Abstract
Background
Acute kidney injury is a common disorder worldwide, occurring in more than 13 million per year, 85% of whom live in developing countries. The high incidence of acute kidney injury among type 2 diabetic patients is a major cause of morbidity and mortality. There is limited data that address the incidence and predictors of acute kidney injury to apply evidence-based interventions in developing countries including Ethiopia specifically in the study area.
Methods
Institution-based retrospective follow-up study was conducted among 420 adults with newly diagnosed type 2 diabetes patients from January 1, 2014, to December 31, 2019. Log rank test and Kaplan–Meier curve were used to compare different categories of survival probability. In a multivariable analysis, variable having a p-value <0.05 in the Cox, proportional hazard model was considered as independent predictors.
Results
Overall, 19.76% (95% CI; 16.2–23.8) of the study population developed acute kidney injury, with a median follow-up period of 30.75 months. Congestive heart failure [adjusted hazard ratio (AHR): 2.89 (95% CI; 1.62, 5.13)], chronic kidney disease [AHR: 2.92 (95% CI; 1.56, 5.48)], hypertension [AHR: 2.87 (95% CI; 1.20, 6.90)], and diabetic nephropathy [AHR: 2.04 (95% CI; 1.13, 3.68)] were found to be predictors of acute kidney injury.
Conclusion
The incidence of acute kidney injury among type 2 diabetes patients was high in the study area. In patients with hypertension congestive heart failure, chronic kidney disease, and diabetic nephropathy efforts should be made to diagnose AKI early and treat it – in addition to better control accordingly among type 2 diabetes mellitus patients.
Keywords: incidence, acute kidney injury, predictors, type 2 diabetes, Gondar, Ethiopia
Background
Diabetes mellitus (DM) is defined as a metabolic disease presented with the chronic raising of blood glucose with the disorder of carbohydrate, fat, and protein metabolism due to problems in insulin production, insulin function, or both.1,2
The overall worldwide magnitude of diabetes in 2019 was estimated to be 9.3% (463 million individuals), rising to 10.2% (578 million individuals) by 2030 and 10.9% (700 million) by 2045 in adults aged 20–79 years.3 A systematic review was done in 2018 showed that the magnitude of type 2 DM in Africa is estimated to be 4.83%.4 Studies conducted in Ethiopia showed that the magnitude of type 2 DM in Ethiopia was found to be 5.3%and complications associated with increasing type 2 DM burden are the major causes of morbidity and premature mortality with the consequence of negative economic impact resulted in a public health challenge.5
Acute kidney injury (AKI), or acute renal failure previously, is characterized by rapid failure of kidney function and is a broad clinical syndrome encompassing various etiologies, including pre-renal azotemia, acute tubular necrosis, acute interstitial nephritis, and acute glomerular renal diseases.6 AKI is diagnosed by a fast increment in serum Creatinine (Cr), also reflected as a quick diminishing in glomerular filtration rate, within a short time. Peoples living with DM are at more risk for AKI than those without diabetes, mainly type 2 DM is one of the major risk factors for the occurrence of AKI.5 Peoples living with type 2 diabetes are eight times more likely to develop acute kidney injury as compared to patients without diabetes.7
Acute kidney injury is a serious disease globally affecting greater than 13 million individuals annually, 85% are living in developing countries.8 International Society of Nephrology’s initiative in Asia showed that the magnitude of AKI among type 2 DM ranges from 19% to 28%.9 A meta-analysis conducted in Africa revealed that the incidence of AKI among type 2 DM patients was around 32% in Africa.10 In a retrospective follow-up study conducted in Harari Region, East Ethiopia, from 2013 to 2017, the total incidence rate of AKI among type 2 DM patients was 14.5%.11
Many studies suggested that the predictors of AKI among type 2 DM patients are old age, congestive heart failure (CHF), chronic kidney disease (CKD), hypertension (HTN), smoking, alcohol consumption, diabetic nephropathy (DN), infections, aminoglycosides, diabetic ketoacidosis, non-steroidal anti-inflammatory drugs (NSAIDs), long duration of DM, diuretics and beta-blockers and delay of follow-up.5
Acute kidney injury is common and its burden has risen over the last two decades among type 2 DM patients.10 Acute kidney injury among type 2 DM patients is associated with significantly increased morbidity and mortality, increased hospitalization days and the risk of hospital-acquired infections, a greater requirement for hospitalization and post-hospital care, and costs need for health-care service.5 The high burden of AKI in type 2 diabetic patients is a significant reason for prolonged hospitalization, kidney failure with a great requirement for dialysis, inability to recover to initial renal function, development, and progression of chronic kidney disease, and finally death.10,12
Increased burden of AKI in type 2 DM patients facilitates harmful consequences leading to end-stage kidney disease (ESKD).13 International Society of Nephrology set a goal of eliminating preventable deaths from AKI by 2025, implementation of this program in developing countries presents major challenges because of the limited data addressing the incidence and causes of AKI in developing countries including Ethiopia and the limited health-care resources to diagnose and treat AKI.8
The available literature in Ethiopia indicated that the Incidence of AKI among type 2 DM patients is becoming significantly increasing and risk factors of AKI are different in different settings. Despite its burden and variability of causes, there is limited data related to the proposed study in the study area to address the goal of the International Society of Nephrology and to apply evidence-based interventions for type 2 DM patients suffer from AKI to reduce its burden.
