Abstract
BACKGROUND
Historically, the receipt of prescription opioids has differed among racial groups in the United States. Research has not sufficiently explored the contribution of individual health systems to these differences by examining within-system prescription opioid receipt according to race.
METHODS
We used 2016 and 2017 Medicare claims data from a random 40% national sample of fee-for-service, Black and White beneficiaries 18 to 64 years of age who were attributed to health systems. We identified 310 racially diverse systems (defined as systems with ≥200 person-years each for Black and White patients). To test representativeness, we compared patient characteristics and opioid receipt among the patients in these 310 systems with those in the national sample. Within the 310 systems, regression models were used to explore the difference between Black and White patients in the following annual opioid measures: any prescription filled, short-term receipt of opioids, long-term receipt of opioids (one or more filled opioid prescriptions in all four calendar quarters of a year), and the opioid dose in morphine milligram equivalents (MME); models controlled for patient characteristics, state, and system.
RESULTS
The national sample included 2,197,153 person-years, and the sample served by 310 racially diverse systems included 896,807 person-years (representing 47.4% of all patients and 56.1% of Black patients in the national sample). The national sample and 310-systems sample differed meaningfully only in the percent of person-years contributed by Black patients (21.3% vs. 25.9%). In the 310-systems sample, the crude annual prevalence of any opioid receipt differed slightly between Black and White patients (50.2% vs. 52.2%), whereas the mean annual dose was 36% lower among Black patients than among White patients (5190 MME vs. 8082 MME). Within systems, the adjusted race differences in measures paralleled the population trends: the annual prevalence of opioid receipt differed little, but the mean annual dose was higher among White patients than among Black patients in 91% of the systems, and at least 15% higher in 75% of the systems.
CONCLUSIONS
Within individual health systems, Black and White patients received markedly different opioid doses. These system-specific findings could facilitate exploration of the causes and consequences of these differences. (Funded by the National Institute on Aging and the Agency for Healthcare Research and Quality.)
The receipt of prescription opioid analgesics in the United States differs according to skin color despite a lack of evidence of racial differences in pain perception or in preferences for pain management.1,2 National, regional, and single-institution studies involving adults have consistently shown that Black and Hispanic patients are less likely to receive opioid analgesics than White patients. When Black and Hispanic patients do receive opioids, they commonly receive a lower dose than their White counterparts.1–14 Studies have suggested that the differences may be narrowing, but their persistence raises disturbing questions about the effect of patient race on physicians’ pain-management decisions and, in turn, the suffering experienced by patients when they are undertreated or overtreated.1,15–18
As a signal of care quality, opioid receipt is especially complex. Although prescription opioid use is discouraged for the management of long-term, non–cancer-related pain, clinicians historically have accepted these drugs as a reasonable first-line therapy for severe, short-term pain and for pain associated with advanced cancer.19–21 Overdose events and death are well-recognized risks associated with prescription opioids.22–24 Prescribers are challenged to balance these risks with their desire to relieve suffering. Historically, this search for balance manifests as more liberal use of opioids for White patients than for Black or Brown patients.1–14 Given the complicated trade-offs, we do not yet know which group has fared better overall, but it is hard to imagine that the influence of race in these decisions — given that there is no known physiologic basis — reflects high-quality, equitable care.2 Steep growth in the incidence of opioid overdose events among White patients in the United States has led some researchers and journalists to characterize unequal opioid receipt as protective for Black and Brown patients.18,23,25 Such characterizations ignore the risks of untreated and undertreated pain.1,26,27
Generally, large studies of prescription opioid receipt examine broad geographic areas or the nation; this approach exposes important trends but largely defies accountability and actionability. To begin addressing this research and reporting gap, we measured and compared prescription opioid receipt among Black and non-Hispanic White Medicare beneficiaries 18 to 64 years of age, across and within individual health systems. This population, which is composed almost entirely of workers with disability, has a high level of prescription opioid receipt and a high rate of opioid overdose events.28 We report the differences between Black and White patients in the receipt of prescription opioids, both overall and within health systems, for this population and present race-specific opioid-receipt measures for each system.
METHODS
DATA AND COHORT
We used Medicare data from a random 40% sample of fee-for-service beneficiaries 18 to 64 years of age who had been fully enrolled for at least 12 months in Medicare Part A (inpatient), Part B (outpatient), and Part D (prescription drug) coverage in 2016 or 2017. We limited our study to beneficiaries who were identified as Black or non-Hispanic White (hereafter, White) by the Research Triangle Institute classification available in the Medicare Master Beneficiary Summary File.29 For health system attribution, we further limited our study to include patients who had at least one primary care service claim in 2016 or 2017. To minimize the inclusion of incomplete claims, we excluded patients living outside the United States, those who were eligible for Medicare owing to end-stage renal disease, those who had any hospice use, and those with no Part D claims. The study was approved by the Dartmouth College Committee for the Protection of Human Subjects. Additional details about the study methods are provided in Supplementary Appendix 1.
