Abstract
The tomato Sw-5b gene confers resistance to tomato spotted wilt virus (TSWV) and encodes a nucleotide-binding leucine-rich repeat (NLR) protein with an N-terminal Solanaceae-specific domain (SD). Although our understanding of how Sw-5b recognizes the viral NSm elicitor has increased significantly, the process by which Sw-5b activates downstream defense signaling remains to be elucidated. In this study, we used a tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS) system to investigate the roles of the SGT1/RAR1, EDS1/NDR1, NPR1, and NRC/ADR1/NRG1 genes in the Sw-5b-mediated signaling pathway. We found that chaperone SGT1 was required for Sw-5b function, but co-chaperone RAR1 was not. Sw-5b-mediated immune signaling was independent of both EDS1 and NDR1. Silencing NPR1, which is a central component in SA signaling, did not result in TSWV systemic infection in Sw-5b-transgenic N. benthamiana plants. Helper NLR NRCs (NLRs required for cell death) were required for Sw-5b-mediated systemic resistance to TSWV infection. Suppression of NRC2/3/4 compromised the Sw-5b resistance. However, the helper NLRs ADR1 and NRG1 may not participate in the Sw-5b signaling pathway. Silencing ADR1, NRG1, or both genes did not affect Sw-5b-mediated resistance to TSWV. Our findings provide new insight into the requirement for conserved key components in Sw-5b-mediated signaling pathways.
Keywords: tomato spotted wilt virus, Sw-5b, NLR receptor, plant innate immunity, defense signaling
1. Introduction
Plants have evolved different layers of defense against pathogen infection [1]. In the first layer, plants use an extracellular pattern recognition receptor (PRR) to recognize a pathogen-associated molecular pattern (PAMP) to trigger PAMP-triggered immunity (PTI). In response, plant pathogens use multiple virulence effectors to suppress PTI. As a second layer of defense, plants have evolved various intracellular nucleotide-binding leucine-rich repeat (NLR) receptors to recognize these effectors and invoke effector-triggered immunity (ETI). NLR proteins are the largest group of resistance genes in plants [2]. Plant NLRs typically have a variable N-terminal domain: a Toll/interleukin-1 receptor (TIR) domain or a coiled-coil (CC) domain, a central NB-ARC (nucleotide binding adaptor and APAF-1, R proteins, and CED-4) domain, and a C-terminal leucine-rich repeat (LRR) domain [3,4]. Hereafter, NLRs with CC domains are referred to as CNLs, and NLRs with TIR domains are referred to as TNLs. Effector-mediated NLR activation results in a robust defense response that is typically associated with a hypersensitive response (HR) and constrains pathogens at the site of infection [1,4,5,6]. However, our understanding of how NLRs activate downstream resistance remains limited. To date, only a few common key downstream immune-signaling components of NLR-mediated resistance have been identified.
Tomato spotted wilt virus (TSWV, genus Orthotospovirus; family Tospoviridae) infects more than 1000 different plants in at least 85 families [7] and is among the most destructive plant viruses [8,9,10], causing more than 1 billion USD in crop losses worldwide each year [11]. The Sw-5b gene is the most effective resistance gene for controlling TSWV in tomato [12,13,14,15]. Since it was introgressed from Peruvian tomato, Solanum peruvianum L., it has been used widely in tomato-breeding projects. Sw-5b encodes a protein belonging to a CNL-type resistance gene. It specifically recognizes the movement protein NSm of TSWV [16,17,18]. Sw-5b has also been found to confer broad-spectrum resistance against various American-type tospoviruses by recognizing a highly conserved 21 amino-acid region in NSm (NSm21) [19]. Sw-5b carries an extended N-terminal Solanaceae-specific domain (SD) and develops a multilayered regulatory mechanism to control its autoinhibition and activation [20]. For activation, Sw-5b uses a two-step recognition mechanism involving both SD and the LRR domain in the detection of viral NSm [21]. This two-step recognition increases the specificity of NSm recognition and also significantly enhances the sensitivity of NSm detection. Although our understanding of how Sw-5b recognizes viral elicitor NSm has increased significantly, the process by which Sw-5b activates downstream defense signaling has yet to be elucidated.
SGT1 (suppressor of the G2 allele of skp1) has been found to be required for the induction of the immunity mediated by many NLRs [22,23]. As a protein conserved in eukaryotes, SGT1 functions in a variety of biological processes by interacting with different protein complexes [24,25,26]. It regulates the protein accumulation levels of NLR immune receptors [27] and participates in the nucleocytoplasmic distribution of tobacco NLR protein N [28]. SGT1 is associated with RAR1, which is required for Mla12 resistance, and heat-shock protein 90 (HSP90), forming a molecular chaperone complex that is required for appropriate accumulation of NLR proteins [23]. Although SGT1 is required for the establishment of the Sw-5b-mediated HR [29], the requirement for SGT1/RAR1/HSP90 in Sw-5b-mediated induction of systemic resistance to TSWV has not been tested.
EDS1 (enhanced disease susceptibility 1) is a lipase-like protein that is needed for all tested TNLs to activate downstream signaling. EDS1 is also necessary for some CNLs, such as the CNL HRT and the CC R-protein RPW8 [30,31]. However, some CNLs, such as RPS2, RPM1, and RPS5, require an alternate protein, NDR1 (non-race-specific disease resistance 1), for immunity [32]. The requirement for EDS1/NDR1 in the Sw-5b/TSWV resistance pathways has not yet been tested.
Salicylic acid (SA) plays an important role in many NLR-mediated resistance mechanisms. In Arabidopsis, NPR1 (non-expresser of pathogenesis-related genes 1) is a central component in SA signaling [33,34]. NPR1 was found to be a receptor of SA [35]. It controls the transcription of approximately 90% of the SA-dependent genes in Arabidopsis [33,36,37,38]. NPR1 shuttles into the nucleus, where it interacts with TGA transcription factors, thereby activating defense genes to establish plant immunity [39]. Tobacco NPR1 is required for N-mediated resistance to tobacco mosaic virus (TMV) [37]. However, HRT-mediated resistance to turnip crinkle virus (TCV) in Arabidopsis is independent of NPR1 [40]. The role of NPR1 in the Sw-5b resistance pathway is not known.
Recently, many “sensor” NLR proteins have been found to require “helper” NLR proteins to activate downstream immune signals [41,42]. Three types of “helper” NLR proteins have been described in plants: the NRC (NB-LRR protein required for HR-associated cell death) family, ADR1 (activated disease resistance 1) family, and NRG1 (N requirement gene 1) family. All of these are CNL-type proteins [42,43]. The NRC family proteins in tobacco and tomato are required for the induction of HR mediated by a variety of NLRs [29,41,44]. Although NRC2/3/4 have been shown to be required for the induction of HR mediated by Sw-5b [29], whether they are required for induction of systemic resistance to TSWV remains to be elucidated. ADR1 family proteins that function downstream of RPS4/RRS1, RPP2, SNC1, CHS1/SOC3, RPP4, and RPS2 are involved in both TNL- and CNL-mediated immunity [45,46,47,48]. NRG1 was first identified in Nicotiana benthamiana L. using virus-induced gene silencing (VIGS) and was found to be required for N-mediated resistance to TMV [49]. NRG1 is essential for Roq1, N, and RPP1-mediated HR and disease resistance [50]. Rysto-dependent extreme resistance to potato virus Y also requires NRG1 [51]. NRG1 is likely a conserved key component of TNL-mediated immune signaling pathways. The ADR1 and NRG1 gene families are function redundant, and their N-terminal domains are related to RPW8-CC [46]. Disease susceptibility was enhanced in an adr1 adr1-L1 adr1-L2 nrg1.1 nrg1.2 nrg1.3 sextuple helperless mutant in comparison to adr1 triple and nrg1 triple mutants [47]. ADR1 and NRG1 were also found to be involved in Rx2-mediated resistance to potato virus X (PVX) [46]. However, the requirement for ADR1 and NRG1 in Sw-5b/TSWV has not yet been tested.
