Abstract
Background:
Food protein-induced allergic proctocolitis (FPIAP) is an early and common manifestation of food allergy, yet its epidemiology and relationship to other allergic diseases remain unclear.
Objective:
To prospectively define the incidence of FPIAP as it is being diagnosed clinically in the community and to identify factors associated with its development.
Methods:
1003 of 1162 eligible serial healthy newborn infants recruited from a single suburban pediatrics practice were followed prospectively for the diagnosis of FPIAP. Investigators reviewed each case to confirm pre-specified inclusion criteria, including documented gross or occult blood in the stool.
Results:
903 infants were analyzed (46% female, 89% term, 32% caesarian-section, 9% neonatal antibiotics). 153 cases met inclusion criteria, a cumulative incidence of 17%, while 63 (7%) had gross blood. Infants initially fed both breastmilk and formula were 61% less likely to develop FPIAP compared to those exclusively formula-fed (HR 0.39, P = .005). Breastmilk and formula at any point during the first 4 months was also associated with lower risk compared to exclusive formula or exclusive breastmilk (HR 0.44, P = .005; HR 0.62, P = .0497). Eczema (OR 1.5 [1.1, 2.2], P = .02) or a first degree relative with food allergies (OR 1.9 [1.2, 2.8], P = .005) were among risk factors for FPIAP development.
Conclusion:
The prospectively defined incidence of FPIAP when diagnosed clinically by community pediatricians without challenge is markedly higher than published estimates. Combination feeding of formula and breastmilk is associated with the lowest rate of FPIAP in this population.
Keywords: Food protein-induced allergic proctocolitis, Cow’s milk protein allergy, non-IgE-mediated milk allergy, formula intolerance
INTRODUCTION
Food protein-induced allergic proctocolitis (FPIAP) is a commonly recognized and burdensome form of non-IgE-mediated food allergy of early infancy whose epidemiology has never been prospectively evaluated.(1-6) While the prevalence of allergic disease is dramatically rising in the United States and other developed nations over recent decades,(7,8) most national data on pediatric allergies either exclude non-IgE-mediated food allergy by design, combine it together with IgE-mediated allergy, or study the subset of non-IgE-mediated allergy exclusively triggered by milk. Furthermore, though there is broad consensus that FPIAP is limited to infants who are generally healthy and growing normally despite a food allergic reaction causing lower GI bleeding, current guidelines(9-11) vary considerably from common accepted practice(12) on the significance of occult blood and level of evidence implicating diet. With these limitations, historical prevalence estimates range widely.(13-23)
FPIAP is typically diagnosed clinically by the presence of bloody or mucoid stools during the first few months of life in a generally healthy infant that resolves with dietary restriction. FPIAP can be seen in both children who are breastfed and formula fed.(12) The most commonly implicated dietary triggers are cow’s milk protein and soy protein,(3) and elimination of these (either by maternal dietary restriction if breastfeeding or by use of hypoallergenic formulas) leads to symptom resolution in most. Most guidelines require gross blood for diagnosis(10) and some suggest that diagnosis requires reintroduction or challenge to confirm symptom recurrence after symptoms resolve with elimination(9,10,24). However, practice in the pediatrics community commonly utilizes fecal occult blood testing (reliability of which has been called into question)(25) and rarely if ever utilizes confirmatory challenge with reintroduction(12,26). Historically, FPIAP was associated with eosinophilic inflammation on histology of rectal biopsy in a subset of those presenting with these symptoms;(6,27) however, sigmoidoscopy with biopsy is also no longer done in routine practice. There are currently no non-invasive biomarkers identified to support the diagnosis of FPIAP. Risk factors for the development of FPIAP are not known. Parental history of atopy has been suggested as a risk factor, but there are conflicting reports.(28) Protective factors have not been described in FPIAP, though factors such as maternal diet, infant diet, siblings, and pets have been associated with lower rates of other pediatric allergic diseases.(29,30)
Despite how little FPIAP has been studied, the burden and impact of FPIAP are quite significant. It is the most frequent indication for referral to pediatric gastroenterology subspecialists in infancy and commonly results in significant maternal dietary restrictions or use of more expensive and hypoallergenic infant formulas. As both the underlying disease pathophysiology of FPIAP itself, as well as current management by dietary restriction, each may increase the risk of IgE-mediated food allergy later in life, we believe that the prospective characterization of FPIAP is an important objective. What follows is a report of primary clinical outcomes from the Gastrointestinal Microbiome and Allergic Proctocolitis (GMAP) Study, the first prospective observational healthy infant cohort study specifically designed to investigate the epidemiology and clinical presentation of FPIAP as it is currently being diagnosed and treated by pediatricians in the community.
