulting in release of phage particles into the environment that then infects the new host. In lysogenic cycle, phage DNA is incorporated into the bacterial host genome, where it is passed on to the subsequent generations. Environmental stressors such as starvation or exposure to toxic substances may cause the prophage to excise and enter the lytic cycle.
In a study by Vinod et al. (2006), the bacteriophage treatment was found to improve the survival of giant tiger prawn, Penaeus monodon, larvae and postlarvae against Vibrio-induced luminous bacterial disease [114]. In another study, bacteriophages are reported to control the growth of pathogenic V. harveyi and improve the survival of P. monodon, against luminous bacterial disease [115]. These studies showed that bacteriophages can be promising alternatives strategies for effective shrimp larval health management and disease control.
Until now, there are only few attempts have been made to control AHPND in shrimp using bacteriophages. Jun et al. (2016) studied bacteriolytic activity of phage pVp-1 (family Siphoviridae phage) against AHPND-causing V. parahaemolyticus strains, the infectivity was tested against 22 strains from geographically diverse regions (5 Asian types and 17 Mexican types). The results showed that the pVp-1 phage can infect 90.9% (20 strains among 22 strains) of V. parahaemolyticus AHPND strains and further demonstrates bacteriolytic activity against three strains, known to be highly pathogenic [116]. In another study, Jun et al. (2018) found that following prophylactic and therapeutic treatment, pVp-1 phage-treated shrimps exhibit significant recovery from AHPND histopathological lesions [108]. These results highlight that phage could be suitable for prophylactic and/or therapeutic use against AHPND-causing V. parahaemolyticus.
Overall, these studies suggest that the usage of lytic phages could be a potential approach to combat AHPND-causing V. parahaemolyticus strains. However, considering that the host range for selected phages was 65–70% and the possibility that bacterial strains may develop resistance [78], phage therapy with a consortium of phages would ensure the efficacy against a wide range of bacterial species/strains reported to cause AHPND in shrimp.
3.3. Plant-Derived and/or Natural Compounds
The use of antimicrobial agents in aquaculture could lead to the emergence of resistance in the microorganism. Hence, alternatives are being sought over the last few years and the plant-based compounds are one of the available options for this purpose. Plants are a rich source of bioactive compounds like alkaloids and glycosides and synthesize aromatic compounds mostly phenols or their oxygen substituted derivates that might serve as potential antimicrobial agent to control pathogenic bacterial infection in shrimp aquaculture. For instance, the plant-based products, e.g., essential oils and phenolic compounds have been tested and used as an efficient and alternative treatment against microbial infection in aquaculture [117,118]. The important function of plant-based compounds as antimicrobial includes binding to substrate or metal ions and making them unavailable for microbial pathogens, microbial cell membrane disruption, binding to bacterial cell adhesins or other proteins and inhibiting the binding of bacteria to cell membranes, inactivating the microbial enzymes, blocking the viral cell fusion or adsorption in host cell, etc. Moreover, the natural or plant-based products are preferred because of their biodegradability in the environment, i.e., the residues from plant derived compound treatment tend to be biodegradable in the water whereas, from antibiotics or other chemical treatment. However, the plant-based products (e.g., essential oils) might also have an effect on non-target organism [119,120].
Few studies have reported that natural/plant-based compounds can minimize the effect of pathogen and improves the immune system and survival of shrimp species against the V. parahaemolyticus AHPND strain. The rose myrtle, Rhodomyrtus tomentosa, seed extract shown significantly high antimicrobial activity against AHPND bacteria. In addition, the extract was found to improve the survival of L. vannamei against AHPND-causing V. parahaemolyticus strain [121]. Later, a study was carried out to determine the effect of plant extract, Phyllanthus amarus, against AHPND-causing V. parahaemolyticus strain in white leg shrimp, L. vannamei. The results showed that both dried and fresh extract from P. amarus, exhibited in vivo antibacterial activity against V. parahaemolyticus AHPND strain [122]. In another study, essential oil mixture prepared from 10 plants, i.e., Lavandula latifolia, Pinus sylvestris, Jasminum officinale, Citrus limon, Prunus avium, Viola odorata, Gardenia jasminoides, Cocos nucifera, Rosa damascene and Eucalyptus globulus, were tested for anti-V. parahaemolyticus activity. The essential oil mixture was found to exhibit antimicrobial activity and significantly improve the survival of L. vanaamei against AHPND-causing V. parahaemolyticus strain [121]. Moreover, seaweeds are also reported to display antimicrobial activity against bacterial pathogen and possess several health-benefiting properties. The protein extract used from red seaweed, Gracilaria fisheri, was evaluated for its anti-bacterial activity and protective role against AHPND-causing V. parahaemolyticus strain in white leg shrimp. The results exhibited that protein extract inhibits the growth of virulent V. parahaemolyticus strain. In addition, the G. fisheri protein extract supplementation significantly improved the survival rate of L. vannamei with normalized histological features of hepatopancreas following V. parahaemolyticus AHPND strain infection [123]. Furthermore, it has been demonstrated that microalgal-bacterial consortia containing microalgae Picochlorum strain S1b and bacteria Labrenzia sp. strain 8, Muricauda sp. strain 50, or Arenibacter sp. strain 61, can significantly inhibit the growth of AHPND-causing V. parahaemolyticus strain and increase the survival of L. vannamei [124]. A synthetic herbal-based polyphenol compound, pyrogallol, demonstrated to exert high in vitro bactericidal efficacy including increased killing rate and degenerative effects against AHPND V. parahaemolyticus cells. The study suggests that pyrogallol based antimicrobial agent could be a promising method to control the AHPND in shrimp producing sectors [125]. Although, the above-mentioned studies have documented that plant-derived compounds exhibit a broad spectrum of pharmacological and health promoting effect, the mechanism of action of these compounds in mediating these effects remain a topic of debate. Therefore, further study to understand the underlying mode of action of these compounds in generating protective responses will be helpful to develop a holistic strategy to control AHPND in shrimp.
Immunostimulatory Properties of Plant-Based Compound
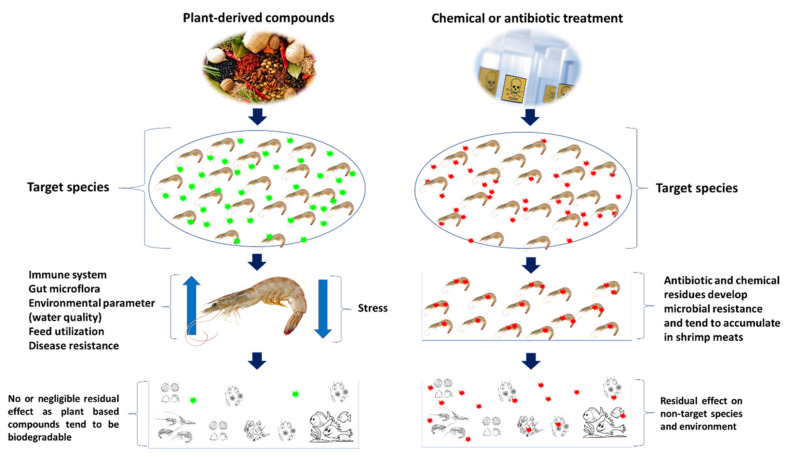
The natural products from medicinal plants and marine seaweeds, are considered as potential alternatives for prevention and treatment of AHPND in shrimp. Apart from antiviral, antibacterial and antiparasitic properties, the plant-based compounds are rich in secondary metabolites and phytochemical compounds that play an important role in feed intake and digestibility and improving growth performance and health of shrimp [126,127]. Plant-derived compounds can be administered as a whole plant or parts (leaf, root or seeds) or extract compound, via water routine or feed additive—either singly or as a combination of extract compounds—or even as a mixture with prebiotics or immunostimulants (Figure 7) [128,129,130].
Figure 7.
Effect of plant based or natural compounds and conventional compounds in shrimp and environment.
Enhancing the immune system of shrimp has gained considerable attention as a potential method that can contribute to protective immunity and help to fight against diseases. The immunostimulatory activity of plant-based compound are contributed in part by phenolics, alkaloids, terpenoids, essential oils, lectins, polypeptides and polyacetylenes (Table 2). There are several reports, which suggest that treatment of crustacean species (like brine shrimp, Macrobrachium spp.) with polyphenols, significantly enhances the innate immune response and provide protection to abiotic (salinity, heat) and biotic (pathogenic bacterial infection) stressors [131,132,133]. In recent years, plant-based compounds are identified to possess the property of inducing heat shock protein within the animal in a non-invasive manner [130,134,135]. These compounds/molecules are also commonly called as heat shock protein inducers (Hspi) [136]. Functionally, the protective function of Hsp70 is documented to be due to its molecular chaperone activity maintaining protein homeostasis by protecting the nascent polypeptides from misfolding, facilitating co- and post-translational folding, assisting in assembly and disassembly of macromolecular complexes and regulating translocation [134]. Additionally, Hsp70 is also reported to confer thermal resistance, protect against osmotic stress, prevent oxidative toxicity and damage and improve tolerance against microbial infection [137,138,139]. These observations suggest that HSP plays important role in host immunity and health. Hence, natural compounds/molecules can be used to induce Hsp70 production in host and provide protection against biotic and abiotic stress.
