Abstract
Purpose
Puerarin (PR), a Chinese medicine rich in natural components, has been reported to display anti-fibrotic, antioxidant, anti-inflammatory and immunomodulatory properties. However, the protective mechanism of PR against unilateral ureteral obstruction (UUO)-mediated renal injury is not fully clarified. Therefore, the aim of this study was to investigate the effects of PR on UUO mice and its possible mechanisms.
Methods
A total of 32 C57BL/6 mice were divided randomly into four groups (n=8): i) sham-operated group (Sham); ii) UUO group (UUO); iii) UUO + PR 50 mg/kg/day (UUO + PRL); and iv) UUO + PR 100 mg/kg/day (UUO + PRH). Continuous gavage administration for 14 days starting one week postoperatively, while the mice in Sham and UUO groups were given equal amounts of vehicle by the same means. All mice were then sacrificed and serum, 24-hour urine and tissue specimens were collected for renal function, histopathology, Western blot, immunohistochemistry.
Results
Renal function and histopathology revealed that PR improved UUO-mediated renal dysfunction and partially reversed tubular injury and tubulointerstitial fibrosis. Additionally, according to the results of Western blot and immunohistochemistry, PR inhibited the expression of inflammatory factors including IL-1β, IL-6, MCP-1 and ECM-related proteins including α-SMA, COL I and VIM. More importantly, the expression of fibrotic pathways TGF-β1, Smad3, p-Smad3 and inflammatory pathways NF-κB p65, NF-κB p-p65, STAT3, p-STAT3 were inhibited to various extents under the PR treatment, while Smad7 was upregulated.
Conclusion
These findings indicate that PR may inhibit the recruitment of inflammatory factors and extracellular matrix (ECM) deposition through the regulation of the NF-κB p65/STAT3 and TGFβ1/Smads pathways, which alleviates the UUO-induced inflammatory and fibrotic response, thereby reversing renal injury.
Keywords: puerarin, UUO, CKD, inflammation, fibrosis
Introduction
According to statistics, the global prevalence of chronic kidney disease (CKD) has reached 13.4% with increasing rates annually,1 which has reduced the patient’s life quality and posed an escalating challenge to public health. Renal fibrosis is a common pathological feature of CKD progression to end-stage renal disease (ESRD), distinguished by ECM sedimentation.2 The ongoing cell death and inflammatory cell infiltration during the evolution of tissue fibrosis is often accompanied by the release of large amounts of cytokines, inflammatory chemokines and growth factors, which promote cell proliferation.3 In addition, free radicals produced by inflammatory cells also contribute to the development of fibrosis by inducing epithelial-mesenchymal transition (EMT).4
Inflammation and fibrosis are protective host cell responses to infection and injury, characterised by the recruitment of pro-inflammatory factors, pro-fibrotic factors and immune cells to the lesion,5,6 resulting the inflammatory and fibrotic cascades to amplify and interplay with other signalling molecules, both of which promote and impact on each other. It is therefore critical to intermediate the progression of inflammation and fibrosis to facilitate the repair of tissue structure and function.
As one of the critical subunits of the NF-κB family, p65 is mainly involved in systemic defence responses, such as inflammation, injury, apoptosis and tumour suppression.7 High activation of p65 can be observed in inflammatory lesions, which is associated with NF-κB-induced expression of inflammatory cytokines, inflammatory enzymes and adhesion molecules, thereby accelerating the inflammatory response.8–10 Regulation of p65 signalling molecules can rescue kidney injury, where the mechanism involves the inhibition of apoptosis, inflammation, and secretion of cytotoxic substances.11,12 Correspondingly, p65 induction exacerbates inflammation.13,14 Consequently, p65 is thought to be a central mediator of inflammatory events and inhibition of the signalling pathways in which it is involved can effectively suppress inflammation and subsequent pathological responses.13–15
As for the TGF-β1/Smads pathway, one of the most classical fibrosis signalling pathways. TGF-β1 restrains and/or synergises with different Smad molecules to affect fibrosis progression. TGF-β1 induction results in fibrosis, conversely, fibrosis and subsequent responses are inhibited.16,17 Despite the developing understanding of TGF-β1 and its downstream molecules, it is still unknown whether PR can regulate TGF-β1 expression in UUO mice.
PR, an extract from Pueraria lobata (Willd.), rich in flavonoids, peptides and other biologically active substances. The multi-bioactive ingredients contribute to its extensive pharmacological effects. In recent years, the anti-inflammatory mechanisms of PR have been extensively studied, which involve the regulation of key signals such as TLR, Nrf2, HDAC and PPARα,18–22 but also the improvement of organelle function.23 However, no researches have yet explored whether PR reverses UUO-mediated renal injury through anti-inflammatory and anti-fibrotic effects. In this context, we conceived the present study and hypothesised that PR could rescue renal function by regulating the NF-κB p65/STAT3 and TGF-β1/Smads pathways to inhibit renal fibrosis and inflammation.
Materials and Methods
Puerarin
PR (98% purity) was purchased from Yuanye Biotechnology Co, Ltd. (Shanghai, China, S30646). Its molecular formula is C21H20O9 and its structural formula is depicted in Figure 1. PR stock solutions were prepared in distilled water containing 0.5% carboxymethylcellulose.
Figure 1.
The structural formula of PR.