METHODS
Study Design and Patient Population
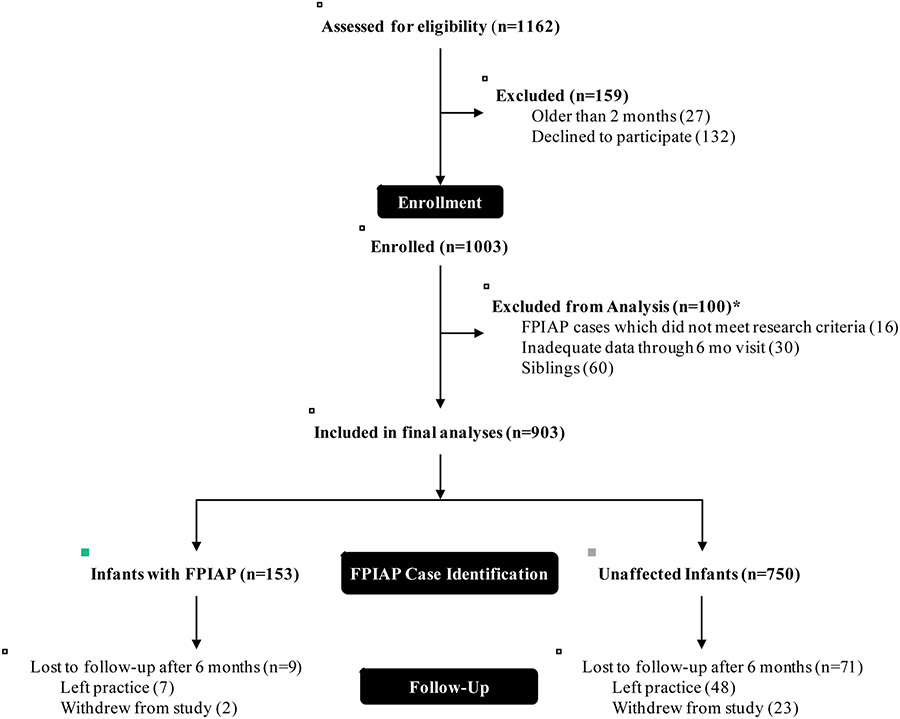
The Gastrointestinal Microbiome and Allergic Proctocolitis (GMAP) Study is a large ongoing prospective observational healthy infant cohort study in suburban Massachusetts. We serially recruited newborn infants at their first office visit at a single private primary pediatrics office (Pediatrics at Newton Wellesley) between April 2014 and February 2017. Any infant born into the practice during that time period was eligible and approached for participation in the study up to 2 months of age (median age at enrollment was 8 days). Infants were followed at all routine well-child visits according to the American Academy of Pediatrics schedule (1 week, 2 weeks, and 1, 2, 4, 6, 9, 12, 15, 18, 24, and 36 months of age), and at unscheduled sick visits. Current median follow-up duration is 24 months with a range of 2 to 45 months. Because our outcome of interest occurred at a median age of 35 days with 95% occurring before 4 months, we excluded from this analysis any infants who withdrew prior to 6 months of age as we were unable to unambiguously define their case status (n = 30, Figure 1). The GMAP Study was approved by the Massachusetts General Hospital Institutional Review Board (IRB) and a parent of all enrolled infants gave written informed consent.
Figure 1. Study population and follow-up.
*there were 6 subjects who met more than one of the analysis exclusion criteria
Exposure Assessment
At the time of enrollment the parents completed an initial questionnaire capturing a broad range of information about the pregnancy, delivery, antibiotic exposures, maternal diet, family structure, race (White, Black, Asian, Other, Multiple Race), ethnicity (Hispanic/Latino or Non-Hispanic/Latino), pets present at birth (cat, dog, other), feeding practices (breastmilk, formula, solid food introduction), and family history of atopic diseases (asthma, environmental allergies, eczema). Maternal antibiotics included any given to the mother at the time of delivery, perinatal antibiotics included any given to the infant prior to discharge, both of which were validated by chart review. Initial diet was defined as the diet the family reported feeding to the infant at their initial visit to the pediatrician. At each subsequent study visit, parents also completed questionnaires reporting on the infant’s current sleeping, feeding, and stooling patterns as well as any gastrointestinal or allergic symptoms. All data were acquired in real time and managed using a set of chart extraction forms and automated patient surveys within an IRB-approved and HIPPA-compliant electronic database. Symptoms and diagnoses of FPIAP were also recorded by their physicians in real time. Inconsistencies or missing data for key variables were chart reviewed by the study team.
Outcome Assessment
The diagnosis of FPIAP in the GMAP study was made by the pediatrician caring for the infant. Research study staff independently and comprehensively reviewed the charts of each child diagnosed with FPIAP. Pre-specified case inclusion criteria were the treating physician’s clinical diagnosis of FPIAP and documented guaiac positive or grossly bloody stool not attributable to another etiology (e.g., constipation with documented or suspected anal fissure, infection, inflammatory bowel disease). We defined a subgroup of infants with “questionable FPIAP” who did not meet inclusion criteria, usually due to lack of documented blood in the stool, but were clinically treated as having FPIAP. These questionable FPIAP cases were excluded from primary analyses (n = 16, Figure 1). The 153 cases meeting our pre-specified inclusion criteria were used for the primary analyses. Sensitivity analyses combining questionable FPIAP with FPIAP cases did not change any of our primary findings. Because the diagnostic criteria for FPIAP diagnosis are debated, we also reported the cumulative incidence with more stringent inclusion criteria described below, which also did not change any of the primary findings. As the study design was observational, treatment of FPIAP was per clinician discretion. Therefore, reintroduction of the suspected offending food to confirm diagnosis was not routinely done and the order of foods eliminated was not standardized. Resolution diets were defined as the diet at the time of symptom resolution. Symptom resolution was based on comprehensive chart review, and if no clear time of resolution was mentioned, was defined as the first visit where the child was no longer reported to be symptomatic.