In this study, we characterized the requirement for SGT1/RAR1, EDS1/NDR1, NPR1, and NRC/ADR1/NRG1 genes in the Sw-5b-mediated immune signaling pathway using a TRV-based virus induced gene silencing (VIGS) approach. We found that SGT1, but not RAR1, was required for Sw-5b-mediated resistance. Sw-5b function was independent of both EDS1 and NDR1. Suppression of NPR1 did not cause TSWV systemic infection in Sw-5b-transgenic N. benthamiana plants. The helper NLRs NRC2/3/4, but not ADR1 and NRG1, were required for Sw-5b-mediated systemic resistance to TSWV infection. These findings provide new insights into the requirement for conserved components in the Sw-5b-mediated signaling pathway.
2. Materials and Methods
2.1. Plasmid Construction
The cDNA fragments corresponding to Sw-5b (AY007366.1), NbHSP90 (Niben101Scf15166g03015.1), NbRAR1 (LC314308.1), NbEDS1 (Niben101Scf06720g01024.1), NbNDR1 (AY438029.1), NbNPR1 (Niben101Scf14780g01001.1), NbNRC2a (KT936525.1), NbNRC2b (KT936526.1), NbNRC3 (MK692736.1), NbNRC4 (MK692737.1), NbNRG1 (DQ054580.1), and NbADR1 (Niben101Scf02422g02015.1) were amplified by polymerase chain reaction (PCR) using PrimeSTAR High-Fidelity DNA Polymerase (TaKaRa, Dalian, China) and cloned into pTRV2 [37]. For constructing TRV-NbNRC2/3/4 and TRV-NbNRG1/NbADR1, gene fragments were fused by overlap PCR. The pTRV2-NbPDS, pTRV2-NbSGT1, and pTRV2-GUS vectors have been described previously [52].
2.2. Host Plant and Virus
Four to 6 week old N. benthamiana plants were used for the VIGS experiments. The source of the TSWV Yunnan isolate has been described previously [53]. TSWV Yunnan isolates were propagated in N. benthamiana. For long-term preservation, fresh systemically infected leaves of N. benthamiana were stored in a refrigerator at −80 °C. Plant leaves containing TSWV were ground in 0.01 M phosphate buffer (pH 7.4). The Sw-5b-transgenic N. benthamiana line has been described previously [20]. The VIGS-treated and TSWV-inoculated plants were maintained in a growth chamber at a temperature of 24 °C under a 16 h light/8 h dark photoperiod.
2.3. VIGS Assay
pTRV1, pTRV2, and their derivatives were individually introduced into Agrobacterium strain GV3101 cells by electroporation. Agrobacterium cultures were resuspended in agroinfiltration buffer (10 mM MgCl2, 10 mM MES pH 5.6, and 100 μM acetosyringone) and adjusted to a final concentration of OD600 = 0.5. The Agrobacteria containing TRV RNA1 and RNA2 were mixed at a 1:1 ratio and incubated for 3 to 5 h in the dark at 28 °C. The mixtures were co-infiltrated into fully expanded leaves of N. benthamiana using a 1 mL needleless syringe. Six plants were used for each treatment and/experiment. Each experiment was repeated three times.
2.4. Total RNA Isolation and Quantitative RT-PCR Analysis
Total RNAs were extracted from N. benthamiana plant leaves using an RNA Easy Fast Isolation kit (catalog no. DP452, Tiangen, Beijing, China). First-strand cDNAs were synthesized using Oligo-dT primer and M-MLV Reverse Transcriptase (Promega, Madison, WI, USA). The expression of genes silenced by TRV-based VIGS was quantified on an ABI 7500 Real-Time PCR system (Life Technologies, Carlsbad, CA, USA) using Power SYBR Green Master Mix (Life Technologies). All of the primers used for quantitative reverse transcriptase (qRT)-PCR are listed in Supplementary Table S1. NbActin and NbEF1a were used as internal controls in the assayed samples.
2.5. Western Blot Assays
Total proteins were extracted from N. benthamiana plant leaves in extraction buffer (10% glycerol, 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 10 mM DTT, 2% polyvinylpolypyrrolidone, 1× protease inhibitor cocktail, 0.2% TritonX-100). The crude plant extracts were centrifuged at 12,600× g for 10 min, and the supernatant was mixed with 3 × SDS loading buffer (150 mM Tris-HCl, pH 6.8, 6% SDS, 0.3% bromophenol blue, 30% glycerol, 300 mM DTT) and boiled for 10 min. Protein samples were separated in 10% SDS-PAGE gels and then transferred to PVDF membranes. The blots were probed with an anti-TSWV N antibody followed by HRP-conjugated goat anti-rabbit antibodies (1:10,000). An ECL Substrate Kit (Thermo Scientific, Hudson, NH, USA) was used to develop the blots. A Bio-Rad ChemiDoc Touch imaging system (Bio-Rad, Hercules, CA, USA) was used to visualize the signals.
3. Results
3.1. Silencing Sw-5b by TRV-Based VIGS Abolished the Resistance of Sw-5b-Transgenic N. benthamiana to TSWV Infection
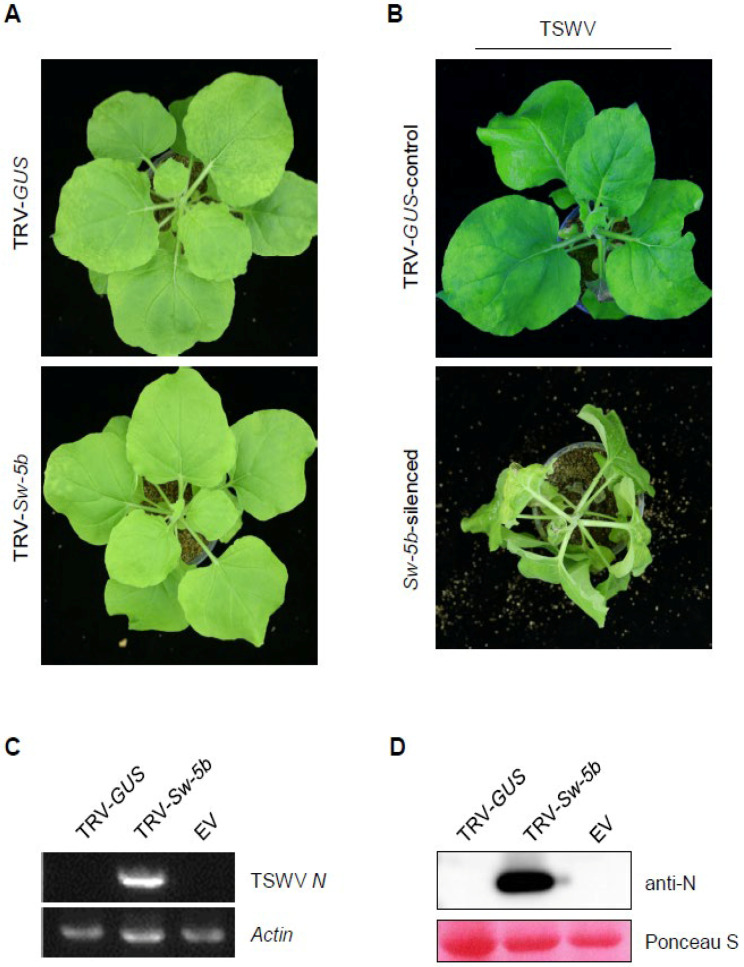
Our laboratory previously generated Sw-5b-transgenic N. benthamiana that confer resistance to TSWV infection [20]. To investigate the downstream defense signaling of Sw-5b-mediated resistance, we adopted a TRV-based VIGS to silence downstream candidate genes in Sw-5b-transgenic N. benthamiana plants, followed by TSWV infection. Before silencing the downstream candidate genes, we first tested silencing the Sw-5b gene in Sw-5b-transgenic plants using a TRV-based VIGS approach, and we examined the process of TSWV infection in the Sw-5b silenced plants. A 500 bp fragment of the Sw-5b gene was amplified by PCR and inserted into the TRV2 vector. As a control, a fragment from the GUS gene was inserted into the TRV2 vector. TRV2 carrying a fragment from NbPDS was also used as a control. Agrobacterium carrying TRV1 mixed equally with Agrobacterium carrying TRV2-Sw-5b, TRV2-GUS, or TRV-NbPDS was co-infiltrated into the leaves of 4–6 week old Sw-5b-transgenic N. benthamiana plants. At 3 weeks post TRV treatment, newly emerged leaves of Sw-5b transgenic N. benthamiana infected with TRV-NbPDS were photobleached (Figure S1A), implying that NbPDS was silenced. At this stage, the corresponding newly emerged leaves of Sw-5b-transgenic N. benthamiana plants pre-treated with TRV-Sw-5b or TRV-GUS control (Figure 1A and Figure S1B) were inoculated with crude sap from TSWV-infected tissue. At 14 days post TSWV inoculation (dpi), typical viral symptoms including leaf curling, stunting, and systemic wilt were observed in the Sw-5b-transgenic N. benthamiana silenced for Sw-5b (Figure 1B). No such symptoms were observed in the plants treated with the TRV-GUS control (Figure 1B).