Table 2.
Role of plant-based compounds in shrimp health.
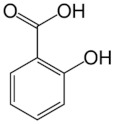
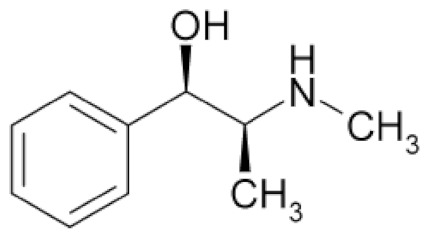
| Class | Chemical Structure | Sub-Class | Example | Role in Aquatic Species |
|---|---|---|---|---|
| Phenolics |
|
Quinones, lavonoids, flavones, tannins, flavonols | Allium spp. (A. cepa, A. sativum, A. tuberosum), Cynodon dactylon, Viscum album, etc. | Immunostimulant, antioxidant, antimicrobial, growth promotor, anti-helminthic, antiviral |
| Alkaloids |
|
Camellia sinensis, Nicotiana tabacum, Aconitum napellus, Atropa belladonna, Conium maculatum, etc. | Immunostimulant, antioxidant, antimicrobial, growth promotor, anti-helminthic, antiviral | |
| Terpenoids and essential oils |
|
Pistacia terebinthus, Lavandula angustifolia, Mentha piperita, Melaleuca alternifolia, etc. | Immunostimulant, antimicrobial, antioxidant, anti-helminthic, growth promotor | |
| Lectins and polypeptides |
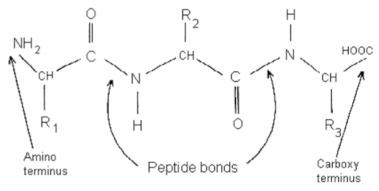
|
Glycine max, Arachis hypogaea, Triticum aestivum, Cocos nucifera, etc. | Antioxidant, antiviral, immunostimulant | |
| Polyacetylenes |
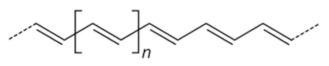
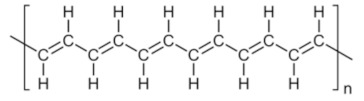
|
Anethum graveolens, Carum, carvi, Daucus carota, etc. | Immunostimulant, antimicrobial, antioxidant |
Recently, it has been demonstrated that polyphenol plant-based compound (phloroglucinol) is a potent in vivo enhancer of Hsp70, and this effect mediates induction of resistance in brine shrimp and Macrobrachium larvae against AHPND-causing V. parahaemolyticus M0904 strain. The ability of polyphenol plant-based compounds to induce resistance in the host and prevent microbial infection has been described to be functionally dependent on antioxidant property, pro-oxidant activity and anti-microbial effects [140,141]. Similarly, the phloroglucinol-induced protective effect in brine shrimp larvae against V. parahaemolyticus were found to be linked to its pro-oxidant activity (e.g., generation of hydrogen peroxide, H2O2). The pro-oxidant action was linked with increased Hsp70 protein production, which stimulate the immune response and induce resistance in brine shrimp and Macrobrachium larvae against AHPND-causing V. parahaemolyticus strain [24,127].
Though, the plant-derived compounds are reported to improve immunity and health of shrimp, some of them are known to carry toxicological properties as well. Few studies indicate that plants used a food source, may have mutagenic or genotoxic potential [142]. The toxicology of plants may originate from chemical compounds originated from either leaf, root or seeds [143]. Hence, before application, the investigation of optimum dose requirements in different species and life stages, mode of application (immersion, feed or injection) and residual effects on non-target species must be carried out in order to achieve a safe treatment with plant products.
3.4. Environmental Manipulation
Aquatic bacteria are often subjected to fluid shear and hydrodynamic forces, created by either natural factors or anthropogenic activities such as the use of aerators and pumping devices frequently used to enhance shrimp productivity [144,145]. Moreover, the microorganisms, including both single-celled and multi-cellular, have evolved to survive in variables and at times extreme conditions and by changing the phenotype it senses and mount effective response to environmental heterogeneity [146,147,148]. Interestingly, V. parahaemolyticus cells are capable of replicating in less than ten minutes, as compared to other Vibrio species takes over one hour [75,149]. Hence, any change in environmental condition might triggers phenotype switching in V. parahaemolyticus that could affect the biological features and induce remodelling of transcription and translational networks requiring to adapt and maintain cellular status. The V. parahaemolyticus cells incubated at constant agitation of 110 rpm (called M0904/110) were demonstrated to develop cellular aggregates or floccules and exhibited significantly higher EPS and biofilm formation (~4 folds). In addition, at M0904/110, the cells produce levan and develop purple colonies. However, cells grown at 120 rpm (called M0904/120) did not produce floccules, had lower EPS and biofilm formation, and produce orange-red colonies. Hence, a critically low shaking frequency might favour the production of self-aggregating biofilm in M0904 like it was described in other species such as Pseudomonas [150]. Furthermore, the study revealed that AHPND-causing V. parahaemolyticus strain, under differential flow conditions (low fluid shear stress) switches to biofilm phenotype causing a major shift in the protein secretome, e.g., alkaline phosphatase PhoX is produced instead of PirAVP/PirBVP toxins [151]. Since, the virulence of AHPND strains is reported to be mediated by the production of PirABVP toxins, the decreased production of virulence related genes including Pir toxins at M0904/110 (biofilm phenotype) results in significantly reduced virulence of AHPND V. parahaemolyticus strain in the host model species, i.e., brine shrimp (A. franciscana) and freshwater prawn (M. rosenbergii) [44]. The study highlights that AHPND V. parahaemolyticus strain has two phenotypic forms (virulent and non-virulent) and shaking condition determines the existence of phenotypic form. Hence, designing methods that can induce phenotype switching in AHPND-causing V. parahaemolyticus in an aquaculture setting will open the possibility for effective management of AHPND in shrimp farming, without necessarily removing the AHPND-causing bacteria from the culture system.
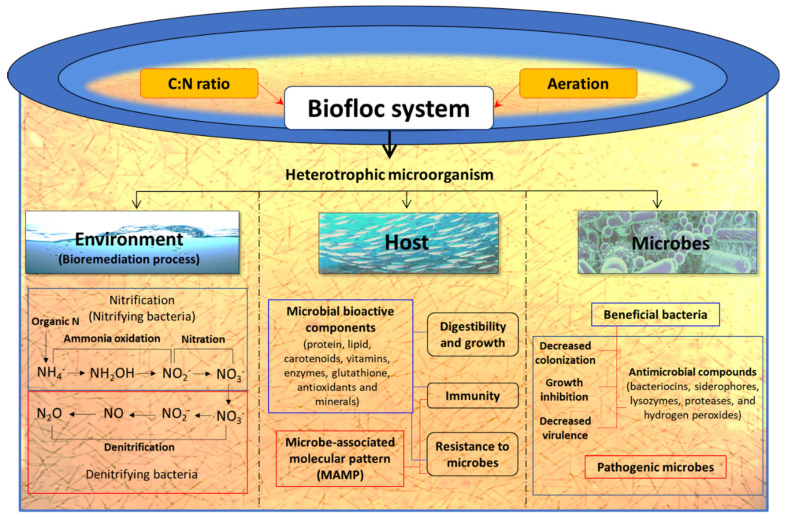
3.4.1. Biofloc Technology
Growing shrimp in a biofloc system can be a promising alternative strategy to improve environmental conditions and health status of cultured animals. The basic principle of the biofloc system is to recycle waste nutrients, in particular, inorganic nitrogen resulting from uneaten feed and faeces into microbial biomass, that can be used in situ by the cultured animals or be harvested and processed into feed ingredients [152,153,154,155]. In fact, the metabolic processes and biochemical transformations take place directly in the water column, which promotes the overall balance of the system and the health of the farmed shrimp [156]. The heterotrophic microbiota is stimulated by steering the C/N ratio of the water through the modification of carbohydrate content in feed or by addition of a carbon source in the water, so that bacteria can assimilate the waste ammonium for new biomass production [155]. Hence, ammonium/ammonia can be maintained at a low and non-toxic concentration so that water replacement is no longer required (Figure 8). For instance, Avnimelech (2007) noted that the use of biofloc in intensive tilapia culture significantly improved the nitrogen recovery from 23% to 43% [153].
Figure 8.
Schematic overview of possible role of biofloc system in host, pathogen and environment in a shrimp aquaculture facility.
