Abstract
At present, the etiology and pathogenesis of major depressive disorder (MDD) are still not clear. Studies have found that the risk of first-degree relatives of MDD is 2–3 times that of the general population. Diffusion tensor imaging (DTI) has been previously used to explore the pathogenesis of MDD. The purpose of this study is to explore the etiology of MDD by DTI and further to explore the correlation between its clinical characteristics and the structural changes of white matter in the brain. The study included 27 first-episode, drug-naive patients with MDD, 16 first-degree relatives without MDD, and 28 healthy control subjects with no family history of MDD (HC). Results showed that the fractional anisotropy (FA) differences among the three groups were mainly in the left anterior thalamic radiation (LATR), right anterior thalamic radiation (RATR), left corticospinal tracts (LCST), forceps major (FMa), right inferior longitudinal fasciculus (RILF), and left superior longitudinal fasciculus (temporal) (LSLF(T)). Among the 6 sites, LCST, FMa, and LSLF(T) showed significant differences between MDD and First-degree relatives compared to HC. MDD patients had significant emotional symptoms, somatic symptoms, and cognitive impairment. FMa FA was significantly positively correlated with delayed memory score (r = 0.43, P = 0.031), and RILF FA was significantly negatively correlated with the FSS score (r = −0.42, P = 0.028). These results revealed that the white matter characteristics of MDD-susceptible patients were LCST, FMa, and LSLF(T) lesions, all of which may be quality indicators of MDD.
1. Introduction
Major depressive disorder (MDD) is characterized by cognitive impairments, functional disability, and mortality. In 2019, the prevalence of MDD in the Chinese population reached 6.8% [1, 2], and 15% of patients had suicidal behavior [3]. However, the pathogenesis of MDD is still unclear.
Genetic studies have shown that depression has familial clustering, and the prevalence of first-degree relatives is 2–3 times that of the general population. Having first-degree relatives with early/repeated episodes may increase the risk of MDD up to 6 times [4]. In the twin study, the heritability of MDD in males and females was 0.41 and 0.49, respectively, and it was found that the age of onset, number of relapses, comorbidities, anxiety, and clinical severity could predict the risk in relatives [5]. According to the high heritability, there are some diathologic changes in first-degree relatives that make them more susceptible to MDD. Meta-analysis of first-degree relatives of MDD patients showed significant differences in cognitive function. We proposed that cognitive impairment is a characteristic marker of familial aggregation of MDD [6]. It can be inferred that first-degree relatives of MDD may have similar characteristics, which may be related to the quality changes of the onset. Therefore, the task of exploring the clinical characteristics of the genetic rules of MDD is one of great significance.
Magnetic resonance imaging (MRI) is a safe and reliable neuroimaging technique. The commonly used MRI mainly includes functional magnetic resonance imaging (fMRI), structural magnetic resonance imaging (sMRI), and diffusion tensor imaging (DTI) [7]. The important principle of DTI is dispersion. The white matter of the brain has a fixed structure, which makes the dispersion of water molecules in each direction different, thereby resulting in an index called fractional anisotropy (FA). FA refers to the proportion of anisotropic components of water molecules in the whole dispersion tensor, and its value is between 0 and 1. Previous studies have shown that numerous changes in white matter fiber integrity are indicative of poor antidepressant efficacy [8]. These studies all showed abnormalities of corpus callosum (CC), capsula interna (CI), and superior longitudinal fasciculus (SLF) in MDD; however, without the ability to distinguish the quality change and the state change, the role they play is still unclear. Therefore, we hypothesized that MDD patients have white matter changes, some of which are quality indicators of MDD. We also hypothesized that the other parts are specific state changes that promote the occurrence of disease, and these white matter changes are closely related to clinical symptoms.
2. Materials and Methods
2.1. Participants
2.1.1. MDD
Inclusion criteria are the following: (1) first-episode, drug-naive patients with MDD admitted to the First Hospital of Shanxi Medical University; (2) 18 ≤ age ≤ 60; (3) conformance to the Diagnostic and Statistical Manual of Disorders Fourth Edition (DSM-IV) MDD diagnostic criteria and through a Structured Clinical Interview for DSM-IV TR Axis I Disorders Patient Edition (SCID-I/P) screening [9]; (4) Hamilton Depression Scale 24 (HAMD‐24) ≥ 20; (5) no regular use of antipsychotics, antidepressants, or sedative and hypnotic drugs in the two weeks before enrollment; and (6) right-handedness. Exclusion criteria are the following: (1) a history of diseases of the nervous system, major physical diseases, or endocrine diseases; (2) a history of brain injury, coma, and other diseases that may interfere with the study; (3) other medical conditions diagnosed by the DSM-IV, including a history of alcohol or drug abuse or dependence; (4) implanted metal materials, pacemakers, etc.; (5) pregnant or lactating women; and (6) a family history of manic episodes or bipolar disorder. A total of 27 cases were enrolled.
