Abstract
Objective
Little is known about temporal changes in nasal bacteria in granulomatosis with polyangiitis (GPA). We examined longitudinal changes in the nasal microbiome in association with relapse in GPA patients.
Methods
Bacterial 16S gene sequencing was performed on nasal swabs of 19 patients with GPA followed longitudinally for a total of 78 visits, including 9 patients who developed a relapse and 10 patients who remained in remission. Relative abundance of bacteria and ratios between bacteria were examined. Generalized estimating equation models evaluated the association between bacterial composition and 1) disease activity and 2) PR3-ANCA level, adjusting for medications.
Results
Corynebacterium and Staphylococcus were the most abundant bacterial genera across all nasal samples. Patients with quiescent disease maintained a stable ratio of Corynebacterium to Staphylococcus across visits. In contrast, in patients who experienced a relapse, a significantly lower ratio occurred at the visit prior to relapse, followed by a higher ratio at time of relapse (adjusted P < 0.01). Species-level analysis identified an association between higher abundance of nasal Corynebacterium tuberculostearicum and relapse (adjusted P = 0.04) and higher PR3-ANCA levels (adjusted P = 0.02).
Conclusion
In GPA, significant changes occur in the nasal microbiome over time and are associated with disease activity. The occurrence of these changes months prior to onset of relapse supports a pathogenic role of nasal bacteria in GPA. Our results uphold existing hypotheses implicating Staphylococcus as an instigator of disease and have generated a novel finding involving Corynebacterium as a potential mediator of disease in GPA.
Keywords: granulomatosis with polyangiitis, microbiome, disease activity, ANCA, longitudinal studies
INTRODUCTION
Granulomatosis with polyangiitis (GPA) is a life- and organ-threatening systemic vasculitis characterized by granulomatosis inflammation and frequent relapses. Rhinosinusitis occurs in up to 90% of patients with GPA and is associated with a higher risk of relapse1. While our understanding about the immunopathogenesis of GPA has advanced, little is known about the triggers of disease activity.
Mechanistic and epidemiologic studies suggest microbes, in particular nasal microbiota, may be an important environmental activator of GPA. Cross-reactivity between host and bacterial peptides may lead to the formation of pathogenic anti-neutrophil cytoplasmic antibodies (ANCA), which are associated with GPA2, 3. Low-grade infections may also evoke inflammatory cytokines which prime neutrophils for activation by ANCA or stimulate neutrophils to release neutrophil extracellular traps (NETs) embedded with ANCA antigens, further breaking immune tolerance and generating autoantibodies4, 5. Nasal colonization with Staphylococcus aureus is associated with a higher risk of relapse in GPA, an observation that led to a randomized, placebo-controlled trial of co-trimoxazole (an anti-Staphylococcal antibiotic) which was found to significantly reduce the risk of relapse in GPA6, 7. These initial culture-dependent studies were limited due to the lack of profiling of the whole community of microbes and evaluating temporally dynamic changes within an individual over time. Furthermore, the mechanism through which S. aureus may instigate relapse in GPA remains unclear8–12.
Advances in culture-independent techniques have enhanced the ability to examine the diversity of microbial species that colonize host sites, known as the human microbiome. While culture-dependent approaches view disease states as exclusively due to a single pathogenic microbe, studies now demonstrate that overall composition of the microbiota strongly influences the behavior of a specific species. In prior work using high-throughput sequencing, our group profiled the entire community of nasal microbiota in GPA and healthy controls using 16S rRNA gene sequencing13. We found a lower abundance of “healthy” commensals in patients with GPA and that patients with GPA off immunosuppressive therapy have the greatest dysbiosis (imbalance in microbiota). However, what changes in the nasal microbiome occur longitudinally within an individual and how these changes relate temporally to disease activity remain unknown. This knowledge is needed to better understand potentially causal relationships between dysbiosis and disease.
The objective of this study was to apply culture-independent sequencing methods to examine longitudinal changes in the nasal microbiome and their association with disease activity in GPA.
PATIENTS AND METHODS
Study Design and Participants
We performed a prospective cohort study at the University of Pennsylvania. Participants were recruited through the Penn Vasculitis Center. Participants with GPA were eligible if they met the modified American College of Rheumatology classification criteria for GPA14, 15. Participants were excluded if they had another systemic inflammatory disorder, known history of human immunodeficiency virus (HIV) infection, primary immunodeficiency, lymphoma, or other malignancy that mimics GPA. This study was approved by the Institutional Review Board of the University of Pennsylvania and written informed consent was obtained from all participants.
Procedures
Nasal mucosa was sampled by swabbing the middle meatus with a sterile flocked specimen collection swab (Copan Diagnostics) which was then transferred to a −80°C freezer. To control for environmental contamination, negative controls (swab exposed to ambient air) were obtained with each participant sampling and a randomly chosen subsample of these controls were processed in parallel. Sampling occurred at every office visit, usually with 3- to 6-month time intervals. Detailed clinical data was also collected at each visit including disease activity and symptoms, infections, topical nasal therapies, and medications. All patients who had received rituximab in the past 6 months were considered to be receiving rituximab at the study visit. Serial serum samples were collected in a subset of patients (29 visits in 11 patients) and ANCA levels tested with direct enzyme-linked immunosorbent assay (ELISA) at a single institutional clinical laboratory. Disease activity was measured using the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG; BVAS/WG > 0 indicates active disease and BVAS/WG = 0 indicates disease remission)16.