Figure 1.
Silencing Sw-5b through VIGS abolished the resistance of Sw-5b-transgenic N. benthamiana to TSWV infection. (A) Sw-5b-transgenic N. benthamiana plants treated with TRV-GUS and TRV-Sw-5b. Agrobacterium harboring TRV1 was mixed equally with Agrobacterium harboring TRV2 with gene fragments of GUS, or Sw-5b, and the mixture was co-infiltrated into 4–6 week old leaves of Sw-5b-transgenic N. benthamiana. The treated plants were photographed at 3 weeks post TRV infection. (B) Analysis of TSWV systemic infection of Sw-5b-transgenic N. benthamiana plants pretreated with TRV-Sw-5b. TRV-GUS treatment was used as the control. The newly emerged leaves of Sw-5b-transgenic N. benthamiana plants silenced for Sw-5b or pretreated with TRV-GUS at 3 weeks post TRV infection were inoculated with crude extract of TSWV-infected tissues. The plants were photographed at 14 days post TSWV inoculation. The systemic infection was evident in all 18 Sw-5b-silenced plants in three repeated experiments. (C) RT–PCR analysis of the expression of TSWV N RNA in systemic leaves of Sw-5b-transgenic N. benthamiana plants pretreated with TRV-GUS or TRV-Sw-5b. The internal reference gene was NbActin. (D) Western blot analysis of TSWV N accumulation in systemic leaves of Sw-5b-transgenic N. benthamiana silenced for Sw-5b and pretreated with TRV-GUS control at 10 dpi post TSWV inoculation. A plant sample expressing empty vector (Vec.) was used as a negative control. Ponceau S staining of Rubisco was used as a protein loading control.
Total RNA was extracted from systemic leaves of TSWV-inoculated transgenic N. benthamiana treated with TRV-Sw-5b or TRV-GUS. The RT-PCR analysis showed that the TSWV N gene was detected in systemically infected leaves of the Sw-5b-silenced transgenic N. benthamiana, but not in the TRV-GUS-treated control plants (Figure 1C). Western blotting assays further confirmed that TSWV N protein accumulated in the systemically infected leaves of Sw-5b-transgenic plants treated with TRV-Sw-5b, but not in those treated with the TRV-GUS control (Figure 1D). These results imply that silencing Sw-5b by TRV-based VIGS abolished Sw-5b-mediated resistance to TSWV infection in transgenic N. benthamiana plants.
3.2. SGT1, But Not Rar1, Was Required for Sw-5b-Mediated Resistance to TSWV
SGT1, RAR1, and HSP90 are required for the induction of disease resistance mediated by a variety of NLR proteins. They form a molecular chaperone complex that is important for regulating the protein stability of plant NLRs [54,55].
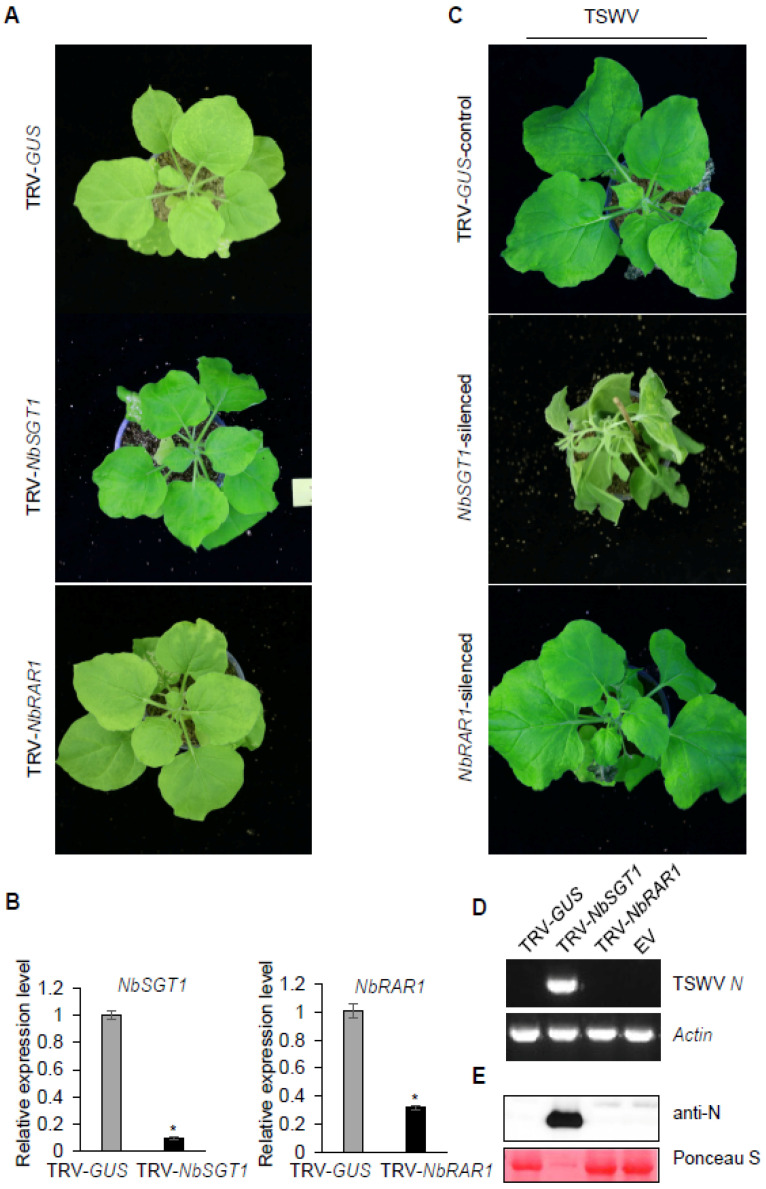
To examine whether SGT1, RAR1, and HSP90 play essential roles in Sw-5b-mediated resistance to TSWV, we used TRV-based VIGS to silence NbSGT1, NbRAR1, and NbHSP90 in Sw-5b-transgenic N. benthamiana. TRV-GUS was used as a negative control. At 3 weeks post TRV treatment, in comparison with the TRV-GUS-treated controls, the transgenic N. benthamiana plants treated with TRV-NbSGT1 and TRV-NbRAR1 did not exhibit significant growth defects (Figure 2A and Figure S2). However, the transgenic N. benthamiana treated with TRV-NbHSP90 were deformed, with extremely small newly emerged leaves and inhibited growth, making it difficult to inoculate them further with TSWV (Figure S3A). The qRT-PCR results showed that mRNA expression of NbSGT1, NbRAR1, and NbHSP90 was significantly reduced in newly emerged leaves of these transgenic plants (Figure 2B and Figure S3B). The newly emerged leaves of transgenic N. benthamiana silenced for NbSGT1 or NbRAR1 were inoculated with TSWV crude extracts from systemically infected tissues. At 14 days post TSWV inoculation, the TSWV spread to the systemic leaves, causing symptoms including systemic wilting in NbSGT1-silenced plants (Figure 2C). No systemic infection was observed in the TRV-GUS-treated control plants (Figure 2C). In the Sw-5b transgenic N. benthamiana silenced for NbRAR1, to our surprise, neither TSWV systemic infection nor viral symptoms were observed (Figure 2C). RT-PCR assays showed that the TSWV N gene was detected in the systemically infected leaves of NbSGT1-silenced transgenic N. benthamiana, but not in the TRV-NbRAR1 and TRV-GUS pretreated plants (Figure 2D). Western blotting assays further confirmed that the TSWV N protein accumulated in the systemically infected leaves of NbSGT1-silenced transgenic N. benthamiana plants, but not in the leaves of TRV-NbRAR1- or TRV-GUS-treated plants (Figure 2E). These findings imply that SGT1, but not RAR1, is involved in Sw-5b-mediated resistance to TSWV.
