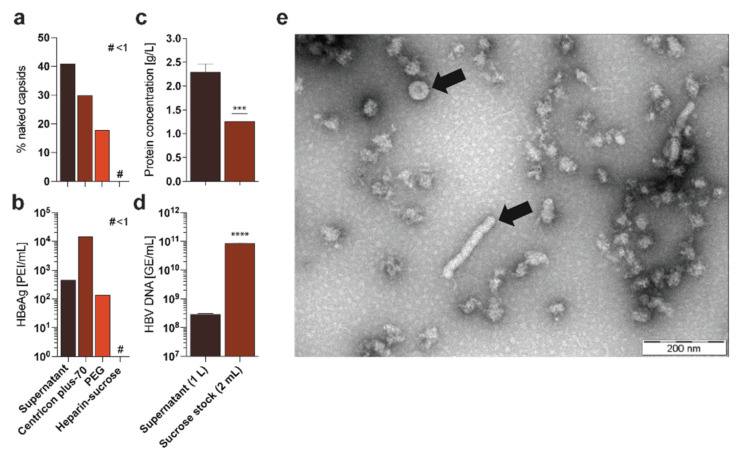
Figure 1.
Comparison of different HBV purification methods. HepAD38 cells were cultured and supernatant was purified according to published protocols via Centricon concentration [29], PEG precipitation [30], or the purification protocol described herein: (a) CsCl ultracentrifugation followed by a fractionated Dot-Blot analysis was performed to determine the frequency of naked capsids in the different preparations [27]; # < 1; (b) HBeAg was quantified via ELISA in the different preparations [4]; # < 1; (c) Protein concentration was determined via Bradford assay in supernatant and purified sucrose stock [31]; (d) Quantitative PCR was performed to analyze the titer of HBV GE in the supernatant and the purified sucrose stock [4]; (e) Electron microscopy was performed after purification of HBV particles via the purification protocol described herein, showing little protein impurity but several virions and filaments (indicated by arrows) [32]. Statistical significance was determined using Student’s t test, *** p < 0.001, **** p ≤ 0.0001.