Microbial DNA Sequencing and Taxonomic Assignment
Nasal swab samples were sequenced and analyzed by the PennCHOP (University of Pennsylvania/Children’s Hospital of Philadelphia) Microbiome Center. Bacterial DNA was extracted from swabs using the DNeasy® PowerSoil® Kit (Qiagen). The V1-V2 variable regions of the bacterial 16S rRNA gene, which has superior taxonomic resolution for the nasal cavity and sinuses17, were PCR amplified using the 27F ( 5’-AGAGTTTGATCCTGGCTCAG-3’) and 338R (5’-TGCTGCCTCCCGTAGGAGT-3’) primers. Each sample was amplified in quadruplicate PCR reactions that consisted of 0.5 uM of each primer, 0.34 U Q5 Pol, 1X Buffer, 0.2 mM dNTPs, and 5 DNA in a total volume of 25 ul. PCR cycling conditions were as follows: 98°C for 1 min, 25 cycles of (98°C for 10 sec, 56°C for 20 sec, 72°C for 20 sec), and 72°C for 8 min. The quadruplicate reactions were pooled together, cleaned using SPRI beads (GE Healthcare), and quantified using the Quant-It™ PicoGreen™ dsDNA Assay Kit (ThermoFisher). Samples were pooled in equimolar amounts and then sequenced on an Illumina MiSeq instrument using a 500 cycle v2 sequencing kit, yielding 250 bp paired-end sequence reads. Environmental and reagent control samples, consisting of air-exposed swabs, DNA free water, and empty wells, and positive control samples were processed and sequenced alongside participant samples.
Sequencing data were processed and analyzed using the QIIME2 pipeline18. The QIIME2 plug-in implementation of DADA2 was used to create a set of Amplicon Sequence Variants (ASVs) from the raw sequence reads19. Taxonomic assignment was performed using a naïve Bayes classifier trained on the reference sequences from GreenGenes 13_8. For diversity metrics including UniFrac distances, a multiple sequence alignment was performed using MAFFT20 and a phylogenetic tree was generated using FastTree21. ASVs were evaluated for consistency with named bacterial species by aligning to the reference sequence from bacterial type strains, and estimating the probability that the full-length 16S rRNA gene similarity diverged by more than 2.5%. The software implementing this algorithm is available at https://github.com/kylebittinger/unassigner.
Statistical Analyses
To evaluate bacterial communities, alpha and beta diversity were assessed and compared between relapsing and non-relapsing patients with GPA. Alpha diversity (within-sample diversity) was measured by the Shannon Diversity Index which accounts for evenness and richness (number) of ASVs within a sample. Beta diversity (between-sample diversity) was calculated using weighted UniFrac distance which estimates the fraction of a sample’s phylogenetic tree that differs from another sample, accounting for the relative abundance of ASVs22, 23. UniFrac distances were visualized on a multidimensional scaling (MDS) plot. Wilcoxon rank-sum test was used to compare continuous variables and chi-square test was used for categorical variables.
To account for repeated measures, we applied generalized estimating equations (GEE) to explore the association between bacterial composition and disease activity, adjusting for antibiotics, immunosuppressive medications, and nasal irrigation. The GEE approach is a semi-parametric model that accounts for the unknown correlations between the longitudinal repeated measurements. We grouped visits based on disease status (stable remission, pre-relapse, relapse, and post-relapse), using stable remission as a reference. We analyzed the data at genus level and normalized the read counts into compositions. Genera with a median relative abundance of greater than 1% were included in the analysis. Relative abundance of individual genera as well as log-ratios between two genera were used as outcomes to address the compositional nature of the microbiome data. Because of the unit-sum constraint on the data, the bacteria components cannot vary freely such that changes in one bacterium must result in the changes of another. Using a log-ratio transformation accounts for the unit-sum constraint and is a well-established approach for relative abundance data24. False discovery rate controlling procedure was used to adjust for multiple comparisons of all pairs of bacterial genera and was set to 10% as the cutoff.
Secondary analyses included exploration of the consistency between ASVs and named bacterial species and were limited to taxa with a mean relative abundance greater than 2% across all samples. Test for trend evaluated for linear associations between relative abundance and disease status (stable remission, pre- pre-relapse, pre-relapse, relapse, and post-relapse).
Analyses were conducted using R V.3.4.1 and code is available upon request.
RESULTS
Participant Characteristics
A total of 78 visits among 19 patients with GPA were included in this study (Table 1). The median total follow-up time was 13.8 months. Median time between all visits was 3.9 months and the median time between the pre-relapse and relapse visits was 3.7 months. As expected, most of the participants were positive for antineutrophil cytoplasmic antibodies with specificity to proteinase-3 (PR3-ANCA) and had sinonasal involvement at some point during their disease course. Nine of the 19 patients developed a relapse of disease during follow-up and the other 10 patients remained in remission. Medications at the first visit, typically when patients were in remission, are listed in Table 1. Among all visits, prednisone was being used at 24 (31%) of patient visits with median dose of 6mg.
Table 1.
Patient Characteristics
| Characteristic [median (IQR) or no. (%)] |
Relapsing GPA (n = 9) |
Non-Relapsing GPA (n = 10) |
P-value |
|---|---|---|---|
| Number of visits per patient | 4 (3 to 5) | 4 (3 to 5) | 0.96 |
| Time between visits, months | 3.3 (2.9 to 4.9) | 4.1 (3.2 to 5.9) | 0.16 |
| Age at enrollment, years | 58 (49 to 67) | 64 (54 to 69) | 0.37 |
| Female | 44% | 40% | 0.85 |
| White race | 100% | 90% | 0.33 |
| Hispanic, Latino, or Spanish origin | 0 | 20% | 0.02 |
| ANCA type | 0.38 | ||
| PR3-ANCA | 78% | 70% | |
| MPO-ANCA | 11% | 30% | |
| Negative ANCA | 11% | 0% | |
| History of relapse prior to enrollment | 67% | 50% | 0.46 |
| Sinonasal involvement prior to enrollment | 67% | 80% | 0.51 |
| Sino-Nasal Outcome Test 22 Score at first visit | 23 (22 to 35) | 29 (8 to 41) | 0.71 |
| Medications at first visit | |||
| Systemic immunosuppressives | 6 (67%) | 10 (100%) | 0.04 |
| Oral prednisone | 3 (33%) | 5 (50%) | 0.46 |
| Rituximab | 2 (22%) | 4 (40%) | 0.41 |
| Azathioprine | 2 (22%) | 4 (40%) | 0.41 |
| Methotrexate | 1 (11%) | 1 (10%) | 0.94 |
| Topical nasal therapies | |||
| Nasal saline irrigation | 5 (56%) | 5 (50%) | 0.81 |
| Topical nasal steroid | 1 (11%) | 2 (20%) | 0.62 |
| Nasal mupirocin | 0 | 1 (10%) | 0.33 |
| Systemic antibiotics | 1 (11%) | 3 (30%) | 0.31 |
| TMP-SMX, full dose | 0 | 0 | -- |
| TMP-SMX, low dose | 1 (11%) | 2 (20%) | 0.60 |
ANCA, antineutrophil cytoplasmic antibody. GPA, granulomatosis with polyangiitis. MPO, myeloperoxidase. PR3, proteinase-3. TMP-SMX, trimethoprim-sulfamethoxazole.
