Abstract
Purpose:
To evaluate the effectiveness and ease of N95 respirator decontamination methods in a clinic setting and to identify the extent of microbial colonization on respirators associated with reuse.
Methods:
In a prospective fashion, N95 respirators (n=15) were randomized to a decontamination process (time, dry heat, or ultraviolet C light [UVC]) in outpatient clinics. Each respirator was re-used up to 5 separate clinic sessions. Swabs on each respirator for SARS-CoV-2, bacteria, and fungi were obtained before clinic, after clinic and post-treatment. Mask integrity was checked after each treatment (n=68). Statistical analyses were performed to determine factors for positive samples.
Results:
All three decontamination processes reduced bacteria counts similarly. On multivariate mixed model analysis, there were an additional 8.1 colonies of bacteria (95% CI 5.7 to 10.5; p<0.01) on the inside compared to the outside surface of the respirators. Treatment resulted in a decrease of bacterial load by 8.6 colonies (95% CI −11.6 to −5.5; p<0.01). Although no decontamination treatment affected the respirator filtration efficiency, heat treatments were associated with the breakdown of thermoplastic elastomer straps. Contamination with fungal and SARS-CoV-2 viral particles were minimal to non-existent.
Conclusions:
Time, heat and UVC all reduced bacterial load on reused N95 respirators. Fungal contamination was minimal. Heat could permanently damage some elastic straps making the respirators nonfunctional. Given its effectiveness against microbes, lack of damage to re-treated respirators and logistical ease, UVC represents an optimal decontamination method for individual N95 respirators when reuse is necessary.
Keywords: SARS-CoV-2, Reuse respirators, N95 respirators, Decontamination
Introduction
Beginning in December 2019, reports suggested that a novel coronavirus, now named SARS-CoV-2, was circulating in the city of Wuhan, China. Since then, the virus has rapidly circulated around the world causing a pandemic.1,2,3
Infections with SARS-CoV-2 concentrate in the oropharynx and nasopharynx, placing otorhinolaryngologists and their staff at high risk for infection.4 Otorhinolaryngology practices must establish a safe and effective way of protecting providers. N95 respirators, which are able to filter out at least 95% of air particulates at 0.3 µm aerodynamic mass median diameter5 are a key part of personal protective equipment (PPE) in the prevention of viral spread. These respirators were in short supply in the early phases of the pandemic,6,7 although supply chains have improved, there has been a surge in the price of the respirators. Supply chains may face shortages again.8,9 As a result, many healthcare workers are reusing respirators.
The US Centers for Disease Control and Prevention (CDC) recommended extending the reuse of respirators with the application of a barrier over the mask such as a face shield and storage of the respirator in a bag.10 Other groups have studied several decontamination methods including the use of ultraviolet C irradiation (UVC), 70°C hot air treatment, steam heat treatment, and vaporized hydrogen peroxide treatment for sterilization.11 Many of the studies for reuse of respirators have been in vitro and focused on efficacy instead of effectiveness. For outpatient otorhinolaryngology practices to maintain both productivity and safety, a sustainable way of reusing respirators needed to be defined based on real-world applications and data. The potential consequences of reuse such as microbial overgrowth pose serious health and safety concerns.
Materials and Methods
Study Design
The primary objectives of this quality improvement study were to determine if N95 respirators used up to five different occasions resulted in an increase in microbial burdens. The effectiveness of the following decontamination methods were compared: UVC irradiation, dry heat, or isolation for 72 hours (the maximum estimated length of time that viable SARS-CoV-2 can remain viable on surfaces4). These methods were chosen based on practicality for clinics. Hence techniques such as hydrogen peroxide requiring specialized equipment were not evaluated. Secondary outcomes included evaluating SARS-CoV-2 burden on outer respirator surfaces and determining the integrity of the reused respirators over time after repeated decontamination treatments. The study was exempt from institutional review board approval at our institution.
Study Population
Otorhinolaryngologists at an academic practice were fit tested, trained and issued respirators (either 3M™ 1860 or 3M™ 8210; Maplewood, MN). The 1860 respirators have braided polyisoprene straps; the 8210 have thermoplastic elastomer straps.12,13 Twelve physicians agreed to utilize their respirators for at least four hours while seeing patients in the clinic from April 14 to June 20, 2020. Of the 12, three otorhinolaryngologists were each issued an additional respirator sequentially such that 15 different respirators were included in the study.