UUO-Induced CKD Mice Model
All animal research was conducted in accordance with the Guiding Opinions on the Treatment of Laboratory Animals issued and the Laboratory Animal Guideline for Ethical Review of Animal Welfare issued by the National Standard GB/T35892-2018 of the People’s Republic of China, and all operations performed on mice have approved the China Medical University Standards for the Laboratory Animals Welfare and Ethical Review (Permit Number: CMU 20211201). The UUO model was established as described previously,24,25 with the following steps: 1. 10% chloral hydrate was injected intraperitoneally at a dose of 0.4 mL/100 g for anaesthesia, after satisfactory anesthesia, the mice were positioned prone and disinfected. 2. A longitudinal surgical incision is made next to the spine at the lower left costal margin, and the subcutaneous fascia and muscles are incised sequentially to expose the perirenal fat. 3. Gently clamp the perirenal fat with ophthalmic forceps and slowly pull the left kidney out of the surgical incision to expose the renal pedicle. 4. Find the left ureter along the renal pedicle and free it with a glass splitting needle, followed by ligating each side of the ureter with 4–0 silk and cutting it at the midpoint. 5. All the organs are incorporated back into the abdominal cavity, and the muscles, skin and incisions are sutured and disinfected in turn. And finally the mice were placed on a thermostatic blanket until awakening. The Sham group of mice were operated identically except that the ureter was not ligated and clipped.
Animal Grouping and PR Treatment
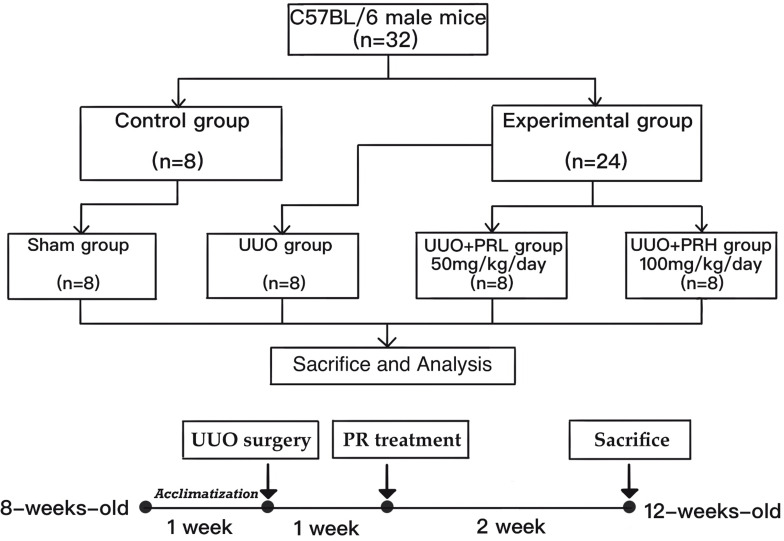
A total of 32 C57BL/6 male mice (age, 8 weeks; weight, 22 ± 2g) were purchased from Beijing HFK Bioscience, Ltd. (Beijing, China) and housed at the Department of Laboratory Animals, China Medical University. The rearing environment is constant temperature (21–23°C) and humidity (45–55%). After 1 week of acclimatization, all mice were divided into Sham, UUO, UUO+PRL and UUO+PRH groups using the random number table method (n=8 per group). Starting 1 week after the UUO operation, PR was administered at 50 mg/kg/day and 100 mg/kg/day to mice in groups UUO + PRL and UUO + PRH respectively for 14 days. While mice in the Sham and UUO groups were gavaged with equal amounts of vehicle. After 2 weeks, all mice were placed in metabolic cages one day in advance to collect urine for 24 hours, after which all mice were sacrificed and left kidney tissues and serum were collected for subsequent assays. Flow chart of the study design as shown in Figure 2.
Figure 2.
Flow chart of the study design.
Biochemical Assays
Retained serum and urine specimens for the assay of serum creatinine (SCr), blood urea nitrogen (BUN) and 24-hour urine protein (24-h UP), the corresponding kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China, C011-2-1, C013-2-1, C035-2-1) and all operations were carried out strictly according to the instructions.
Histopathological Analysis
The detached left kidney were rapidly fixed in 10% paraformaldehyde solution for 24 hours, then tissues was dehydrated in gradient alcohol, embedded in paraffin, sectioned (4 μm), stained with Hematoxylin-Eosin (HE) kit (Solarbio, Beijing, China, G1120) and Masson’s Trichrome (Masson) kit (Solarbio, Beijing, China, G1340). Sections were placed under an optical microscope (Olympus Corporation, Japan) at 400x magnification for imaging and photographed. Renal injury including tubular and glomerular deformation and tubular epithelial cell detachment and assessed by semi-quantitative analysis as described by Choi et al.26 With the following criteria: 0: no abnormality; 1: impairment less than 10%; 2: impairment less than 25%; 3: impairment less than 50%; 4: impairment less than 75%; 5: impairment greater than 75%. Semi-quantitative analysis of fibrotic area was assessed by Masson staining, ie calculating the percentage of blue staining over the entire view.27 Both semi-quantitative assessments were performed on 10 randomly selected non-overlapping areas under the optical microscope.
Immunohistochemistry (IHC)
Paraffin sections with a tissue thickness of 4um were sequentially dewaxed, hydrated and subsequently placed in xylene and graded alcohol, followed by antigen repair. The sections were stained according to the instructions of the immunohistochemistry kits (MXB, Fuzhou, China, KIT-9710, DAB-0031), and the dilution ratios of the primary antibody were as follows: IL-1β (1:100; cat. no. 516288; ZEN-BIOSCIENCE, Inc.), MCP-1 (1:200, cat. no. 66272-1-Ig; ProteinTech Group, Inc.), IL-6 (1:200; cat. no. 66146-1-Ig; ProteinTech Group, Inc.), α-SMA (1:800, cat. no. 14395-1-AP; ProteinTech Group, Inc.), VIM (1:150; cat. no. 5741; Cell Signaling Technology, Inc.), COLI (1:500, cat. no. 14695-1-AP; ProteinTech Group, Inc.). The sections are finally photographed under a 400x optical microscope (Olympus Corporation, Japan). The integrated optical density (IOD) of α-SMA, VIM, COLI, IL-1β, MCP-1 and IL-6 was measured using Image-Pro Plus 6.0, and the mean density was calculated by IOD/area.