There were 12 active disease visits among the 9 relapsing patients with GPA. Of these 12 visits with active disease, 10 visits included manifestations outside of the upper respiratory tract and 8 had sinonasal inflammation. At 9 of the 12 visits, patients were receiving immunosuppressive therapy: 6 on oral prednisone (3 on a stable prednisone dose of 5mg and 3 who initiated higher dose prednisone 1–3 weeks prior due to concern for relapse), 4 on rituximab, 1 on azathioprine, 1 on methotrexate, and 2 on leflunomide. Patients were performing home nasal saline irrigation prior to 7 of the 12 active disease visits and 2 patients were using nasal antibiotics (mupirocin and gentamicin). Notably, only 2 patients were receiving a systemic antibiotic at time of relapse visit: one patient was on full-dose trimethoprim-sulfamethoxazole and the other patient was on azithromycin. All patients responded to escalation of systemic immunosuppression except for one patient who received only topical nasal steroids for sinonasal symptoms; this patient initially improved but later developed a multi-system relapse requiring oral prednisone and rituximab.
Common nasal bacteria identified in patients with GPA
The heatmap in Figure 1A depicts the 20 most abundant bacteria found in the samples, grouped by disease status (remission, pre-relapse, relapse, and post-relapse visit) and outcome (relapser vs non-relapser). The most abundant bacteria at the genus-level were (in decreasing order of abundance) Corynebacterium, Staphylococcus, Propionibacterium, Streptococcus, and Alloiococcus (Figure 1A). These 5 bacteria comprised a large majority of the composition of the samples (mean [standard deviation] of total combined abundance of 5 bacteria was 86% [SD 13%] in relapsing group and 83% [SD 18%] in non-relapsing group) (Figure 1B). No significant differences were found in Shannon diversity (Figure 1C) or weighted UniFrac (data not shown) between patients with relapsing vs non-relapsing disease; no difference was seen in these diversity measures when comparing disease status (remission, pre-relapse, relapse, and post-relapse visits).
Figure 1. Bacterial composition of patients with GPA.
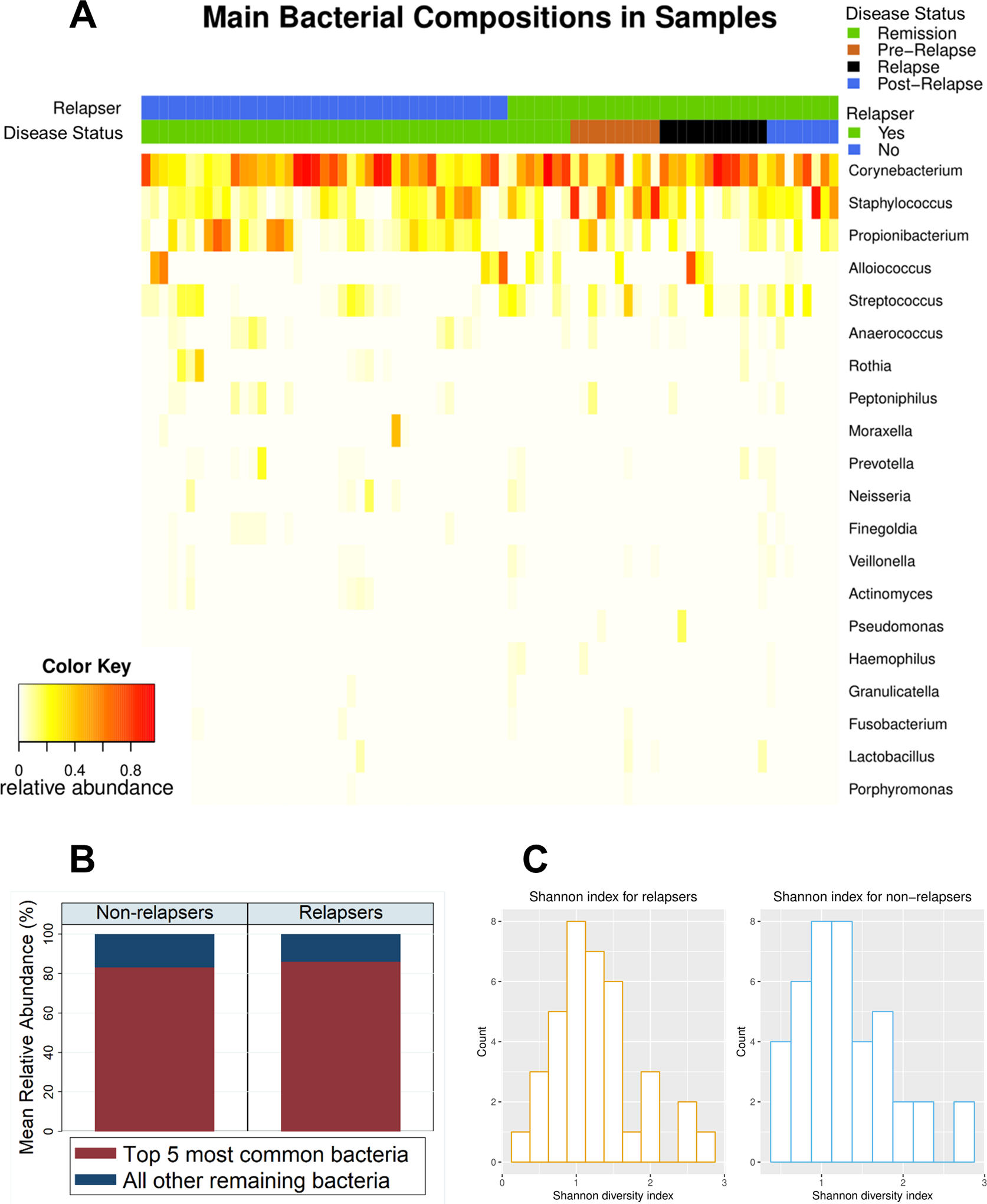
(A) A heatmap illustrating the relative abundance of top 20 most abundant bacterial genera across all samples, grouped by outcome (relapsing vs non-relapsing GPA) and disease status (remission, pre-relapse, relapse, and post-relapse) among all samples (n = 78). The samples were primarily comprised of 5 bacteria (in descending order of abundance): Corynebacterium, Staphylococcus, Propionibacterium, Alloiococcus, and Streptococcus. (B) Bar plot showing mean relative abundance of 5 most abundant bacteria vs all other remaining bacteria. Over 80% of bacteria in samples were represented by the 5 most abundant bacteria. (C) Comparison of alpha diversity measured by Shannon diversity index among all visits grouped by relapsing (left) and non-relapsing (right) GPA (P-value = 0.78).