The biofloc are rich in free amino acids such as alanine, glutamate, arginine and glycine, which are reported to serve as diet attractants for shrimp [157]. Hence, it is noted that shrimp in biofloc system consume up to 29% flocculating particles of their daily feed intake [158]. Moreover, apart from serving as protein and lipid sources these aggregates flocs can contain microbe-associated molecular pattern (MAMP) and microbially bioactive components such as carotenoids, vitamins, glutathione, antioxidants and minerals, which nutritionally modulate the shrimp health and immune response and result in better growth performance and increased resistance against pathogenic microbial infections (Figure 8) [157,159,160,161]. For instance, in situ utilization of microbial flocs in biofloc system by aquaculture organism as well as the utilization of processed biofloc as a feed ingredient has been reported to improve growth performance and the health of shrimp [161,162,163,164,165,166]. There are few reports that have illustrated the role of biofloc in stimulating the non-specific immunity and resistance of shrimp against microbial pathogens including AHPND V. parahaemolyticus [167,168]. Hostin et al. (2019) designed an experiment to investigate the effect of autotrophic (with or without probiotics) and heterotrophic bioflocs (with or without probiotics) on L. vannamei against AHPND bacterial strain. The results showed that heterotrophic bioflocs (with and without probiotics) and autotrophic bioflocs (with probiotics) can decrease the impact of AHPND-causing V. parahaemolyticus and the highest survival of L. vannamei was observed when challenged in the presence of their respective biofloc suspensions (shrimp grown in biofloc environment but challenged in clear water were not protected). So, the protective effect in shrimp was depending on operational parameters of the biofloc system, namely C/N ratio [91]. Recently, Kumar et al. (2020) demonstrated that the biofloc system regulates the expression of bacterial virulence genes resulting in enhanced survival of L. vannamei upon AHPND-causing V. parahaemolyticus challenge. The study showed that, in the biofloc system, AHPND-causing V. parahaemolyticus possibly switch from free-living virulent planktonic phenotype to a non-virulent biofilm phenotype, as demonstrated by a decreased transcription of flagella-related motility genes (flaA, CheR and fliS), Pir toxin (PirBVP) and AHPND plasmid genes (ORF14) and increased expression of the phenotype switching marker AlkPhoX gene in both in vitro and in vivo conditions [46]. Taken together, the ability of the biofloc system to boost the water quality, growth performance and resistance of L. vannamei against V. parahaemolyticus AHPND strain makes it a potent aquaculture technology that will be valuable to prevent microbial infection including AHPND and increase the shrimp production with high-density and minimal or no water exchange culture.
3.4.2. Pond Management
The above-mentioned management practices including probiotics, phage therapy and plant-derived compounds have shown promising results to control the outbreak of AHPND in shrimp. However, most of these studies are based on laboratory trials and further validation of dose, route of delivery and associated risk factors are still needed to establish the effectiveness in shrimp farm conditions. Moreover, recently Putth and Polchana (2016) demonstrated that by adopting a better farm management practice, shrimp farmers can control AHPND and avoid production losses. The study showed that pre-stocking and post-stocking measures, including evaluation and screening of the health status of post-larvae, feed quality assessment and disinfection of input materials (e.g., sea water) is helpful to control AHPND in shrimp farms [169].
Apart from management measures, the polyculture system has been identified as a potential strategy to control AHPND in shrimp farms. Tran et al. (2014) studied the effect of polyculture system, including tilapia and L. vannamei, in controlling AHPND infection and mortality. The results showed that tilapia induced beneficial algal and bacterial blooms in water, promote healthy and balanced biota communities that confer positive effects in controlling AHPND in shrimps [170]. In another study, Boonyawiwat et al. (2017) evaluated factors related with farm characteristics, farm management, pond and water preparation, feed management, post-larvae and stock management in occurrence of AHPND in shrimp. The results demonstrated that the presence of predator fish, multiple shrimp species or high stocking density in culture system contribute to increased risk of AHPND infections. However, alternative approaches like polyculture, water ageing (≥ 7 days long) and delay in feeding after stocking were likely to promote protection against AHPND in shrimp [171].
4. Conclusions and Future Perspective
Shrimp aquaculture is one of the fastest growing food producing sectors in the world. However, an outbreak of acute hepatopancreatic necrosis disease (AHPND) has caused significant economic losses in the shrimp farming industry since 2009. The disease is caused by a specific virulent strain of bacteria, including V. parahaemolyticus, V. punensis, V. harveyi, V. owensii, V. campbelli and Shewanella sp. that contains pVA1 plasmid (63–70 kb) encoding the binary PirAVP and PirBVP toxins. Interestingly, the AHPND affected shrimp show unique histopathological changes, including massive sloughing of hepatopancreatic epithelial cells without any accompanying signs of a pathogen, which demonstrates the involvement of bacterial secreted binary PirAVP and PirBVP toxins in inducing AHPND. Moreover, recent studies have demonstrated that, apart from PirABVP toxins, the AHPND associated strains have other specific virulence factors that might be involved in virulence of AHPND-causing bacteria and disease pathology. Hence, by using the host-pathogen model, further molecular, microbiological and histopathological studies are still needed for effective characterization of virulence factors of AHPND-causing bacteria and diagnosis of AHPND in shrimp.
In the past, chemical and antibiotics have been commonly used in the shrimp culture system to control bacterial diseases including AHPND. However, the excessive and indiscriminate use of antibiotic has resulted in the development of antibiotic-resistant microbes, which may have potential risks for consumer health globally. Moreover, the above-mentioned management approach discussed in this review, including, probiotics, phage therapy, use of plant-based compounds and environmental manipulation might be applicable in shrimp culture system to control the AHPND (alone or in combination). However, even with these developments, the industry is continuously confronted with the devastating impacts of AHPND. Hence, the quest for alternative methods to control AHPND-causing Vibrio spp. is an important challenge for the sustainable development of shrimp aquaculture.
Acknowledgments
The authors acknowledge the technical assistant provided by Brigitte Van Moffaert and Anita De Haese.
Author Contributions
Conceptualization, V.K. and S.R.; methodology, V.K. and S.R.; writing—original draft preparation, V.K.; writing—review and editing, S.R., B.K.B., P.B. and B.K.D.; visualization, B.K.B., P.B. and B.K.D.; project administration, P.B. and B.K.D.; funding acquisition, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by international doctoral fellowship from the Indian Council of Agricultural Research (ICAR), New Delhi.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
In this paper, first an overview of the current knowledge on acute hepatopancreatic necrosis disease (AHPND) is given, including the disease associated gross signs and histopathology changes. Later, the current status on management/mitigation solutions for AHPND with respect to shrimp aquaculture are summarized.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roy S., Kumar V., Manna R.K., Suresh V.R. Sundarbans mangrove deltaic system—An overview of its biodiversity with special reference to fish diversity. J. Appl. Nat. Sci. 2016;8:1090–1099. doi: 10.31018/jans.v8i2.926. [DOI] [Google Scholar]
- 2.Soltani M., Ghosh K., Hoseinifar S.H., Kumar V., Lymbery A.J., Roy S., Ringø E. Genus bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev. Fish. Sci. Aquac. 2019;27:331–379. doi: 10.1080/23308249.2019.1597010. [DOI] [Google Scholar]
- 3.FAO . State of Fisheries and Aquaculture in the World. Food and Agriculture Organization of the United Nations; Rome, Italy: 2019. [Google Scholar]