Although the International Society of Nephrology set a goal of eliminating preventable deaths from AKI by 2025, implementing this program in developing countries presents major challenges because of the limited data that address the incidence and predictors of AKI in developing countries including Ethiopia specifically in the study area.
The high morbidity and mortality associated with AKI in Ethiopia represent a major challenge to the health of the community. Quantifying the burden of the disease and early detection of the risk factors is paramount in the prevention of AKI results reduction of the growing burden of end-stage kidney disease and fewer incidences of dialysis dependency and mortality. Determining the risk factors of AKI will help health-care providers to identify high-risk patients for urgent management including more intensive monitoring results in the reduction of health-care costs related to the care needed and reduce the rapid progression of AKI to end-stage kidney disease which needs continuous renal replacement therapies to survive.
Despite the growing incidence of AKI worldwide, there is little data on the burden and predictors of AKI among adult type 2 DM patients in Ethiopia including in the study area. Therefore, conducting this study provides information on the incidence and predictors of AKI among adults with type 2 DM patients. Also, the results of this study provide information for health-care providers, health institutions, health administrative offices, and policymakers to maximize efforts on the prevention and risk minimization of AKI in type 2 DM patients and death due to complications through strict follow-up and regular monitoring of their health conditions mainly related to kidney function and also this study used as baseline information for further study.
Methods
Study Design
An institution-based retrospective follow-up study was conducted at chronic follow-up clinic of University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia among newly diagnosed type 2 diabetes patients who are enrolled between January 1, 2014, and December 31, 2019.
Study Area and Period
The study was conducted at the chronic follow-up clinic of the University of Gondar Comprehensive Specialized Hospital, Northwest, Ethiopia, which is found in Gondar town. Gondar town is located in the North Gondar zone of Amhara Nation Regional State. Gondar town is located 738 km from Addis Ababa, the capital city of Ethiopia, and 113 km from Bahir Dar, the capital city of Amhara Nation Regional State. Based on the 2007 national census conducted by the Central Statistical Agency of Ethiopia (CSA), Gondar had a total population of 207,044, of whom 98,120 were men and 108,924 women.14 Gondar town has one Comprehensive Specialized Hospital, eight public health centers. The hospital has more than 500 beds capacity and serves as a tertiary level referral center for over five million people in and around Gondar town. The hospital gives different inpatient and outpatient services, including chronic clinic follow-up. Around 24,552 patients have chronic disease follow-up per year and the hospital has to provide DM follow-up service for around 8800 DM patients who come from different areas, and from this figure around 5000 are adult type 2 DM adults aged 18 years and above. The study was conducted from February 15 - March 15, 2020, among adults with newly diagnosed type 2 diabetes patients.
Operational Definitions
Newly Diagnosed Type 2 Diabetic (T2D) Patients–Patients who were diagnosed with Type 2 Diabetes after December 31, 2014.
Acute Kidney Injury: This is a rapid decrease in kidney function characterized by an increase in Serum Creatinine (SCr) by ≥0.3mg/dl (≥26.5μmol/L) versus baseline or increase in SCr ≥1.5 times baseline value or based on the clinical decision of the physician.11,15–17
Event: Was the development of AKI.
Censored: Patients who were not experiencing AKI until the end of the study or died or lost to follow up before experiencing AKI within the study period.
Data Collection Procedures and Data Quality Control
The study used secondary data. A data extraction checklist was prepared to collect the data. The checklist contains socio-demographic, behavioral, baseline clinical, and treatment-related factors. Forms used for laboratory requests, follow-up cards, DM registration logbooks, and patient cards were reviewed. All newly diagnosed type 2 diabetes patients aged 18 years and above and those who are registered into the adult chronic clinic follow-up between January 1, 2014, and December 31, 2019, were included in the study but Patients initially present with AKI, or no baseline record (serum Creatinine) or patients who were missing key predictors such as hypertension, chronic kidney disease, congestive heart failure, and diabetic nephropathy were excluded. The outcome of each patient was dichotomized as censored or develops AKI. For data quality, a checklist from a study conducted in Ethiopia was used. The data collection checklist was pre-tested to check the completeness of data items before the actual data collection. The training was given to data collectors for the description of the questionnaire and the way they collect data from the patient chart. Each component of the checklist was discussed clearly for data collectors. The data collection process was monitored closely by the supervisor. Finally, the completeness of the checklist was checked.