PRESCRIPTION OPIOID MEASURES
Using Medicare Part D data, we constructed four annual measures of patient-level prescription opioid receipt: any opioid receipt, short-term receipt (at least one filled opioid prescription in one to three quarters of the calendar year), long-term receipt (at least one filled opioid prescription in four calendar quarters of a year), and the total morphine milligram equivalents (MME) per person-year (the MME value of all filled opioid prescriptions summed for each person-year). Definitions are provided in Tables S1 and S2 in Supplementary Appendix 1.30,31
COVARIATES AND PATIENT CHARACTERISTICS
Medicare data include information on patients’ age, sex, and state of residence. We classified patients as long-term care residents if 50% or more of their prescriptions in a calendar year had been filled by a long-term care pharmacy (according to the Pharmacy Characteristics File).32 We used contemporaneous claims to calculate patients’ annual Hierarchical Condition Category (HCC) scores (scores generally range from 0.7 to 1.5, with higher scores indicating higher morbidity and predicting higher medical spending).33 To align with the Institute of Medicine on disparities measurement, we did not include poverty indicators in our study.34 For secondary analyses, we identified patients with cancer diagnoses and used Part D data to calculate the annual nonopioid prescription drug 30-day supply count for each patient.33 (Definitions are provided in Tables S1, S3, and S4.)
HEALTH SYSTEM ATTRIBUTION
We used the IQVIA OneKey database to link providers on claims to health systems and practices using the National Provider Identifier.35,36 We used claims to attribute each patient to a health system in each calendar year on the basis of the plurality of primary care visits.37 Data on patients who had been treated primarily outside health systems (i.e., in independent practices) were retained for the national-sample analyses only. For system-level analyses, we identified 310 health systems that included at least 200 attributed person-years each for Black patients and White patients in our study period. This threshold ensured compliance with the Centers for Medicare and Medicaid Services reporting requirements that are designed to protect the confidentiality of Medicare beneficiaries.38 Beneficiaries who had been attributed to these 310 diverse-population systems composed the sample used in the regression analyses.
STATISTICAL ANALYSIS
We explored each prescription-opioid measure and the Black–White measure differences in both the national sample and the sample attributed to the 310 health systems with sufficient cohorts of Black and White patients, and we examined differences between the two samples to test representativeness. We then modeled the mean person-level race differences in opioid measures in the sample of patients attributed to the 310 systems. Next, we estimated adjusted race differences in measures for each of the 310 individual systems. We graphed these adjusted system-level race differences.
To obtain the mean person-level race differences in opioid measures among the patients attributed to the 310 systems, we fit the following linear regression model (linear probability model for binary outcomes) for each outcome for the years 2016 and 2017:
where Y is the prescription opioid measure for individual i attributed to system k in year t residing in state s. In this equation, “Black” indicates Black race, “System” is a vector of indicators for the system delivering the plurality of primary care visits to patient i, “CY16” indicates 2016 observations, “State” is a vector of state of residence indicators, and “Covariates” is a vector of patient characteristics. We used a robust sandwich variance estimator, specifying clusters as systems. We report β1, an estimate of adjusted Black–White differences for each outcome, with 95% confidence intervals. Because the confidence intervals were not adjusted for multiplicity, inferences drawn from these intervals may not be reproducible.
To obtain measures for each of the 310 individual systems and to provide insight into the source of mean differences between populations, we estimated within-system Black–White differences in opioid measures. For this analysis, we modified the model above by replacing the single “Black” race indicator with “Black⋆System” (an interaction term multiplying the Black race indicator by each system indicator) and omitted the constant term. In these models, β1 is a vector of system-specific estimates of the Black–White difference in Y, as compared with β2, a vector of estimated adjusted means of Y among White patients within each system. We used these models to create, for each health system, estimates of Black and White measure values, differences, and ratios.
We conducted four sets of secondary analyses. First, we reestimated the main models among patients who had at least one cancer diagnosis code in both years in order to explore the effect of cancer diagnoses on differences in opioid receipt and measures according to race. Second, to assess whether observed differences in opioid receipt would reflect a lower annual prevalence of receipt of all prescription drugs among Black patients than among White patients, we reestimated the main models, controlling for the 30-day supply count of the nonopioid prescriptions for each patient. Third, to explore sources of differences in the annual MME according to race, we examined the characteristics of the filled opioid prescriptions: the MME per filled prescription, the MME per unit dispensed, and the number of units dispensed, according to race, among long-term recipients and short-term recipients. Finally, we explored how estimated Black–White differences varied according to the fraction of person-years contributed by Black patients in a system.
RESULTS
POPULATION
Table 1 presents the characteristics of the patients in the national sample and of those in the sample attributed to the 310 systems included in our system-level analysis. The national sample included 2,197,153 person-years (from 1,297,519 unique patients) and differed meaningfully from the sample attributed to the 310 studied systems only in the percent of the person-years contributed by Black patients (21.3% vs. 25.9%).
Table 1.