- 4.Tacon A.G.J. Trends in Global Aquaculture and Aquafeed Production: 2000–2017. Rev. Fish. Sci. Aquac. 2020;28:43–56. doi: 10.1080/23308249.2019.1649634. [DOI] [Google Scholar]
- 5.FAO . State of Fisheries and Aquaculture in the World. Food and Agriculture Organization of the United Nations; Rome, Italy: 2018. [Google Scholar]
- 6.Sui J., Luan S., Yang G., Xia Z., Luo K., Tang Q., Lu X., Meng X., Kong J. Genetic parameters and selection response for the harvest body weight of the giant freshwater prawn (Macrobrachium rosenbergii) in a breeding program in China. PLoS ONE. 2019;14:e0218379. doi: 10.1371/journal.pone.0218379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson J.L., Valderrama D., Jory D.E. GOAL 2019: Global Shrimp Production Review. GOAL; Portsmouth, NH, USA: 2019. [Google Scholar]
- 8.Roy S. Ph.D. Thesis. University of Ghent; Ghent, Belgium: 2020. Modulating Innate Immune Memory in Brine Shrimp (Artemia franciscana) and in Giant Freshwater Prawn (Macrobrachium rosenbergii) [Google Scholar]
- 9.Verdegem M.C.J. Nutrient discharge from aquaculture operations in function of system design and production environment. Rev. Aquac. 2013;5:158–171. doi: 10.1111/raq.12011. [DOI] [Google Scholar]
- 10.Lee K.K., Liu P.C., Chuang W.H. Pathogenesis of gastroenteritis caused by Vibrio carchariae in cultured marine fish. Mar. Biotechnol. 2002;4:267–277. doi: 10.1007/s10126-002-0018-9. [DOI] [PubMed] [Google Scholar]
- 11.Aguirre-Guzmán G., Ruíz H.M., Ascencio F. A review of extracellular virulence product of Vibrio species important in diseases of cultivated shrimp. Aquac. Res. 2004;35:1395–1404. doi: 10.1111/j.1365-2109.2004.01165.x. [DOI] [Google Scholar]
- 12.Defoirdt T., Boon N., Sorgeloos P., Verstraete W., Bossier P. Alternatives to antibiotics to control bacterial infections: Luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007;25:472–479. doi: 10.1016/j.tibtech.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Kesarcodi-Watson A., Kaspar H., Lategan M.J., Gibson L. Two pathogens of greenshellTM mussel larvae, perna canaliculus: Vibrio splendidus and a V. coralliilyticus/neptunius-like isolate. J. Fish Dis. 2009;32:499–507. doi: 10.1111/j.1365-2761.2009.01006.x. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S., Haldar S. Vibrio Related Diseases in Aquaculture and Development of Rapid and Accurate Identification Methods. J. Mar. Sci. Res. Dev. 2012;s1:1–7. [Google Scholar]
- 15.Kumar V., Roy S. Aquaculture Drugs: Sources, Active Ingredients, Pharmaceutic Preparations and Methods of Administration. J. Aquac. Res. Dev. 2017;8:1–13. doi: 10.4172/2155-9546.1000510. [DOI] [Google Scholar]
- 16.Tran P.T.N., Kumar V., Bossier P. Do acute hepatopancreatic necrosis disease-causing PirABVP toxins aggravate vibriosis? Emerg. Microbes Infect. 2020;9:1919–1932. doi: 10.1080/22221751.2020.1811778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C.-T., Chen I.-T., Yang Y.-T., Ko T.-P., Huang Y.-T., Huang J.-Y., Huang M.-F., Lin S.-J., Chen C.-Y., Lin S.-S., et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran L., Nunan L., Redman R.M., Mohney L.L., Pantoja C.R., Fitzsimmons K., Lightner D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Organ. 2013;105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- 19.Dong X., Bi D., Wang H., Zou P., Xie G., Wan X., Yang Q., Zhu Y., Chen M., Guo C., et al. pirABvp-Bearing Vibrio parahaemolyticus and Vibrio campbellii pathogens isolated from the Same AHPND-affected pond possess highly similar pathogenic plasmids. Front. Microbiol. 2017;8:1859. doi: 10.3389/fmicb.2017.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi M., Stevens A.M., Smith S.A., Taylor D.P., Kuhn D.D. Strain and dose infectivity of Vibrio parahaemolyticus: The causative agent of early mortality syndrome in shrimp. Aquac. Res. 2017;48:3719–3727. doi: 10.1111/are.13197. [DOI] [Google Scholar]
- 21.Kumar R., Ng T.H., Wang H.C. Acute hepatopancreatic necrosis disease in penaeid shrimp. Rev. Aquac. 2020;12:1867–1880. doi: 10.1111/raq.12414. [DOI] [Google Scholar]
- 22.Kumar V. Ph.D. Thesis. University of Ghent; Ghent, Belgium: 2020. Acute Hepatopancreatic Necrosis Disease (AHPND) in Shrimp: Virulence, Pathogenesis and Mitigation Strategies. [Google Scholar]
- 23.Leung T.L.F., Bates A.E. More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J. Appl. Ecol. 2013;50:215–222. doi: 10.1111/1365-2644.12017. [DOI] [Google Scholar]
- 24.Kumar V., Baruah K., Nguyen D.V., Smagghe G., Vossen E., Bossier P. Phloroglucinol mediated Hsp70 production in crustaceans: Protection against Vibrio parahaemolyticus in Artemia franciscana and Macrobrachium rosenbergii. Front. Immunol. 2018;9:1091. doi: 10.3389/fimmu.2018.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinn A.P., Pratoomyot J., Griffiths D., Jiravanichpaisal J., Briggs M. Asian shrimp production and the economic costs of disease. Asian Fish. Sci. J. 2018;31S:29–58. doi: 10.33997/j.afs.2018.31.S1.003. [DOI] [Google Scholar]
- 26.FAO . The State of World Fisheries and Aquaculture. Volume 2014. Food and Agriculture Organization of the United Nations; Rome, Italy: 2014. [Google Scholar]
- 27.Flegel T.W. A future vision for disease control in shrimp aquaculture. J. World Aquac. Soc. 2019;50:249–266. doi: 10.1111/jwas.12589. [DOI] [Google Scholar]
- 28.Hong X., Lu L., Xu D. Progress in research on acute hepatopancreatic necrosis disease (AHPND) Aquac. Int. 2016;24:577–593. doi: 10.1007/s10499-015-9948-x. [DOI] [Google Scholar]
- 29.Roy S., Kumar V., Bossier P., Norouzitallab P., Vanrompay D. Phloroglucinol treatment induces transgenerational epigenetic inherited resistance against Vibrio infections and thermal stress in a brine shrimp (Artemia franciscana) model. Front. Immunol. 2019;10:2745. doi: 10.3389/fimmu.2019.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Defoirdt T., Crab R., Wood T.K., Sorgeloos P., Verstraete W., Bossier P. Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscana from pathogenic Vibrio harveyi, Vibrio campbellii, and Vibrio parahaemolyticus Isolates. Appl. Environ. Microbiol. 2006;72:6419–6423. doi: 10.1128/AEM.00753-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crab R., Lambert A., Defoirdt T., Bossier P., Verstraete W. The application of bioflocs technology to protect brine shrimp (Artemia franciscana) from pathogenic Vibrio harveyi. J. Appl. Microbiol. 2010;109:1643–1649. doi: 10.1111/j.1365-2672.2010.04791.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar V., Bels L.D., Couck L., Baruah K., Bossier P., Van den Broeck W. PirABVP toxin binds to epithelial cells of the digestive tract and produce pathognomonic AHPND lesions in germ-free brine shrimp. Toxins. 2019;11:717. doi: 10.3390/toxins11120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han J.E., Tang K.F.J., Tran L.H., Lightner D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Organ. 2015;113:33–40. doi: 10.3354/dao02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campa-Córdova A.I., León-Gallo A.F., Romero-Maldonado A., Ibarra-Serrano A.C., Rosales-Mendoza S., Hirono I., Angulo C. Recombinant PirA-like toxin protects shrimp against challenge with Vibrio parahaemolyticus, the aetiological agent of acute hepatopancreatic necrosis disease. J. Fish Dis. 2017;40:1725–1729. doi: 10.1111/jfd.12625. [DOI] [PubMed] [Google Scholar]
- 35.Soto-Rodriguez S.A., Gomez-Gil B., Lozano-Olvera R., Betancourt-Lozano M., Morales-Covarrubias M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in northwestern Mexico. Appl. Environ. Microbiol. 2015;81:1689–1699. doi: 10.1128/AEM.03610-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Rodriguez S.A., Gomez-Gil B., Lozano-Olvera R., Bolanmejia C., Aguilar-Rendon K.G., Enciso-Ibarra J. Pathological, genomic and phenotypical characterization of Vibrio parahaemolyticus, causative agent of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Asian Fish. Sci. 2018;31:102–111. doi: 10.33997/j.afs.2018.31.S1.007. [DOI] [Google Scholar]
- 37.Sirikharin R., Taengchaiyaphum S., Sanguanrut P., Chi T.D., Mavichak R., Proespraiwong P., Nuangsaeng B., Thitamadee S., Flegel T.W., Sritunyalucksana K. Characterization and PCR detection of binary, pir-like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE. 2015;10:e0126987. doi: 10.1371/journal.pone.0126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P., Kinch L.N., Ray A., Dalia A.B., Cong Q., Nunan L.M., Camilli A., Grishin N.V., Salomon D., Orth K. Acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus strains maintain an antibacterial type VI secretion system with versatile effector repertoires. Appl. Environ. Microbiol. 2017:83. doi: 10.1128/AEM.00737-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar V., Viet D., Baruah K., Bossier P. Probing the mechanism of VP AHPND extracellular proteins toxicity purified from Vibrio parahaemolyticus AHPND strain in germ-free Artemia test system. Aquaculture. 2019;504:414–419. doi: 10.1016/j.aquaculture.2019.02.029. [DOI] [Google Scholar]