Data Processing and Analysis
Data were coded and entered into Epi info version 7.2.2.6 by the principal investigator and was exported to Stata 14 for analysis. A statistical summary was used to describe socio-demographic, behavioral, clinical, and treatment-related variables of the study. The occurrence of AKI Incidence was calculated and the rate was calculated for the study period. AKI incidence rate was calculated by dividing the numbers of new AKI cases occurring during the follow-up period to total person-time observation and expressed as per 100 person-year observation. Log rank test and Kaplan–Meier curve were used to compare different categories of survival probability.
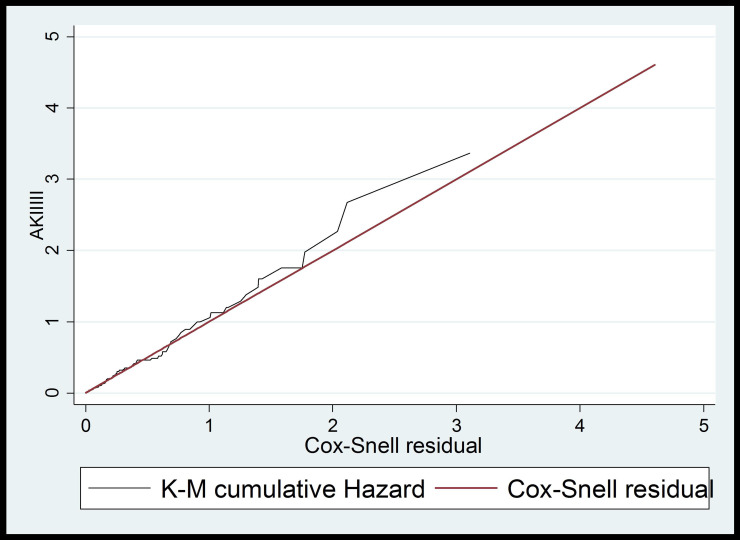
PH assumption was checked graphically and with Schoenfeld residual test (p-value = 0.2333) and the Goodness-of-fit of the model was assessed by using the Cox-Snell residual technique. Multicollinearity was checked using the variance inflation factor (VIF). After applying bivariable analysis, those variables with a p-value < 0.2 were entered into the multivariable Cox proportional hazard model to identify predictors of AKI. 95% confidence interval of hazard ratio was computed and variable having p-value < 0.05 in the multivariable Cox proportional hazards model was considered as independent predictors of AKI.
Results
Baseline Characteristics of Study Participants
Out of the total of 420 newly diagnosed type 2 DM patients, 252 (60%) were males. The median and standard deviation of the age of the study participants was 50 ±13.67. Among the study participants 243 (57.9%were urban dwellers. Among study participants, 24 (5.71%) had a history of smoking. About 31.4% of participants had a history of alcohol use. About 26.67% of participants had a family history of DM. The median baseline serum Creatinine was 0.81 mg/dl. Regarding the clinical factors that were identified about 54.05% had a history of hypertension, about 25% had congestive heart failure, 16.20% had a history of diabetic nephropathy, about 9.05% had chronic kidney disease and about 57.86% experienced different types of infections (Table 1).
Table 1.