Characteristics of the National Sample and the Sample Attributed to 310 Large, Diverse Health Systems.*
| Characteristic | All Beneficiaries | Black Beneficiaries | White Beneficiaries | |||
|---|---|---|---|---|---|---|
| National Sample | Sample in 310 Health Systems | National Sample | Sample in 310 Health Systems | National Sample | Sample in 310 Health Systems | |
| No. of beneficiaries | 1,297,519 | 615,089 | 283,881 | 159,363 | 1,013,638 | 455,726 |
| Person-yr | ||||||
| Total no. | 2,197,153 | 896,807 | 468,577 | 232,587 | 1,728,576 | 664,220 |
| Contributed by Black beneficiaries (%) | 21.3 | 25.9 | 100 | 100 | 0 | 0 |
| Years contributed (%) | ||||||
| Only 2016 | 10.2 | 18.9 | 12.0 | 19.3 | 9.7 | 18.8 |
| Only 2017 | 7.9 | 18.3 | 9.1 | 17.7 | 7.5 | 18.5 |
| Both 2016 and 2017 | 81.9 | 62.8 | 78.8 | 63.0 | 82.7 | 62.8 |
| Age | ||||||
| Mean (yr) | 50.5 | 50.4 | 50.0 | 49.9 | 50.7 | 50.6 |
| Distribution (%) | ||||||
| 18–24 yr | 1.4 | 1.4 | 1.7 | 1.7 | 1.3 | 1.4 |
| 25–29 yr | 3.1 | 3.2 | 3.9 | 4.0 | 2.9 | 3.0 |
| 30–34 yr | 5.1 | 5.2 | 5.6 | 5.7 | 4.9 | 5.1 |
| 35–39yr | 6.9 | 7.0 | 7.2 | 7.2 | 6.9 | 6.9 |
| 40–44 yr | 8.6 | 8.6 | 8.6 | 8.6 | 8.6 | 8.7 |
| 45–49 yr | 12.1 | 12.2 | 11.8 | 11.9 | 12.2 | 12.3 |
| 50–54 yr | 18.1 | 18.1 | 17.9 | 17.9 | 18.2 | 18.1 |
| 55–59 yr | 24.1 | 24.0 | 23.7 | 23.6 | 24.2 | 24.1 |
| 60–64 yr | 20.5 | 20.2 | 19.7 | 19.5 | 20.7 | 20.5 |
| Female sex (%) | 52.8 | 53.7 | 56.2 | 57.0 | 51.9 | 52.6 |
| Long-term care (%)† | 9.2 | 9.3 | 8.2 | 7.4 | 9.5 | 9.9 |
| HCC score‡ | 1.10±1.15 | 1.15±1.20 | 1.19±1.25 | 1.22±1.27 | 1.08±1.12 | 1.13±1.17 |
Plus–minus values are means ±SD. The unit of analysis is the person-year. The overall national sample included all the person-years that were contributed by beneficiaries who met the following study criteria: at least 12 months of fee-for-service inpatient, outpatient, and prescription plan enrollment in 2016, 2017, or both years; at least one visit for primary care services (for attribution to a single health care organization in the calendar year); and Black or non-Hispanic White race indicator derived from the Medicare Beneficiary Summary file. The 310 health systems studied were those with at least 200 person-years contributed by Black patients and at least 200 person-years contributed by White patients across the 2016–2017 study period. These systems were included in the system-level analyses. These 310 systems included 47.4% of all beneficiaries in the national sample and 56.1% of Black beneficiaries.
Long-term care indicates that a long-term care pharmacy dispensed at least half the beneficiary’s annual prescriptions. Data regarding the pharmacy-type variable are from the Medicare Part D Pharmacy Characteristics file.
The Hierarchical Condition Category (HCC) score is a risk-score assignment that is used by the Centers for Medicare and Medicaid Services to calculate risk-adjusted payments. Scores generally range from 0.7 to 1.5, with higher scores indicating higher morbidity and predicting higher medical spending.
Of 3686 health systems, 310 (8.4%) met our inclusion criteria for within-system analyses (i.e., ≥200 person-years contributed by Black patients and ≥200 person-years contributed by White patients in that system). The 310 systems included 896,807 attributed person-years and represented 47.4% of all the patients in the national sample and 56.1% of the Black patients in the national sample. In the 310-systems sample, 53.7% of the patients were women, and the mean age of the patients was 50.4 years. Black patients had more coexisting conditions than White patients (mean HCC score, 1.22 vs. 1.13) and were less likely to be in long-term care (7.4% vs. 9.9%) (Table 1).
Among the 310 systems, individual-system cohorts ranged from 433 to 34,845 total person-years (mean [±SD], 2893±3625), from 200 to 7236 person-years (mean, 750±785) contributed by Black patients, and from 212 to 27,609 person-years (mean, 2143±3004) contributed by White patients. The system-level percent of person-years that were contributed by Black patients ranged from 5.6 to 79.5% (mean, 32.7±15.7) (Table S5). In a comparison of systems that met the inclusion criteria for system-level analyses with those that did not meet these criteria, the characteristics of the patients were similar except for the percent of patients within each sample who were Black (Table S6). The included systems were larger, were more commonly not-for-profit, and were more likely to own hospitals than the systems that were not included (Table S7).
CRUDE OPIOID MEASURES
Crude opioid measures among patients who were attributed to the 310 health systems did not differ from the measures among those in the national sample (Table 2). Among patients served by the 310 systems, the annual prevalence of any filled opioid prescription was 50.2% among Black patients and 52.2% among White patients; the annual prevalence of long-term opioid receipt was 21.8% and 26.4%, respectively. Black patients received lower annual opioid doses than White patients, regardless of the receipt pattern. Among all patients (including nonrecipients), the doses were 5190 MME among Black patients and 8082 MME among White patients (36% lower among Black patients); among short-term recipients, 1241 MME and 1835 MME, respectively (33% lower among Black patients); and among long-term recipients, 22,211 MME and 28,860 MME, respectively (23% lower among Black patients).
Table 2.
