Abstract
p38 mitogen-activated protein kinase (p38) has been extensively studied as a stress-responsive kinase, but its role in development remains unknown. The fruit fly, Drosophila melanogaster, has two p38 genes, D-p38a and D-p38b. To elucidate the developmental function of the Drosophila p38’s, we used various genetic and pharmacological manipulations to interfere with their functions: expression of a dominant-negative form of D-p38b, expression of antisense D-p38b RNA, reduction of the D-p38 gene dosage, and treatment with the p38 inhibitor SB203580. Expression of a dominant-negative D-p38b in the wing imaginal disc caused a decapentaplegic (dpp)-like phenotype and enhanced the phenotype of a dpp mutant. Dpp is a secretory ligand belonging to the transforming growth factor β superfamily which triggers various morphogenetic processes through interaction with the receptor Thick veins (Tkv). Inhibition of D-p38b function also caused the suppression of the wing phenotype induced by constitutively active Tkv (TkvCA). Mosaic analysis revealed that D-p38b regulates the Tkv-dependent transcription of the optomotor-blind (omb) gene in non-Dpp-producing cells, indicating that the site of D-p38b action is downstream of Tkv. Furthermore, forced expression of TkvCA induced an increase in the phosphorylated active form(s) of D-p38(s). These results demonstrate that p38, in addition to its role as a transducer of emergency stress signaling, may function to modulate Dpp signaling.
The mitogen-activated protein kinase (MAPK) is a conserved eukaryotic factor which is integral to various signal transduction pathways. Three subgroups of the MAPK superfamily have been identified (5, 12, 33, 41): the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK, also referred to as stress-activated protein kinase [SAPK]), and p38 (also called Mpk2). MAPKs are activated through phosphorylation by specific MAPK kinases (MAPKKs), which are themselves phosphorylated and activated by specific MAPKK kinases (MAPKKKs), thus comprising a series of separate MAPK cascades. The ERK cascade plays a central role in the transduction of mitogenic signals and regulation of development, while the JNK and p38 cascades have been implicated in the responses to stresses and inflammation. To elucidate the developmental functions of MAPK cascades, we have used the fruit fly, Drosophila melanogaster, an excellent organism for the study of cell signaling pathways through genetic analysis (2). All three types of MAPK have been identified in Drosophila: an ERK homolog, Rolled (Rl [4, 10]), a Drosophila homolog of JNK (DJNK [46, 50]), and Drosophila homologs of p38 (D-p38a and D-p38b [23, 24]).
The pleiotropic functions of Rl in development have been extensively characterized (4, 10, 16, 17). Rl is expressed in most tissues and mediates various receptor tyrosine kinase (RTK)-initiated morphogenetic and mitogenic signaling events throughout development. At least five RTKs, Btl (Breathless, a Drosophila homolog of fibroblast growth factor [FGF] receptor), DER (Drosophila homolog of epidermal growth factor [EGF] receptor), Htl (Heartless, another Drosophila homolog of FGF receptor), Sev (Sevenless, a Drosophila homolog of c-ros), and Tor (Torso), are known to activate the Rl cascade in the processes of tracheal elaboration, cell proliferation, mesodermal patterning, R7 photoreceptor cell differentiation, and differentiation of embryonic terminal structures, respectively. Furthermore, immunohistochemical experiments using an antibody against the phosphorylated form of Rl (16, 17) revealed an unexpected spatiotemporal pattern of distribution, which could not be accounted for by known RTK pathways.
Recent studies on DJNK have demonstrated its role in cell morphogenesis during dorsal closure in the embryo (18, 19, 27, 46, 47, 50). An unknown trigger activates DJNK cascade in the leading-edge cell of the epithelial cell sheet, which in turn induces cell shape changes and maintenance of production of Decapentaplegic (Dpp [43]), a secretory ligand belonging to the transforming growth factor β (TGF-β) superfamily. Both responses, i.e., cell shape changes and maintenance of Dpp production, are required for cell sheet movement prior to dorsal closure. As has been well investigated for mammalian JNK, DJNK is also activated by stress-inducing and inflammatory stimuli, such as UV irradiation and lipopolysaccharide (LPS).
The role of D-p38’s in inhibiting antimicrobial peptide production in cultured cells has been reported recently (24). Like mammalian p38 and the yeast homolog HOG1, D-p38’s are also activated by stress-inducing and inflammatory stimuli, such as UV irradiation, high osmolarity, heat, serum starvation, H2O2, and LPS (23, 24). D-p38a and D-p38b function redundantly in these responses. However, the possible role of D-p38’s in development has remained unknown. Here we show that D-p38b functions in mediating Dpp signaling in wing morphogenesis. Expression of a dominant-negative mutant of D-p38b resulted in a dpp-like wing aberration in wild-type flies and enhanced the aberrant wing phenotype of a dpp mutant. Moreover, inhibition of D-p38b function by various means resulted in the suppression of the phenotype caused by expression of a constitutively active Dpp receptor. D-p38b was also found to be involved in controlling Dpp-dependent transcription and was activated by signaling from constitutively active Dpp receptor.
MATERIALS AND METHODS
Complementation of the yeast MAPK hog1Δ mutant.
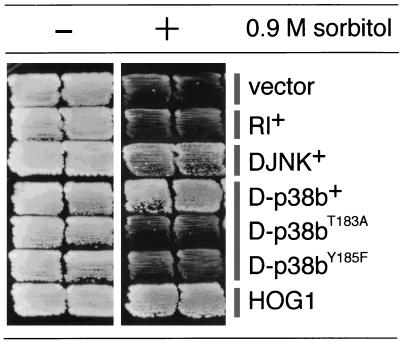
A Saccharomyces cerevisiae hog1Δ::LEU2 strain (7) was transformed with various plasmids. Transformants were streaked onto yeast extract-peptone-dextrose (YPD) plates containing 0.9 M sorbitol and were incubated at 30°C (see Fig. 1).
