Abstract
Undoubtedly, the development of COVID-19 vaccines displays a critical step towards ending this devastating pandemic, considering their protective benefits in the general population. Yet, data regarding their efficacy and safety in cancer patients are limited. Herein we provide the initial analysis of immune responses after the first dose of vaccination in 21 breast cancer patients receiving cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors. The levels of neutralizing antibodies post vaccination were similar to the matched healthy controls, whereas no safety issues have been raised. Further exploration is needed to reduce the uncertainty of SARS-CoV-2 immunity among cancer patients under treatment.
Keywords: COVID-19, Vaccination, Breast cancer, CDK4/6 inhibitors
Highlights
-
•
Data on the efficacy and safety of COVID-19 vaccines in cancer patients are limited.
-
•
21 breast cancer patients under CDK4/6 inhibition enrolled to our prospective study.
-
•
Nearly 30% of them developed favorable viral inhibition after the first dose.
-
•
Immune responses did not differ between patient cohort and healthy controls.
1. Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, has a severe impact in every country of the world [1]. Vaccination process represents an effective mitigation measure [2]. Cancer patients face a higher risk of both severe infection and death [3] and have been prioritised to receive COVID-19 vaccination in several countries, including Greece. However, their exclusion from the confirmatory clinical trials [4] creates a gap in clinical data regarding the vaccines’ efficacy and safety in this group of immunocompromised patients. In this context, we undertook a prospective study (NCT047443388) in order to investigate the immune response to COVID-19 vaccination in patients with hematological malignancies, solid tumours and healthy volunteers [5]. Herein, we present the analysis of SARS-CoV-2 neutralizing antibodies (NAbs) kinetics in breast cancer patients, receiving cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors.
2. Materials & methods
Inclusion criteria for the patient cohort included: (i) patients with histologically confirmed breast cancer under treatment with CDK4/6 inhibitors; (ii) age above 18 years; (iii) eligibility for vaccination. Both patients and healthy controls, known to be previously infected with COVID-19 virus, were excluded from the analysis.
Using an FDA approved assay (ELISA, cPass™ SARS-CoV-2 NAb Detection Kit; GenScript, Piscataway, NJ, USA) to measure SARS-CoV-2 NAbs, we analyzed serial blood samples, collected on day 1 (D1), prior to vaccination, and on day 22 (D22) post vaccination. The same ELISA plate was used for serum samples of the same patient or control subjects. The study was approved by the relevant Ethical Committees and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. Written informed consent was provided by each subject prior to enrollment. Baseline demographics, comorbidities, and the SARS-CoV-2 NAb levels have been compared between the study group and the control subjects; Chi-square test and Wilcoxon signed-rank test were applied for categorical variables or unpaired t-test and for continuous variables, respectively. To adjust for potential confounding variables, we used case-control matching to match the two groups for age and type of vaccine with the calipmatch command in Stata. All data extraction and analyses were conducted using Stata 16.0 (Stata Corp 2019, Stata Statistical Software: Release 16. College Station, TX: Stata Corp LLC). Two-sided p value < 0.05 was used for statistical significance.
3. Results
For this analysis of immunogenicity and safety, 21 female breast cancer patients with median age of 63 years (IQR: 46–76 years) and 160 controls (median age: 68 years; IQR: 58–82 years; p = 0.101 for age compared with patients), vaccinated during the same period, were enrolled. 20/21 (95.2%) patients and 135/160 (84.4%) controls were vaccinated with a mRNA vaccine (BNT162b2 and mRNA-1273), while one patient and 25 controls received the AZD1222 vaccine (p = 0.18). There was no significant difference in body mass index (BMI) between the two groups (mean BMI: 26.47 kg/m2 in the study group and 26.48 kg/m2 in the control group; p = 0.99). With regards to CDK4/6 inhibitor, 11 (52.4%) patients were treated with ribociclib, 7 (33.3%) with palbociclib, and the remaining 3 (14.3%) with abemaciclib. Comorbidities in the study group included diabetes mellitus in 9.52%, cardiovascular disease in 38.1%, and pulmonary disease in 4.76%. Summary of the main characteristics of the 21 patients included are demonstrated in Table 1.
Table 1.
