Abstract
Objective:
Eosinophils are tissue-dwelling immune cells. Accumulating evidence indicates that a type of cell death termed ETosis is an important cell fate involved in the pathophysiology of inflammatory diseases. Although the critical role of eosinophils, in eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg-Strauss syndrome) is well established, the presence of eosinophil ETosis (EETosis) is poorly understood.
Methods:
In vitro studies using blood-derived eosinophils were conducted to characterize EETosis. The occurrence of EETosis in tissues from patients with EGPA was studied by immunostaining and electron microscopy. Serum concentrations of eosinophil-derived proteins in healthy controls, asthmatics, and EGPA patients in active and remission states (n=15 per group) were examined.
Results:
EETosis was dependent on reactive oxygen species and peptidylarginine deiminase 4-dependent histone citrullination, resulting in the cytolytic release of net-like eosinophil extracellular traps, free galectin-10, and membrane-bound intact granules. The signature of EETosis including loss of cytoplasmic galectin-10 and deposition of granules was observed in eosinophils infiltrating into various tissues from EGPA patients. Serum eosinophil granule proteins and galectin-10 levels were increased in EGPA and positively correlated with disease activity assessed by Birmingham vasculitis activity score (galectin-10; r=0.8531, p<0.0001). When normalized by blood eosinophil count, this correlation remained for galectin-10 (r=0.7168, p<0.0001) but not for granule proteins. Galectin-10 levels in active EGPA positively correlated with serum IL-5.
Conclusion:
Eosinophils infiltrating diseased tissues in EGPA undergo EETosis. Considering the exclusive expression and large pool of cytoplasmic galectin-10 in eosinophils, elevated serum galectin-10 in patients with EGPA might reflect the systemic occurrence of cytolytic EETosis.
Keywords: ETosis, eosinophilic granulomatosis with polyangiitis, extracellular traps, eosinophils, galectin-10
Capsule summary:
Eosinophil ETosis results in the lytic release of cytoplasmic galectin-10 and citrullinated histone-containing extracellular traps, and was observed in patients with eosinophilic granulomatosis with polyangiitis.
Introduction
Eosinophils are tissue-dwelling immune cells that play an important role in type-2 inflammation. As end-stage effector cells, eosinophils may mediate cytotoxic effects on parasites or allergic tissue. The pleiotropic effects of recruited eosinophils were recently reported to affect immunomodulation, and tissue homeostasis and repair (1,2). Many of these functions rely upon the capacity of eosinophils to release a group of granule-derived proteins, including major basic protein (MBP), eosinophil peroxidase (EPO), eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN) (1,3). Understanding eosinophil activation and the process of degranulation remains one of the central quests related to the pathophysiology of eosinophilic diseases.
Detailed transmission electron microscopy (TEM) studies demonstrated that eosinophils have multiple degranulation mechanisms. Preformed, granule-stored proteins can be released by three main secretory processes: exocytosis, piecemeal degranulation, and cytolysis (1,4,5). Exocytosis is the release of whole granule contents as individual granules that fuse with the cell membrane, although this has rarely been observed in vivo. Secretory vesicle-mediated release of granule contents, termed piecemeal degranulation, is important for the selective secretion of various proteins contained in the granules. Cytolysis, or lytic degranulation, releases cytoplasmic proteins and intact eosinophil granules. This process has been reported in numerous pathological conditions, ranging from 10%–80% of all degranulation modes in vivo (1,6–8).
Recent findings revealed that lytic degranulation represents a process of active cell death, termed eosinophil ETosis (EETosis) (3,9), which is characterized by the release of filamentous chromatin structures called eosinophil extracellular traps (EETs) (10). EETs, mediators of eosinophil innate immune function, may capture pathogens; however, in excess, they can be pathogenic. EETs form stable aggregates that contribute to the viscosity of secretions observed in eosinophilic chronic rhinosinusitis (11), eosinophilic otitis (12), and allergic bronchopulmonary aspergillosis (13). EETosis is also associated with the crystallization of galectin-10 (also known as Charcot-Leyden protein or lysophospholipase) to form Charcot-Leyden crystals, a classical hallmark of eosinophilic inflammation (14,15). EETosis is the cell fate of lytic “whole cell degranulation” and therefore is crucial to understanding the pathophysiology of allergic diseases.
Eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg-Strauss syndrome) is a rare form of anti-neutrophil cytoplasm antibody-associated vasculitis that affects multiple organs. Clinical features of EGPA include various combinations of neuropathy, pulmonary infiltrates, myocarditis, ear, nose and throat, skin, gastrointestinal, and renal involvement (16). Eosinophil-rich granulomatous inflammation and small-to-medium sized vessel vasculitis characterize the pathological findings of EGPA. The critical role of eosinophils in EGPA is well established, as reported by the clinical benefit of eosinophil-targeted anti-IL-5 antibody therapy (3,17). A recent study indicated that isolated eosinophils are prone to undergo EETosis in response to autoimmune antibodies (18). Several studies indicated that neutrophil extracellular traps (NETs) might contribute to the pathogenesis of EGPA (16,19), although the presence of EETs/EETosis is less well understood.
Because there is no gold standard for detecting EETs and EETosis in vivo, we performed a series of in vitro studies to better understand the characteristics of EETosis. We studied the occurrence of EETosis in various organ tissues obtained from EGPA patients. Finally, we measured serum concentrations of eosinophil-derived proteins from healthy controls, stable asthmatics, and EGPA patients in active and remission states.
Materials and methods
Study subjects and ethical approval
Written informed consent was obtained from all participants in accordance with the principles laid out in the Declaration of Helsinki. The study used institutional review board-approved protocols (Akita University: permission No. 994; National Hospital Organization Sagamihara: 2017–048). Experimental protocols requiring purified blood eosinophils used samples obtained from patients with mild eosinophilia. Biopsy tissues and blood samples were obtained from EGPA patients treated at the Clinical Research Center for Allergy and Rheumatology, National Hospital Organization, Sagamihara, Kanagawa, Japan. Collected blood was left for 30 min at room temperature, centrifuged (10 min at 1700 ×g), and then serum was stored at −30°C. All biopsy specimens were fixed in 10% formaldehyde and embedded in paraffin. The control and asthmatic groups comprised 15 healthy subjects per group matched to the EGPA group by sex, age, and body mass index. The patients with asthma were assessed as well controlled, based on GINA guidelines, with exclusion criteria of non-steroidal anti-inflammatory drugs exacerbated respiratory disease and allergic bronchopulmonary mycosis. EGPA patients were diagnosed using the American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome with reference to the CHCC systems (20,21). Active EGPA was characterized by increased eosinophil counts (>10% eosinophils or >1000 eosinophils/μL) and symptoms listed in the Birmingham vasculitis activity score (BVAS) in at least one involved organ as shown by histological, clinical or laboratory data. All active EGPA subjects were newly diagnosed and have not been used systemic steroids except for the purpose of treating asthma. Active EGPA subjects whose symptoms flared up during treatment were not included. Remission was defined as the absence of any clinical signs or symptoms of active vasculitis for at least 3 months after these treatments. Persistent and unchanged symptoms were not defined as vasculitis in this study. BVAS (version 3) was used to capture all current symptoms, meaning a combination of active disease and previous damage. The disease features which have been present for > 1 month were counted as “persistent” score. The exclusion criterion was receiving antibody-based therapy.