FIG. 1.
Complementation of the S. cerevisiae MAPK hog1Δ mutant by Drosophila members of the MAPK superfamily. The D-p38b+ clone clearly complements the high-osmolarity (0.9 M sorbitol)-sensitive growth phenotype of the hog1Δ mutant, while the DJNK+ clone does so only weakly. The Rl+ clone fails to complement this phenotype. When the TGY sequence of D-p38b, a putative MAPKK phosphorylation site, was mutated (T183A and Y185F), complementation was abolished.
Establishment of transgenic flies.
The UAS–D-p38bantisense construct was made by inserting the full-length D-p38b cDNA inverted in the P element vector pUAST (6). UAS-tkvCA and UAS-tkv+ constructs were made by using the cDNA encoding the short isoform of Tkv (42). Transformation was carried out as described elsewhere (2).
Clonal analysis of omb expression in the AyGAL4 system.
AyGAL4 designates a transgene consisting of Actin5C promoter-yeast FLP recombinase target (FRT)-transcriptional termination signal-yellow+-FRT-GAL4 (28). Larvae carrying both the AyGAL4 and hsFlp (yeast FLP recombinase gene driven by the heat shock promoter) transgenes were produced. GAL4-expressing clones were induced by heat treatment (at 37°C for 30 min) 48 to 72 h after egg laying, and omb expression was observed 48 h later (see also the legend to Fig. 6B).
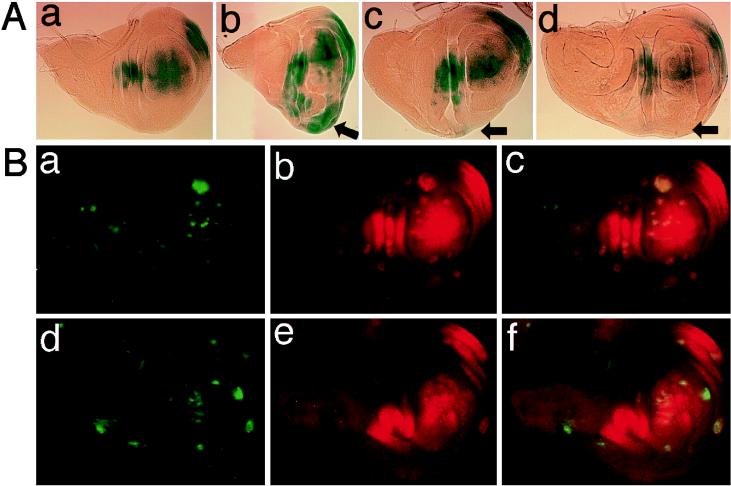
FIG. 6.
D-p38b regulates early Dpp-Tkv signaling-dependent omb expression in the wing disc. (A) The omb expression pattern visualized by a lacZ reporter, ombP1 (51). Anterior is to the top and dorsal to the left. (a) ombP1/+; 69B-GAL4/+ (control). (b) ombP1/+; 69B-GAL4/UAS-tkvCA-S. TkvCA induces ectopic expression of omb (arrow) and overgrowth. This photograph is reduced to 75% the size of the others. (c) ombP1/UAS-D-p38bDN-W; 69B-GAL4/UAS-tkvCA-S. (d) ombP1/UAS-D-p38bantisense; 69B-GAL4/UAS-tkvCA-S. Expression of either D-p38bDN or D-p38bantisense reduced TkvCA-induced ectopic omb expression (arrows) and overgrowth (c or d, respectively). (B) D-p38bDN-expressing clones generated outside the domain in which dpp is expressed display reduced sensitivity of omb induction to TkvCA. Note that dpp expression at this stage in the wing disc lies in a narrow belt just anterior to the anteroposterior boundary (20, 24). Clones of cells that expressed various UAS-transgenes under the control of Actin5C-GAL4 were generated by the flp-out technique and marked by the presence of green fluorescent protein (GFP) (28) (a and d, green). omb expression was revealed by staining with an antibody raised against β-galactosidase (Promega) (b and e, red). (a through c) Wing disc-carrying clones expressing both D-p38b+ and tkvCA as controls (hsFlp/ombP1 UAS-D-p38b+; AyGAL4 UAS-GFP/+; UAS-tkvCA-S/+). (d through f) Wing disc-carrying clones expressing both D-p38bDN and tkvCA (hsFlp/ombP1 UAS-D-p38bDN-S; AyGAL4 UAS-GFP/+; UAS-tkvCA-S/+). (c and f) Superimposed images.
Immunoprecipitation by anti-p-Tyr antibody.
Drosophila extract preparation and immunoprecipitation methods were essentially as described previously (29). Three volumes of extraction buffer were added to flies pulverized in liquid N2. Anti-phosphotyrosine (anti-p-Tyr) antibody (4G10) was purchased from Upstate Biotechnology Incorporated. Immunoprecipitates from extracts equivalent to 25 individuals were loaded in each lane for electrophoresis.
Western blot analysis of Drosophila extracts.
Extracts were prepared in the presence of 4% sodium dodecyl sulfate (SDS) as described elsewhere (4). Extract from the equivalent of 0.2 individual per lane was loaded for electrophoresis and then subjected to Western blotting. Anti-D-p38b antibody was prepared by immunization of a rabbit with recombinant glutathione S-transferase (GST)–D-p38b fusion protein.
Nucleotide sequence accession number.
The DDBJ accession no. for D-p38b cDNA is AB006364.
RESULTS
Identification and characterization of D-p38b.