Socio-Demographic, Clinical and Treatment-Related Factors of Adults with Newly Diagnosed Type 2 Diabetes Patients at Chronic Follow-Up Clinic of University of Gondar Comprehensive Specialized Hospital, Northwest, Ethiopia, 2020
| Variable | Category | Frequency | Percent (%) |
|---|---|---|---|
| Sex | Male | 252 | 60 |
| Female | 168 | 40 | |
| Age | < 40 | 109 | 25.95 |
| 40–49 | 77 | 18.33 | |
| 50–59 | 97 | 23.10 | |
| 60–69 | 86 | 20.48 | |
| 70–79 | 44 | 10.48 | |
| ≥80 | 7 | 1.67 | |
| Marital status | Single | 27 | 6.43 |
| Married | 326 | 78.10 | |
| Divorced | 26 | 6.19 | |
| Widowed | 41 | 9.76 | |
| Residence | Urban | 243 | 57.9 |
| Rural | 177 | 42.1 | |
| Occupation | Gov’t employee | 73 | 17.38 |
| Private work | 91 | 21.67 | |
| Farmer | 85 | 20.24 | |
| Unemployed | 12 | 2.86 | |
| Housewife | 100 | 23.81 | |
| Retired | 51 | 12.14 | |
| Others | 8 | 1.90 | |
| Hypertension | Yes | 232 | 55.05 |
| No | 188 | 45.95 | |
| Congestive heart failure | Yes | 105 | 25 |
| No | 315 | 75 | |
| Chronic kidney disease | Yes | 38 | 9.05 |
| No | 382 | 90.95 | |
| Infection | Yes | 243 | 57.86 |
| No | 177 | 42.14 | |
| Stroke | Yes | 33 | 7.9 |
| No | 387 | 92.1 | |
| Diabetic nephropathy | Yes | 68 | 16.2 |
| No | 352 | 83.8 | |
| Diabetic foot ulcer | Yes | 82 | 19.5 |
| No | 338 | 80.5 | |
| Diabetic retinopathy | Yes | 34 | 8.1 |
| No | 386 | 91.9 | |
| Diabetic ketoacidosis | Yes | 86 | 20.5 |
| No | 334 | 79.5 | |
| Family history of DM | Yes | 112 | 26.7 |
| No | 308 | 73.3 | |
| Total cholesterol, mg/dl | < 200 | 300 | 71.43 |
| ≥ 200 | 120 | 28.57 | |
| Triglyceride, mg/d | < 150 | 303 | 72.14 |
| ≥ 150 | 117 | 27.86 | |
| LDL cholesterol, mg/dl | < 100 | 241 | 57.38 |
| ≥ 100 | 179 | 42.62 | |
| HDL cholesterol, mg/dl | < 40 | 169 | 40.24 |
| ≥ 40 | 251 | 59.76 | |
| Beta-blockers | Yes | 31 | 7.4 |
| No | 389 | 92.6 | |
| Calcium channel blockers | Yes | 102 | 24.3 |
| No | 318 | 75.7 | |
| ACE inhibitor/ARBS | Yes | 175 | 41.7 |
| No | 245 | 58.3 | |
| Diuretics | Yes | 78 | 18.6 |
| No | 342 | 81.4 | |
| Aminoglycosides exposure | Yes | 36 | 8.6 |
| No | 384 | 91.4 | |
| NSAIDs | Yes | 113 | 26.9 |
| No | 303 | 73.1 | |
| Statin exposure | Yes | 192 | 45.7 |
| No | 228 | 54.3 |
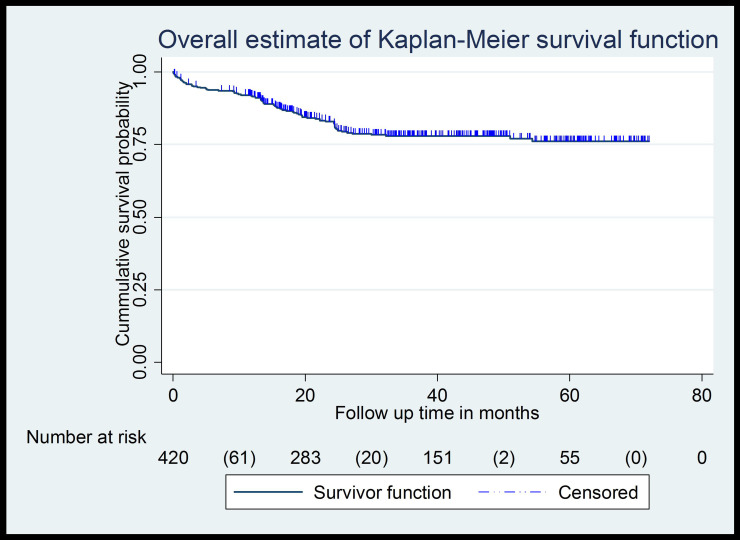
A total of 420 patients were followed for 1150.33 person-years. The median follow-up period of patients to experience the first episode of AKI was 30.75 months (19.56SD) with a minimum and maximum of 0.0333 and 71.867 months follow-up, respectively. During the follow-up, a total of 83 patients developed AKI. 19.76% (95% CI; 16.2–23.8) developed acute kidney injury. Overall, incidence density was 7.22 per 100 person-year observation (Figure 1).
Figure 1.
Kaplan–Meier curve of AKI-free survival probability among type 2 diabetes patients at chronic follow-up clinic of University of Gondar comprehensive specialized hospital from January 1, 2014, to December 31, 2019.