2.1.2. First-Degree Relatives
Inclusion criteria are the following: (1) biological parents, children, or siblings of above patients; (2) 18 ≤ age ≤ 60; (3) HAMD‐24 < 8; and (4) right-handedness. Exclusion criteria are the following: (1) meeting the inclusion or exclusion criteria for “MDD”; (2) severe head trauma or neonatal diseases; and (3) having a high fever convulsion in childhood or infancy. A total of 16 cases were enrolled.
2.1.3. HC
Inclusion criteria are the following: (1) 18 ≤ age ≤ 60; (2) age, gender, and education level match the above two groups; (3) HAMD‐24 < 8; and (4) right-handedness. Exclusion criteria are the following: (1) meeting the inclusion or exclusion criteria for “MDD”; (2) a clear family history of mental or neurological diseases; (3) severe head trauma or neonatal diseases; and (4) having a high fever convulsion in childhood or infancy. A total of 28 cases were enrolled.
This study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University.
2.2. Methods
2.2.1. Diagnosis and Scale Evaluation
The general demographic data of the patients were collected: gender, age, education, family history, history of tobacco/alcohol use, and substance abuse. All of the scales were evaluated by the same experienced psychological evaluator. MDD should not be observed from a single perspective but must be observed from multiple perspectives of emotional experience, physical experience, and cognition [10]. We collected the following data from MDD and HC: the HAMD-24 for the patient's condition, the Snaith-Hamilton Pleasure Scale (SHAPS) for affective symptoms, the Fatigue Severity Scale (FSS) for somatic symptoms, and the Assessment of Neuropsychological Status (RBANS) for cognitive function.
2.2.2. fMRI Scanning
The data were collected by Siemens 3.0 T MRI scanner and 12-channel phased array surface head coil in Shanxi Provincial People's Hospital. During the scan, subjects were asked to remain awake, lie flat at rest, breathe calmly, and keep their heads in a fixed position. First, an MRI plain scan of conventional structural images was performed to exclude subjects with brain organic lesions. The DTI was collected with a single spin echo planar imaging sequence, axial scanning, scanning a total of 45 continuous level, 12 diffusion sensitive gradient direction, the diffusion sensitive coefficient b = 1000, while at the same time getting an axis a scan for the best tonsure diffusion weighted imaging b = 0, repetition time/echo time (TR/TE) = 3600/90 ms, matrix = 128∗128, field of view (FOV) = 24∗24 cm, flip angle=90°, thickness = 0 mm. The scanning time was 4 minutes and 14 seconds.
2.2.3. DTI Data Processing
The original image was converted from DICOM to NIFTI by the MRIconvert software. Based on the Matlab platform, using the PANDA to process the NIFTI data, the nonbrain tissues 3 mm away from the upper and lower, front and rear, and left and right directions of the scalp were all cut. FSL software was used for scalp stripping. The head movement correction and eddy current correction were performed on the subjects' head movements to obtain the brain template and calculate FA, based on the JHU white matter tractography atlas templates and to calculate the average 20 white matters in the region of interest (ROI) FA [11]. The raw DTI data were observed by the naked eye, and no obvious artifacts were found. The average FA in 20 ROI was extracted and placed in SPSS 23.0 for statistical analysis.
2.2.4. Statistical Analysis
This study used SPSS 23.0 ANOVA was performed for age, years of education, and HAMD-24 among the three groups, and the chi-squared test was used for gender. Measurement data were expressed as mean ± SD. The test level was set at α = 0.05. P < 0.05 indicated that the difference was statistically significant. The FA extracted from PANDA was placed in SPSS 23.0 and analyzed by ANOVA, and the regions with significant differences were compared in pairs under the Least—Significant Difference (LSD). The results were considered statistically significant when P < 0.01. Through SPSS 23.0, a Two-sample T-test was used to compare the differences of SHAPS/FSS/RBANS between MDD and HC. Pearson correlation analysis was used to analyze the correlation between abnormal FA with statistical differences and clinical characteristics in MDD. The results in P < 0.05 were considered statistically significant.