Eosinophil isolation
Human eosinophils were purified by CD16 negative selection as previously described (22). Briefly, venous blood was collected into tubes containing 0.1 M ethylenediaminetetraacetic acid (EDTA)-dextran solution to sediment erythrocytes. Supernatants were collected, layered onto 1.085 g/mL Percoll (P1644, Sigma, St. Louis, MO) density gradients, and centrifuged (740 ×g, 30 min, 20°C) to separate mononuclear cells. We collected the cell pellet and added ice-cold distilled water to lyse erythrocytes. The remaining granulocytes were incubated with anti-CD16 microbeads (#130–045-701, Miltenyi Biotec, Bergisch Gladbach, Germany) for 40 min at 4–8°C. The cell suspension was applied to a magnetic column at 4°C to remove neutrophils. Eosinophil purity was >98% and viability assessed by trypan-blue exclusion was >99%.
Induction of cell death
EETosis was induced as previously described (11). Briefly, eosinophils were stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng/mL; #P1585, Sigma), immobilized IgG and IgA (1 mg/mL, coated on plates for 3 h, Sigma), or platelet-activating factor (1 μM; Enzo Life Sciences, Farmingdale, NY) and IL-5 (10 ng/mL; 205-IL, R&D Systems, Minneapolis, MN) in phenol-red free RPMI 1640 medium containing 0.3% bovine serum albumin (BSA; Sigma) at 37°C for 180 min. Necrotic cell death was induced by brief heating in medium containing 0.3% BSA (60°C for 7 min followed by 37°C for 60 min). Apoptosis was induced using an anti-Fas antibody (100 ng/mL in medium containing 10% fetal bovine serum; clone CH11, Millipore, Temecula, CA) for the indicated times. To quantify cell death, SYTOX green (1:5000; #S7020, Life Technologies, Carlsbad, CA) was added to the medium. Bright field and fluorescence images were randomly obtained and SYTOX positive cells were counted amongst at least 200 cells in a blinded manner. In some experiments, diphenyleneiodonium chloride (DPI; 20 μM; Sigma) was added to the culture medium. Indicated concentrations of Cl-Amidine (Cayman Chemical, Ann Arbor, MI) were added 15 min prior to each stimulus.
TEM
For conventional TEM, eosinophils isolated from peripheral blood (treated to induce cell death as described above) and nerve tissues obtained from six EGPA patients at the Kyoto Konoe Rehabilitation Hospital were fixed and prepared as previously described (22,23). Details were described in Supplemental Methods.
Immunofluorescence staining
Human eosinophils (1×106/mL) were seeded in eight-well LAB-TEK II chamber slides (Nunc, Roskilde, Denmark), stimulated for the indicated durations, and then fixed with 4% paraformaldehyde for 10 min. Cells were blocked with PBS containing 3% BSA at 4°C overnight and then permeabilized with PBS containing 10% BSA and 0.1% saponin. For pathological tissue analyses, samples were fixed with 10% formalin and embedded in paraffin. For MBP and galectin-10 staining, deparaffinized sections were treated for antigen retrieval with 0.1% proteinase K at room temperature for 6 min. The samples were incubated with the following primary antibodies as follows: rabbit anti-human MBP (10 μg/mL; a kind gift from Dr. Hirohito Kita, Mayo Clinic Arizona) for 30 min at 37°C and mouse anti-galectin-10 antibody (1:50; B-F42, ab27417, Abcam, Cambridge, UK) for 90 min at room temperature. Subsequently, the samples were incubated with Alexa-488 conjugated goat anti-mouse IgG (1:200; A11001, Life Technologies), Alexa-594 goat anti-rabbit IgG antibody (1:200; A11072, Life Technologies), and Hoechst 33342 (1:5000; H3570, Invitrogen, Carlsbad, CA) for 30 min at room temperature. The cytolysis index was calculated from images immunostained for galectin-10 and MBP: a greater loss of intracellular galectin-10 compared with cell-retained MBP yielded an elevated cytolysis index. Assessment of the cytolysis index was conducted as described in Supplemental Fig. 1. The non-cytolytic control value was set at 0.7. For galectin-10 and MBP staining, 23 tissue samples were obtained from 16 EGPA patients (Supplemental Table 1).
For CitH3 staining, antigen retrieval of human eosinophil samples was performed by incubation for 15 min in Tris-EDTA buffer in a microwave oven. The slides were subsequently incubated with primary rabbit anti-CitH3 monoclonal antibody (10 μg/mL, 90 min at room temperature; Abcam). Alexa-488–conjugated secondary antibodies (Life Technologies) were then added for 30 min at room temperature. Isotype-matched control antibodies and Hoechst 33342 were used in each experiment. Samples were mounted using Prolong Diamond (Life Technologies) and images were obtained using a LSM 780 confocal microscope (Carl Zeiss, Oberkochen, Germany). In some experiments, coverslips were removed and samples were stained with hematoxylin-eosin (H&E) (22). For CitH3 staining, 14 tissue samples were obtained from 10 EGPA patients (lung, n=3; skin, n=6; upper digestive tract, n=4; lower digestive tract, n=1). Tissue samples with nonspecific staining were excluded.
Western blotting and measurement of galectin-10, EDN, ECP, lactate dehydrogenase (LDH) and IL-5 levels.
Details were described in Supplemental Methods.
Statistical analyses
Data were analyzed using GraphPad Prism 5.04 (GraphPad Software, San Diego, CA). Differences between groups were assessed using unpaired t-tests and one-way analysis of variance (ANOVA) followed by the Newman-Keuls test. Blood sample data were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparison post-hoc test. One-way ANOVA with post hoc Tukey’s test were used for normally distributed data. The correlation was analyzed using Spearman’s correlation analysis. Values of P<0.05 were considered statistically significant.
Results
EETosis is characterized by reactive oxygen species (ROS)-dependent histone citrullination and release of EETs
To better characterize the different types of eosinophil cell death, we assessed the ultrastructural morphologies of EETosis, apoptosis, and necrosis in vitro (Fig. 1A–C). Each type of cell death was induced in purified human eosinophils using previously established conditions (9). Anti-Fas antibody-stimulated eosinophils showed the classical morphology of apoptosis, including cytoplasmic and nuclear condensation (Fig. 1B). Heat-treated eosinophils showed morphology typical of necrosis, including bleb formation and organ swelling. Notably, most electron-dense granular contents remained in apoptotic cells, but were lost from necrotic cells (Fig. 1C). PMA-stimulated EETotic cells showed chromatolysis and plasma membrane disintegration (Fig. 1A). In line with previous reports (9,14), membrane-bound cell-free granules filled with granular contents were associated with the originating EETotic cell. Similar morphologies were observed using other known physiological EETosis stimuli (Supplemental Fig. 2).
Figure 1. Citrullinated histone H3-loaded EETs are released during EETosis.