Western Blot (Wb)
Tissue proteins are extracted and protein concentrations are routinely quantified by using the BCA kit (Beyotime Biotechnology, P0010S). 30ug on each sample by 8% or 12% SDS-PAGE, and subsequently by semi-dry transfer to PVDF membrane (Millipore, USA). After blocking for 1 hour at room temperature in 5% skimmed milk prepared in TBST, the membrane is incubated overnight at 4° in pre-diluted primary antibody. The dilution ratios for all antibodies are as follows: α-SMA (1:6000, cat. no. 14395-1-AP; ProteinTech Group, Inc.), VIM (1:1000; cat. no. 5741; Cell Signaling Technology, Inc.), COLI (1:2000, cat. no. 14695-1-AP; ProteinTech Group, Inc.), TGF-β1 (1:1000; cat. no. ab215715; abcam, Inc.), Smad3 (1:5000; cat. no. ab40854; abcam, Inc.), p-Smad3 (1:1000; cat. no. ab52903; abcam, Inc.), Smad7 (1:1000; cat. no. 516288; ZEN-BIOSCIENCE, Inc.), IL-1β (1:2000; cat. no. 516288; ZEN-BIOSCIENCE, Inc.), MCP-1 (1:2000, cat. no. 66272-1-Ig; ProteinTech Group, Inc.), IL-6 (1:2000; cat. no. 66146-1-Ig; ProteinTech Group, Inc.), NF-κB p65 (1:1000; cat. no. 8242; Cell Signaling Technology, Inc.), NF-κB p-p65 (1:1000; cat. no. ab76302; abcam, Inc.), STAT3 (1:1500; cat. no. ab68153; abcam, Inc.), p-STAT3 (1:6000; cat. no. ab32143; abcam, Inc.), GAPDH (1:25000, cat. no. 60004-1-Ig; ProteinTech Group, Inc.). After the membrane were washed and incubated in secondary antibody for 1 hour at room temperature. Finally, the grey scale values were analysed using ImageJ software.
Statistical Analysis
All data are presented as median (min-max) and were analyzed using SPSS 20 software. To determine differences among the groups, data were analyzed by the Kruskal–Wallis H-test followed by a Steel–Dwass post hoc test. P < 0.05 was deemed to be a statistical difference.
Results
PR Improves UUO-Mediated Renal Dysfunction
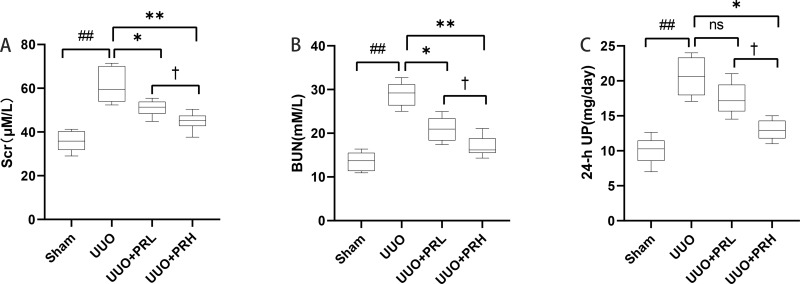
As shown in Figure 3A–C, after 2 weeks of treatment, the differences between the UUO group and the Sham group were statistically significant in terms of Scr, BUN and 24-h UP. With the exception of PRL, which was ineffective in reducing 24-hour UP, PR alleviated UUO-mediated renal dysfunction to varying degrees, and statistical differences were observed between the UUO+PRH and UUO+PRL groups, suggesting that PR was effective in suppressing UUO-mediated renal function deterioration and that PRH was more effective than PRL.
Figure 3.
PR improves UUO-mediated renal dysfunction. (A–C) PR reduced SCr, BUN and 24-h UP in UUO mice model. ##P<0.01, vs Sham group; *P<0.05, **P<0.01, vs UUO group; †P<0.05, vs UUO + PRL group; ns: p>0.05, no significance.
PR Alleviates UUO-Mediated Renal Injury and Fibrosis
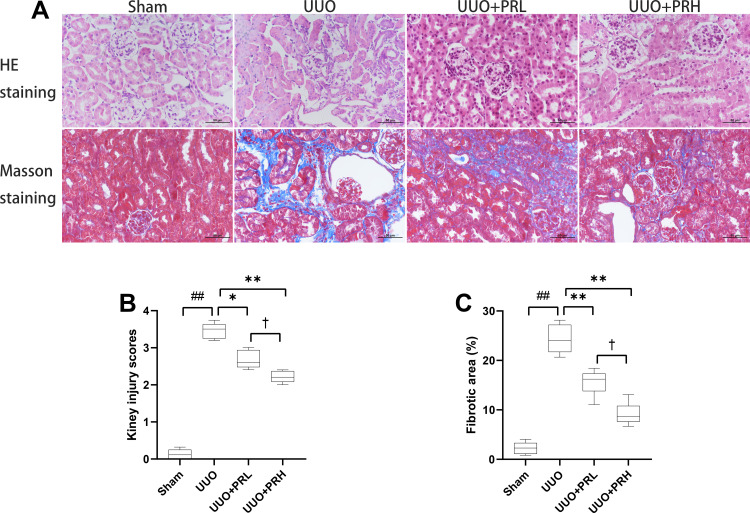
As shown in Figure 4A, kidney injuries and fibrosis were assessed by HE and Masson staining. The Sham group was structurally normal, without inflammatory cell infiltration and interstitial fibrosis. In the UUO group, the tubules were greatly dilated, with massive inflammatory cell infiltration and extensive interstitial fibrosis. After PR intervention, interstitial fibrosis and histopathological damage were alleviated and tubular and glomerular malformations were reduced in a dose-dependent manner. The kidney injury scores and area of fibrosis in each group were shown in Figure 4B and C. The above indicates that PR is effective in alleviating UUO-mediated renal pathological injury and fibrosis.
