Abstract
Objective:
To estimate the impact of the PediBIRN (Pediatric Brain Injury Research Network) 4-variable clinical decision rule (CDR) on abuse evaluations and missed abusive head trauma in pediatric intensive care settings.
Study design:
Cluster randomized trial. Participants were 8 pediatric intensive care units (PICUs) in US academic medical centers; PICU and child abuse physicians; and consecutive acutely head-injured patients <3 years (n=183 and n=237, intervention vs. control). PICUs were stratified by patient volumes, pair matched, and randomized equally to intervention or control conditions. Randomization was concealed from the biostatistician. Physician-directed, cluster level interventions included initial and booster training, an abusive head trauma probability calculator, and information sessions. Outcomes included “higher risk” patients evaluated thoroughly for abuse (with skeletal survey and retinal examination), potential cases of missed abusive head trauma (patients lacking either evaluation), and estimates of missed abusive head trauma (among potential cases). Group comparisons were performed using generalized linear mixed-effects models.
Results:
Intervention physicians evaluated a greater proportion of higher risk patients thoroughly (81% vs. 73%, P=. 11) and had fewer potential cases of missed abusive head trauma (21% vs. 32%, P=.05), although estimated cases of missed abusive head trauma did not differ (7% vs 13%, P=.22). From baseline (in prior studies) to trial, the change in higher risk patients evaluated thoroughly (67%→81% vs. 78%→73%, P=.01), and potential cases of missed abusive head trauma (40%→21% vs. 29%→32%, P=.003), diverged significantly. We did not identify a significant change in the number of estimated cases of missed abusive head trauma (15%→7% vs. 11%→13%, P=.22).
Conclusion:
PediBIRN-4 CDR application facilitated changes in evaluations that reduced potential cases of missed abusive head trauma in PICU settings.
Trial registration.
Keywords: child abuse, screening test, clinical decision rule
Abusive head trauma is the leading cause of traumatic death and disability during early childhood, with an estimated annual incidence of 20–30 cases per 100,000 children under two years of age.1–7 Survivors are frequently burdened with lifelong physical and cognitive deficits.8–11 In their landmark single institutional study, Jenny et al found that 15 (28%) of 54 patients with unrecognized abusive head trauma were reinjured when returned to their abusive caregivers, and that 5 (10%) subsequently died or were killed.12 Unfortunately, physicians continue to miss this diagnosis.13
To reduce missed abusive head trauma, Pediatric Brain Injury Research Network (PediBIRN) investigators derived and validated an abusive head trauma screening tool that comes in the form of a directive clinical decision rule (CDR).14,15 The “PediBIRN-4” CDR recommends thorough abuse evaluations for young, acutely head-injured, “higher risk” patients who present with any one or more of its four predictor variables: acute respiratory compromise; bruising of the torso, ear(s), or neck; bilateral or interhemispheric subdural hemorrhage(s); and complex skull fracture(s)(Table I).
Table 1.
The PediBIRN 4-variable clinical decision rule for abusive head trauma.
| To minimize missed cases, every acutely head-injured infant or young child hospitalized for intensive care who presents with one or more of these 4 predictor variables should be considered higher risk and thoroughly evaluated for abuse. |
|
|
| Any clinically-significant respiratory compromise at the scene of injury, during transport, in the Emergency Department, or prior to admission |
| Any bruising involving the child’s torso, ear(s), or neck |
| Any subdural hemorrhage(s) or fluid collection(s) that are bilateral or involve the interhemispheric space |
| Any skull fracture(s) other than an isolated, unilateral, nondiastatic, linear, parietal skull fracture |
Abbreviations: PediBIRN=pediatric brain injury research network
Observational studies15,17,18 suggest that, if applied perfectly, the CDR will miss (stratify as lower risk) approximately 4% of abusive head trauma cases. However, these data do not account for real-life implementation variability.19,20 It follows that widespread CDR implementation must await an efficacy study that demonstrates physician behavior can be altered to reduce missed abusive head trauma.