Figure 2.
Silencing expression of SGT1, but not RAR1, compromised the Sw-5b-mediated resistance to TSWV. (A) Sw-5b-transgenic N. benthamiana plants infected with TRV-NbSGT1 and TRV-NbRAR1 at 3 weeks post TRV treatment. TRV-GUS treatment was used as a control. Agrobacterium harboring TRV1 was mixed equally with Agrobacterium harboring TRV2-NbSGT1 or TRV2-NbRAR1 and co-infiltrated into leaves of 4–6 week old Sw-5b-transgenic N. benthamiana. The TRV-treated plants were photographed at 3 weeks post agroinfiltration. (B) Quantitative RT-PCR analysis of the expression of NbSGT1 and NbRAR1 mRNA in the newly emerged leaves of TRV-treated transgenic N. benthamiana plants at 3 weeks post agroinfiltration. The NbActin and NbEF1a genes were used as internal reference genes. Asterisks indicate significant differences (Student’s t-test, * p < 0.05). (C) Analysis of TSWV systemic infection of Sw-5b-transgenic N. benthamiana plants silenced for NbSGT1 and NbRAR1. TRV-GUS was used as a control. The gene-silenced newly emerged leaves of Sw-5b-transgenic N. benthamiana plants were inoculated with crude extract of TSWV-infected tissues. The TSWV challenged plants were photographed at 14 days post viral inoculation. The systemic infection was evident in all 18 NbSGT1-silenced plants. (D) RT–PCR detection of TSWV N RNA in systemic leaves of Sw-5b-transgenic N. benthamiana plants silenced for NbSGT1 and NbRAR1 at 14 days post TSWV inoculation. The internal reference gene was NbActin. (E) Western blot analysis of TSWV N protein accumulation in systemic leaves of Sw-5b-transgenic N. benthamiana plants silenced for NbSGT1 and NbRAR1 using a TSWV N-specific antibody. The leaves were collected at 14 days post TSWV inoculation. A plant sample expressed with p2300 empty vector (Vec.) was used as a negative control. Ponceau S staining was used as a protein loading control.
3.3. Suppression of EDS1 and NDR1 Did Not Disrupt Sw-5b-Triggered Immunity
EDS1 is a common signal component in the TNL-mediated ETI process [56], in which it plays an important role downstream of TIR NADase activity [57,58]. EDS1 is also involved in the immunity mediated by some CNLs [30,31]. NDR1 is a key signal component of the plant immune response mediated by some CNLs [59,60,61]. To investigate whether EDS1 and NDR1 are involved in Sw-5b-mediated resistance to TSWV infection, Agrobacterium cultures containing TRV1 were mixed with Agrobacterium carrying TRV-NbEDS1 or TRV-NbNDR1 and co-infiltrated into leaves of Sw-5b-transgenic N. benthamiana. The TRV-GUS was used as a control. At 3 weeks post TRV treatment, the transgenic N. benthamiana treated with TRV-NbEDS1 and TRV-NbNDR1 did not show any differences compared to the plant treated with the TRV-GUS control (Figure 3A and Figure S4). qRT-PCR confirmed that expression of NbEDS1 and NbNDR1 mRNA transcripts in the corresponding silenced plants was significantly reduced (Figure 3B). Subsequently, gene-silenced newly emerged leaves of transgenic N. benthamiana plants were inoculated with TSWV. At 14 days post TSWV inoculation, no obvious virus systemic infection was observed in the NbEDS1- or NbNDR1-silenced transgenic N. benthamiana plants (Figure 3C). RT-PCR and Western blot assays confirmed that TSWV was absent in the systemic leaves of the NbEDS1- and NbNDR1-silenced transgenic N. benthamiana plants (Figure 3D,E). These results imply that silencing expression of the NbEDS1 or NbNDR1 genes did not disrupt the resistance of Sw-5b-transgenic N. benthamiana plants to TSWV infection.
Figure 3.
Sw-5b-mediated resistance to TSWV was independent of EDS1 and NDR1. (A) Sw-5b-transgenic N. benthamiana plants treated with TRV-NbEDS1 and TRV-NbNDR1. TRV-GUS was used as a control. Agrobacterium harboring TRV1 was mixed equally with Agrobacterium harboring TRV-NbEDS1 or TRV-NbNDR1 and co-infiltrated into leaves of 4–6 week old Sw-5b-transgenic N. benthamiana. The TRV-treated plants were photographed at 3 weeks post agroinfiltration. (B) qRT-PCR analysis of expression of NbEDS1 and NbNDR1 mRNA in newly emerged leaves of Sw-5b-transgenic N. benthamiana treated with TRV-NbEDS1, TRV-NbNDR1, or TRV-GUS. Samples were collected at 3 weeks post TRV treatment. Values were normalized using NbActin and NbEF1a genes as a reference. Student’s t-test was used for statistical analysis (* p < 0.05). (C) Analysis of TSWV systemic infection in Sw-5b-transgenic N. benthamiana plants silenced for NbEDS1 and NbNDR1. TRV-GUS was used as the control. The gene-silenced newly emerged leaves of Sw-5b-transgenic N. benthamiana plants were inoculated with crude extract of TSWV-infected tissues. The plants challenged with TSWV were photographed at 14 days post inoculation. (D) RT–PCR analysis of expression of TSWV N RNA in systemic leaves of Sw-5b-transgenic N. benthamiana plants silenced for NbSGT1 and NbRAR1 at 14 days post TSWV inoculation. A TSWV-infected N. benthamiana sample was used as a positive control. The reference gene was NbActin. (E) Western blot analysis of TSWV N protein accumulation in systemic leaves of Sw-5b-transgenic N. benthamiana plants silenced for NbSGT1 and NbRAR1 in panel C using specific antibodies against N. A plant sample expressed with p2300 empty vector (Vec.) was used as a negative control. Ponceau S staining was used as a protein loading control.
3.4. Silencing Expression of NPR1 Did Not Result in TSWV Systemic Infection in Sw-5b-Transgenic N. benthamiana Plants
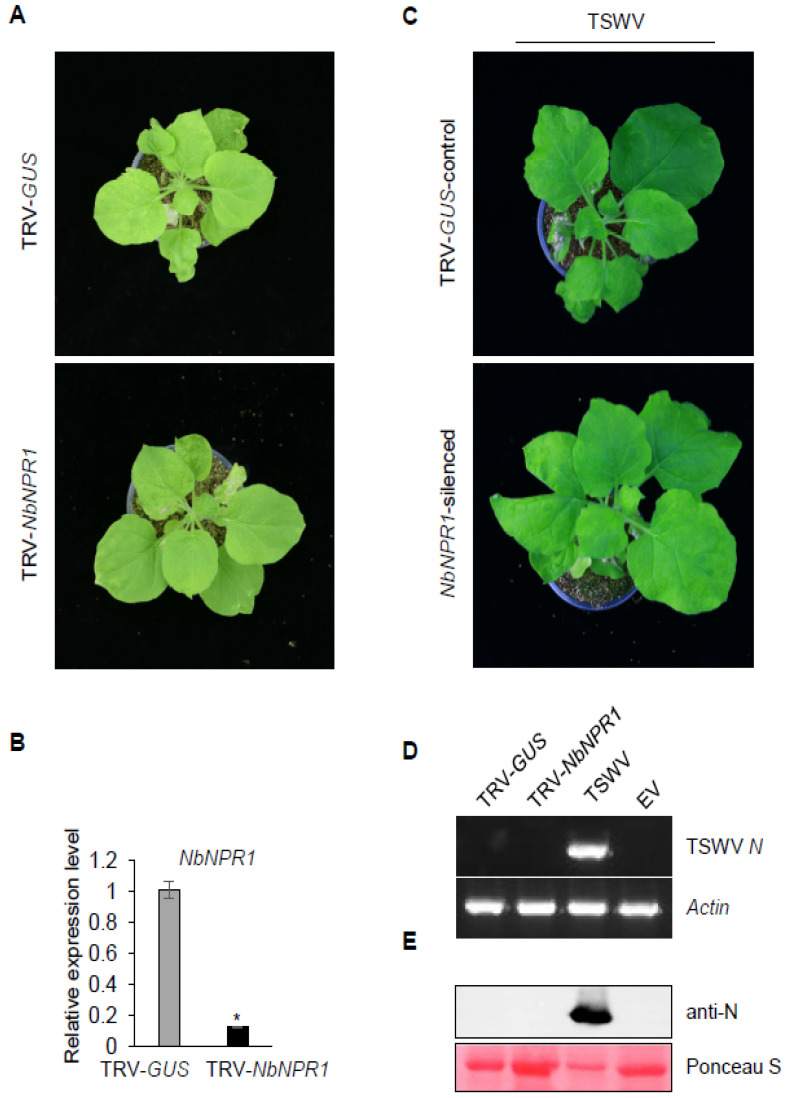
NPR1 acts as a key regulator of SA-signaling pathways [33]. To examine whether NPR1 plays a role in Sw-5b-mediated resistance to TSWV, the mixed Agrobacteria carrying TRV1 and TRV-NbNPR1 were co-infiltrated into Sw-5b-transgenic N. benthamiana. The Agrobacterium carrying the TRV-GUS was used as a control. At 3 weeks post TRV treatment, the growth state of transgenic N. benthamiana plants treated with TRV-NbNPR1 was comparable to that of plants treated with TRV-GUS (Figure 4A and Figure S5). qRT-PCR assays confirmed that expression of NbNPR1 mRNA was silenced in TRV-NbNPR1-treated plants (Figure 4B). The silenced plant leaves were inoculated with TSWV. At 14 days post TSWV inoculation, no obvious symptoms were observed in the systemic leaves of NbNPR1-silenced N. benthamiana plants (Figure 4C). RT-PCR and Western blot assays further confirmed the absence of TSWV in systemic leaves of NbNPR1-silenced Sw-5b-transgenic plants (Figure 4D,E). These data imply that silencing expression of NbNPR1 did not result in TSWV systemic infection in Sw-5b-transgenic N. benthamiana.