Dynamic changes in Corynebacterium and Staphylococcus begin prior to onset of relapse in GPA
Since most of the bacteria had very low abundance, we focused on the top 5 most abundant bacteria genera (the only genera with a median relative abundance of greater than 1%) and recalculated the relative abundance of these 5 bacteria. Temporally dynamic changes in the relative abundance of the 5 bacteria were observed with change in disease status (remission, pre-relapse, relapse, and post-relapse). Specifically, there is a greater abundance of Staphylococcus at the pre-relapse visit followed by an increase in the abundance of Corynebacterium at time of relapse (Figure 2). This is best demonstrated when examining the log-ratio of Corynebacterium to Staphylococcus which was significantly lower at the pre-relapse visit even after adjusting for antibiotics, immunosuppressive medications, and nasal irrigation (adjusted P < 0.01; Figure 3). This log-ratio subsequently increased at the relapse visit (adjusted P < 0.01), similar to remission levels (compared to remission visits, P > 0.05). No significant changes were observed at pre-relapse or relapse visits among the ratios of the other bacteria (data not shown). A sensitivity analysis excluding patients with more than 1 active disease visit yielded similar results (data not shown).
Figure 2. Relative abundance of 5 most abundant bacteria grouped by disease status.

Boxplot demonstrates differences in relative abundance associated with disease status with most pronounced variations seen in the abundance of Corynebacterium (blue) and Staphylococcus (orange). Each box spans the first to the third quartile and the middle line indicates the median abundance; the whiskers below and above the box demonstrate the minimum and maximum abundance in the group with the dots representing outliers.
Figure 3. Dynamic changes in ratio of Corynebacterium to Staphylococcus occur across visits of relapsing GPA.

Median relative abundance of Corynebacterium (black) and Staphylococcus (white) in non-relapsing (left) and relapsing (right) GPA. Consecutive visits are shown separately to display temporal shifts. Patients with non-relapsing GPA (n=10) had a relatively stable ratio of Corynebacterium to Staphylococcus across 3 consecutive visits (all P > 0.05); in contrast, patients with relapsing GPA (n=9) had significantly lower ratio at pre-relapse visit (adjusted P < 0.01) followed by an increase in ratio at relapse (adjusted P < 0.01), even after adjusting for antibiotics, immunosuppressive medications, and nasal irrigation.
Species-level analysis reveals associations between nasal Corynebacterium tuberculostearicum and relapse in GPA
We further analyzed the 16S rRNA marker gene sequences to assess whether the partial gene sequences observed in our study were compatible with named bacterial species. Although we were not able to conclusively determine that the observed sequences arose from a particular named species, we were in many cases able to rule out all potential species assignments but one. When this occurred, we used the observed sequence, representing a fraction of the 16S gene, to compute the probability that the full-length 16S gene sequence would be compatible with the named bacterial species.
We found that 16S sequences compatible with Corynebacterium tuberculostearicum featured prominently in this cohort. C. tuberculostearicum, which has previously been shown to have a pathogenic role in chronic rhinosinusitis25, had the highest mean relative abundance in all the samples (relative abundance 12.7%) followed by C. propinquum (relative abundance 11.9%) and Cutibacterium acnes (formerly known as Propionibacterium acnes; relative abundance 10.6%). Corynebacterium tuberculostearicum, Cutibacterium acnes, and Staphylococcus epidermidis were the only 3 bacterial species detected in all 78 samples. Staphylococcus aureus was present in 33 (42%) of samples and had a mean relative abundance of 3.5%.
When we evaluated associations between nasal bacteria and relapse in GPA, we found that an increasing relative abundance of C. tuberculostearicum was associated with disease status, categorized as stable remission, pre-pre-relapse, pre-relapse, relapse, and post-relapse visits even after adjusting for medications (test for trend, adjusted P = 0.04; Figure 4). Given the historical interest of S. aureus in GPA and prior studies demonstrating interactions between S. aureus and Corynebacterium spp., we also adjusted for presence of S. aureus in the sample and found that the presence of S. aureus was independently associated with a higher abundance of C. tuberculostearicum (adjusted P = 0.02). We found similar results involving C. tuberculostearicum when examining only relapses with sinonasal involvement (test for trend, adjusted P < 0.01). No significant associations were found between individual bacteria and patient-reported symptoms of rhinosinusitis (the Sino-Nasal Outcome Test-22 [SNOT-22] score).
Figure 4. Increasing abundance of nasal Corynebacterium tuberculostearicum is associated with relapse in GPA.