Data Management and Statistical Analysis
All data were managed in REDCap (Research Electronic Data Capture) hosted at Partners Heath Care. (31) To preserve the assumption of independence of observations, one infant from any sibling group (for those families who enrolled more than one child in GMAP) was randomly selected and the other sibling(s) excluded from in our final analyses (n=60, Figure 1). Prespecified analysis of primary and secondary outcomes was done by univariable logistic regression using the statistical programming language, R (v3.5.0). (32) Exploratory survival analysis of the impact of diet on FPIAP development was done in Stata/SE 13.1 (StataCorp LP, College Station, TX). We used a Cox regression model for analysis of initial diet and an extended Cox regression model for non-proportional hazards to analyze the diet over time. In both logistic and survival analyses, p-values for significance were calculated using the Wald test with a priori levels of significance set at P < .05. Group comparisons were performed using Chi square, Mann-Whitney U, and Kruskal-Wallis rank sum tests of significance as appropriate. Sensitivity analysis was performed by repeating the logistic regression analyses to assess alterations in the primary and secondary outcomes specified above when combining the questionable FPIAP cases with the FPIAP cases.
RESULTS
Characteristics of the GMAP Cohort
Of the 1162 infants approached, 1003 (86%) enrolled in GMAP (Figure 1). After exclusion of siblings, subjects with inadequate data through 6 months, and FPIAP cases not meeting research criteria, 903 infants were included in the final analysis (Figure 1). Over the course of the study, 80 (9%) infants withdrew participation, 55 (69%) of whom relocated or left the pediatrics practice and 25 (31%) of whom chose to no longer participate. We found no statistically significant differences between infants who withdrew before 6 months and the remainder of the cohort across any of our exposures of interest (not shown).
Of the main cohort of 903 infants, a majority were White, with a slight male predominance (Figure 2). Most were born at term (89%), delivered vaginally (68%), and 50% were exposed to intrapartum maternal antibiotics, while a small proportion (9%) were directly exposed to antibiotics postnatally (Figure 2). The median age at enrollment was 8 days (IQR: 5, 14). At their first pediatrics visit, most infants (62%) were being fed only breastmilk, 7% only formula, and 32% a mixed diet (both breastmilk and cow’s milk – based formula). 47% were first born children and 41% had pets living in their home at birth. 45% had first degree family members with atopic diseases and 15% had first degree relatives with food allergies.
Figure 2. Early life risk factors for FPIAP development.
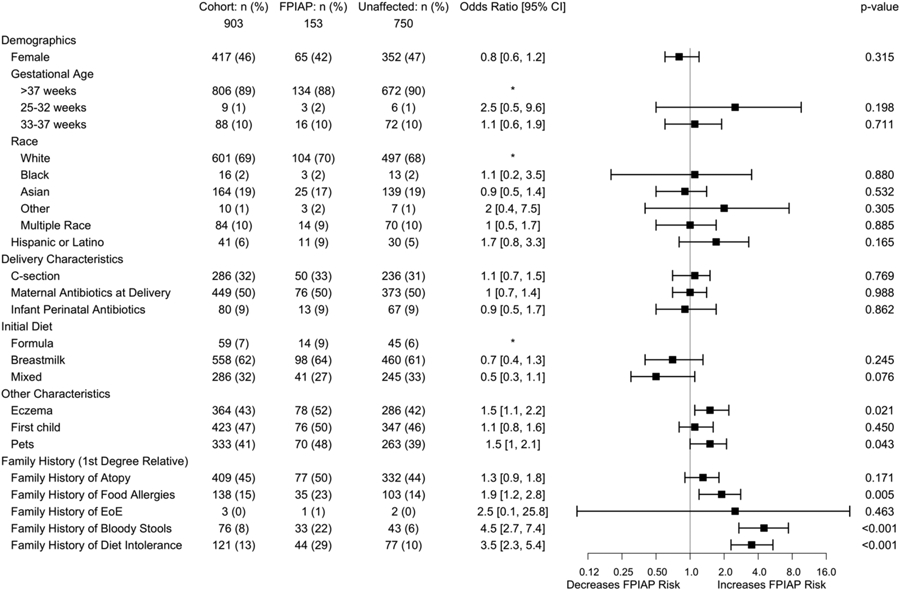
*denotes hazard ratio reference group for polytomous variables. Reference group chosen was the largest by default, except for infant initial diet for which, given our a priori hypothesis that any breastmilk would be protective, formula is the appropriate reference.