Figure 4.
Analysis of the requirement for NPR1 in Sw-5b-mediated resistance to TSWV. (A) TRV-NbNPR1- and TRV-GUS-treated Sw-5b transgenic N. benthamiana plants at 3 weeks post TRV agroinfiltration. (B) qRT-PCR analysis of the expression of NbNPR1 mRNA in newly emerged leaves of Sw-5b transgenic N. benthamiana plant treated with NPR1-silenced transgenic plants. The NbActin and NbEF1a genes were used as internal controls. Student’s t-test was used for statistical analysis (* p < 0.05). (C) Analysis of systemic infection of TSWV in Sw-5b-transgenic N. benthamiana plants silenced for NbNPR1. TRV-GUS plants were used as a control. The gene-silenced newly emerged leaves of Sw-5b-transgenic N. benthamiana plants were inoculated with TSWV. The photographs of treated plants were taken at 14 days post TSWV inoculation. (D) RT–PCR detection of TSWV N RNA in systemic leaves of Sw-5b-transgenic N. benthamiana plants silenced for NbNPR1 at 14 days post TSWV inoculation. A sample from TSWV-infected N. benthamiana tissues was used as a positive control. The NbActin gene was used as an internal reference gene. (E) Western blot analysis of TSWV N protein accumulation in the systemic leaves of Sw-5b-transgenic N. benthamiana plants silenced for NbNPR1 in panel C using specific antibodies against TSWV N. A plant sample expressed with p2300 empty vector (Vec.) was used as a negative control. Ponceau S staining was used as a protein loading control.
3.5. The Helper NLRs NRC2/3/4 Were Required for Sw-5b-Mediated Systemic Resistance to TSWV Infection
In N. benthamiana, the NRC family contains NRC2a, NRC2b, NRC3, and NRC4. NRC2, NRC3, and NRC4 are functionally redundant. Simultaneous silencing of NRC2, NRC3, and NRC4 expression in N. benthamiana suppressed the HR function of Sw-5b [29]. To examine the role of the helper NLRs NRC2/3/4 in systemic resistance mediated by Sw-5b to TSWV, we silenced NbNRC2a, NbNRC2b, NbNRC3, and NbNRC4 simultaneously (NbNRC2/3/4) in Sw-5b-transgenic N. benthamiana by TRV-based VIGS (Figure 5A and Figure S6). The TRV-GUS vector was used as a control. At 3 weeks after TRV treatment, expression of NbNRC2a, NbNRC2b, NbNRC3, and NbNRC4 was significantly reduced in the newly emerged leaves of N. benthamiana pretreated with TRV-NbNRC2/3/4 (Figure 5B). These silenced leaves were challenged with TSWV. At 14 days post TSWV inoculation, TSWV was found to have infected the NbNRC2/3/4-silenced Sw-5b-transgenic N. benthamiana plants, causing systemic wilting (Figure 5C–E). These data imply that the NbNRC2/3/4 genes are required for Sw-5b-mediated systemic resistance to TSWV infection, further supporting the essential role of helper NLR NRCs in downstream signaling of the sensor NLR Sw-5b.
Figure 5.
Requirement for the helper NLRs NRC2/3/4, NRG1, ADR1, and NRG1/ADR1 in Sw-5b-mediated resistance to TSWV infection. (A) TRV-based NbNRC2/3/4, NbNRG1, NbADR1, and NbNRG1/NbADR1 gene-silenced plants at 3 weeks post TRV treatment. (B) qRT-PCR analysis of the expression of NbNRC2a, 2b, 3, and 4 or NbNRG1 and NbADR1 mRNA transgenic plants treated with TRV-NbNRC2/3/4, TRV-NbNRG1, TRV-NbADR1, and TRV-NbNRG1/NbADR1. The NbActin and NbEF1a genes were used as reference genes. Student’s t-test was used for statistical analysis (* p < 0.05). (C) Analysis of systemic TSWV infection of Sw-5b-transgenic N. benthamiana plants silenced for NbNRC2/3/4, NbNRG1, NbADR1, or NbNRG1/NbADR1. TRV-GUS-treated plants were used as a control. The gene-silenced newly emerged leaves of Sw-5b-transgenic N. benthamiana plants were inoculated with TSWV. The photographs of TSWV-challenged plants were taken at 14 days post TSWV inoculation. The systemic infection was evident in all 18 NbNRC2/3/4-silenced plants in three repeated experiments. (D) RT–PCR detection of TSWV N RNA in systemic leaves of plants in panel C at 14 days post TSWV infection. A sample from a TRV-GUS-treated plant was used as a negative control. The NbActin gene was used as an internal reference gene. (E) Western blot analysis of the accumulation of TSWV N protein in systemic leaves of plants in panel C using N-specific antibodies. The samples were harvested at 14 dpi. A plant leaf sample expressed with p2300 empty vector (Vec.) was used as a negative control. Ponceau S staining was used as a protein loading control.
3.6. Silencing Expression of the Helper NLRs NRG1 and ADR1 Did Not Affect Sw-5b-Mediated Systemic Resistance to TSWV
ADR1 and NRG1 play important roles in the regulation of ETI mediated by multiple NLRs [47,62]. To examine whether NRG1 and ADR1 are involved in Sw-5b-mediated resistance, we silenced NbNRG1, NbADR1, and both genes (NbNRG1/NbADR1) in Sw-5b-transgenic N. benthamiana plants using TRV-based VIGS. At 3 weeks post TRV treatment, the growth states of NbNRG1-, NbADR1-, and NbNRG1/NbADR1-silenced N. benthamiana plants were similar to that of TRV-GUS-treated plants (Figure 5A and Figure S6). The TSWV crude extract was inoculated onto the gene-silenced newly emerged plant leaves. At 14 days post TSWV inoculation, no systemic viral infection was found in systemic leaves of NbNRG1-, NbADR1-, or NbNRG1/NbADR1-silenced plants (Figure 5C). RT-PCR and Western blot assays confirmed the absence of TSWV in the systemic leaves of the NbNRG1-, NbADR1-, and NbNRG1/NbADR1-silenced plants (Figure 5D,E). These results imply that the helper NLRs NRG1 and ADR1 may not be involved in Sw-5b-mediated resistance.