Characteristics of the 21 breast cancer patients enrolled in the study.
| # | Age | BMI | CDK4/6 inhibitor | CDK4/6 inhibitor dosage | Endocrine therapy | Months of treatment | Comorbidities | Lymphocytes (/μL) | Neutrophils (/μL) | Vaccine | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | 20.2 | Palbociclib | 75 mg x1 | Fulvestrant | 26 | Hypertension | 1620 | 1500 | BNT162b2 | None |
| 2 | 79 | 25.8 | Palbociclib | 125 mg x1 | Letrozole | 21 | None | 1100 | 1710 | mRNA-1273 | None |
| 3 | 79 | 30.1 | Palbociclib | 75 mg x1 | Letrozole | 26 | Hypertension, dyslipidemia | 1500 | 990 | BNT162b2 | Pyrexia, arthralgia |
| 4 | 64 | 36.1 | Ribociclib | 400 mg x1 | Letrozole | 30 | Hypertension, dyslipidemia | 1700 | 2000 | AZD1222 | Pyrexia |
| 5 | 76 | 32.6 | Ribociclib | 600 mg x1 | Letrozole | 26 | Diabetes mellitus, hypertension | 700 | 2300 | BNT162b2 | None |
| 6 | 39 | 24.8 | Ribociclib | 200 mg x1 | Letrozole | 13 | None | 1530 | 1350 | BNT162b2 | Pain at injection site |
| 7 | 76 | 28.9 | Palbociclib | 125 mg x1 | Fulvestrant | 31 | None | 1700 | 1170 | BNT162b2 | None |
| 8 | 45 | 23 | Abemaciclib | 150 mg x2 | Tamoxifen | 26 | Hashimoto's thyroiditis | 1600 | 2970 | mRNA-1273 | None |
| 9 | 58 | 23.4 | Ribociclib | 600 mg x1 | Letrozole | 4 | Hyperthyroidism | 940 | 1640 | BNT162b2 | None |
| 10 | 45 | 22.6 | Abemaciclib | 150 mg x2 | Letrozole | 24 | Hypothyroidism | 1550 | 2040 | BNT162b2 | None |
| 11 | 67 | 30.2 | Ribociclib | 600 mg x1 | Letrozole | 2 | Asthma | 3200 | 2910 | BNT162b2 | Pyrexia |
| 12 | 69 | 21.48 | Palbociclib | 100 mg x1 | Fulvestrant | 7 | None | 750 | 1950 | mRNA-1273 | Pyrexia |
| 13 | 75 | 21.9 | Ribociclib | 400 mg x1 | Letrozole | 2 | Hypertension, hypothyroidism, dyslipidemia, osteoporosis | 1100 | 1770 | BNT162b2 | None |
| 14 | 42 | 21.23 | Ribociclib | 600 mg x1 | Letrozole | 3 | None | 1240 | 780 | BNT162b2 | Headache |
| 15 | 59 | 27.61 | Ribociclib | 600 mg x1 | Fulvestrant | 2 | Diabetes mellitus, hypertension | 1100 | 1170 | BNT162b2 | None |
| 16 | 75 | 31.32 | Ribociclib | 600 mg x1 | Letrozole | 2 | Systemic lupus erythematosus, hypertension, atrial fibrillation | 2160 | 4180 | BNT162b2 | Fatigue, headache |
| 17 | 76 | 26.86 | Palbociclib | 100 mg x1 | Letrozole | 15 | None | 850 | 940 | BNT162b2 | None |
| 18 | 38 | 33.5 | Ribociclib | 400 mg x1 | Letrozole | 34 | Hypothyroidism, Hodgkin lymphoma | 1260 | 2560 | BNT162b2 | None |
| 19 | 74 | 25 | Palbociclib | 125 mg x1 | Fulvestrant | 11 | Myasthenia gravis, hypothyroidism, dyslipidemia | 600 | 1410 | BNT162b2 | Fatigue |
| 20 | 55 | 23.4 | Ribociclib | 600 mg x1 | Fulvestrant | 8 | Hypothyroidism | 2680 | 3250 | BNT162b2 | None |
| 21 | 46 | 25.92 | Abemaciclib | 50 mg x2 | Tamoxifen | 25 | None | 1300 | 2810 | BNT162b2 | None |
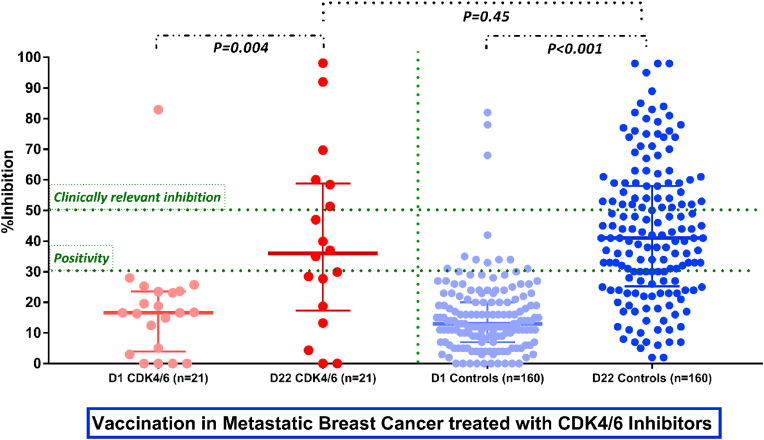
On D1, no difference regarding the NAb titers between the two groups was observed (p = 0.42); 1 (4.76%) patient and 11 (6.9%) controls had a NAb titer of ≥30% (positivity cut-off). None had known history of COVID-19 infection.
