Abstract
Uremic toxins (UTs) are mainly produced by protein metabolized by the intestinal microbiota and converted in the liver or by mitochondria or other enzymes. The accumulation of UTs can damage the intestinal barrier integrity and cause vascular damage and progressive kidney damage. Together, these factors lead to metabolic imbalances, which in turn increase oxidative stress and inflammation and then produce uremia that affects many organs and causes diseases including renal fibrosis, vascular disease, and renal osteodystrophy. This article is based on the theory of the intestinal–renal axis, from bench to bedside, and it discusses nonextracorporeal therapies for UTs, which are classified into three categories: medication, diet and supplement therapy, and complementary and alternative medicine (CAM) and other therapies. The effects of medications such as AST-120 and meclofenamate are described. Diet and supplement therapies include plant-based diet, very low-protein diet, probiotics, prebiotics, synbiotics, and nutraceuticals. The research status of Chinese herbal medicine is discussed for CAM and other therapies. This review can provide some treatment recommendations for the reduction of UTs in patients with chronic kidney disease.
Keywords: chronic kidney disease, diet control, dietary supplement, complementary and alternative medicine, uremic toxin, conventional medical therapy
1. Introduction
Chronic kidney disease (CKD) is characterized by a gradual decrease in the glomerular filtration rate and proteinuria. CKD is a global health problem, and its incidence has been increasing. The estimated global prevalence of CKD is 8–14% [1,2]. When kidney function deteriorates gradually, many metabolites accumulate in the body. These accumulated substances, termed as uremic toxins (UTs), can result in adverse pathophysiological outcomes [3]. UTs can affect multiple organs and cause renal fibrosis, vascular calcification, anemia, peripheral arterial disease [4], adynamic bone disease [5], adipocyte dysfunction with insulin resistance [6], impaired immune system [7], uremic pruritus [8], and impaired valsartan-induced neovascularization [9].
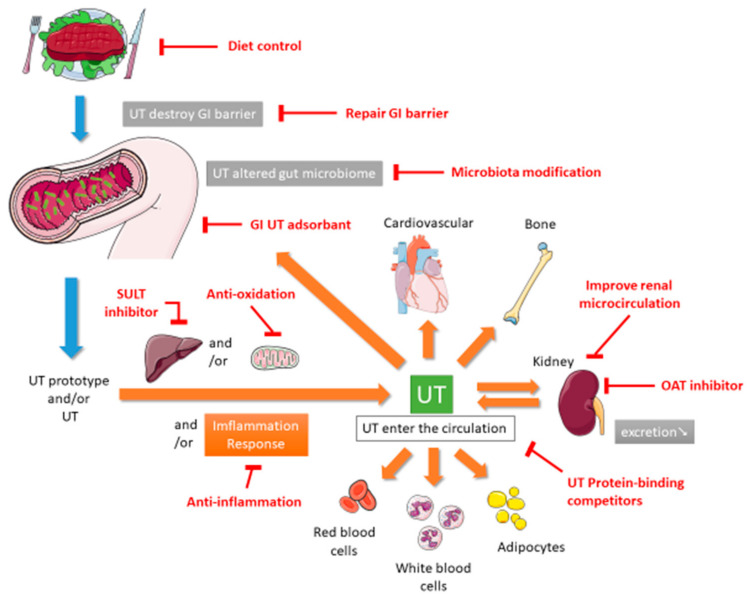
In 1999, Vanholder and other researchers established the European Uremic Toxin (EUTox) Work Group and divided UTs into three categories based on their solubility, molecular weight, and ability to bind to serum proteins. These categories are small water-soluble compounds such as creatinine and uric acid (UA), middle molecules such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, and compounds that bind to proteins such as indoxyl sulfate (IS) [10]. The EUTox Work Group continues to identify new UTs and specify their standard and uremic concentrations [3]. Esmeralda et al. revised some UTs items based on several inflammatory markers that would increase the deterioration of CKD [11]. Currently, many hundreds of UTs including small water-soluble solutes, which can be removed through dialysis, and larger and protein-bound molecules, which are less likely to be removed during dialysis [12]. The pathophysiological mechanisms through which UTs cause multiple organ damage are complex and not completely understood. These mechanisms may include inflammation, reactive oxidative stress, cellular transdifferentiation, impaired mitochondria function, intestinal barrier destruction, and changes in intestinal microbiota [12,13,14]. Figure 1 shows the proposed mechanism of UTs generation. After food enters the intestine, it is not only digested by exocrine glands such as the liver, gallbladder, intestines, and pancreas but also decomposed by intestinal microbiota. Protein catabolism produces amino acids. If many ingredients that are favored by harmful intestinal microbiota are consumed, including tryptophan and tyrosine, then they will be converted into UTs through the proteolysis of intestinal microbiota [13,15]. Precursors such as indole and p-cresol are absorbed by human intestinal villi cells into the portal blood circulation of the liver. Subsequently, the liver metabolizes them into IS and p-tolyl sulfate [16]. CKD patients are more prone to constipation than ordinary people. The retention of feces will change the intestinal microbiota and promote the production and accumulation of more UTs. When the anaerobic bacteria that can produce short-chain fatty acids (SCFAs) are reduced, SCFAs such as butyrate and acetate, that promote intestinal contraction are also reduced, which in turn makes constipation worse [17,18]. The accumulation of UTs destroys the protective barrier of the intestinal epithelium, leading to the transfer of microbiota from the intestine to the body [13,19]. After IS produced in the liver enters the blood circulatory system, which is absorbed by proximal renal tubular cells through organic anion transporters (OAT) 1 and OAT3 at the basolateral membrane. The accumulated IS induces oxidative stress and then activates nuclear factor E2-related factor 2 (Nrf2) [20]. Some of these UTs can destroy mitochondrial function. Mitochondria may also be the source of UTs because mitochondria contain certain enzymes involved in the UTs synthesis pathway; thus, they may be the site of UTs synthesis. Moreover, because mitochondria are the key regulators of cellular redox homeostasis, they may directly affect the production of UTs. In addition, because many metabolites can be degraded in mitochondria, mitochondrial dysfunction might cause the accumulation of this toxin in organisms. Therefore, CKD can lead to the appearance of compounds that destroy mitochondria, and subsequent mitochondrial damage can cause further accumulation of UTs; the synthesis of UTs is related to mitochondria [14]. CKD reduces the ability to excrete UTs, leading to the accumulation of UTs in blood. At the same time, UTs can accelerate the deterioration of kidney function, leading to a vicious circle. All these factors together lead to the typical destruction of normal metabolic balance and uremic homeostasis that results in inflammation and uremia, causing multiple organ damage [12]. According to the review articles, due to the use of a highly permeable membrane with a greater pores radius and better preservation of the residual renal function, peritoneal dialysis (PD) could be anticipated that some uremic toxins are more efficiently cleared across the peritoneal membrane [21], and that the plasma levels of p-Cresol (protein-bound uremic toxin) are lower than in hemodialysis patients [22].
Figure 1.
Proposed mechanism of UT generation and therapeutic methods. GI, gastrointestinal; OAT, organic anion transporter; SULT, sulfotransferase; UT, uremic toxin.
This review describes the potential nonextracorporeal methods from bench to bedside that can be used to reduce UTs levels and improve renal function based on the aforementioned mechanisms. The PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure, Airiti library, and Wanfang databases were searched by using the term “uremic toxin”. To broaden the search, we further reviewed the included articles and citations utilizing the “related articles” facility on PubMed. This paper is organized as follows. The first section focuses on medications including intestinal sorbents, UA modulators, and enzyme inhibitors. The second section focuses on diet control and supplement therapies such as probiotics, prebiotics, synbiotics, and nutraceuticals. The final section describes complementary and alternative medicine (CAM) and other therapies.
2. Conventional Medication Therapy
In conventional medical therapies, the serum concentration of UTs is mainly reduced using drugs that control underlying diseases to slow down the deterioration of the kidneys, like decrease hypertension, neutralize catecholamines, combat fluid overload, combat dyslipidemia and anemia [23], exert antioxidative effects [24], cause the metabolic degradation of UTs [25], change the bacterial amino acid metabolism [26], inhibit UA synthesis [27], inhibit sulfotransferase [28], reduce amino acid degradation [29], inhibit renal OAT [30], and combine or reduce the intake of toxins or their precursors to adjust the intestinal absorption capacity [31]. In addition, dialysis is performed for extracorporeal removal of UTs; however, the removal of middle and protein-bound UTs through conventional dialysis is inadequate [12]. The following is a brief discussion of treatment strategies that can be used to reduce UTs levels (Table 1).
Table 1.
Medications for the control of UTs.
| Intervention | Route, Dosage and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Acarbose | Oral, 100 mg, TID | Evenepoel et al., 2006 [26] | Changes in bacterial amino acid metabolism | Clinical trial | Healthy people | 9 | PCS ↘ |
| AST-120 | Oral, 2.7 to 9 g/day | Chen et al., 2019 [32] | UT adsorbent | Meta-analysis | Patients with CKD | 3349 | IS ↘ |
| L-carnitine | i.v., 20 mg/kg, 3 times/week | Fatouros et al., 2010 [33] | Antioxidation | Clinical trial | Patients undergoing HD | 12 | MDA ↘ |
| Folate | Oral, 10 mg, QD | Trimarchi et al., 2002 [34] | Metabolic degradation of UT | RCT | Patients undergoing HD | 62 | Hcy ↘ |
| Folate and Methylcobami | i.v. methylcobalami 500 µg, 3 times/week and oral folate 15 mg, QD | Koyama et al., 2010 [25] | Metabolic degradation of UT | RCT | Patients undergoing HDs | 40 | ADMA ↘, Hcy ↘ |
| Ketoacid and LPD | Oral, 1 pill/5 kg, QD | Marzocco et al., 2013 [35] | Decreased amino acid degradation/protein carbamylation | RCT | CKD stage 3 adults | 32 | IS ↘ |
| Ketoacid and LPD | Oral, 0.1 g/kg, TID | Garibotto et al., 2018 [29] | Decreased amino acid degradation/protein carbamylation | RCT | Patients with CKD | 17 | Urea ↘ |
| Reduced glutathione | Oral, 400 mg, TID | Wang et al., 2016 [36] | Antioxidation | RCT | Patients undergoing HD | 150 | IL-6 ↘, TNF-α ↘ |
| Animal Studies | |||||||
| AST-120 | Oral, 8% w/w, QD | Sato et al., 2017 [37] | UT adsorbent | Animal | Adenine-induced CKD mice | 24 | IS ↘, PCS ↘ |
| L-carnitine | i.p., 500 mg/kg, QD | Sener et al., 2004 [38] | Antioxidation | Animal | Right nephrectomy rats | 16 | BUN ↘, Cr ↘, MDA ↘ |
| Cilastatin | i.v., 200 mg/kg, once | Huo et al., 2019 [30] | OAT inhibitor | Animal | Imipenem-induced nephrotoxicity rabbits | 4 | BUN ↘, Cr ↘ |
| cyclosporine | i.v., 3 mg/kg, once | Lemoine et al., 2015 [24] | Antioxidation | Animal | I/R mice | 22 | BUN ↘, Cr ↘ |
| Enalapril | Oral, 12.6 mg/kg, QD | Marek et al., 2018 [39] | ACEI, increased glomerular filtration, and urine output | Animal | Wistar rats | 27 | TMAO ↘ |
| meclofenamate | i.v., 10 mg/kg, TID | Saigo et al., 2014 [28] | SULT inhibitors | Animal | Renal I/R rats | 9 | BUN ↘, Cr ↘, IS ↘ |
| Probenecid | i.v., 50 mg/kg, once | Huo et al., 2019, [30] | OAT inhibitor | Animal | Imipenem-induced nephrotoxicity rabbits | 12 | BUN ↘, Cr ↘ |
↘, decrease; ACEI, angiotensin converting enzyme inhibitor; ADMA, asymmetric dimethylarginine; BUN, blood urea nitrogen; CKD, chronic kidney disease; Cr, creatinine; Hcy, homocysteine; HD, hemodialysis; i.p., intraperitoneal; i.v., intravenous; I/R, ischemia/reperfusion; IL-6, interleukin-6; IS, indoxyl sulfate; LPD, low protein diet; MDA, malondialdehyde; OAT, organic anion transporter; PCS, p-cresyl sulfate; QD, quaque die; RCT, randomized controlled trial; SULT, sulfotransferase; TID, ter in die; TMAO, trimethylamine N-oxide; TNF-α, tumor necrosis factor alpha; UA, uric acid.