Crude Measures of Prescription Opioid Receipt.*
| Variable | All Patients | Black Patients | White Patients | Black-White Difference† |
|---|---|---|---|---|
| Sample served by 310 diverse-population health systems | ||||
| Prescription opioid receipt (%) | ||||
| Any receipt | 51.7 | 50.2 | 52.2 | −2.0 |
| Short-term receipt | 26.5 | 28.4 | 25.9 | 2.5 |
| Long-term receipt | 25.2 | 21.8 | 26.4 | −4.6 |
| Annual MME | ||||
| Overall, including nonrecipients | 7,332±22,193 | 5,190±17,697 | 8,082±23,519 | −2892 |
| Among short-term recipients | 1,670±5,359 | 1,241±3,821 | 1,835±5,835 | −594 |
| Among long-term recipients | 27,368±37,256 | 22,211±32,359 | 28,860±38,428 | −6649 |
| National sample | ||||
| Prescription opioid receipt (%) | ||||
| Any receipt | 51.8 | 51.2 | 52.0 | −0.8 |
| Short-term receipt | 25.5 | 27.5 | 25.0 | 2.5 |
| Long-term receipt | 26.3 | 23.7 | 27.0 | −3.2 |
| Annual MME | ||||
| Overall, including nonrecipients | 7,838±23,025 | 5,634±18,120 | 8,435±24,149 | −2801 |
| Among short-term recipients | 1,779±5,709 | 1,320±3,960 | 1,915±6,127 | −595 |
| Among long-term recipients | 28,118±37,782 | 22,235±31,690 | 29,521±38,963 | −7286 |
Plus–minus values are means ±SD. The measures from the national sample are presented to permit a comparison and test of representativeness. The unit of analysis is the person-year. Any receipt of a prescription opioid was defined as at least one filled prescription, short-term receipt as at least one filled prescription in one to three quarters in a calendar year, and long-term receipt as at least one filled prescription in all four quarters of a calendar year. Values for the same outcomes that were measured at the level of the person (rather than person-year) among Black and White patients, respectively, were as follows: in the national sample, any opioid receipt (51.1% vs. 52.1%), short-term receipt (28.1% vs. 25.5%), long-term receipt (23.0% vs. 26.5%), morphine milligram equivalents (MME) for all patients (5428 MME vs. 8258 MME), MME for patients with short-term receipt (1375 MME vs. 2013 MME), and MME for patients with long-term receipt (20,871 MME vs. 27,991 MME); and in the sample attributed to the 310 systems studied, any opioid receipt (50.3% vs. 52.4%), short-term receipt (28.9% vs. 26.4%), long-term receipt (21.4% vs. 26.1%), MME for all patients (5081 MME vs. 8024 MME), MME for patients with short-term receipt (1286 MME vs. 1907 MME), and MME for patients with long-term receipt (21,152 MME vs. 27,978 MME).
Differences in percentages are shown in percentage points.
MODEL RESULTS IN SAMPLE OF PATIENTS ATTRIBUTED TO 310 SYSTEMS
Tables 3 and S8 show the results of patient-level models estimating the mean annual Black–White differences in opioid measures in the 310-systems sample. The models included patient characteristics and indicators for each patient’s state of residence and health system. In these models, Black race, as compared with White race, mirrored the crude differences and was associated with a lower annual rate of any opioid receipt (difference, −3.3 percentage points; 95% confidence interval [CI], −4.0 to −2.6), a higher annual rate of short-term opioid receipt (difference, 1.7 percentage points; 95% CI, 1.3 to 2.0), and a lower annual rate of long-term opioid receipt (difference, −5.0 percentage points; 95% CI, −5.7 to −4.2). In models examining the annual MME, the differences in dose between Black patients and White patients were also similar to the crude differences: −3140 MME (95% CI, −3465 to −2815) overall, −588 MME (95% CI, −656 to −520) among short-term recipients, and −7232 MME (95% CI −7938 to −6525) among long-term opioid recipients.
Table 3.
Adjusted Estimates of Black–White Difference in Prescription Opioid Measures in 310 Health Systems.*
| Variable | Black-White Difference (95% CI) | R2† |
|---|---|---|
| Prescription opioid receipt (percentage points) | ||
| Any receipt | −3.3 (−4.0 to −2.6) | 0.115 |
| Short-term receipt | 1.7 (1.3 to 2.0) | 0.021 |
| Long-term receipt | −5.0 (−5.7 to −4.2) | 0.081 |
| Annual MME | ||
| Overall, including nonrecipients | −3140 (−3465 to −2815) | 0.038 |
| Among short-term recipients | −588 (−656 to −520) | 0.020 |
| Among long-term recipients | −7232 (−7938 to −6525) | 0.049 |
Each estimate represents the adjusted difference between Black patients and White patients in a linear regression model. The unit of analysis is the person-year. The population sample in these models included 896,807 person-years (from 615,089 unique patients) that were attributed to 310 health systems with at least 200 person-years contributed by Black patients and at least 200 person-years contributed by White patients in the study period. Models included the following person-year–specific patient characteristics: age category, sex, HCC score, long-term care residence status, and indicators for year, the patient’s state of residence, and the health system at which the patient received the plurality of primary care services in the year. Confidence intervals (CIs) were computed on the basis of robust sandwich variance estimators, with specification for clusters as systems, but were not adjusted for multiple comparisons.