3. Results
3.1. General Demographic Data
There was no statistically significant difference in gender, age, or years of education among the three groups (P < 0.05), but there was a statistically significant difference in the HAMD-24 score (P < 0.05) (see Table 1).
Table 1.
General demographic data of each group.
| Items | MDD (n = 27) | First-degree relatives (n = 16) | HC (n = 28) | F/χ2 | P |
|---|---|---|---|---|---|
| Sex (female/male) | 19/8 | 11/5 | 17/11 | 0.306 | 0.738 |
| Age | 28.92 ± 8.72 | 30.93 ± 4.15 | 26.78 ± 6.91 | 1.643 | 0.201 |
| Education (year) | 13.77 ± 2.48 | 13.56 ± 2.12 | 14.85 ± 2.42 | 2.038 | 0.138 |
| HAMD-24 | 26.92 ± 4.15 | 4.56 ± 1.71 | 5.39 ± 1.68 | 475.877 | <0.01 |
3.2. White Matter FA
3.2.1. Overall White Matters FA
There were six differences in white matter in MDD, First-degree relatives, and HC, and these included LATR, RATR, LCST, FMa, RILF, and LSLF(T) (P < 0.01) (see Table 2).
Table 2.
White matter fiber values and differences of MDD, First-degree relatives, and HC.
| Fiber | MDD FA | First-degree relatives FA | HC FA | F | t |
|---|---|---|---|---|---|
| Left anterior thalamic radiation | 0.39 ± 0.01 | 0.40 ± 0.02 | 0.41 ± 0.01 | 6.089 | 0.004∗ |
| Right anterior thalamic radiation | 0.38 ± 0.02 | 0.39 ± 0.02 | 0.40 ± 0.01 | 8.754 | 0.000∗ |
| Left corticospinal tracts | 0.56 ± 0.02 | 0.55 ± 0.02 | 0.58 ± 0.02 | 8.436 | 0.001∗ |
| Right corticospinal tracts | 0.57 ± 0.02 | 0.57 ± 0.03 | 0.58 ± 0.02 | 3.358 | 0.041 |
| Left cingulated | 0.52 ± 0.02 | 0.53 ± 0.03 | 0.54 ± 0.03 | 1.468 | 0.238 |
| Right cingulated | 0.48 ± 0.04 | 0.49 ± 0.04 | 0.48 ± 0.03 | 0.399 | 0.673 |
| Left hippocampus | 0.40 ± 0.03 | 0.41 ± 0.03 | 0.41 ± 0.03 | 0.495 | 0.611 |
| Right hippocampus | 0.37 ± 0.02 | 0.43 ± 0.05 | 0.42 ± 0.04 | 0.604 | 0.549 |
| Forceps major | 0.57 ± 0.02 | 0.57 ± 0.02 | 0.59 ± 0.01 | 7.621 | 0.001∗ |
| Forceps minor | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.45 ± 0.01 | 2.494 | 0.090 |
| Left inferior frontal occipital tract | 0.42 ± 0.02 | 0.43 ± 0.02 | 0.44 ± 0.02 | 3.363 | 0.040 |
| Right inferior frontal occipital tract | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.45 ± 0.02 | 4.820 | 0.011 |
| Left inferior longitudinal fasciculus | 0.44 ± 0.02 | 0.43 ± 0.03 | 0.44 ± 0.02 | 4.327 | 0.017 |
| Right inferior longitudinal fasciculus | 0.44 ± 0.02 | 0.45 ± 0.03 | 0.47 ± 0.02 | 6.675 | 0.002∗ |
| Left superior longitudinal fasciculus | 0.37 ± 0.02 | 0.38 ± 0.01 | 0.38 ± 0.01 | 5.235 | 0.008 |
| Right superior longitudinal fasciculus | 0.39 ± 0.02 | 0.40 ± 0.03 | 0.40 ± 0.01 | 4.813 | 0.011 |
| Left uncinate fasciculus | 0.41 ± 0.02 | 0.40 ± 0.03 | 0.41 ± 0.01 | 0.178 | 0.838 |
| Right uncinate fasciculus | 0.41 ± 0.02 | 0.41 ± 0.02 | 0.42 ± 0.02 | 2.429 | 0.096 |
| Left superior longitudinal fasciculus (temporal) | 0.47 ± 0.03 | 0.47 ± 0.03 | 0.50 ± 0.04 | 5.996 | 0.004∗ |
| Right superior longitudinal fasciculus (temporal) | 0.52 ± 0.04 | 0.53 ± 0.05 | 0.56 ± 0.05 | 4.345 | 0.017 |
∗P < 0.01.