2.1. Acarbose
Acarbose is a small intestinal alpha-glucosidase inhibitor that can increase the number of undigested carbohydrates reaching the colon, increase the production of short-chain fatty acid butyrate in the colon, and reduce intraluminal power of hydrogen (pH) value, thereby inhibiting bacterial deamination and increasing the use of ammonia. In a clinical trial, nine healthy people were treated with acarbose for 3 weeks, and their serum p-cresol declined significantly through changes in bacterial amino acid metabolism [26].
2.2. AST-120
AST-120 can absorb UTs and their precursors in the gastrointestinal tract and then excrete them in feces, reducing the absorption of UTs into the blood. For example, indole is produced through tryptophan metabolism by bacteria in the gastrointestinal tract. AST-120 absorbs indole and reduces its conversion into IS [31]. In an adenine-induced CKD mouse study, Sato et al. demonstrated that the administration of AST-120 led to a decline in accumulated IS and p-cresol sulfate (PCS) in multiple organs, such as the kidneys, heart, brain, and skeletal muscles [37]. The effect of AST-120 on renal disease progression had been reported in some large-scale clinical trials. The Carbonaceous Oral Adsorbent’s Effects on Progression of Chronic Kidney Disease (CAP-KD) trial demonstrated administration of AST-120 can significantly suppress the decrease in estimated glomerular filtration rate (eGFR) for a short observation period of one year [40]. However, it failed to suppress the composite endpoint consisting of doubling of serum creatinine (Scr), Scr ≥ 6.0 mg/dL, end-stage renal disease, and death. In Evaluating Prevention of Progression in CKD (EPPIC)-1 and EPPIC-2 trails, AST-120 was administered to 2035 adults with moderate to severe CKD. AST-120 administration was unable to suppress the primary endpoint which is the initiation of dialysis, transplantation, and doubling of Scr levels [32]. However, the secondary endpoint, the eGFR decline rate was significantly suppressed. The Kremezin study against renal disease progression in Korea (K-STAR) trial included measurement of plasma IS concentration. The primary endpoint was not suppressed in the AST-120 group [41]. Similarly, the eGFR decline tended to be suppressed. Finally, a meta-analysis including eight studies revealed that AST-120 can effectively lower IS levels but still controversial in terms of slowing disease progression and all-cause mortality [32]. Additional large-scale randomized controlled trials (RCTs) with longer follow-up durations and standardized outcomes are still necessary to clarify the clinical efficacy of AST-120.
2.3. L-Carnitine
Patients undergoing hemodialysis (HD) may develop L-carnitine deficiency due to intestinal malabsorption, reduced carnitine synthesis, and reduced carnitine clearance during dialysis. In a double-blind, placebo-controlled, crossover trial of patients undergoing HD, supplementation of L-carnitine, an antioxidant, for 8 weeks reduced the malondialdehyde (MDA) level, increased reduced/oxidized glutathione, and enhanced glutathione peroxidase activity and protein carbonyl concentration without adverse clinical effects [33]. The intravenous injection of carnitine supplementation has been shown to have a nephroprotective effect on contrast-medium nephropathy in an open-label, crossover study, it can reduce the neutrophil gelatinase associated lipocalin and SCr [42]. An animal study suggested that the nephroprotective effect of L-carnitine could reduce blood urea nitrogen (BUN), Scr, and MDA levels in CKD rats following right nephrectomy, possibly through antioxidative properties and free radical scavenging [38].
2.4. Cilastatin
Imipenem is a carbapenem antibiotic with nephrotoxicity. However, cilastatin, the renal dehydropeptidase-I (DHP-1) inhibitor, can prevent imipenem-induced nephrotoxicity by inhibiting the OAT-mediated transport of imipenem [43]. Cilastatin or probenecid ameliorated kidney injury and reduced BUN, Scr, and the renal secretion of imipenem in an imipenem-induced nephrotoxicity rabbit model through inhibition of renal OATs [30].
2.5. Cyclosporine A
Cyclosporine A (CsA), a calcineurin inhibitor, is used as an immunosuppressant. Although CsA is considered to be a nephrotoxic agent, its single injection can protect the kidneys during ischemia and reperfusion. In an ischemia-reperfusion (I/R) renal injury mice model, Lemoine et al. demonstrated that the administration of CsA improved mitochondrial respiration and reduced BUN and Scr probably through the inhibition of mitochondrial permeability transition [24].
2.6. Enalapril
Enalapril, an angiotensin-converting enzyme inhibitor, is used to treat cardiovascular diseases. Marek et al. found that in Wistar rats, enalapril treatment reduced the plasma trimethylamine-N-oxide (TMAO) level by increasing glomerular filtration and urine output; however, it did not reduce the plasma level of IS or change the bacterial composition in the gut [39].
2.7. Folate and Methylcobalamin
Folic acid (FA) is the main cofactor of total homocysteine (Hcy) metabolism, and methylcobalamin (Me-Cbl) is the methylated form of cobalamin. Both play key roles in the conversion of Hcy to methionine. The combined administration of oral FA and intravenous Me-Cbl can reduce plasma Hcy in HD patients [44], which may prevent cardiovascular disease and stroke [45]. In a prospective RCT of patients undergoing HD, both oral FA supplementation and intravenous (i.v.) injection of Me-Cbl plus oral FA normalized plasma total Hcy; however, i.v. injection of Me-Cbl showed no change [34]. Another RCT reported that patients undergoing HD who received i.v. injection of Me-Cbl plus oral FA showed a reduction in Hcy and asymmetric dimethylarginine (ADMA) levels; this reduction possibly resulted from an increase in the metabolic degradation of UTs [25].
2.8. Ketoacids
Ketoanalogues have been used in chronic kidney disease for many years. It can combine the excess amino acids to synthesize essential amino acids (EAAs) through transamination, which can reduce the formation of nitrogenous waste and urinary toxins, and reduce the degradation of protein, which in turn, can improve the patient’s renal function and prognosis. The transamination of ketoanalogues is bidirectional and is related to the concentration of amino acids. When the concentration of amino acids in the body was high, the body will tend to decompose ketonanalogues instead of synthesizing them into new EAAs [46]. In a pilot study published in 2013, researchers analyzed the physiological data of 32 patients with stage 3 CKD. These patients were randomly divided into two groups: very low protein diet (VLPD) with ketoacid (KA) analog supplementation for 1 week, followed by low protein diet (LPD) administration in the second week, and LPD administration for 1 week, followed by VLPD with KA analog supplementation in the second week. The results indicated that restricted protein consumption with KA analog supplementation reduced the production and intestinal absorption of IS [35]. In another clinical trial, 17 patients were included and randomly divided into two groups: LPD and VLPD with EAAs and KA supplementation. After the 24-week trial, forearm protein metabolism was affected in both groups. Overall, skeletal muscle may adapt to restricted protein intake by combining the responses of decreased protein degradation and increased amino acid recycling [29].
2.9. Meclofenamate
Meclofenamate is a type of nonsteroidal anti-inflammatory drug (NSAID) that inhibits hepatic IS production. Saigo et al. used an I/R rat kidney model to demonstrate that meclofenamate considerably improved renal function and reduced BUN, Scr, and IS. In addition, they indicated that meclofenamate could inhibit sulfotransferase (SULT) and reverse the downregulation of rat OAT1 and rat OAT3 expression by preventing prostaglandin E2 generation [28].
2.10. Reduced Glutathione
Oxidative stress arises when there is an imbalance between free radical production and antioxidant defense. Uremic toxins can be a source of oxidative stress in CKD patients. Retention of these toxins promotes systemic inflammation via priming polymorphonuclear leukocytes and stimulating CD-8+ cells [47]. Additional associative factors of oxidative stress in CKD include low serum selenium concentration, low platelet glutathione peroxidase activity [48], and lower serum levels of glutathione [48]. Although glutathione is critical to fight against oxidative stress, some evidences disclosed that glutathione is poorly absorbed by oral route mainly due to the action of an intestinal enzyme, the γ-glutamyl transpeptidase which degrades glutathione [49]. Wang et al. ever found that the oral supplementation of reduced glutathione in patients undergoing HD significantly reduced their IL-6 and TNF-α levels but not their Scr or BUN levels in their RCT trial [36]. Although the very low oral bioavailability limits the interest of oral glutathione supplementation, Schmitt et al. demonstrated the superiority of a new sublingual form of glutathione over the oral form in terms of glutathione supplementation [50]. A new sublingual form may be a promising antioxidant therapy in CKD patients in the future.
3. Diet Control and Diet Supplements
UTs are usually produced through the metabolism of amino acids by intestinal microbiota microbiota, and accumulated UTs can change the composition of intestinal microbiota. Increasing evidence shows that intestinal microbiota plays a crucial role in the development of CKD [51]. Therefore, the current methods of reducing UTs through diet control and supplement therapy include reducing protein intake through a low-protein or plant-based diet [52] and supplementing probiotics, prebiotics, and synbiotics to change the composition of intestinal microbiota; some supplements could exert anti-inflammatory and antioxidative effects [53]. These treatment strategies are briefly discussed below (Table 2).
Table 2.
Diet treatments for the control of UTs.
| Intervention | Route, Dosage and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Prebiotics—OF-IN | Oral, 10 g, BID | Meijers et al., 2010 [54] | Modulating intestinal microbiota | Open-label phase I/II study | Patients undergoing HD | 22 | PCS ↘ |
| Probiotics: L. acidophilus KB27, B. longum KB31, and S. thermophilus KB19 | Oral, 2 capsules, TID | Ranganathan et al., 2010 [55] | Modulating intestinal microbiota | RCT | Patients with CKD stages 3 and 4 | 46 | BUN ↘, Cr ↘, UA ↘ |
| Probiotics: B. longum | Oral, 3–12 × 109 CFU/day | Taki et al., 2005 [56] | Modulating intestinal microbiota | Case series | Patients undergoing HD | 27 | Hcy ↘, IS ↘ |
| SCFA: sodium propionate | Oral, 1 g, QD | Marzocco et al., 2018 [57] | Anti-inflammation and antioxidation | Clinical trial | Patients undergoing HD | 20 | IS ↘, MDA ↘, PCS ↘ |
| Synbiotic: L. casei, B. breve, and galactooligosaccharides | Oral, 1 pack, TID | Nakabayashi el et al., 2011 [58] | Modulating intestinal microbiota | Clinical trial or case series | Patients undergoing HD | 9 | p-Cresol ↘ |
| Vegetarian | Oral | Kandouz et al., 2016 [59] | Improvement of metabolic acidosis, modification of intestinal microbiota | Cohort | Patients in hemodiafiltration | 138 | IS ↘, PCS ↘, Urea ↘ |
| Vitamin D | Oral, 300,000 IU, QD | Kumar et al., 2017 [53] | Anti-inflammation | RCT | Patients with nondiabetic CKD and vitamin D deficiency | 120 | IL-6 ↘, UA ↘ |
| Animal Studies | |||||||
| Diet® k/d® | Oral, 1.6 RER, QD | Hall et al., 2018 [60] | Anti-inflammation | Animal | CKD dogs | 36 | BUN ↘, Cr ↘, SDMA ↘ |
| Lingonberry | Oral, 5% w/w, QD | Madduma Hewageet al., 2020 [61] | Anti-inflammation | Animal | HFD-induced kidney injury mice | 30 | BUN ↘, Cr ↘, IL-6 ↘, TNF-α ↘ |
| MitoQ | i.v., 4 mg/kg, once | Hu et al., 2018 [62] | Antioxidation through reducing mitochondrial ROS | Animal | I/R mice | 24 | Cr ↘, IL-1β ↘, IL-6 ↘, TNF-α ↘ |
| Soluble Fiber and Omega-3 | Oral, 3666 kcal/kg, QD | Ephraim et al., 2020 [63] | Modulating intestinal microbiota | Animal | Dogs aged older than 7 years | 36 | phenolic UTs ↘, SDMA ↘ |
↘, decrease; BUN, blood urea nitrogen; BID, bis in die; CFU, colony-forming units; CKD, chronic kidney disease; Cr, creatinine; Hcy, homocysteine; HD, hemodialysis; HFD, high-flux dialysis; I/R, ischemia/reperfusion; IL-1β, interleukin-1 beta; IL-6, interleukin 6; IS, indoxyl sulfate; IU, International unit; i.v., intravenous; MDA, malondialdehyde; MitoQ, mitoquinone; OF-IN, oligofructose-enriched inulin; PCS, p-cresyl sulfate; QD, quaque die; RER, resting energy requirement; RCT, randomized controlled trial; SCFA, short-chain fatty acids; SDMA, symmetric dimethylarginine; UA, uric acids; UTs, uremic toxins.
3.1. Diet® k/d®
Prescription Diet® k/d®, an antioxidant-enriched food, includes the supplementation of alpha-linolenic acid (ALA) (18:3 [n-3]) to manage CKD in aging dogs. Dogs with International Renal Interest Society (IRIS) Stage 1 CKD fed with Prescription Diet k/d for 12 months exhibited decreased BUN, Scr, and symmetric dimethylarginine (SDMA) [60].
