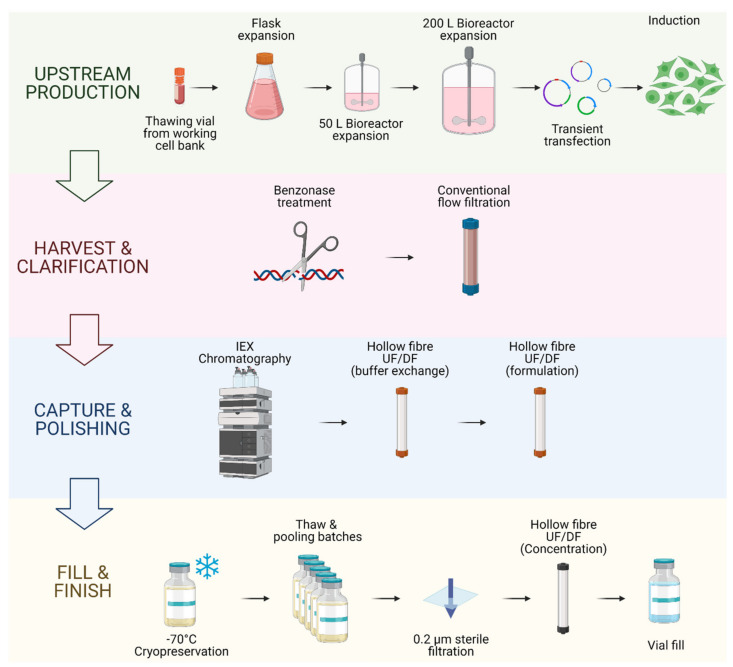
Figure 2.
Example of an end-to-end upstream and downstream bioprocess for GMP-grade LV vector production. This figure describes the bioprocess patented by Oxford Biomedica (Oxford, UK) for the production of their GMP-grade lentiviral vector using their suspension-adapted, serum-free HEK293T producer cell line. This process includes an inducible plasmid system dependent on sodium butyrate. Each batch is individually cryopreserved and stored until enough material is produced, to then be combined, filtered and concentrated for final formulation [14,45,46].