4. Discussion
Here, we used the TRV-based VIGS system to characterize the roles of the SGT1/RAR1/HSP90, EDS1/NDR1, NPR1, and NRC/ADR1/NRG1 genes in Sw-5b-mediated resistance to TSWV. We found that chaperone SGT1 was essential for Sw-5b function; however, co-chaperone RAR1 was not. Suppression of both EDS1 and NDR1 did not affect Sw-5b-mediated resistance to TSWV. Moreover, suppression of NPR1 did not result in TSWV systemic infection in Sw-5b-transgenic plants. However, silencing the helper NLRs NRC2/3/4 compromised systemic resistance to TSWV infection in Sw-5b-transgenic N. benthamiana plants. Silencing another two helper NLRs, ADR1 and NRG1, did not affect Sw-5b-mediated immune signaling.
We explored the role of the SGT1–RAR1–HSP90 chaperone complex in Sw-5b-mediated immunity. Scofield et al. found that all three chaperone molecules, SGT1–RAR1–HSP90, were required for Lr21–mediated resistance. Silencing any of the three compromised resistance [63]. In our study, silencing HSP90 resulted in significant morphological defects in the transgenic N. benthamiana, which led us to discontinue further investigation into the role of HSP90. Silencing SGT1 compromised Sw-5b function; however, silencing RAR1 did not. SGT1–RAR1–HSP90 forms a chaperone complex. However, Sw-5b-mediated immune signaling is independent of RAR1. A previous study on Mi-1.2 also reported that Mi-1.2-mediated pest resistance required SGT1 but not RAR1 [64]. SGT1 has diverse roles in cellular pathways. For example, it plays an important role in nucleoplasm partitioning of tobacco N NLR [28]. We hypothesized that SGT1 may help in stabilizing Sw-5b immune receptor. In addition to functioning as a chaperone, SGT1 may play other roles in Sw-5b- and Mi-1.2-mediated defense signaling, and these roles of SGT1 may be independent of RAR1.
We tested the role of EDS1/NDR1 in Sw-5b-mediated resistance to TSWV. Early studies revealed that EDS1 was necessary for some CNLs. We found that the Sw-5b-mediated immune response was independent of EDS1. Arabidopsis CNLs such as RPS2, RPM1, and RPS5 are dependent on NDR1 for immunity. Our Sw-5b-mediated immunity was not dependent on NDR1. Arabidopsis CNLs, including HRT, ZAR1, RPP7, and RPP8, have also been found to be NDR1-independent [65]. In addition, the CNLs RPP13 and ZAR1 were independent of both EDS1 and NDR1 [66,67]. These findings imply that Sw-5b may induce EDS1- and NDR1-independent immune signaling pathways that remain to be uncovered.
We also explored the role of NPR1 in Sw-5b-mediated resistance. SA plays important roles in plant innate immunity and system acquired resistance (SAR). NPR1 acts as a receptor of SA and is a core player in SA signaling pathways. In our study, silencing expression of NPR1 did not disrupt Sw-5b resistance to TSWV. However, NPR1 has been shown to be required for the function of the tobacco N gene. Our results imply that Sw-5b-mediated resistance may not rely on the NPR1-dependent SA signaling pathway.
Lastly, we tested the function of “helper” NLR proteins in Sw-5b-mediated systemic resistance to TSWV infection. NRCs are required for HR mediated by various NLRs in Solanaceae and disease resistance mediated by Rpi-blb2 and Rx [29]. Here, we found that silencing the helper NLRs NRC2/3/4 compromised Sw-5b-mediated resistance to TSWV, further supporting the essential role of NRC in NLR-mediated resistance in Solanaceae. ADR1 proteins are involved in both TNL- and CNL-mediated immunity [45,46,47,48]. NRG1 is a key component involved in TNL-mediated immune signaling pathways. ADR1 and NRG1 function redundantly in mediating NLR immune signaling [62]. Silencing ADR1 and NRG1 simultaneously partially compromised resistance to PVX [46]. We found that silencing NRG1, ADR1, or both had no effect on Sw-5b-mediated resistance to TSWV.
Mixed infection by two different viruses may have synergistic or suppressive effects. Our presented data are based on the use of TRV for gene silencing, followed by TSWV infection. We cannot rule out the possibility that infection of Sw-5b transgenic plants by TRV constructs may have enhanced or reduced the resistance to TSWV infection. In the future, gene knockout mediated by CRISPR/Cas9 in Sw-5b transgenic plant is needed to confirm the results obtained in this study.
In summary, we systematically investigated the requirement for the SGT1/RAR1/HSP90, EDS1/NDR1, NPR1, and NRC/ADR1/NRG1 genes in Sw-5b mediated immune signaling. We demonstrated that SGT1 and NRCs are conserved essential components of Sw-5b-mediated resistance to TSWV. Our results showing the independence of EDS1/NDR1 and NPR1 also indicate that Sw-5b has some unique and undiscovered defense signaling pathway that remains to be elucidated. As Sw-5b-mediated resistance is dependent on NRCs, we proposed that Sw-5b may either directly recruit downstream helper NLRs or indirectly transfer the upstream pathogen perception signals to downstream helper NLRs to amply and induce robust defense signaling.
Acknowledgments
We thank Yu Zhang and Chongkun Zuo for assisting with greenhouse management.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13081447/s1. Figure S1. Silencing NbPDS and Sw-5b by TRV-based VIGS in Sw-5b-transgenic N. benthamiana plants. Figure S2. Sw-5b-transgenic N. benthamiana plants infected with TRV-GUS, TRV-NbSGT1, and TRV-NbRAR1 at 3 weeks post TRV treatment. Figure S3. Silencing the expression of NbHSP90 in Sw-5b-transgenic N. benthamiana plants. Figure S4. Sw-5b-transgenic N. benthamiana plants infected with TRV-GUS, TRV- NbEDS1, and TRV-NbNDR1 at 3 weeks post TRV treatment. Figure S5. Sw-5b-transgenic N. benthamiana plants infected with TRV-GUS and TRV-NbNPR1 at 3 weeks post TRV treatment. Figure S6. Sw-5b-transgenic N. benthamiana plants infected with TRV-GUS, TRV-NbNRC2/3/4, TRV-NbNRG1, TRV-NbADR1, and TRV-NbNRG1/ADR1 at 3 weeks post TRV treatment. Table S1. List of primers used in this study.