3.2. Lingonberry
Lingonberry (Vaccinium vitis-idaea L.) is an evergreen dwarf shrub rich in anthocyanins, which has anti-inflammatory, anti-diabetic, and renal protection properties [64,65]. In another study, mice were fed with a high-fat diet (HFD) to induce chronic kidney injury for investigating the effects of lingonberry on CKD. They found that the serum levels of inflammatory cytokines such as TNF-α and IL-6 decreased and renal injury improved in the HFD group that received lingonberry supplementation. In addition, lingonberry supplementation attenuated palmitic acid-induced nuclear factor-kappa B (NF-κB) activation and inflammatory cytokine expression in proximal tubule epithelial cells. Lingonberry supplementation may protect the kidney from metabolic abnormality-induced injury by preventing an inflammatory response [61].
3.3. Mitoquinone
Mitoquinone (MitoQ), a synthesized mitochondrially targeted antioxidant that contains a lipophilic triphenylphosphonium cation and coenzyme Q10, can block reactive oxygen species (ROS) and prevent mitochondrial oxidative damage which was applied to treat various diseases including liver fibrosis and neurodegenerative disease [66,67]. MitoQ reduced Scr, IL-1β, IL-6, and TNF-α levels in an I/R CKD mouse model. Moreover, MitoQ recovered intestinal barrier dysfunction by ameliorating mitochondrial deoxyribonucleic acid (DNA) damage [62].
3.4. Prebiotic Oligofructose-Enriched Inulin
Oligofructose, a type of oligosaccharide fructans, was used as an alternative sweetener. Probiotic bacteria such as Bifidobacteria and Lactobacilli are saccharolytic in nature. They cause oligofructose to produce SCFAs that exert a positive immunomodulatory effect, maintaining the symbiosis of intestinal microbiota [68]. In a nonrandomized, open-label trial of patients undergoing HD, administration of prebiotics composed of prebiotic oligofructose-enriched inulin (OF-IN) for 4 weeks reduced the generation rate and serum concentration of PCS but not that of IS. Another study proposed that prebiotics possessed the ability to modify intestinal uremic metabolites [54].
3.5. Probiotics
Probiotics were defined as “live microorganisms” by the World Health Organization (WHO) and Food and Agriculture Organization in 2002. Emerging evidence suggests that an appropriate supplementation of probiotics can improve gastrointestinal health, strengthen the immune system, lower the incidence of allergies, alleviate the symptoms of lactose intolerance, and reduce the risks of cancer [69,70]. The mechanisms by which probiotics provide their effects may involve modifying the pH of the intestine, defeating pathogens by producing antibacterial compounds, competitively excluding pathogens at the binding site, and combining of harmful mutagens and carcinogens [71]. Many studies have suggested that oral supplementation of a probiotic formula of selected microorganisms may exert a nephroprotective effect by extracting UTs in patients with CKD. In an RCT, the administration of Kibow Biotics probiotic formulation (L. acidophilus KB27, B. longum KB31, and S. thermophilus KB19) in patients with CKD reduced BUN, Scr, and UA levels; enhanced patients’ quality of life; and resulted in no adverse effects [55]. Another study demonstrated that the administration of a probiotic containing Bifidobacterium longum in gastric-resistant seamless capsules could effectively reduce serum Hcy and IS levels in patients with HD [56].
3.6. Short-Chain Fatty Acids
In CKD and end-stage renal disease (ESRD), gut-derived UTs can cause systemic inflammation, oxidative stress, and cause dysbiosis of the gut. When the anaerobic bacteria that can produce SCFA declines, resulting in insufficient production of SCFA [72]. SCFAs was mainly composed of acetate, propionate, and butyrate and exerted a positive immunomodulatory, anti-inflammatory, anti-oxidative, antibacterial, and anti-diabetic effects effect [73,74,75]. In a nonrandomized pilot study, the food additive sodium propionate (SP), an SCFA, was administered in the form of capsules, and this effectively reduced IS, MDA, and PCS in patients undergoing HD. Moreover, SP reduced pro-inflammatory parameters and oxidative stress and improved insulin resistance and iron metabolism [57].
3.7. Soluble Fiber and Omega-3 Fatty Acids
Omega-3 fatty acids (O3FAs) are a group of polyunsaturated fatty acids, among which eicosapentaenoic acid (EPA), ALA, and docosahexaenoic acid (DHA) are closely related to human physiology. O3FAs can be gained primarily from vegetable seed oils or aquatic organisms [76]. O3FA plays a crucial role in the eicosanoid biosynthesis. EPA competes with arachidonic acid to switch the synthesis pathway to promote the production of anti-inflammatory eicosanoids [77]. In addition, eicosanoids derived from O3FA have shown clinical benefits for cardiovascular disease, diabetes, and nephropathies due to their anti-inflammatory effects [77,78].
Omega-3 supplementation could improve oxidative stress in patients with CKD [79]. Eating fiber-rich foods can reduce the risk of kidney disease, inflammation, and death [80]. Soluble fiber and omega-3 fatty acids may reduce IS, PCS, and SDMA by modulating the gut microbiota [63].
3.8. Synbiotic
Synbiotics, a combination of probiotics and prebiotics, has immune system regulation and anti-inflammatory effects. It can improve the intestinal environment and reduce concentration of nitrogen-containing metabolites [81,82], was applied to treat intestinal chronic diseases [82], allergic rhinitis and asthma [83]. An uncontrolled trial showed that a synbiotic consisting of Lactobacillus casei, Bifidobacterium breve, and galactooligosaccharides reduced the PCS level but did not change IS or phenol levels in in patients undergoing HD [58].
3.9. Vegetarian Diet
Plants are the dietary source of fibers, which shifts the gut microbiota to increased generation of anti-inflammatory compounds and reduced production of uremic toxins [84]. Vegetarian diets have a low endogenous acid load, which could reduce metabolic acidosis in CKD patients and potentially slow CKD progression [84,85]. Moreover, in the primary prevention of CKD, our previous study showed that vegetarian diets a strong negative association between vegetarian diets and the prevalence of CKD [86].
Another review supports this observation and revealed that plant-based foods and Mediterranean (MD) diet or Dietary Approaches to Stop Hypertension (DASH) diets could reduce many types of UTs; however, the potential risk of hyperkaliemia should be examined [87]. A cohort study revealed that vegetarian diet patients in hemodiafiltration have not only lower plasma levels of IS and PCS, but also lower serum level of urea [59].
3.10. Vitamin D
CKD patients are prone to vitamin D deficiency, which can cause a variety of diseases. Higher 25-hydroxyvitamin D (25[OH]D) levels can significantly improve the survival rate of CKD patients [88].
Case–control studies have shown that vitamin D supplementation could improve endothelial dysfunction in patients with CKD [89]. An RCT reported that vitamin D supplementation for 8 weeks could reduce the IL-6 and UA levels in patients with nondiabetic CKD stage 3–4 and vitamin D deficiency. In addition, vitamin D supplementation could improve vascular function, as determined by significant positive changes in pulse wave velocity [53].
4. Complementary and Alternative Medicine Therapy
CAM therapy is widely used in CKD treatment. A meta-analysis including 20 RCTs showed that in patients with diabetic kidney disease (DKD), traditional Chinese medicine (TCM) as an adjunctive treatment significantly reduced BUN and Scr levels, and improved uremic protein excretion and quality of life compared to the placebo group with less adverse events [90]. The clinical data of high-quality CAM therapy is indeed scarce. Prescriptions and acupuncture have been studied in RCT grade [91,92], and the evidence of moxibustion belongs to meta-analysis grade [93]. However, most of the CAM therapy studies are still animal experiments. Fortunately, it is indeed observed that CAM therapy has the effect of reducing urinary toxins and improving renal function in animal experiments [94]. Increasing evidence has revealed that CAM therapy can slow the progression of renal damage through mechanisms such as improving renal microcirculation, exerting anti-inflammatory and antioxidative effects, and inhibiting programmed cell death [94]. Therefore, this section briefly describes the nephroprotective effects of CAM therapies and their ability to control UTs. CAM therapeutic methods applied for CKD include TCM preparations, herbal active principle, acupuncture, and moxibustion (Table 3).
Table 3.
CAM treatments for the control of UTs.
| Intervention | Route, Dosage, and Frequency | Author/Year | Mechanism/Usage | Study Design | Subjects | Subject Number | Result |
|---|---|---|---|---|---|---|---|
| Clinical Studies | |||||||
| Curcuma longa and Boswellia serrata | Oral, 1 capsule, BID | Moreillon et al., 2013 [91] | Anti-inflammation, inhibition of NF-Κb and MAPK | RCT | Patients with CKD | 16 | IL-6 ↘ |
| UCG | Oral, 5 g, TID and 10 g HS | Zheng et al., 2017 [95] | Anti-inflammation and antifibrosis | RCT | Patients with CKD | 292 | Cr ↘ |
| Acupuncture | External, LI4, ST36 and KI3, 1 time/week | Yu et al., 2017 [92] | Improving renal local microcirculation | RCT | Patients with CKD | 59 | Cr ↘ |
| Moxibustion | External, 0.5~7 sessions/week | Zhou et al., 2020 [93] | Dilating local renal capillaries, alleviating kidney podocyte injury | MA | Patients with CKD | 1571 | BUN ↘, Cr ↘ |
| Animal Studies | |||||||
| DFD | Gastric gavage, 2.5 g/kg, QD | Tu et al., 2014 [96] | Inhibiting apoptosis by blocking TGF-b1-JNK | Animal | Adenine-induced renal injury rats | 27 | BUN ↘, Cr ↘, UA ↘ |
| DHI and salvianolic acids | Extracorporeal, DHI 4.16 mL/kg or LA 24.69 mg/kg, once | Li et al., 2019 [97] | Protein-binding competitors | Animal | CKD rats with accumulated IS and pCS | 16 | Enhanced dialysis removal of IS and pCS |
| UCG | Gastric gavage, 5 g/kg, QD | Huang et al., 2014 [98] | Antifibrosis, regulation of ECM degradation | Animal | Adenine and UUO-induced renal failure rats | 26 | BUN ↘, Cr ↘, UA ↘ |
| ZDW | i.p., 2 g/kg, once | Hsu et al., 2014 [99] | Attenuation of apoptosis through limiting of caspase-3 activation | Animal | Gentamicin-induced renal injury rat |
12 | BUN ↘Cr ↘ |
| ZDW | Embryo exposure, 100 ppm, once | Lu et al., 2020 [100] | Suppression of proinflammatory gene expression | Animal | AA-intoxicated zebrafish embryos | 150 | tnf-α ↘ |
| Catechin | Oral, 100 mg/kg, QD | Korish et al., 2008 [101] | Antioxidation | Animal | 5/6 nephrectomy rats | 40 | ADMA ↘ |
| Cyanidin-3-O-glucoside (C3G) | i.p., 20 mg/kg, QD | Qin et al., 2018 [102] | Antioxidation | Animal | db/db mice with DN | 60 | BUN ↘, Cr ↘ |
| EGCG | i.p., 50 mg/kg, QD | Wang et al., 2015 [103] | Anti-inflammation and antioxidation through inhibition of the NF-κB signaling pathway and activation of the Nrf2-Keap1 pathway | Animal | UUO mice | 24 | BUN ↘, Cr ↘ |
| Gypenoside (GP) | i.v., 50 mg/kg, once | Ye et al., 2016 [104] | Attenuating inflammatory and oxidative stress by inhibiting ERK signaling | Animal | I/R-induced renal injury mice | 30 | BUN ↘, Cr ↘, IL-1β ↘, IL-6 ↘, MDA ↘, TNF-α ↘ |
| Huangkui capsule | Gastric gavage, 0.75 g/kg, QD | Cai et al., 2017 [105] | Inhibition of the NADPH oxidase/ROS/ERK pathway | Animal | Adenine-induced CRF Rats | 18 | BUN ↘, Cr ↘ |
| Huangkui capsule | Gastric gavage, 0.675 g/kg, QD | Wang et al., 2019 [106] | Inhibition of the transformation of Trp to indole | Animal | 5/6 nephrectomy Rats | 21 | IS ↘ |
| Leonurine (LEO) | i.v., 50 mg/kg, QD | Xu et al., 2014 [107] | Inhibition of inflammatory and oxidative stress through downregulation of NF-kB | Animal | LPS-induced renal injury mice | 120 | BUN ↘, Cr ↘, IL-1 ↘, IL-6 ↘, IL-8 ↘, MDA ↘, TNF-α ↘ |