Figure 4.
PR alleviates UUO-mediated renal injury. (A) Representative HE and Masson staining of kidney tissue sections. Scale bar = 50 μm. (B and C) Results of semi-quantitative analysis of renal injury scores and fibrotic area. ##P<0.01, vs Sham group; *P<0.05, **P<0.01, vs UUO group; †P<0.05, vs UUO + PRL group.
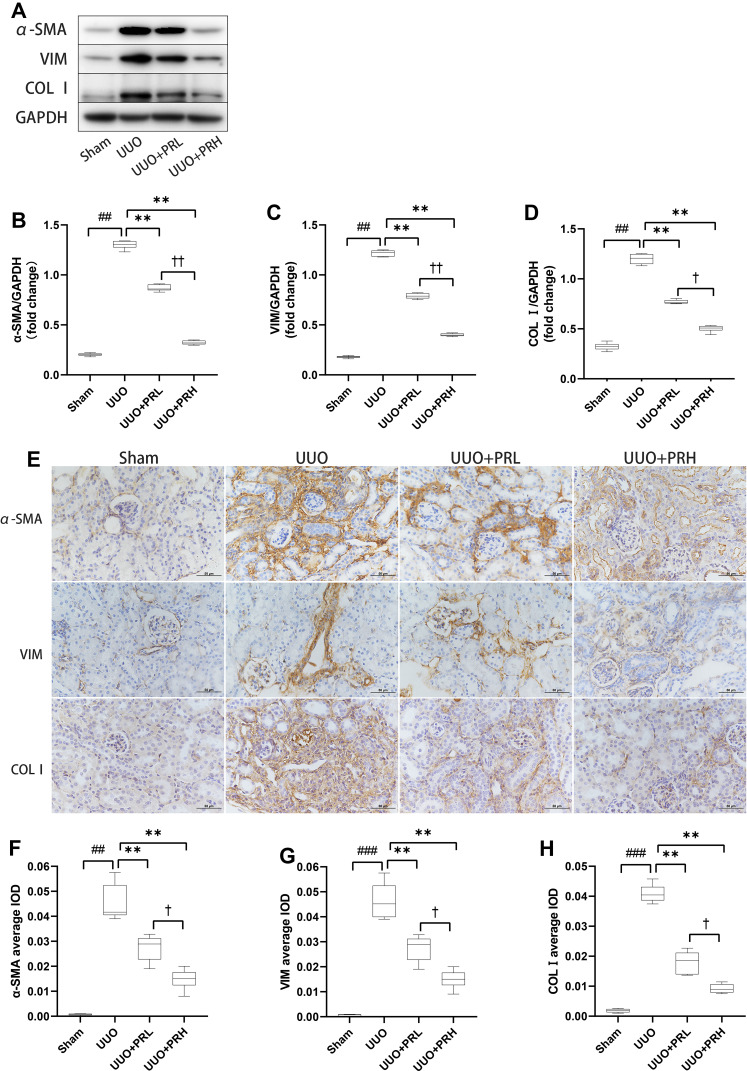
PR Inhibits ECM Accumulation in UUO Mice
α-SMA, VIM and COLI are classic ECM proteins, and their accumulation is one of the central parts of fibrogenesis. As shown in Figure 5A–D, ECM proteins was significantly upregulated in the UUO group, which was in distinct contrast to the Sham group, while PR intervention prevented UUO-mediated ECM protein upregulation and PRH was more effective than PRL. The results of IHC were consistent with Wb’s trend, as shown in Figure 5E–H, the ECM proteins were deposited most abundantly in the renal interstitium, with the UUO group being the most pronounced, followed by the UUO+PRL, UUO+PRH, and Sham groups. The above indicates that PR can effectively inhibit the ECM accumulation in the kidney of UUO mice and thus protect against fibrosis.
Figure 5.
PR inhibits ECM accumulation in UUO mice. (A–H) According to the results of Wb and IHC, PR significantly inhibited the expression levels of the fibrosis markers α-SMA, VIM and COL I in the kidney of UUO mice. Scale bar = 50μm. ##P<0.01, ###P<0.001, vs Sham group; **P<0.01, vs UUO group; †P<0.05, ††P<0.01, vs UUO + PRL group.
PR Regulates UUO-Induced TGF-β1/Smads Signaling Pathway Transduction
As presented in Figure 6A–D, PR inhibited the upregulation of TGF-β1 and its downstream molecules Smad3 and p-Smad3 in the kidney of UUO mice. Smad7, another downstream molecule of TGF-β1, plays an opposite role to Smad3 and is lowly expressed in response to fibrosis. As shown in Figure 6A and E, PR reversed the UUO-mediated low expression of Smad7, and the impact of PRH was more pronounced than PRL. The above data suggest that PR exerts a regulatory effect on the TGF-β1/Smad pathway, which may be an essential reason why PR inhibits ECM deposition and kidney fibrosis progression.
Figure 6.
PR regulates UUO-induced TGF-β1/smads signaling pathway transduction. (A–E) According to the results of Wb, PR significantly inhibited the activation of the fibrotic pathway TGF-β1/smad3 in the kidney of UUO mice, while smad7 was upregulated. ##P<0.01, ###P<0.001, vs Sham group; *P<0.05, **P<0.01, vs UUO group; †P<0.05, ††P<0.01, vs UUO + PRL group.