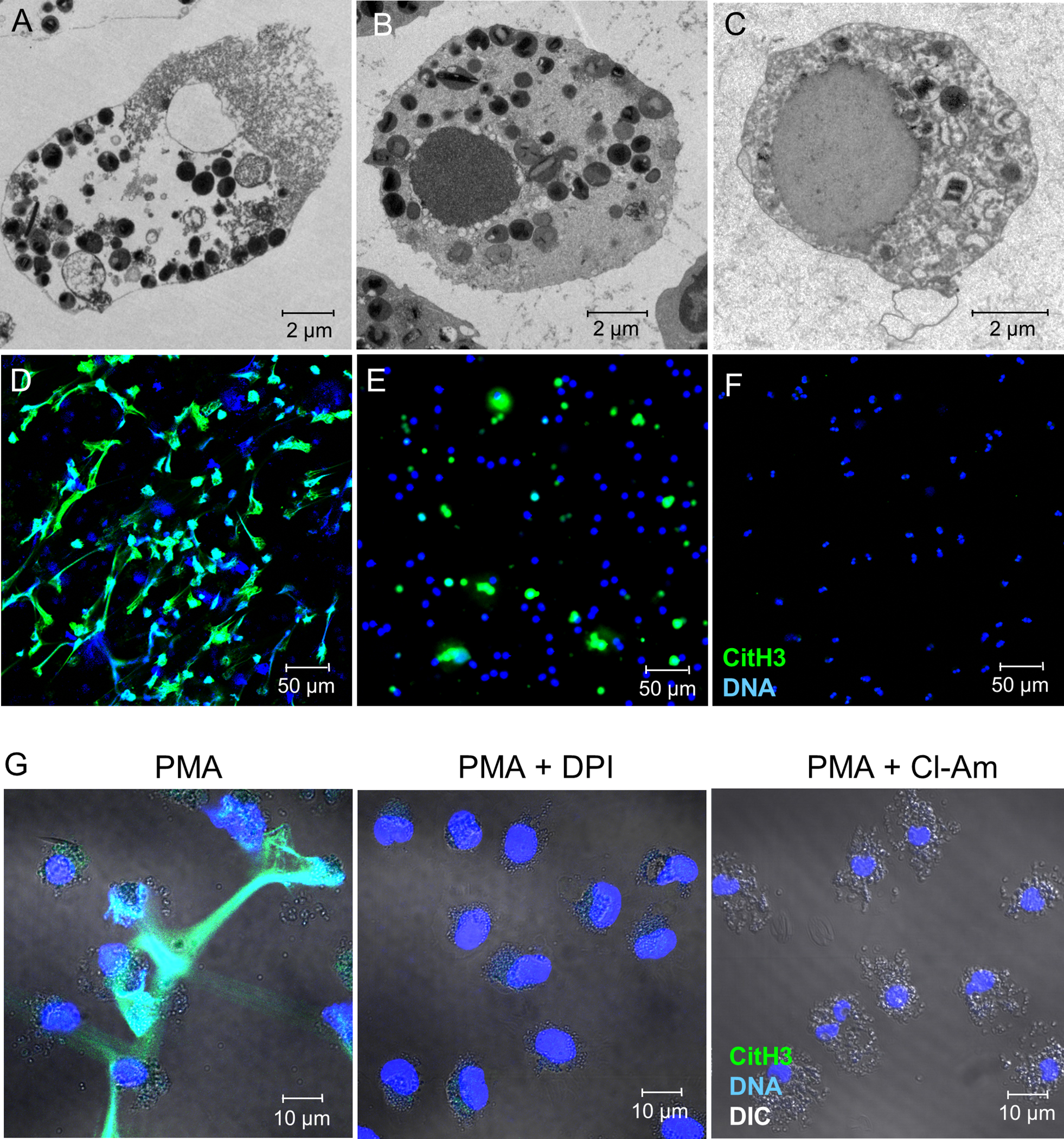
Using purified human eosinophils, EETosis was induced by stimulation with PMA for 180 min (A, D), apoptosis was induced by treatment with anti-Fas antibody for 48 h (B, E), and necrosis was induced by heat treatment for 7 min followed by incubation at 37°C for 60 min (C, F). Cells were assessed by transmission electron microscopy (A–C) and immunofluorescence staining of citrullinated histone H3 (CitH3; green) and DNA (blue) (D–F) was visualized by confocal microscopy (20× objective). (G) Eosinophils were stimulated with PMA for 3 h in the presence of vehicle, DPI, or Cl-Amidine (Cl-Am) and assessed by immunofluorescence staining. Differential interference contrast (DIC) images were merged.
In neutrophils, peptidylarginine deiminase 4 (PAD4)-mediated histone hyper-citrullination was reported to have an essential role in the formation of NETs by relaxing the chromatin structure (24,25). We assessed the presence of CitH3 in each type of cell death using immunostaining. As expected, EETotic cells released CitH3-stained net-like EETs (Fig. 1D). CitH3-positive EETs were observed regardless of the EETosis stimulus (Supplemental Fig. 2). CitH3 was also detected in apoptotic cells, although EETs were not observed (Fig. 1E). Necrotic cells did not show any CitH3 (Fig. 1F). These results were confirmed by western blotting for CitH3 and total histone H3 (Supplemental Fig. 3). Taken together, these data suggest that EETosis is characterized by the cytolytic release of CitH3-positive EETs and cell-free granules.
EETosis involves the NADPH oxidase-dependent production of ROS (9). To examine the role of ROS in histone hyper-citrullination, eosinophils were stimulated with or without DPI, an NADPH-oxidase inhibitor. As shown in Fig. 1G and Supplemental Figs. 3, 4, EETosis-mediated cytolysis and CitH3-positive EETs were completely inhibited by DPI. We also confirmed PAD4-mediated histone hyper-citrullination using Cl-Amidine, a pharmacological PAD4 inhibitor. Histone citrullination and formation of EETs were completely inhibited by Cl-Amidine (Fig. 1G, Supplemental Figs. 3,4). Interestingly, varying concentrations of Cl-amidine did not inhibit cell death, even at higher concentration (Supplemental Fig. 5). These results indicated that cytolytic cell death was mediated by enzymatic activation of NADPH-oxidase but release of net-like EETs was dependent on the NADPH-oxidase-PAD4 pathway.
Cytolytic release of cytoplasmic galectin-10 and intact granules
Human eosinophils contain a large pool of galectin-10, an S-type lectin that comprises 10% of total eosinophil cytoplasmic protein (26). During EETosis, cytoplasmic galectin-10 is released extracellularly (14). To assess the precise subcellular localization of galectin-10, we used a nanogold-conjugated antibody and TEM (27). In blood eosinophils, galectin-10 was consistently localized in the peripheral cytoplasm but not within granules (Fig. 2A). We stimulated eosinophils to induce ETosis, fixed them at 15 and 180 min, then immunostained for galectin-10 (green) and MBP (red) (Fig. 2B, Supplemental Fig. 6). In unstimulated cells, galectin-10 and MBP were localized in the cytoplasm and in granules, respectively. Cells undergoing EETosis and releasing net-like DNA (blue) were only stained with MBP, indicating the cytolytic loss of galectin-10 was not accompanied by loss of granular contents. We quantified the immunostaining images further using ImageJ software. The MBP/galectin-10 ratio was considered to reflect the cytolysis index whereby the greater loss of intracellular galectin-10 over that of cell-retained MBP yielded an elevated cytolysis index (Supplemental Fig. 1). The cytolysis index was comparable following stimulation for 15 min, but was significantly increased by stimulation for 180 min with various EETosis-inducing stimuli (Fig. 2C).