| Ligustrazine (LIG) | i.p., 80 mg/kg, once | Feng et al., 2011 [108] | Downregulation of oxidative stress and apoptosis, decrease in neutrophil infiltration | Animal | I/R-induced renal injury mice | 48 | MDA ↘, TNF-α ↘ |
| Notoginsenoside R1 (NR1) | i.p., 80 mg/kg, once | Liu et al., 2010 [109] | Blocking apoptosis and inflammatory response by suppressing p38 and NF-kB | Animal | I/R-induced renal injury rats | 24 | Cr ↘, TNF-α ↘ |
| Osthole | i.p., 40 mg/kg, once | Luo et al., 2016 [110] | Abrogating inflammation by suppressing JAK2/STAT3 signaling, activating PI3K/Akt signaling | Animal | I/R-induced renal injury rats | 70 | BUN ↘, Cr ↘, IL-6 ↘, TNF-α ↘ |
| Paeoniflorin (PF) | i.p., 30 mg/kg, once | Liu et al., 2015 [111] | Attenuation of inflammatory response by inhibiting CXCR3/CXCL | Animal | ConA-induced renal injury mice | 60 | BUN ↘, Cr ↘, IL-1β ↘ |
| Resveratrol | Gastric Gavage, 1 mg/kg, QD | Chen et al., 2016 [112] | Modulation of intestinal microbiota | Animal | ApoE(-/-) mice | 20 | TMAO ↘ |
| Tanshinone I | i.p., 120 mg/kg, QD | Feng et al., 2013 [113] | Enhancement of AAI metabolism by induction of CYP1A | Animal | AAI-induced renal injury mice | 40 | BUN ↘, Cr ↘ |
| Rhubarb | Enema, 0.5 g, QD | Lu et al., 2015 [114] | Antioxidation, anti-inflammation | Animal | 5/6 nephrectomy rats | 28 | Cr ↘, IS ↘ |
| Rhubarb | Enema, 2.12 g/kg, QD | Ji et al., 2020 [115] | Modulation of intestinal microbiota, improving the intestinal barrier, anti-inflammation |
Animal | 5/6 nephrectomy rats | 30 | IL-1β ↘, IL-6 ↘ |
| SkQR1 | i.p., 400 nmol/kg, once | Plotnikov et al., 2011 [116] | Antioxidation | Animal | Glycerol-induced rhabdomyolysis rats | 36 | BUN ↘, MDA ↘ |
↘, decrease; AA, aristolochic acid; ADMA, asymmetric dimethylarginine; ApoE, apolipoprotein E; BID, bis in die; BUN, blood urea nitrogen; CKD, chronic kidney disease; ConA, concanavalin A; Cr, creatinine; CRF, chronic renal failure; CXCR3/CXCL, c-X-c motif chemokine receptor 3/C-X-C motif Chemokine ligand; CYP1A, cytochrome P450 1A; DFD, Dahuang Fuzi Decoction; DHI, Danhong injection; DN, diabetic nephropathy; ECM, extracellular matrix; EGCG, eepigallocatechin-3-gallate; ERK, extracellular signal-regulated kinase; HS, hora somni; i.p., intraperitoneal injection; I/R, ischemia/reperfusion; IL-1, interleukin-1; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IS, indoxyl sulfate; JAK2/STAT3, Janus kinase 2/signal transducer and activator of transcription 3; LA, lithospermic acid; MA, meta-analysis; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; NADPH, nicotinamide adenine dinucleotide phosphate; NFκB, nuclear factor kappa B; Nrf2-keap1, nuclear factor E2-related factor 2-Kelch-like ECH-associated protein 1; PCS, p-cresyl sulphate; PI3K/Akt, phosphatidylinositol 3-kinase/Ak strain transforming; QD, quaque die; RCT, randomized controlled trial; ROS, reactive oxygen species; SkQR1, 10-(6′-plastoquinonyl) decylrhodamine; TGF β1-JNK, transforming growth factor-beta-1-c-Jun N-terminal kinase; TID, ter in die; TMAO, trimethylamine N-oxide; TNF-α, tumor necrosis factor alpha; UA, uric acid; UCG, uremic clearance granule ; UUO, unilateral ureteral obstruction; ZDW, Zhibai Dihuang Wan.
4.1. Curcuma Longa and Boswellia Serrata
Dietary turmeric, rich in curcumin, has anti-inflammatory, anti-oxidant, and chemotherapeutic effects [117]. Curcumin has been shown to reduce inflammation in patients with type 2 diabetes [118]. CKD rat models have confirmed the anti-inflammatory effects of curcumin [119], and Boswellia serrata could improve the inflammatory symptoms of colitis and knee osteoarthritis [120,121]; however, their efficacy in patients with CKD has not been examined. An RCT including patients with CKD revealed a significant decrease in plasma IL-6 following treatment with oral C. longa and B. serrata for 8 weeks compared with a placebo group; however, other variables such as TNF-α and CRP exhibited no significant differences. Moreillon et al. indicated that C. longa and B. serrata reduce IL-6 possibly through inhibition of the pathways of NF-κB and mitogen-activated protein kinase [91].
4.2. Dahuang Fuzi Decoction
Dahuang Fuzi Decoction (DFD), a well-known traditional Chinese prescription, consists Dahuang (Radix et Rhizoma Rhei), Paofuzi (Radix Aconiti Lateralis Preparata), and Xixin (Radix et Rhizoma Asari), originated from the Synopsis of Golden Chamber [96]. DFD is often used to treat gynecological diseases and CKD [122], it could also reduce BUN, Scr, and UA levels in an adenine-induced renal injury rat model. Moreover, DFD could block the activation of transforming growth factor beta 1 c-Jun N-terminal kinase (TGF-b1-JNK) pathways to mitigate renal damage and tubular epithelial apoptosis [96].
4.3. Danhong Injection and Salvianolic Acids
Danhong injection (DHI) contains the extraction of two Chinese medicines, the radix and rhizome of Salvia miltiorrhiza Bunge (Labiatae) and the flower of Carthamus tinctorius L. (Asteraceae) [123]. Salvianolic acid B, rosmarinic acid, and lithospermic acid are powerful protein-binding ligands, and DHI and salvianolic acids are rich in these substances [123]. In a nephrectomized CKD rat model, injection of DHI and its salvianolic acids through the caudal vein increased the dialysis removal of IS and PCS. DHI and its salvianolic acids showed favorable ability as protein-bound competitors [97].
4.4. Uremic Clearance Granule
Uremic clearance granule (UCG), also called as NiaoDuQing (NDQ), created based on the TCM theory consisting of 16 herbs, such as Rheum officinale, Glycyrrhiza uralensis, Astragalus membranaceus, Poria cocos, Sophora flavescens, Chrysanthemum morifolium, and so forth, is the first Chinese medicine approved by the China Food and Drug Administration for the treatment of CKD. UCG has been clinically demonstrated to slow CKD progression [124]. Its effects include improving systemic micro-inflammation [125], reducing Scr [95], and calibrating calcium and phosphorus metabolic disorders [126]. However, its mechanism has not been thoroughly studied [95]. A multicenter double-blind, placebo-controlled, and randomized clinical trial recruited 292 patients with stage 3b-4 CKD proved that UCG could delay CKD progression safely and effectively [95]. In an adenine and unilateral ureteral obstruction (UUO)-induced CKD rat model, Huang et al. reported that the administration of UCG reduced BUN, Scr, and UA levels. In addition, UCG exhibited antifibrosis ability through regulation of extracellular matrix (ECM) degradation and associated signaling pathway activity [98].
4.5. Zhibai Dihuang Wan
Zhibai Dihuang Wan (ZDW) is a TCM preparation consisting of Cornus officinalis Siebold and Zucc, Rehmannia glutinosa (Gaertn.) DC., root, baked, Dioscorea oppositifolia L., Phellodendron amurense Rupr., bark, Anemarrhena asphodeloides Bunge, rhizome, Paeonia suffruticosa Andrews, root bark, Alisma plantago-aquatica L., rhizome and Poria cocos (Schw.)Wolf, that has been used to treat CKD and diabetes for thousands of years [127]. ZDW reduced BUN and Scr levels in rats with gentamicin-induced renal injury and exerted a protective effect on renal tubular cells through antiapoptotic effects by limiting caspase-3 activation [99]. Moreover, a study of aristolochic acid (AA)-intoxicated zebrafish demonstrated that ZDW treatment could attenuate AA-induced kidney malformations and suppress the expression levels of TNF-α [100].
4.6. Catechin Combined with Vitamin C and Vitamin E
Catechin, a type of flavonoid, is found in green tea and other plant foods, which has the benefit of protecting against congestive heart failure and reducing the incidence of myocardial ischemia [128,129]. Catechin, vitamin C, and vitamin E all have the favorable antioxidant capacity [130,131,132]. A study performed 5/6 nephrectomy in aged Wistar rats to explore the mechanism through which catechin protects against renal dysfunction. Catechin with vitamin C and E supplementation could reduce oxidative stress resulting from aging and renal failure and prevent ADMA accumulation [101].
4.7. Cyanidin-3-O-Glucoside
Anthocyanins are polyphenol compounds that are abundant in blueberries and purple corn [133], which can reduce the risk of cardiovascular disease-related mortality [134]. Through in vitro and in vivo investigations, Qin et al. found that cyanidin-3-O-glucoside (C3G), the most widespread anthocyanin, acts as an antioxidative agent and regulates glutathione metabolism, resulting in the reduction of BUN, Scr, and urine albumin–creatinine ratio (UACR) and the amelioration of pathological changes in kidney biopsy in db/db mice [102].
4.8. Epigallocatechin-3-Gallate
Green tea is rich in a variety of catechin polyphenols, which has anti-inflammatory, anti-oxidant, anti-viral, and anti-cancer effects [135,136,137]. Epigallocatechin-3-gallate (EGCG), which is extracted from green tea, is the richest and the most active catechin polyphenol. A UUO-induced CKD mouse study suggested that EGCG exerts anti-inflammatory and antioxidative effects by inhibiting the NF-κB signaling pathway and activating the nuclear factor E2-related factor 2-Kelch-like ECH-associated protein 1 (Nrf2-Keap1) pathway [103].
4.9. Gypenoside
Gynostemma pentaphyllum is a widely used and safe TCM. Recent studies have shown that it has anti-cancer, cardioprotective, anti-diabetic, and anti-inflammatory activities [138]. Gypenoside (GP), a major component of Gynostemma pentaphyllum, exerts anti-inflammatory and antioxidative effects [139]. However, whether GP can attenuate CKD remains unclear. In mice with renal I/R injury, intravenous GP injection reduced BUN, Scr, TNF-α IL-1β, IL-6, and MDA by inhibiting extracellular signal-regulated kinase (ERK) signaling [104].
4.10. Huangkui Capsule
Huangkui capsule, which is prepared using the extract of the flower Abelmoschus manihot (Linn.) Medicus (A. manihot), was approved by China’s State Food and Drug Administration in 1999 for treating chronic nephritis. Studies have confirmed the renal protective effect of the Huangkui capsule in vivo, in vitro, and clinically [105,140]. Flavonoids, the main chemical component of the Huangkui capsule, can be converted into glucuronic acid-sulfate conjugates in vivo, which may contribute to the nephroprotective effects [141]. A multicenter RCT showed that Huangkui capsule has antiproteinuric effect for patients with early stage primary glomerulonephritis, and the other multicenter, double-blind, double-dummy RCT also showed that it can effectively reduce proteinuria in patients with IgA nephropathy. Both RCTs showed that Huangkui capsule treatment has no obvious side effects [142,143]. Cai et al. demonstrated that the Huangkui capsule reduced BUN and Scr in adenine-induced chronic renal failure (CRF) rats. It exerted a preventive effect on tubulointerstitial fibrosis, which participates in the mechanism that inhibits the nicotinamide adenine dinucleotide phosphate oxidase (NADPH)/ROS/ERK pathway [105]. Another 5/6 nephrectomy rat model study revealed that the Huangkui capsule inhibited the synthesis of indole by gut bacteria by interfering with the transport of tryptophan, thereby effectively inhibiting the accumulation of IS in CKD rat [106].
4.11. Leonurine
Leonurus cardiac has antibacterial, antioxidant, and anti-inflammatory activities. For thousands of years, it has been traditionally used in China for gynecological diseases such as uterotonic action and postpartum blood stasis. Leonurus cardiac has been used in modern times to treat nervous system dysfunction and cardiovascular disease [144]. Leonurine (LEO), an alkaloid isolated from Leonurus cardiac, exerts antioxidative effects [145]. LEO reduced BUN and Scr levels and downregulated TNF-α, IL-1, IL-6, IL-8, and kidney injury molecule-1 (KIM-1) expression by inhibiting the ROS-mediated NF-kB signaling pathway in a mouse model of lipopolysaccharide (LPS)-induced renal injury [107].