The R2 statistic conveys the share of variation in each prescription opioid–receipt measure that is explained by the regression model. The range is 0 to 1. Higher values of R2 indicate that the regression explains more of the variation in opioid prescription receipt across patients.
ADJUSTED RACE DIFFERENCES WITHIN EACH OF THE 310 SYSTEMS
Among the 310 diverse-population systems, within-system differences in adjusted race-specific measures varied, but a dominant trend emerged. White patients received a higher annual MME than Black patients in 91% of the 310 systems, and the MME was at least 15% higher for White patients in 75% of the systems (Tables S9 and S10). For each of the 310 systems, Figure 1 shows the adjusted mean annual MME among Black and White patients for both short-term and long-term receipt of opioids.
Figure 1. Within-System Differences between Black and White Patients in Mean Annual Morphine Milligram Equivalents.
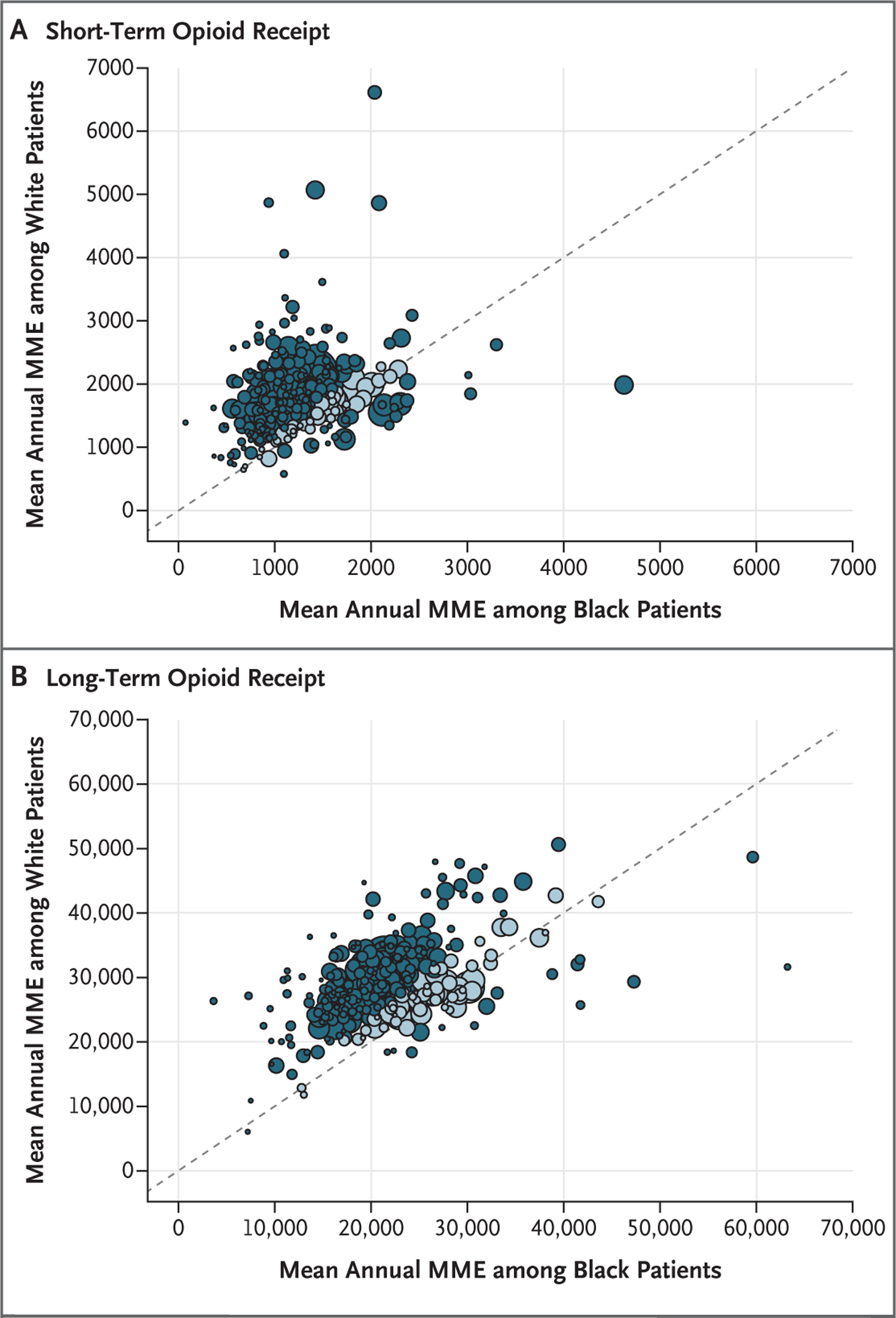
Each dot represents 1 of the 310 health systems analyzed and shows, within each health system, the mean annual morphine milligram equivalents (MME) among Black and White patients, with adjustment for age category, female sex, Hierarchical Condition Category score, long-term care status (defined as ≥50% prescriptions having been dispensed by a long-term care pharmacy), and indicators for state of residence and year (2016). The 45-degree line in each panel represents equal values among Black patients and White patients (i.e., a ratio of 1). Dots above the line represent greater MME among White patients than among Black patients, and dots below the line represent the opposite. Distance from the line signals the magnitude of the race difference. Darker dots indicate systems in which the Black-to-White ratio for the MME was less than or equal to 0.85 or was greater than or equal to 1.15, whereas lighter dots indicate systems in which the ratio was more than 0.85 and less than 1.15. The size of the dots is relative to the number of attributed patients. Note the scale difference between Panels A (short-term receipt) and B (long-term receipt).