Cumulative Incidence of FPIAP
Of the 903 healthy infants included in the final analysis, 169 of them were diagnosed with FPIAP and 153 met the prespecified inclusion criteria (Figure 1). This identifies a cumulative incidence of FPIAP of 17% over three years in this healthy unselected population. The median age at symptom onset was 26 days (IQR:14, 34) and the median age at FPIAP diagnosis was 35 days (IQR: 26, 81).
Risk Factors for FPIAP Development
A family history in a first degree relative of food allergy (OR = 1.9 [1.2, 2.8], P = .005), bloody stools (OR = 4.5 [2.7, 7.4], P < .001), and diet intolerance (OR =3.5 [2.3, 5.4], P < .001) were risk factors for the development of FPIAP, all of which were assessed at the time of enrollment (prior to disease onset). FPIAP was also associated with a history of eczema (OR = 1.5 [1.1, 2.2], P = .02) and pets in the home at birth (OR=1.5 [1.01, 2.1], P = .043). We did not find mode of delivery or perinatal antibiotic exposure to be risk factors for the development of FPIAP (Figure 2). FPIAP development was also not associated with sex, race or ethnicity, gestational age, siblings, family history of atopy, or family history of eosinophilic esophagitis (EoE). Sensitivity analysis using more (grossly bloody stools, n = 63) or less (all MD diagnosed, n = 169) case definitions confirmed the findings above, with the exception of the association with pets, which was lost with either more or less stringent criteria.
Lower Rates of FPIAP in Infants Fed Both Breastmilk and Formula
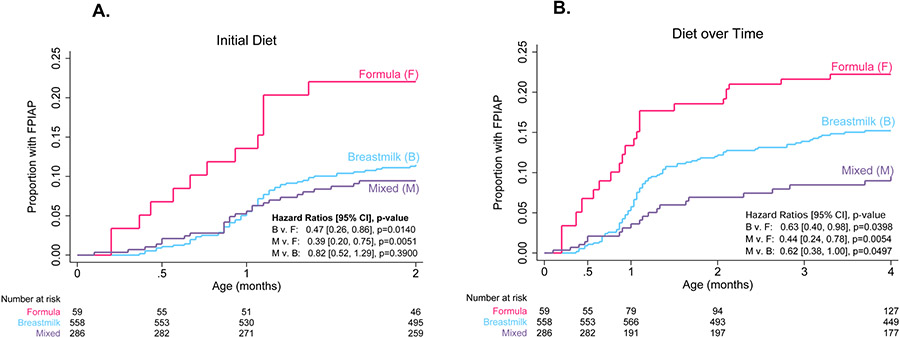
In the initial logistic regression analysis of baseline variables, there was a trend toward association between an initial diet (infant diet reported at first visit) of mixed feeding (breastmilk and cow’s milk-based formula) and decreased rates of FPIAP compared to formula alone (OR = 0.5 [0.3, 1.1], P = .07) (Figure 2). This association was present for those FPIAP cases with documented grossly bloody stools as well (n = 63; mixed feeding OR = 0.3 [0.1, 0.8], P = .02). Recognizing that infant diet can change throughout infancy, we used survival analyses to explore the effect of infant diet over time on the development of FPIAP. Infants initially fed both breastmilk and cow’s milk-based formula were 61% less likely to develop FPIAP and those who were fed only breastmilk were 53% less likely to develop FPIAP compared to those exclusively formula-fed over the first 2 months (HR 0.39, P = .005; HR 0.47, P = .01) (Figure 3A). Infants fed both breastmilk and formula at any point during the first 4 months were 56% less likely than infants fed exclusively formula and 38% less likely than infants fed exclusively breastmilk to develop FPIAP (HR 0.44, P = .005; HR 0.62, P = .0497) (Figure 3B). Exclusively formula fed infants developed FPIAP at the highest rates (Figure 3) and at younger ages (median 15 days v. 26 days, P < .001) than those who received breastmilk.
Figure 3. Effect of infant diet on FPIAP development.
(A) Kaplan-Meier curves of time to FPIAP diagnosis by infant initial diet. (B) Kaplan-Meier curves of time to FPIAP diagnosis by infant diet as a time-varying covariate over the first four months (during which 95% of FPIAP cases presented).