- 40.Dong X., Wang H., Zou P., Chen J., Liu Z., Wang X., Huang J. Complete genome sequence of Vibrio campbellii strain 20130629003S01 isolated from shrimp with acute hepatopancreatic necrosis disease. Gut Pathog. 2017;9:1–5. doi: 10.1186/s13099-017-0180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L., Xiao J., Xia X., Pan Y., Yan S. Draft Genome Sequence of Vibrio owensii Strain SH-14, Which Causes. Genome Announc. 2015;3:3354. doi: 10.1128/genomeA.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Restrepo L., Bayot B., Arciniegas S., Bajaña L., Betancourt I., Panchana F., Muñoz A.R. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-30903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wechprasit P., Panphloi M., Thitamadee S., Sritunyalucksana K., Prachumwat A. Complete Genome Sequence of Shewanella sp. strain TH2012, isolated from shrimp in a cultivation pond exhibiting early mortality syndrome. Microbiol. Resour. Announc. 2019;8:e01703–18. doi: 10.1128/MRA.01703-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar V., Roy S., Baruah K., Van Haver D., Impens F., Bossier P. Environmental conditions steer phenotypic switching in acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus, affecting PirAVP/PirBVP toxins production. Environ. Microbiol. 2020;22:4212–4230. doi: 10.1111/1462-2920.14903. [DOI] [PubMed] [Google Scholar]
- 45.Tran L., Redman R.M., Lightner D.V. EMS/AHPNS: Infectious Disease Caused by Bacteria. Glob. Aquac. Advocate. 2013;20:19–20. [Google Scholar]
- 46.Kumar V., Wille M., Lourenço T.M., Bossier P. Biofloc-based enhanced survival of Litopenaeus vannamei Upon AHPND-causing Vibrio parahaemolyticus challenge is partially mediated by reduced expression of its virulence genes. Front. Microbiol. 2020;11:1270. doi: 10.3389/fmicb.2020.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunan L., Lightner D., Pantoja C., Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis. Aquat. Organ. 2014;111:81–86. doi: 10.3354/dao02776. [DOI] [PubMed] [Google Scholar]
- 48.Dhar A.K., Piamsomboon P., Caro L.F.A., Kanrar S., Adami R., Juan Y.S. First report of acute hepatopancreatic necrosis disease (AHPND) occurring in the USA. Dis. Aquat. Organ. 2019;132:241–247. doi: 10.3354/dao03330. [DOI] [PubMed] [Google Scholar]
- 49.Eshik M.M.E., Abedin M.M., Punom N.J., Begum M.K., Rahman M.S. Molecular identification of AHPND positive Vibrio parahaemolyticus causing an outbreak in south-west shrimp farming regions of Bangladesh. J. Bangladesh Acad. Sci. 2017;41:127–135. doi: 10.3329/jbas.v41i2.35492. [DOI] [Google Scholar]
- 50.Kondo H., Van P.T., Dang L.T. Draft genome sequence of non- Vibrio parahaemolyticus acute diseased shrimp in Vietnam. Genome Announc. 2015;3:2014–2015. doi: 10.1128/genomeA.00978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muthukrishnan S., Defoirdt T., Ina-Salwany M.Y., Yusoff F.M., Shariff M., Ismail S.I., Natrah I. Vibrio parahaemolyticus and Vibrio harveyi causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei (Boone, 1931) isolated from Malaysian shrimp ponds. Aquaculture. 2019;511:734227. doi: 10.1016/j.aquaculture.2019.734227. [DOI] [Google Scholar]
- 52.Prachumwat A., Wechprasit P., Srisala J., Kriangsaksri R., Flegel T.W., Thitamadee S. Shewanella khirikhana sp. nov.—A shrimp pathogen isolated from a cultivation pond exhibiting early mortality syndrome. Microb. Biotechnol. 2020;13:781–795. doi: 10.1111/1751-7915.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ffrench-Constant R.H., Waterfield N., Burland V., Perna N.T., Daborn P.J., Bowen D., Blattner F.R. A genomic sample sequence of the entomopathogenic bacterium Photorhabdus luminescens W14: Potential implications for virulence. Appl. Environ. Microbiol. 2000;66:3310–3329. doi: 10.1128/AEM.66.8.3310-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duchaud E., Rusniok C., Frangeul L., Buchrieser C., Givaudan A., Taourit S., Bocs S., Boursaux-Eude C., Chandler M., Charles J.F., et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- 55.Waterfield N., Kamita S.G., Hammock B.D., Ffrench-Constant R. The Photorhabdus Pir toxins are similar to a developmentally regulated insect protein but show no juvenile hormone esterase activity. FEMS Microbiol. Lett. 2005;245:47–52. doi: 10.1016/j.femsle.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn M.B., Farrar R.R., Novak N.G., Lawrence S.D. Remarkable susceptibility of the diamondback moth (Plutella xylostella) to ingestion of Pir toxins from Photorhabdus luminescens. Entomol. Exp. Appl. 2006;121:31–37. doi: 10.1111/j.1570-8703.2006.00457.x. [DOI] [Google Scholar]
- 57.Ahantarig A., Chantawat N., Waterfield N.R., Ffrench-Constant R., Kittayapong P. PirAB toxin from Photorhabdus asymbiotica as a larvicide against dengue vectors. Appl. Environ. Microbiol. 2009;75:4627–4629. doi: 10.1128/AEM.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo H., Tinwongger S., Proespraiwong P., Mavichak R., Unajak S., Nozaki R., Hirono I. Draft genome sequences of six strains of Vibrio parahaemolyticus isolated from early mortality syndrome/acute hepatopancreatic necrosis disease shrimp in Thailand. Genome Announc. 2014;2:e00221–14. doi: 10.1128/genomeA.00221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin S.J., Hsu K.C., Wang H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp toxins. Mar. Drugs. 2017;15:9. doi: 10.3390/md15010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Almanza-Martínez N., Francisco Martínez Díaz S., Flores-Ramírez G., Zuñiga-Navarrete F., Gómez I., Cardona-Félix C.S. An α-amylase-like protein interacts with PirB toxin from Vibrio parahaemolyticus in digestive tract tissue of white shrimp Litopenaeus vannamei. Aquac. Res. 2020;51:3910–3914. doi: 10.1111/are.14688. [DOI] [Google Scholar]
- 61.De Los Santos M.V., Vibanco-Pérez N., Soto-Rodriguez S., Pereyra A., Zenteno E., Cano-Sánchez P. The B subunit of PirABvp toxin secreted from Vibrio parahaemolyticus causing AHPND is an amino sugar specific lectin. Pathogens. 2020;9:1–15. doi: 10.3390/pathogens9030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai H.C., Ng T.H., Ando M., Te Lee C., Chen I.T., Chuang J.C., Mavichak R., Chang S.H., De Yeh M., Chiang Y.A., et al. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish Shellfish. Immunol. 2015;47:1006–1014. doi: 10.1016/j.fsi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-gil B., Soto-rodríguez S., Lozano R., Betancourt-lozano M. Draft genome sequence of Vibrio parahaemolyticus strain M0605, which causes severe mortalities of shrimps in Mexico. Genome Announc. 2014;2:e00055–14. doi: 10.1128/genomeA.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osei-Adjei G., Huang X., Zhang Y. The extracellular proteases produced by Vibrio parahaemolyticus. World J. Microbiol. Biotechnol. 2018;34:1–7. doi: 10.1007/s11274-018-2453-4. [DOI] [PubMed] [Google Scholar]
- 65.Pérez-Acosta J.A., Martínez-Porchas M., Elizalde-Contreras J.M., Leyva J.M., Ruiz-May E., Gollas-Galván T., Martínez-Córdova L.R., Huerta-Ocampo J.Á. Proteomic profiling of integral membrane proteins associated to pathogenicity in Vibrio parahaemolyticus strains. Microbiol. Immunol. 2018;62:14–23. doi: 10.1111/1348-0421.12556. [DOI] [PubMed] [Google Scholar]
- 66.Phiwsaiya K., Charoensapsri W., Taengphu S., Dong H.T., Sangsuriya P., Nguyen G.T., Pham H.Q., Amparyup P., Sritunyalucksana K., Taengchaiyaphum S., et al. A Natural Vibrio parahaemolyticus ΔpirAVp pirBVp+ Mutant Kills Shrimp but Produces neither PirVp Toxins nor Acute Hepatopancreatic Necrosis Disease Lesions. Appl. Environ. Microbiol. 2017;83:e00680–17. doi: 10.1128/AEM.00680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao J., Liu L., Ke Y., Li X., Liu Y., Pan Y., Yan S., Wang Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci. Rep. 2017;7:1–11. doi: 10.1038/srep42177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srikanth C.V., McCormick B.A. Interactions of the Intestinal Epithelium with the Pathogen and the Indigenous Microbiota: A Three-Way Crosstalk. Interdiscip. Perspect. Infect. Dis. 2008;2008:1–14. doi: 10.1155/2008/626827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almagro-Moreno S., Pruss K., Taylor R.K. Intestinal Colonization Dynamics of Vibrio cholerae. PLoS Pathog. 2015;11:e1004787. doi: 10.1371/journal.ppat.1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quintana-Hayashi M., Padra M., Padra J., Benktander J., Lindén S. Mucus-pathogen interactions in the gastrointestinal tract of farmed animals. Microorganisms. 2018;6:55. doi: 10.3390/microorganisms6020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dharmani P., Srivastava V., Kissoon-Singh V., Chadee K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009;1:123–135. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rekecki A., Ringø E., Olsen R., Myklebust R., Dierckens K., Bergh O., Laureau S., Cornelissen M., Ducatelle R., Decostere A., et al. Luminal uptake of Vibrio (Listonella) anguillarum by shed enterocytes—A novel early defence strategy in larval fish. J. Fish Dis. 2013;36:419–426. doi: 10.1111/jfd.12009. [DOI] [PubMed] [Google Scholar]