Figure 2. Cytoplasmic galectin-10, but not granule proteins, are released during EETosis.
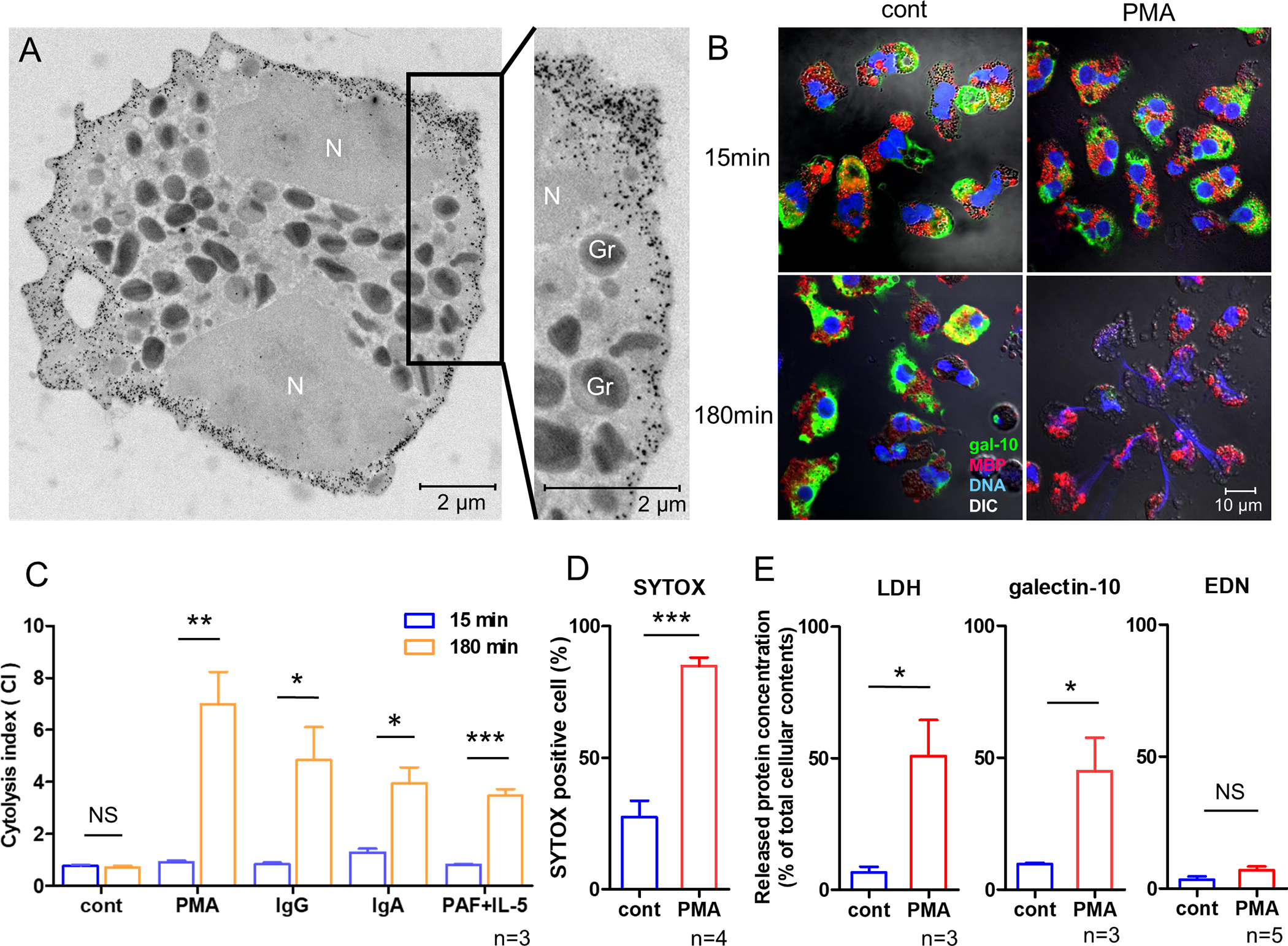
(A) Ultrastructural immunolabeling of galectin-10 in unstimulated eosinophils. Black dots indicate nanogold-conjugated antibody. N, nucleus. Secretory granules (Gr) show typical morphology (an electron-dense core surrounded by an electron-lucent matrix). (B) Merged immunofluorescence staining of galectin-10 (green), MBP (red), and DNA (blue) and differential interference contrast (DIC) images obtained by confocal microscopy (100× objective). (C) Cytolysis index (the ratio of intracellular MBP- and galectin-10 stained areas) was assessed as described in Supplemental Fig. 1. (D) EETosis was induced by treatment with PMA for 180 min. Membrane permeability was assessed using SYTOX. (E) Following induction of EETosis (PMA, 180 min), culture supernatants were obtained by centrifugation at 10,000 ×g for 10 min. LDH, galectin-10, and EDN concentrations were measured and normalized to levels in cell lysates as 100%. *P<0.05, **P<0.01, ***P<0.005, n=3–5 from different donors. NS, not significant. Bar graph represents the mean ± SD.
The cell-impermeable DNA-specific dye, SYTOX, can detect cells whose plasma and nuclear membranes have been compromised. Following induction of EETosis by treatment with PMA for 180 min, approximately 85% of eosinophils were SYTOX-positive (Fig. 2D). To quantify the proteins released by EETosis, levels of LDH (a cytoplasmic protein), galectin-10, and EDN (a granular protein) in culture medium were measured (Fig. 2E). Culture medium was centrifuged at 10,000 ×g to remove contaminating cellular components including major free vesicles and granules. EETosis resulted in the release of 51% total cellular LDH and 45% galectin-10, but only 7% EDN. These results indicated that the release of intracellular galectin-10 was closely associated with cytolysis, rather than the classical secretory mechanisms of degranulation in accordance with recent studies (14,23).