Clinical Presentation, Treatment, and Resolution of FPIAP
Of the 153 cases of FPIAP, 63 (41%) had gross blood in their stool (either by physician documentation or parent report) and 56 (37%) had gross mucous in their stool (either by physician documentation or parent report) in addition to microscopic blood (physician documented), meaning that 119 (63+56) or 78% had either gross blood or mucous in their stool as one of their presenting symptoms. The remaining 34 had other symptoms (e.g. irritability, feeding difficulty, gagging, vomiting, diarrhea) which prompted and revealed positive occult blood testing and resulted in diagnosis of FPIAP by their treating clinician. Compared to unaffected infants, parents of infants diagnosed with FPIAP were more likely to report irritability, gagging, food refusal, and blood or mucous in the stool (Figure E1, online repository). Infants with FPIAP were also significantly more likely than those unaffected to have been treated with proton pump inhibitor or histamine H2-receptor antagonist medications (45% v. 14%, P < .001) for symptoms of reflux (Figure E1, online repository). Those symptoms and the use of acid suppression were significantly different even when a post hoc stricter definition of FPIAP was used, requiring gross mucous or blood in the stool (Table E1, online repository). The median age of infants at the time of symptom resolution was 123 days (IQR: 63, 174), with a median time to documented resolution (days from diagnosis to symptom resolution) of 50 days (IQR: 31, 99). Of the 87 infants (57% of FPIAP cases) whose FPIAP resolved with maternal dietary restrictions, 41 (47%) resolved with maternal diet restriction of milk alone, 35 (40%) resolved on maternal elimination of milk and soy, 11(13%) resolved on maternal elimination of milk, soy, and egg. Of the 62 infants (41% of FPIAP cases) with FPIAP who resolved while consuming formula, either exclusively or mixed feeding with breastmilk, 48 (77.4%) resolved on extensively hydrolyzed formulas and 14 (22.6%) resolved on amino acid-based formulas. Twenty resolved with a combination of maternal diet elimination and hypoallergenic formula. Of note, 23 infants’ parents (15% of FPIAP cases) elected not to restrict the diet at all, and all of those children’s FPIAP symptoms resolved with a combination of watchful waiting, acid suppression, and/or probiotic therapy per clinician discretion. All 23 of those infants continued to tolerate cow’s milk protein throughout infancy. There was no significant association between time to resolution of FPIAP symptoms and therapy used (maternal diet restriction, hypoallergenic formula, probiotics). Among all cases who restricted cow’s milk, the median age at which cow’s milk was successfully reintroduced in any form was 11 months (IQR: 9, 12), with 90 infants (59%) with FPIAP tolerating milk by 12 months of age.
DISCUSSION
In this prospective observational cohort study of unselected healthy infants, we found that 17% (153 of 903) were diagnosed by their pediatrician with food protein-induced allergic proctocolitis (FPIAP) and had confirmed evidence of gastrointestinal bleeding. 63 (41%) of the 153 infants diagnosed with FPIAP had documented gross blood and 119 (78%) had either grossly bloody or mucoid stools (with microscopic blood).
This cumulative incidence is higher than published estimates, though the cumulative incidence of FPIAP, regardless of antigenic trigger, has not been previously defined. Host et al.,(17) focusing solely on milk-triggered cases, but including both IgE- and non-IgE-mediated allergy, states from a review of primary sources from the 1970-90s(18-23) that, “symptoms suggestive of CMPA have been reported in 5 to 15% of infants. However, when controlled elimination/challenge procedures were used, a diagnosis of CMPA was only confirmed in approximately 1 of 3 of the infants resulting in an incidence of CMPA of approximately 2 to 5%.” We cannot ascertain what percent of infants would prove to be reactive to milk elimination and provocation in this observational setting, but the lack of confirmation by reintroduction likely led to overdiagnosis.
Prevalence estimates of this disorder vary by 100-fold in the literature, likely due to differences in the methodology of case identification, particularly whether one includes only those with milk-triggered disease, and the lack of accepted consensus on diagnostic criteria. Cow’s milk is thought to be the single most common culprit, and restriction followed by dietary provocation is recommended when FPIAP is suspected. However, similar to eosinophilic esophagitis, to which FPIAP has been associated(33), in some patients milk may not be a trigger (or it may not be the only trigger). Unlike oral food challenges for suspected immediate hypersensitivity or FPIES, which are routine procedures in allergy offices with unambiguous outcomes within minutes to hours, there is no standardized protocol for the conduct of (and no agreed upon endpoint for the interpretation of) food challenges in the setting of suspected FPIAP. While guidelines typically recommend re-introduction of the suspected food shortly after symptom resolution to confirm diagnosis (9-11,24), our study and others(12,26) show that this is generally not done in the pediatrics community. Instead, empiric diet restriction for most of infancy followed by reintroduction around a year of age (11 months [IQR: 9, 12] in this population) is common practice.
Elizur et al., conducted one of the most thorough population (n > 13,000) assessments of both IgE- and non-IgE-mediated milk allergy, by first ascertaining any report of adverse reaction to milk, of which there were 381 (3%). Of those, 25 had bloody stools, 21 of whom were determined to have FPIAP due to milk, for an overall prevalence of 0.16%. We draw two observations from this study. First, the large majorty (84%) of infants with blood in stool were found to have FPIAP – other etiologies (FPIES, n = 2; actue gastroenteritis, n = 1; anal fissure, n = 1) were less common and easily excluded. Second, either rectal bleeding, an objective and readily observed source of anxiety among parents, was very rare in their population (incidence not reported), or more likely it is not a presentation that families readily perceive (unlike immediate adverse reactions) as being a reaction to milk or another food. Had our study been designed similarly: first ascertaining cases with parent-reported adverse reactions to milk, and then following up on the most likely cause of each, we would also likely find a very small number of cases of FPIAP.
Some experts have restricted analysis to those with the classic presentation of visible blood, as is suggested by some practice guidelines(10,11), and there is literature that evaluation for microscopic blood may result in false positives(25) in asymptomatic healthy populations. However, other widely cited sources recommend testing for occult blood in suspected cases. (12,26) While this practice has not been validated, likely leads to overdiagnosis, and is not supported by formal national guidelines(9-11,24), our interest here was to prospectively identify cases of FPIAP as it is being done in general pediatrics clinical practice. In our study, restricting the analysis to only those with gross blood would decrease the estimated incidence from 17% to 7%, but did not change the risk factors or feeding practices associated with FPIAP.