- 73.Rekecki A., Gunasekara R.A.Y.S.A., Dierckens K., Laureau S., Boon N., Favoreel H., Cornelissen M., Sorgeloos P., Ducatelle R., Bossier P., et al. Bacterial host interaction of GFP-labelled Vibrio anguillarum HI-610 with gnotobiotic sea bass, Dicentrarchus labrax (L.), larvae. J. Fish Dis. 2012;35:265–273. doi: 10.1111/j.1365-2761.2011.01342.x. [DOI] [PubMed] [Google Scholar]
- 74.Reyes-Becerril M., Maldonado-García M., Guluarte C., León-Gallo A., Rosales-Mendoza S., Ascencio F., Hirono I., Angulo C. Evaluation of ToxA and Vibrio parahaemolyticus lysate on humoral immune response and immune-related genes in Pacific red snapper. Fish Shellfish Immunol. 2016;56:310–321. doi: 10.1016/j.fsi.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Makino K., Oshima K., Kurokawa K., Yokoyama K., Uda T., Tagomori K., Iijima Y., Najima M., Nakano M., Yamashita A., et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V. cholerae. Lancet. 2003;361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 76.Thompson F., Iida T., Swings J. Biodiversity of Vibrios. Microbiol. Mol. Biol. Rev. 2004;68:403–431. doi: 10.1128/MMBR.68.3.403-431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karunasagar I., Karunasagar I., Raghunath P. Ecology, Virulence, and Detection of Pathogenic and Pandemic Vibrio parahaemolyticus. Front. Microbiol. 2016;7:156. doi: 10.3389/fmicb.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karunasagar I., Karunasagar I. Ecology, virulence factors and global spread of vibrio parahaemolyticus. Asian Fish. Sci. 2018;31:15–28. doi: 10.33997/j.afs.2018.31.S1.002. [DOI] [Google Scholar]
- 79.Beuchat L.R. Environmental Factors Affecting Survival and Growth of Vibrio parahaemolyticus. A Review. J. Milk Food Technol. 1975;38:476–480. doi: 10.4315/0022-2747-38.8.476. [DOI] [Google Scholar]
- 80.Jayasree L., Janakiram P., Madhavi R. Characterization of Vibrio spp. associated with diseased shrimp from culture ponds of Andhra Pradesh (India) J. World Aquac. Soc. 2006;37:523–532. doi: 10.1111/j.1749-7345.2006.00066.x. [DOI] [Google Scholar]
- 81.Chonsin K., Matsuda S., Theethakaew C., Kodama T., Junjhon J., Suzuki Y., Suthienkul O., Iida T. Genetic diversity of Vibrio parahaemolyticus strains isolated from farmed pacific white shrimp and ambient pond water affected by acute hepatopancreatic necrosis disease outbreak in Thailand. FEMS Microbiol. Lett. 2015;363 doi: 10.1093/femsle/fnv222. [DOI] [PubMed] [Google Scholar]
- 82.Vicente A., Taengphu S., Hung A.L., Mora C.M., Dong H.T., Senapin S. Detection of Vibrio campbellii and V. parahaemolyticus carrying full-length pirABVp but only V. campbellii produces PirVp toxins. Aquaculture. 2020;519:734708. doi: 10.1016/j.aquaculture.2019.734708. [DOI] [Google Scholar]
- 83.Tinwongger S., Nochiri Y., Thawonsuwan J., Nozaki R., Kondo H., Awasthi S.P., Hinenoya A., Yamasaki S., Hirono I. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. J. Appl. Microbiol. 2016;121:1755–1765. doi: 10.1111/jam.13256. [DOI] [PubMed] [Google Scholar]
- 84.Okada N., Iida T., Park K.S., Goto N., Yasunaga T., Hiyoshi H., Matsuda S., Kodama T., Honda T. Identification and characterization of a novel type III secretion system in trh-positive Vibrio parahaemolyticus strain TH3996 reveal genetic lineage and diversity of pathogenic machinery beyond the species level. Infect. Immun. 2009;77:904–913. doi: 10.1128/IAI.01184-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santos H.M., Tsai C.Y., Maquiling K.R.A., Tayo L.L., Mariatulqabtiah A.R., Lee C.W., Chuang K.P. Diagnosis and potential treatments for acute hepatopancreatic necrosis disease (AHPND): A review. Aquac. Int. 2020;28:169–185. doi: 10.1007/s10499-019-00451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.González-Gómez J.P., Soto-Rodriguez S., López-Cuevas O., Castro-del Campo N., Chaidez C., Gomez-Gil B. Phylogenomic Analysis Supports Two Possible Origins for Latin American Strains of Vibrio parahaemolyticus Associated with Acute Hepatopancreatic Necrosis Disease (AHPND) Curr. Microbiol. 2020;77:3851–3860. doi: 10.1007/s00284-020-02214-w. [DOI] [PubMed] [Google Scholar]
- 87.De Schryver P., Defoirdt T., Sorgeloos P. Early mortality syndrome outbreaks: A microbial management issue in shrimp farming? PLoS Pathog. 2014;10:10–11. doi: 10.1371/journal.ppat.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith P. Antimicrobial Resistance: The Use of Antimicrobials in the livestock sector. Rev. Sci. Tech. 2008;27:243–264. doi: 10.20506/rst.27.1.1799. [DOI] [PubMed] [Google Scholar]
- 89.Han J.E., Mohney L.L., Tang K.F.J., Pantoja C.R., Lightner D.V. Plasmid mediated tetracycline resistance of Vibrio parahaemolyticus associated with acute hepatopancreatic necrosis disease (AHPND) in shrimps. Aquac. Rep. 2015;2:17–21. doi: 10.1016/j.aqrep.2015.04.003. [DOI] [Google Scholar]
- 90.Roy S., Bossier P., Norouzitallab P., Vanrompay D. Trained immunity and perspectives for shrimp aquaculture. Rev. Aquac. 2020;12:2351–2370. doi: 10.1111/raq.12438. [DOI] [Google Scholar]
- 91.Hostin B., Wasielesky W., Decamp O., Bossier P., De Schryver P. Managing input C/N ratio to reduce the risk of Acute Hepatopancreatic Necrosis Disease (AHPND) outbreaks in biofloc systems—A laboratory study. Aquaculture. 2019;508:60–65. doi: 10.1016/j.aquaculture.2019.04.055. [DOI] [Google Scholar]
- 92.Defoirdt T. Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Rev. Aquac. 2014;6:100–114. doi: 10.1111/raq.12030. [DOI] [Google Scholar]
- 93.Kumar V., Roy S., Meena D.K., Sarkar U.K. Application of probiotics in shrimp aquaculture: Importance, mechanisms of action, and methods of administration. Rev. Fish. Sci. Aquac. 2016;24:342–368. doi: 10.1080/23308249.2016.1193841. [DOI] [Google Scholar]
- 94.Aguilera-Rivera D., Prieto-Davó A., Escalante K., Chávez C., Cuzon G., Gaxiola G. Probiotic effect of FLOC on Vibrios in the pacific white shrimp Litopenaeus vannamei. Aquaculture. 2014;424–425:215–219. doi: 10.1016/j.aquaculture.2014.01.008. [DOI] [Google Scholar]
- 95.Wang H., Wang C., Tang Y., Sun B., Huang J., Song X. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture. 2018;494:30–36. doi: 10.1016/j.aquaculture.2018.05.020. [DOI] [Google Scholar]
- 96.Pinoargote G., Flores G., Cooper K., Ravishankar S. Effects on survival and bacterial community composition of the aquaculture water and gastrointestinal tract of shrimp (Litopenaeus vannamei) exposed to probiotic treatments after an induced infection of acute hepatopancreatic necrosis disease. Aquac. Res. 2018;49:3270–3288. doi: 10.1111/are.13791. [DOI] [Google Scholar]
- 97.Kewcharoen W., Srisapoome P. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains) Fish Shellfish. Immunol. 2019;94:175–189. doi: 10.1016/j.fsi.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 98.Chomwong S., Charoensapsri W., Amparyup P., Tassanakajon A. Two host gut-derived lactic acid bacteria activate the proPO system and increase resistance to an AHPND-causing strain of Vibrio parahaemolyticus in the shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2018;89:54–65. doi: 10.1016/j.dci.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Lim S.Y., Loo K.W., Wong W.L. Synergistic antimicrobial effect of a seaweed-probiotic blend against acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Probiotics Antimicrob. Proteins. 2019;12:906–917. doi: 10.1007/s12602-019-09616-8. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y.B. Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture. 2007;269:259–264. doi: 10.1016/j.aquaculture.2007.05.035. [DOI] [Google Scholar]
- 101.Loh J. The role of probiotics and their mechanisms of action: An aquaculture perspective. World Aquac. 2017;48:19–23. [Google Scholar]
