Abstract
Background.
Polymorphisms in GRM3, the gene encoding the mGlu3 metabotropic glutamate receptor, are associated with impaired cognition and neuropsychiatric disorders such as schizophrenia. Limited availability of selective genetic and molecular tools has hindered progress in developing a clear understanding of the mechanisms through which mGlu3 receptors regulate synaptic plasticity and cognition.
Methods.
We examined associative learning in mice with trace fear conditioning, a hippocampal-dependent learning task disrupted in patients with schizophrenia. Underlying cellular mechanisms were assessed using ex vivo hippocampal slice preparations with selective pharmacological tools and selective genetic deletion of mGlu3 receptor expression in specific neuronal subpopulations.
Results.
mGlu3 receptor activation enhanced trace fear conditioning and reversed deficits induced by subchronic phencyclidine. Mechanistic studies revealed that mGlu3 receptor activation induced metaplastic changes, biasing afferent stimulation to induce long-term potentiation through a mGlu5 receptor-dependent, endocannabinoid-mediated, disinhibitory mechanism. Selective genetic deletion of either mGlu3 or mGlu5 from hippocampal pyramidal cells eliminated effects of mGlu3 activation, revealing a novel mechanism by which mGlu3 and mGlu5 interact to enhance cognitive function.
Conclusions.
These data demonstrate that activation of mGlu3 receptors in hippocampal pyramidal cells enhances hippocampal-dependent cognition in control and impaired mice by inducing a novel form of metaplasticity to regulate circuit function – providing a clear mechanism through which genetic variation in GRM3 can contribute to cognitive deficits. Developing approaches to positively modulate mGlu3 receptor function represents an encouraging new avenue for treating cognitive disruption in schizophrenia and other psychiatric diseases.
Keywords: mGlu3, mGlu5, hippocampus, schizophrenia, cognition, synaptic plasticity
Introduction
Several analyses, including a 37,000-patient genome-wide association study, have implicated allelic variants in the GRM3 gene with cognitive deficits and an increased likelihood of schizophrenia diagnosis (1-4). In particular, GRM3 variation has been associated with performance on memory-based tasks in schizophrenia patients and neurotypical controls (5-7). GRM3 codes for the mGlu3 subtype of metabotropic glutamate receptor. One well-studied GRM3 polymorphism is associated with splice variants that translate to truncated, non-functional mGlu3 receptor protein (8, 9), suggesting decreased mGlu3 receptor function contributes to schizophrenia etiology in some individuals. But until recently, the paucity of selective tools has hindered the clear delineation of mechanisms that link mGlu3 receptor function and hippocampal cognition.
A large body of preclinical work has prompted great interest in targeting mGlu3 (and mGlu2) receptors for the treatment of schizophrenia (reviewed in (3)). Efforts in developing efficacious mGlu2/3 agonists culminated in a mixture of promising and disappointing phase II clinical trials. These trials were generally motivated by research showing that mGlu2 receptor activation attenuates behaviors that model positive symptoms and psychosis (10-13). On the other hand, mGlu2 receptor agonism suppresses rapid eye movement sleep (12) and can impair learning and memory on hippocampal-dependent tasks (14). These findings suggest that, in clinical trials with non-selective compounds, activating mGlu2 receptors may have induced cognitive side effects that obscured latent benefits of mGlu3 receptor activation. Collectively, the clinical and translational findings with mGlu2/3 agonists, along with genetic studies implicating GRM3, raise the exciting possibility that selective activation of mGlu3 could improve cognition, particularly in models of schizophrenia-like deficits.
Here, we leverage the recent development of highly selective negative allosteric modulators (NAMs) to demonstrate that mGlu3 receptor activation enhances cognition. Moreover, we report that mGlu3 receptors regulate hippocampal plasticity by a novel mechanism requiring co-activation of another mGlu receptor, mGlu5. Finally, using newly developed genetic mouse models to selectively delete mGlu3 and mGlu5 from pyramidal cells, we relate these changes in synaptic plasticity to associative learning. These data build on exciting advances in human genetics and reveal mGlu3 receptors as novel targets for ameliorating cognitive symptoms in schizophrenia.
Methods and Materials
Animals.
Mice were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Studies were approved by the Vanderbilt University School of Medicine IACUC and occurred during the light phase. Young adult (6-8-week-old) C57BL/6J (Jackson Laboratories), Grm5Fl/Fl, Grm5-CaMKII KO, Grm3Fl/Fl, Grm3-CMV KO, and Grm3-CaMKII KO mice (Key Resources Table) were housed on a standard light cycle (on at 6:00) and provided ad libitum food and water. Strains were backcrossed to congenic C57BL/6J mice for more than 5 generations. Both male and female mice were used.
Key Resource Table
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| rabbit anti-mGlu3 antibody (1:500) | Alomone | AGC-012 |
| mouse anti- GAPDH antibody (1:5000) | ThermoFisher | MA5-15738 |
| goat anti-rabbit 800 (1:5000) | LiCor | 925-32211 |
| goat anti-mouse 680 (1:5000) | LiCor | 926-68070 |
| RNAscope in situ Hybridization Probes | ||
| Grm3 (target region 875-1676) | ACDBio | Mm-Grm3-O (NM_181850.2) |
| Grm5 (target region 2409-3336) | ACDBio | Mm-Grm5-O1 (NM_001081414.2) |
| Slc17a7 (target region 464-1415) | ACDBio | Mm-Slc17a7-C3 (NM_182993.2) |
| Syn2 (target region 585-1482) | ACDBio | Mm-Syn2-C2 (NM_013681.3) |
| Slc1a3 (target region 1122-2237) | ACDBio | Mm-Slc1a3-C3 (NM_148938.3) |
| Slc32a1 (target region 894-2037) | ACDBio | Mm-Slc32a1-C2 (NM_009508.2) |
| Chemicals | ||
| LY379268 (3 mg/kg; 100-300 nM) | Tocris | 5064 |
| D-AP5 (50 μM) | Tocris | 106 |
| DHPG (25-50 μM) | Tocris | 805 |
| CNQX (20 μM) | Tocris | 1045 |
| MTEP (1 μM) | Tocris | 2921 |
| AM251 (2 μM) | Tocris | 1117 |
| Phencyclidine (10 mg/kg) | Sigma | P3029 |
| VU0469650 (10 μM) | CW Lindsley (Lovell et al., 2013) | N/A |
| VU0650786 (30 mg/kg; 20 μM) | CW Lindsley (Engers et al., 2017) | N/A |
| VU6001966 (10 mg/kg; 10 μM) | CW Lindsley (Bollinger et al., 2017) | N/A |
| Experimental Models | ||
| C57BL/6J | The Jackson Laboratory | 664 |
| Grm5 Fl/Fl | The Jackson Laboratory | 28626 |
| Grm3 Fl/Fl | See Methods | N/A |
| CaMKII Cre | The Jackson Laboratory | 5359 |
| CMV Cre | The Jackson Laboratory | 6054 |
Drugs.
Most drugs were purchased from Tocris (Key Resources Table). PCP was purchased from Sigma. VU0469650, VU0650786, VU6001966 were synthesized in-house (15-17). For ex vivo experiments, concentrations were selected to be 30-fold higher than each NAM’s IC50. For in vivo experiments, NAMs were delivered by intraperitoneal injection (10% Tween-80, 10 μL/kg) at behaviorally active doses that achieve unbound brain concentrations 3-fold above each compound’s IC50. (16-19).
Subchronic phencyclidine (scPCP) treatment.
5-week-old mice received daily subcutaneous injections with 10 mg/kg PCP (0.9% saline, 10 μL/kg) for 7 days. Separate cohorts of mice were used for electrophysiology or behavior following a 7-day washout period.
Extracellular field potential recordings.
Experiments were performed as described previously (20, 21). In brief, mice were anesthetized with isoflurane and coronal slices (400 μm) containing the dorsal hippocampus were prepared in N-methyl-D-glucamine-based solution. Slices were held at room temperature in ACSF containing (in mM): 126 NaCl, 1.25 NaH2PO4, 2.5 KCl, 10 D-glucose, 26 NaHCO3, 2 CaCl2, and 1 MgSO4. We recorded field excitatory postsynaptic potentials (fEPSPs) with glass electrodes (1-3 MΩ) in the stratum radiatum of CA1 and a bipolar stimulating electrode near the CA3-CA1 border. Stimuli were constantly delivered at 0.05 Hz unless otherwise noted. Theta burst stimulation (TBS) long-term potentiation (LTP) was evoked using 9 bursts of 4 stimuli at 100 Hz, repeated every 230 ms (20). Afferent ‘priming’ consisted of 2 bursts of 10 stimuli at 10 Hz, separated by 20 seconds (22). Long-term depression (LTD) was evoked with DHPG or with 15 minutes of paired pulse 1 Hz stimulation, each in the presence of the mGlu1 NAM VU0469650 (10 μM). Data were digitized using a Multiclamp 700B, Digidata 1322A, and pClamp 10 software (Molecular Devices). Slices from at least 3 mice are included in each group.
Whole-cell voltage-clamp recordings.