Our objective was to apply the rigor of a cluster randomized trial (CRT) to estimate the actual impact of the PediBIRN-4 on abuse evaluations and missed abusive head trauma when applied prospectively in pediatric intensive care unit (PICU) settings. Cluster randomization was chosen for practical reasons (administrative convenience, cost savings, the ability to study interventions best applied at the PICU level) and to minimize contamination resulting from physician selection bias.20–22 Stratification was imposed on randomization in an attempt to minimize imbalance between intervention groups.
Hypotheses were generated and outcomes measured at the individual patient participant level. We hypothesized that our physician-directed, cluster level interventions would trigger more frequent “thorough” abuse evaluations of higher risk patients (with skeletal survey and retinal examination), less frequent evaluations of the remaining lower risk patients (with either or both abuse evaluations), fewer potential cases of missed abusive head trauma (patients lacking skeletal survey and/or retinal examination), and lower estimates of missed abusive head trauma (among potential cases), at intervention vs. control PICUs, respectively.
Methods
Design.
Clusters were eight PICUs (4 intervention, 4 control) in academic medical centers from across the United States (Table 2; available at www.jpeds.com). Stratification was based on projected patient volumes. The CRT was extended from 24 months to 32 based on lower than projected eligible patients at intervention PICUs. No other changes in trial design or procedures were required. Data and safety were monitored by a data safety monitoring board.
PICU recruitment and stratification.
Candidate PICUs included 18 that had participated in prior observational studies to derive and validate the CDR.14,15 The seven PICUs whose lead PediBIRN investigator was no longer available were eliminated from initial consideration. The remaining PICUs were stratified based on each PICU’s mean monthly count of eligible patients in prior studies (<1, 1–2, 2–3, or >3). The two PICUs within each stratification with the highest mean counts were invited and agreed to participate.
Participants.
Physician participants were the licensed, credentialed, PICU and child abuse pediatric (CAP) physicians in active practice who made decisions to launch or forgo child abuse evaluations in their young, acutely head-injured patients. Patient participants were consecutive children under three years of age admitted for intensive care of symptomatic, acute, closed, traumatic, cranial or intracranial injuries confirmed on initial neuroimaging. Victims of motor vehicle collisions and patients with pre-existing brain abnormalities were excluded.
Randomization.
PICUs served as the units of randomization. The study principal investigator (PI) matched participating PICUs into four pairs based on projected patient volumes. A senior biostatistician randomized one PICU from each pair to the intervention arm. Randomization was concealed from a second biostatistician responsible for data analysis. At intervention PICUs, physician participants were not blind to allocation. At control PICUs, the CRT was strictly observational.
Interventions.
Interventions designed to support CDR application as an abusive head trauma screening tool were targeted toward physician participants at the cluster level. They included: (1) an initial online 15 minute training that provided an overview regarding abusive head trauma; missed abusive head trauma; and the CDR’s derivation, validation, application, and potential impact; (2) CDR badge cards; (3) prompts by local research coordinators to apply the CDR; (4) monthly “booster training” emails; (5) access to an online “abusive head trauma probability calculator” (accessible at www.pedibirn.com) that applied the PediBIRN-4 as an prediction tool16 rather than a directive decision rule; (6) progress reports (every 6 months x4) that compared PICU-specific measures of CRT progress, provider engagement, and adherence to the CDR’s recommendations; and (7) local “information sharing sessions” (every 6 months x4) led by the site PI (x3) or study PI (x1) to discuss barriers to CDR acceptance and utilization. Strict compliance with the CDR’s recommendations was not required.
Outcomes measures.
Primary and secondary measures of patient safety, practice efficiency, and clinical outcomes are described in Table 3. All were measured at the individual patient participant level. To enhance understanding of methods and results, one outcome measure (potential cases of missed abusive head trauma) was added after trial launch.