Predictors of Acute Kidney Injury
In bivariable analysis variables having a P-value <0.2 were entered in a multivariable Cox proportional hazards model to identify predictors of acute kidney injury. The bivariable Cox regression analysis showed that alcohol consumption, residence, smoking, congestive heart failure, chronic kidney disease, hypertension, stroke, diabetic retinopathy, family history of DM, statin exposure, diabetic nephropathy, infections, aminoglycosides, diabetic ketoacidosis, non-steroidal anti-inflammatory drugs, ACE inhibitors, calcium channel blockers, diuretics and beta-blockers were significant predictors of AKI. However, after adjusting for confounders in multivariable analysis using Cox regression model Congestive heart failure [AHR: 2.89 (95% CI; 1.62, 5.13)], chronic kidney disease [AHR: 2.92 (95% CI; 1.56, 5.48)], hypertension [AHR: 2.87 (95% CI; 1.20, 6.90)], and diabetic nephropathy [AHR: 2.04 (95% CI; 1.13, 3.68)] were found to be predictors of acute kidney injury among type 2 adult DM patients (Table 2 and Figure 2).
Table 2.
Cox Regression Analysis of Predictors of Acute Kidney Injury Among Cohorts of Type 2 Diabetes Patients at Chronic Follow-Up Clinic of University of Gondar Comprehensive Specialized Hospital, from January 1, 2014, to December 31, 2019 (n= 420)
| Variables | Survival Status | CHR(95% CI) | Adjusted AHR(95% CI) | |
|---|---|---|---|---|
| Event | Censored | |||
| Residence | ||||
| Urban | 55 | 188 | 1.5(0.95, 2.36) | 0.33(0.78, 2.17) |
| Rural | 28 | 149 | 1.00 | 1.00 |
| Smoking habit | ||||
| Never smoker | 10 | 14 | 0.45(0.23, 0.86) | 1.06(0.49, 2.30) |
| Smoker | 73 | 323 | 1.00 | 1.00 |
| Alcohol use | ||||
| Yes | 44 | 88 | 2.746(1.783, 4.23) | 1.60(0.97, 2.65) |
| No | 39 | 249 | 1.00 | 1.00 |
| Hypertension | ||||
| Yes | 73 | 154 | 7.08(3.65, 13.71) | 2.87(1.20, 6.90)* |
| No | 10 | 181 | 1.00 | 1.00 |
| Congestive heart failure | ||||
| Yes | 53 | 52 | 6.61(4.22, 10.37) | 2.89(1.62, 5.13)* |
| No | 30 | 285 | 1.00 | 1.00 |
| Chronic kidney disease | ||||
| Yes | 30 | 8 | 9.03(5.71, 14.27) | 2.92(1.56, 5.48)* |
| No | 53 | 329 | 1.00 | 1.00 |
| Infection | ||||
| Yes | 68 | 175 | 3.71(2.12, 6.50) | 1.82(0.95, 3.52) |
| No | 15 | 162 | 1.00 | 1.00 |
| Stroke | ||||
| Yes | 14 | 19 | 2.71(1.53, 4.82) | 1.85(0.95, 3.58) |
| No | 69 | 318 | 1.00 | 1.00 |
| Diabetic nephropathy | ||||
| Yes | 43 | 25 | 7.13(4.62, 10.99) | 2.04(1.13, 3.68)* |
| No | 40 | 312 | 1.00 | 1.00 |
| Diabetic ketoacidosis | ||||
| Yes | 24 | 62 | 1.83(1.14, 2.95) | 1.58(0.91, 2.73) |
| No | 59 | 275 | 1.00 | 1.00 |
| Diabetic retinopathy | ||||
| Yes | 14 | 20 | 2.27(1.28, 4.04) | 0.80(0.38, 1.67) |
| No | 69 | 317 | 1.00 | 1.00 |
| Diabetic foot ulcer | ||||
| Yes | 26 | 56 | 2.03(1.28, 3.23) | 1.10(0.61, 1.97) |
| No | 57 | 281 | 1.00 | 1.00 |
| Family history of DM | ||||
| Yes | 31 | 81 | 1.84(1.18, 2.88) | 0.79(0.46, 1.36) |
| No | 52 | 256 | 1.00 | 1.00 |
| Beta blockers | ||||
| Yes | 13 | 18 | 2.59(1.43, 4.69) | 1.05(0.53, 2.08) |
| No | 70 | 319 | 1.00 | 1.00 |
| Calcium channel blocker | ||||
| Yes | 41 | 61 | 3.70(2.40, 5.70) | 1.22(0.72, 2.08) |
| No | 42 | 276 | 1.00 | 1.00 |
| ACE inhibitor/ARBs | ||||
| Yes | 51 | 124 | 2.37(1.52, 3.69) | 0.85(0.48, 1.48) |
| No | 32 | 213 | 1.00 | 1.00 |
| Diuretics | ||||
| Yes | 25 | 53 | 1.96(1.22, 3.13) | 0.66(0.37, 1.17) |
| No | 58 | 284 | 1.00 | 1.00 |
| Aminoglycosides | ||||
| Yes | 14 | 21 | 2.24(1.26, 3.97) | 1.14(0.59, 2.22) |
| No | 69 | 315 | 1.00 | 1.00 |
| NSAIDs | ||||
| Yes | 41 | 72 | 2.83(1.84, 4.35) | 1.17(0.64, 2.14) |
| No | 42 | 265 | 1.00 | 1.00 |
| Statin exposure | ||||
| Yes | 53 | 139 | 2.24(1.43, 3.51) | 0.94(0.51, 1.72) |
| No | 30 | 197 | 1.00 | 1.00 |
Note: *Significant at p<0.05.