Whole-cell recordings were made from hippocampal CA1 pyramidal neuron somata. The pipette was filled with intracellular solution (mM): 125 CsCl, 4 NaCl, 0.5 MgCl2, 10 TEA, 10 HEPES, 0.5 EGTA, 5 QX-314, 5 Tris-phosphocreatine, 4 ATP-Mg and 0.3 GTP-Na, adjusted to pH 7.3 and 290-295 mOsm. Inhibitory postsynaptic currents (IPSCs) were evoked at 0.1 Hz and recorded at −70 mV in CNQX (20 μM) and AP-5 (50 μM).
Trace fear conditioning.
Design for experiments was modified from previous studies (22). Vehicle or LY379268 was injected 30 minutes before the session. All mGlu NAMs were injected 20 minutes prior to LY379268/Vehicle treatment. Mice were placed in a conditioning chamber with a shock grid (Med Associates) in the presence of 10% vanilla odor. Mice were acclimated for 60 seconds before conditioning and a 15-second tone (90dB, 2900Hz) was applied preceding a 1-second foot shock (0.5-mA mild shock or 0.7-mA strong shock). A precise 30-second interval, or ‘trace’, separated the tone and shock. Three tone-trace-shock pairings (four pairings for scPCP study) were applied, 240-seconds apart. Freezing was quantified during each trace by video software (VideoFreeze) and confirmed by a blinded observer.
Generation of floxed Grm3 mice.
We generated Grm3Fl/Fl mice using embryonic stem cell-mediated gene targeting on the C57BL/6J genetic background (Ingenious Targeting Laboratory) in which exon 3 of Grm3 is flanked by LoxP sites (Supplemental Methods).
RNA and cDNA preparation.
Tissues were homogenized and total RNA was prepared using standard Trizol-chloroform methodology. RNA concentration was measured using Nanodrop and cDNA was synthesized from 2 μg total RNA with the Superscript VILO kit (Thermo Fisher). Primers targeted Grm3: F 5’-TGTGGTGAATGCAGTGTACG-3’, R 5’-CATCCCGTCTCCAAAAGTGT-3’ and Actin: F 5'-GTGGGCCGCTCTAGGCACCAA-3’, R 5'-CTCTTTGATGTCACGCACGATTTC-3’.
Western blotting.
Tissues were snap-frozen in liquid nitrogen and stored at −80 °C. Tissues were homogenized in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Sigma Aldrich). Homogenized samples were spun at 12000 g at 4°C for 20 minutes and western bots were run using 50 μg of protein electro-transferred to polyvinyl difluoride membrane. mGlu3 receptor primary antibody (Key Resources Table) was added overnight at 4°C followed by incubation with fluorescent secondary antibody for 30 minutes at room temperature. mGlu3 receptor protein expression was quantified relative to GAPDH and normalized to values from control mice.
RNAscope in situ hybridization.
RNAscope was conducted according to the Advanced Cell Diagnostics user manual (Supplemental Methods). Thin brain slices (16 μm) were incubated with cell type-specific probes and with probes we designed to recognize sequences targeted for excision in Grm3Fl/Fl and Grm5Fl/Fl mice (Key Resources Table).
Statistics.
Sample sizes were determined based on previous experiments (21). Analyses were performed using GraphPad Prism 8. Data are represented as mean ± SEM. Significance between groups was determined using Student’s t-test or ANOVA with appropriate post-hoc tests, as specified in the figure legends.
Results
mGlu3 receptor activation enhances trace fear conditioning
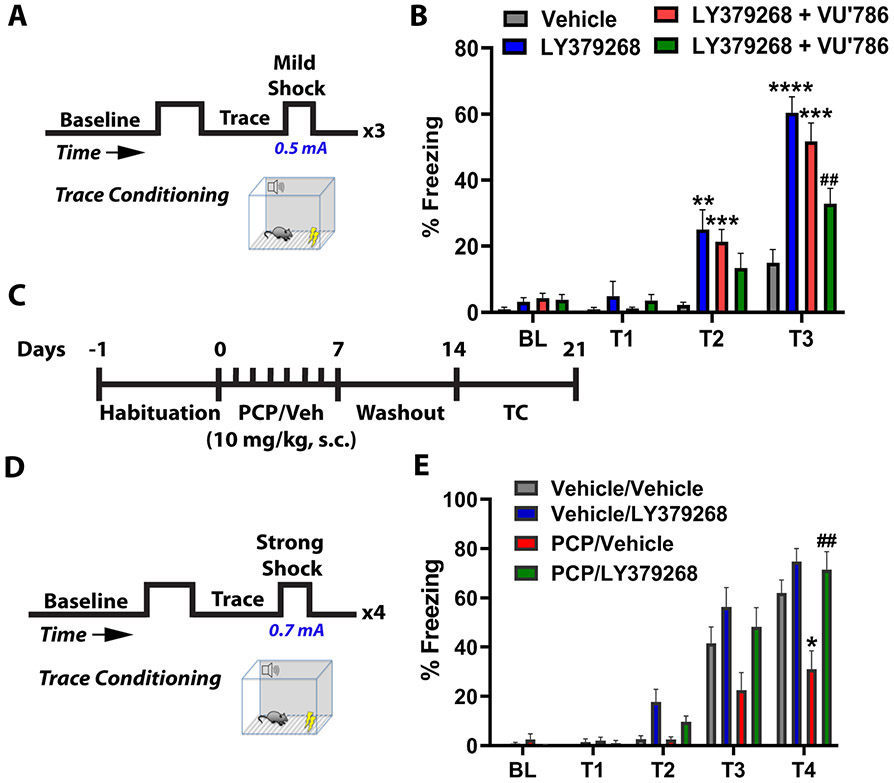
Genetic studies have revealed an association between GRM3 variation and cognition (5-7). We therefore asked whether activating the mGlu3 receptor would improve trace conditioning, a high-demand associative learning task disrupted in patients with schizophrenia (23). Mice were placed in fear conditioning chambers and received three pairings of a tone cue, 30-second “inactive” trace, and mild foot shock (Figure 1A). Mice rapidly associate the trace period with the upcoming shock and express this association by conditioned freezing. We activated mGlu3 by systemic delivery of the mGlu2/3 agonist LY379268 (3 mg/kg). LY379268 treatment enhanced freezing during the traces proceeding shock presentation (Figure 1B). We next separated the contributions of mGlu2 and mGlu3 receptors using selective NAMs. NAM doses were selected based on previously published pharmacokinetic (16, 17) and behavioral studies (18, 19). Either the mGlu2 NAM VU6001966 (10 mg/kg, i.p.) or the mGlu3 NAM VU0650786 (30 mg/kg, i.p.) was injected 20 minutes prior to LY379268 treatment. Administration of VU6001966 did not block the LY379268-induced increase in trace fear conditioning (Figure 1B). In contrast, administration of the selective mGlu3 NAM VU0650786 (30 mg/kg) blocked the LY379268-induced trace fear conditioning enhancement (Figure 1B). No drug combinations affected freezing during the baseline or first trace, indicating specificity for the learned components of the task. Together, these data suggest that mGlu3 receptor activation enhances associative learning in mice.
Figure 1. Activation of mGlu3 receptors enhances associative learning and rescues schizophrenia-like cognitive deficits.
(A) Behavioral schematic. Mice received 3 pairings of a tone and mild foot-shock (0.5 mA), each separated by a 30-second trace period. Freezing time was quantified across the 3 traces. (B) Administration of the mGlu2/3 agonist LY379268 (3 mg/kg, i.p., blue bars) 30 minutes prior to conditioning increased freezing at trace 2 and trace 3 relative to vehicle treated mice (grey bars) (**p<0.01, ****p<0.0001). Mice were treated with the mGlu2 negative allosteric modulator (NAM) VU6001966 (10 mg/kg, i.p., red bars) or the mGlu3 NAM VU0650786 (30 mg/kg, i.p., green bars) to isolate the effects of mGlu2 and mGlu3 receptor activation. LY379268 enhanced trace conditioning in VU6001966-treated mice (red bars, **p<0.01, ****p<0.0001 compared to vehicle (gray bars)), whereas the effect was blocked by the mGlu3 NAM VU0650786 (##p<0.01 compared to LY379268, F(3,72)=12.73 by two-way repeated measures ANOVA with Tukey’s post-hoc test, N=17-24 mice). (C) Schematic representing subchronic phencyclidine (scPCP) treatment regimen. After 7 days of habituation, mice were injected with scPCP (10 mg/kg) for 7 days. Trace fear conditioning experiments were performed 7 days after last PCP injection. (D) Behavioral schematic. Mice received 4 pairings of a tone, trace, and strong foot-shock (0.7 mA). (E) scPCP-treated mice (orange bars) froze less than vehicle controls (*p<0.05 compared to vehicle/vehicle; gray bars). Acute LY379268 treatment (30 minutes prior to the experiment; green bars) rescued scPCP-induced deficits in freezing (##p<0.01 compared to PCP/vehicle, red bars, F(3,35)=8.027 by two-way repeated measures ANOVA with Tukey’s post-hoc test, N=8-13). Data are presented as mean ± SEM.
mGlu3 agonism rescues PCP-induced deficits in cognition and synaptic plasticity
Subchronic NMDA receptor hypofunction can generate lasting impairments in synaptic plasticity and cognitive function and is thought to model schizophrenia-like forebrain pathophysiology (24, 25). Mice received daily injections of the psychotomimetic NMDA receptor antagonist PCP or Vehicle for one week (scPCP, Figure 1C) and all experiments were performed following a one-week washout. For this experiment, the number of tone-shock pairings was increased to four and stronger shocks (0.7 mA) were applied to increase freezing in control mice (Figure 1D). Under these conditions, LY379268 no longer enhanced freezing in control mice, likely due to a ceiling effect. Mice treated with scPCP, however, displayed decreased freezing during trace presentations as compared to Vehicle controls. Acute treatment with LY379268 rescued this deficit (Figure 1E), suggesting that mGlu3 receptor activation exerts pro-cognitive effects in a model related to schizophrenia-like pathophysiology.