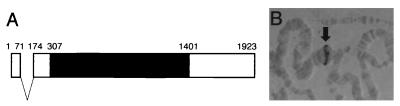
Mammalian p38 has been shown to complement the high-osmolarity-sensitive growth phenotype of the budding yeast hog1Δ mutant (7, 22). To isolate the cDNA for the Drosophila homolog of p38, we conducted genetic screens to isolate suppressors of the hog1Δ mutation (36). A total of 16 positive clones were obtained and assigned to two classes (Fig. 1). The cDNAs from one class were identical to DJNK (46, 50). cDNAs from the other class were identical to D-p38b, recently reported to be the Drosophila homolog of p38 (24) based on the genomic sequence deposited by the Berkeley Drosophila Genome Project. We subsequently carried out hybridization screening with another cDNA library (8) to obtain a longer D-p38b cDNA clone. Comparison of the cDNA and genomic sequences revealed that the D-p38b gene is organized into two exons, although its coding region is continuous within a single exon (Fig. 2A). The D-p38b locus was mapped to the 34D region on the second chromosome by in situ chromosomal hybridization (Fig. 2B), whereas the D-p38a locus was reported to map to the 95E4-to-95F1 region on the third chromosome (24). Expression of D-p38b is known to occur in most of the tissues throughout the Drosophila life span (1, 24).
FIG. 2.
Exon organization and mapping of the D-p38b gene. (A) Exon organization of the D-p38b gene. Two exons are indicated by boxes. The coding region is filled. Nucleotide 1 is assigned to the 5′ end of the longest D-p38b cDNA. (B) In situ chromosomal hybridization. Salivary gland polytene chromosomes were derived from the hemizygote of Df(2L)b82a2 which had lost the region from 34D1 to -2 through 34E1 to -2 (37). The hybridization signal (arrow) was observed at the 34D region. This region expands somewhat owing to the compression caused by pairing of the wild-type chromosome with the deficiency chromosome. The method used for digoxigenin-labelled in situ hybridizations has been described previously (2).
Inhibition of D-p38 function leads to induction of a dpp-related wing phenotype.
The chromosomal region around the D-p38b locus has been well characterized genetically (53). However, the D-p38b+ transgene was unable to rescue any of the known mutations mapping to this region [l(2)34Da (kuz), l(2)34Db, and l(2)34Dc]. Likewise, attempts to isolate a mutant of D-p38b from 3,000 chromosomes mutagenized by ethyl methanesulfonate were unsuccessful. These failures were possibly due to the functional redundancy of the two p38 homologs. We therefore used methods to interfere with endogenous D-p38(s) in order to investigate its function. A dominant-negative allele of D-p38b, designated D-p38bDN, was generated by replacing the Thr-183 of the MAPKK target site with Ala, analogous to the change in ERK2 which produces a dominant-negative allele (44). This recombinant mutant protein lost its ability to suppress the yeast hog1Δ mutant phenotype (Fig. 1).
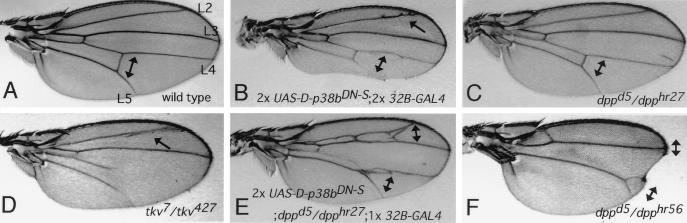
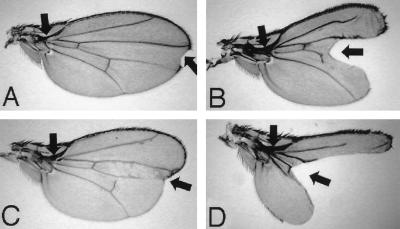
Expression of D-p38bDN was induced in a wild-type background by using the GAL4–upstream activation sequence (UAS) system (6). We isolated two lines which express D-p38bDN at different levels: D-p38bDN-S (Strong), which expresses high levels, and D-p38bDN-W (Weak), which expresses low levels. When two copies of the D-p38bDN-S transgene were expressed in the wing by using two copies of the 32B-GAL4 enhancer trap transgene (6), a certain fraction of adult flies that escaped death exhibited ectopic vein fragments around the end of the longitudinal vein L2 and a reduction in the distance between L4 and L5 (Fig. 3B). Both of these features were also observed with some mutant alleles of dpp (Fig. 3C) and tkv (thick veins [Fig. 3D]), a gene encoding a type I receptor for Dpp (9, 39, 42, 45). This wing phenotype was rescued by coexpression of the D-p38b+ transgene but not by coexpression of a DJNK+ transgene (1), indicating that the effect of D-p38bDN expression is specific. When two copies of the D-p38bDN-S transgene were weakly expressed in the wing of a dpp mutant by using one copy of the 32B-GAL4 transgene, the vein phenotype of dpp was strongly enhanced (Fig. 3E). These phenotypes suggest the involvement of D-p38(s) in Dpp function in the early and late stages of wing pattern development. Dpp is known to play a dual role during wing development, acting as a morphogen (34, 40) and mitogen (14) in the early stage, while activating vein differentiation in the late stage (13).
FIG. 3.
Effect of D-p38bDN on wing phenotype. (A) Wild-type Canton-S. L2, L3, L4, and L5 indicate the four longitudinal veins. (B) An individual expressing high levels of D-p38bDN. The distance between L4 and L5 (double-headed arrow) is reduced, similar to what is seen in the dpp mutant (panel C). Ectopic veins around distal L2 (arrow) are reminiscent of those in various tkv mutants (see panel D) (13, 37, 45). A notched wing margin is occasionally observed with other dpp alleles (see panel F). (C) An example of a very hypomorphic dpp allele (dppd5/dpphr27) (37). (D) An example of a hypomorphic tkv allele (tkv7/tkv427). (E) A dpp mutant expressing moderate levels of D-p38bDN. L4 and L5 were partially fused, and the distance between L2 and L3 was also reduced, similar to that seen with the severe dpp alleles (9) (see panel F). (F) An example of a severe dpp allele (dppd5/dpphr56) (37).