4.12. Ligustrazine
Ligustrazine (LIG), an alkaloid extracted from Ligusticum wallichii, could reduce I/R-induced hepatic and endothelial cell damage by scavenging cytotoxic oxygen free radicals [146,147]. LIG reduced MDA and TNF-α levels in I/R-induced renal injury mice by downregulating oxidative stress and apoptosis and reducing neutrophil infiltration [108].
4.13. Notoginsenoside R1
Panax notoginseng is a TCM that promotes blood circulation and removes blood stasis. It is widely used in China to treat cardiovascular diseases. A study reported that notoginsenoside R1 (NR1) is the main component of Panax notoginseng that possesses anti-inflammatory and anticoagulant properties [148]. NR1 mitigated I/R-induced kidney dysfunction as determined by the reduced levels of serum Cr and TNF-α, possibly through suppressing the p38/NF-kB pathway in a rat model of I/R-induced renal injury [109].
4.14. Osthole
Osthole isolated from Cnidium moonnieri (L.) Cussion, which is a coumarin derivative, protects cerebral artery occlusion injury by exerting anti-inflammatory effects [149]. In an I/R-induced renal injury model, Luo et al. demonstrated that the administration of osthole reduced TNF-α, IL-8, and IL-6 levels. Osthole prevents renal injury by suppressing the Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway and activating PI3K/Akt signaling pathway [110].
4.15. Paeoniflorin
Paeoniflorin (PF) is a bioactive monoterpene glycoside extracted from Radix Paeoniae Rubra, which could inhibit colitis by inhibiting the expression of Toll-like receptor 4 (TLR4) [150] and liver fibrosis by inhibiting macrophage activation [151]. PF reduced BUN, Scr, and IL-1β levels and attenuated inflammatory responses by inhibiting CXCR3/CXCL activation in mice with concanavalin A (ConA)-induced renal injury [111].
4.16. Resveratrol
Resveratrol (RSV), a natural polyphenol that originates in grapes, berries, and other dietary constituents, exerts antioxidant activity and anti-atherosclerotic effects by modulating the growth of certain gut microbiota, such as Lactobacillus and Bifidobacterium [152,153]. RSV can promote the release of nitric oxide and prostacyclin to maintain endothelial function and adjust vascular tone [154]. RCT confirmed that RSV has cardioprotective effects in patients with stable coronary artery disease [155]. Chen et al. reported that RSV attenuated TMAO in TMAO-induced atherosclerosis mice through the remodeling of gut microbiota [112].
4.17. Rhubarb
Rhubarb is a common herbal medicine composed of the dried rhizomes and roots of Rheum palmatum L, Rheum tanguticum Maxim that is broadly used in sepsis and pancreatitis to protect the intestinal mucosal barrier [156,157]. Rhubarb enema could alleviate IS overload to ameliorate renal tubulointerstitial fibrosis in the 5/6 nephrectomy renal injury rats model by reducing kidney oxidative stress and inflammatory damage [114]. Furthermore, Ji et al. reported that rhubarb enema could reduce IL-1β and IL-6 levels in 5/6 nephrectomy renal injury rats by modifying intestinal microbiota [115].
4.18. 10-(6′-Plastoquinonyl) Decylrhodamine 19
Mitochondria play an important role in the pathogenesis of uremia, and their damage will lead to the accumulation of UTs [14]. The compartment of the mitochondria is negatively charged in the cell, the design of active molecules combined with cationic carriers can accumulate in mitochondria due to their positive charges [158]. 10-(6′-plastoquinonyl) decylrhodamine 19 (SkQR1), a mitochondrial-targeted antioxidant conjugated with plastoquinone and cationic decylrhodamine 19, possesses high antioxidant capacity in animal models and in vitro [159]. SkQR1 exerted renal protection by increasing erythropoietin levels in gentamicin-induced renal injury rats. In addition, SkQR1 could reduce BUN and MDA levels [116].
4.19. Tanshinone I
Danshen, also named Salvia miltiorrhiza, is a traditional Chinese medicine often used to treat cardiovascular diseases. Tanshinone I, an active component of Salvia miltiorrhiza Bunge, has the effects of anti-inflammation, cardiovascular protection, anti-tumor, and anti-hepatic fibrosis [160,161]. Tanshinone I exerted a renal protective effect, as indicated by reduced BUN and Scr levels, through the enhancement of AA metabolism by inducing CYP1A in a mouse model of AA-induced renal injury [113].
4.20. Acupuncture
Acupuncture is a popular CAM therapeutic method that can effectively treat certain diseases such as pain and insomnia; this method is recognized by WHO [162]. Acupuncture can stimulate the production of endomorphin-1, encephalin, β-endorphin, and serotonin in plasma and brain tissue, thereby achieving the effects of analgesia, sedation, and immune regulation [163]. In an RCT including 53 patients with CKD, once-weekly electroacupuncture at bilateral Hegu (LI4), Zusanli (ST36), and Taixi (KI3) for 12 weeks improved renal function; the reduction in Scr levels induced by reduced eGFR was greater in the acupuncture group than in the sham acupuncture group [92]. Acupuncture might improve renal function by regulating sympathetic nerves and activating biologically active chemicals [164].
4.21. Moxibustion
Moxibustion, a CAM therapy that consists of burning dried moxa on particular acupoints on the body, is used to treat a variety of diseases, such as malposition, diarrhea, soft tissue damage, and dysmenorrhea [165]. Moxibustion methods can be divided into direct moxibustion and indirect moxibustion. Direct moxibustion is to place moxa sticks on acupuncture points, while indirect moxibustion is defined as placing medicinal materials (e.g., mugwort, ginger, etc.) between moxa sticks and acupuncture points [166]. Moreover, the moxa sticks are placed on acupuncture needles inserted into the skin to improve the curative effect [167]. The pharmacological effects of moxibustion are the heat and light radiation of moxibustion burning and aromatherapy [168,169]. Moxibustion may improve CKD by exerting anti-inflammatory effects [170]. A meta-analysis including 23 RCTs revealed that in patients with CKD, moxibustion therapy significantly reduced BUN and Scr levels and improved uremic protein excretion and quality of life. In patients with CKD, moxibustion causes expansion of local renal capillaries and reduces renal podocyte damage [93].
5. Conclusions
Different treatments, such as medicine, diet control, diet supplement, and CAM, employ different mechanisms of action to improve kidney function or reduce different UTs, including the inhibition of inflammation, cell apoptosis, removal of toxins, regulation of intestinal bacteria, and inhibition of oxidative stress. This evidence supports the potential applications of these therapies. Growing evidence has revealed that the intestine is the main source of UTs. Regulating diet and modulating intestinal microbiota can reduce the accumulation of UTs. Recent studies have indicated that reducing the production of UTs in mitochondria can protect mitochondria, thus reducing the pathological consequences of CKD. Some Chinese medicines have shown the ability to protect the kidneys and reduce UTs; however, certain Chinese medicines are harmful to the kidneys and increased attention should be focused on the use of Chinese medicines. The scale of clinical trials on Chinese medicines is small, and many treatments are still in the animal experiment stage; however, additional large-scale trials are needed to examine their effects on reducing UTs or improving residual renal functions.
Acknowledgments
We greatly appreciate technical support from the Core Laboratory of the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation.
Author Contributions
P.-H.L. and K.-L.K.: contributed to concept generation, data interpretation, drafting of the manuscript, critical revision of the manuscript, and approval of the article; M.-C.Y. and M.-J.W.: contributed to data interpretation, drafting of the manuscript, and approval of the article. K.-L.K.: contributed to supervision and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology (MOST 108-2314-B-303-006-MY3), Taipei Tzu Chi Hospital (TCRD-TPE-MOST-110-08 and TCRD-TPE-110-03), Buddhist Tzu Chi Medical Foundation, Taiwan (TCMF-EP 109-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This article integrates nonextracorporeal therapies for uremic toxins from bench to bedside based on the intestinal-renal axis theory of uremic toxins generation. We classified the nonextracorporeal therapies for uremic toxins into three categories: medication, diet and supplement therapies, as well as complementary and alternative medicine therapies.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coresh J., Selvin E., Stevens L.A., Manzi J., Kusek J.W., Eggers P., Van Lente F., Levey A.S. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C., Agodoa L.Y., Bhave N., Bragg-Gresham J., Balkrishnan R., Dietrich X., Eckard A., Eggers P.W. US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duranton F., Cohen G., De Smet R., Rodriguez M., Jankowski J., Vanholder R., Argiles A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung S.C., Kuo K.L., Huang H.L., Lin C.C., Tsai T.H., Wang C.H., Chen J.W., Lin S.J., Huang P.H., Tarng D.C. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016;89:574–585. doi: 10.1016/j.kint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki Y., Kazama J.J., Yamato H., Shimoda H., Fukagawa M. Accumulated uremic toxins attenuate bone mechanical properties in rats with chronic kidney disease. Bone. 2013;57:477–483. doi: 10.1016/j.bone.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Soulage C.O., Koppe L., Fouque D. Protein-bound uremic toxins… new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease. J. Ren. Nutr. 2013;23:464–466. doi: 10.1053/j.jrn.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Lau W.L., Savoj J., Nakata M.B., Vaziri N.D. Altered microbiome in chronic kidney disease: Systemic effects of gut-derived uremic toxins. Clin. Sci. 2018;132:509–522. doi: 10.1042/CS20171107. [DOI] [PubMed] [Google Scholar]
- 8.Lu P.-H., Tai Y.-C., Yu M.-C., Lin I.-H., Kuo K.-L. Western and complementary alternative medicine treatment of uremic pruritus: A literature review. Tzu Chi Med. J. 2021 doi: 10.4103/tcmj.tcmj_151_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo K.-L., Zhao J.-F., Huang P.-H., Guo B.-C., Tarng D.-C., Lee T.-S. Indoxyl sulfate impairs valsartan-induced neovascularization. Redox Biol. 2020;30:101433. doi: 10.1016/j.redox.2020.101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanholder R., Glorieux G., De Smet R., Lameire N., European Uremic Toxin Work Group New insights in uremic toxins. Kidney Int. Suppl. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 11.Castillo-Rodríguez E., Pizarro-Sánchez S., Sanz A., Ramos A., Sanchez-Niño M., Martin-Cleary C., Fernandez-Fernandez B., Ortiz A. Inflammatory Cytokines as Uremic Toxins: “Ni Son Todos Los Que Estan, Ni Estan Todos Los Que Son”. Toxins. 2017;9:114. doi: 10.3390/toxins9040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanholder R.C., Eloot S., Glorieux G.L. Future Avenues to Decrease Uremic Toxin Concentration. Am. J. Kidney Dis. 2016;67:664–676. doi: 10.1053/j.ajkd.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Koppe L., Fouque D., Soulage C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins. 2018;10:155. doi: 10.3390/toxins10040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popkov V.A., Silachev D.N., Zalevsky A.O., Zorov D.B., Plotnikov E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019;20:3094. doi: 10.3390/ijms20123094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010;20:S2–S6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Saito H., Yoshimura M., Saigo C., Komori M., Nomura Y., Yamamoto Y., Sagata M., Wakida A., Chuman E., Nishi K., et al. Hepatic sulfotransferase as a nephropreventing target by suppression of the uremic toxin indoxyl sulfate accumulation in ischemic acute kidney injury. Toxicol. Sci. 2014;141:206–217. doi: 10.1093/toxsci/kfu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumida K., Yamagata K., Kovesdy C.P. Constipation in CKD. Kidney Int. Rep. 2020;5:121–134. doi: 10.1016/j.ekir.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Yu Y.-B. Intestinal microbiota and chronic constipation. SpringerPlus. 2016;5:1–8. doi: 10.1186/s40064-016-2821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijers B., Glorieux G., Poesen R., Bakker S.J. Nonextracorporeal methods for decreasing uremic solute concentration: A future way to go? Semin. Nephrol. 2014;34:228–243. doi: 10.1016/j.semnephrol.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto A., Takeda M., Taki K., Takayama F., Noshiro R., Niwa T., Endou H. Interactions of human organic anion as well as cation transporters with indoxyl sulfate. Eur. J. Pharmacol. 2003;466:13–20. doi: 10.1016/S0014-2999(03)01530-9. [DOI] [PubMed] [Google Scholar]