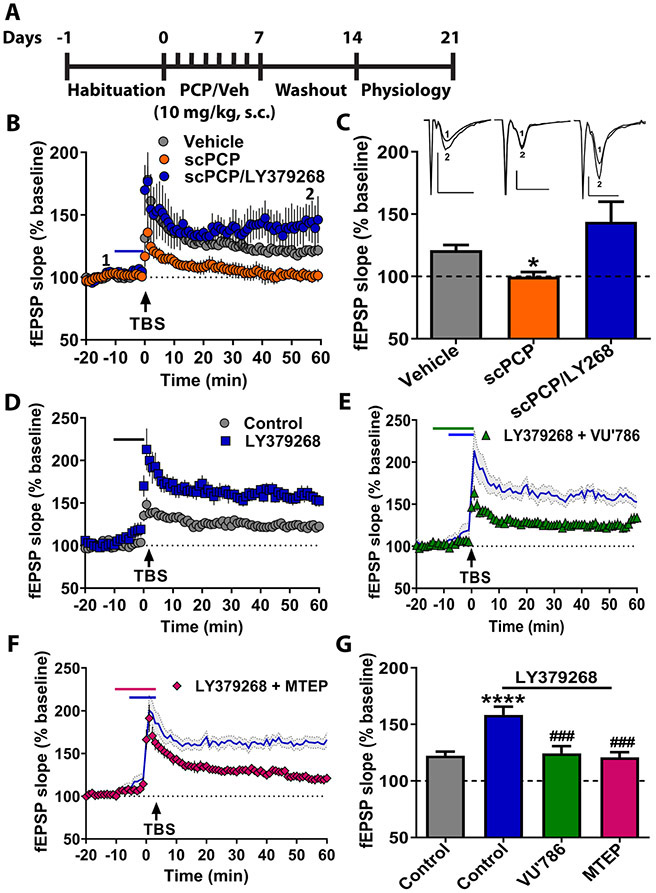
Trace conditioning has been associated with hippocampal function and molecular processes related to synaptic plasticity (25, 26). We therefore evaluated whether scPCP treatment alters LTP at the CA3-CA1 synapse (Figure 2A). TBS induced modest LTP in Vehicle-treated control conditions, but slices from scPCP mice did not display LTP (Figure 2B/2C). Acute slice perfusion with LY379268 (100 nM) completely reversed the plasticity deficit. Together, these findings indicate that mGlu3 receptor activation ameliorates coincidental deficits in cognition and hippocampal synaptic plasticity, suggesting that shared molecular mechanisms contribute to both processes. Further, we performed a series of studies to isolate mGlu3 receptors and investigate hippocampal LTP. Acute bath application of the mGlu2/3 agonist LY379268 enhanced TBS-LTP in slices from control mice (Figure 2D/2G). The selective mGlu2 NAM VU6001966 (10 μM) had no effect on the LY379268- induced increase in LTP (Figure S1). By contrast, the selective mGlu3 NAM VU0650786 (20 μM) completely blocked the actions of LY379268 (Figure 2E/2G), confirming that mGlu3 receptors are essential for the LTP enhancement.
Figure 2. mGlu3 receptor activation enhances hippocampal long-term potentiation (LTP) through concerted signaling with mGlu5 receptors.
(A) Schematic representing scPCP treatment regimen. After 7 days of habituation, mice were injected with scPCP for 7 days. Electrophysiology experiments were performed 7 days after last PCP injection. (B) Field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum radiatum of CA1 after electrical stimulation of the Schaffer collateral. Time course showing moderate LTP after a single application of theta burst stimulation (TBS) in slices from vehicle-treated mice (grey bar, n=7 slices). Slices from mice treated with scPCP displayed impaired LTP (orange bar n=6). Bath application of the mGlu2/3 agonist LY379268 (100 nM) rescued TBS-LTP in slices from scPCP-treated mice (blue bar n=6). (C) Summary of averaged fEPSP slope of last 5 minutes of recordings from panel B (*p<0.05, compared to Vehicle, F(2,16)=5.11, one-way ANOVA with Tukey’s post-hoc test). Insets for (C) are representative fEPSP traces for the various experimental conditions from (B) measured during baseline (1) and 55 min post stimulation (2). Scale bars represent 1 mV and 100 ms for all traces. Traces for each experimental condition are placed over respective bar graphs. (D) In control mice, LY379268 application enhanced LTP in response to TBS (blue squares, n=10). (E) Co-application of mGlu3 NAM VU0650786 (VU786; 10 μM) blocked the enhanced LTP induced by LY379268 (green triangles, n=11) and had no effect on its own at baseline. Blue line displays LY379268 data from panel D. (F) The mGlu5 NAM MTEP (1 μM) blocked enhanced LTP when co-applied with LY379268 (magenta diamonds, n=8) and had no effect on its own at baseline. (G) Summary of averaged fEPSP slope of last 5 minutes of recordings from panels D-F (****p<0.0001 compared to control, ***p<0.001 compared to LY379268+control, F(3,42)=10.11, one-way ANOVA with Tukey’s post-hoc test). Data are presented as mean ± SEM.
mGlu3 activation enhances LTP through mGlu5 receptors
The finding that the mGlu2/3 agonist can reverse scPCP-induced deficits in LTP was somewhat surprising since previous studies more commonly implicate group II mGlu receptors in reducing excitatory synaptic transmission. However, previous studies reveal that mGlu3 receptors can potentiate or recruit mGlu5 receptors (27, 28), which are critical for trace fear conditioning and associative learning (21, 22, 29). We therefore hypothesized that mGlu3 agonism potentiates hippocampal LTP through a mechanism that involves co-activation of mGlu5 receptors. Consistent with this, the selective mGlu5 NAM, MTEP (1 μM), inhibited the ability of LY379268 to potentiate TBS-LTP, without any effect on TBS-LTP alone (Figure 2F/2G). Importantly, all compounds were applied at concentrations that maintain functional selectivity over other mGlu receptor subtypes (28, 30-32). Together, these results suggest that mGlu3 receptor activation potentiates TBS-LTP through a mechanism that requires co-activation of mGlu5 receptors.
mGlu3 receptor activation biases plasticity towards LTP and away from LTD
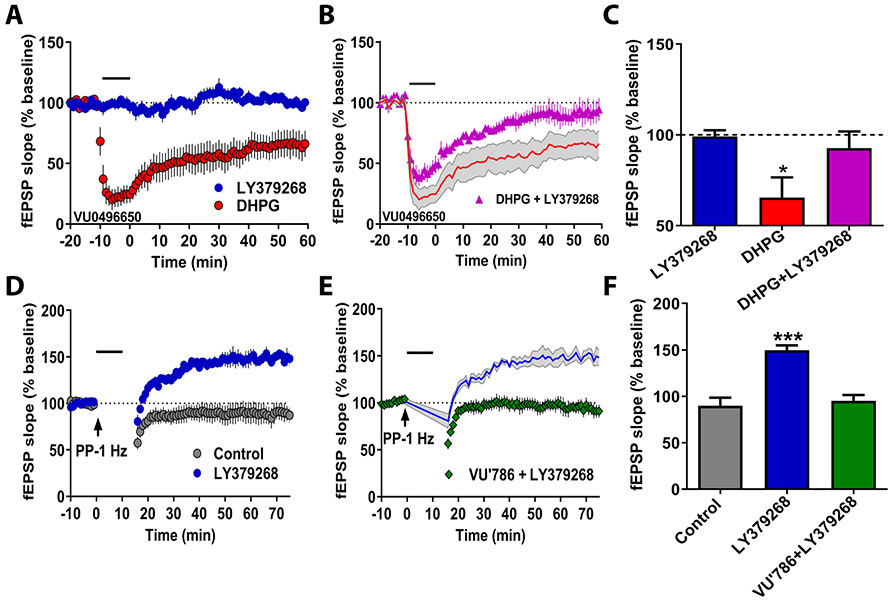
We aimed to leverage the extensive literature describing how mGlu5 receptors regulate hippocampal plasticity to better understand mGlu3 receptor function. The CA3-CA1 synapse is bidirectionally modulated by mGlu5 receptors. Modest activation of mGlu5 receptors facilitates LTP induction, while stronger activation can promote LTD through distinct signaling cascades (33-35). We reasoned that, if mGlu3 receptor activation potentiates all mGlu5 receptor-dependent signaling pathways, mGlu3 agonists should also enhance LTD. We elicited LTD using the mGlu1/5 agonist DHPG (50 μM) co-applied with the mGlu1 NAM VU0469650 (10 μM) (Figure 3A/3C). LY379268 (100 nM) impaired LTD (Figure 3B/3C) and did not alter the response to a threshold concentration of DHPG (25 μM) (Figure S2). These data suggest that mGlu3 receptor activation induces a qualitative shift in mGlu5 receptor signaling to impair LTD. We corroborated these findings using paired-pulse 1-Hz stimulation, which also induces mGlu5 receptor-dependent LTD. Remarkably, LY379268 application converted the response to paired-pulse 1-Hz stimulation of Schaffer collateral afferents from induction of LTD to induction of LTP (Figure 3D/3F), fundamentally altering the form of synaptic plasticity induced by low-frequency synaptic activation. This effect was normalized by the mGlu3 NAM, VU0650786 (Figure 3E/3F), confirming the involvement of mGlu3 receptors. Overall, these results demonstrate that mGlu3 activation induces metaplasticity to favor induction of LTP relative to LTD at the CA3-CA1 synapse. We thus aimed to investigate how mGlu3 receptors regulate other forms of LTP.