Intervention
Once enrolled, each respirator was randomized into one of three decontamination protocols (room temperature for 72 hours (“time”), dry oven heat at 70°C for 30 minutes (“heat”), or ultraviolet C light for 5 minutes on each side (“UVC”)) based on a list randomizer. For the time group, respirators were left at the end of clinic in a brown paper bag for >72 hours, a time selected based on the natural decay of SARS-CoV-2 on surfaces below levels of detection.4 Heating to 70°C for 30 minutes has been shown to disinfect >99% of E. coli, a surrogate for SARS-CoV-2 as it is less susceptible to physical processes, from contaminated N95 respirators.11 Respirators were set on a wooden block and treated with dry heat using a toaster oven. A thermometer was placed inside the oven to ensure an accurate temperature of 70°C/158°F. For the UVC treatment, each side of the respirator was treated with a 5-minute exposure of 330.0 mJ/cm2 of UVC light via the 3B Medical Lumin CPAP mask and accessories cleaner (3B Medical, Inc.; Winter Haven, FL). SARS-CoV-2 is inactivated by very low doses of UV ranging from 2–6 mJ/cm2.14 Manufacturer specifications cite that a 5-minute cycle will deliver between 330.0 mJ/cm2 at its weakest point up to 500 mJ/cm2 at the direct line of sight.13
Each respirator was swabbed prior to the start of each clinic day and again at the end of the clinic day. The respirator then underwent the assigned treatment and a third swab was performed (Figure 1). The respirator was swabbed separately for SARS-CoV-2 virus, bacteria and fungi on the outside surface (side away from the user’s mouth) and for bacteria and fungi on the inside surface. The swabs were stored at 4°C and transferred to respective labs for processing within 48 hours (swab processing details are available in the online supplement). After each treatment and prior to the next clinic use, the integrity of each N95 respirator was tested quantitatively and qualitatively. Filter efficiency was assessed by TSI Port-a-count and a user based fit test, and qualitative evaluation by visual inspection. If the respirator was deemed a good fit and integrity not compromised, then the user was permitted to re-use the respirator. Each respirator remained in its initial randomized group. The respirator was re-used up to 5 times or until loss of integrity.
Figure 1. Study Protocol.
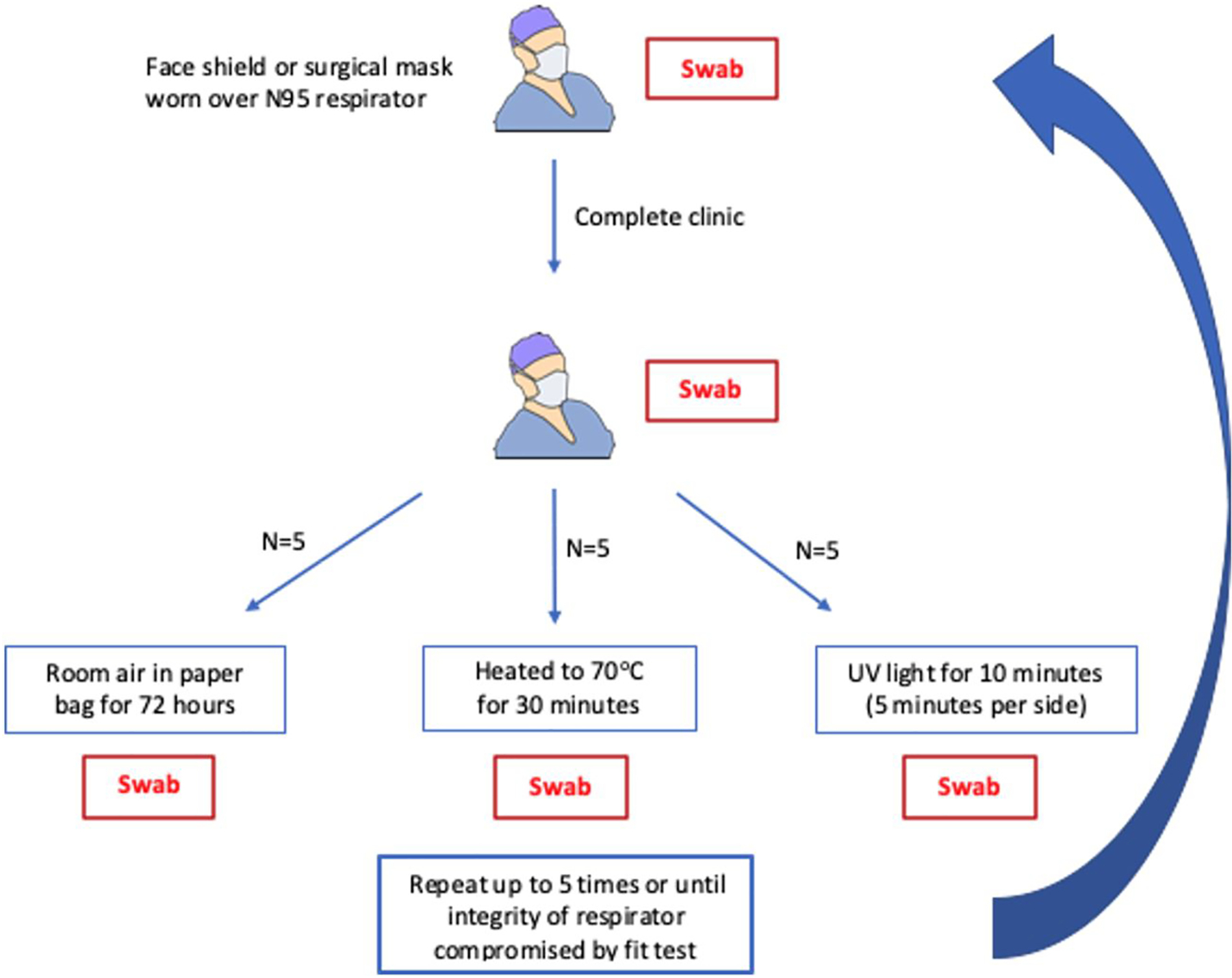
Timing of the swabs during each clinic cycle. Fungal and bacterial swabs were performed on both the inside and outside of the respirators. Viral swabs were only done on the outside of the respirators.