In addition to classic symptoms of mucous and blood in the stool, infants diagnosed with FPIAP were more likely to exhibit symptoms characteristic of upper GI tract disease such as gagging, irritability, and feeding refusal (see Figure E1 in the Online Repository) when compared to unaffected infants. This remained true even when post hoc analyses restricted the cases of FPIAP to those with gross blood or mucous in the stool (see Table E1 in the Online Repository). They did not have growth failure, severe vomiting, or severe diarrhea. We hypothesize that either these infants had other diagnoses like reflux and were incorrectly diagnosed with FPIAP, or that the allergic inflammation characteristic of FPIAP is not limited to the distal colon. Retrospective evidence shows that symptoms of FPIAP are associated with increased odds of eosinophilic esophagitis(33) suggesting a potential shared pathophysiology, which we can now prospectively evaluate. A recent report(34) suggested an association between acid suppression in infancy and subsequent childhood risk of allergic diseases, including eczema. However, we suspect that FPIAP confounded that association, given that infants with FPIAP in our cohort were both more likely to have eczema and much more likely to have been treated with acid suppression early in infancy.(35) We hypothesized, based on our retrospective association of EoE with mode of delivery and perinatal antibiotics,(33) that these factors might also increase risk of FPIAP, but that was not the case. This is, however, consistent with several other prospective cohorts which have failed to find such an association with IgE-mediated food allergy(36,37) and other allergic diseases. (38)
We found that diagnosis of FPIAP was associated with eczema and exclusive milk-based formula feeding. We found that infants fed a combination of breastmilk and formula were the least likely to be diagnosed with FPIAP. Neither restricting our analyses to those with the most severe findings (gross blood), nor the inclusion of the 16 questionable cases of MD-diagnosed FPIAP without verified GI bleeding, altered the observed associations of FPIAP with other atopic conditions and infant diet.
FPIAP is typically the first clinical presentation of food allergy with a median age of onset of one month in our cohort, often even before eczema presents or is diagnosed, suggesting that it may represent a first step of the allergic march for some infants. Eczema was associated with FPIAP in this cohort, and is a strong risk factor for IgE-mediated food allergies in other population-based cohorts.(38) A subset of children with FPIAP have been observed to develop IgE-mediated allergy to cow’s milk and other food and environmental allergens, though the strength of those associations is currently unclear.(17) The extent to which FPIAP is independently associated with IgE sensitization and/or IgE-mediated food allergy later in childhood can now be carefully evaluated in this cohort. Given that early introduction of other dietary antigens to high risk infant groups has been shown to lower subsequent food allergy risk and is now recommended,(38-42) the potential effects of sustained milk avoidance for this non-IgE-mediated food allergy should also be carefully studied. Growing observational data show that early exposure to cow’s milk is associated with lower rates of IgE-mediated milk allergy.(43,44)
There are several important limitations to this study. First, as already discussed, the diagnosis of FPIAP was made clinically following commonly referenced sources,(12) but formal national guidelines were often not followed (e.g. fecal occult blood testing was often used despite lack of validation for this indication, and confirmatory challenges recommended by the guidelines were rarely if ever performed)(9-11,24) which may have led to significant overdiagnosis of FPIAP. Second, the low number of infants exposed to perinatal antibiotics may make our study underpowered to assess this association. Third, the single recruitment center in a predominantly Caucasian and affluent community limits generalizability, however we did not find associations between disease presentation and any demographic variables. Finally, the study was not designed to evaluate the effect of infant diet on the prevention or treatment of FPIAP. Therefore, while the negative association between mixed feeding and FPIAP is suggestive of protection, this study should not be used to justify dietary recommendations. We do believe, especially given other data supporting a beneficial role of dietary antigen exposure on immune health,(38) that prospective and randomized studies to verify these findings may be warranted.
The association between mixed feeding (breastmilk and formula) and lower rates of FPIAP diagnosis in this cohort generates several hypotheses about the pathogenesis of FPIAP. We suspect that the intestinal microbiota (influenced by diet and other factors) coupled with significant oral exposure to dietary antigen (e.g. cow’s milk proteins in formula) promote infant immune development and antigen specific tolerance acquisition. The role of the intestinal microbiome in food sensitization, tolerance acquisition, and food allergy is being actively investigated,(45,46) and an early diet consisting of both breastmilk and cow’s milk formula has been shown to be associated with lower rates of IgE-mediated cow’s milk allergy.(43,44) Longitudinal microbiome, metabolome, and immune response analyses are ongoing within this cohort to prospectively evaluate these hypotheses.
Supplementary Material
HIGHLIGHTS.
What is already known about this topic?
Food protein-induced allergic proctocolitis (FPIAP) is commonly diagnosed by pediatricians leading to dietary alterations during infancy, yet its prevalence, presentation, risk associations have never been prospectively defined.