Table 3.
Outcome measures.
| Outcome Measure | Description | Category |
|---|---|---|
| Higher risk patients evaluated thoroughly for abuse | Patients who presented with any one or more of the CDR’s four predictor variables and were evaluated with skeletal survey AND retinal exam. | A primary measure of patient safety |
| Lower risk patients evaluated even partially for abuse | Patients who presented with none of the CDR’s four predictor variables and were nevertheless evaluated with skeletal survey AND/OR retinal exam. | A primary measure of practice efficiency |
| Eligible patients evaluated at least partially for abuse | A composite representation of abusive head trauma evaluation practices, capturing all patients evaluated with skeletal survey AND/OR retinal exam. | A secondary measure of patient safety |
| Corroborated cases of abusive head trauma | Patients whose completed skeletal survey and/or retinal exam revealed findings considered moderately or highly specific for abuse.a A measure of overall diagnostic yield. | A secondary measure of practice efficiency |
| Potential cases of missed abusive head trauma | Patients lacking skeletal survey AND retinal exam, and, patients whose partial abuse evaluation (with skeletal survey OR retinal exam) revealed no corroborating findings of abuse. | A primary clinical outcome measure |
| Mean estimate of abusive head trauma probability | Calculated using the patient-specific estimates of abusive head trauma probability accessed by applying the PediBIRN- 4 CDR as a prediction tool (“abusive head trauma probability calculator”). Needed/Used to calculate estimated cases of missed abusive head trauma among potential cases of missed abusive head trauma. | A secondary clinical outcome measure |
| Estimated cases of missed abusive head trauma | An estimate of missed abusive head trauma cases among potential cases of missed abusive head trauma. Calculated by multiplying [potential cases of missed abusive head trauma] x [their mean estimate of abusive head trauma probability].b Needed/Used to calculate estimated rates of missed abusive head trauma among all cases of abusive head trauma. | A secondary clinical outcome measure |
| Estimated rate of missed abusive head trauma | An estimate of cases of missed abusive head trauma among all cases of abusive head trauma. Calculated by dividing [estimated cases of missed abusive head trauma] by [estimated cases of missed abusive head trauma + corroborated cases of missed abusive head trauma]. | A primary clinical outcome measure |
Abbreviations: CDR=clinical decision rule, PediBIRN=pediatric brain injury research network
Including rib fracture(s), classic metaphyseal lesion fracture(s), epiphyseal separation(s), fracture(s) of the scapula or sternum, fracture(s) of digit(s), vertebral body fracture(s) or dislocation(s), fracture(s) of spinous process(es), retinoschisis, and/or retinal hemorrhages described as dense, extensive, covering a large surface area, and/or extending to the ora serrata.
The PediBIRN-4 CDR’s patient-specific estimates of abuse probability were previously shown to correlate positively and strongly (Pearson’s R = 0.71) with the overall diagnostic yield of PediBIRN patients’ completed skeletal surveys and/or retinal examinations (see reference 16).
Our selection of clinical outcome measures was based on four assumptions adopted to facilitate estimation of missed abusive head trauma in the absence of a gold standard: (1) patients who underwent both skeletal survey and retinal examination had been thoroughly evaluated for abuse; (2) the discovery of moderately or highly specific findings of abuse on either abuse evaluation corroborated a patient’s head trauma as abusive; (3) patients lacking skeletal survey and/or retinal examination represented potential cases of missed abusive head trauma; and (4) actual cases of missed abusive head trauma—among potential cases—could be estimated as (potential cases of missed abusive head trauma) x (their mean estimate of abuse probability).