Statistical Analysis
Shapiro-Wilk test for normality was utilized to check for normality of data in the subgroups. As data was nonparametric for some subgroups, sign rank testing was used to compare the impact on bacterial counts of leaving all respirators overnight after treatment in a shifted time format where the pre-clinic counts (in the subsequent use) were compared to the post-treatment counts of the previous use. Multivariate analysis was conducted to determine factors impacting bacterial counts and the odds ratio for a positive culture result. A mixed model of fixed and random effects was utilized. The individual respirators were considered a random effect.
In vitro fungal assays
Given the low fungal burden on the clinical used N95 respirators, post-in vitro assays were designed to evaluate the three different decontamination methods on fungi. Separate, unused autoclaved N95 respirators were sectioned into 2×2 cm squares. Candida albicans strain SC5314 was deposited on the skin-facing surface of each respirator section at a lower and higher concentration range and allowed to dry for 30 minutes. The control group (“Untreated”) was plated immediately onto solid YPD (Yeast extract, Peptone, Dextrose) media containing penicillin (100 IU/ml) and streptomycin (100 µg/ml) to suppress bacterial growth. The other sections were treated with UVC, dry heat at 70°C for 30 minutes (“70°C/30 min”) or room temperature for 72 hours (“RT/72 hrs”) as described above before plating.
Results
Fifteen respirators were included in the study (5 in each of the 3 treatment arms for 5 cycles each). A cycle is comprised of respirator testing before use, after use, and after decontamination treatment. 68 of the 75 theoretical cycles were completed and available for analysis. The following failures were observed: 1) one respirator in the time group failed fit testing prior to the fifth use and 2) two of the respirators in the heat group were discontinued after the second use due to breakage in the head straps (both thermoplastic elastomer straps). All other respirators passed both quantitative and qualitative integrity testing. No samples were positive for the SARS-CoV-2 virus.
Overall, despite multiple reuse, bacterial contamination on respirators was minimal with colony counts ranging from 0–81 colonies, median of 0 (interquartile range of 0–3). Only 25% of swabs had more than three colonies. Most colonies were gram-positive organisms, only three samples had isolated gram-negative organisms. The distribution of colonies among the different treatment groups and time points are shown in Figure 2. By univariate subgroup analysis, on the inside of the post-treatment respirator, bacterial counts appeared statistically different among the treatment groups (with the rank from highest to lowest: time, UVC, and heat, p=0.003). Sign-rank of matched pairs suggested that UVC and heat may be more effective in decreasing bacterial counts than time (data not shown). This trend is illustrated in Figure 2 comparing post-treatment to post-clinic levels among the 3 decontamination methods. Although multivariate analysis does not reflect a significant difference among the treatment groups in bacterial counts, the point estimates for heat and UVC were −2.5 and −2.0, respectively, and their 95% CI skewed towards lower bacterial counts associated with these treatments as compared to treatment with time (Table 1). Taken together, post-treatment swabs of a respirator was associated with a decrease in bacterial load by 8.6 colonies (95% CI −11.6 to −5.5, p<0.01). This decrease in bacterial count was maintained in the pre-clinic counts at 7.1 colonies (95% CI −10.0 to −4.2, p<0.01). The inside of the respirator had on average 8.1 more colonies of bacteria (95% CI 5.7 to 10.5; p<0.01) compared to the outside. Repeated use and total mask use time did not appear to impact bacterial counts. Associated fungal growth similarly did not impact bacterial counts. The random effects part of the model suggested that individual respirator/users did play a significant role in the estimation of bacterial counts.
Figure 2. Box-and-whisker Plot of Bacterial Count.