- 21.Fülöp T., Zsom L., Tapolyai M.B., Molnar M.Z., Salim S.A., Arany I., Hamrahian M., Rosivall L. Peritoneal dialysis: The unique features by compartmental delivery of renal replacement therapy. Med. Hypotheses. 2017;108:128–132. doi: 10.1016/j.mehy.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Lameire N., Vanholder R., De Smet R. Uremic toxins and peritoneal dialysis. Kidney Int. Suppl. 2001;78:S292–S297. doi: 10.1046/j.1523-1755.2001.59780292.x. [DOI] [PubMed] [Google Scholar]
- 23.Vanholder R., Baurmeister U., Brunet P., Cohen G., Glorieux G., Jankowski J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008;19:863–870. doi: 10.1681/ASN.2007121377. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine S., Pillot B., Rognant N., Augeul L., Rayberin M., Varennes A., Laville M., Ovize M., Juillard L. Postconditioning with cyclosporine a reduces early renal dysfunction by inhibiting mitochondrial permeability transition. Transplantation. 2015;99:717–723. doi: 10.1097/TP.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 25.Koyama K., Ito A., Yamamoto J., Nishio T., Kajikuri J., Dohi Y., Ohte N., Sano A., Nakamura H., Kumagai H., et al. Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am. J. Kidney Dis. 2010;55:1069–1078. doi: 10.1053/j.ajkd.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Evenepoel P., Bammens B., Verbeke K., Vanrenterghem Y. Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: A pilot study. Kidney Int. 2006;70:192–198. doi: 10.1038/sj.ki.5001523. [DOI] [PubMed] [Google Scholar]
- 27.Goicoechea M., de Vinuesa S.G., Verdalles U., Ruiz-Caro C., Ampuero J., Rincón A., Arroyo D., Luño J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saigo C., Nomura Y., Yamamoto Y., Sagata M., Matsunaga R., Jono H., Nishi K., Saito H. Meclofenamate elicits a nephropreventing effect in a rat model of ischemic acute kidney injury by suppressing indoxyl sulfate production and restoring renal organic anion transporters. Drug Des. Dev. Ther. 2014;8:1073–1082. doi: 10.2147/DDDT.S67456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garibotto G., Sofia A., Parodi E.L., Ansaldo F., Bonanni A., Picciotto D., Signori A., Vettore M., Tessari P., Verzola D. Effects of Low-Protein, and Supplemented Very Low-Protein Diets, on Muscle Protein Turnover in Patients With CKD. Kidney Int. Rep. 2018;3:701–710. doi: 10.1016/j.ekir.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huo X., Meng Q., Wang C., Zhu Y., Liu Z., Ma X., Ma X., Peng J., Sun H., Liu K. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs) Acta Pharm. Sin. B. 2019;9:986–996. doi: 10.1016/j.apsb.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asai M., Kumakura S., Kikuchi M. Review of the efficacy of AST-120 (KREMEZIN(®)) on renal function in chronic kidney disease patients. Ren. Fail. 2019;41:47–56. doi: 10.1080/0886022X.2018.1561376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y.-C., Wu M.-Y., Hu P.-J., Chen T.-T., Shen W.-C., Chang W.-C., Wu M.-S. Effects and safety of an oral adsorbent on chronic kidney disease progression: A systematic review and meta-analysis. J. Clin. Med. 2019;8:1718. doi: 10.3390/jcm8101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fatouros I.G., Douroudos I., Panagoutsos S., Pasadakis P., Nikolaidis M.G., Chatzinikolaou A., Sovatzidis A., Michailidis Y., Jamurtas A.Z., Mandalidis D., et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med. Sci. Sports Exerc. 2010;42:1809–1818. doi: 10.1249/MSS.0b013e3181dbacab. [DOI] [PubMed] [Google Scholar]
- 34.Trimarchi H., Schiel A., Freixas E., Díaz M. Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron. 2002;91:58–63. doi: 10.1159/000057605. [DOI] [PubMed] [Google Scholar]
- 35.Marzocco S., Dal Piaz F., Di Micco L., Torraca S., Sirico M.L., Tartaglia D., Autore G., Di Iorio B. Very low protein diet reduces indoxyl sulfate levels in chronic kidney disease. Blood Purif. 2013;35:196–201. doi: 10.1159/000346628. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y.F. Analysis on the effects of reduced glutathione intervening in microinflammation of uremia patients with maintenance hemodialysis. Chin. J. Front. Med. Sci. 2016;8:101–104. [Google Scholar]
- 37.Sato E., Saigusa D., Mishima E., Uchida T., Miura D., Morikawa-Ichinose T., Kisu K., Sekimoto A., Saito R., Oe Y., et al. Impact of the Oral Adsorbent AST-120 on Organ-Specific Accumulation of Uremic Toxins: LC-MS/MS and MS Imaging Techniques. Toxins. 2017;10:19. doi: 10.3390/toxins10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sener G., Paskaloglu K., Satiroglu H., Alican I., Kaçmaz A., Sakarcan A. L-Carnitine Ameliorates Oxidative Damage due to Chronic Renal Failure in Rats. J. Cardiovasc. Pharmacol. 2004;43:698–705. doi: 10.1097/00005344-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Konop M., Radkowski M., Grochowska M., Perlejewski K., Samborowska E., Ufnal M. Enalapril decreases rat plasma concentration of TMAO, a gut bacteria-derived cardiovascular marker. Biomarkers. 2018;23:380–385. doi: 10.1080/1354750X.2018.1432689. [DOI] [PubMed] [Google Scholar]
- 40.Akizawa T., Asano Y., Morita S., Wakita T., Onishi Y., Fukuhara S., Gejyo F., Matsuo S., Yorioka N., Kurokawa K. Effect of a carbonaceous oral adsorbent on the progression of CKD: A multicenter, randomized, controlled trial. Am. J. Kidney Dis. 2009;54:459–467. doi: 10.1053/j.ajkd.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Cha R.-H., Kang S.W., Park C.W., Cha D.R., Na K.Y., Kim S.G., Yoon S.A., Han S.Y., Chang J.H., Park S.K. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin. J. Am. Soc. Nephrol. 2016;11:559–567. doi: 10.2215/CJN.12011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armaly Z., Artol S., Jabbour A.R., Saffouri A., Habashi N., Abd Elkadir A., Ghattas N., Farah R., Kinaneh S., Nseir W. Impact of pretreatment with carnitine and tadalafil on contrast-induced nephropathy in CKD patients. Ren. Fail. 2019;41:976–986. doi: 10.1080/0886022X.2019.1669459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornik C.P., Herring A.H., Benjamin D.K., Jr., Capparelli E.V., Kearns G.L., van den Anker J., Cohen-Wolkowiez M., Clark R.H., Smith P.B. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatric Infect. Dis. J. 2013;32:748. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koyama K., Usami T., Takeuchi O., Morozumi K., Kimura G. Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high-dose folic acid supplementation. Nephrol. Dial. Transplant. 2002;17:916–922. doi: 10.1093/ndt/17.5.916. [DOI] [PubMed] [Google Scholar]
- 45.Martí-Carvajal A.J., Solà I., Lathyris D., Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2017;8:CD006612. doi: 10.1002/14651858.CD006612.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah A.P., Kalantar-Zadeh K., Kopple J.D. Is there a role for ketoacid supplements in the management of CKD? Am. J. Kidney Dis. 2015;65:659–673. doi: 10.1053/j.ajkd.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 47.Ling X.C., Kuo K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018;4:1–9. doi: 10.1186/s41100-018-0195-2. [DOI] [Google Scholar]
- 48.Ceballos-Picot I., Witko-Sarsat V., Merad-Boudia M., Nguyen A.T., Thévenin M., Jaudon M.C., Zingraff J., Verger C., Jingers P., Descamps-Latscha B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic. Biol. Med. 1996;21:845–853. doi: 10.1016/0891-5849(96)00233-X. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H., Forman H.J., Choi J. γ-Glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005;401:468–483. doi: 10.1016/S0076-6879(05)01028-1. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt B., Vicenzi M., Garrel C., Denis F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015;6:198–205. doi: 10.1016/j.redox.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snelson M., Biruete A., McFarlane C., Campbell K. A Renal Clinician’s Guide to the Gut Microbiota. J. Ren. Nutr. 2020;30:384–395. doi: 10.1053/j.jrn.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Iorio B.R., Rocchetti M.T., De Angelis M., Cosola C., Marzocco S., Di Micco L., di Bari I., Accetturo M., Vacca M., Gobbetti M., et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study) J. Clin. Med. 2019;8:1424. doi: 10.3390/jcm8091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar V., Yadav A.K., Lal A., Kumar V., Singhal M., Billot L., Gupta K.L., Banerjee D., Jha V. A randomized trial of vitamin D supplementation on vascular function in CKD. J. Am. Soc. Nephrol. 2017;28:3100–3108. doi: 10.1681/ASN.2017010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meijers B.K., De Preter V., Verbeke K., Vanrenterghem Y., Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010;25:219–224. doi: 10.1093/ndt/gfp414. [DOI] [PubMed] [Google Scholar]
- 55.Ranganathan N., Ranganathan P., Friedman E.A., Joseph A., Delano B., Goldfarb D.S., Tam P., Rao A.V., Anteyi E., Musso C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010;27:634–647. doi: 10.1007/s12325-010-0059-9. [DOI] [PubMed] [Google Scholar]
- 56.Taki K., Takayama F., Niwa T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J. Ren. Nutr. 2005;15:77–80. doi: 10.1053/j.jrn.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 57.Marzocco S., Fazeli G., Di Micco L., Autore G., Adesso S., Dal Piaz F., Heidland A., Di Iorio B. Supplementation of Short-Chain Fatty Acid, Sodium Propionate, in Patients on Maintenance Hemodialysis: Beneficial Effects on Inflammatory Parameters and Gut-Derived Uremic Toxins, A Pilot Study (PLAN Study) J. Clin. Med. 2018;7:315. doi: 10.3390/jcm7100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakabayashi I., Nakamura M., Kawakami K., Ohta T., Kato I., Uchida K., Yoshida M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2010;26:1094–1098. doi: 10.1093/ndt/gfq624. [DOI] [PubMed] [Google Scholar]
- 59.Kandouz S., Mohamed A.S., Zheng Y., Sandeman S., Davenport A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 2016;20:610–617. doi: 10.1111/hdi.12414. [DOI] [PubMed] [Google Scholar]
- 60.Hall J.A., Fritsch D.A., Yerramilli M., Obare E., Yerramilli M., Jewell D.E. A longitudinal study on the acceptance and effects of a therapeutic renal food in pet dogs with IRIS-Stage 1 chronic kidney disease. J. Anim. Physiol. Anim. Nutr. 2018;102:297–307. doi: 10.1111/jpn.12692. [DOI] [PubMed] [Google Scholar]
- 61.Madduma Hewage S., Prashar S., Debnath S.C., Karmin O., Siow Y.L. Inhibition of Inflammatory Cytokine Expression Prevents High-Fat Diet-Induced Kidney Injury: Role of Lingonberry Supplementation. Front. Med. 2020;7:80. doi: 10.3389/fmed.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu Q., Ren J., Li G., Wu J., Wu X., Wang G., Gu G., Ren H., Hong Z., Li J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9:403. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ephraim E., Jackson M.I., Yerramilli M., Jewell D.E. Soluble fiber and omega-3 fatty acids reduce levels of advanced glycation end products and uremic toxins in senior dogs by modulating the gut microbiome. J. Food Sci. Nutr. Res. 2020;3:18–33. doi: 10.26502/jfsnr.2642-11000036. [DOI] [Google Scholar]
- 64.Isaak C.K., Wang P., Prashar S., Karmin O., Brown D.C., Debnath S.C., Siow Y.L. Supplementing diet with Manitoba lingonberry juice reduces kidney ischemia-reperfusion injury. J. Sci. Food Agric. 2017;97:3065–3076. doi: 10.1002/jsfa.8200. [DOI] [PubMed] [Google Scholar]