Author Contributions
Z.C., Q.W. and X.T. conceptualized and designed the experiments; Z.C., Q.W., C.T., H.C., D.M., X.Q., X.Z. and L.J. performed the experiments; Z.C., Q.W. and X.T. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the National Natural Science Foundation of China (31630062, 31925032 and 31870143), the Fundamental Research Funds for the Central Universities (JCQY202104 and KYXK202012), Youth Science and Technology Innovation Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data set analyzed for the current study is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Kourelis J., van der Hoorn R.A.L. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell. 2018;30:285–299. doi: 10.1105/tpc.17.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Biezen E.A., Jones J.D. The NB-ARC domain: A novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr. Biol. 1998;8:R226–R227. doi: 10.1016/S0960-9822(98)70145-9. [DOI] [PubMed] [Google Scholar]
- 4.Jones J.D., Vance R.E., Dangl J.L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016:354. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- 5.Bonardi V., Cherkis K., Nishimura M.T., Dangl J.L. A new eye on NLR proteins: Focused on clarity or diffused by complexity? Curr. Opin. Immunol. 2012;24:41–50. doi: 10.1016/j.coi.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dangl J.L., Horvath D.M., Staskawicz B.J. Pivoting the plant immune system from dissection to deployment. Science. 2013;341:746–751. doi: 10.1126/science.1236011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parrella G., Gognalons P., Gebre-Selassie K., Vovlas C., Marchoux G. An update of the host range of tomato spotted wilt virus. J. Plant. Pathol. 2003;85:227–264. [Google Scholar]
- 8.Kormelink R., Garcia M.L., Goodin M., Sasaya T., Haenni A.L. Negative-strand RNA viruses: The plant-infecting counterparts. Virus Res. 2011;162:184–202. doi: 10.1016/j.virusres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Scholthof K.B., Adkins S., Czosnek H., Palukaitis P., Jacquot E., Hohn T., Hohn B., Saunders K., Candresse T., Ahlquist P., et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011;12:938–954. doi: 10.1111/j.1364-3703.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver J.E., Whitfield A.E. The genus tospovirus: Emerging bunyaviruses that threaten food security. Annu. Rev. Virol. 2016;3:101–124. doi: 10.1146/annurev-virology-100114-055036. [DOI] [PubMed] [Google Scholar]
- 11.Prins M., Goldbach R. The emerging problem of tospovirus infection and nonconventional methods of control. Trends MicroBiol. 1998;6:31–35. doi: 10.1016/S0966-842X(97)01173-6. [DOI] [PubMed] [Google Scholar]
- 12.Boiteux L.S., Giordano L.D. Genetic-basis of resistance against 2 tospovirus species in tomato (Lycopersicon-Esculentum) Euphytica. 1993;71:151–154. doi: 10.1007/BF00023478. [DOI] [Google Scholar]
- 13.Brommonschenkel S.H., Frary A., Frary A., Tanksley S.D. The broad-spectrum tospovirus resistance gene Sw-5 of tomato is a homolog of the root-knot nematode resistance gene Mi. Mol. Plant Microbe Interact. 2000;13:1130–1138. doi: 10.1094/MPMI.2000.13.10.1130. [DOI] [PubMed] [Google Scholar]
- 14.Spassova M.I., Prins T.W., Folkertsma R.T., Klein-Lankhorst R.M., Hille J., Goldbach R.W., Prins M. The tomato gene Sw5 is a member of the coiled coil, nucleotide binding, leucine-rich repeat class of plant resistance genes and confers resistance to TSWV in tobacco. Mol. Breed. 2001;7:151–161. doi: 10.1023/A:1011363119763. [DOI] [Google Scholar]
- 15.Bendahmane A., Farnham G., Moffett P., Baulcombe D.C. Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 2002;32:195–204. doi: 10.1046/j.1365-313X.2002.01413.x. [DOI] [PubMed] [Google Scholar]
- 16.Hallwass M., de Oliveira A.S., de Campos Dianese E., Lohuis D., Boiteux L.S., Inoue-Nagata A.K., Resende R.O., Kormelink R. The Tomato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in Sw-5-containing resistant tomato lines and in Nicotiana benthamiana transformed with the functional Sw-5b resistance gene copy. Mol. Plant Pathol. 2014;15:871–880. doi: 10.1111/mpp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiro A., Canizares M.C., Rubio L., Lopez C., Moriones E., Aramburu J., Sanchez-Navarro J. The movement protein (NSm) of Tomato spotted wilt virus is the avirulence determinant in the tomato Sw-5 gene-based resistance. Mol. Plant Pathol. 2014;15:802–813. doi: 10.1111/mpp.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W.Y., Jiang L., Feng Z.K., Chen X.J., Huang Y., Xue F., Huang C.J., Liu Y., Li F., Liu Y.T., et al. Plasmodesmata targeting and intercellular trafficking of Tomato spotted wilt tospovirus movement protein NSm is independent of its function in HR induction. J. Gen. Virol. 2016;97:1990–1997. doi: 10.1099/jgv.0.000496. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M., Jiang L., Bai B.H., Zhao W.Y., Chen X.J., Li J., Liu Y., Chen Z.Q., Wang B.T., Wang C.L., et al. The Intracellular Immune Receptor Sw-5b confers broad-spectrum resistance to tospoviruses through recognition of a conserved 21-amino acid viral effector epitope. Plant Cell. 2017;29:2214–2232. doi: 10.1105/tpc.17.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Zhu M., Jiang L., Zhao W., Li J., Wu J., Li C., Bai B., Lu G., Chen H., et al. A multilayered regulatory mechanism for the autoinhibition and activation of a plant CC-NB-LRR resistance protein with an extra N-terminal domain. New Phytol. 2016;212:161–175. doi: 10.1111/nph.14013. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Huang H., Zhu M., Huang S., Zhang W., Dinesh-Kumar S.P., Tao X. A plant immune receptor adopts a two-step recognition mechanism to enhance viral effector perception. Mol. Plant. 2019;12:248–262. doi: 10.1016/j.molp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Azevedo C., Sadanandom A., Kitagawa K., Freialdenhoven A., Shirasu K., Schulze-Lefert P. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science. 2002;295:2073–2076. doi: 10.1126/science.1067554. [DOI] [PubMed] [Google Scholar]
- 23.Kadota Y., Shirasu K., Guerois R. NLR sensors meet at the SGT1-HSP90 crossroad. Trends Biochem. Sci. 2010;35:199–207. doi: 10.1016/j.tibs.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z.Q., Liu Y.Y., Shi L.P., Yang S., Shen L., Yu H.X., Wang R.Z., Wen J.Y., Tang Q., Hussain A., et al. SGT1 is required in PcINF1/SRC2-1 induced pepper defense response by interacting with SRC2-1. Sci. Rep. 2016;6:21651. doi: 10.1038/srep21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su H., Chen G., Yang L., Zhang Y., Wang Y., Fang Z., Lv H. Proteomic variations after short-term heat shock treatment reveal differentially expressed proteins involved in early microspore embryogenesis in cabbage (Brassica oleracea) PeerJ. 2020;8:e8897. doi: 10.7717/peerj.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman A.V.E., Hunt M., Surana P., Velasquez-Zapata V., Xu W., Fuerst G., Wise R.P. Disruption of barley immunity to powdery mildew by an in-frame Lys-Leu deletion in the essential protein SGT1. Genetics. 2021;217:iyaa026. doi: 10.1093/genetics/iyaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azevedo C., Betsuyaku S., Peart J., Takahashi A., Noel L., Sadanandom A., Casais C., Parker J., Shirasu K. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 2006;25:2007–2016. doi: 10.1038/sj.emboj.7601084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoser R., Zurczak M., Lichocka M., Zuzga S., Dadlez M., Samuel M.A., Ellis B.E., Stuttmann J., Parker J.E., Hennig J., et al. Nucleocytoplasmic partitioning of tobacco N receptor is modulated by SGT1. New Phytol. 2013;200:158–171. doi: 10.1111/nph.12347. [DOI] [PubMed] [Google Scholar]
- 29.Wu C.H., Abd-El-Haliem A., Bozkurt T.O., Belhaj K., Terauchi R., Vossen J.H., Kamoun S. NLR network mediates immunity to diverse plant pathogens. Proc. Natl. Acad. Sci. USA. 2017;114:8113–8118. doi: 10.1073/pnas.1702041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra-Shekara A.C., Navarre D., Kachroo A., Kang H.G., Klessig D., Kachroo P. Signaling requirements and role of salicylic acid in HRT- and rrt-mediated resistance to turnip crinkle virus in Arabidopsis. Plant J. 2004;40:647–659. doi: 10.1111/j.1365-313X.2004.02241.x. [DOI] [PubMed] [Google Scholar]