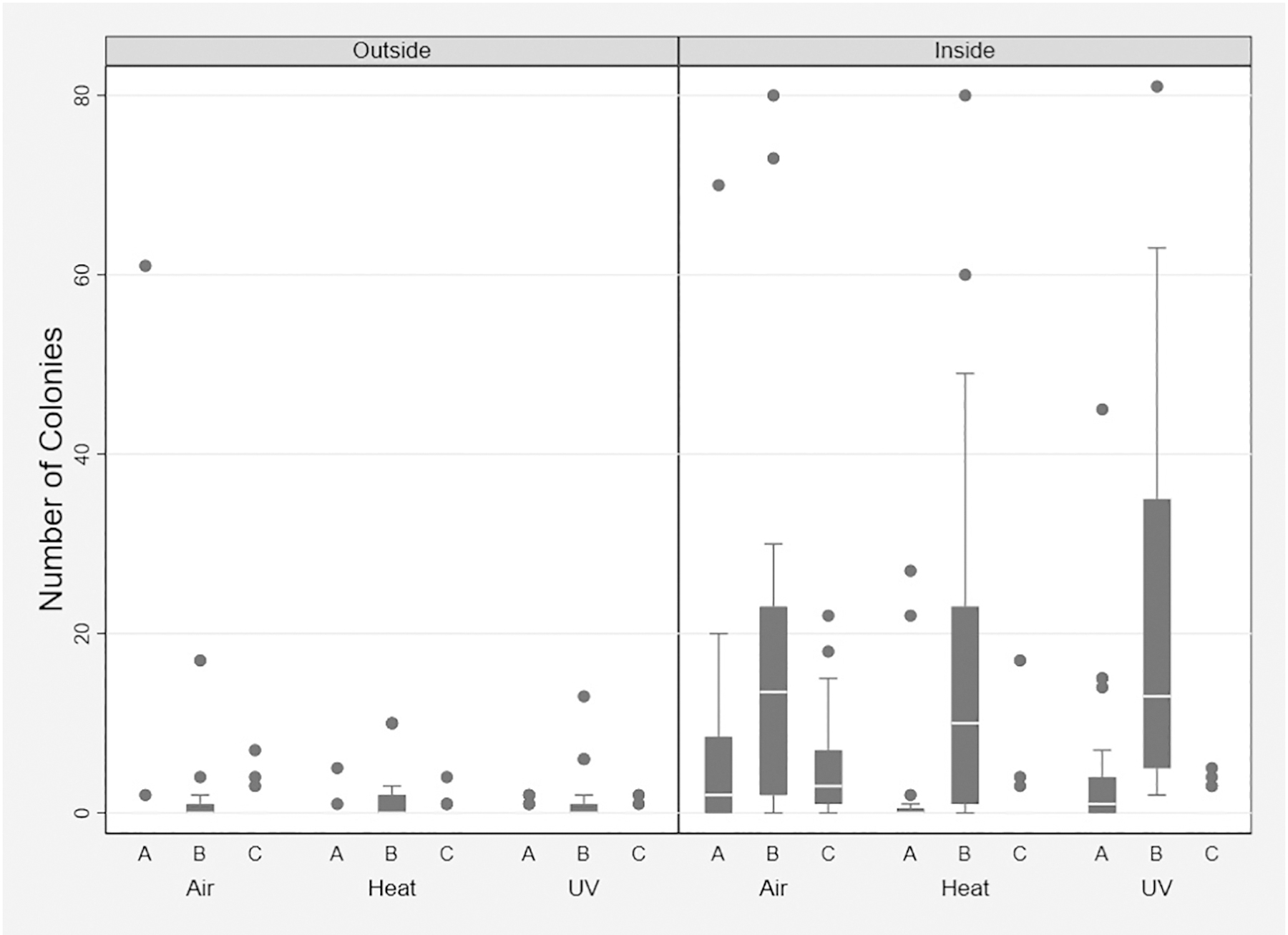
Comparison of bacterial counts in each treatment arm on the inside and outside of the masks at different time points in the clinic cycle. A= pre-clinic; B = post-clinic; C = post-treatment
Table 1.