Figure 3. mGlu3 receptor activation induces hippocampal metaplasticity to promote LTP.
(A) Application of the mGlu1/5 agonist DHPG (50 μM) induced long-term depression (LTD) of fEPSP slope (red circles, n=6 slices), while the mGlu2/3 agonist LY379268 (100 nM) had no acute or long-term effects (blue circles, n=5). (B) Co-application of LY379268 abrogated DHPG-induced LTD (pink triangles, n=5). (C) Summary of averaged fEPSP slope of last 5 minutes of recordings from panels A-B (*p<0.05, compared to LY379268 alone, F(2,13)=4.087, one-way ANOVA with Tukey’s post-hoc test). (D) Paired-pulse (PP) 1-Hz stimulation for 15 minutes induces mGlu5-dependent LTD in control slices (grey circles, n=6). Coincidental application of LY379268 (100 nM) changes PP 1-Hz LTD into a long-term enhancement of fEPSP slope (blue circles, n=6). (E) The mGlu3 NAM VU0650786 (VU786; 10 μM) blocked the metaplastic effects of LY379268 application (green diamonds, n=6). (F) Summary of averaged fEPSP slope of last 5 minutes of recordings from panels D and E (***p<0.001, compared to Control, F(2,13)=17.13, p<0.001, one-way ANOVA with Tukey’s post-hoc test). All experiments were performed in presence of mGlu1 NAM, VU0469650 (10 μM). Data are expressed as mean ± SEM.
mGlu3 receptors regulate multiple facets of hippocampal LTP
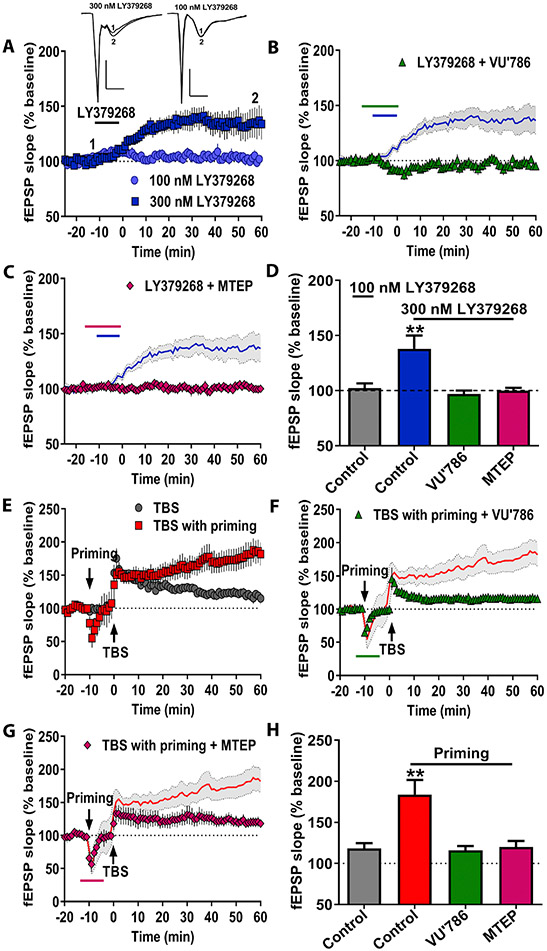
mGlu receptor activation is necessary and sufficient to induce some forms of LTP at the CA3-CA1 synapse (33, 36-38). Consistent with prior findings, a high concentration of LY379268 (300 nM) induced a slow onset LTP (Figure 4A/4D). Paired-pulse ratios were not altered at any time point (data not shown), suggesting these effects are mediated by postsynaptic mechanisms reminiscent of TBS-LTP. Co-application of either the selective mGlu3 NAM VU0650786 (Figure 4B/4D) or the mGlu5 NAM, MTEP (Figure 4C/4D) blocked chemically-induced LTP. These data indicate that sustained activation of mGlu3 receptors induces LTP, fundamentally contrasting with mGlu5 receptor-dependent LTD.
Figure 4. mGlu3 receptor signaling regulates multiple modes of LTP.
(A) A high concentration of LY379268 (300 nM) and sustained 0.5 Hz stimulation enhanced fEPSP slope in acute hippocampal slices (dark blue squares, n=6 slices). A modest concentration of LY379268 (100 nM) did not affect fEPSP slope on its own (light blue circles, n=8). Insets for (A) are representative fEPSP traces for the various experimental conditions measured during baseline (1) and 55 min post stimulation (2). Scale bars represent 1 mV and 50 ms for all traces. (B) The mGlu3 NAM VU0650786 (10 μM) blocked the fEPSP enhancement induced by LY379268 application (green triangles, n=5). Blue line displays LY379268 (300 nM) data from panel A. (C) The mGlu5 NAM MTEP (1 μM) blocked LY379268-induced LTP (pink diamonds, n=5). (D) Summary of averaged fEPSP slope of last 5 minutes of recordings from panels A-C (**p<0.01, compared to Control, F(3,20)=5.672, p<0.01 one-way ANOVA with Tukey’s post-hoc test). (E) Brief priming stimulation (2, 10-second, 10 Hz trains) followed by TBS (red squares, n=5) enhanced fEPSP slope compared to TBS alone (gray circles, n=5). (F) The mGlu3 NAM VU0650786 (10 μM) perfused before, during, and after priming stimulation blocked the enhanced LTP (green triangles, n=4). Red lines display TBS with priming data from panel E. (G) The mGlu5 NAM MTEP (1 μM) perfused throughout priming stimulation blocked the enhanced LTP (pink diamonds, n=4). (H) Summary of averaged fEPSP slope of last 5 minutes of recordings from E-G (**p<0.01, compared to Control, F(3,14)=9.136, p<0.01 one-way ANOVA with Tukey’s post-hoc test). Data are presented as mean ± SEM.
Interestingly, the effects of mGlu3 receptor activation outlined above are reminiscent of a previously reported form of mGlu5 receptor-dependent metaplasticity, referred to as LTP priming (22). Priming is a phenomenon in which modest activation of mGlu5 receptors with a brief stimulation of glutamatergic afferents exerts minimal acute effects on excitatory transmission but dramatically facilitates LTP induction by subsequent TBS afferent stimulation. We primed CA3-CA1 synapses with brief afferent stimulation (2 bursts of 10 stimuli at 10 Hz), and observed a robust increase in subsequent TBS-induced LTP relative to control slices (Figure 4E/4H). In light of the finding that mGlu3 activation potentiates TBS-LTP, we hypothesized that endogenous mGlu3 receptors might be involved in this process. Consistent with this hypothesis, the mGlu3 NAM VU0650786 blocked priming metaplasticity and returned TBS-LTP to control levels (Figure 4F/4H). In addition, consistent with previous studies (22), the mGlu5 NAM MTEP (Figure 4G/4H) also prevented the priming enhancement of TBS-LTP. Together, these results demonstrate that mGlu3 receptor activation induces this form of metaplasticity through coordinated signaling with mGlu5 receptors.