Involvement of D-p38b in a Dpp-Tkv signaling pathway leading to vein formation.
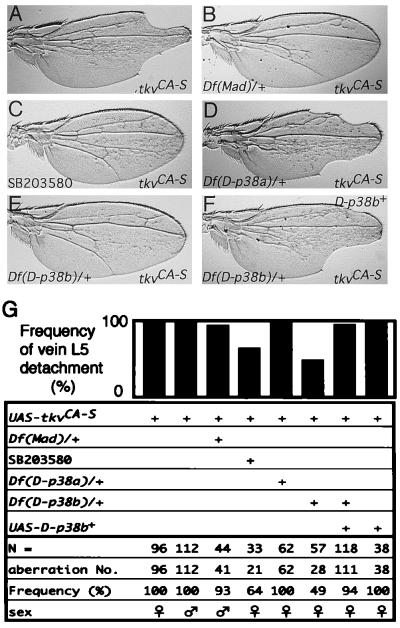
To examine whether D-p38(s) functions in the Dpp signaling pathway, we tested the genetic interaction between D-p38(s) and a constitutively active mutant of Tkv (TkvCA), in which a Gln in the GS domain has been replaced by an acidic residue (34, 40, 52). Two classes of tkvCA insertions, tkvCA-S (Strong) and tkvCA-W (Weak), were used. When tkvCA-S was driven by 71B-GAL4 (6), normal wing venation was severely distorted and extensive production of fragments of vein material was observed (Fig. 4A) (13). The abdominal-cuticle pattern also appeared irregular (1). This wing phenotype suggested that TkvCA may influence Dpp action during vein formation. Ectopic coexpression of dpp+ and tkv+ under the control of 71B-GAL4 caused similar phenotypes (1), indicating that these TkvCA-induced aberrations were indeed the result of an increase in Dpp signaling. We thus expected that reducing the levels of downstream components would suppress tkvCA. In fact, reducing the gene dosage of Mothers against dpp (Mad), a well-documented Dpp-signaling factor (25, 49), by one-half significantly suppressed the tkvCA wing phenotype (Fig. 4B and G). Similar phenomena have been reported previously (11, 26).
FIG. 4.
Effects of reduced Mad gene dosage, the p38 inhibitor SB03580, and reduced D-p38 gene dosage on the adult wing phenotype induced by constitutively active Tkv (TkvCA). (A through F) Adult wing phenotypes. All these wings are from individuals carrying one copy each of UAS-tkvCA-S and 71B-GAL4. (A) Wild-type background for other genes. Incision of wing margins was frequently observed. (B) Hemizygote for Df(2L)C28 (49) that uncovers the Mad locus. Reduction of the Mad gene dosage suppresses the tkvCA wing phenotype. (C) An individual fed a standard diet supplemented by 120 nM SB203580 (a p38 inhibitor; Calbiochem/Novabiochem). SB203580 suppresses the tkvCA wing phenotype. (D) Hemizygote of Df(3R)crb-F89-4 that uncovers the D-p38a locus. Reduction of D-p38a gene dosage does not suppress the tkvCA wing phenotype. (E) Hemizygote of Df(2L)b82a2 that uncovers the D-p38b locus. Reduction of D-p38b gene dosage suppresses the tkvCA wing phenotype. (F) Hemizygote of Df(2L)b82a2 carrying one copy of UAS-D-p38b+. Suppression by reduced D-p38b gene dosage was abrogated by reintroducing the D-p38b+ transgene. (G) Quantitative representation. Histograms represent percentages of wings in which L5 is detached from the wing margin.
We first tested the effect of the imidazole compound SB203580, a p38 inhibitor (35), on the tkvCA wing phenotype. SB203580 has been reported to inhibit both D-p38a and D-p38b (24), and penetration of various imidazole compounds through the insect epidermis is well known (32). Consequently, exposure of growing larvae to SB203580 resulted in suppression of the phenotype (Fig. 4C and G). We further tested whether endogenous D-p38 genes are involved in the tkvCA wing phenotype by reducing endogenous gene dosage using chromosomal hemizygosity. Interestingly, reduction of D-p38b suppressed the tkvCA wing phenotype (Fig. 4E and G), while reduction of D-p38a was not effective (Fig. 4D and G). Suppression by reduction of the D-p38b gene dosage was abrogated by the introduction of a transgene for D-p38b+ (Fig. 4F and G). Thus, the gene within the deficiency that suppresses tkvCA is indeed D-p38b. These results suggest that D-p38b plays a major role in this morphogenetic process, and we focused on this gene in further analyses.