3.2.2. MDD, First-Degree Relatives, and HC Were Compared Pair-Wise
Multiple comparisons and corrections of brain regions showed that there were significant differences between MDD/HC and First-degree relatives/HC in three regions: LCST, FMa, and LSLF(T); however, there were no significant differences between MDD/First-degree relatives. The values of MDD and First-degree relatives FA were both lower than that of the HC (see Table 3 and Figure 1).
Table 3.
Comparison between MDD, First-degree relatives, and HC.
| Fiber | MDD/HC | MDD/first-degree relatives | First-degree relatives/HC |
|---|---|---|---|
| LATR | 0.001∗ | 0.190 | 0.100 |
| RATR | 0.000∗ | 0.234 | 0.022 |
| LCST | 0.003∗ | 0.262 | 0.000∗ |
| FMa | 0.001∗ | 0.813 | 0.005∗ |
| RILF | 0.001∗ | 0.363 | 0.033 |
| LSLF(T) | 0.003∗ | 0.878 | 0.007∗ |
∗P < 0.01.
Figure 1.
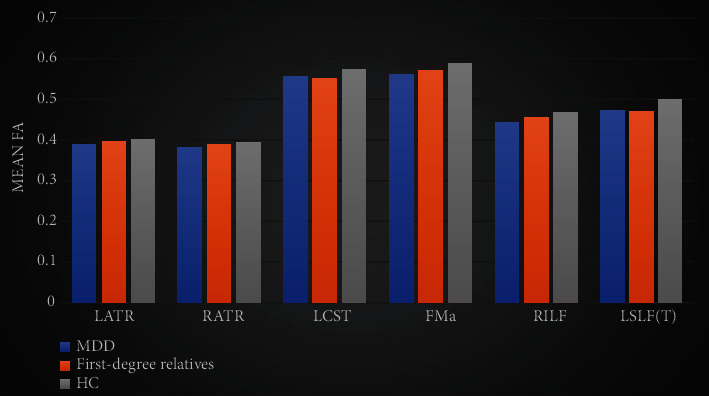
Differences in MDD, First-degree relatives and HC white matter. The bar graph represents the FA mean ± 2SD. ∗P < 0.01.
3.3. Correlation Analysis of White Matter Changes and Clinical Manifestations
3.3.1. Differences in Clinical Manifestations between MDD and HC
MDD was significantly increased in SHAPS and FSS when compared to HC. The scores of immediate memory, visual span, speech function, attention, and delayed memory in the RBANS test of MDD were significantly lower than those of HC, and the difference was statistically significant (P < 0.05) (see Table 4).
Table 4.
MDD and HC clinical symptoms difference.
| Items | MDD | HC | t |
|---|---|---|---|
| Affective symptoms | 23.15 ± 6.304 | 4.59 ± 4.29 | 0.000∗ |
| Physical symptom | 46.185 ± 13.12 | 25.84 ± 5.79 | 0.000∗ |
| Spatial span | 74.20 ± 15.16 | 96.19 ± 14.85 | 0.038∗ |
| Visual span | 90.88 ± 20.10 | 103.42 ± 13.03 | 0.033∗ |
| Speech function | 88.20 ± 17.88 | 97.27 ± 11.41 | 0.000∗ |
| Attentional function | 99.76 ± 16.01 | 119.38 ± 13.29 | 0.004∗ |
| Delayed memory | 84.12 ± 16.47 | 95.19 ± 6.87 | 0.000∗ |
∗P < 0.05.
3.3.2. Correlation between Abnormal White Matter FA and Clinical Manifestations
There was a significant positive correlation between FMa FA and delayed memory score (r = 0.43, P = 0.031), as well as a significant negative correlation between RILF FA and FSS total score (r = −0.42, P = 0.028). No significant correlation was found for the rest (see Table 5 and Figures 2 and 3).
Table 5.