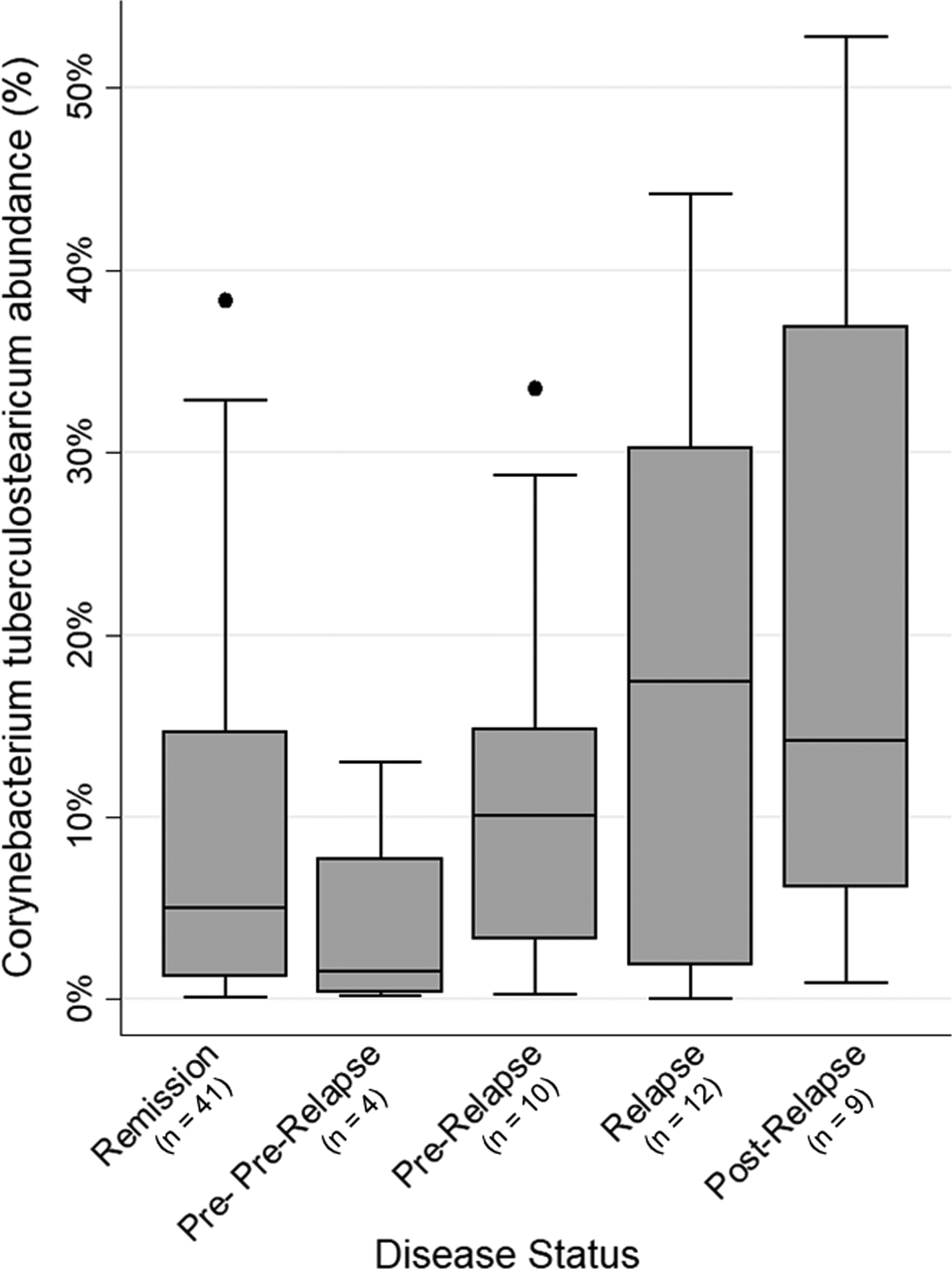
Box plot depicts median relative abundance of C. tuberculostearicum by disease status: stable remission, pre- pre-relapse, pre-relapse, relapse, and post-relapse. Test for trend detected a significant linear increase in nasal C. tuberculostearicum abundance across visits even after adjusting for antibiotics, immunosuppressives, nasal irrigation, and presence of Staphylococcus aureus (adjusted P = 0.04).
To investigate relationships between bacteria, we examined the association between ratios of bacterial abundance and clinical outcomes. An increasing ratio of C. tuberculostearicum to S. caprae was associated with disease status (stable remission, pre-pre-relapse, pre-relapse, relapse, and post-relapse visits) when examining any relapse (test for trend, adjusted P < 0.01) as well as only relapses including the sinonasal area (test for trend, adjusted P < 0.01). Disease status was also associated with an increasing ratio of C. pseudodiphtheriticum to S. caprae (test for trend, adjusted P < 0.01). No bacterial ratios were associated with patient-reported symptoms of rhinosinusitis (SNOT-22 score).
Nasal Corynebacterium tuberculostearicum is associated with higher PR3-ANCA levels
Due to the potential pathogenicity of PR3-ANCA in GPA26, 27, we assessed the association between nasal bacteria and PR3-ANCA levels in a subgroup of 11 patients with 29 visits with available ANCA levels. The median PR3-ANCA level was 19 (interquartile range 6 to 56). No significant association between PR3-ANCA and the abundance of bacterial genera or ratios of bacterial genera were found. When examining bacterial species, we found that an increasing abundance of nasal C. tuberculostearicum was associated with higher levels of PR3-ANCA even after adjusting for immunosuppressive medications, antibiotics, and nasal rinse (adjusted P = 0.02; Figure 5). No other nasal bacterial species were associated with PR3-ANCA levels, including S. aureus.
Figure 5. Relative abundance of nasal Corynebacterium tuberculostearicum is associated with PR3-ANCA level in GPA.

Scatterplot demonstrates the association between abundance of nasal C. tuberculostearicum and serum PR3-ANCA levels in 11 patients with a total of 29 visits (unadjusted P < 0.01). The association remained significant even after adjusting for use of antibiotics, immunosuppressives, and nasal irrigation (adjusted P = 0.02). Reference range for normal PR3-ANCA < 20 units.