Eosinophils infiltrating into various tissues show the EETosis signature in EGPA patients
We studied affected tissues from patients with active EGPA to confirm our in vitro results. H&E staining showed the infiltration of intact eosinophils as well as chromatolytic cells and free eosinophil granules (Fig. 3A and Supplemental Fig. 7, arrows). Immunostaining of identical fields indicated these lytic eosinophils contained net-like CitH3 and DNA (Fig. 3B and Supplemental Fig. 7, arrows). CitH3-stained lytic eosinophils were detected in 12 of 14 biopsy samples from 10 patients with EGPA. The characteristic ultrastructural morphology of EETosis was also confirmed by TEM (Fig. 3C). We did not observe eosinophils with apoptotic or necrotic morphology. Next, biopsy samples were immunostained for galectin-10 and MBP. Low magnification images were typical of inflamed tissues and showed diffuse staining of MBP and cellular staining of galectin-10 (Fig. 3D, E). High magnification images showed intact eosinophils positive for galectin-10 and MBP (Fig. 3F, arrowheads), whereas lytic cells and free granules were only positive for MBP (Fig. 3F, arrows). Cytolytic eosinophils were detected in 21 of 23 samples from 15 patients and were often associated with small punctate galectin-10 labeling (Fig, 3F, open arrowheads), which were probably eosinophil extracellular vesicles released from EETotic cells (9,14). No cells were positively stained for cytoplasmic galectin-10 alone. Additional representative images are shown in Supplemental Fig. 8. A summary of detailed clinical data obtained from EGPA patient biopsy samples is shown in Supplemental Table. 1. The cytolysis index of affected tissue from EGPA patients exceeded 0.7 (the control value obtained from intact cells) except for one of 23 samples. These results show for the first time that eosinophils infiltrating into various tissues undergo EETosis in EGPA patients.
Figure 3. Presence of EETosis in affected tissues in patients with EGPA.

(A) Hematoxylin-eosin staining shows chromatolytic eosinophils and adjacent cell-free granules (arrows) in a lung biopsy from a patient with EGPA. (B) Immunostaining image (identical field to A) for CitH3 (green) and DNA (Hoechst 33342, blue) indicates mesh-like extracellular traps (arrows). Note that most intact cells are not stained with CitH3 (100× objective). (C) Typical EETosis morphology, characterized by plasma/nuclear membrane disintegration and chromatin decondensation, is present in nerve tissue from an EGPA patient, observed by TEM. Chromatolytic nucleus (N) and free granules (Gr) are observed in nerve tissue. (D) Confocal image of tissues from EGPA patients stained with two isotype-matched control antibodies show no fluorescence (skin biopsy; DNA: blue, 20× objective). (E) Serial sections of (D) were stained for galectin-10 (green), MBP (red), and DNA (blue) and observed using identical parameters. (F) Under higher magnification (100× objective), intact eosinophils retained MBP and galectin-10 (arrowheads). Separate distinct and focal extracellular punctate MBP (arrows) and galectin-10 stainings (open arrowheads) are indicative of free extracellular granules and vesicles, respectively. Cytolytic eosinophils were observed in 21 samples from 16 patients.
Serum galectin-10 levels in EGPA are associated with disease activity
Given the difference in the release of cytoplasmic galectin-10 and granule proteins, we investigated their serum concentrations in healthy individuals, stable asthmatics, and EGPA patients whose disease was active and in remission (Table 1, Supplemental Table 2). Elevated galectin-10 was detected in all active EGPA patients but only seven of 15 EGPA subjects in remission (cutoff level: 0.312 ng/mL). Levels of galectin-10 and granule proteins (EDN and ECP) were significantly elevated in patients with active EGPA (Fig. 4A). To exclude the possibility that blood eosinophil density affected these results (28,29), we compared serum concentrations of these proteins normalized to blood eosinophil counts (Fig. 4B). Normalized levels of galectin-10, but not of granule proteins, were significantly elevated in patients with active EGPA. The detailed correlation with eosinophil counts in each group are shown in Supplemental Table 3.
Table 1.
Patient characteristics and laboratory data.
| Parameters | Active EGPA | Remission EGPA | Asthma | Healthy | P value |
|---|---|---|---|---|---|
| Sample size | 15 | 15 | 15 | 15 | |
| Age,yr | 50 (46.5–65.5) | 59.5 (48.5–66.5) | 49 (42.0–57.0) | 50 (41.5–59.5) | 0.5264 |
| Male / Female | 8 / 7 | 6 / 9 | 7/8 | 6 / 9 | 0.8636 |
| Body mass index (kg/m2) | 21.4 (18.0–23.6) | 21.4 (18.5–23.9) | 23.7 (20.8–25.4) | 21.1 (20.3–22.2) | 0.3367 |
| Atopy, n (%) | 10 (66.67) | 8 (53.33) | 11 (73.33) | 5 (33.33) | 0.1271 |
| GINA Step 1/2/3/4/5 | 2/1/0/5/7 | 0/1/0/4/10 | 0/1/3/8/3 | - | 0.0548 |
| Smoking never/ex/current | 10/5/0 | 12/3/0 | 11/4/0 | 12/3/0 | 0.8066 |
| Basic laboratory tests | |||||
| White blood cells (103/μl) | 12.6 (11.1–24.5) | 8.1 (6.5–8.5) | 5.4 (4.67–6.34) | 6.0 (5.1–7.0) | <0.0001 |
| Blood eosinophilia (103/μl) | 5.0 (3.4–15.5) | 0.5 (0.4–0.7) | 0.39 (0.34–0.54) | 0.2 (0.1–0.3) | <0.0001 |
| Blood platelet count (103/μl) | 299 (236–394) | 245 (214–261) | 237 (186–267) | 212 (195–249) | 0.03 |
| IgE (IU/ml) | 1060 (702–2530) | 134 (68–375) | 341 (199–574) | - | 0.0002 |
| MPO-ANCA positive, n (%) | 5 (33.3) | 6 (40.0) | - | - | >0.9999 |
| BVAS | 12.0 (10.0–30.0) | 4.0 (4.0–5.0) | - | - | <0.0001 |
| Diagnostic disease characteristics at onset of EGPA, n (%) | |||||
| Asthma with eosinophilia | 15 (100) | 15 (100) | - | - | >0.9999 |
| Biopsy evidence * | 8 (53.3) | 12 (80.0) | - | - | 0.2451 |
| Neuropathy | 13 (86.7) | 15 (100) | - | - | 0.4828 |
| Pulmonary infiltrates | 7 (46.7) | 6 (40.0) | - | - | >0.9999 |
| Sinonasal abnormality | 14 (93.3) | 13 (86.7) | - | - | >0.9999 |
| Cardiomyopathy | 1 (6.7) | 4 (26.7) | - | - | 0.3295 |
| Palpable purpura | 5 (33.3) | 4 (26.7) | - | - | >0.9999 |
Categorical variables are presented as numbers (percentages) and continuous variables as median and interquartile range. For diagnostic disease characteristics at the onset of EGPA, we referred to the BVAS; for more details about BVAS, see the Materials and methods section.
Biopsy evidence was defined as a biopsy specimen showing histopathological evidence of eosinophilic vasculitis, perivascular eosinophilic infiltration, or eosinophil-rich granulomatous inflammation.
Figure 4. Increased serum galectin-10 levels in patients with EGPA.

(A) Levels of eosinophil-derived proteins in serum. Galectin-10, EDN, and ECP levels were measured by ELISA. Data are presented as box plots showing median values, interquartile ranges, and minima/maxima. (B) Levels of eosinophil-derived proteins were normalized to blood eosinophil counts. Fifteen samples were analyzed for each group. (C) Correlation between eosinophil-derived proteins and disease activity score in patients with EGPA (n=30). For more details about BVAS, see Materials and methods section. (D) Correlation between normalized eosinophil-derived proteins and disease activity score in patients with EGPA (n=30).