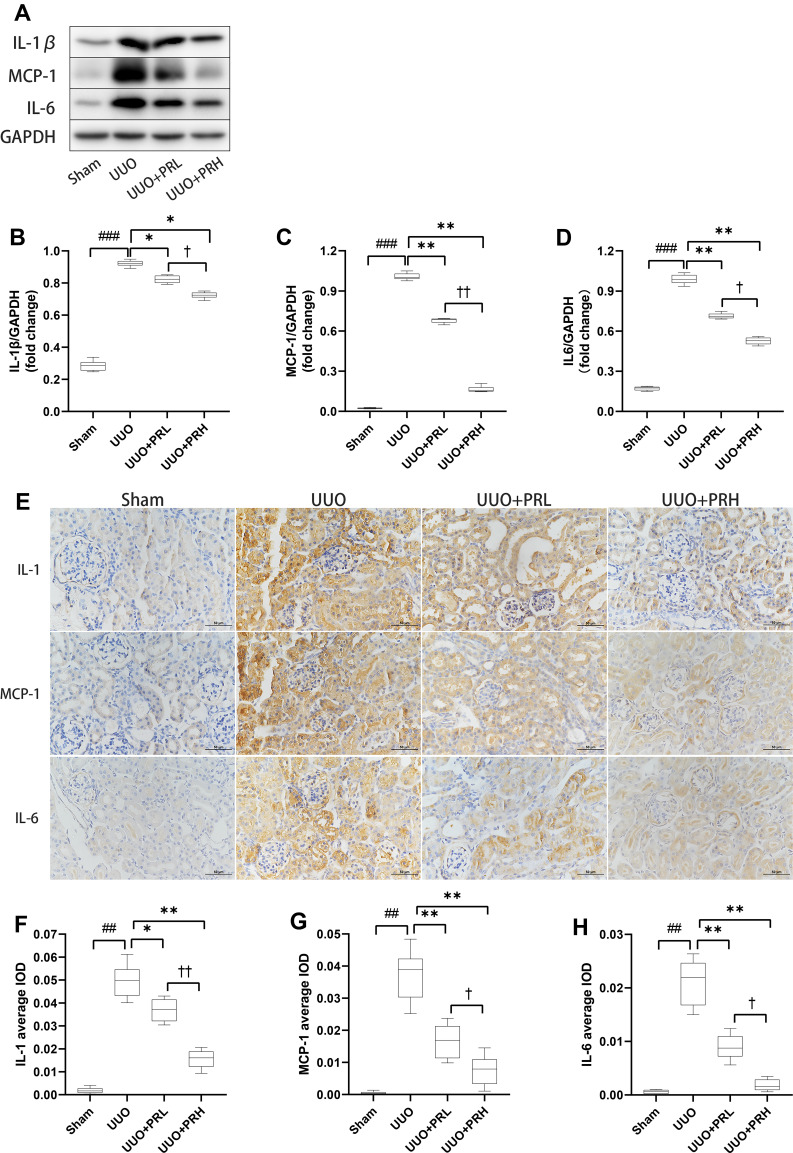
PR Suppresses Inflammatory Factor Recruitment in UUO Mice
The Wb trend is shown in Figure 7A–D, PR suppressed the upregulation of UUO-induced inflammatory factors including IL-1β, MCP-1 and IL-6. The outcomes of IHC were consistent with the trend of Wb. The results of IHC are shown in Figure 7E–H, inflammatory factors were widely expressed in renal interstitial and tubular epithelial cells and were most extensive and pronounced in the UUO group, followed by the PRL, PRH and Sham groups. The above indicates that PR can inhibit the recruitment of inflammatory factors in the kidney of UUO mice, thereby mitigating inflammation and that PRH is more effective than PRL.
Figure 7.
PR suppresses inflammatory factor recruitment in UUO mice. (A–H) According to the results of Wb and IHC, PR significantly inhibited the expression levels of the inflammatory factor IL-1β, MCP-1 and IL-6 in the kidney of UUO mice. Scale bar = 50 μm. ##P<0.01, ###P<0.001, vs Sham group; *P<0.05, **P<0.01, vs UUO group; †P<0.05, ††P<0.01, vs UUO + PRL group.
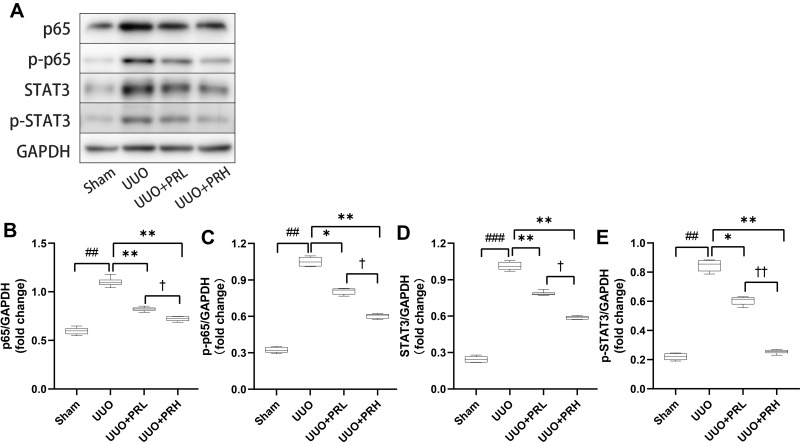
PR Suppresses UUO-Induced NF-κB P65/STAT3 Signaling Pathway Activation
As illustrated in Figure 8A–E, two weeks after PR treatment, the expression of p65 and STAT3 was significantly upregulated and statistically different from the Sham group. We similarly focused on the expression of p-p65 and p-STAT3, and consistent with this, there was similar expression of p-p65, p-STAT3 and p65, STAT3. However, PR significantly inhibited the UUO-mediated upregulation of p65/STAT3 expression, and the effect of PRH was more pronounced than that of PRL. These data demonstrate that PR can inhibit UUO-induced NF-κB p65/STAT3 signaling pathway activation, which may be an important mechanism for the inhibition of inflammatory factor recruitment.