Linear Multivariate Analysis Bacterial Counts
| Variable | Coefficient/Estimation | p-value | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Swab Location | ||||
| Inside of Respirator | 8.1 | <0.01 | 5.7 | 10.5 |
| Swab timing | ||||
| Pre-Clinic | −7.1 | <0.01 | −10.0 | −4.2 |
| Post-Treatment | −8.6 | <0.01 | −11.6 | −5.5 |
| Use | ||||
| 2 | 2.0 | 0.35 | −2.1 | 6.1 |
| 3 | −1.0 | 0.72 | −6.7 | 4.6 |
| 4 | −1.2 | 0.77 | −9.3 | 6.9 |
| 5 | 0.8 | 0.89 | −10.2 | 11.7 |
| Treatment | ||||
| Heat Oven | −2.5 | 0.31 | −7.5 | 2.4 |
| Ultraviolet Light | −2.0 | 0.40 | −6.7 | 2.7 |
| Days | −0.1 | 0.43 | −0.5 | 0.2 |
| Fungus | 3.4 | 0.14 | −1.2 | 7.9 |
| Fixed Effects Intercept | 9.1 | <0.001 | 4.7 | 13.5 |
| Random Effects (Intercept variability): Respirator | 8.3 | 0.02 | 2.0 | 34.2 |
Baseline for comparison is the outside of the mask, post-clinic, on the first use, in the 72 hour air dry group, at day 0, with no fungal elements
To investigate the possibility of bacterial growth between decontamination treatment and the subsequent use, a sign rank analysis for bacterial counts after treatment to respirator matched pre-clinic bacteria counts showed no significant difference in bacterial counts between these time points suggesting no significant bacterial growth (Table 2). This was confirmed with multivariate analysis with shifted uses where the bacterial counts were found to be −0.3 on the pre-clinic swab vs the post-treatment swab from the previous use, (p=0.83, 95% CI −3.5 to 2.8). Similarly, logistic regression suggested no difference in proportion of gram-positive bacteria between the pre-clinic swab vs the post-treatment swab from the previous use (OR 0.6 p=0.22, 95% CI 0.3 to 1.4).
Table 2.
Sign Rank Test Bacterial Counts Between Uses, Matched Masks
| Between Post-Treatment -> Pre-Clinic the next use | Cycle | p-value |
|---|---|---|
| Treatment Time | 1 | 0.40 |
| 2 | 1.00 | |
| 3 | 0.65 | |
| 4 | 0.32 | |
| Treatment Heat | 1 | 0.22 |
| 2 | n/a | |
| 3 | n/a | |
| 4 | n/a | |
| Treatment UV | 1 | 0.40 |
| 2 | 0.84 | |
| 3 | 0.56 | |
| 4 | n/a |
No p-value is possible if all observation with a subgroup showed 0
Fungal counts were similarly low ranging from 0–120 colonies with a median of 0 (interquartile range of 0–0). Only 8% of the samples demonstrated any fungal growth (7%, 9%, 7% in the respective time, heat, and UVC treatment groups, p=0.89) with most swabs culturing only one colony (5%). No factors were statistically significant in influencing fungal counts. In vitro studies suggested that at low levels (123 – 689 CFUs/mask segment), fungal burdens were significantly reduced after UVC treatment by a mean of 43% but were eliminated altogether after incubation at 70°C or after 72 hours at room temperature (eFigure 1). To further evaluate these decontamination methods, respirator fragments were inoculated with supra-physiological fungal burdens. As seen in Figure 3, treatment at 70°C for 30 minutes completely sterilized the respirators at all concentrations, while room temperature for 72 hours still retained some viable fungal counts but was more effective than UVC exposure.
Figure 3. In-vitro Fungal Cultures.
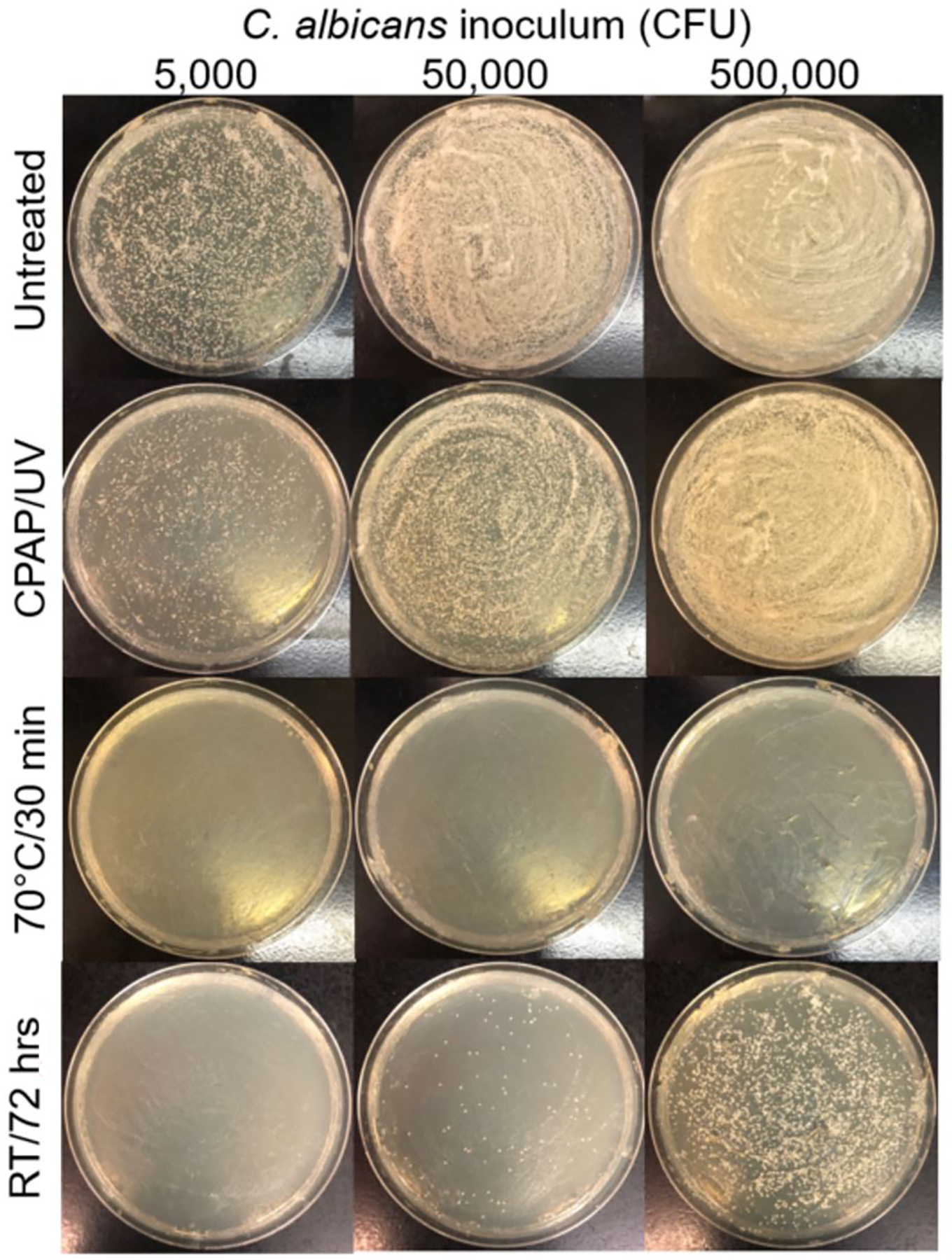