On D22, after the first vaccine dose, NAb titers increased significantly in both breast cancer patients and controls (median NAb inhibition titer of 39.5% for patients and 42.83% for controls; p = 0.45). More specifically, 10/18 (55.6%) patients and 115/160 (71.9%) control subjects developed a NAb titer ≥30% on D22 (p = 0.15). In addition, the number of patients and controls who developed clinically relevant viral inhibition (NAb titers ≥50% [6] was 6/18 (33.3%) and 58/160 (36.2%) respectively. Of note, lymphopenia grade 1/2 and/or neutropenia grade 2/3 occurred in 5/21 (23.8%) patients prior to vaccination and were not associated with the D22 NAb titers (Fig. 1).
Fig. 1.
Kinetics of the neutralizing antibodies in breast cancer patients receiving CDK4/6 inhibitors and matched controls, following the first dose of the BNT162b2, AZD1222, mRNA-1273 vaccines.
No safety issues linked targeted therapy administration was noted and the vaccines were well tolerated. In particular, 61.9% of patients reported no toxicities, while fever was the most common adverse effect of the vaccination, recorded in 19.1% of patients. No unexpected adverse events regarding the treatment with CDK4/6 inhibitors was noted during the post-vaccination follow-up period.
4. Discussion
Breast cancer represents a common malignancy of significant epidemiologic relevance among women. While, endocrine therapy (ET) has been historically the backbone of hormone receptor (HR)-positive disease, the recent advent of CDK4/6 inhibitors has transformed the therapeutic landscape of HR-positive and human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer [7]. Thus, these novel targeted therapies in combination with ET or fulvestrant are nowadays considered the standard of care for this subgroup of patients [8].
Yet, their administration during the pandemic remains an open debate, given the limited and inconsistent literature data regarding their safety. At first, a case-report of a middle-aged breast cancer patient with liver metastatic disease under CDK4/6 inhibition suggested that the short-term myelotoxic effect of palbociclib linked to delayed presentation of COVID-19 infection [9]. Later on, a Spanish retrospective study conducted on 79 breast cancer patients demonstrated that either withdrawal or dose modification of CDK4/6 inhibitors might lead to a non-significant reduction in SARS-CoV-2 disease risk [10]. Recently, the experience of European cancer centers is indicative of a rather safe use of these targeted treatment modalities during the COVID-19 pandemic [11,12].
To the best of our knowledge, we provide the first insights into the immunogenicity and safety of COVID-19 vaccination in breast cancer patients receiving the first dose of BNT162b2, mRNA-1273, and AZD1222 vaccines, while on treatment with CDK4/6 inhibitors. Generally, all three vaccines were well tolerated in the study population and immune response up to day 22 was similar to the general population. It should be noted that almost one out of three breast cancer patients on CDK4/6 had developed clinical significantly immunity (NAb titers ≥50%) 3 weeks after vaccination. These results differ from the poor one-dose vaccine efficacy in cancer patients, reported by Monin et al. and Terpos et al. [13,14].