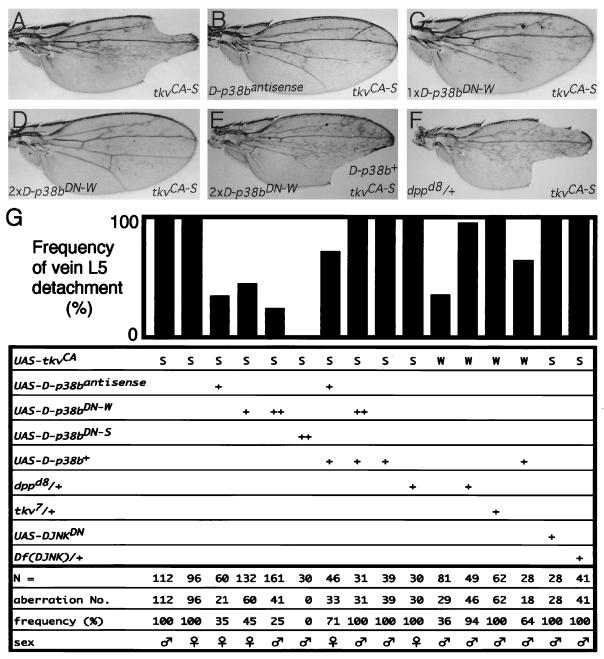
When antisense D-p38b RNA was coexpressed with TkvCA-S, the tkvCA-S phenotype was markedly suppressed (Fig. 5B and G). Four of five independently established D-p38bantisense lines showed significant suppression (1). Suppression affected the various pleiotropic phenotypes associated with the tkvCA allele, including wing blade morphology, abdominal-cuticle morphology, and wing posture. Similar suppression of the tkvCA phenotype was also achieved by coexpression of D-p38bDN-W in a dose-dependent manner (Fig. 5C, D, and G). This suppression was greater when D-p38bDN-S was coexpressed (Fig. 5G). Furthermore, this suppression was abrogated by simultaneous coexpression of D-p38b+ (Fig. 5E and G), demonstrating that D-p38bDN and D-p38b+ competitively sequester endogenous factors essential to signaling. These results suggest either that D-p38b functions downstream of Tkv or that inhibition of D-p38b causes a reduction in endogenous dpp activity. Since the expression pattern of dpp in the developing wing of the D-p38bDN-S producer was found to be indistinguishable from that of the wild type (1), and reduction in the gene dosage of dpp was not effective in suppressing the tkvCA phenotype (Fig. 5F and G), we conclude that D-p38b does not affect Dpp production per se but rather acts as a downstream component of the Dpp-Tkv signaling pathway, operating late in wing development. The fact that the weak phenotype of tkvCA-W was significantly enhanced by D-p38b+ (Fig. 5G) is also consistent with this conclusion. In contrast, attempts to interfere with the function of DJNK, such as by coexpression of DJNKDN (T181A nonactivatable mutant) or generation of chromosomal hemizygosity, did not result in suppression of the tkvCA phenotype (Fig. 5G). Thus, the role of D-p38b in Dpp signaling appears to be specific. Moreover, expression of D-p38bDN did not have any significant effect on the wing phenotypes caused by expression of other dominant active receptors such as DER (ElpB1 [37]) or Notch (Ax1 [37]) (1), further indicating the specificity of D-p38b function for Dpp signaling.
FIG. 5.
Effects of altering D-p38b function and reducing DJNK, Dpp, and Tkv functions on the wing phenotypes caused by either UAS-tkvCA-W (W) or UAS-tkvCA-S (S) driven by 71B-GAL4. (A through F) Adult wing phenotypes. All these wings are from individuals carrying one copy each of UAS-tkvCA-S and 71B-GAL4. (A) Wild-type background for other genes. (B) An individual carrying one copy of UAS–D-p38bantisense. D-p38bantisense suppresses the tkvCA wing phenotype. (C and D) Individuals carrying one copy (C) or two copies (D) of UAS–D-p38bDN-W. D-p38bDN suppresses the tkvCA wing phenotype in a dose-response manner. (E) An individual carrying two copies of UAS–D-p38bDN-W together with one copy of UAS–D-p38b+. Suppression by D-p38bDN was abrogated by coexpression of D-p38b+. (F) Heterozygote of dppd8 (37). Reduction of the dpp gene dosage does not suppress the tkvCA wing phenotype. (G) Quantitative representation. Histograms represent percentages of wings in which L5 is detached from the wing margin. ++, two introduced copies of the transgenes. Df(DJNK), Df(2L)flp147E (46, 50).
Effect of D-p38b on Tkv-dependent omb transcription.
To examine the involvement of D-p38b in the Dpp signaling pathway, we tested the effect of D-p38b on omb transcription. The omb (optomotor-blind) gene encodes a T-box family transcription factor, and its expression in the wing imaginal disc is dependent on early Dpp-Tkv signaling (20, 34, 40). In the wing discs of UAS-tkvCA-S/69B-GAL4 (6) individuals, omb expression domain is greatly expanded and overgrowth of the disc is evident (Fig. 6A, panel b), as previously reported (40). Expression of D-p38bDN or D-p38bantisense markedly suppressed both omb expression and disc overgrowth (Fig. 6A, panel c or d, respectively). Induction of omb in the tkvCA-expressing clones in regions outside those where dpp is expressed was also inhibited by coexpression of D-p38bDN (Fig. 6B), consistent with the possibility that D-p38b functions downstream of Tkv. Furthermore, while D-p38bDN slightly affected omb expression in a tkv+ genetic background (1), the wing phenotype of a hypomorphic omb allele was clearly enhanced by expression of D-p38bDN or D-p38bantisense (Fig. 7), as observed in the wing phenotype of the severe omb alleles such as omb3198 and omb282 (20, 34). These results suggest that D-p38b is also involved in early Dpp-Tkv signaling in wing development to activate omb transcription.
FIG. 7.
Enhancement of ombbi by D-p38bDN or by D-p38bantisense. (A) A wing of an ombbi/Y male. Arrows indicate the aberrations typically observed: fusion of veins at the base of the wing and small notching at the wing tip. (B) A wing of an ombbi individual expressing D-p38bantisense (ombbi/Y; UAS-D-p38bantisense/69B-GAL4). (C and D) Wings of ombbi individuals expressing D-p38bDN weakly (ombbi UAS–D-p38bDN-W/Y; 69B-GAL4/+) (C) or strongly (ombbi UAS–D-p38bDN-S/Y; 69B-GAL4/+) (D). Arrows point to the ombbi phenotypes.
D-p38 is activated by Tkv signaling in vivo.