mGlu3 receptor activation disinhibits CA1 pyramidal cells
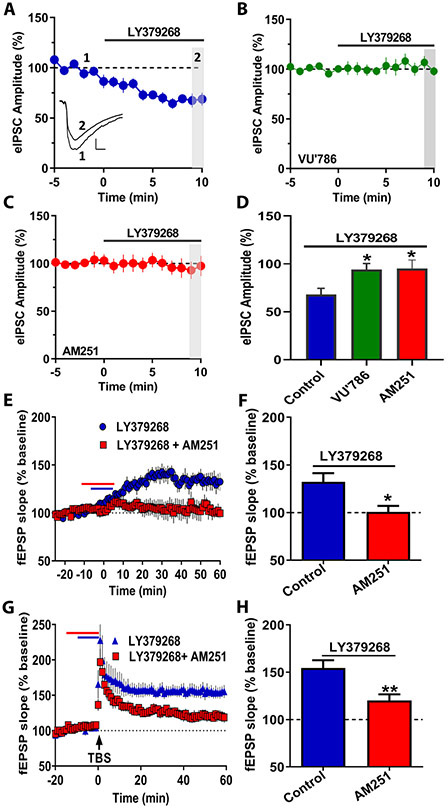
mGlu5 receptor-dependent metaplasticity requires release of endocannabinoids, activation of CB1 endocannabinoid receptors, and the inhibition of GABAergic transmission onto pyramidal cells (22). We directly tested whether mGlu3 receptors induce similar CB1-dependent effects on inhibitory transmission by isolating evoked IPSCs (eIPSCs) on CA1 pyramidal cells. LY379268 (300 nM) acutely depressed eIPSCs in control recordings (Figure 5A/5D), which were blocked in the presence of the mGlu3 NAM VU0650786 (20 μM; Figure 5B/5D). The CB1 receptor antagonist AM251 (2 μM) also blocked the decrease in eIPSCs (Figure 5C/5D). Further, we returned to field potential recordings to test the hypothesis that activation of CB1 receptors is required for mGlu3 receptor-induced metaplasticity. AM251 (2 μM) co-application blocked LTP induced by high concentration LY379268 (Figure 5E/5F) and prevented effects on TBS-LTP (Figure 5G/5H). Together, these results suggest that mGlu3 receptors regulate hippocampal synaptic plasticity by recruiting endocannabinoid-mediated disinhibition of GABAergic transmission onto pyramidal cells following receptor activation.
Figure 5. mGlu3 receptor activation decreases evoked inhibitory transmission onto hippocampal pyramidal neurons.
(A) Electrically-evoked inhibitory postsynaptic currents (eIPSCs) were recorded from pyramidal cells in CA1. A high concentration of the mGlu2/3 agonist LY379268 (300 nM) reduced the amplitude of eIPSCs (blue circles, n=6 cells). Insets for (A) are representative eIPSCs traces measured during baseline (1) and last 2 min of the recordings from the time course (2). Scale bars represent 200 pA and 50 ms. (B) Co-application of the mGlu3 NAM, VU0650786 (20 μM) blocked the LY379268-induced decrease in eIPSCs (green squares, n=10). (C) The CB1 antagonist AM251 (2 μM) blocked the effects of LY379268 on eIPSC amplitude (red circles, n=6). (D) Summary of the last 2 min of the recordings from the time course experiments in panels A-C (*p<0.05, **p<0.01 compared to control, F(2,19)= 8.042, one-way ANOVA with Tukey’s post-hoc test). (E) In field potential configuration, AM251 (2 μM) blocked LTP (red squares, n=5 slices) induced by high concentration of LY379268 (300 nM) (blue circles, n=5). (F) Summary of averaged fEPSP slope of last 5 minutes of recordings from panel E (*p<0.05, compared to Control, t(8)=2.86, Student’s t-test). (G) AM251 (red squares, n=10) blocked the ability of LY379268 (100 nM) to enhance LTP following TBS (blue circles, n=5). (H) Summary of averaged fEPSPs slope of last 5 minutes of recordings from panel H. (**p<0.01, compared to Control, t(13)=3.617, Student’s t-test). Data are expressed as mean ± SEM.
Neuronal mGlu3 receptors mediate hippocampal metaplasticity
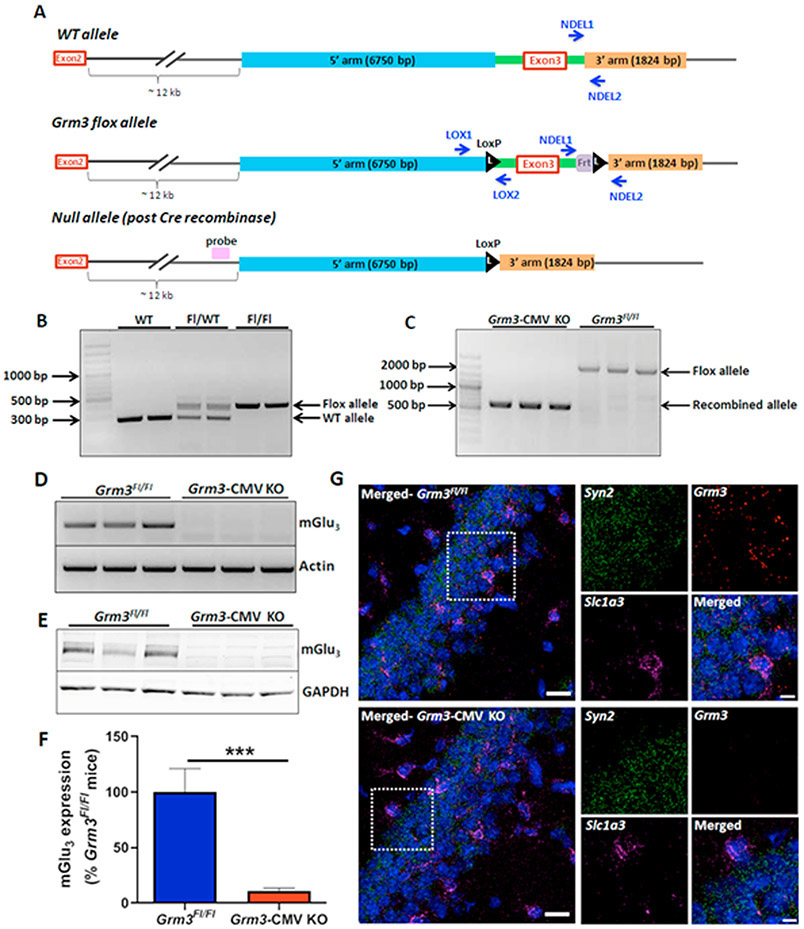
Previous studies suggest that mGlu5-induced effects on hippocampal LTP are mediated by activation of mGlu5 expressed in hippocampal pyramidal cells (22). Based on this, the simplest hypothesis to explain our findings is that mGlu3 interacts with mGlu5 in hippocampal pyramidal cells to regulate LTP and trace fear conditioning. However, the mGlu3 receptor is expressed in multiple neuronal populations and is heavily expressed in astrocytes, and it is possible that mGlu3 expressed in any of these cell populations could be required for enhancing LTP and trace fear conditioning. We therefore generated conditional Grm3Fl/Fl mice (Figure 6A/6B and Supplementary Methods) and validated the Grm3Fl/Fl construct by introducing Cre recombinase under the ubiquitously expressed cytomegalovirus (CMV) minimal promotor. We detected excision of Grm3 exon 3 (Figure 6C), indicating Cre-mediated recombination readily occurs in vivo. We also performed RT-PCR, RNAscope, and western blots from hippocampal tissues and detected minimal transcript and protein in Grm3-CMV-KO mice (Figure 6D-6G).
Figure 6. Generation and characterization of conditional Grm3Fl/Fl mice.
(A) Schematic of the procedure for generating the floxed Grm3 clones. (Top) Wild-type Grm3 locus surrounding exon 3. (Middle) Floxed Grm3 locus where LoxP sites are inserted flanking exon 3 of Grm3. (Bottom) Cre-mediated recombination leading to loss of Grm3 exon 3 and Frt site. (B) PCR from the DNA of mice homozygous for the WT allele (277 bp) and mice heterozygous and homozygous for the floxed allele (413 bp). (C) CMV-Cre mediated recombination in Grm3Fl/Fl mice excises Grm3 exon 3, leading to loss of WT allele (~1900 bp) and generation of a smaller recombination allele (671 bp). (D) RT-PCR from the hippocampi of WT and Grm3-CMV KO mice. (E) Western blot depicting loss of mGlu3 protein from the hippocampus of Grm3-CMV KO mice (red bar) relative to Grm3Fl/Fl controls (blue bar). (F) Bar graph depicting quantification of Western blots. Data are presented as mean ± SEM, N=3-6 mice. (***p<0.001 compared to Grm3Fl/Fl mice, t(7)= 7.018, Student’s t-test). (G) Confocal 40X RNAscope in situ hybridization images showing loss of Grm3 mRNA (red) from both neurons (Syn2; Synapsin, green) and astrocytes (Slc1a3; GLAST, magenta) in Grm3-CMV KO mice. Scale bar = 20 μm for the merged left panel image, and 10 μm for the 3X images.