What does this article add to our knowledge?
In this prospective observational cohort study of healthy newborn infants, 17% were diagnosed with FPIAP by their pediatrician with occult or gross blood in their stool, while only 7% had gross blood. Exclusive formula feeding, eczema, and a family history of food allergies were among statistically significant risk factors for its development.
How does this study impact current management guidelines?
The high rate of clinically diagnosed FPIAP and the array of associated symptoms and treatments in this cohort identifies a need for more discerning biomarkers and suggests that interventional studies to evaluate the impact of cow’s milk exposure may be warranted.
ACKNOWLEDGEMENTS
We are grateful for the tremendous support from the entire study site staff and physicians of Pediatrics at Newton Wellesley and we’d like to extend a special thank you to all of the participating GMAP families. This work was conducted with support from Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
There are no conflicts of interest to disclose. Funding for this study was provided by the Gerber Foundation (1685-3680), the Demarest Lloyd Jr Foundation (230465), and the Food Allergy Science Initiative (229711). Y.V.V. was supported by a grant from the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (K23AI130408).
ABBREVIATIONS
- FPIAP
Food protein-induced allergic proctocolitis
- GMAP
the Gastrointestinal Microbiome and Allergic Proctocolitis Study
- IRB
Institutional Review Board
- IQR
Interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lake AM, Whitington PF, Hamilton SR. Dietary protein-induced colitis in breast-fed infants. J Pediatr. 1982December;101(6):906–10. [DOI] [PubMed] [Google Scholar]
- 2.Machida HM, Catto Smith AG, Gall DG, Trevenen C, Scott RB. Allergic colitis in infancy: clinical and pathologic aspects. J Pediatr Gastroenterol Nutr. 1994July;19(1):22–6. [DOI] [PubMed] [Google Scholar]
- 3.Goldman H, Proujansky R. Allergic proctitis and gastroenteritis in children. Clinical and mucosal biopsy features in 53 cases. Am J Surg Pathol. 1986February;10(2):75–86. [DOI] [PubMed] [Google Scholar]
- 4.Katz AJ, Twarog FJ, Zeiger RS, Falchuk ZM. Milk-sensitive and eosinophilic gastroenteropathy: similar clinical features with contrasting mechanisms and clinical course. J Allergy Clin Immunol. 1984July;74(1):72–8. [DOI] [PubMed] [Google Scholar]
- 5.Winter HS, Antonioli DA, Fukagawa N, Marcial M, Goldman H. Allergy-related proctocolitis in infants: diagnostic usefulness of rectal biopsy. Mod Pathol. 1990January;3(1):5–10. [PubMed] [Google Scholar]
- 6.Odze RD, Wershil BK, Leichtner AM, Antonioli DA. Allergic colitis in infants. J Pediatr. 1995February;126(2):163–70. [DOI] [PubMed] [Google Scholar]
- 7.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998April25;351(9111):1225–32. [PubMed] [Google Scholar]
- 8.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief. 2013May;(121):1–8. [PubMed] [Google Scholar]
- 9.Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. Vol. 55, Journal of pediatric gastroenterology and nutrition. 2012. pp. 221–9. [DOI] [PubMed] [Google Scholar]
- 10.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. 2010. pp. 1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson HA, James J, Jones S, Perry TT, Sicherer SH, Vickery BP, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014November;134(5):1016–43. [DOI] [PubMed] [Google Scholar]
- 12.Liacouras CA. Food Protein-Induced Allergic Proctocolitis of infancy. In Sicherer SH, Hoppin AG (Ed), UpToDate; 2018. Available at https://www.uptodate.com/contents/food-protein-induced-allergic-proctocolitis-of-infancy.