Figure 8.
PR inhibits UUO-induced NF-κB p65/STAT3 signaling pathway activation. (A–E) According to the results of Wb, PR significantly inhibited the activation of the inflammatory pathway NF-κB p65/STAT3 in the kidney of UUO mice. ##P<0.01, ###P<0.001, vs Sham group; *P<0.05, **P<0.01, vs UUO group; †P<0.05, ††P<0.01, vs UUO + PRL group.
Discussion
UUO is one of the well-established modeling method for mediating CKD because it well stimulates the compensatory and decompensatory events of the residual kidney forced to maintain physiological activity caused by the loss or dysfunction of substantial nephron. Even the ensuing interstitial fibrosis, inflammation, oxidative stress, endothelial cell dysfunction, and a host of other disorders are consistent with an unfavorable microenvironment in the kidney of CKD patients. Although the occurrence order of these disorders in CKD is still not well defined, anti-inflammation and anti-fibrosis remain the recognised targets for the treatment of CKD. Therefore, the suppression of local inflammation and fibrosis after the initiation of UUO events, or rather in the early stages of CKD, is of great importance to improve renal function and microcirculation as well as to restore metabolic homeostasis.
Including both congenital and acquired etiologies, the renal dysfunction and structural abnormalities that accompany CKD patients often result in abnormal protein filtration and excretion. Decreasing urinary protein, optimizing microcirculation and delaying the deterioration of renal function are the main means and objectives of the management of CKD. Agents used clinically to lower urinary protein levels primarily include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, glucocorticoids, and immunosuppressants. These medications have obvious side effects and contraindications and are costly to maintain. Unlike the above mentioned pharmaceuticals, PR is coming into the limelight with the advantage of immunomodulation and affordability. Our study indicated that PR attenuates UUO-induced chronic kidney injury and suppressed the progradation of inflammatory response and fibrosis in the kidney, whereas the NF-κB p65/STAT3 and TGF-β1/Smads pathways are key signaling pathways involved in these effects.
TGF-β1 is recognised as a key target for intervention in fibrosis, as it cascades the complete fibrosis event and can lead to phenotypic changes in fibroblasts when its over-induction occurs. In Wu Tao’s study, PR was found to inhibit TGF-β1/Smad3 expression and down-regulate fibronectin and E-cadherin expression in an in vitro EMT model.28 Besides, in the streptozotocin (STZ-)-induced diabetic mouse model, She et al. found that PR inhibited IFN-γ and IL-4 levels to exert anti-inflammatory effects and that TGF-β1/Smad2 expression was down-regulated, which is consistent with our conclusion that PR has anti-inflammatory and anti-fibrotic effects, but they did not further explore the specific mechanism of PR’s anti-inflammatory effects.29 Although we did not study the regulatory effect of PR on Smad2, the available data still demonstrate a regulatory effect of PR on the TGF-β1/Smads pathway, but such regulatory effect is related to factors such as animal models, tissue specificity, and administration mode and dosage. It has also been indicated that the reversal of myocardial fibrosis, hepatic fibrosis and pulmonary fibrosis by PR is inextricably linked to the modulation of TGF-β1 and its downstream pathways and the inhibition of EMT and/or interstitial ECM deposition.30–33 And our study reveals for the first time that PR protects the kidney against UUO by mechanisms other than oxidative stress, in which, not coincidentally, TGF-β1 still plays an indispensable role.
The inflammatory troubles that accompany CKD are indisputable, and an increasing number of inflammatory factors have been found to induce activation of NF-κB family members, either directly or indirectly. The initiation of the inflammatory response arises from the sensitivity of immune cells and lymphocytes to tissue damage, where the surveillance mechanism involves pattern recognition receptors on the cell surface and in the cytoplasm, most of which can respond to pathogen-associated molecular patterns or host-derived damage-associated molecular patterns via NF-κB induction.34 While STAT3 serves an essential part in nuclear translocation phosphorylation, and involves in multiple signalling pathways, through dimerization or targeted phosphorylation activation.35 The NF-κB/STAT3 signaling pathway regulates acute phase proteins in response to stress, inflammation, as well as injury, from which the interaction determines whether IL-1β, IL-6-mediated gene expression is inhibited or enhanced.36–38 Although the interaction between NF-κB family members and STAT3 is not yet fully understood, it has been suggested that p65 can compete with STAT3 for binding, resulting in coverage of the corresponding binding site and thus inhibiting IL-6-induced γ-fibrinogen expression.39,40 Additionally, IL-1 can induce the p65/STAT3 protein complex, which is associated with the recruitment of unphosphorylated STAT3 into the transcriptionally active region by p65.41 These data suggest that inflammatory factors are perhaps the real molecular switches in the inflammatory response which determine the trajectory of the NF-κB/STAT3 signalling pathway as well as their interactions, phosphorylation modifications, etc. This is also consistent with our data that the group with the most active expression of inflammatory factors showed the most pronounced NF-κB p65/STAT3 activation.
In the diabetic nephropathy mouse model induced by tail vein injection of STZ, the nephroprotective effect of PR was confirmed by an 8-week PR gavage, and NF-κB expression was also downregulated, which, mechanistically, is related to the downregulation of the SIRT1/FOXO1 pathway and the suppression of mitochondrial oxidative stress.32 Although modelling methods, interventions, and the like are not entirely consistent, our study describes the effects of PR on inflammation and fibrosis in a relatively more complete way. In the study by Ma et al, PR reversed CCL4-induced renal injury, downregulated TNF-α and IL-6 levels in renal tissues, and NF-κB p65 and oxidative damage were inhibited.42 As their experimental period was only 5 days, again, the effect of PR on renal interstitial fibrosis was difficult to be noticed. Collectively, our study presents a more comprehensive picture of the effects of PR on renal fibrosis and inflammation in CKD than previous studies that focused on the renoprotective effects of PR via antioxidant mechanisms.