To clarify, actual cases of missed abusive head trauma, among larger cohorts of potential cases, were estimated by applying the PediBIRN-4 as a prediction tool (the “abusive head trauma probability calculator”). PediBIRN investigators had demonstrated previously that the PediBIRN-4’s patient-specific estimates of abuse probability correlated positively and strongly (Pearson R=.71) with the overall diagnostic yield of their patients’ completed skeletal surveys and/or retinal examinations.16 Thus, the estimates of missed abusive head trauma cases at intervention and control PICUs were estimates of the proportion of their potential cases whose missing abuse evaluations would have yielded corroborating findings of abuse.
Human subjects protections.
At every participating PICU, local institutional review boards (IRB) approved CRT participation with waivers of parental informed consent for eligible patients. At control PICUs, where the CRT was to be strictly observational, local IRBs also approved waivers of informed consent for PICU and CAP physicians. At intervention PICUs, PICU and CAP physicians provided written informed consent for participation. Consent was secured after randomization prior to trial launch.
Data capture and management.
PICUs were required to capture complete data regarding >90% of eligible patients based on monthly audits. Patient-specific data were abstracted from medical records. Intervention physicians self-reported their abusive head trauma screening and evaluation logic and decisions. Captured data were uploaded into access-controlled Research Electronic Data Capture (REDCap) software hosted at Penn State Hershey Medical Center. Data integrity rules programmed in REDCap facilitated identification of data inconsistencies that were tracked until resolution. Completed data forms regarding 15% of eligible patients were selected at random for upload and reviewed to verify the accuracy of data entry.
Power and sample size calculations.
Matched PICUs were assigned randomly to intervention and control groups in a 1:1 allocation ratio (4 intervention, 4 control). In prior strictly observational studies15,16, physicians at the same eight PICUs evaluated 73% of their higher risk patients thoroughly for abuse. We predicted that intervention physicians would increase thorough abuse evaluations of higher risk patients (with both skeletal survey and retinal examination) from 73% to 90%. Thus, we powered our primary hypothesis to detect a 17% increase. To test the hypothesis, we utilized the simulation technique for power analysis. Generalized linear mixed-effects models were adopted via SAS PROC GLIMMIX, version 9.4 to analyze 1,000 Monte Carlo datasets from simulation. For the target sample size of 304 higher risk patients (152 in each arm), the proportion of simulated datasets that yielded a statistically significant result for the primary hypothesis was 95.7%, indicating sufficient statistical power.
Post hoc analyses.
We completed two post hoc analyses. First, using equivalent data from the same eight PICUs (captured prospectively between 2010 and 2013 in comparable patient cohorts), we compared outcome measures in the current study to equivalent measures in prior observational studies14,15, at intervention vs. control PICUs, respectively. Second, to re-verify the CDR’s potential abusive head trauma screening performance over time and across trial arms, we: (1) combined cases of corroborated abusive head trauma and estimates of missed abusive head trauma to estimate abusive (and non-abusive) head trauma prevalence in higher and lower risk subpopulations, (2) entered values into 2×2 contingency tables, and (3) calculated CDR test characteristics (sensitivity, predictive values, likelihood ratios), assuming physicians evaluated every higher risk patient thoroughly for abuse and deferred abuse evaluations in all lower risk patients.
Statistical analyses.
Intent-to-treat analyses were used for all statistical analyses examining group differences, making use of all available data from all randomized patient participants. Data regarding categorical variables were characterized as frequencies with proportions. Group comparisons (intervention vs. control) of outcome measures (including pre-post changes from baseline to CRT) were analyzed using generalized linear mixed-effects models. In particular, the random intercept in the statistical model captured the PICU-level heterogeneity. The covariates in the statistical model included a group indicator, pre-post interventions, and their interactions. Results were characterized using P values and odds ratios (OR) with 95% confidence intervals (CI). Analyses were undertaken using Statistical Analysis Software (SAS), version 9.4 (Cary, NC). For additional information regarding model fitting and parameter set-ups, see Methods in the Appendix (available at www.jpeds.com).
Results
Patients.