- 13.Fiocchi A, Brozek J, Schünemann H, Bahna SL, Berg von A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. World Allergy Organ J. 2010April;3(4):57–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venter C, Pereira B, Voigt K, Grundy J, Clayton CB, Higgins B, et al. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy. 2008March;63(3):354–9. [DOI] [PubMed] [Google Scholar]
- 15.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. Journal of Allergy and Clinical Immunology. 2007September;120(3):638–46. [DOI] [PubMed] [Google Scholar]
- 16.Elizur A, Cohen M, Goldberg MR, Rajuan N, Cohen A, Leshno M, et al. Cow's milk associated rectal bleeding: a population based prospective study. Pediatr Allergy Immunol. 2012October11;23(8):765–9. [DOI] [PubMed] [Google Scholar]
- 17.Høst A Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol. 2002December;89(6 Suppl 1):33–7. [DOI] [PubMed] [Google Scholar]
- 18.Halpern SR, Sellars WA, Johnson RB, Anderson DW, Saperstein S, Reisch JS. Development of childhood allergy in infants fed breast, soy, or cow milk. Journal of Allergy and Clinical Immunology. 1973March;51(3):139–51. [DOI] [PubMed] [Google Scholar]
- 19.Gerrard JW, MacKenzie JW, Goluboff N, Garson JZ, Maningas CS. Cow's milk allergy: prevalence and manifestations in an unselected series of newborns. Acta Paediatr Scand Suppl. 1973;234:1–21. [PubMed] [Google Scholar]
- 20.Jakobsson I, Lindberg T. A prospective study of cow's milk protein intolerance in Swedish infants. Acta Paediatr Scand. 1979November;68(6):853–9. [DOI] [PubMed] [Google Scholar]
- 21.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987May;79(5):683–8. [PubMed] [Google Scholar]
- 22.Høst A, Husby S, Osterballe O. A prospective study of cow“s milk allergy in exclusively breast-fed infants. Incidence, pathogenetic role of early inadvertent exposure to cow”s milk formula, and characterization of bovine milk protein in human milk. Acta Paediatr Scand. 1988September;77(5):663–70. [DOI] [PubMed] [Google Scholar]
- 23.Schrander JJ, van den Bogart JP, Forget PP, Schrander-Stumpel CT, Kuijten RH, Kester AD. Cow's milk protein intolerance in infants under 1 year of age: a prospective epidemiological study. Eur J Pediatr. 1993August;152(8):640–4. [DOI] [PubMed] [Google Scholar]
- 24.Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, et al. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Archives of Disease in Childhood. 2007October;92(10):902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Concha S, Cabalín C, Iturriaga C, Pérez-Mateluna G, Gomez C, Cifuentes L, et al. [Diagnostic validity of fecal occult blood test in infants with food protein-induced allergic proctocolitis]. Rev Chil Pediatr. Sociedad Chilena de Pediatría; 2018October;89(5):630–7. [DOI] [PubMed] [Google Scholar]
- 26.Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2005July;41(1):16–22. [DOI] [PubMed] [Google Scholar]
- 27.Odze RD, Bines J, Leichtner AM, Goldman H, Antonioli DA. Allergic proctocolitis in infants: a prospective clinicopathologic biopsy study. Hum Pathol. 1993June;24(6):668–74. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg M, Eisenberg E, Elizur A, Rajuan N, Rachmiel M, Cohen A, et al. Role of parental atopy in cow's milk allergy: a population-based study. Ann Allergy Asthma Immunol. 2013April;110(4):279–83. [DOI] [PubMed] [Google Scholar]
- 29.Havstad S, Wegienka G, Zoratti EM, Lynch SV, Boushey HA, Nicholas C, et al. Effect of prenatal indoor pet exposure on the trajectory of total IgE levels in early childhood. J Allergy Clin Immunol. Elsevier; 2011October;128(4):880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ege MJ, Mayer M, Normand A-C, Genuneit J, Cookson WOCM, Braun-Fahrländer C, et al. Exposure to environmental microorganisms and childhood asthma. Yearbook of Medicine. 2011February24;364(8):701–9. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009April;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- 33.Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. The Journal of Allergy and Clinical Immunology: In Practice. 2014July;2(4):475–477.e1. [DOI] [PubMed] [Google Scholar]
- 34.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood. JAMA Pediatr. 2018April2;:e180315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin V, Keet C, Yuan Q. Antibiotics and Acid-Suppressing Medications in Early Life and Allergic Disorders. JAMA Pediatr. 2018October1;172(10):989–90. [DOI] [PubMed] [Google Scholar]
- 36.Koplin JJ, Dharmage SC, Ponsonby AL, Tang MLK, Lowe AJ, Gurrin LC, et al. Environmental and demographic risk factors for egg allergy in a population-based study of infants. Allergy. 2012November;67(11):1415–22. [DOI] [PubMed] [Google Scholar]
- 37.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O'Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009May8;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin PE, Eckert JK, Koplin JJ, Lowe AJ, Gurrin LC, Dharmage SC, et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin Exp Allergy. 2015January;45(1):255–64. [DOI] [PubMed] [Google Scholar]
- 39.Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan ES, Muraro A, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Allergy, Asthma & Clinical Immunology. Allergy, Asthma & Clinical Immunology; 2015August17;:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. Yearbook of Medicine. 2016May5;374(18):1733–43. [DOI] [PubMed] [Google Scholar]
- 41.Fisher HR, Toit Du G, Bahnson HT, Lack G. The challenges of preventing food allergy: Lessons learned from LEAP and EAT. Ann Allergy Asthma Immunol. 2018September;121(3):313–9. [DOI] [PubMed] [Google Scholar]
- 42.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N Engl J Med. 2015February26;372(9):803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow“s milk protein is protective against IgE-mediated cow”s milk protein allergy. J Allergy Clin Immunol. 2010July;126(1):77–82.e1. [DOI] [PubMed] [Google Scholar]
- 44.Peters RL, Koplin JJ, Dharmage SC, Tang MLK, McWilliam VL, Gurrin LC, et al. Early exposure to cow“s milk protein is associated with a reduced risk of cow”s milk allergic outcomes. The Journal of Allergy and Clinical Immunology: In Practice. 2018September26. [DOI] [PubMed] [Google Scholar]
- 45.Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015March;45(3):632–43. [DOI] [PubMed] [Google Scholar]
- 46.Ho H-E, Bunyavanich S. Role of the Microbiome in Food Allergy. Curr Allergy Asthma Rep. 2018April5;18(4):27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.