Of course, there may be some limitations to the study. For example, how inflammation and fibrosis interact in this study has not been explored. Furthermore, in terms of some issues outside of this study, it is also worth considering and discussing. The clinical application of PR is limited by its complex composition and pharmacokinetic instability. There is an urgency for some advanced approaches such as nanotechnology to help it achieve better activity and structural stability, and thus higher bioavailability. Based on the multi-component, multi-target and multi-effect characteristics of Chinese medicine, there is still a need for more in-depth research on its pharmacological effects and related mechanisms in modern medicine.
Conclusion
Our data suggest that PR can attenuate UUO-mediated fibrosis and inflammation through pathways other than oxidative stress, and that the main mechanisms may be related to regulation of the TGFβ1/Smads and NF-κB p-65/STAT3 pathways. Moreover, Our study provides a new entry point for PR in the treatment of CKD and contributes to assess its pharmacological effects better.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of China (No. 81870505), the Guiding Program of Liaoning Natural Science Foundation in China (No. 20180550610), the Program for Liaoning Innovative Talents in University, and the Scientific Research of the First Hospital of China Medical University (FHCMU-FSR0816). Thanks to Prof. Hailong Wang for statistical help.
Abbreviations
PR, puerarin; UUO, unilateral ureteral obstruction; α-SMA, α-smooth muscle actin; COLI, collagen type I; VIM, vimentin; IL-1β, interleukin-1β; IL-6, interleukin-6; MCP-1, monocyte chemotactic protein-1; ECM, extracellular matrix; CKD, chronic kidney disease; ESRD, end-stage renal disease; EMT, epithelial-mesenchymal transition; TGF-β1, transforming growth factor-β1; NF-κB, nuclear factor κ-B; STAT3, signal transducer and activator of transcription 3; TLR, toll-like-receptor; Nrf2, nuclear factor E2-related factor 2; HDAC, histone deacetylase; PPARα, peroxisome proliferator-activated receptor α; SCr, serum creatinine; BUN, blood urea nitrogen; 24-h UP, 24-hour urine protein; STZ, streptozotocin; IFN-γ, interferon-γ; SIRT1, silent information regulator factor 2-related enzyme 1; FOXO1, forkhead box O1; TNF-α, tumour necrosis factor α.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. [DOI] [PubMed] [Google Scholar]
- 2.Bülow RD, Boor P. Extracellular matrix in kidney fibrosis: more than just a scaffold. J Histochem Cytochem. 2019;67(9):643–661. doi: 10.1369/0022155419849388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mack M. Inflammation and fibrosis. Mat Biol. 2018;68–69:106–121. doi: 10.1016/j.matbio.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 4.Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J Mol Med. 2021;99(1):1–20. doi: 10.1007/s00109-020-01988-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Sun SC. NF-κB in inflammation and renal diseases. Cell Biosci. 2015;5(1):63. doi: 10.1186/s13578-015-0056-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12(1):86. doi: 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324. [DOI] [PubMed] [Google Scholar]
- 10.Zhong L, Simard MJ, Huot J. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J. 2018;32(8):4070–4084. doi: 10.1096/fj.201701536R [DOI] [PubMed] [Google Scholar]
- 11.Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother. 2020;122:109772. doi: 10.1016/j.biopha.2019.109772 [DOI] [PubMed] [Google Scholar]
- 12.Karuppagounder V, Arumugam S, Thandavarayan RA, et al. Naringenin ameliorates daunorubicin induced nephrotoxicity by mitigating AT1R, ERK1/2-NFκB p65 mediated inflammation. Int Immunopharmacol. 2015;28(1):154–159. doi: 10.1016/j.intimp.2015.05.050 [DOI] [PubMed] [Google Scholar]
- 13.Tian L, Li W, Yang L, et al. Cannabinoid receptor 1 participates in liver inflammation by promoting M1 macrophage polarization via rhoA/NF-κB p65 and ERK1/2 pathways, respectively, in mouse liver fibrogenesis. Front Immunol. 2017;8:1214. doi: 10.3389/fimmu.2017.01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu YJ, Xu B, Huang SW, et al. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42(1):88–96. doi: 10.1038/s41401-020-0411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chade AR, Williams ML, Engel JE, Williams E, Bidwell GL 3rdMolecular targeting of renal inflammation using drug delivery technology to inhibit NF-κB improves renal recovery in chronic kidney disease. Am J Physiol Renal Physiol. 2020;319(1):F139–f148. doi: 10.1152/ajprenal.00155.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li ZL, Shi Y, Le G, et al. 24-week exposure to oxidized tyrosine induces hepatic fibrosis involving activation of the MAPK/TGF-β1 signaling pathway in sprague-dawley rats model. Oxid Med Cell Longev. 2016;2016:3123294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KK, Sheppard D, Chapman HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293. doi: 10.1101/cshperspect.a022293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni SY, Zhong XL, Li ZH, et al. Puerarin alleviates lipopolysaccharide-induced myocardial fibrosis by inhibiting PARP-1 to prevent HMGB1-mediated TLR4-NF-κB signaling pathway. Cardiovasc Toxicol. 2020;20(5):482–491. [DOI] [PubMed] [Google Scholar]