Increasing concentrations of C. albicans were inoculated onto sterile segments of respirators. Segments were plated immediately (“Untreated”) or subjected to one of three decontamination methods prior to plating. Shown are representative images.
Discussion
In this pragmatic trial, we found that reusing N95 respirators up to five separate clinic days with decontamination between uses with either time (waiting 72 hours prior to next use), dry heat, or UVC resulted in controlling bacteria and fungi growth. Although several methods have been validated for decontaminating respirators of viable SAR-CoV-2 virus,16 to our knowledge this is the first study to evaluate the potential contamination of reusing N95 respirators by bacteria and fungi and the ability of these small-scale decontamination methods to control for these microbes. All three decontamination methods did not affect the filtration effectiveness of the respirators, but logistically, UVC treatment was the most efficient. Repeated heat was associated with breakage on the thermoplastic elastomer straps making them unusable. Using time to decontaminate was feasible but required multiple respirators to be used during a week in the clinic for each user.
We found that after at least four hours of use, the highest bacteria counts were detected on the respirator’s inner surface, which contacts the user’s skin. These bacterial levels dropped significantly following each decontamination treatment. Post-clinic swabs inside the respirators had the highest bacterial burden with very few bacteria cultured from the outside of the respirators. This observation suggests the user’s normal flora as the source of most of the bacteria found on respirators. As the same user reused the respirators in this clinic setting, this bacterial contamination may not be clinically concerning.
According to the National Institute for Occupational Safety and Health (NIOSH), data on vaporous hydrogen peroxide, UVC, and moist heat suggest these are the most promising methods to decontaminate respirators for reuse.10 However, in choosing a decontamination method for outpatient clinics, cost effectiveness and logistics are critical factors. Unfortunately, these CDC preferred decontamination methods do not meet these criteria as they often require expensive machines or consumable reagents. Several studies have also shown that 70°C dry heat can be an effective inactivator of viruses including SARS-CoV-2.16,17 However, there are concerns over the filtration degradation with dry heat exposure.16 In our study, we found that the dry heat did not affect the filtration efficiency or fit test of the respirators after five treatments. However, the thermoplastic elastomer straps but not the braided polyisoprene straps were vulnerable to breakage from the heat. Thirty-minute treatment cycles needed for the heat treatment may also pose time restraints for a busy outpatient clinic. As such, despite its effectiveness against microbes and its relative ease of incorporation into an outpatient clinic, the potential damage to certain straps, duration of treatment, and negative effects noted on some N95 materials16 makes dry heat a less than ideal decontamination method.