- 65.Eid H.M., Ouchfoun M., Brault A., Vallerand D., Musallam L., Arnason J.T., Haddad P.S. Lingonberry (Vaccinium vitis-idaea L.) exhibits antidiabetic activities in a mouse model of diet-induced obesity. Evid. Based Complementary Altern. Med. 2014;2014:645812. doi: 10.1155/2014/645812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Escribano-Lopez I., Diaz-Morales N., Rovira-Llopis S., de Marañon A.M., Orden S., Alvarez A., Bañuls C., Rocha M., Murphy M.P., Hernandez-Mijares A. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016;10:200–205. doi: 10.1016/j.redox.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dare A.J., Bolton E.A., Pettigrew G.J., Bradley J.A., Saeb-Parsy K., Murphy M.P. Protection against renal ischemia–reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163–168. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montemurno E., Cosola C., Dalfino G., Daidone G., De Angelis M., Gobbetti M., Gesualdo L. What would you like to eat, Mr CKD microbiota? A Mediterranean diet, please! Kidney Blood Press. Res. 2014;39:114–123. doi: 10.1159/000355785. [DOI] [PubMed] [Google Scholar]
- 69.Murthy M., Venkitanarayan K., Rangavajhyala N., Shahani K. Delineation of beneficial characteristics of effective probiotics. JAMA. 2000;3:38–43. [Google Scholar]
- 70.Lee Y.-K., Salminen S. The coming of age of probiotics. Trends Food Sci. Technol. 1995;6:241–245. doi: 10.1016/S0924-2244(00)89085-8. [DOI] [Google Scholar]
- 71.Reddy B.S. Possible mechanisms by which pro-and prebiotics influence colon carcinogenesis and tumor growth. J. Nutr. 1999;129:1478S–1482S. doi: 10.1093/jn/129.7.1478S. [DOI] [PubMed] [Google Scholar]
- 72.Puddu A., Sanguineti R., Montecucco F., Viviani G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat. Inflamm. 2014;2014:162021. doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food Nutr. Pap. 1998;66:1–140. [PubMed] [Google Scholar]
- 74.Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pisano A., D’Arrigo G., Coppolino G., Bolignano D. Biotic Supplements for Renal Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018;10:1224. doi: 10.3390/nu10091224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman A., Moe S. Review of the effects of omega-3 supplementation in dialysis patients. Clin. J. Am. Soc. Nephrol. 2006;1:182–192. doi: 10.2215/CJN.00740805. [DOI] [PubMed] [Google Scholar]
- 77.Shahidi F., Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 78.Uwaezuoke S.N., Muoneke U.V., Mbanefo N.R. The supportive treatment of IgA nephropathy and idiopathic nephrotic syndrome: How useful are omega-3 polyunsaturated fatty acids? Int. J. Nephrol. Renov. Dis. 2020;13:27. doi: 10.2147/IJNRD.S237527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouzidi N., Mekki K., Boukaddoum A., Dida N., Kaddous A., Bouchenak M. Effects of omega-3 polyunsaturated fatty-acid supplementation on redox status in chronic renal failure patients with dyslipidemia. J. Ren. Nutr. 2010;20:321–328. doi: 10.1053/j.jrn.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Krishnamurthy V.M.R., Wei G., Baird B.C., Murtaugh M., Chonchol M.B., Raphael K.L., Greene T., Beddhu S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300–306. doi: 10.1038/ki.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Vrese M., Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Food Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 82.Plaza-Díaz J., Ruiz-Ojeda F.J., Vilchez-Padial L.M., Gil A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients. 2017;9:555. doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meirlaen L., Levy E.I., Vandenplas Y. Prevention and Management with Pro-, Pre and Synbiotics in Children with Asthma and Allergic Rhinitis: A Narrative Review. Nutrients. 2021;13:934. doi: 10.3390/nu13030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chauveau P., Koppe L., Combe C., Lasseur C., Trolonge S., Aparicio M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transplant. 2019;34:199–207. doi: 10.1093/ndt/gfy164. [DOI] [PubMed] [Google Scholar]
- 85.Carrero J.J., González-Ortiz A., Avesani C.M., Bakker S.J., Bellizzi V., Chauveau P., Clase C.M., Cupisti A., Espinosa-Cuevas A., Molina P. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020;16:525–542. doi: 10.1038/s41581-020-0297-2. [DOI] [PubMed] [Google Scholar]
- 86.Liu H.W., Tsai W.H., Liu J.S., Kuo K.L. Association of Vegetarian Diet with Chronic Kidney Disease. Nutrients. 2019;11:279. doi: 10.3390/nu11020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cases A., Cigarran-Guldris S., Mas S., Gonzalez-Parra E. Vegetable-Based Diets for Chronic Kidney Disease? It is Time to Reconsider. Nutrients. 2019;11:1263. doi: 10.3390/nu11061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pilz S., Iodice S., Zittermann A., Grant W.B., Gandini S. Vitamin D status and mortality risk in CKD: A meta-analysis of prospective studies. Am. J. Kidney Dis. 2011;58:374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Q.-Y., Jiang C.-M., Sun C., Tang T.-F., Jin B., Cao D.-W., He J.-S., Zhang M. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J. Nephrol. 2015;28:471–476. doi: 10.1007/s40620-014-0167-8. [DOI] [PubMed] [Google Scholar]
- 90.Zhang L., Yang L., Shergis J., Zhang L., Zhang A.L., Guo X., Qin X., Johnson D., Liu X., Lu C. Chinese herbal medicine for diabetic kidney disease: A systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open. 2019;9:e025653. doi: 10.1136/bmjopen-2018-025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moreillon J.J., Bowden R.G., Deike E., Griggs J., Wilson R., Shelmadine B., Cooke M., Beaujean A. The use of an anti-inflammatory supplement in patients with chronic kidney disease. J. Complement. Integr. Med. 2013;10:143–152. doi: 10.1515/jcim-2012-0011. [DOI] [PubMed] [Google Scholar]
- 92.Yu J.S., Ho C.H., Wang H.Y., Chen Y.H., Hsieh C.L. Acupuncture on Renal Function in Patients with Chronic Kidney Disease: A Single-Blinded, Randomized, Preliminary Controlled Study. J. Altern. Complement. Med. 2017;23:624–631. doi: 10.1089/acm.2016.0119. [DOI] [PubMed] [Google Scholar]
- 93.Zhou X., Wu Q., Wang Y., Ren Q., Zhu W., Yao Z., Chen J. Moxibustion as an Adjuvant Therapy for Chronic Kidney Disease: A Systematic Review and Meta-Analysis of 23 Randomized Controlled Trials. Evid. Based Complementary Altern. Med. 2020;2020:6128673. doi: 10.1155/2020/6128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H.-D., Meng X.-M., Huang C., Zhang L., Lv X.-W., Li J. Application of Herbal Traditional Chinese Medicine in the Treatment of Acute Kidney Injury. Front. Pharmacol. 2019;10:379. doi: 10.3389/fphar.2019.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng Y., Cai G.-Y., He L.-Q., Lin H.-L., Cheng X.-H., Wang N.-S., Jian G.-H., Liu X.-S., Liu Y.-N., Ni Z.-H. Efficacy and safety of Niaoduqing particles for delaying moderate-to-severe renal dysfunction: A randomized, double-blind, placebo-controlled, multicenter clinical study. Chin. Med. J. 2017;130:2402. doi: 10.4103/0366-6999.216407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tu Y., Sun W., Wan Y.G., Gao K., Liu H., Yu B.Y., Hu H., Huang Y.R. Dahuang Fuzi Decoction ameliorates tubular epithelial apoptosis and renal damage via inhibiting TGF-beta1-JNK signaling pathway activation in vivo. J. Ethnopharmacol. 2014;156:115–124. doi: 10.1016/j.jep.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 97.Li J., Wang Y., Xu X., Cao W., Shen Z., Wang N., Leng J., Zou N., Shang E., Zhu Z., et al. Improved dialysis removal of protein-bound uremic toxins by salvianolic acids. Phytomedicine. 2019;57:166–173. doi: 10.1016/j.phymed.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 98.Huang Y.-R., Wei Q.-X., Wan Y.-G., Sun W., Mao Z.-M., Chen H.-L., Meng X.-J., Shi X.-M., Tu Y., Zhu Q. Ureic clearance granule, alleviates renal dysfunction and tubulointerstitial fibrosis by promoting extracellular matrix degradation in renal failure rats, compared with enalapril. J. Ethnopharmacol. 2014;155:1541–1552. doi: 10.1016/j.jep.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 99.Hsu Y.-H., Chen T.-H., Wu M.-Y., Lin Y.-F., Chen W.-L., Cheng T.-H., Chen C.-H. Protective effects of Zhibai Dihuang Wan on renal tubular cells affected with gentamicin-induced apoptosis. J. Ethnopharmacol. 2014;151:635–642. doi: 10.1016/j.jep.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 100.Lu P.-H., Lee H.-Y., Liou Y.-L., Tung S.-F., Kuo K.-L., Chen Y.-H. Nephroprotective Role of Zhibai Dihuang Wan in Aristolochic Acid-Intoxicated Zebrafish. BioMed Res. Int. 2020;2020:5204348. doi: 10.1155/2020/5204348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Korish A.A., Arafah M.M. Catechin combined with vitamins C and E ameliorates insulin resistance (IR) and atherosclerotic changes in aged rats with chronic renal failure (CRF) Arch. Gerontol. Geriatr. 2008;46:25–39. doi: 10.1016/j.archger.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Qin Y., Zhai Q., Li Y., Cao M., Xu Y., Zhao K., Wang T. Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomed. Pharm. 2018;103:1223–1230. doi: 10.1016/j.biopha.2018.04.137. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Wang B., Du F., Su X., Sun G., Zhou G., Bian X., Liu N. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin. Pharm. Toxicol. 2015;117:164–172. doi: 10.1111/bcpt.12383. [DOI] [PubMed] [Google Scholar]
- 104.Ye Q., Zhu Y.I., Ye S., Liu H., She X., Niu Y., Ming Y. Gypenoside attenuates renal ischemia/reperfusion injury in mice by inhibition of ERK signaling. Exp. Ther. Med. 2016;11:1499–1505. doi: 10.3892/etm.2016.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai H.-D., Su S.-L., Qian D.-W., Guo S., Tao W.-W., Cong X.D., Tang R., Duan J.-A. Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol. 2017;206:152–159. doi: 10.1016/j.jep.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y.-Y., Li J.-P., Lu J.-B., Li C.-X., Yu J.-G., Zhang S., Jiang S., Guo J.-M., Duan J.-A. Effect and mechanism of Huangkui capsule on reduction of uremic toxin accumulation in an animal model of chronic kidney disease. Acta Pharm. Sin. 2019;54:10. [Google Scholar]
- 107.Xu D., Chen M., Ren X., Ren X., Wu Y. Leonurine ameliorates LPS-induced acute kidney injury via suppressing ROS-mediated NF-kappaB signaling pathway. Fitoterapia. 2014;97:148–155. doi: 10.1016/j.fitote.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Feng L., Ke N., Cheng F., Guo Y., Li S., Li Q., Li Y. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J. Surg. Res. 2011;166:298–305. doi: 10.1016/j.jss.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 109.Liu W.J., Tang H.T., Jia Y.T., Ma B., Fu J.F., Wang Y., Lv K.Y., Xia Z.F. Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock. 2010;34:314–320. doi: 10.1097/SHK.0b013e3181ceede4. [DOI] [PubMed] [Google Scholar]
- 110.Luo L.-N., Xie D.Q., Zhang X.G., Jiang R. Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp. Ther. Med. 2016;12:2009–2014. doi: 10.3892/etm.2016.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu C., Cheng Z., Wang Y., Dai X., Zhang J., Xue D. Paeoniflorin exerts a nephroprotective effect on concanavalin A-induced damage through inhibition of macrophage infiltration. Diagn. Pathol. 2015;10:120. doi: 10.1186/s13000-015-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen M.L., Yi L., Zhang Y., Zhou X., Ran L., Yang J., Zhu J.D., Zhang Q.Y., Mi M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio. 2016;7:e02210-15. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feng C., Xie X., Wu M., Li C., Gao M., Liu M., Qi X., Ren J. Tanshinone I protects mice from aristolochic acid I-induced kidney injury by induction of CYP1A. Environ. Toxicol. Pharmacol. 2013;36:850–857. doi: 10.1016/j.etap.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 114.Lu Z., Zeng Y., Lu F., Liu X., Zou C. Rhubarb Enema Attenuates Renal Tubulointerstitial Fibrosis in 5/6 Nephrectomized Rats by Alleviating Indoxyl Sulfate Overload. PLoS ONE. 2015;10:e0144726. doi: 10.1371/journal.pone.0144726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ji C., Deng Y., Yang A., Lu Z., Chen Y., Liu X., Han L., Zou C. Rhubarb Enema Improved Colon Mucosal Barrier Injury in 5/6 Nephrectomy Rats May Associate With Gut Microbiota Modification. Front. Pharmacol. 2020;11:1092. doi: 10.3389/fphar.2020.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plotnikov E.Y., Chupyrkina A.A., Jankauskas S.S., Pevzner I.B., Silachev D.N., Skulachev V.P., Zorov D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta. 2011;1812:77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 117.Hatcher H., Planalp R., Cho J., Torti F., Torti S. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Usharani P., Mateen A., Naidu M., Raju Y., Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus. Drugs R D. 2008;9:243–250. doi: 10.2165/00126839-200809040-00004. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh S.S., Massey H.D., Krieg R., Fazelbhoy Z.A., Ghosh S., Sica D.A., Fakhry I., Gehr T.W. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am. J. Physiol. Ren. Physiol. 2009;296:F1146–F1157. doi: 10.1152/ajprenal.90732.2008. [DOI] [PubMed] [Google Scholar]