DISCUSSION
Using unbiased molecular methods, we examined temporal changes in the nasal bacterial community of patients with GPA over multiple consecutive visits. At the genus level, we found that dynamic changes in the relative abundance of two common nasal commensals, Corynebacterium and Staphylococcus, precedes the development of relapse in GPA, whereas the abundance of these two bacteria remains stable in patients with quiescent disease. At the species level, an increasing abundance of nasal Corynebacterium tuberculostearicum, a bacterium previously found to be a potential pathogenic mediator of chronic rhinosinusitis25, was associated both with relapse as well as PR3-ANCA levels in GPA. Our results are consistent with prior interest in Staphylococcus species in GPA and additionally suggest that: 1) the totality of the microbiome, not just individual bacteria, may be critical; 2) the composition at time points preceding disease activity may have a role in inciting disease; and 3) another nasal commensal bacteria that has not been previously studied in GPA may be an important mediator of disease.
The possibility of microbes as instigators of the immune response has been a longstanding theory for GPA as well as other autoimmune diseases. We chose to study the nasal cavity for two primary reasons: (1) the nasal mucosa is an active site of immunity28, and (2) sinonasal inflammation is a destructive feature of GPA and associated with relapse. The overall bacterial composition in our cohort is similar to the nasal microbiome described in other non-GPA populations17, 29, 30 as well as other cohorts of GPA8, 13, 31 and is consistent with previous studies in demonstrating that the nasal cavity is colonized by a restricted number of microbes in contrast to other mucosal surfaces such as the intestine29, 32. The longitudinal design of our study offers the added advantage of assessing intra-individual changes temporally over time.
Prior studies of the nasal microbiota in GPA largely focused on Staphylococcus aureus, a pathogenic bacterium with a well-known predilection for the nasal cavity. S. aureus has been found in a greater proportion of patients with relapsing GPA6, but the potential mechanism of S. aureus pathogenicity in GPA is unknown. Staphylococcus superantigens (SAg) have been proposed as a link between S. aureus and relapse in GPA12 but has not been replicated by subsequent studies8–10. Differences in S. aureus strains and genetic loci have been found between PR3-ANCA and MPO-ANCA vasculitis, although no direct mechanistic links between strain heterogeneity and disease have been shown10. A prior report showed a potential role for complementary PR3 peptide in the formation of PR3-ANCA and identified sequence homology between complementary PR3 peptide and several microbes, including S. aureus3. However, there still remains no direct evidence demonstrating that S. aureus colonization or infection leads to PR3-ANCA formation in GPA. More recently, a S. aureus plasmid that has homology to an MPO T-cell epitope was shown to induce MPO-ANCA formation and glomerulonephritis in mice11; whether this explains ANCA formation in all patients with GPA including those with PR3-ANCA type still remains unclear.
In this study, we found a significant increase in the abundance of the genus Staphylococcus prior to onset of a relapse; however, interrogation of species-level identity of bacteria did not show any relationship between Staphylococcus aureus and relapse. The lack of an association between S. aureus and relapse in GPA may be due to constraints of 16S gene sequencing which is limited in resolving species-level classification. Differences in methodology may also explain disparate results compared to prior studies which relied on repeated nasal cultures to define chronic carriers6, 33. We chose to use sequence-based culture-independent methods which, compared to culture-dependent methods, has the added advantage of comprehensively evaluating all nasal microbes, including unculturable or difficult-to-culture organisms. Lastly, we sampled the middle meatus which is lined by mucosa with ciliated pseudostratified columnar epithelium, closer in proximity to sinuses, and a site of active inflammation in GPA; prior studies sampled the anterior nares which is lined by skin-like squamous epithelium and the main reservoir for S. aureus, but not typically an area of active disease in GPA. Differences in sampling site may also account for differences in results between this study and prior studies.
Unexpectedly, we identified a novel finding involving a lesser known and poorly-studied nasal commensal, corynebacteria. Corynebacteria are aerobic, gram-positive bacilli that populates the human nose and skin and was previously thought to be harmless commensals. However, emerging evidence implicates a role for Corynebacterium in diseases of the lower respiratory tract (such as asthma), upper respiratory tract (such as chronic rhinosinusitis), and skin (such as atopic dermatitis) as well as granulomatous diseases25, 34–37. Both in our cohort as well as others, corynebacteria are often among the most abundant genera in the nasal cavity and prior studies have shown it is also the most species-rich taxon with one study reporting at least 23 different species of corynebacteria in the nose of almost every patient sampled38. Corynebacteria typically have a complex cell wall with an outer layer of mycolic acids which is similar to the cell wall of mycobacteria (another granuloma-forming pathogen) and functionally equivalent to the outer layer of Gram-negative bacteria in respect to its permeability barrier and host-pathogen interactions39. While the immunogenicity of the Corynebacterium cell wall has not been well-studied, the mycolic acid-containing cell wall of Mycobacterium tuberculosis is known to contribute to its pathogenicity40. For example trehalose dimycolate (TDM), which is a constituent of the mycolic acid layer in M. tuberculosis, induces inflammatory responses and granuloma formation41. Trehalose 6,6’-dicorynomycolate (TDCM) in corynebacteria is comparable to TDM in mycobacteria; TDCM has been shown to activate murine macrophages and induce inflammatory cytokines such as tumor necrosis factor alpha (TNFα)42. Therefore, it is plausible corynebacteria may play a role in the granulomatous inflammation characteristic of GPA. Interestingly, most species of Corynebacterium are susceptible to trimethoprim-sulfamethoxazole, an antibiotic found to prevent relapses in GPA.