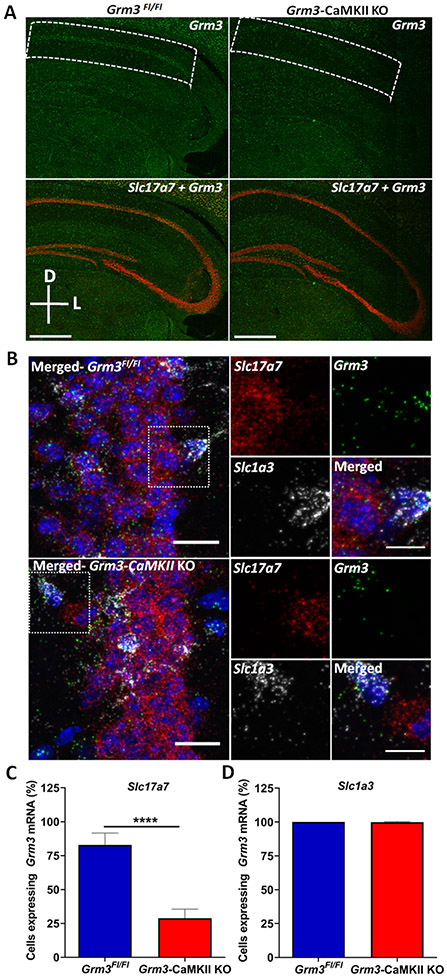
To test the hypothesis that postsynaptic mGlu3 receptors on pyramidal cells are required for enhancing hippocampal synaptic plasticity, we crossed Grm3Fl/Fl with CaMKII Cre mice, a selective marker for glutamatergic neurons in the cortex and hippocampus (22, 39) and performed experiments at an age that displays preferential receptor ablation from the CA1 area of hippocampus (Figure 7A). We assessed cell type-specificity using RNAscope and observed Grm3 ablation from cells co-expressing transcript for vGluT1(Slc17a7) but not GLAST (Slc1a3) in hippocampus from Grm3-CaMKII KO mouse (Figure 7B). CaMKII Cre mice display recombination in the striatum during late adulthood (40); however, we observed no changes in Grm3 in the nucleus accumbens of 7-8-week-old Grm3-CaMKII KO mice (Figure S3). We performed similar experiments to validate Grm5 deletion from Grm5-CaMKII KO mice (Figure S4) (see also (22)).
Figure 7. Grm3-CamKII KO mice display selective ablation of mGlu3 receptor transcript in pyramidal cells of the hippocampus.
(A) Characterization of Grm3-CamKII KO mice. Merged confocal 20X tile scan image of a coronal section highlighting decreased Grm3 mRNA expression in the CA1 of 7-8-week-old Grm3-CaMKII KO mice. Scale bars denote 500 μm. (B) Representative confocal 40X RNAscope in situ hybridization images showing loss of Grm3 mRNA (green) from pyramidal neurons (Slc17a7; vGluT1, red) of CA1, while Grm3 mRNA expression is intact in astrocytes (Slc1a3; GLAST, gray). Scale bars denote 20 μm for the left panel image and 10 μm for the right panels (per genotype: Grm3Fl/Fl=3; Grm3-CaMKII KO=4). (C) The percentage of Slc17a7-positive cells (vGluT1) with Grm3 mRNA was decreased in Grm3-CaMKII KO mice (red bar, n/N = 16/4 slices/mice) as compared to WT controls (blue bar, n/N = 15/3) (****p<0.0001 compared to Grm3Fl/Fl mice, t(29) = 19.40, Student’s t-test). (D) The percentage of Slc1a3-positive cells (GLAST) with Grm3 mRNA was not different between Grm3-CaMKII KO mice (red bar, n/N = 14/4) and controls (blue bar, n/N = 9/3) (t(21) = 0.7951, Student’s t-test, p=0.4355).
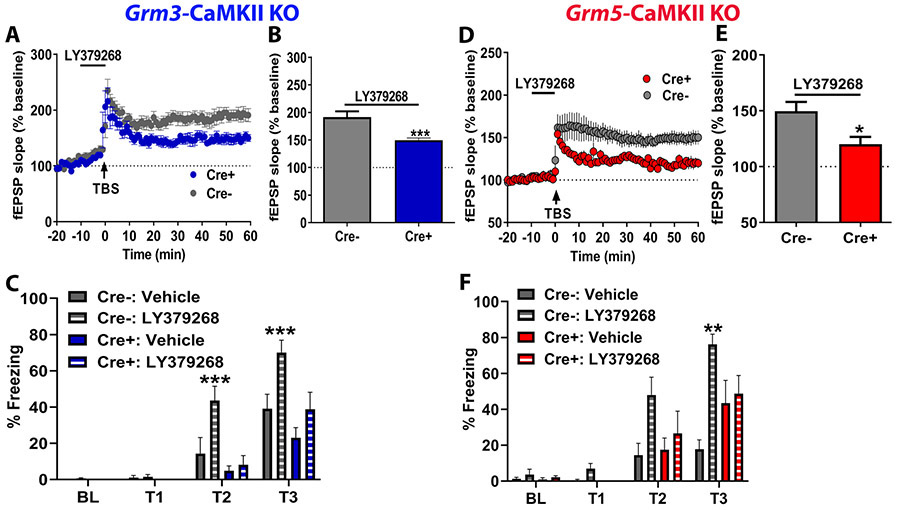
With these new transgenic tools, we proceeded to test the hypothesis that neuronal mGlu3 receptors mediate hippocampal metaplasticity. Consistent with our hypothesis, LY379268 (100 nM) potentiated TBS-LTP in slices from Cre− (littermate controls) but not Cre+ (Grm3-CaMKII KO) mice (Figure 8A/8B), suggesting mGlu3 receptors expressed in the pyramidal neurons are necessary for LTP enhancement. As hippocampal metaplasticity is important for associative learning (22), we reasoned that neuronal mGlu3 receptors would be essential for cognitive enhancement following mGlu3 receptor activation. To test this hypothesis, we performed trace fear conditioning experiment using Grm3-CaMKII KO mice and littermate controls. We observed increased freezing in female Grm3-CaMKII KO mice relative to controls (data not shown), therefore, we only used male Grm3-CaMKII KO mice to examine potentiation by LY379268. Systemic LY379268 administration potentiated trace fear conditioning in littermate control mice, however no difference in freezing was observed between Vehicle- and LY379268-treated Grm3-CaMKII KO male mice (Figure 8C). We obtained similar physiological and behavioral results using Grm5-CaMKII KO mice (Figure 8D-8F), indicating that concerted interactions between neuronal mGlu3 and mGlu5 receptors mediate hippocampal metaplasticity and pro-cognitive effects. In sum, the data obtained from transgenic mice corroborate our pharmacological findings and provide new evidence that mGlu3 receptor activation enhances cognition through direct actions on hippocampal pyramidal cells.
Figure 8. Neuronal mGlu receptors mediate cognitive enhancement following mGlu3 receptor activation.
(A) LY379268 (100 nM) failed to enhance LTP after TBS in slices from Cre+ knockout mice (blue circles, n=6) relative to Cre− littermate controls (grey circles, n=5). (B) Summary of averaged fEPSPs of last 5 minutes of recordings from panel C (*p<0.05 compared to Cre−, t(9)= 2.506, Student’s t-test). (C) Systemic treatment with LY379268 (3 mg/kg) enhances the expression of trace fear conditioning in Cre− littermate controls (hatched grey bars, N=9 mice) but not in Cre+ knockout mice (hatched blue bars, N=7 mice) as compared to treatment with vehicle (gray bars, Cre−, N=7 mice; blue bars, Cre+, N=7 mice) (***p<0.001 compared to Cre− Vehicle, F(3,104)=60.24, two-way repeated measures ANOVA with Tukey’s post-hoc test). (D) LY379268 (100 nM) potentiates LTP after TBS stimulation in Cre− controls (grey circles, n=5) but not Cre+ slices (red circles, n=5). (E) Summary of averaged fEPSPs of last 5 minutes of recordings (*p<0.05 compared to Cre−, t(8)= 2.7, Student’s t-test). (F) Loss of enhanced freezing expression following LY379268 treatment (hatched bars) in Cre+ mice (red hatched bars, 7 mice) as compared to Cre− littermate controls (grey hatched bars, 8 mice) (**p<0.01 compared to Cre− Vehicle, F(3,72)=2.788, two-way repeated measures ANOVA with Tukey’s post-hoc test, N=4-9 mice). Data are expressed as mean ± SEM.
Discussion
Genetic studies have revealed associations between GRM3 variations and cognitive function in patients with schizophrenia (2, 5, 41, 42). Despite these links, the hypothesis that mGlu3 receptors promote cognition has not been evaluated in mechanistic preclinical studies. Here, we demonstrated that activating mGlu3 receptors enhances associative learning in mice and in a model of schizophrenia-like cognitive dysfunction. We then found that hippocampal mGlu3 receptor activation rescues plasticity deficits by recruiting mGlu5 receptor-dependent, endocannabinoid-mediated disinhibition. Finally, we generated a new line of transgenic mice and found that mGlu3 receptors that are co-expressed with mGlu5 receptors in hippocampal projection neurons are required for the cognitive enhancing properties of mGlu3 receptor activation.