Single-stranded RNA viruses like SARS-CoV-2 are inactivated at very low doses of UV ranging from 2–6 mJ/cm2.14 In a study commissioned by the FDA, the integrity of the N95 respirator strap, fit, and filtration efficiency was tested over several doses of UV irradiation ranging from 1 to 2000 mJ/cm2 and found no negative effects on the N95 models tested.20 Similarly, we found no negative effects on filtration efficiency or user fit with 5-minute exposures of 330 mJ/cm2 UVC via a commercially available CPAP supply cleaner to each side of the respirators. UVC also represented the most logistically convenient method among the three studied, taking only five minutes to treat each respirator side.
Based on data noting that viable virus can only survive on various surfaces for up to 72 hours,4 the CDC also suggested assigning healthcare workers with five respirators that could be used on a rotation basis during the week and stored in a breathable bag between use.10 In addition to viral load, we found that bacterial and fungal counts also decrease with time. Although bacteria and fungi can survive up to months on dry surfaces,18 objects such as medical records, if left alone, show decreases in colony forming units starting at 24 hours.19 In our study, bacterial counts were statistically no different among treatment groups on multivariate analysis, possibly due to insufficient power or the random effects of each user. However, confidence intervals suggest that UVC and heat treatments were more effective than time alone in reducing bacterial burdens. Univariate analyses with matched sign-rank tests suggested that the UVC and heat group may decrease bacterial loads more effectively than the time group. Decontamination of respirators with time is a reasonable option but requires multiple respirators, which may strain already limited supplies, and may not be as effective as the other decontamination options. In the reused N95 respirators, a majority had little or no fungal growth. Using in vitro fungal assays with a supraphysiologic density of fungi, the data suggested that heat followed by time seemed most effective at controlling fungal growth. According to the white paper on the device used in this study, the 5-minute UVC exposure cycle of 330 mJ/cm2 resulted in a log 2 reduction of Aspergillus niger, the hardiest mold evaluated in their study.15 At dense concentrations of C. albicans, a yeast commonly found within oral cavities that can cause opportunistic infections, we found that exposure to 330 mJ/cm2 for five minutes reduced initial yeast inoculum ranging from 123–659 CFU by about 60%. As such, at physiologically relevant levels, the UVC decontamination technique represents a reasonable option for managing reused respirators. UVC may not be adequate in the rare user with high respiratory levels of fungi such as during an active fungal infection.
There are several limitations of this study. This study may have been underpowered to detect the differences in bacterial load with the different decontamination techniques, as the microbial burden was unexpectedly low. In addition, as a pragmatic study, the mixed effects model demonstrated that much of the variability in the bacterial counts came from the users. Users had different microbial flora, different clinical schedules with varying times of respirator use and of time between uses, as well as performing different numbers of aerosol-generating procedures. However, respirators were used at least four hours during each use. This study was designed to determine feasibility within an outpatient clinical practice. Each of the decontamination methods tested in this study are accessible and affordable for a small practice: brown paper bags for the air group, a toaster oven for the heat group, and an UV disinfectant machine originally intended for CPAP masks that cost less than $300 at the time of this publication.21
The resources and needs of a larger hospital system are different from that of smaller outpatient clinical practices. Results cannot be applied to sharing of the mask in a common decontamination pool. The study only broadly identified the presence of fungi and bacteria without speciation. Further investigation into organism species was beyond the scope of this study. No SARS-CoV-2 viruses were detected in our samples. This study cannot directly comment on the effectiveness of these decontamination methods on the virus, but other papers, as previously mentioned, have found that these are effective methods for viral decontamination.
Conclusion
Among the three evaluated decontamination techniques known to be effective against SAR-CoV-2, UVC exposure at 330 mJ/cm2 for five minutes on each side seemed equally able to control bacterial burdens on reused respirators without negatively impacting their filtration efficiency and represented the most logistically convenient method to incorporate into an outpatient clinic practice. Although effective at decontaminating re-used N95 respirators, dry heat required more time and damaged certain but not all straps. With re-use, bacteria and fungi contamination seemed low with minimal clinical significance.
Supplementary Material
Acknowledgement
This study was supported in part by NIH award R01AI143304 to M.C.L. We would like to thank MicroGenDx (Lubbock, TX) for providing swab collection and transport material and processing these swabs for SARs-CoV-2. We thank Drs. Ron Karni, Soham Roy and Arturo Eguia for their participation in this study. Finally, we thank Dr. W. Katherine Kao for her recommendations on the study protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
MJC serves as a consultant for Acclarent (Irvine, CA), BioMed ENT (San Antonio, TX), Inquis Medical (Atherton, CA), Iodphor (Philadelphia, TX), MicroGenDx (Lubbock, TX), and Stryker (Kalamazoo, MI)
AL serves as a consultant for Inquis Medical (Atherton, CA), Medtronic (Jacksonville, FL), and Stryker (Kalamazoo, MI). She is on the advisory board for ENTvantage (Austin, TX) and has served on advisory boards for Genetech (San Francisco, CA), AstraZeneca (Wilmington, DE) and Sanofi (Paris, France).