The more common side effects of CDK4/6 inhibitors palbociclib and ribociclib - due to their mechanism of action – are neutropenia and leukopenia [15]. However, this did not preclude immune response in these patients and no difference in the NAb titers among the three types of CDK4/6 inhibitors administered in our patients was noted. Noteworthy, there were neither specific timing issues nor treatment schedule changes; indeed, the included patients received the first dose of COVID-19 vaccine at any timepoint, during their treatment cycle, yet, every patient underwent a complete blood count the day prior to vaccination.
Despite the small size sample of our study, our data provide significant information regarding the optimal management of breast cancer patients treated with CDK4/6 inhibitors during vaccination for COVID-19. Ongoing recruitment and additional follow-up will allow further investigation of safety and efficacy of the vaccination as well as possible associations with factors related to the treatment or the disease.
5. Conclusions
Patients with breast cancer receiving CDK4/6 inhibitors develop SARS-CoV-2 NAbs in response to the first dose of COVID-19 vaccines, similarly to the general population.
Ethical approval
The study was approved by the respective Ethical Committees (Alexandra Hospital Ethics Committee, reference number: 900/24-12-2020) in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. All patients and controls provided written informed consent prior enrollment in the study.
Funding
No funding was received for this study.
Declaration of competing interest
The authors report no conflict of interest.
Acknowledgments
We would like to thank Ioanna Charitaki, RN; Tina Bagratuni, PhD; Christine Ivy Liacos, PhD; Nikoletta-Aikaterini Kokkali, RN; Nefeli Mavrianou-Koutsoukou, PhD; Dimitrios Patseas, PhD and Mrs Stamatia Skourti for administrative, technical, or material support; Sentiljana Gumeni, PhD for acquisition, analysis, or interpretation of data. We also thank SYN-ENOSIS (Greece), AEGEAS (Greece) and IEMBITHEK (Greece) for partially funding this study, as well as all of the study participants for donating their time and samples.
References
- 1.https://covid19.who.int/
- 2.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021 Feb;27(2):205–211. doi: 10.1038/s41591-021-01230-y. [DOI] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 Jun 20;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corti C., Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol. 2021 Apr;32(4):569–571. doi: 10.1016/j.annonc.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E., Trougakos I.P., Apostolakou F., et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021 Apr 10 doi: 10.1002/ajh.26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020 Dec 17;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrahennadi S., Sami A., Manna M., et al. Current landscape of targeted therapy in hormone receptor-positive and HER2-negative breast cancer. Curr Oncol. 2021 May 11;28(3):1803–1822. doi: 10.3390/curroncol28030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso F., Senkus E., Costa A., et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018 Aug 1;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinshpun A., Merlet I., Fruchtman H., Nachman D. A protracted course of COVID19 infection in a metastatic breast cancer patient during CDK4/6 inhibitor therapy. Front Oncol. 2020 Jun 9;10:1085. doi: 10.3389/fonc.2020.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolosa P., Sanchez-Torre A., Bote de Cabo H., et al. Impact of CDK 4/6i withdrawal or dose adjustment on COVID-19 incidence in HR+/HER2− mBC patients during the pandemic. Abst 020 Present AACR COVID-19 Canc. 2020 www.onlineevent.com/aacrcovid19andcancer/courses/21657/video presentations/166985 [Google Scholar]
- 11.Barba M., Krasniqi E., Pizzuti L., et al. COVID-19 risk in breast cancer patients receiving CDK4/6 inhibitors: literature data and a monocentric experience. Breast J. 2021;27(4):359–362. doi: 10.1111/tbj.14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelis V., McFarlane P., Cunningham N., et al. 97P Is continuing CDK4-6 inhibitor therapy safe during the COVID-19 pandemic? A UK cancer centre experience. Ann Oncol. 2021;32:S64–S65. doi: 10.1016/j.annonc.2021.03.111. [DOI] [Google Scholar]
- 13.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021 Apr 27;(21):S1470–S2045. doi: 10.1016/S1470-2045(21)00213-8. 00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terpos E., Zagouri F., Liontos M., et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021 May 31;14(1):86. doi: 10.1186/s13045-021-01099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onesti C.E., Jerusalem G. CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. A systematic review and meta-analysis. Expert Rev Anticancer Ther. 2021 Mar;21(3):283–298. doi: 10.1080/14737140.2021.1852934. [DOI] [PubMed] [Google Scholar]