- 102.Nguyen Thi Truc L., Trinh Ngoc A., Tran Thi Hong T., Nguyen Thanh T., Huynh Kim H., Pham Kim L., Huynh Truong G., Truong Quoc P., Nguyen Thi Ngoc T. Selection of Lactic Acid Bacteria (LAB) Antagonizing Vibrio parahaemolyticus: The Pathogen of Acute Hepatopancreatic Necrosis Disease (AHPND) in Whiteleg Shrimp (Penaeus vannamei) Biology. 2019;8:91. doi: 10.3390/biology8040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang D., Li J., Zhu G., Zhao K., Jiang W., Li H., Wang W., Kumar V., Dong S., Zhu W., et al. Mechanism of the Potential therapeutic candidate Bacillus subtilis BSXE-1601 against shrimp pathogenic vibrios and multifunctional metabolites biosynthetic capability of the strain as predicted by genome analysis. Front. Microbiol. 2020;11:581802. doi: 10.3389/fmicb.2020.581802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Twort F.W. An Investigation on the Nature of Ultra-Microscopic Viruses. Lancet. 1915;186:1241–1243. doi: 10.1016/S0140-6736(01)20383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clark J.R., March J.B. Bacteriophages and biotechnology: Vaccines, gene therapy and antibacterials. Trends Biotechnol. 2006;24:212–218. doi: 10.1016/j.tibtech.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 106.Abedon S.T., Kuhl S.J., Blasdel B.G., Kutter E.M. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gutiérrez D., Martínez B., Rodríguez A., García P. Isolation and characterization of bacteriophages infecting Staphylococcus epidermidis. Curr. Microbiol. 2010;61:601–608. doi: 10.1007/s00284-010-9659-5. [DOI] [PubMed] [Google Scholar]
- 108.Jun J.W., Han J.E., Giri S.S., Tang K.F.J., Zhou X., Aranguren L.F., Kim H.J., Yun S., Chi C., Kim S.G., et al. Phage Application for the protection from acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2018;58:114–117. doi: 10.1007/s12088-017-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Angulo C., Loera-Muro A., Trujillo E., Luna-González A. Control of AHPND by phages: A promising biotechnological approach. Rev. Aquac. 2019;11:989–1004. doi: 10.1111/raq.12275. [DOI] [Google Scholar]
- 110.Oakey H.J., Owens L. A new bacteriophage, VHML, isolated from a toxin-producing strain of Vibrio harveyi in tropical Australia. J. Appl. Microbiol. 2000;89:702–709. doi: 10.1046/j.1365-2672.2000.01169.x. [DOI] [PubMed] [Google Scholar]
- 111.Shivu M.M., Rajeeva B.C., Girisha S.K., Karunasagar I., Krohne G., Karunasagar I. Molecular characterization of Vibrio harveyi bacteriophages isolated from aquaculture environments along the coast of India. Environ. Microbiol. 2007;9:322–331. doi: 10.1111/j.1462-2920.2006.01140.x. [DOI] [PubMed] [Google Scholar]
- 112.Crothers-Stomps C., Høj L., Bourne D.G., Hall M.R., Owens L. Isolation of lytic bacteriophage against Vibrio harveyi. J. Appl. Microbiol. 2010;108:1744–1750. doi: 10.1111/j.1365-2672.2009.04578.x. [DOI] [PubMed] [Google Scholar]
- 113.Yang M., Liang Y., Huang S., Zhang J., Wang J. Isolation and Characterization of the Novel Phages vB_VpS_BA3 and vB_VpS_CA8 for Lysing Vibrio parahaemolyticus. Front. Microbiol. 2020;11:259. doi: 10.3389/fmicb.2020.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vinod M.G., Shivu M.M., Umesha K.R., Rajeeva B.C., Krohne G., Karunasagar I., Karunasagar I. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture. 2006;255:117–124. doi: 10.1016/j.aquaculture.2005.12.003. [DOI] [Google Scholar]
- 115.Karunasagar I., Shivu M.M., Girisha S.K., Krohne G., Karunasagar I. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture. 2007;268:288–292. doi: 10.1016/j.aquaculture.2007.04.049. [DOI] [Google Scholar]
- 116.Jun J.W., Han J.E., Tang K.F.J., Lightner D.V., Kim J., Seo S.W., Park S.C. Potential application of bacteriophage pVp-1: Agent combating Vibrio parahaemolyticus strains associated with acute hepatopancreatic necrosis disease (AHPND) in shrimp. Aquaculture. 2016;457:100–103. doi: 10.1016/j.aquaculture.2016.02.018. [DOI] [Google Scholar]
- 117.Citarasu T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010;18:403–414. doi: 10.1007/s10499-009-9253-7. [DOI] [Google Scholar]
- 118.Wunderlich A.C., Zica É.D.O.P., dos Santos Ayres V.F., Guimarães A.C., Takeara R. Plant-Derived Compounds as an Alternative Treatment Against Parasites in Fish Farming: A Review. IntechOpen; London, UK: 2016. pp. 115–135. [Google Scholar]
- 119.Ramudu K.R., Dash G. A review on herbal drugs against harmful pathogens in aquaculture. Am. J. Drug Discov. Dev. 2013;3:209–219. doi: 10.3923/ajdd.2013.209.219. [DOI] [Google Scholar]
- 120.Sivasankar P., Santhiya A.V., Kanaga V. A review on plants and herbal extracts against viral diseases in aquaculture. J. Med. Plants Stud. 2015;3:75–79. [Google Scholar]
- 121.Jha R.K., Babikian H.Y., Babikian H.Y., Khoa L.V., Wisoyo D., Srisombat S., Jiaravanon B. Efficacy of natural herbal formulation against acute hepatopancreatic necrosis disease (AHPND) causing Vibrio parahaemolyticus in Penaeus vannamei. Vet. Med. Open J. 2017;2:1–6. doi: 10.17140/VMOJ-2-109. [DOI] [Google Scholar]
- 122.Phuong T.V., Hai Yen P.T., Linh N.Q. Antibacterial activity of extracts from dried and fresh herbal plant (Phyllanthus amarus) against pathogens causing acute hepatopancreatic necrosis disease (ahpnd) in white leg shrimp (Litopenaeus vannamei) at Thua Thien Hue province, Vietnam. Asploro J. Biomed. Clin. Case Rep. 2019;2:120–128. [Google Scholar]
- 123.Boonsri N., Rudtanatip T., Withyachumnarnkul B., Wongprasert K. Protein extract from red seaweed Gracilaria fisheri prevents acute hepatopancreatic necrosis disease (AHPND) infection in shrimp. J. Appl. Phycol. 2017;29:1597–1608. doi: 10.1007/s10811-016-0969-2. [DOI] [Google Scholar]
- 124.Chang Y.H., Kuo W.C., Wang H.C., Chen Y.M. Biocontrol of acute hepatopancreatic necrosis disease (AHPND) in shrimp using a microalgal-bacterial consortium. Aquaculture. 2020;521:734990. doi: 10.1016/j.aquaculture.2020.734990. [DOI] [Google Scholar]
- 125.Tinh T.H., Elayaraja S., Mabrok M., Gallantiswara P.C.D., Vuddhakul V., Rodkhum C. Antibacterial spectrum of synthetic herbal-based polyphenols against Vibrio parahaemolyticus isolated from diseased Pacific whiteleg shrimp (Penaeus vannamei) in Thailand. Aquaculture. 2020;533:736070. doi: 10.1016/j.aquaculture.2020.736070. [DOI] [Google Scholar]
- 126.Kumar V., Bossier P. Importance of plant-derived compounds and/or natural products in aquaculture. Aquafeed. 2018;10:28–31. [Google Scholar]
- 127.Kumar V., Bossier P. Novel plant-based compounds could be useful in protecting shrimp species against AHPND Vibrio parahaemolyticus. J. Inl. Fish. Soc. India. 2019;51:03–05. [Google Scholar]
- 128.Dong H., Zheng X., Kumar V., Roy S., Duan Y., Gao H., Zhang J. Dietary supplementation of teprenone potentiates thermal and hypoxia tolerance as well as cellular stress protection of Epinephelus coioides juveniles reared under multiple stressors. Aquaculture. 2020;514:734413. doi: 10.1016/j.aquaculture.2019.734413. [DOI] [Google Scholar]
- 129.Kumar V., Kumar S., Pandey P.K., Raman R.P., Prasad K.P., Roy S., Kumar A., Kumar K. Growth and Hemato-Immunological Response to Dietary i-Carrageenan in Labeo rohita (Hamilton, 1822) Juveniles. Isr. J. Aquac. 2014;66:20742. [Google Scholar]
- 130.Dong H., Roy S., Zheng X., Kumar V., Das B.K., Duan Y., Sun Y., Zhang J. Dietary teprenone enhances non-specific immunity, antioxidative response and resistance to hypoxia induced oxidative stress in Lateolabrax maculatus. Aquaculture. 2020;533:736126. doi: 10.1016/j.aquaculture.2020.736126. [DOI] [Google Scholar]
- 131.Baruah K., Norouzitallab P., Linayati L., Sorgeloos P., Bossier P. Reactive oxygen species generated by a heat shock protein (Hsp) inducing product contributes to Hsp70 production and Hsp70-mediated protective immunity in Artemia franciscana against pathogenic vibrios. Dev. Comp. Immunol. 2014;46:470–479. doi: 10.1016/j.dci.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 132.Baruah K., Huy T.T., Norouzitallab P., Niu Y., Gupta S.K., De Schryver P., Bossier P. Probing the protective mechanism of poly-ß-hydroxybutyrate against vibriosis by using gnotobiotic Artemia franciscana and Vibrio campbellii as host-pathogen model. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep09427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baruah K., Norouzitallab P., Roberts R.J., Sorgeloos P., Bossier P. A novel heat-shock protein inducer triggers heat shock protein 70 production and protects Artemia franciscana nauplii against abiotic stressors. Aquaculture. 2012;334:152–158. doi: 10.1016/j.aquaculture.2011.12.015. [DOI] [Google Scholar]
- 134.Niu Y., Norouzitallab P., Baruah K., Dong S., Bossier P. A plant-based heat shock protein inducing compound modulates host-pathogen interactions between Artemia franciscana and Vibrio campbellii. Aquaculture. 2014;430:120–127. doi: 10.1016/j.aquaculture.2014.04.001. [DOI] [Google Scholar]