We then investigated whether the clinical symptoms in EGPA patients were associated with levels of galectin-10 and granule proteins. Disease activity was characterized using the BVAS scoring system. As expected, there was a positive correlation between BVAS score and serum eosinophil-derived proteins (Fig. 4C). When serum concentrations were normalized by blood eosinophils, galectin-10 was positively correlated with BVAS score but granule proteins showed a negative correlation (Fig. 4D). These results indicated that galectin-10 is a unique biomarker for patients with EGPA.
Serum IL-5 levels are associated with galectin-10 in active EGPA
Since the IL-5-eosinophil axis plays a critical role in the pathogenesis of EGPA (3,16,30), we measured serum IL-5 in all subjects. As expected, serum IL-5 levels significantly increased in active EGPA patients compared with other subjects (Fig. 5A). In addition, a positive correlation between galectin-10 and IL-5 was observed (Fig. 5B), indicating the causal role of IL-5 in increased galectin-10. We further assessed the relationship between IL-5 and blood eosinophil count, galectin-10, ECP, and EDN in each group (Supplemental Table. 4). Of note, a positive correlation was observed only with galectin-10 in the active EGPA group.
Figure 5. Serum IL-5 and galectin-10 levels.
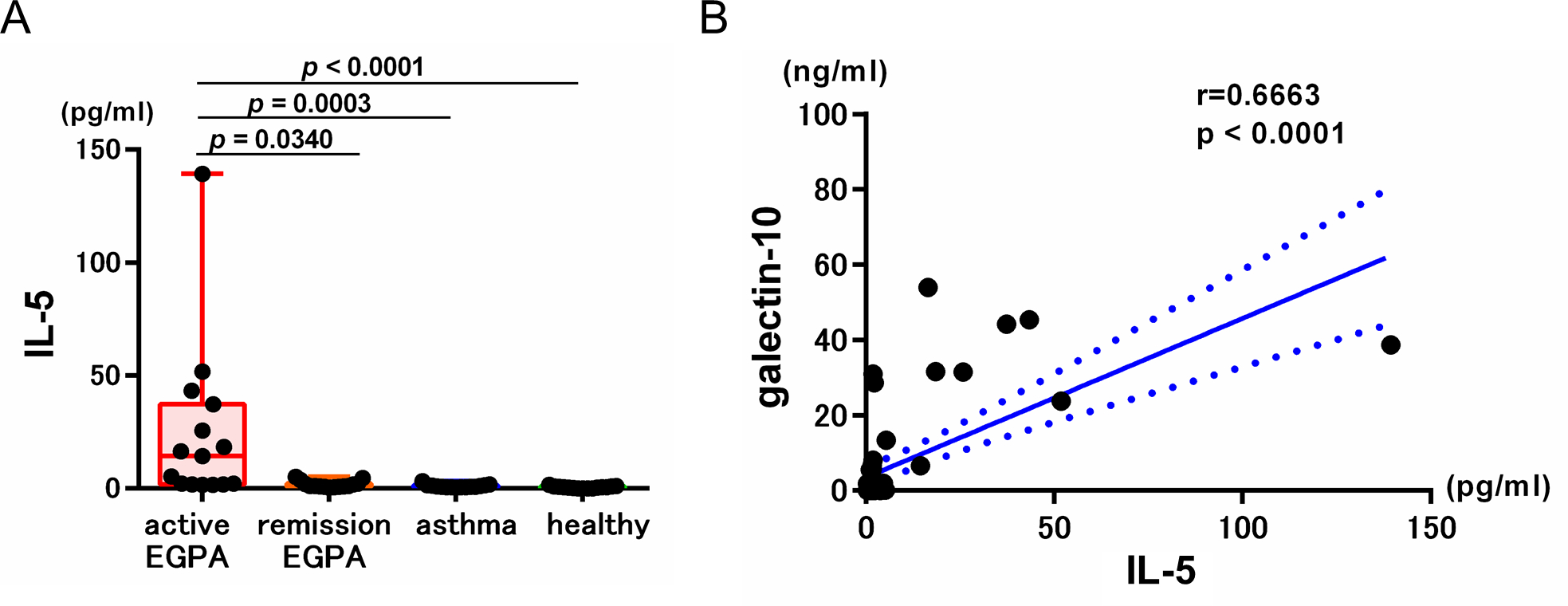
(A) Serum IL-5 levels were measured by ELISA. Data are presented as box plots showing median values, interquartile ranges, and minima/maxima. (B) Correlation between serum IL-5 and galectin-10 in all subjects (n=60).
Discussion
ETosis, the controlled release of chromatin from inflammatory cells, is considered an evolutionarily conserved mechanism of the innate immune system (31). Accumulating studies of neutrophils have indicated that uncontrolled ETosis can lead to end-organ dysfunction; however, the relationship with eosinophils is less clear. Similar to neutrophils, eosinophils terminally differentiate in the bone marrow and are non-dividing cells. Evaluation of eosinophil cell death is therefore essential to our understanding of eosinophilic inflammatory diseases. Eosinophils are the major cells responsible for EGPA, as opposed to neutrophils in other vasculitides including microscopic polyangiitis and granulomatosis with polyangiitis (32). The present study is the first demonstration that EETosis occurs in patients with EGPA. Galectin-10, a non-secreted, lectin-like protein that is highly abundant in the cytoplasm of human (and not other animal) eosinophils (33), was detected in the serum of EGPA patients and was associated with disease activity. Our study suggests that galectin-10 might be a novel biomarker for systemic eosinophilic inflammation.
Using isolated human eosinophils, we found several characteristics of EETosis that were clearly different from apoptosis and necrosis. EETosis does not cause DNA fragmentation but disintegrates the plasma and nuclear membranes through NADPH oxidase activation (9,22) and eventually releases PAD4-mediated CitH3-positive EETs. Indeed, patients with chronic granulomatosis diseases lacking ROS production in phagocytes and PAD4 deficient mice were susceptible to bacterial infection related to a lack of NET formation (25,34). PAD4 is a calcium-dependent enzyme responsible for histone hypercitrullination (24) and calcium ionophore is a potent EETosis inducer (9). Adversely, physiological stimuli-induced EETosis was completely inhibited by a calcium chelator EDTA (9,11). These signaling pathways might have an important role in future therapeutic modalities by regulating EETs.
Lytic eosinophils in inflamed tissue are not caused by necrosis or related to artifacts caused by the handling of sample preparation (35). We did not observe morphologically identifiable apoptotic or necrotic eosinophils in EGPA tissues using TEM. In contrast, EETotic cells were consistently observed in diseased tissues. This is likely because of the rapid process (0.5–3 h in vitro) of EETosis and lack of surface phosphatidylserine redistribution typical during apoptosis (9). Phosphatidylserine is recognized by macrophages as an “find-me” signal (36); however, extracellular traps (ETs) remain in the tissue without macrophage processing. Apoptotic eosinophils are rapidly engulfed by phagocytes, protecting tissues from harmful exposure to the inflammatory contents (37). A previous electron microscopic study revealed that the occurrence of apoptosis in eosinophils in allergic airway tissues was rare (6).