Multiple mGlu receptors modulate hippocampal physiology (3), but assigning specific roles to individual receptor subtypes has been hampered by low availability of tools to differentially modulate their functions. We recently discovered molecular probes that comprise the first selective mGlu3 receptor ligands (17, 43). Using these tools and global knockout mice, we found that mGlu3 receptors regulate synaptic plasticity in the prefrontal cortex through pathways that are also regulated by mGlu5 receptors (27, 28). Here, we observed a similar functional interaction between mGlu3 and mGlu5 receptors in area CA1 of the hippocampus, where extensive previous studies indicate that mGlu5 receptors bidirectionally modulate synaptic plasticity (22, 29, 33) and multiple aspects of cognition (33, 44-46). Interestingly, mGlu5 receptors separately promote LTP through calcium-dependent endocannabinoid-mediated disinhibition (23, 37) and LTD via phosphoinositide-3-kinase (PI3K), Akt, and mTOR signaling (50). Here, we showed that mGlu3 receptors selectively bias mGlu5 cascades towards LTP by facilitating endocannabinoid signaling to attenuate GABA transmission (Fig S5). We posit that these actions were responsible for reversing deficits in mice treated with scPCP, which has been shown to elevate inhibitory tone onto CA1 pyramidal cells (25). While the current studies relied on AM251 as a single tool to evaluate CB1 function, extensive previous research has demonstrated that CA1 mGlu5 receptors facilitate LTP through the endocannabinoid molecule 2-arachidonoyl glycerol and CB1 receptors (47-49). Nonetheless, it remains possible that additional or alternative endocannabinoid species might be involved following mGlu3 receptor stimulation and future studies would help to tease apart this complex signaling. Furthermore, the present findings suggest that hippocampal mGlu3 receptors also bias mGlu5 signaling away from the PI3K cascade that induces LTD. These findings contrast with observations in prefrontal cortex pyramidal cells, where mGlu3 receptors direct synaptic plasticity through mGlu5-PI3K-Akt signaling (27). We suspect this metaplastic shift is guided by Homer proteins, which are key elements in mGlu5-dependent plasticity (27, 50) that can bias mGlu5 receptors towards specific signaling pathways (51, 52). Interactions between mGlu receptors and NMDA receptors and other synaptic proteins are also likely to shape the metaplastic landscape and merit further investigation.
The coordinated signaling between mGlu3 and mGlu5 receptors has exciting ramifications given that systemic administration of selective mGlu5 PAMs improve performance in a broad range of animal models of cognitive function that are dependent on intact function of the hippocampus or prefrontal cortex (PFC) (33, 45, 46, 53-62). Furthermore, mGlu5 PAMs reverse deficits in animal models in which synaptic plasticity and cognitive function are impaired (54, 55, 61, 63, 64). The mGlu5 PAMs have not reached clinical trials and multiple companies have halted efforts to advance mGlu5 PAMs. One contributing factor was concern that stemmed from excitotoxicity induced by specific, structural classes of related mGlu5 modulators that have allosteric agonist activity and potentiate NMDA receptor currents. Recent efforts have generated new biased mGlu5 PAMs that do not directly modulate NMDA receptor activity but retain the ability to modulate discrete aspects of mGlu5 actions in the CNS (65, 66). For example, Balu et al. demonstrated that these biased mGlu5 PAMs can rescue schizophrenia-like phenotypes in a genetic model of NMDA receptor hypofunction (67). These studies raise the possibility that selective activators of mGlu3 may share the ability of mGlu5 PAMs to enhance cognition across multiple domains. In support of that hypothesis, LY379268 has been shown to rescue deficits in novel object recognition following acute NMDA receptor antagonism (68), but it remains unclear whether mGlu3 and mGlu5 receptors underlie that effect. Finally, we recently reported that activation of mGlu3 also potentiates mGlu5 signaling and interacts with mGlu5 to induce long-term depression in the PFC (27, 28, 69). However, mGlu3 is also expressed in astrocytes and other cell populations, and undoubtedly has other actions that are not related to the modulation of mGlu5 receptor signaling. For example, while adult astrocytes do not express mGlu5 receptors (70), astrocytic mGlu3 receptors exert neuroprotective effects (71, 72). Also, selective mGlu3 PAMs might also exert beneficial effects not related to the modulation of mGlu5 receptor signaling. Thus, in future studies it will be important to determine whether mGlu3 regulation of mGlu5 signaling plays a broad role in regulating different aspects of cognitive function or if this is restricted to a more limited range of cognitive tasks. Furthermore, previous studies revealed deficits in reference memory and working memory following constitutive deletion of Grm3 in mice (73, 74), but we observed no basal cognitive deficits in CaMKII-mGlu3−/− mice, suggesting a critical role for astrocytic mGlu3 receptor signaling unrelated to mGlu5 receptors. Continued research interrogating mechanisms of neuronal versus astrocytic mGlu3 receptors should yield insight into the biological psychiatry of schizophrenia and have exciting ramifications for mGlu3 PAM development.
Collectively, these combined electrophysiological and behavioral studies highlight that mGlu3 receptors regulate hippocampal plasticity and promote associative learning. Further translational studies will be needed to fully scrutinize this hypothesis, but the current results suggest that mGlu3 receptors are well-suited for ameliorating cognitive symptoms in schizophrenia.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Margarita Behrens for providing the initial breeders for the Grm5Fl/Fl mice used in these studies and Dr. Xiaoyan Zhan and Dr. Hyekyung Plumley for assistance establishing the Grm3Fl/Fl mice. Behavioral experiments were performed through the Murine Neurobehavior Core lab at the Vanderbilt University Medical Center. Confocal imaging was performed in part through the Vanderbilt Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126). This work was supported by NIH grants F32MH111124 (B.J.S), K01MH112983 (R.G.G.), K99AA027806 (M.E.J.), R01MH062646 (P.J.C.), and R37NS031373 (P.J.C.) and rettsyndrome.org grant #3503 (C.M.N). A pre-print of this manuscript was posted on bioRxiv prior to submission to this journal (https://www.biorxiv.org/content/10.1101/2020.10.27.356196v1).
Footnotes
Declaration of interests
P.J.C., C.W.L, and C.M.N. receive research support from Acadia Pharmaceuticals and Boehringer Ingelheim and C.W.L. also receives support from Ono Pharmaceutical. P.J.C., C.W.L., and C.M.N. are inventors on multiple patents for allosteric modulators of metabotropic glutamate receptors. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finan C, Gaulton A, Kruger FA, Lumbers RT, Shah T, Engmann J, et al. (2017): The druggable genome and support for target identification and validation in drug development. Science translational medicine. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA (2008): The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 22:308–322. [DOI] [PubMed] [Google Scholar]
- 3.Maksymetz J, Moran SP, Conn PJ (2017): Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol Brain. 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schizophrenia Working Group of the Psychiatric Genomics C (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature. 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, et al. (2004): Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 101:12604–12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini SM, Mancuso SG, Mostaid MS, Liu C, Pantelis C, Everall IP, et al. (2017): Meta-analysis supports GWAS-implicated link between GRM3 and schizophrenia risk. Transl Psychiatry. 7:e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Quervain DJ, Papassotiropoulos A (2006): Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci U S A. 103:4270–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartorius LJ, Weinberger DR, Hyde TM, Harrison PJ, Kleinman JE, Lipska BK (2008): Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology. 33:2626–2634. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Bea A, Bermudez I, Harrison PJ, Lane TA (2017): A group II metabotropic glutamate receptor 3 (mGlu3, GRM3) isoform implicated in schizophrenia interacts with canonical mGlu3 and reduces ligand binding. J Psychopharmacol. 31:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fell MJ, Svensson KA, Johnson BG, Schoepp DD (2008): Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J Pharmacol Exp Ther. 326:209–217. [DOI] [PubMed] [Google Scholar]
- 11.Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R (2000): Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol. 397:R1–2. [DOI] [PubMed] [Google Scholar]
- 12.Wood CM, Wafford KA, McCarthy AP, Hewes N, Shanks E, Lodge D, et al. (2018): Investigating the role of mGluR2 versus mGluR3 in antipsychotic-like effects, sleep-wake architecture and network oscillatory activity using novel Han Wistar rats lacking mGluR2 expression. Neuropharmacology. 140:246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN (2008): The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl). 196:431–440. [DOI] [PubMed] [Google Scholar]
- 14.Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, Adam G, et al. (2004): Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 46:907–917. [DOI] [PubMed] [Google Scholar]
- 15.Lovell KM, Felts AS, Rodriguez AL, Venable DF, Cho HP, Morrison RD, et al. (2013): N-Acyl-N'-arylpiperazines as negative allosteric modulators of mGlu1: identification of VU0469650, a potent and selective tool compound with CNS exposure in rats. Bioorg Med Chem Lett. 23:3713–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bollinger KA, Felts AS, Brassard CJ, Engers JL, Rodriguez AL, Weiner RL, et al. (2017): Design and Synthesis of mGlu2 NAMs with Improved Potency and CNS Penetration Based on a Truncated Picolinamide Core. ACS Med Chem Lett. 8:919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engers JL, Rodriguez AL, Konkol LC, Morrison RD, Thompson AD, Byers FW, et al. (2015): Discovery of a Selective and CNS Penetrant Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 3 with Antidepressant and Anxiolytic Activity in Rodents. J Med Chem. 58:7485–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, et al. (2019): mGlu2 and mGlu3 Negative Allosteric Modulators Divergently Enhance Thalamocortical Transmission and Exert Rapid Antidepressant-like Effects. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joffe ME, Santiago CI, Engers JL, Lindsley CW, Conn PJ (2017): Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalocortical function. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noetzel MJ, Gregory KJ, Vinson PN, Manka JT, Stauffer SR, Lindsley CW, et al. (2013): A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol Pharmacol. 83:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stansley BJ, Fisher NM, Gogliotti RG, Lindsley CW, Conn PJ, Niswender CM (2017): Contextual Fear Extinction Induces Hippocampal Metaplasticity Mediated by Metabotropic Glutamate Receptor 5. Cereb Cortex.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A (2014): Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci. 34:16762–16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marenco S, Weinberger DR, Schreurs BG (2003): Single-cue delay and trace classical conditioning in schizophrenia. Biol Psychiatry. 53:390–402. [DOI] [PubMed] [Google Scholar]