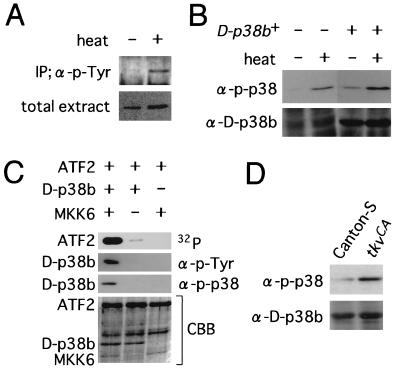
To investigate whether D-p38b is activated by Tkv signaling, we conducted a preliminary biochemical characterization of D-p38b. Immediately after heat treatment of flies, the amount of D-p38b immunoprecipitated by anti-p-Tyr antibody was found to increase considerably (Fig. 8A), demonstrating that D-p38b is tyrosine phosphorylated following heat shock, like mammalian p38 (12, 33). The site of tyrosine phosphorylation was expected to be in the “activation loop” region recognized by MAPKK, as is the case in mammalian p38 (12, 33). Thus, we tested whether an anti-phospho-p38 (anti-p-p38) antibody (New England Biolabs, Inc.) raised against a phosphorylated peptide from the activation loop of mammalian p38 could cross-react with D-p38b (Fig. 8B). This anti-p-p38 antibody detected a protein with a calculated size of 42 kDa whose amount increased immediately after heat shock (Fig. 8B, left half). This protein was also more abundant in the flies overproducing D-p38b regardless of heat treatment (Fig. 8B, right half). Therefore, we concluded that anti-p-p38 can cross-react with the phosphorylated from of D-p38b and can be used to assay recombinant D-p38b phosphorylation in vitro (Fig. 8C). Treatment of D-p38b with recombinant human MKK6 (38), a MAPKK which activates p38, caused a marked increase in the level of D-p38b, as detected with anti-p-Tyr and anti-p-p38 antibodies, and a drastic increase in the level of D-p38-dependent phosphorylation of recombinant human activating transcription factor 2 (ATF2; Santa Cruz Biotechnology), a physiological substrate for mammalian p38 (21). The correlation between the phosphorylation state and kinase activity of D-p38b indicates that the anti-p-p38 antibody recognizes the active form of D-p38b. This allowed activation of D-p38b by TkvCA to be examined in vivo. The amount of active D-p38b was found to be slightly but significantly higher in larvae carrying UAS-tkvCA and 71B-GAL4 relative to that in wild-type Canton-S larvae (Fig. 8D). However, it has been reported that D-p38a protein expressed in yeast, which was presumed to have the same molecular mass as D-p38b, is also recognized by anti-p-p38 antibody (23). It is therefore possible that D-p38b, or both D-p38’s, may be activated by Tkv signaling in vivo.
FIG. 8.
Phosphorylation and activation of D-p38b. (A) D-p38b is tyrosine phosphorylated after heat shock. Extracts from adult flies left untreated or treated with heat (37°C for 1 h) were immunoprecipitated (IP) with anti-p-Tyr antibody (α-p-Tyr) and immunoblotted with anti-D-p38b antibody after separation by SDS-polyacrylamide agarose gel electrophoresis (PAGE) (upper panel). Total extracts were also immunoblotted with anti-D-p38b antibody (lower panel). In this experiment, flies carrying one copy each of UAS-D-p38b+ and hs-GAL4 (6) were used to facilitate detection. The total amount of D-p38b did not change immediately after heat shock. The basal activity of the heat shock promoter was sufficient to achieve constitutive expression of GAL4; thus, D-p38 was overexpressed even in the absence of heat treatment. (B) Anti-p-p38 antibody recognizes phosphorylated D-p38b in fly extract. Flies harboring both UAS-D-p38b+ and hs-GAL4 (right half) or only hs-GAL4 (left half) were heat shocked as described above, and extracts were immunoblotted with either anti-p-p38 or anti-D-p38b antibody after separation by SDS-PAGE. The intensities of bands recognized by anti-p-p38 increased as a result of either heat treatment or D-p38b overexpression. (C) The phosphorylation level of D-p38b correlates with its enzymatic activity. Recombinant D-p38b protein was pretreated with recombinant MKK6 in the presence of cold ATP. Then ATF2 and [γ-32P]ATP were added to aliquots of reaction mixtures, and incubation was continued. The resulting mixtures were resolved by SDS-PAGE and subjected to autoradiography for detection of kinase activities (top panel) or to immunoblotting with anti-p-Tyr and anti-p-p38 antibodies (two middle panels). The bottom panel represents the gel stained with Coomassie brilliant blue (CBB). The kinase assay method used has been described elsewhere (30). (D) Activation of D-p38(s) by TkvCA. Extracts from larvae of Canton-S (wild type) or from larvae carrying two copies each of UAS-tkvCA-S and 71B-GAL4 were resolved by SDS-PAGE and immunoblotted with anti-p-p38 or anti-D-p38b antibody.
DISCUSSION
To investigate the role of the D-p38b gene in development, we used reverse genetic techniques. Our genetic analyses showed that D-p38b is integrally involved in Dpp signaling in the process of wing morphogenesis. Various manipulations which interfere with D-p38b function enhanced or suppressed the phenotypes caused by decreased or increased Dpp signaling, respectively. However, the present study did not elucidate any function of D-p38b in normal Dpp signaling; rather, its effects were apparent only when Dpp signaling was enhanced or inhibited. For example, D-p38bDN slightly affected omb expression in a tkv+ background but clearly suppressed it in a tkvCA background. Thus, it is possible that D-p38 is involved in unusual Dpp signaling events, consistent with the known role of mammalian p38 as an emergency signaling factor.
D-p38b and upstream kinases can be involved in the Dpp signaling pathway.
Both D-p38a and D-p38b were reported to be activated by D-MKK3, a MAPKK (24). Thus, it is likely that D-p38 is part of a kinase cascade similar to the mammalian p38 cascade. In fact, genetic manipulation of D-MKK3 function has revealed an interaction with Dpp signaling similar to that found here for D-p38b (1). However, a corresponding MAPKKK functioning in the D-p38 cascade has yet to be identified. A mammalian member of the MAPKKK family, TAK1 (TGF-β-activated kinase 1), has been reported to mediate signals elicited by TGF-β superfamily members through activation of MKK3 (a MAPKK), MKK6, and p38 (38, 54). The activation of D-p38b by TkvCA correlates with that of p38 by TGF-β stimulus (38). Thus, we currently hypothesize that a D-p38b cascade exists which can be involved in Dpp signaling and which includes D-MKK3 and a homolog of TAK1.