- 120.Sengupta K., Alluri K.V., Satish A.R., Mishra S., Golakoti T., Sarma K.V., Dey D., Raychaudhuri S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008;10:1–11. doi: 10.1186/ar2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Madisch A., Miehlke S., Eichele O., Mrwa J., Bethke B., Kuhlisch E., Bästlein E., Wilhelms G., Morgner A., Wigginghaus B. Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int. J. Colorectal Dis. 2007;22:1445–1451. doi: 10.1007/s00384-007-0364-1. [DOI] [PubMed] [Google Scholar]
- 122.Liu G., Zhou Q., Tong X. The clinical application and pharmacological research progress of Dahuang fuzi Decoction. Chin. Arch. Tradit. Chin. Med. 2010;28:1848–1851. [Google Scholar]
- 123.Li J.-P., Guo J.-M., Hua Y.-Q., Zhu K.Y., Tang Y.-P., Zhao B.-C., Jia L.-F., Zhao J., Tang Z.-S., Duan J.-A. The mixture of Salvia miltiorrhiza–Carthamus tinctorius (Danhong injection) alleviates low-dose aspirin induced gastric mucosal damage in rats. Phytomedicine. 2016;23:662–671. doi: 10.1016/j.phymed.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Wang X., Yu S., Jia Q., Chen L., Zhong J., Pan Y., Shen P., Shen Y., Wang S., Wei Z. NiaoDuQing granules relieve chronic kidney disease symptoms by decreasing renal fibrosis and anemia. Oncotarget. 2017;8:55920. doi: 10.18632/oncotarget.18473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yin X.-F., Han L.-L. Effect of uremic clearance granule on the systemic micro-inflammatory state of patients after peritoneal dialysis. Pract. Pharm. Clin. Remedies. 2013;2:125–126. [Google Scholar]
- 126.Ye M.-Y., Zheng J., Zhang J. The Influence of Niaoduqing Particle on the Calcium and Phosphorus Metabolism and FGF23 in Patients with Chronic Kidney Disease. Med. Innov. China. 2015;12:16–18. [Google Scholar]
- 127.Wojcikowski K., Johnson D.W., Gobe G. Herbs or natural substances as complementary therapies for chronic kidney disease: Ideas for future studies. J. Lab. Clin. Med. 2006;147:160–166. doi: 10.1016/j.lab.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 128.Ishikawa T., Suzukawa M., Ito T., Yoshida H., Ayaori M., Nishiwaki M., Yonemura A., Hara Y., Nakamura H. Effect of tea flavonoid supplementation on the susceptibility of low-density lipoprotein to oxidative modification. Am. J. Clin. Nutr. 1997;66:261–266. doi: 10.1093/ajcn/66.2.261. [DOI] [PubMed] [Google Scholar]
- 129.Arts I.C., Hollman P.C., Feskens E.J., Bueno de Mesquita H.B., Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 130.Chander V., Singh D., Chopra K. Catechin, a natural antioxidant protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Pharmacol. Res. 2003;48:503–509. doi: 10.1016/S1043-6618(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 131.Sumien N., Forster M.J., Sohal R.S. Supplementation with vitamin E fails to attenuate oxidative damage in aged mice. Exp. Gerontol. 2003;38:699–704. doi: 10.1016/S0531-5565(03)00068-8. [DOI] [PubMed] [Google Scholar]
- 132.Mahfouz M., Kummerow F. Vitamin C or vitamin B6 supplementation prevent the oxidative stress and decrease of prostacyclin generation in homocysteinemic rats. Int. J. Biochem. Cell Biol. 2004;36:1919–1932. doi: 10.1016/j.biocel.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 133.He J., Giusti M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 134.McCullough M.L., Peterson J.J., Patel R., Jacques P.F., Shah R., Dwyer J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y.Q., Li Q.S., Zheng X.Q., Lu J.L., Liang Y.R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules. 2021;26:3962. doi: 10.3390/molecules26133962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodges J.K., Sasaki G.Y., Bruno R.S. Anti-inflammatory activities of green tea catechins along the gut-liver axis in nonalcoholic fatty liver disease: Lessons learned from preclinical and human studies. J. Nutr. Biochem. 2020;85:108478. doi: 10.1016/j.jnutbio.2020.108478. [DOI] [PubMed] [Google Scholar]
- 137.Filippini T., Malavolti M., Borrelli F., Izzo A.A., Fairweather-Tait S.J., Horneber M., Vinceti M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020;3:CD005004. doi: 10.1002/14651858.CD005004.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nguyen N.H., Ha T.K.Q., Yang J.L., Pham H.T.T., Oh W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021;268:113574. doi: 10.1016/j.jep.2020.113574. [DOI] [PubMed] [Google Scholar]
- 139.Quan Y., Yang Y., Wang H., Shu B., Gong Q.H., Qian M. Gypenosides attenuate cholesterol-induced DNA damage by inhibiting the production of reactive oxygen species in human umbilical vein endothelial cells. Mol. Med. Rep. 2015;11:2845–2851. doi: 10.3892/mmr.2014.3095. [DOI] [PubMed] [Google Scholar]
- 140.Li N., Tang H., Wu L., Ge H., Wang Y., Yu H., Zhang X., Ma J., Gu H.F. Chemical constituents, clinical efficacy and molecular mechanisms of the ethanol extract of Abelmoschus manihot flowers in treatment of kidney diseases. Phytother. Res. 2021;35:198–206. doi: 10.1002/ptr.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen Y., Cai G., Sun X., Chen X. Treatment of chronic kidney disease using a traditional Chinese medicine, Flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae) Clin. Exp. Pharmacol. Physiol. 2016;43:145–148. doi: 10.1111/1440-1681.12528. [DOI] [PubMed] [Google Scholar]
- 142.Zhang L., Li P., Xing C.Y., Zhao J.Y., He Y.N., Wang J.Q., Wu X.F., Liu Z.S., Zhang A.P., Lin H.L., et al. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: A prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 2014;64:57–65. doi: 10.1053/j.ajkd.2014.01.431. [DOI] [PubMed] [Google Scholar]
- 143.Li P., Lin H., Ni Z., Zhan Y., He Y., Yang H., Fang J., Wang N., Li W., Cai G., et al. Efficacy and safety of Abelmoschus manihot for IgA nephropathy: A multicenter randomized clinical trial. Phytomedicine. 2020;76:153231. doi: 10.1016/j.phymed.2020.153231. [DOI] [PubMed] [Google Scholar]
- 144.Wojtyniak K., Szymański M., Matławska I. Leonurus cardiaca L. (motherwort): A review of its phytochemistry and pharmacology. Phytother. Res. 2013;27:1115–1120. doi: 10.1002/ptr.4850. [DOI] [PubMed] [Google Scholar]
- 145.Liu X.H., Pan L.L., Yang H.B., Gong Q.H., Zhu Y.Z. Leonurine attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: Involvement of reactive oxygen species and NF-κB pathways. Eur. J. Pharmacol. 2012;680:108–114. doi: 10.1016/j.ejphar.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Z.-H., Yu S.-Z., Wang Z.-T., Zhao B.-L., Hou J.-W., Yang F.-J., Xin W.-J. Scavenging effects of tetramethylpyrazine on active oxygen free radicals. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1994;15:229–231. [PubMed] [Google Scholar]
- 147.Wu W., Qiu F. Experimental study on ischemia and reperfusion injury of rat liver and effects of ligustrazine and salvia compound. Chin. Med. Sci. J. = Chung-Kuo I Hsueh K’o Hsueh Tsa Chih. 1994;9:162–166. [PubMed] [Google Scholar]
- 148.Feng L., Xiong Y., Cheng F., Zhang L., Li S., Li Y. Effect of ligustrazine on ischemia-reperfusion injury in murine kidney. Transpl. Proc. 2004;36:1949–1951. doi: 10.1016/j.transproceed.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 149.Li F., Gong Q., Wang L., Shi J. Osthole attenuates focal inflammatory reaction following permanent middle cerebral artery occlusion in rats. Biol. Pharm. Bull. 2012;35:1686–1690. doi: 10.1248/bpb.b12-00133. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J., Dou W., Zhang E., Sun A., Ding L., Wei X., Chou G., Mani S., Wang Z. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G27–G36. doi: 10.1152/ajpgi.00465.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen X., Liu C., Lu Y., Yang Z., Lv Z., Xu Q., Pan Q., Lu L. Paeoniflorin regulates macrophage activation in dimethylnitrosamine-induced liver fibrosis in rats. BMC Complementary Altern. Med. 2012;12:1–11. doi: 10.1186/1472-6882-12-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chung J.H., Manganiello V., Dyck J.R.B. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Larrosa M., Yañéz-Gascón M.J., Selma M.V., González-Sarrías A., Toti S., Cerón J.J., Tomás-Barberán F., Dolara P., Espín J.C. Effect of a Low Dose of Dietary Resveratrol on Colon Microbiota, Inflammation and Tissue Damage in a DSS-Induced Colitis Rat Model. J. Agric. Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 154.Rakici O., Kiziltepe U., Coskun B., Aslamaci S., Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int. J. Cardiol. 2005;105:209–215. doi: 10.1016/j.ijcard.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 155.Magyar K., Halmosi R., Palfi A., Feher G., Czopf L., Fulop A., Battyany I., Sumegi B., Toth K., Szabados E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- 156.Xiong Y., Chen L., Fan L., Wang L., Zhou Y., Qin D., Sun Q., Wu J., Cao S. Free total rhubarb anthraquinones protect intestinal injury via regulation of the intestinal immune response in a rat model of severe acute pancreatitis. Front. Pharmacol. 2018;9:75. doi: 10.3389/fphar.2018.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L., Cui Y.-L., Zhang Z., Lin Z.-F., Chen D.-C. Rhubarb monomers protect intestinal mucosal barrier in sepsis via junction proteins. Chin. Med. J. 2017;130:1218. doi: 10.4103/0366-6999.205855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fetisova E.K., Avetisyan A.V., Izyumov D.S., Korotetskaya M.V., Chernyak B.V., Skulachev V.P. Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress. FEBS Lett. 2010;584:562–566. doi: 10.1016/j.febslet.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 159.Silachev D.N., Plotnikov E.Y., Pevzner I.B., Zorova L.D., Balakireva A.V., Gulyaev M.V., Pirogov Y.A., Skulachev V.P., Zorov D.B. Neuroprotective effects of mitochondria-targeted plastoquinone in a rat model of neonatal hypoxic–ischemic brain injury. Molecules. 2018;23:1871. doi: 10.3390/molecules23081871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cai Y., Zhang W., Chen Z., Shi Z., He C., Chen M. Recent insights into the biological activities and drug delivery systems of tanshinones. Int. J. Nanomed. 2016;11:121. doi: 10.2147/IJN.S84035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou J., Jiang Y.Y., Chen H., Wu Y.C., Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53:e12739. doi: 10.1111/cpr.12739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 162.World Health Organization . Acupuncture: Review and Analysis of Reports on Controlled Clinical Trials. World Health Organization; Geneve, Switzerland: 2002. [Google Scholar]
- 163.Cabýoglu M.T., Ergene N., Tan U. The mechanism of acupuncture and clinical applications. Int. J. Neurosci. 2006;116:115–125. doi: 10.1080/00207450500341472. [DOI] [PubMed] [Google Scholar]
- 164.Xiong W., He F.F., You R.Y., Xiong J., Wang Y.M., Zhang C., Meng X.F., Su H. Acupuncture Application in Chronic Kidney Disease and its Potential Mechanisms. Am. J. Chin. Med. 2018;46:1169–1185. doi: 10.1142/S0192415X18500611. [DOI] [PubMed] [Google Scholar]
- 165.Huang Q.-F., Wu H.-G., Liu J., Hong J. Bibliometric analysis of diseases spectrum of moxibustion therapy. J. Acupunct. Tuina Sci. 2012;10:342–348. doi: 10.1007/s11726-012-0633-6. [DOI] [Google Scholar]
- 166.Lin J.-G., Li T., Hsu S. Newly Edited Color Book of Acupuncture and Moxibustion. JYIN Publishing Company; Taipei, Taiwan: 2009. pp. 107–108. [Google Scholar]
- 167.Cardini F., Weixin H. Moxibustion for correction of breech presentation: A randomized controlled trial. JAMA. 1998;280:1580–1584. doi: 10.1001/jama.280.18.1580. [DOI] [PubMed] [Google Scholar]
- 168.Chiu J.-H. How does moxibustion possibly work? Evid. Based Complementary Altern. Med. 2013;2013:198584. doi: 10.1155/2013/198584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deng H., Shen X. The mechanism of moxibustion: Ancient theory and modern research. Evid. Based Complementary Altern. Med. 2013;2013:379291. doi: 10.1155/2013/379291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Y., Sun Y., Zhang C., Wang K., Shen P., Huang D., Ma W., Zhang J., Li L., He L. Moxibustion alleviates injury in a rat focal segmental glomerulosclerosis model. Evid. Based Complementary Altern. Med. 2017;2017:7169547. doi: 10.1155/2017/7169547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.