- 24.Ghoshal A, Rook JM, Dickerson JW, Roop GN, Morrison RD, Jalan-Sakrikar N, et al. (2016): Potentiation of M1 Muscarinic Receptor Reverses Plasticity Deficits and Negative and Cognitive Symptoms in a Schizophrenia Mouse Model. Neuropsychopharmacology. 41:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nomura T, Oyamada Y, Fernandes HB, Remmers CL, Xu J, Meltzer HY, et al. (2016): Subchronic phencyclidine treatment in adult mice increases GABAergic transmission and LTP threshold in the hippocampus. Neuropharmacology. 100:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ (1986): Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 100:729–744. [DOI] [PubMed] [Google Scholar]
- 27.Joffe ME, Santiago CI, Stansley BJ, Maksymetz J, Gogliotti RG, Engers JL, et al. (2019): Mechanisms underlying prelimbic prefrontal cortex mGlu3/mGlu5-dependent plasticity and reversal learning deficits following acute stress. Neuropharmacology. 144:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, et al. (2018): Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology. 128:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Zhu Y, Contractor A, Heinemann SF (2009): mGluR5 has a critical role in inhibitory learning. J Neurosci. 29:3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engers JL, Bollinger KA, Weiner RL, Rodriguez AL, Long MF, Breiner MM, et al. (2017): Design and Synthesis of N-Aryl Phenoxyethoxy Pyridinones as Highly Selective and CNS Penetrant mGlu3 NAMs. ACS Med Chem Lett. 8:925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoepp DD, Jane DE, Monn JA (1999): Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 38:1431–1476. [DOI] [PubMed] [Google Scholar]
- 32.Cosford ND, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, et al. (2003): 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 46:204–206. [DOI] [PubMed] [Google Scholar]
- 33.Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, et al. (2009): mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 34:2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Z, Lv X, Maksymetz J, Stansley BJ, Ghoshal A, Gogliotti RG, et al. (2019): mGlu5 Positive Allosteric Modulators Facilitate Long-Term Potentiation via Disinhibition Mediated by mGlu5-Endocannabinoid Signaling. ACS Pharmacol Transl Sci. 2:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manahan-Vaughan D (1997): Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 17:3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg N, Gerber U, Ster J (2016): Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. J Neurosci. 36:11521–11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, et al. (1993): Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 363:347–350. [DOI] [PubMed] [Google Scholar]
- 38.Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, et al. (1998): The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology. 37:1445–1458. [DOI] [PubMed] [Google Scholar]
- 39.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, et al. (1996): Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 87:1317–1326. [DOI] [PubMed] [Google Scholar]
- 40.Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, et al. (2013): GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 16:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Brien NL, Way MJ, Kandaswamy R, Fiorentino A, Sharp SI, Quadri G, et al. (2014): The functional GRM3 Kozak sequence variant rs148754219 affects the risk of schizophrenia and alcohol dependence as well as bipolar disorder. Psychiatric genetics. 24:277–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedogni F, Cobolli Gigli C, Pozzi D, Rossi RL, Scaramuzza L, Rossetti G, et al. (2016): Defects During Mecp2 Null Embryonic Cortex Development Precede the Onset of Overt Neurological Symptoms. Cereb Cortex. 26:2517–2529. [DOI] [PubMed] [Google Scholar]
- 43.Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, et al. (2015): Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci U S A. 112:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, et al. (2015): Biased mGlu5-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu5 Modulation of NMDAR Currents. Neuron. 86:1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, et al. (2009): Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 57:531–538. [DOI] [PubMed] [Google Scholar]
- 46.Stefani MR, Moghaddam B (2010): Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol. 639:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevaleyre V, Castillo PE (2003): Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 38:461–472. [DOI] [PubMed] [Google Scholar]
- 48.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE (2007): Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 54:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madronal N, Gruart A, Valverde O, Espadas I, Moratalla R, Delgado-Garcia JM (2012): Involvement of cannabinoid CB1 receptor in associative learning and in hippocampal CA3-CA1 synaptic plasticity. Cereb Cortex. 22:550–566. [DOI] [PubMed] [Google Scholar]
- 50.Ronesi JA, Huber KM (2008): Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 28:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, et al. (2001): Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 411:962–965. [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Molinaro G, Collins KA, Hays SA, Paylor R, Worley PF, et al. (2016): Selective Disruption of Metabotropic Glutamate Receptor 5-Homer Interactions Mimics Phenotypes of Fragile X Syndrome in Mice. J Neurosci. 36:2131–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilmour G, Broad LM, Wafford KA, Britton T, Colvin EM, Fivush A, et al. (2013): In vitro characterisation of the novel positive allosteric modulators of the mGlu(5) receptor, LSN2463359 and LSN2814617, and their effects on sleep architecture and operant responding in the rat. Neuropharmacology. 64:224–239. [DOI] [PubMed] [Google Scholar]
- 54.Lin CW, Chen CY, Cheng SJ, Hu HT, Hsueh YP (2014): Sarm1 deficiency impairs synaptic function and leads to behavioral deficits, which can be ameliorated by an mGluR allosteric modulator. Frontiers in cellular neuroscience. 8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhardwaj SK, Ryan RT, Wong TP, Srivastava LK (2015): Loss of dysbindin-1, a risk gene for schizophrenia, leads to impaired group 1 metabotropic glutamate receptor function in mice. Frontiers in behavioral neuroscience. 9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clifton NE, Morisot N, Girardon S, Millan MJ, Loiseau F (2013): Enhancement of social novelty discrimination by positive allosteric modulators at metabotropic glutamate 5 receptors: adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology. 225:579–594. [DOI] [PubMed] [Google Scholar]
- 57.Horio M, Fujita Y, Hashimoto K (2013): Therapeutic effects of metabotropic glutamate receptor 5 positive allosteric modulator CDPPB on phencyclidine-induced cognitive deficits in mice. Fundamental & clinical pharmacology. 27:483–488. [DOI] [PubMed] [Google Scholar]
- 58.Moghaddam B (2004): Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology. 174:39–44. [DOI] [PubMed] [Google Scholar]
- 59.Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004): Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 29:1259–1269. [DOI] [PubMed] [Google Scholar]
- 60.Darrah JM, Stefani MR, Moghaddam B (2008): Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behavioural pharmacology. 19:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, et al. (2016): mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Human molecular genetics. 25:1990–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, et al. (2015): Biased mGlu-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu Modulation of NMDAR Currents. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J, et al. (2012): Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 486:261–265. [DOI] [PubMed] [Google Scholar]
- 64.Waung MW, Huber KM (2009): Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Current opinion in neurobiology. 19:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gregory KJ, Herman EJ, Ramsey AJ, Hammond AS, Byun NE, Stauffer SR, et al. (2013): N-aryl piperazine metabotropic glutamate receptor 5 positive allosteric modulators possess efficacy in preclinical models of NMDA hypofunction and cognitive enhancement. J Pharmacol Exp Ther. 347:438–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellyer SD, Albold S, Sengmany K, Singh J, Leach K, Gregory KJ (2019): Metabotropic glutamate receptor 5 (mGlu5 )-positive allosteric modulators differentially induce or potentiate desensitization of mGlu5 signaling in recombinant cells and neurons. J Neurochem. 151:301–315. [DOI] [PubMed] [Google Scholar]
- 67.Balu DT, Li Y, Takagi S, Presti KT, Ramikie TS, Rook JM, et al. (2016): An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia. Neuropsychopharmacology. 41:2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wieronska JM, Slawinska A, Stachowicz K, Lason-Tyburkiewicz M, Gruca P, Papp M, et al. (2013): The reversal of cognitive, but not negative or positive symptoms of schizophrenia, by the mGlu(2)/(3) receptor agonist, LY379268, is 5-HT(1)A dependent. Behavioural brain research. 256:298–304. [DOI] [PubMed] [Google Scholar]
- 69.Joffe ME, Santiago CI, Engers JL, Lindsley CW, Conn PJ (2019): Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalocortical function. Mol Psychiatry. 24:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, et al. (2013): Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 339:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, et al. (2007): The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci. 27:8297–8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spampinato SF, Copani A, Nicoletti F, Sortino MA, Caraci F (2018): Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection? Front Mol Neurosci. 11:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lainiola M, Procaccini C, Linden AM (2014): mGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behavioural brain research. 266:94–103. [DOI] [PubMed] [Google Scholar]
- 74.Fujioka R, Nii T, Iwaki A, Shibata A, Ito I, Kitaichi K, et al. (2014): Comprehensive behavioral study of mGluR3 knockout mice: implication in schizophrenia related endophenotypes. Mol Brain. 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.