The signaling hierarchy involving D-p38b in wing disc morphogenesis is different from that of DJNK in embryonic dorsal closure.
p38 is known to share many characteristics with JNK (12, 33), such as activation by various stress-inducing and inflammatory stimuli. In Drosophila, mutations in DJNK (46, 50) and its activator MAPKK Hemipterous (Hep [18]) cause a “dorsal open phenotype,” reminiscent of that observed in dpp and tkv mutants. It is also known that the DJNK signal regulates dpp expression in the leading-edge cells during embryonic dorsal closure (19, 27, 47). However, our mosaic analysis shows that the suppression of tkvCA by D-p38bDN occurs even in cells which do not produce Dpp (Fig. 6B). This indicates that, in contrast to the case of dorsal closure, the MAPK functioning in the wing disc morphogenetic pathway, i.e., D-p38b, acts downstream of Tkv. The role of D-p38b proposed here may be more analogous to that of mammalian JNK, which also functions as a downstream component of the TGF-β and Smad (mammalian homolog of Mad) signaling pathways in the mammalian cultured cell line MDCK (3).
Possible relationship of the D-p38b cascade and other signaling factors.
A Dpp-signaling factor, Mad, is known to be directly phosphorylated by Tkv and then migrates into the nucleus, where it functions as a DNA-binding transcriptional factor (25). It is unclear at present if there is any functional interaction between Mad and D-p38b. While Mad is directly phosphorylated by Tkv (25), the activation of D-p38 by TkvCA may be indirect. Alternatively, it is possible that one or more transcription factors functioning in the Dpp response require prior phosphorylation mediated by the D-p38 cascade, a process which is regulated independently of Tkv. In fact, one of the mammalian MAPKs, ERK, which is regulated independently of the BMP (a mammalian homolog of Dpp) signaling pathway and activated by the EGF signaling pathway, is known to phosphorylate Smad1 and prevent its nuclear localization (31). One class of transcription factors which might function downstream of D-p38 is the ATF-cyclic AMP responsive element binding protein (CREB) family, members of which are known to be targets for the p38 cascade (21). For example, Mad and ATF-CREB might interact as components in a transcriptional activator complex which induce expression of Dpp signaling target genes. Correspondingly, a CREB binding site has been reported to mediate the response to Dpp signaling during Drosophila endoderm induction (15). Although we have not yet determined whether D-p38(s) is involved in Dpp-regulated endoderm induction, we know that the function of D-p38b in the Dpp response is not restricted to the wing because we have observed that forced (by use of a constitutive GAL4 driver) expression of TkvCA throughout the body leads to D-p38b activation in regions outside the wing (1). In addition, interfering with D-p38b also suppresses the abdominal phenotype induced by TkvCA, as mentioned above. Further examination of the extent of the functional relationship between D-p38 and Dpp signaling in various aspects of Drosophila development should be useful in identifying targets of D-p38 in Dpp signaling.
ACKNOWLEDGMENTS
We thank Karin Ekstrom, Shigeo Hayashi, Yasushi Hiromi, Kathy Matthews, Shigeru Morimura, Gert O. Pflugfelder, and Tetsuya Tabata for providing fly stocks, Nicholas H. Brown and Patrick H. O’Farrell for cDNA libraries, Won-Jae Lee and Norbert Perrimon for plasmid DNA, Masatoshi Hagiwara for recombinant MKK6 protein, and Yutaka Inaguma, Kanefusa Kato, Michael B. O’Connor, Akira Mizoguchi, Eisuke Nishida, and Masamitsu Yamaguchi for technical advice. We are also grateful to Marc Lamphier, Enrique Martín-Blanco, Shin Sugiyama, Tetsuya Tabata, and Takashi Takabatake for advice on the manuscript and to Kana Dohmoto and Tomiko Tsuboi for technical assistance.
This work was supported by grants from The Kurata Foundation, The Ministry of Education, Science, Sports, and Culture of Japan, and the Japan Science and Technology Corporation.
REFERENCES
- 1.Adachi-Yamada, T. Unpublished data.