- 135.Baruah K., Phong H.P.P.D., Norouzitallab P., Defoirdt T., Bossier P. The gnotobiotic brine shrimp (Artemia franciscana) model system reveals that the phenolic compound pyrogallol protects against infection through its prooxidant activity. Free Radic. Biol. Med. 2015;89:593–601. doi: 10.1016/j.freeradbiomed.2015.10.397. [DOI] [PubMed] [Google Scholar]
- 136.Baruah K., Norouzitallab P., Phong H.P.P.D., Smagghe G., Bossier P. Enhanced resistance against Vibrio harveyi infection by carvacrol and its association with the induction of heat shock protein 72 in gnotobiotic Artemia franciscana. Cell Stress Chaperones. 2017;22:377–387. doi: 10.1007/s12192-017-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lindquist S. The heat-shock response. Annu. Rev. Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 138.Richter K., Haslbeck M., Buchner J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 139.Roberts R.J., Agius C., Saliba C., Bossier P., Sung Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010;33:789–801. doi: 10.1111/j.1365-2761.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 140.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 141.Ozcan T., Akpinar-Bayizit A., Yilmaz-Ersan L., Delikanli B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014;5:393–396. doi: 10.7763/IJCEA.2014.V5.416. [DOI] [Google Scholar]
- 142.Çelik T.A., Aslantürk Ö.S. Cytotoxic and genotoxic effects of Lavandula stoechas aqueous extracts. Biologia. 2007;62:292–296. doi: 10.2478/s11756-007-0051-2. [DOI] [Google Scholar]
- 143.Hamidi R.M., Jovanova B., Kadifkova Panovska T. Toxicological evaluation of the plant products using brine shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014;60:9–18. doi: 10.33320/maced.pharm.bull.2014.60.01.002. [DOI] [Google Scholar]
- 144.Sultana T., Haque M., Salam M., Alam M. Effect of aeration on growth and production of fish in intensive aquaculture system in earthen ponds. J. Bangladesh Agric. Univ. 2017;15:113–122. doi: 10.3329/jbau.v15i1.33536. [DOI] [Google Scholar]
- 145.Welker T.L., Overturf K., Abernathy J. Effect of aeration and oxygenation on growth and survival of rainbow trout in a commercial serial-pass, flow-through raceway system. Aquac. Rep. 2019;14:100194. doi: 10.1016/j.aqrep.2019.100194. [DOI] [Google Scholar]
- 146.Guo M., Gross C.A. Stress induced remodeling of the bacterial proteome. Curr. Biol. 2014;24:R424–R434. doi: 10.1016/j.cub.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fonseca A.P., Sousa J.C. Effect of shear stress on growth, adhesion and biofilm formation of Pseudomonas aeruginosa with antibiotic-induced morphological changes. Int. J. Antimicrob. Agents. 2007;30:236–241. doi: 10.1016/j.ijantimicag.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 148.Kent A.G., Garcia C.A., Martiny A.C., Biology E. Increased biofilm formation due to high temperature adaptation in marine Roseobacter. Nat. Microbiol. 2018;3:989–995. doi: 10.1038/s41564-018-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cui Y., Yang C., Qiu H., Wang H., Yang R., Falush D. The landscape of coadaptation in Vibrio parahaemolyticus. bioRxiv. 2019;9:373936. doi: 10.7554/eLife.54136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Franken J., Brandt B.A., Tai S.L., Bauer F.F. Biosynthesis of Levan, a Bacterial Extracellular Polysaccharide, in the Yeast Saccharomyces cerevisiae. PLoS ONE. 2013;8:e77499. doi: 10.1371/journal.pone.0077499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar V., Baruah K., Bossier P. Bamboo powder protects gnotobiotically-grown brine shrimp against AHPND-causing Vibrio parahaemolyticus strains by cessation of PirABVP toxin secretion. Aquaculture. 2021;5539:736624. doi: 10.1016/j.aquaculture.2021.736624. [DOI] [Google Scholar]
- 152.Ekasari J., Hanif M., Surawidjaja E.H., Nuryati S., De Schryver P., Bossier P. Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish Immunol. 2014;41:332–339. doi: 10.1016/j.fsi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 153.Avnimelech Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture. 1999;176:227–235. doi: 10.1016/S0044-8486(99)00085-X. [DOI] [Google Scholar]
- 154.Crab R., Defoirdt T., Bossier P., Verstraete W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture. 2012;356–357:351–356. doi: 10.1016/j.aquaculture.2012.04.046. [DOI] [Google Scholar]
- 155.Kumar V.S., Pandey P.K., Anand T., Bhuvaneswari G.R., Dhinakaran A., Kumar S. Biofloc improves water, effluent quality and growth parameters of Penaeus vannamei in an intensive culture system. J. Environ. Manag. 2018;215:206–215. doi: 10.1016/j.jenvman.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 156.Bossier P., Ekasari J. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol. 2017;10:1012–1016. doi: 10.1111/1751-7915.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ju Z.Y., Forster I., Conquest L., Dominy W. Enhanced growth effects on shrimp (Litopenaeus vannamei) from inclusion of whole shrimp floc or floc fractions to a formulated diet. Aquac. Nutr. 2008;14:533–543. doi: 10.1111/j.1365-2095.2007.00559.x. [DOI] [Google Scholar]
- 158.Burford M.A., Thompson P.J., Bauman H., Pearson D.C. Microbial communities affect water quality, shrimp performance at Belize Aquaculture. Glob. Aquac. Advocate. 2003;6:64–65. [Google Scholar]
- 159.Wasielesky W., Atwood H., Stokes A., Browdy C.L. Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture. 2006;258:396–403. doi: 10.1016/j.aquaculture.2006.04.030. [DOI] [Google Scholar]
- 160.Trichet V.V. Nutrition and immunity: An update. Aquac. Res. 2010;41:356–372. doi: 10.1111/j.1365-2109.2009.02374.x. [DOI] [Google Scholar]
- 161.Cardona E., Saulnier D., Lorgeoux B., Chim L., Gueguen Y. Rearing effect of biofloc on antioxidant and antimicrobial transcriptional response in Litopenaeus stylirostris shrimp facing an experimental sub-lethal hydrogen peroxide stress. Fish Shellfish Immunol. 2015;45:933–939. doi: 10.1016/j.fsi.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 162.Kuhn D.D., Boardman G.D., Lawrence A.L., Marsh L., Flick G.J. Microbial floc meal as a replacement ingredient for fish meal and soybean protein in shrimp feed. Aquaculture. 2009;296:51–57. doi: 10.1016/j.aquaculture.2009.07.025. [DOI] [Google Scholar]
- 163.Anand P.S.S., Kumar S., Kohli M.P.S., Sundaray J.K., Sinha A., Pailan G.H., Roy S. Dietary biofloc supplementation in black tiger shrimp, Penaeus monodon: Effects on immunity, antioxidant and metabolic enzyme activities. Aquac. Res. 2017;48:4512–4523. doi: 10.1111/are.13276. [DOI] [Google Scholar]
- 164.Cohen J.M., Samocha T.M., Fox J.M., Gandy R.L., Lawrence A.L. Characterization of water quality factors during intensive raceway production of juvenile Litopenaeus vannamei using limited discharge and biosecure management tools. Aquac. Eng. 2005;32:425–442. doi: 10.1016/j.aquaeng.2004.09.005. [DOI] [Google Scholar]
- 165.Azim M.E., Little D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus) Aquaculture. 2008;283:29–35. doi: 10.1016/j.aquaculture.2008.06.036. [DOI] [Google Scholar]
- 166.Lee C., Kim S., Lim S., Lee K. Supplemental effects of biofloc powder on growth performance, innate immunity, and disease resistance of Pacific white shrimp Litopenaeus vannamei. Fish. Aquat. Sci. 2017;20:1–7. doi: 10.1186/s41240-017-0059-7. [DOI] [Google Scholar]
- 167.Shinn A.P., Jiravanichpaisal J., Grifffiths D., Pokharatsiri A., Burana P., Sumon T., Tongmee C., Decamp O., Galli L. Effect of Biofloc on the Survival of Whiteleg Shrimp, Penaeus vannamei Boone 1931, When challenged with a pathogenic strain of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease. Asian Fish. Sci. 2018;31:210–225. [Google Scholar]
- 168.Sajali U.S.B.A., Atkinson N.L., Desbois A.P., Little D.C., Murray F.J., Shinn A.P. Prophylactic properties of bio floc- or Nile tilapia-conditioned water against Vibrio parahaemolyticus infection of whiteleg shrimp (Penaeus vannamei) Aquaculture. 2019;498:496–502. doi: 10.1016/j.aquaculture.2018.09.002. [DOI] [Google Scholar]
- 169.Putth S., Polchana J. Current status and impact of early mortality syndrome (EMS)/acute hepatopancreatic necrosis disease (AHPND) and hepatopancreatic microsporidiosis (HPM) outbreaks on Thailand s shrimp farming. Southeast Asian Fish. Dev. 2016:79–87. [Google Scholar]
- 170.Tran L.H., Ftizsimmons K.M., Lightner D.V. Tilapia could enhance water conditions, help control EMS in shrimp ponds. Glob. Aquac. Advocate. 2014;40:11–12. [Google Scholar]
- 171.Boonyawiwat V., Patanasatienkul T., Kasornchandra J., Poolkhet C., Yaemkasem S., Hammell L., Davidson J. Impact of farm management on expression of early mortality syndrome/acute hepatopancreatic necrosis disease (EMS/AHPND) on penaeid shrimp farms in Thailand. J. Fish Dis. 2017;40:649–659. doi: 10.1111/jfd.12545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.