Figure 2.
Cox-Snell residuals for Cox- regression PH models of newly diagnosed type 2 diabetes patients at chronic follow-up clinic of University of Gondar comprehensive specialized hospital, from January 2014 to December 2019.
Discussion
Acute kidney injury is a serious problem and the leading cause of morbidity and mortality among adults with type 2 diabetes patients. This study investigated the incidence and predictors of acute kidney injury among type 2 DM patients at the University of Gondar Comprehensive Specialized Hospital, Ethiopia. A total of four factors were identified as the predictors of AKI in type 2 diabetes patients. These factors included hypertension, chronic kidney disease, congestive heart failure, and diabetic nephropathy. The current study showed that the cumulative incidence of AKI among type 2 diabetes patients was 19.76% (95% CI; 16.2–23.8) with a median follow-up period of 30.75 months. The finding of this study was in line with a study conducted in Scotland at 19.7%,17 in Cameroon at 17.6%,18 in Congo at 20.4%,19 and South Africa at 17.8%.20
However, the finding of this study revealed that the cumulative incidence was lower than in a study done in Pakistan (27.9%), Southern India (34.7%) Egypt (23.7%), and the USA (29%) the discrepancy might be, due to the difference in sampling method, socio-economic status, study setting, study design, duration of follow-up time, and sample size.21 The result is lower than a cohort study done in Brazil which shows the cumulative incidence found to be 28.2%, the discrepancy might be, due to the difference in study design (prospective study), socio-economic difference, the difference in sampling method, and study area that was conducted in Brazil may increase the incidence cases by the availability of specialized health profession with a special diagnostic tool.22 The result is lower than a retrospective follow-up study conducted in England indicated the cumulative incidence was 27%, the variation might be, due to difference in demographic characteristics of study participants (age>65 years), sample size, in median follow-up time, and socio-economic status.5
The finding of this study was higher than the findings of studies done in India 11.22%, the variation might be, due to the presence of quality of care delivered for type 2 DM patients might contribute to a lower incidence of acute kidney injury, the difference in study design with short duration of follow-up study (prospective 1 year follow-up study) and contextual differences between the two countries.23 The result is higher than a retrospective follow-up study conducted in Ethiopia 14.5%, the difference might be, the difference in sample size, outcome ascertainment, and short follow-up the time used (5-year multi-center retrospective study).11 The result also is higher than a study done in Iran 16.4%, the inconsistency might be due to difference in late diagnosis and initiation of follow up of diabetic patients, poor quality in health-care systems and lack of strict monitoring of complications in those African countries including Ethiopia may increase the incidence of AKI and due to contextual differences present between developing and developed countries.24
This study assessed the risk of acute kidney injury related to socio-demographic, clinical, and treatment-related characteristics of the patients based on the records taken from their medical follow-up chart. Factors hypertension, congestive heart failure, diabetic nephropathy, and chronic kidney disease were found to be significant predictors of acute kidney injury. In this study, we have found that hypertension was independently associated with AKI. Patients with hypertension had a 2.87 times higher risk to develop AKI than patients without hypertension [AHR: 2.87 (95% CI; 1.20, 6.90)] after adjusting multiple confounders. The finding was consistent with studies conducted in Taiwan,5 England,25 England,7 India,16 and Ethiopia.11
This might be because hypertension in diabetes patients causes total peripheral blood vessel resistance, an increase in extracellular fluid volume, and nephron and tubular necrosis and leads to impairment of renal excretory function finally causing elevation of serum Creatinine causing a sign of acute kidney injury.26