- 31.Xiao S., Calis O., Patrick E., Zhang G., Charoenwattana P., Muskett P., Parker J.E., Turner J.G. The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J. 2005;42:95–110. doi: 10.1111/j.1365-313X.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 32.Day B., Dahlbeck D., Staskawicz B.J. NDR1 interaction with RIN4 mediates the differential activation of multiple disease resistance pathways in Arabidopsis. Plant Cell. 2006;18:2782–2791. doi: 10.1105/tpc.106.044693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao H., Glazebrook J., Clarke J.D., Volko S., Dong X.N. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/S0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- 34.Ryals J., Weymann K., Lawton K., Friedrich L., Ellis D., Steiner H.Y., Johnson J., Delaney T.P., Jesse T., Vos P., et al. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Z.Q., Yan S., Saleh A., Wang W., Ruble J., Oka N., Mohan R., Spoel S.H., Tada Y., Zheng N., et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486:228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao H., Bowling S.A., Gordon A.S., Dong X.N. Characterization of an arabidopsis mutant that is nonresponsive to inducers of systemic acquired-resistance. Plant Cell. 1994;6:1583–1592. doi: 10.2307/3869945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Schiff M., Marathe R., Dinesh-Kumar S.P. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002;30:415–429. doi: 10.1046/j.1365-313X.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y., Detchemendy T.W., Pajerowska-Mukhtar K.M., Mukhtar M.S. NPR1 in JazzSet with pathogen effectors. Trends Plant Sci. 2018;23:469–472. doi: 10.1016/j.tplants.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Janda M., Ruelland E. Magical mystery tour: Salicylic acid signalling. Environ. Exp. Bot. 2015;114:117–128. doi: 10.1016/j.envexpbot.2014.07.003. [DOI] [Google Scholar]
- 40.Kachroo P., Yoshioka K., Shah J., Dooner H.K., Klessig D.F. Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell. 2000;12:677–690. doi: 10.1105/tpc.12.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C.H., Belhaj K., Bozkurt T.O., Birk M.S., Kamoun S. Helper NLR proteins NRC2a/b and NRC3 but not NRC1 are required for Pto-mediated cell death and resistance in Nicotiana benthamiana. New Phytol. 2016;209:1344–1352. doi: 10.1111/nph.13764. [DOI] [PubMed] [Google Scholar]
- 42.Jubic L.M., Saile S., Furzer O.J., El Kasmi F., Dangl J.L. Help wanted: Helper NLRs and plant immune responses. Curr. Opin. Plant Biol. 2019;50:82–94. doi: 10.1016/j.pbi.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Van Wersch S., Tian L., Hoy R., Li X. Plant NLRs: The Whistleblowers of Plant Immunity. Plant Commun. 2020;1:100016. doi: 10.1016/j.xplc.2019.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabriels S.H., Takken F.L., Vossen J.H., de Jong C.F., Liu Q., Turk S.C., Wachowski L.K., Peters J., Witsenboer H.M., de Wit P.J., et al. CDNA-AFLP combined with functional analysis reveals novel genes involved in the hypersensitive response. Mol. Plant Microbe Interact. 2006;19:567–576. doi: 10.1094/MPMI-19-0567. [DOI] [PubMed] [Google Scholar]
- 45.Bonardi V., Tang S., Stallmann A., Roberts M., Cherkis K., Dangl J.L. Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc. Natl. Acad. Sci. USA. 2011;108:16463–16468. doi: 10.1073/pnas.1113726108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collier S.M., Hamel L.P., Moffett P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol. Plant Microbe Interact. 2011;24:918–931. doi: 10.1094/MPMI-03-11-0050. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z., Li M., Dong O.X., Xia S., Liang W., Bao Y., Wasteneys G., Li X. Differential regulation of TNL-mediated immune signaling by redundant helper CNLs. New Phytol. 2019;222:938–953. doi: 10.1111/nph.15665. [DOI] [PubMed] [Google Scholar]
- 48.Castel B., Ngou P.M., Cevik V., Redkar A., Kim D.S., Yang Y., Ding P., Jones J.D.G. Diverse NLR immune receptors activate defence via the RPW8-NLR NRG1. New Phytol. 2019;222:966–980. doi: 10.1111/nph.15659. [DOI] [PubMed] [Google Scholar]
- 49.Peart J.R., Mestre P., Lu R., Malcuit I., Baulcombe D.C. NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr. Biol. 2005;15:968–973. doi: 10.1016/j.cub.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 50.Qi T., Seong K., Thomazella D.P.T., Kim J.R., Pham J., Seo E., Cho M.J., Schultink A., Staskawicz B.J. NRG1 functions downstream of EDS1 to regulate TIR-NLR-mediated plant immunity in Nicotiana benthamiana. Proc. Natl. Acad. Sci. USA. 2018;115:E10979–E10987. doi: 10.1073/pnas.1814856115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grech-Baran M., Witek K., Szajko K., Witek A.I., Morgiewicz K., Wasilewicz-Flis I., Jakuczun H., Marczewski W., Jones J.D.G., Hennig J. Extreme resistance to Potato virus Y in potato carrying the Rysto gene is mediated by a TIR-NLR immune receptor. Plant Biotechnol. J. 2020;18:655–667. doi: 10.1111/pbi.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian X., Xiang Q., Yang T.Q., Ma H.Y., Ding X.S., Tao X.R. Molecular co-chaperone SGT1 is critical for cell-to-cell movement and systemic infection of tomato spotted wilt virus in Nicotiana benthamiana. Viruses. 2018;10:647. doi: 10.3390/v10110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu Z.Z., Feng Z.K., Zhang Z.J., Liu Y.B., Tao X.R. Complete genome sequence of a tomato spotted wilt virus isolate from China and comparison to other TSWV isolates of different geographic origin. Arch. Virol. 2011;156:1905–1908. doi: 10.1007/s00705-011-1078-9. [DOI] [PubMed] [Google Scholar]
- 54.Shirasu K. The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 2009;60:139–164. doi: 10.1146/annurev.arplant.59.032607.092906. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M., Kadota Y., Prodromou C., Shirasu K., Pearl L.H. Structural basis for assembly of Hsp90-Sgt1-CHORD protein complexes: Implications for chaperoning of NLR innate immunity receptors. Mol. Cell. 2010;39:269–281. doi: 10.1016/j.molcel.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cui H., Tsuda K., Parker J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015;66:487–511. doi: 10.1146/annurev-arplant-050213-040012. [DOI] [PubMed] [Google Scholar]
- 57.Horsefield S., Burdett H., Zhang X., Manik M.K., Shi Y., Chen J., Qi T., Gilley J., Lai J.S., Rank M.X., et al. NAD(+) cleavage activity by animal and plant TIR domains in cell death pathways. Science. 2019;365:793–799. doi: 10.1126/science.aax1911. [DOI] [PubMed] [Google Scholar]
- 58.Wan L., Essuman K., Anderson R.G., Sasaki Y., Monteiro F., Chung E.H., Osborne Nishimura E., DiAntonio A., Milbrandt J., Dangl J.L., et al. TIR domains of plant immune receptors are NAD(+)-cleaving enzymes that promote cell death. Science. 2019;365:799–803. doi: 10.1126/science.aax1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Century K.S., Holub E.B., Staskawicz B.J. Ndr1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc. Natl. Acad. Sci. USA. 1995;92:6597–6601. doi: 10.1073/pnas.92.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Century K.S., Shapiro A.D., Repetti P.P., Dahlbeck D., Holub E., Staskawicz B.J. NDR1, a pathogen-induced component required for Arabidopsis disease resistance. Science. 1997;278:1963–1965. doi: 10.1126/science.278.5345.1963. [DOI] [PubMed] [Google Scholar]
- 61.Selote D., Shine M.B., Robin G.P., Kachroo A. Soybean NDR1-like proteins bind pathogen effectors and regulate resistance signaling. New Phytol. 2014;202:485–498. doi: 10.1111/nph.12654. [DOI] [PubMed] [Google Scholar]
- 62.Saile S.C., Jacob P., Castel B., Jubic L.M., Salas-Gonzales I., Backer M., Jones J.D.G., Dangl J.L., El Kasmi F. Two unequally redundant “helper” immune receptor families mediate Arabidopsis thaliana intracellular “sensor” immune receptor functions. PLoS Biol. 2020;18:e3000783. doi: 10.1371/journal.pbio.3000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scofield S.R., Huang L., Brandt A.S., Gill B.S. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 2005;138:2165–2173. doi: 10.1104/pp.105.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhattarai K.K., Li Q., Liu Y., Dinesh-Kumar S.P., Kaloshian I. The MI-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 2007;144:312–323. doi: 10.1104/pp.107.097246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapos P., Devendrakumar K.T., Li X. Plant NLRs: From discovery to application. Plant Sci. 2019;279:3–18. doi: 10.1016/j.plantsci.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Bittner-Eddy P.D., Beynon J.L. The Arabidopsis downy mildew resistance gene, RPP13-Nd, functions independently of NDR1 and EDS1 and does not require the accumulation of salicylic acid. Mol. Plant Microbe Interact. 2001;14:416–421. doi: 10.1094/MPMI.2001.14.3.416. [DOI] [PubMed] [Google Scholar]
- 67.Lewis J.D., Wu R., Guttman D.S., Desveaux D. Allele-specific virulence attenuation of the Pseudomonas syringae HopZ1a type III effector via the Arabidopsis ZAR1 resistance protein. PLoS Genet. 2010;6:e1000894. doi: 10.1371/journal.pgen.1000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set analyzed for the current study is available from the corresponding author upon reasonable request.