At the species level, we uncovered associations between nasal Corynebacterium tuberculostearicum abundance and both GPA disease relapse and PR3-ANCA levels. C. tuberculostearicum has been implicated in the pathogenesis of chronic rhinosinusitis25. That study found that only nasal C. tuberculostearicum was significantly increased in abundance in patients with chronic rhinosinusitis compared to healthy subjects and inoculating the nasal cavity of antibiotic-treated mice with C. tuberculostearicum induced pathologic features of chronic rhinosinusitis. In GPA, studies have shown evidence for induction of PR3-ANCA by autoreactive B cells in granulomatous lesions and chronic B-cell activation in inflamed mucosa of patients with GPA, indicating a local antigen driven process which promotes formation of autoantibodies that are associated with the life-threatening systemic features of GPA43, 44. We postulate that Corynebacterium (and more specifically, Corynebacterium tuberculostearicum) may contribute to the initiation of local granulomatous lesions and PR3-ANCA formation in sinonasal mucosa of patients with GPA. Our use of unbiased profiling methods has uncovered the potential role of this previously-disregarded bacteria in the pathogenesis of GPA.
We propose two possible explanations for the dynamic changes observed between Corynebacterium and Staphylococcus in this study. One potential hypothesis is that Corynebacterium regulates Staphylococcus through inter-species interactions, consistent with prior studies, but when this balance is lost an outgrowth in Staphylococcus incites host inflammation. By the time of the relapse visit, the balance between these two bacteria may be restored but ongoing inflammation ensues due to a dysregulated immune response in the host. Alternatively, Staphylococcus may promote the outgrowth of a pathogenic strain of Corynebacterium which promotes inflammation. It is notable that the relative abundance of Staphylococcus, which had significantly increased at the pre-relapse visit, returned to baseline at the relapse visit, which was contrary to what we had expected to find. While our data does not show direct interactions between Corynebacterium and Staphylococcus, many prior studies have demonstrated species-specific interactions between these two bacteria29, 45–47. In the context of GPA, this may involve a type of 3-way relationship that has been previously described in the nasal cavity48, 49. In this process, referred to as within-host competition, negative competition between bacteria is mediated by the host. The median time interval between the pre-relapse visit (in which Staphylococcus predominated) and the relapse visit (in which Corynebacterium predominated) was 3.7 months; this relatively long time interval supports the possibility that Corynebacterium may be a more important mediator of disease than Staphylococcus. Furthermore, aberrant immune responses in patients with GPA may explain why these ubiquitous commensals are associated with disease in only a small fraction of the general population.
There are several limitations of this study to consider. While these novel findings raise new questions about the pathogenesis of GPA, they are associative and do not demonstrate causality. It is possible that disruption of the nasal microbiota is occurring secondary to mucosal inflammation; however, identification of changes in nasal bacteria 3 months prior to onset of disease relapse makes this less likely. Similarly, other confounders such as unmeasured environmental or medication exposures not accounted for in the analyses may explain these results. While sequencing of the highly-conserved 16S bacterial gene is a commonly-used initial approach to evaluating the microbiome, this sequencing method is limited in its ability to determine species identity50. Additionally, 16S rRNA gene sequencing allows taxonomic identification but not enumeration of functional content; it is possible that metabolic pathways and virulence-related genes are the key to understanding pathogenesis of disease32. Additional studies are needed to investigate mechanisms mediating microbe-microbe and host-microbial relationships and the current work suggests that greater attention should be paid to both Corynebacterium and Staphylococcus. Lastly, results of this study may not be generalizable to other populations of GPA including patients residing in different geographic regions or with different genetic predisposition for disease.
In conclusion, this longitudinal study of the nasal microbiome in GPA identified changes in the nasal commensal bacteria several months prior to disease relapse. In addition to supporting the possibility of Staphylococcus as an instigator of disease activity, this work has uncovered a novel finding implicating Corynebacterium as a potential mediator of host-microbial interactions and specifically C. tuberculostearicum as a species of particular interest. These findings support the long-standing theory that overgrowth of pathogenic bacteria are potentially involved in the disease process of GPA and identify specific new targets for mechanistic investigation. Understanding how nasal bacteria activate disease in GPA may potentially lead to novel therapeutic targets, new measures to predict relapse, and better precision medicine approaches in GPA.
ACKNOWLEDGEMENTS:
We thank all participants for their involvement in the study.
Data is accessible via NCBI Sequence Read Archive.
FUNDING: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award K23AR071514.
Footnotes
CONFLICTS OF INTEREST: None
REFERENCES
- 1.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–98. [DOI] [PubMed] [Google Scholar]
- 2.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14(10):1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pendergraft WF 3rd, Preston GA, Shah RR, Tropsha A, Carter CW Jr., Jennette JC, et al. Autoimmunity is triggered by cPR-3(105–201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10(1):72–9. [DOI] [PubMed] [Google Scholar]
- 4.van Dam LS, Kraaij T, Kamerling SWA, Bakker JA, Scherer UH, Rabelink TJ, et al. Intrinsically Distinct Role of Neutrophil Extracellular Trap Formation in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Compared to Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71(12):2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadema H, Abdulahad WH, Lepse N, Stegeman CA, Kallenberg CG, Heeringa P. Bacterial DNA motifs trigger ANCA production in ANCA-associated vasculitis in remission. Rheumatology (Oxford). 2011;50(4):689–96. [DOI] [PubMed] [Google Scholar]
- 6.Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120(1):12–7. [DOI] [PubMed] [Google Scholar]
- 7.Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335(1):16–20. [DOI] [PubMed] [Google Scholar]