Differences in clinical symptoms between MDD and HC.
| Tests | Correlation between white matter and test scores | |||||
|---|---|---|---|---|---|---|
| LATR | RATR | LCST | FMa | RILF | LSLF(T) | |
| Affective symptoms | 0.17 | 0.06 | 0.22 | 0.22 | -0.13 | 0.03 |
| Physical symptom | -0.03 | -0.10 | -0.12 | -0.20 | -0.42∗ | 0.01 |
| Spatial span | 0.13 | 0.05 | 0.06 | -0.37 | 0.18 | -0.16 |
| Visual span | -0.22 | -0.15 | -0.20 | 0.09 | 0.17 | -0.04 |
| Speech function | -0.11 | -0.20 | -0.03 | 0.22 | 0.21 | -0.17 |
| Attentional function | -0.79 | -0.34 | -0.03 | 0.13 | 0.21 | -0.17 |
| Delayed memory | 0.24 | 0.35 | 0.15 | 0.43∗ | 0.34 | -0.05 |
| Severity | 0.22 | 0.15 | 0.36 | 0.21 | 0.27 | -0.03 |
∗P < 0.05.
Figure 2.
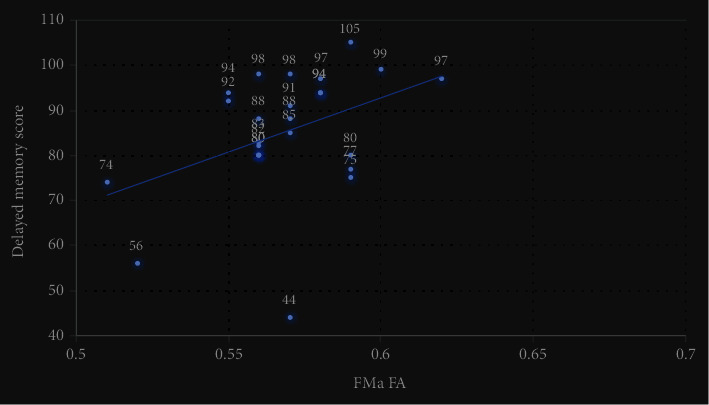
Dispersion of FMa FA and delayed memory score. FMa FA was positively correlated with a delayed memory score (r = 0.43, P = 0.031).
Figure 3.
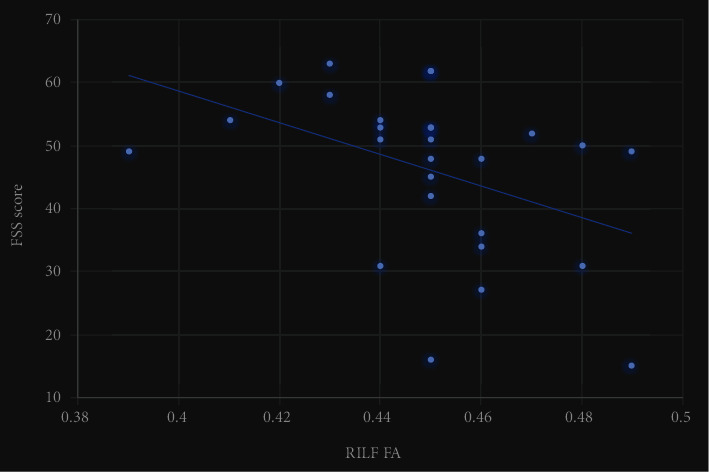
Dispersion of RILF FA and FSS. RILF FA was negatively correlated with total FSS score (r = −0.42, P = 0.028).
4. Discussion
4.1. About the DTI
This study used the JHU white matter tractography atlas, based on the parameters of ROI. The JHU white matter tractography atlas divides white matter fiber tracts into 20 regions. Although this method is less sensitive than voxel-based and white matter skeleton-based, the obtained results are reliable. At the same time, rather than just carrying out correlation analysis on a certain lump of differences, our study used a ROI-based analysis method to ascertain that each brain region had clear anatomical significance [12].
4.2. About the Results
These results indicated that while LCST (P = 0.262), FMA (P = 0.813), and LSLF(T) (P = 0.878) had the same white matter characteristics in patients with MDD as in first-degree relatives, they were not found in healthy controls. Therefore, we speculate that the impairment of LCST, FMA, and LSLF(T) is a quality indicator of MDD and that the first-degree relatives of MDD patients need more state changes to develop the disease. We also found that FMa was associated with cognitive function and that RILF was associated with physical symptom.