Table S10, which is in Supplementary Appendix 2 (available at NEJM.org), provides system-specific cohort details and adjusted measures according to race for each of the 310 systems analyzed. Within relatively small geographic areas, system-level race differences varied. For example, an exploration of the Black-to-White ratio of the annual MME per person-year overall revealed the following findings. In the Chicago area, the ratios at three health systems were 0.98 at Northshore University Health, 0.55 at Northwestern, and 0.36 at Cook County. In Massachusetts, the ratios at four health systems were 0.95 at Cambridge Health Alliance, 0.71 at Baystate Health, 0.62 at Beth Israel Deaconess, and 0.60 at Boston Medical Center.
SECONDARY ANALYSES
In secondary analyses examining opioid receipt among 5893 patients with cancer diagnoses, Black–White differences in opioid measures mirrored those of the overall study population (Table S11). Measurement of crude nonopioid prescription receipt revealed that the mean annual 30-day supply count was 52.3±42.5 among Black patients and 62.9±45.9 among White patients. In secondary analyses, we repeated our main models with additional adjustment for the annual count of nonopioid prescription 30-day supplies. In these secondary models, estimates of Black–White differences in opioid-receipt measures were slightly smaller in magnitude than the estimates shown in Table 3 (Table S12). Secondary analyses examining the characteristics of the filled opioid prescriptions showed that prescriptions received by Black patients, on average, had lower values for all components — the fill count, MME, units dispensed, and MME per unit — than those received by White patients (Table S13).
In secondary analyses exploring how estimated Black–White differences varied according to the fraction of person-years contributed by Black patients in a system, we decomposed the racial differences in opioid receipt into between-system differences (i.e., the effect of Black patients receiving care in lower opioid-prescribing systems as compared with higher opioid-prescribing systems) and within-system racial differences. We found that Black patients disproportionately received care from lower-prescribing systems, but the effect of being served by different health systems was small as compared with the within-system effect. For example, in a comparison of the annual MME overall among Black patients and White patients, the between-system differences, on average, accounted for a dose that was 537 MME lower, whereas within-system differences accounted for a dose that was 2817 MME lower (Fig. S1A through S1F and Table S14). Overall, the discrepancies in opioid receipt according to race stemmed from within-system differences and not from differences among health systems serving relatively more Black patients than White patients.
DISCUSSION
In this population of Medicare beneficiaries with disability, the annual prevalence of prescription opioid receipt was similar among Black and White patients (approximately 50%), but Black patients received 36% fewer MME annually. For each opioid measure, crude differences according to race shifted minimally in adjusted models, which suggests that observed differences did not result from Black and White patients having different health conditions or demographic characteristics or living in different states. Almost all the population-average race gaps stemmed from within-system differences; that is, Black patients and White patients who were served by the same system had very different patterns of opioid receipt. These opioid-receipt patterns probably reflect both overtreatment of White patients and under-treatment of Black patients. The findings should prompt systems to explore the causes and consequences of these biased patterns and to develop and test efforts to eliminate the influence of race on the receipt of pain treatment.
Could these findings result from something other than racial bias? We do not have the nuanced clinical data necessary to assess the appropriateness of the observed patterns of opioid receipt. Even when clinical data are available, the quality of pain management is hard to assess owing to the complex nature of this care. Despite the limitations of our data, it is hard to imagine that, within each of the 310 health systems that we studied, the differences in need, preferences, and opioid-associated risks among White patients and Black patients explain such substantial inequality in opioid receipt. Yet, most of the systems (75%) were characterized by clinically meaningful differences, with the opioid dose received by White patients being at least 15% higher than that received by Black patients. Research from clinical settings suggests that such differences in practice may result from clinicians’ conscious and unconscious racial bias.1,2,6,7,18,39,40 Such bias could reflect mistaken beliefs that Black patients experience less pain and are more likely to misuse prescription opioids than White patients.7,41 It may also reflect patient–physician racial discordance and the related potential for lower levels of empathy, trust, physician perception of patient’s pain, and effective communication.42–45 We expect that systemic structural racism contributes as well.46 Such systemic factors may include, for example, racially segregated neighborhoods and a lower density of pharmacies and continuity care clinics in predominantly Black neighborhoods than in predominantly White neighborhoods.47,48
The finding that the differences between races in the opioid dose was also large among patients with cancer diagnoses emphasizes the need to understand observed opioid-receipt patterns and associated outcomes. Given the broad range of cancer diagnoses used, and in the absence of any information on whether opioids were intended for cancer-related or other pain, we interpret these secondary analyses with caution.
Our study has important limitations. We used filled prescriptions as a measure of opioid receipt. We did not have information on prescriptions that were written but unfilled. The decision to fill an opioid prescription may vary according to race and contribute unmeasured confounding. Our finding that Black patients, on average, received lower doses and smaller quantities in each filled prescription indicates that unfilled prescriptions cannot fully explain our findings. We did not adjust models for visit intensity because prescription receipt and visits are tightly correlated. If visit intensity and care setting (e.g., emergency department or continuity clinic) vary according to race, this difference in care intensity may influence observed differences regarding opioid receipt.49 Although differences in access to clinicians may explain some racial differences in opioid measures, a similar annual prevalence of opioid receipt overall among Black patients and White patients suggests that access did not differ dramatically in these groups. Our attribution method holds the health system delivering the plurality of a patient’s care accountable for the annual opioid receipt. If the receipt of opioids from outside providers differed according to race, we would have misattributed differences in the opioid measures to the assigned system.