This study found that the expected hazard ratio of acute kidney injury was 2.89 times higher in patients with congestive heart failure among type 2 DM patients than in patients without congestive heart failure while keeping other covariates constant [AHR: 2.89 (95% CI; 1.62, 5.13)]. This finding was in line with studies done in Taiwan,5 England,7 and Ethiopia.11 This might be associated with congestive heart failure among diabetes patients who face altered renal hemodynamics resulting in hypoperfusion to the kidney due to low cardiac output state and impaired venous return due to blood stasis leads to ischemic injury, often termed acute renal tubular necrosis finally results in GFR declines and elevation of serum Creatinine due to glomerular ultrafiltration pressure is reduced as renal blood flow falls, obstruction by casts comprised of shed epithelial cells and necrotic debris, and back leak of filtrate through the injured tubular epithelium.27 This study found that patients with chronic kidney disease were 2.92 times at risk of developing AKI among type 2 DM patients than patients without chronic kidney disease [AHR: 2.92 (95% CI; 1.56, 5.48)]. This is similar to a study conducted in Taiwan,5 England,7 and Ethiopia.11 This might be related to diseased kidneys’ reduced renal reserve and inability to handle stress such as abnormally low blood pressure or nephrotoxic drugs and patients with chronic kidney disease experience more acute medical illnesses and procedures that increase the risk of exposure to nephrotoxic insults causing AKI.28 In this study, patients with diabetic nephropathy were 2.04 times at higher risk of developing acute kidney injury among patients without diabetic nephropathy [AHR: 2.04 (95% CI; 1.13, 3.68)]. This finding was in concordance with studies done in India,16 India,23 and Ethiopia.11 This might be due to dysfunction of endothelial cells involves interstitial peritubular capillary vessels as well as the glomerular afferent and efferent arterioles causes of reduction in relaxation factor produced by endothelial nitric oxide synthase (eNOS) renders the renal vasculature more sensitive to stimuli leading to vasoconstriction and makes the vascular resistance even worse causing retention of serum Creatinine causing AKI.13
Limitation
Because of the retrospective nature of the study, some important predictors that had a significant association with acute kidney injuries like behavioral factors, post-renal factors, and volume depletion factors were missed since we used chart review to obtain the data and making it difficult to assess possible confounders. The absence of a central database is also another limitation.
Conclusion
In this retrospective follow-up study, findings showed that the incidence of acute kidney injury among type 2 diabetes patients at the chronic follow-up clinic of the University of Gondar Comprehensive Specialized Hospital was high. Hypertension, congestive heart failure, chronic kidney disease, and diabetic nephropathy, were predictors of acute kidney injury among type 2 DM patients.
It would be better if health professionals in the DM follow-up clinics provide targeted intervention through close follow-up and screening of AKI in patients with hypertension, congestive heart failure, chronic kidney disease, and diabetic nephropathy insult and manage accordingly. in patients with hypertension congestive heart failure, chronic kidney disease, and diabetic nephropathy efforts should be made to diagnose AKI early and treat it - in addition to better control accordingly among type 2 diabetes mellitus patients.
Acknowledgments
We would like to thank the University of Gondar Referral Hospital administrative bodies and card room workers for their cooperation and permission to conduct the study. We are also thankful to the data collectors who participated in the study for their commitment.
Funding Statement
There is no funding to report.
Abbreviations
ACEI, Angiotensin-Converting Enzyme Inhibitor; AHR, Adjusted Hazard Ratio; AKI, Acute Kidney Injury; AOR, Adjusted Odds Ratio; ARBs, Angiotensin Receptor Blockers; ATR, Adjusted Time Ratio; CHF, Congestive Heart Failure; CHR, Crude Hazard Ratio; CKD, Chronic Kidney Disease; CI, Confidence Interval; Cr, Creatinine; DKA, Diabetic Ketoacidosis; DM, Diabetes Mellitus; ESKD, End-Stage Kidney Disease; HTN, Hypertension; KDIGO, Kidney Disease Improving Global Outcomes; NSAID, Non-steroidal Anti-inflammatory Drugs; PVD, Peripheral Vascular Disease; SRS, Simple Random Sampling; UOGCSH, University of Gondar Comprehensive Specialized Hospital; USA, United States of America.
Data Sharing Statement
All data about this study are contained and presented in this document.
Ethical Approval and Consent
Ethical clearance and approval were obtained from the ethical review committee of the School of Nursing to behaving with the Institutional Review Board (IRB) of the University of Gondar. Upon this clearance, additional written permission to conduct the study on medical records of DM patients was obtained from the coordinator of chronic clinic follow-up of the University of Gondar Comprehensive Specialized Hospital. Confidentiality of information was maintained through refrain from the recording of the patient’s name of the used chart and keeping data anonymous. The information didn’t use other than the study purpose. This study was conducted following the Declaration of Helsinki.
Author Contributions
Authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing of interest.