- 8.Wagner J, Harrison EM, Martinez Del Pero M, Blane B, Mayer G, Leierer J, et al. The composition and functional protein subsystems of the human nasal microbiome in granulomatosis with polyangiitis: a pilot study. Microbiome. 2019;7(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fijołek J, Wiatr E, Petroniec V, Augustynowicz-Kopec E, Bednarek M, Gawryluk D, et al. The presence of staphylococcal superantigens in nasal swabs and correlation with activity of granulomatosis with polyangiitis in own material. Clin Exp Rheumatol. 2018;36 Suppl 111(2):40–5. [PubMed] [Google Scholar]
- 10.Glasner C, de Goffau MC, van Timmeren MM, Schulze ML, Jansen B, Tavakol M, et al. Genetic loci of Staphylococcus aureus associated with anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides. Sci Rep. 2017;7(1):12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooi JD, Jiang JH, Eggenhuizen PJ, Chua LL, van Timmeren M, Loh KL, et al. A plasmid-encoded peptide from Staphylococcus aureus induces anti-myeloperoxidase nephritogenic autoimmunity. Nat Commun. 2019;10(1):3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popa ER, Stegeman CA, Abdulahad WH, van der Meer B, Arends J, Manson WM, et al. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener’s granulomatosis. Rheumatology (Oxford). 2007;46(6):1029–33. [DOI] [PubMed] [Google Scholar]
- 13.Rhee RL, Sreih AG, Najem CE, Grayson PC, Zhao C, Bittinger K, et al. Characterisation of the nasal microbiota in granulomatosis with polyangiitis. Ann Rheum Dis. 2018;77(10):1448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leavitt RY, Fauci AS, Bloch DA, Michel BA, Hunder GG, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum. 1990;33(8):1101–7. [DOI] [PubMed] [Google Scholar]
- 15.Wegener’s Granulomatosis Etanercept Trial Research G. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352(4):351–61. [DOI] [PubMed] [Google Scholar]
- 16.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001;44(4):912–20. [DOI] [PubMed] [Google Scholar]
- 17.Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): a Resource for the Microbiome of the Human Aerodigestive Tract. mSystems. 2018;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73(5):1576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aitchison J The statistical analysis of compositional data. Journal of the Royal Statistical Society. Series B (Methodological) 1982;44.2:139–60. [Google Scholar]
- 25.Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4(151):151ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Primo VC, Marusic S, Franklin CC, Goldmann WH, Achaval CG, Smith RN, et al. Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis. Clin Exp Immunol. 2010;159(3):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millet A, Martin KR, Bonnefoy F, Saas P, Mocek J, Alkan M, et al. Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. The Journal of Clinical Investigation. 2015;125(11):4107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riese P, Sakthivel P, Trittel S, Guzmán CA. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv. 2014;11(10):1619–34. [DOI] [PubMed] [Google Scholar]
- 29.Yan M, Pamp SJ, Fukuyama J, Hwang PH, Cho DY, Holmes S, et al. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe. 2013;14(6):631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rom D, Bassiouni A, Eykman E, Liu Z, Paramasivan S, Alvarado R, et al. The Association Between Disease Severity and Microbiome in Chronic Rhinosinusitis. Laryngoscope. 2019;129(6):1265–73. [DOI] [PubMed] [Google Scholar]
- 31.Lamprecht P, Fischer N, Huang J, Burkhardt L, Lütgehetmann M, Arndt F, et al. Changes in the composition of the upper respiratory tract microbial community in granulomatosis with polyangiitis. J Autoimmun. 2019;97:29–39. [DOI] [PubMed] [Google Scholar]
- 32.Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmela A, Rasmussen N, Tervaert JWC, Jayne DRW, Ekstrand A. Chronic nasal Staphylococcus aureus carriage identifies a subset of newly diagnosed granulomatosis with polyangiitis patients with high relapse rate. Rheumatology (Oxford). 2017;56(6):965–72. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42(4):756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridaura VK, Bouladoux N, Claesen J, Chen YE, Byrd AL, Constantinides MG, et al. Contextual control of skin immunity and inflammation by Corynebacterium. The Journal of experimental medicine. 2018;215(3):785–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Jackson D, Bacharier LB, Mauger D, Boushey H, Castro M, et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun. 2019;10(1):5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Co M, Cheng VCC, Wei J, Wong SCY, Chan SMS, Shek T, et al. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50(7):742–7. [DOI] [PubMed] [Google Scholar]
- 38.Kaspar U, Kriegeskorte A, Schubert T, Peters G, Rudack C, Pieper DH, et al. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ Microbiol. 2016;18(7):2130–42. [DOI] [PubMed] [Google Scholar]
- 39.Burkovski A Cell envelope of corynebacteria: structure and influence on pathogenicity. ISRN Microbiol. 2013;2013:935736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Indrigo J, Hunter RL, Actor JK. Cord factor trehalose 6,6’-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149(Pt 8):2049–59. [DOI] [PubMed] [Google Scholar]
- 41.Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190(11):5722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chami M, Andréau K, Lemassu A, Petit JF, Houssin C, Puech V, et al. Priming and activation of mouse macrophages by trehalose 6,6’-dicorynomycolate vesicles from Corynebacterium glutamicum. FEMS Immunol Med Microbiol. 2002;32(2):141–7. [DOI] [PubMed] [Google Scholar]
- 43.Voswinkel J, Mueller A, Kraemer JA, Lamprecht P, Herlyn K, Holl-Ulrich K, et al. B lymphocyte maturation in Wegener’s granulomatosis: a comparative analysis of VH genes from endonasal lesions. Ann Rheum Dis. 2006;65(7):859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Odell E, Choong LM, Barone F, Fields P, Wilkins B, et al. Granulomatosis with polyangiitis involves sustained mucosal inflammation that is rich in B-cell survival factors and autoantigen. Rheumatology (Oxford). 2012;51(9):1580–6. [DOI] [PubMed] [Google Scholar]
- 45.Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species. Frontiers in microbiology. 2016;7:1230-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardy BL, Dickey SW, Plaut RD, Riggins DP, Stibitz S, Otto M, et al. Corynebacterium pseudodiphtheriticum Exploits Staphylococcus aureus Virulence Components in a Novel Polymicrobial Defense Strategy. MBio. 2019;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemon KP, Klepac-Ceraj V, Schiffer HK, Brodie EL, Lynch SV, Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. MBio. 2010;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown SP, Le Chat L, Taddei F. Evolution of virulence: triggering host inflammation allows invading pathogens to exclude competitors. Ecology letters. 2008;11(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(9):3429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poretsky R, Rodriguez RL, Luo C, Tsementzi D, Konstantinidis KT. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One. 2014;9(4):e93827. [DOI] [PMC free article] [PubMed] [Google Scholar]