We relied on diagnostic codes to adjust for medical conditions; the underrecording and over-recording of diagnoses would affect the validity of this approach. Limited patient numbers prohibited the study of other minority populations. The racial composition of our selected systems (large systems serving racially diverse populations) is not nationally representative. The 310 systems that we studied served 47.4% of all patients and 56.1% of the Black patients in the national sample but represent only 8.4% of the systems identified. Our findings may not reflect patterns in unstudied health systems or independent practices. However, a comparison of the patients in our 310 systems with patients in smaller, less diverse organizations showed remarkably similar opioid-receipt patterns and race differences in opioid receipt. Finally, our population of Medicare enrollees with disability, who were younger than 65 years of age, is complex; the patterns that we observed may not be generalizable.50
In this population of beneficiaries with disability, we found substantial racial inequality in the receipt of prescription opioids, especially with regard to opioid dose. Although others have found evidence that differences in health care intensity and quality result from Black patients and White patients receiving care at different health care facilities, we found that race gaps within systems were generally as large as race differences overall.51,52 We do not know whether or how these differences affect patient outcomes, because both opioid underuse and overuse can cause harm. We do know that skin color should not influence the receipt of pain treatment. Our overall observations and system-specific reporting should prompt action by providers, health system administrators, and policymakers to explore root causes, consequences, and effective remediation strategies for racially unequal opioid receipt.
Supplementary Material
Acknowledgments
Supported by a grant (P01 AG019783, to Drs. Morden and Meara) from the National Institute on Aging and by a grant (1U19HS024075) from the AHRQ Comparative Health System Performance Initiative.
Footnotes
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from IQVIA information services (OneKey subscription information services 2010–18, IQVIA; all rights reserved). The statements, findings, conclusions, views, and opinions contained and expressed in this article are not necessarily those of IQVIA or any of its affiliated or subsidiary entities. The American Medical Association (AMA) was the source for the raw physician data; the statistics, tables, and tabulations were prepared by the authors using data from the AMA Physician Masterfile. The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of the Agency for Healthcare Research and Quality (AHRQ).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain 2009; 10: 1187–204. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A 2016; 113: 4296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P, Le Saux M, Siegel R, et al. Racial and ethnic disparities in the management of acute pain in US emergency departments: meta-analysis and systematic review. Am J Emerg Med 2019; 37: 1770–7. [DOI] [PubMed] [Google Scholar]
- 4.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA 2008; 299: 70–8. [DOI] [PubMed] [Google Scholar]
- 5.Singhal A, Tien Y-Y, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One 2016; 11(8): e0159224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ly DP. Racial and ethnic disparities in the evaluation and management of pain in the outpatient setting, 2006–2015. Pain Med 2019; 20: 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staton LJ, Panda M, Chen I, et al. When race matters: disagreement in pain perception between patients and their physicians in primary care. J Natl Med Assoc 2007; 99: 532–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain 2010; 151: 625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J Pain 2006; 7: 225–35. [DOI] [PubMed] [Google Scholar]
- 10.Hausmann LRM, Gao S, Lee ES, Kwoh KC. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain 2013; 154: 46–52. [DOI] [PubMed] [Google Scholar]
- 11.Joynt M, Train MK, Robbins BW, Halterman JS, Caiola E, Fortuna RJ. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med 2013; 28: 1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisniewski AM, Purdy CH, Blondell RD. The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. J Addict Dis 2008; 27: 1–11. [DOI] [PubMed] [Google Scholar]
- 13.Todd KH, Deaton C, D’Adamo AP, Goe L. Ethnicity and analgesic practice. Ann Emerg Med 2000; 35: 11–6. [DOI] [PubMed] [Google Scholar]
- 14.Romanelli RJ, Shen Z, Szwerinski N, Scott A, Lockhart S, Pressman AR. Racial and ethnic disparities in opioid prescribing for long bone fractures at discharge from the emergency department: a cross-sectional analysis of 22 centers from a health care delivery system in northern California. Ann Emerg Med 2019; 74: 622–31. [DOI] [PubMed] [Google Scholar]
- 15.Harrison JM, Lagisetty P, Sites BD, Guo C, Davis MA. Trends in prescription pain medication use by race/ethnicity among US adults with noncancer pain, 2000–2015. Am J Public Health 2018; 108: 788–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med 2006; 9: 1454–73. [DOI] [PubMed] [Google Scholar]
- 17.Bleich SN, Findling MG, Casey LS, et al. Discrimination in the United States: experiences of Black Americans. Health Serv Res 2019; 54: Suppl 2: 1399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soares WE III, Knowles KJ II, Fried-mann PD. A thousand cuts: racial and ethnic disparities in emergency medicine. Med Care 2019; 57: 921–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep 2016; 65: 1–49. [DOI] [PubMed] [Google Scholar]