References
- 1.Organization WH. Classification of Diabetes Mellitus; 2019. [Google Scholar]
- 2.Alberti KGMM, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complications. Part 2: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 2012;15(7):539–553. doi: [DOI] [PubMed] [Google Scholar]
- 3.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 4.Asmelash D, Asmelash Y. The burden of undiagnosed diabetes mellitus in adult African Population: a systematic review and meta-analysis. J Diabetes Res. 2019;2019:1–8. doi: 10.1155/2019/4134937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.doi: 10.4314/ejhs.v28i4.11 [DOI]
- 6.Kellum JA, Lameire N; Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girman C, Kou T, Brodovicz K, et al. Risk of acute renal failure in patients with type 2 diabetes mellitus. Diabet Med. 2012;29(5):614–621. doi: 10.1111/j.1464-5491.2011.03498.x [DOI] [PubMed] [Google Scholar]
- 8.Ponce D, Balbi A. Acute kidney injury: risk factors and management challenges in developing countries. Int J Nephrol Renovasc Dis. 2016;9:193–200. doi: 10.2147/IJNRD.S104209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L. Acute kidney injury in Asia. Kidney Dis. 2016;2(3):95–102. doi: 10.1159/000441887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regassa LD, Gete YK, Mekonnen FA, Remuzzi G. Time to acute kidney injury and its predictors among newly diagnosed type 2 diabetic patients at government hospitals in Harari Region, East Ethiopia. PLoS One. 2019;14(5):e0215967. doi: 10.1371/journal.pone.0215967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu C-Y, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891–898. doi: 10.2215/CJN.05571008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu SM-W, Bonventre JV. Acute kidney injury and progression of diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25(2):166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikipedia. Gondar. Available from:https://en.wikipedia.org/wiki/South_Gondar_Zone. Accessed May1, 2012.
- 15.Harzallah A, Kaaroud H, Hajji M, et al. Drug-induced acute kidney injury in diabetes mellitus. Open J Nephrol. 2016;6(04):176. doi: 10.4236/ojneph.2016.64023 [DOI] [Google Scholar]
- 16.Paul FK, Kannath M. Aetiology and outcome of acute kidney injury in type 2 diabetes patients. J Evol Med Dent Sci. 2018;7(47):5878–5884. doi: 10.14260/jemds/2018/1129 [DOI] [Google Scholar]
- 17.Bell S, Farran B, McGurnaghan S, et al. Risk of acute kidney injury and survival in patients treated with metformin: an observational cohort study. BMC Nephrol. 2017;18(1):163. doi: 10.1186/s12882-017-0579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halle MPE, Chipekam NM, Beyiha G, et al. Incidence, characteristics and prognosis of acute kidney injury in Cameroon: a prospective study at the Douala general hospital. Ren Fail. 2018;40(1):30–37. doi: 10.1080/0886022X.2017.1419970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masewu A, Makulo J-R, Lepira F, et al. Acute kidney injury is a powerful independent predictor of mortality in critically ill patients: a multicenter prospective cohort study from Kinshasa, the democratic Republic of Congo. BMC Nephrol. 2016;17(1):118. doi: 10.1186/s12882-016-0333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dlamini TA, Heering PJ, Chivese T, Rayner B. A prospective study of the demographics, management and outcome of patients with acute kidney injury in Cape Town, South Africa. PLoS One. 2017;12(6):e0177460. doi: 10.1371/journal.pone.0177460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan SA, Mirbahar A-U, Qadir M. Acute kidney injury in hospitalized patients frequency of various etiologies. Prof Med J. 2019;26(11):1952–1957. doi: 10.29309/TPMJ/2019.26.11.3544 [DOI] [Google Scholar]
- 22.Mahmood N, Rahman MF, Rahman MM, Shahid SH, Ahman Siddiqui MM. Acute kidney injury in patients of intensive care unit. Anwer Khan Mod Med Coll J. 2017;8(1):38–44. doi: 10.3329/akmmcj.v8i1.31656 [DOI] [Google Scholar]
- 23.Kohli HS, Bhat A, Jairam A, et al. Predictors of mortality in acute renal failure in a developing country: a prospective study. Ren Fail. 2007;29(4):463–469. doi: 10.1080/08860220701260651 [DOI] [PubMed] [Google Scholar]
- 24.Safari S, Hashemi B, Forouzanfar MM, Shahhoseini M, Heidari M. Epidemiology and outcome of patients with acute kidney injury in emergency department; a Cross-Sectional Study. Emergency. 2018;6(1):e30. [PMC free article] [PubMed] [Google Scholar]
- 25.Jain A, McDonald HI, Nitsch D, Tomlinson L, Thomas SL. Risk factors for developing acute kidney injury in older people with diabetes and community-acquired pneumonia: a population-based UK cohort study. BMC Nephrol. 2017;18(1):142. doi: 10.1186/s12882-017-0566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3):625–633. doi: 10.1161/01.HYP.0000052314.95497.78 [DOI] [PubMed] [Google Scholar]
- 27.Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. Vol. 2012. New York: Mcgraw-hill; 2012. [Google Scholar]
- 28.Hsu RK, Hsu C-Y. The role of acute kidney injury in chronic kidney disease. Paper presented at: Seminars in nephrology; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]