WCY is a consultant for Stryker (Kalamazoo, MI) and speaker for Optinose (Yardley, PA). The Department of Otorhinolaryngology - Head & Neck Surgery, McGovern Medical School, The University of Texas Health Science Center at Houston, has received funding from Genetech/Roche (San Francisco, CA), AstraZeneca (Cambridge, England) and Optinose (Yardley, PA).
References
- 1.WHO. Rolling updates on coronavirus disease. UpdatedJuly 31, 2020. AccessedAugust 8, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 2.Balkhair AA. COVID-19 pandemic: a new chapter in the history of infectious diseases. Oman Medical J. 2020;35(2): e123. doi: 10.5001/omj.2020.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones DS. History in a crisis - lessons for Covid-19. NEJM. 2020;382(18):1681–83. doi: 10.1056/NEJMp2004361 [DOI] [PubMed] [Google Scholar]
- 4.Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. NEJM. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC-NIOSH. 42 CFR Part 84 Respiratory Protective Devices. AccessedAugust 8, 2020. https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html
- 6.Miroff N Protective gear in national stockpile is nearly depleted. Washington Post. AccessedApril 28, 2020. https://www.washingtonpost.com/national/coronavirus-protective-gear-stockpile-depleted/2020/04/01/44d6592a-741f-11ea-ae50-7148009252e3_story.html [Google Scholar]
- 7.Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. NEJM. 2020;382(18): e41. doi: 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
- 8.Anderson M Supply chain task force says US will have enough N95 masks to last through October. Becker’s Hospital Review. AccessedAugust 08, 2020. https://www.beckershospitalreview.com/supply-chain/supply-chain-task-force-says-us-will-have-enough-n95-masks-to-last-through-october.html
- 9.Rosalsky G Are high mask prices the problem or the solution? NPR Planet Money. AccessedAugust 08, 2020. https://www.npr.org/sections/money/2020/03/03/811181309/are-high-mask-prices-the-problem-or-the-solution
- 10.CDC. Decontamination and reuse of filtering facepiece respirators. UpdatedAugust 4, 2020. AccessedAugust 8, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- 11.Price A, Chu L. COVID-19 evidence service report: addressing COVID-19 face mask shortages. The Learnly Anesthesia/Stanford AIM Lab. UpdatedMarch 22, 2020. AccessedApril 22, 2020. https://stanfordmedicine.app.box.com/v/covid19-PPE-1-1 [Google Scholar]
- 12.3M Health Care Particulate Respirator and Surgical Mask, 1860S, N95, Small Technical Specification Sheet. 3M; 2017. AccessedAugust 11, 2020. https://multimedia.3m.com/mws/media/1425066O/3m-health-care-particulate-respirator-and-surgical-mask-1860s-n95-small-technical-specifications.pdf
- 13.3M Health Care Particulate Respirator 8210, N95, Technical Specification Sheet. 3M; 2018. AccessedAugust 11, 2020. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- 14.Tseng C, Li C. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J Occup Environ Hyg. 2007;4(6):400–5. doi: 10.1080/15459620701329012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman L, Jones KL. White paper: 3B Lumin and cleaning of CPAP accessories. AccessedApril 1, 2020. https://www.3blumin.com/assets/uploads/2019/11/Lumin-White-Paper.pdf
- 16.Fischer RJ, Morris DH, van Doremalen N, et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv : the preprint server for health sciences. UpdatedApril 24, 2020. AccessedApril 30, 2020. doi: 10.1101/2020.04.11.20062018 [DOI]
- 17.Xiang Y, Song Q, Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am J Infect Control. 2020;48(8):880–2. doi: 10.1016/j.ajic.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hübner N, Hübner C, Kramer A, Assadian O. Survival of bacterial pathogens on paper and bacterial retrieval from paper to hands: preliminary results. Am J Nurs. 2011;111(12):30–36. doi: 10.1097/01.NAJ.0000408181.37017.82 [DOI] [PubMed] [Google Scholar]
- 20.Heimbuch B, Harnish D. Research to mitigate a shortage of respiratory protection devices during public health emergencies. Applied Research Associates, Inc. Sept2019. AccessedApril 23, 2020. https://www.ara.com/sites/default/files/MitigateShortageofRespiratoryProtectionDevices_3.pdf [Google Scholar]
- 21.3B Lumin Product information. https://www.amazon.com/stores/page/0D42717C-68F1-482D-AA0A-5E501BE16235?ingress=2&visitId=ae769b17-2e82-4fa9-aaf1-cb00f7d234ee&ref_=ast_bln. AccessedDecember 9, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