- 20.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med 2003; 349: 1943–53. [DOI] [PubMed] [Google Scholar]
- 21.Barnett ML. Opioid prescribing in the midst of crisis — myths and realities. N Engl J Med 2020; 382: 1086–8. [DOI] [PubMed] [Google Scholar]
- 22.Dart RC, Severtson SG, Bucher-Bartelson B. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015; 372: 1573–4. [DOI] [PubMed] [Google Scholar]
- 23.Lippold KM, Jones CM, Olsen EO, Giroir BP. Racial/ethnic and age group differences in opioid and synthetic opioid-involved overdose deaths among adults aged ≥18 years in metropolitan areas — United States, 2015–2017. MMWR Morb Mortal Wkly Rep 2019; 68: 967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011; 305: 1315–21. [DOI] [PubMed] [Google Scholar]
- 25.Frakt A, Monkovic T. A ‘rare case where racial biases’ protected African-Americans. New York Times. November25, 2019. (https://www.nytimes.com/2019/11/25/upshot/opioid-epidemic-blacks.html). [Google Scholar]
- 26.Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer: the Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 1997; 127: 813–6. [DOI] [PubMed] [Google Scholar]
- 27.Frantsve LME, Kerns RD. Patient-provider interactions in the management of chronic pain: current findings within the context of shared medical decision making. Pain Med 2007;8: 25–35. [DOI] [PubMed] [Google Scholar]
- 28.Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med 2016; 375: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Research Triangle Institute (RTI) race code. Research Data Assistance Center; (https://www.resdac.org/cms-data/variables/research-triangle-institute-rti-race-code). [Google Scholar]
- 30.Narcotic equivalence converter. 2001. (http://www.medcalc.com/narcotics.html).
- 31.Opioid dose calculator. Olympia, WA: Agency Medical Directors’ Group, 2015. (http://agencymeddirectors.wa.gov/Calculator/DoseCalculator.htm). [Google Scholar]
- 32.Part D pharmacy characteristics file. Research Data Assistance Center; (https://www.resdac.org/cms-data/files/part-d-pharmacy-characteristics). [Google Scholar]
- 33.Risk adjustment. 2016model software (https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/2016-Midyear-Final-Model.zip).
- 34.Data collection and monitoring. In: Smedley BD, Stith AY, Nelson AR, eds. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: National Academies Press, 2003: 215–34. [PubMed] [Google Scholar]
- 35.Cohen GR, Jones DJ, Heeringa J, et al. Leveraging diverse data sources to identify and describe U.S. health care delivery systems. EGEMS (Wash DC) 2017; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compendium of U.S. health systems, 2018. Rockville, MD: Agency for Healthcare Research and Quality, 2019. (https://www.ahrq.gov/chsp/data-resources/compendium-2018.html). [Google Scholar]
- 37.Centers for Medicare and Medicaid Services. Medicare Shared Savings Program: shared savings and losses and assignment methodology. Version 7.February2019. (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/Shared-Savings-Losses-Assignment-Spec-V7.pdf).
- 38.CMS cell size suppression policy. Research Data Assistance Center, 2017. (https://resdac.org/articles/cms-cell-size-suppression-policy#:~:text=The%20policy%20stipulates%20that%20no,the%20minimum%20cell%20size%20policy).
- 39.Sabin J, Nosek BA, Greenwald A, Rivara FP. Physicians’ implicit and explicit attitudes about race by MD race, ethnicity, and gender. J Health Care Poor Under-served 2009; 20: 896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med 2013; 28: 1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santoro TN, Santoro JD. Racial bias in the US opioid epidemic: a review of the history of systemic bias and implications for care. Cureus 2018; 10(12): e3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diversity in medicine: facts and figures 2019. Washington, DC: Association of American Medical Colleges, 2019. (https://www.aamc.org/data-reports/workforce/interactive-data/figure-18-percentage-all-active-physicians-race/ethnicity-2018). [Google Scholar]
- 43.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med 2003; 139: 907–15. [DOI] [PubMed] [Google Scholar]
- 44.Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA 1993; 269: 1537–9. [PubMed] [Google Scholar]
- 45.Mende-Siedlecki P, Qu-Lee J, Backer R, Van Bavel JJ. Perceptual contributions to racial bias in pain recognition. J Exp Psychol Gen 2019; 148: 863–89. [DOI] [PubMed] [Google Scholar]
- 46.Boyd RW, Lindo EG, Weeks LD, McLemore MR. On racism: a new standard for publishing on racial health inequities. Health Affairs Blog, July2, 2020. (https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/). [Google Scholar]
- 47.Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B. ‘Pharmacy deserts’ are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Mill-wood) 2014; 33: 1958–65. [DOI] [PubMed] [Google Scholar]
- 48.Ryvicker M, Sridharan S. Neighborhood environment and disparities in health care access among urban Medicare beneficiaries with diabetes: a retrospective cohort study. Inquiry 2018; 55: 46958018771414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: characteristics of prescriptions and association with long-term use. Ann Emerg Med 2018; 71(3): 326–336.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morden NE, Munson JC, Colla CH, et al. Prescription opioid use among disabled Medicare beneficiaries: intensity, trends, and regional variation. Med Care 2014; 52: 852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation 2005; 112: 2634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bynum JPW, Fisher ES, Song Y, Skinner J, Chandra A. Measuring racial disparities in the quality of ambulatory diabetes care. Med Care 2010; 48: 1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