Nuclear histones and DNA have been known to act as alarmins (8,38). To identify EETs/EETosis in tissue samples, we utilized multiple methods including TEM, conventional H&E staining, and immunofluorescence confocal microscopy targeting relevant indicators of EETs/EETosis. Detecting EETs only in conventional thin sections of solid tissues is challenging because limited cross-sectional planes of view preclude the recognition of eosinophils and EETs extending into contiguous but unexamined sections (37). Because NETs contain neutrophil elastase and myeloperoxidase, double immunostaining techniques for DNA (histones) and these neutrophil-specific granule proteins can be utilized to identify NETs (25). In contrast, eosinophil granule proteins were not uniformly co-localized with EETs, because EETosis releases intact granules but not free granule-derived proteins (9,22). A recent electron microscopy study reported the ongoing release of free eosinophil granules in a sural nerve biopsy of an EGPA subject (39). The extracellular granules remained in the tissues and secreted their contents (9,37,40).
Eosinophil granule proteins have marked potential toxicity for host tissues and significant functions relevant to the mechanisms of pathogenesis in EGPA. For example, MBP contributed to increased epithelial permeability, smooth muscle contraction, and liberation of molecules related to tissue remodeling and fibrosis (41). EDN can recruit dendritic cells and enhance antigen-specific immune responses (42). In agreement with our data, the deposition of extracellular eosinophil granule proteins was prominently associated with lesional tissues from patients with EGPA (43). The cationic nature of granule proteins suggests they are adsorbed to negatively-charged tissues and likely contribute to the local inflammatory response (2,44). Previous studies reported increased serum eosinophil granule proteins in patients with EGPA: higher concentrations of serum ECP (45), MBP (43,46), and EDN (43,46) were observed in active EGPA compared with healthy controls.
To the best of our knowledge, there have been no reports of the clinical significance of serum galectin-10. Eosinophils contain large amounts of galectin-10 in the cytoplasm, which is not secreted by piecemeal degranulation (14,23). Our current data indicate galectin-10 as a unique marker with different characteristics compared with granule-derived proteins. Cationic eosinophil granule proteins bind strongly to tissue elements and aggregate with long half-lives and thus may fail to readily enter the peripheral circulation (2,47). The rapid release of extracellular galectin-10 and membrane-bound, cell-free intact eosinophil granules (with their contained cationic granule proteins) from human eosinophils might be associated with differing serum concentrations of galectin-10 and eosinophil granule proteins.
A proposed mechanism of increased galectin-10 in EGPA is shown in Supplemental Fig. 9. In patients with EGPA, serum IL-5 has been shown to be associated with disease severity (48,49) and anti-IL-5 treatment is an important therapeutic modality (50). Taken together with the fact that IL- 5 induces EETosis in the presence of additional stimulus (9), IL-5-elicited eosinophil activation cascades might lead to the induction of EETosis and the release of galectin-10. Unlike stable asthmatics, increased serum galectin-10 was observed in patients with active EGPA, likely due to systemic occurrence of EETosis.
Although our results suggest serum galectin-10 as a surrogate marker for EETosis, there are limitations in this study. Galectin-10 levels could be affected by the various factors including eosinophil production, distribution, and activation status and also by blood half-life of galectin-10. Galectin-10 may also be expressed by basophils and some T-cell subsets (33). We speculate that these cells were not contributory because galectin-10-positive cells were consistently stained for eosinophil-specific MBP in our histological analysis. Elevated galectin-10 might not specific to EGPA since the occurrence of EETosis is observed in other diseases (8,51). Future studies on galectin-10 levels from various eosinophilic diseases in different conditions are required to provide cogent evidence between EETosis and galectin-10.
Eosinophils, granulocytes of the innate immune system, contain distinct proteins in their granules and cytoplasm. Proteomic analysis of human peripheral blood revealed that galectin-10 was the fifth most abundant human eosinophil protein (after actin, a non-secretory ribonuclease and histones) (52). Another earlier proteomic study found galectin-10 was the second most prevalent protein in eosinophil cytoplasmic subcellular fractions (53). Considering the exclusive expression and large pool of galectin-10 in eosinophils, elevated serum galectin-10 levels in EGPA patients likely reflects systemic eosinophil cytolysis/EETosis.
Supplementary Material
Acknowledgments
The authors are grateful to Noriko Tan for outstanding technical assistance and Satomi Misawa for outstanding assistance in drawing the figure. We thank Ludovic Croxford and Dr Gillian Campbell from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Funding statement:
This study was funded in part by a Research Grant on Allergic Disease and Immunology from the Japan Agency for Medical Research and Development (JP20ek0410055 to SU), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (SU), the Japanese Society of Laboratory Medicine Fund for Promotion of Scientific Research (SU), JSPS KAKENHI (16K08926, 15KK0329, and 20K08794 to SU; 19K17898 to YK), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (309734/2018–5 and 434914/2018–5 to RCNM), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (CBB-APQ-03647–16 to RCNM), and the National Institutes of Health (R37AI020241 to PFW).
Conflict of interest disclosure: MF and Y. Konno received GlaxoSmithKline (GSK) Japan Research Grants 2017 (Y. Konno) and 2018 (MF); Y. Kamide received honoraria for lectures from AstraZeneca (AZ); SU received honoraria for lectures from AZ and GSK and grant support from AZ, Novartis, Sanofi and Maruho Co. Ltd; NO received honoraria for lectures from AZ; HT received grant support from Teijin; and MT received grant support from AZ and GSK. PFW received advisory board honoraria from GSK. The remaining authors declare no competing financial interests.