ATR is an important component of the cortical-thalamic-cortical circuit and is mainly involved in the execution and planning of complex behaviors, which can explain why ATR changes lead to the onset of MDD [13]. Our study showed the presence of bilateral ATR damage in MDD. Previous studies showed that the FA decrease of ATR was also found in bipolar disorder (BD), indicating that ATR plays an important role in the onset of affective disorders [14], though this may be related to the different participants. The FA reduction in LCST has been widely reported in previous studies on BD, which is similar to the findings located in MDD in our study [15]. Chhetry et al. found that MDD remisses were associated with increased LCST FA [16]. Decreased FA of CST were also found in studies of patients with schizophrenia [17, 18]. There were also studies inconsistent with our results. Sacchet Matthew et al. obtained the MDD bilateral CST with a higher FA [19]. Meta-analysis showed that FMa reduction was a common feature of affective disorders [20]. Studies on MDD have also found that FA of FMa may be related to anhedonia [21], but our study did not find that, and this may be related to the heterogeneity of samples and different data processing methods. Previous studies have found ILF changes in MDD. Maurizio et al. found that ILF was significantly abnormal in MDD [22, 23]. FA abnormalities in LILF also exist in adolescent depression [24]. Reduced FA in LILF was found in all psychiatric disorders without distinguishing the disease types, and this change was related to the severity of the disease [25]. Our research also shows that first-degree relatives as high-risk groups have LILF anomaly, this may be related to the MDD recurrence. Studies have shown that the FA value of LSLF in MDD decreases, which is similar to our results [26]. FA changes in SLF may be related to the NETRIN1 signaling pathway [27]. Reduced FA in RSLF was found in individuals with a family history of BD [28], thereby suggesting a degree of heritability in RSLF changes. A previous review indicated that a lower FA value of ILF in Parkinson's disease patients leads to poor cognitive function, but our study did not show similar results [29].
There are many shortcomings in this study: the sample size should be expanded, and multiple methods were not used to verify the results. Of course, we did find brain imaging changes associated with the onset of MDD, and this provides the foundation for further research work.
Acknowledgments
This study was supported by the National Natural Science Youth Fund Project (81601192 and 81701345), National Natural Science Foundation of China (81471379), National Key Research and Development Program of China (2016YFC1307103), Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, and 136 Medical Rejuvenation Project of Shanxi Province.
Contributor Information
Kerang Zhang, Email: atomsxmu@vip.163.com.
Ning Sun, Email: sunning@sxmu.edu.cn.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Ziwei Liu and Lijun Kang are co-first authors.
References
- 1.Huang Y., Wang Y., Wang H., et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. The Lancet Psychiatry. 2019;6:211–224. doi: 10.1016/s2215-0366(18)30511-x. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R. C., Bromet E. J. The epidemiology of depression across cultures. Annual Review of Public Health. 2013;34(1):119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y. W., Dilsaver S. C. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry. 1996;39(10):896–899. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]
- 4.Weissman M. M. Psychiatric disorders in the relatives of probands with affective disorders. Archives of General Psychiatry. 1984;41(1):13–21. doi: 10.1001/archpsyc.1984.01790120015003. [DOI] [PubMed] [Google Scholar]
- 5.Kendler Kenneth S., Henrik O., Paul L. The genetic epidemiology of treated major depression in Sweden. American Journal of Psychiatry. 2018;175(11):1137–1144. doi: 10.1176/appi.ajp.2018.17111251. [DOI] [PubMed] [Google Scholar]
- 6.MacKenzie Lynn E., Rudolf U., Barbara P. Cognitive performance in first-degree relatives of individuals with vs without major depressive disorder. JAMA Psychiatry. 2019;76(3):297–305. doi: 10.1001/jamapsychiatry.2018.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambeitz J., Cabral C., Sacchet M. D., et al. Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biological Psychiatry. 2017;82(5):330–338. doi: 10.1016/j.biopsych.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Bollettini I., Poletti S., Locatelli C., et al. Disruption of white matter integrity marks poor antidepressant response in bipolar disorder. Journal of Affective Disorders. 2015;174:233–240. doi: 10.1016/j.jad.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Validity S. I. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II) Comprehensive Handbook of Psychological Assessment. 2004;2 [Google Scholar]