- 2.Ashburner M. Drosophila, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 3.Atfi A, Buisine M, Mazars A, Gespach C. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor-β through stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway. J Biol Chem. 1997;272:24731–24734. doi: 10.1074/jbc.272.40.24731. [DOI] [PubMed] [Google Scholar]
- 4.Biggs W H, III, Zavitz K H, Dickson B, van der Straten A, Brunner D, Hafen E, Zipursky S L. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994;13:1628–1636. doi: 10.1002/j.1460-2075.1994.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumer K J, Johnson G L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 6.Brand A H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotype. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 7.Brewster J L, de Valoir T, Dwyer N D, Winter E, Gustin M C. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- 8.Brown N H, Kafatos F C. Functional cDNA libraries from Drosophila embryo. J Mol Biol. 1988;203:425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- 9.Brummel T J, Twombly V, Marqués G, Wrana J L, Newfeld S J, Attisano L, Massagué J, O’Connor M B, Gelbart W M. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–261. doi: 10.1016/0092-8674(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 10.Brunner D, Oellers N, Szabad J, Biggs III W H, Zipursky S L, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 11.Das P, Maduzia L L, Wang H, Finelli A L, Cho S-H, Smith M M, Padgett R W. The Drosophila gene Medea demonstrates the requirement for different classes of Smads in dpp signaling. Development. 1998;125:1519–1528. doi: 10.1242/dev.125.8.1519. [DOI] [PubMed] [Google Scholar]
- 12.Davis R J. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 13.de Celis J F. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 1997;124:1007–1018. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- 14.Edgar B A, Lehner C F. Developmental control of cell cycle regulators: a fly’s perspective. Science. 1996;274:1646–1652. doi: 10.1126/science.274.5293.1646. [DOI] [PubMed] [Google Scholar]
- 15.Eresh S, Riese J, Jackson D B, Bohmann D, Bienz M. A CREB-binding site as a target for decapentaplegic signalling during Drosophila endoderm induction. EMBO J. 1997;16:2014–2022. doi: 10.1093/emboj/16.8.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabay L, Seger R, Shilo B-Z. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- 17.Gabay L, Seger R, Shilo B-Z. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997;124:3535–3541. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- 18.Glise B, Bourbon H, Noselli S. hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 19.Glise B, Noselli S. Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 1997;11:1738–1747. doi: 10.1101/gad.11.13.1738. [DOI] [PubMed] [Google Scholar]
- 20.Grimm S, Pflugfelder G O. Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science. 1996;271:1601–1604. doi: 10.1126/science.271.5255.1601. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Campbell D, Dérijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Lee J-D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 23.Han S-J, Choi K-Y, Brey P T, Lee W-J. Molecular cloning and characterization of a Drosophila p38 mitogen-activated protein kinase. J Biol Chem. 1998;279:369–374. doi: 10.1074/jbc.273.1.369. [DOI] [PubMed] [Google Scholar]
- 24.Han Z S, Enslen H, Hu X, Meng X, Wu I-H, Barrett T, Davis R J, Ip Y T. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol Cell Biol. 1998;18:3527–3539. doi: 10.1128/mcb.18.6.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heldin C-H, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 26.Hoodless P A, Haerry T, Abdollah S, Stapleton M, O’Connor M B, Attisano L, Wrana J L. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- 27.Hou X S, Goldstein E S, Perrimon N. Drosophila Jun relays the Jun amino-terminal kinase signal transduction pathway to the Decapentaplegic signal transduction pathway in regulating epithelial cell sheet movement. Genes Dev. 1997;11:1728–1737. doi: 10.1101/gad.11.13.1728. [DOI] [PubMed] [Google Scholar]
- 28.Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurons and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- 29.Kosako H, Gotoh Y, Matsuda S, Ishikawa M, Nishida E. Xenopus MAP kinase activator is a serine/threonine/tyrosine kinase activated by threonine phosphorylation. EMBO J. 1992;11:2903–2908. doi: 10.1002/j.1460-2075.1992.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosako H, Nishida E, Gotoh Y. cDNA cloning of MAP kinase kinase reveals kinase cascade pathways in yeasts to vertebrates. EMBO J. 1993;12:787–794. doi: 10.1002/j.1460-2075.1993.tb05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 32.Kuwano E, Takeya R, Eto M. Synthesis and anti-juvenile hormone activity of 1-substituted-5-[(E)-2,6-dimethyl-1,5-heptadienyl]imidazoles. Agric Biol Chem. 1985;49:483–486. [Google Scholar]
- 33.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 34.Lecuit T, Brrok W J, Ng M, Calleja M, Sun H, Cohen S M. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- 35.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Stickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 36.Léopold P, O’Farrell P H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991;66:1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsley D L, Zimm G G. The genome of Drosophila melanogaster. San Diego, Calif: Academic Press, Inc.; 1992. [Google Scholar]
- 38.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 39.Nellen D, Affolter M, Basler K. Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell. 1994;78:225–237. doi: 10.1016/0092-8674(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 40.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 41.Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 42.Okano H, Yoshikawa S, Suzuki A, Ueno N, Kaizu M, Okabe M, Takahashi T, Matsumoto M, Sawamoto K, Mikoshiba K. Cloning of a Drosophila melanogaster homologue of the mouse type-I bone morphogenetic protein-2/-4 receptor: a potential decapentaplegic receptor. Gene. 1994;148:203–209. doi: 10.1016/0378-1119(94)90690-4. [DOI] [PubMed] [Google Scholar]
- 43.Padgett R W, St Johnston D D, Gelbart W M. A transcript from Drosophila pattern gene predicts a protein homologous to the transforming growth factor-β family. Nature. 1987;325:81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- 44.Pagès G, Lenormand P, L’Allemain G, Chambard J-C, Meloche S, Pouysségur J. Mitogen-activated protein kinase p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8309–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penton A, Chen T, Staehling-Hampton K, Wrana J L, Attisano L, Szidonya J, Cassill J A, Massagué J, Hoffman F M. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell. 1994;78:239–250. doi: 10.1016/0092-8674(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 46.Riesgo-Escovar J R, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for Djun-dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- 47.Riesgo-Escovar J R, Hafen E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor Djun during dorsal closure. Genes Dev. 1997;11:1717–1727. doi: 10.1101/gad.11.13.1717. [DOI] [PubMed] [Google Scholar]
- 48.Schulze K L, Bellen H J. Drosophila syntaxin is required for cell viability and may function in membrane formation and stabilization. Genetics. 1996;144:1713–1724. doi: 10.1093/genetics/144.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekelsky J J, Newfeld S J, Raftery L A, Chartoff E H, Gelbart W M. Genetic characterization and cloning of Mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sluss H K, Han Z, Barrett T, Davis R J, Ip Y T. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y H, Tsai C-J, Green M M, Chao J-L, Yu C-T, Jaw T J, Yeh J-Y, Bolshakov V N. white as a reporter gene to detect transcriptional silencers specifying position-specific gene expression during Drosophila melanogaster eye development. Genetics. 1995;141:1075–1086. doi: 10.1093/genetics/141.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wieser R, Wrana J L, Massagué J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodruff R C, Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. II. Lethal mutations in the region. Genetics. 1979;92:133–149. doi: 10.1093/genetics/92.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]