Abbreviations
- BVAS
Birmingham vasculitis activity score
- CitH3
citrullinated histone H3
- DIC
differential interference contrast
- ECP
eosinophil cationic protein
- EDN
eosinophil-derived neurotoxin
- EETosis
eosinophil ETosis
- EETs
eosinophil extracellular traps
- ETs
extracellular traps
- ETosis
extracellular trap cell death
- EGPA
eosinophilic granulomatosis with polyangiitis
- LDH
lactate dehydrogenase
- MBP
major basic protein
- NETs
neutrophil ETs
- PAD4
peptidylarginine deiminase 4
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- TEM
transmission electron microscopy
References
- 1.Melo RCN, Liu L, Xenakis JJ, Spencer LA. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy 2013;68:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klion AD, Ackerman SJ, Bochner BS. Contributions of Eosinophils to Human Health and Disease. Annu Rev Pathol 2020;15:179–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int 2020;69:178–86. [DOI] [PubMed] [Google Scholar]
- 4.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol 2017;17:746–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo RCN, Weller PF. Contemporary understanding of the secretory granules in human eosinophils. J Leukoc Biol 2018;104:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erjefalt JS, Persson CG. New aspects of degranulation and fates of airway mucosal eosinophils. Am J Respir Crit Care Med 2000;161:2074–85. [DOI] [PubMed] [Google Scholar]
- 7.Saffari H, Hoffman LH, Peterson KA, Fang JC, Leiferman KM, Pease LF 3rd, et al. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol 2014;133:1728–34 e1. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee M, Lacy P, Ueki S. Eosinophil Extracellular Traps and Inflammatory Pathologies-Untangling the Web! Front Immunol 2018;9:2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueki S, Melo RCN, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013;121:2074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal 2008;1:pe25. [DOI] [PubMed] [Google Scholar]
- 11.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol 2016;137:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta N, Ueki S, Konno Y, Hirokawa M, Kubota T, Tomioka-Matsutani S, et al. ETosis-derived DNA trap production in middle ear effusion is a common feature of eosinophilic otitis media. Allergol Int 2018;67:414–6. [DOI] [PubMed] [Google Scholar]
- 13.Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, et al. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol 2018;141:571–85 e7. [DOI] [PubMed] [Google Scholar]
- 14.Ueki S, Tokunaga T, Melo RCN, Saito H, Honda K, Fukuchi M, et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood 2018;132:2183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson EK, Verstraete K, Heyndrickx I, Gevaert E, Aegerter H, Percier J-M, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 2019;364. [DOI] [PubMed] [Google Scholar]
- 16.Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int 2019;68:430–6. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmark T, Ohlsson S, Pettersson A, Hansson M, Johansson ACM. Eosinophils in anti-neutrophil cytoplasmic antibody associated vasculitis. BMC Rheumatol 2019;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natorska J, Zabczyk M, Siudut J, Krawiec P, Mastalerz L, Undas A. Neutrophil extracellular traps formation in patients with eosinophilic granulomatosis with polyangiitis: association with eosinophilic inflammation. Clin Exp Rheumatol 2017;35Suppl 103:27–32. [PubMed] [Google Scholar]
- 20.Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094–100. [DOI] [PubMed] [Google Scholar]
- 21.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 22.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol 2016;137:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melo RCN, Wang H, Silva TP, Imoto Y, Fujieda S, Fukuchi M, et al. Galectin-10, the protein that forms Charcot-Leyden crystals, is not stored in granules but resides in the peripheral cytoplasm of human eosinophils. J Leukoc Biol 2020:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol 2012;3:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 2012;198:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo RCN, Wang H, Silva TP, Imoto Y, Fujieda S, Fukuchi M, et al. Galectin-10, the protein that forms Charcot-Leyden crystals, is not stored in granules but resides in the peripheral cytoplasm of human eosinophils. J Leukoc Biol 2020;108:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo RCN, Morgan E, Monahan-Earley R, Dvorak AM, Weller PF. Pre-embedding immunogold labeling to optimize protein localization at subcellular compartments and membrane microdomains of leukocytes. Nat Protoc 2014;9:2382–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai C, Yamazaki H, Tanaka Y, Matsunaga M, Numata O, Torigoe K. Ratio of eosinophil cationic protein/eosinophil count as a new marker in children with acute asthma. Pediatr Int 1999;41:142–6. [DOI] [PubMed] [Google Scholar]
- 29.Koh YY, Kang H, Kim CK. Ratio of serum eosinophil cationic protein/blood eosinophil counts in children with asthma: comparison between acute exacerbation and clinical remission. Allergy Asthma Proc 2003;24:269–74. [PubMed] [Google Scholar]
- 30.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. New England Journal of Medicine 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robb CT, Dyrynda EA, Gray RD, Rossi AG, Smith VJ. Invertebrate extracellular phagocyte traps show that chromatin is an ancient defence weapon. Nat Commun 2014;5:4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaigne B, Guillevin L. Vasculitis for the internist: focus on ANCA-associated vasculitis. Intern Emerg Med 2017;12:577–85. [DOI] [PubMed] [Google Scholar]
- 33.Weller PF, Wang H, Melo RCN. The Charcot-Leyden crystal protein revisited-A lysopalmitoylphospholipase and more. J Leukoc Biol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 2010;207:1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson CG, Erjefalt JS. “Ultimate activation” of eosinophils in vivo: lysis and release of clusters of free eosinophil granules (Cfegs). Thorax 1997;52:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata S Apoptosis and Clearance of Apoptotic Cells. Annu Rev Immunol 2018;36:489–517. [DOI] [PubMed] [Google Scholar]
- 37.Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, et al. Eosinophil ETosis and DNA Traps: a New Look at Eosinophilic Inflammation. Curr Allergy Asthma Rep 2016;16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011;118:1952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishi R, Koike H, Ohyama K, Fukami Y, Ikeda S, Kawagashira Y, et al. Differential clinicopathologic features of EGPA-associated neuropathy with and without ANCA. Neurology 2020;94:e1726–e37. [DOI] [PubMed] [Google Scholar]
- 40.Neves JS, Perez SA, Spencer LA, Melo RCN, Reynolds L, Ghiran I, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A 2008;105:18478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, Fillon S, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2013;62:1395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med 2008;205:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peen E, Hahn P, Lauwers G, Williams RC Jr., Gleich G, Kephart GM. Churg-Strauss syndrome: localization of eosinophil major basic protein in damaged tissues. Arthritis Rheum 2000;43:1897–900. [DOI] [PubMed] [Google Scholar]
- 44.Adamko DJ, Wu Y, Ajamian F, Ilarraza R, Moqbel R, Gleich GJ. The effect of cationic charge on release of eosinophil mediators. J Allergy Clin Immunol 2008;122:383–90, 90 e1–4. [DOI] [PubMed] [Google Scholar]
- 45.Guilpain P, Auclair JF, Tamby MC, Servettaz A, Mahr A, Weill B, et al. Serum eosinophil cationic protein: a marker of disease activity in Churg-Strauss syndrome. Ann N Y Acad Sci 2007;1107:392–9. [DOI] [PubMed] [Google Scholar]
- 46.Drage LA, Davis MD, De Castro F, Van Keulen V, Weiss EA, Gleich GJ, et al. Evidence for pathogenic involvementof eosinophils and neutrophilsin Churg-Strauss syndrome. J Am Acad Dermatol 2002;47:209–16. [DOI] [PubMed] [Google Scholar]
- 47.Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E, et al. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell 2015;57:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kataoka H, Tomita T, Kondo M, Mukai M. Presence of purpura is related to active inflammation in association with IL-5 in eosinophilic granulomatosis with polyangiitis. Rheumatol Int 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellmich B, Csernok E, Gross WL. Proinflammatory cytokines and autoimmunity in Churg-Strauss syndrome. Ann N Y Acad Sci 2005;1051:121–31. [DOI] [PubMed] [Google Scholar]
- 50.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N Engl J Med 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuchi M, Miyabe Y, Furutani C, Saga T, Moritoki Y, Yamada T, et al. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol Int 2021;70:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, Jarjour NN, et al. The Peripheral Blood Eosinophil Proteome. J Proteome Res 2016;15:1524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Straub C, Pazdrak K, Young TW, Stafford SJ, Wu Z, Wiktorowicz JE, et al. Toward the Proteome of the Human Peripheral Blood Eosinophil. Proteomics Clin Appl 2009;3:1151–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