- 19.Niu L, Luo R, Zou M, et al. Puerarin inhibits mycoplasma gallisepticum (MG-HS)-induced inflammation and apoptosis via suppressing the TLR6/MyD88/NF-κB signal pathway in chicken. Int Immunopharmacol. 2020;88:106993. doi: 10.1016/j.intimp.2020.106993 [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Huang C, Sun H. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. 2021;12(5):2075–2089. doi: 10.1039/D0FO03076G [DOI] [PubMed] [Google Scholar]
- 21.Guo CJ, Xie JJ, Hong RH, Pan HS, Zhang FG, Liang YM. Puerarin alleviates streptozotocin (STZ)-induced osteoporosis in rats through suppressing inflammation and apoptosis via HDAC1/HDAC3 signaling. Biomed Pharmacother. 2019;115:108570. doi: 10.1016/j.biopha.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 22.He L, Wang T, Chen BW, Lu FM, Xu J. Puerarin inhibits apoptosis and inflammation in myocardial cells via PPARα expression in rats with chronic heart failure. Exp Ther Med. 2019;18(5):3347–3356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Chang X, Zhang T, Liu D, et al. Puerarin attenuates LPS-induced inflammatory responses and oxidative stress injury in human umbilical vein endothelial cells through mitochondrial quality control. Oxid Med Cell Longev. 2021;2021:6659240. doi: 10.1155/2021/6659240 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Zhang L, Xu X, Yang R, et al. Paclitaxel attenuates renal interstitial fibroblast activation and interstitial fibrosis by inhibiting STAT3 signaling. Drug Des Devel Ther. 2015;9:2139–2148. doi: 10.2147/DDDT.S81390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Sun L, Xian W, et al. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/smad activity. Lab Invest. 2010;90(3):436–447. doi: 10.1038/labinvest.2009.149 [DOI] [PubMed] [Google Scholar]
- 26.Choi DE, Jeong JY, Lim BJ, et al. Aliskiren ameliorates renal inflammation and fibrosis induced by unilateral ureteral obstruction in mice. J Urol. 2011;186(2):694–701. doi: 10.1016/j.juro.2011.03.122 [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Lee AS, Jung YJ, et al. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor α-mediated transforming growth factor-β1/smad signaling pathway. Nephrol Dial Transplant. 2014;29(11):2043–2053. doi: 10.1093/ndt/gfu240 [DOI] [PubMed] [Google Scholar]
- 28.Wu T, Liu T, Xing L, Ji G. Baicalin and puerarin reverse epithelial-mesenchymal transition via the TGF-β1/smad3 pathway in vitro. Exp Ther Med. 2018;16(3):1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.She S, Liu W, Li T, Hong Y. Effects of puerarin in STZ-induced diabetic rats by oxidative stress and the TGF-β1/smad2 pathway. Food Funct. 2014;5(5):944–950. doi: 10.1039/C3FO60565E [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Xue J, Xie M. Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-β1 expression. J Nutr Biochem. 2012;23(9):1080–1085. doi: 10.1016/j.jnutbio.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 31.Jin YG, Yuan Y, Wu QQ, et al. Puerarin protects against cardiac fibrosis associated with the inhibition of TGF-β1/smad2-mediated endothelial-to-mesenchymal transition. PPAR Res. 2017;2017:2647129. doi: 10.1155/2017/2647129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Zheng N, Chen Z, Huang W, Liang T, Kuang H. Puerarin, isolated from Pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene. 2016;591(2):411–416. doi: 10.1016/j.gene.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 33.Liu MW, Liu R, Wu HY, et al. Radix puerariae extracts ameliorate paraquat-induced pulmonary fibrosis by attenuating follistatin-like 1 and nuclear factor erythroid 2p45-related factor-2 signalling pathways through downregulation of miRNA-21 expression. BMC Complement Altern Med. 2016;16(1):11. doi: 10.1186/s12906-016-0991-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3):a006049–a006049. doi: 10.1101/cshperspect.a006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calò V, Migliavacca M, Bazan V, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197(2):157–168. doi: 10.1002/jcp.10364 [DOI] [PubMed] [Google Scholar]
- 36.Albrecht U, Yang X, Asselta R, et al. Activation of NF-kappaB by IL-1beta blocks IL-6-induced sustained STAT3 activation and STAT3-dependent gene expression of the human gamma-fibrinogen gene. Cell Signal. 2007;19(9):1866–1878. doi: 10.1016/j.cellsig.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 37.Bode JG, Fischer R, Häussinger D, Graeve L, Heinrich PC, Schaper F. The inhibitory effect of IL-1 beta on IL-6-induced alpha 2-macroglobulin expression is due to activation of NF-kappa B. J Immunol. 2001;167(3):1469–1481. doi: 10.4049/jimmunol.167.3.1469 [DOI] [PubMed] [Google Scholar]
- 38.Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2012;91(6–7):496–505. doi: 10.1016/j.ejcb.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Fuller GM. The competitive binding of STAT3 and NF-kappaB on an overlapping DNA binding site. Biochem Biophys Res Commun. 1997;237(1):90–94. doi: 10.1006/bbrc.1997.7082 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Fuller GM. Interleukin 1beta inhibits interleukin 6-mediated rat gamma fibrinogen gene expression. Blood. 2000;96(10):3466–3472. doi: 10.1182/blood.V96.10.3466 [DOI] [PubMed] [Google Scholar]
- 41.Yoshida Y, Kumar A, Koyama Y, et al. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem. 2004;279(3):1768–1776. doi: 10.1074/jbc.M311498200 [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Yan L, Zhu Q, Shao F. Puerarin attenuates cisplatin-induced rat nephrotoxicity: the involvement of TLR4/NF-κB signaling pathway. PLoS One. 2017;12(2):e0171612. doi: 10.1371/journal.pone.0171612 [DOI] [PMC free article] [PubMed] [Google Scholar]