- 10.Christopher H. Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychological Bulletin. 2015;141:311–363. doi: 10.1037/a0038101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua K., Zhang J., Wakana S., et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Z., Zhong S., Xu P., He Y., Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gemmell C. Plasticity in the projection from the anterior thalamic nuclei to the anterior cingulate cortex in the rat _in vivo_ : paired-pulse facilitation, long-term potentiation and short-term depression. Neuroscience. 2002;109(3):401–406. doi: 10.1016/S0306-4522(01)00554-1. [DOI] [PubMed] [Google Scholar]
- 14.Niida R., Yamagata B., Niida A., Uechi A., Matsuda H., Mimura M. Aberrant anterior thalamic radiation structure in BD: a diffusion tensor tractography study. Frontiers in Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise T., Radua J., Nortje G., Cleare A. J., Young A. H., Arnone D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biological Psychiatry. 2016;79(4):293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Chhetry B. T., Hezghia A., Miller J. M., et al. Omega-3 polyunsaturated fatty acid supplementation and white matter changes in major depression. Journal of Psychiatric Research. 2016;75:65–74. doi: 10.1016/j.jpsychires.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamah D., Ji A., Rutlin J., Shimony J. S. White matter integrity in schizophrenia and BD: tract- and voxel-based analyses of diffusion data from the Connectom scanner. NeuroImage: Clinical. 2019;21 doi: 10.1016/j.nicl.2018.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repple J., Meinert S., Grotegerd D., et al. A voxel-based diffusion tensor imaging study in unipolar and bipolar depression. Bipolar Disorders. 2017;19:23–31. doi: 10.1111/bdi.12465. [DOI] [PubMed] [Google Scholar]
- 19.Sacchet M. D., Prasad G., Foland-Ross L. C., et al. Structural abnormality of the corticospinal tract in major depressive disorder. Biology of Mood & Anxiety Disorders. 2014;4 doi: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins L. M., Barba A., Campbell M., et al. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. NeuroImage: Clinical. 2016;12:1022–1034. doi: 10.1016/j.nicl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X.-h., Wang Y., Wang D.-f., et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Research: Neuroimaging. 2017;264:29–34. doi: 10.1016/j.pscychresns.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Bergamino M., Kuplicki R., Victor T. A., Cha Y.-H., Paulus M. P. Comparison of two different analysis approaches for DTI free-water corrected and uncorrected maps in the study of white matter microstructural integrity in individuals with depression. Human Brain Mapping. 2017;38:4690–4702. doi: 10.1002/hbm.23694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olvet Doreen M., Lauren D., Fang-Cheng Y., et al. A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depression and Anxiety. 2016;33(1):56–65. doi: 10.1002/da.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessette K. L., Nave A. M., Caprihan A., Stevens M. C. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging and Behavior. 2014;8(4):531–541. doi: 10.1007/s11682-013-9274-8. [DOI] [PubMed] [Google Scholar]
- 25.Hatton S., Lagopoulos J., Hermens D., Hickie I., Scott E., Bennett M. White matter tractography in early psychosis: clinical and neurocognitive associations. Journal of Psychiatry & Neuroscience. 2014;39(6):417–427. doi: 10.1503/jpn.130280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota M., Noda T., Sato N., et al. White matter abnormalities in major depressive disorder with melancholic and atypical features: a diffusion tensor imaging study. Psychiatry and Clinical Neurosciences. 2015;69:360–368. doi: 10.1111/pcn.12255. [DOI] [PubMed] [Google Scholar]
- 27.Barbu Miruna C., Yanni Z., Xueyi S. Association of whole-genome and NETRIN1 signaling pathway-derived polygenic risk scores for major depressive disorder and white matter microstructure in the UK Biobank. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4 doi: 10.1016/j.bpsc.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versace A., Ladouceur C. D., Graur S., et al. Diffusion imaging markers of bipolar versus general psychopathology risk in youth at-risk. Neuropsychopharmacology. 2018;43(11):2212–2220. doi: 10.1038/s41386-018-0083-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haghshomar M., Dolatshahi M., Sherbaf F. G., Moghaddam H. S., Shandiz M. S., Aarabi M. H. Disruption of inferior longitudinal fasciculus microstructure in Parkinson's disease: a systematic review of diffusion tensor imaging studies. Frontiers in Neurology. 2018;9 doi: 10.3389/fneur.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