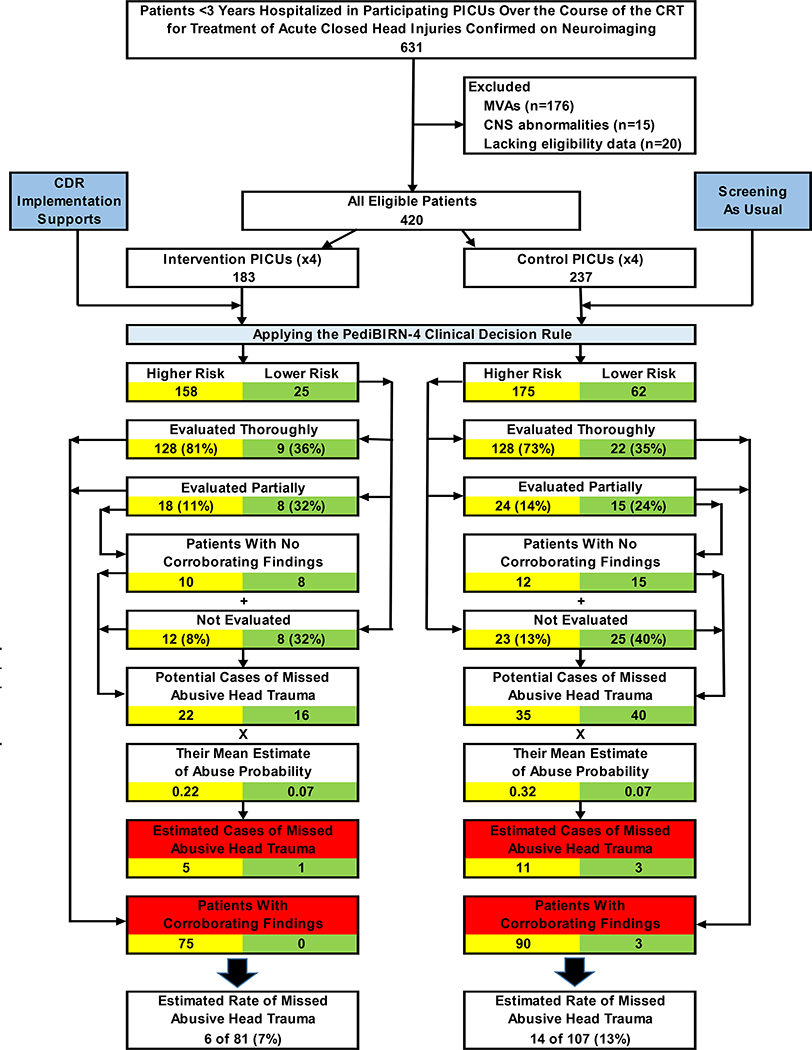
Prospective data capture began August 1, 2017, and ended March 31, 2020, when enrollment targets based on power analysis were reached. Over these 32 months, 631 acutely head-injured patients under 3 years of age were hospitalized for intensive care in a participating PICU. Of these, 211 (33%) were excluded, including 176 victims of motor vehicle accidents, 15 patients with preexisting brain abnormalities, and 20 patients lacking data to confirm eligibility. Investigators captured complete requested data regarding all of the remaining 420 eligible patients. Of these, 183 (44%) were hospitalized in intervention PICUs; the remaining 237 (56%) in control PICUs (Figure 1). Significant differences in patient race/ethnicity were noted across trial arms (Table 4; available at www.jpeds.com).
Figure 1. Patient attrition and the impacts of abusive head trauma screening and evaluation practices at intervention vs. control sites over the course of the CRT.
Of the 631 acutely head-injured patients under 3 years of age hospitalized in a participating PICU over the course of the 32-month CRT, 211 (33%) were excluded from study participation. 183 (44%) of the remaining 420 eligible patients were admitted to an intervention PICU, where clinicians were encouraged to apply the PediBIRN-4 CDR as an abusive head trauma screening tool. The remaining 237 patients (56%) were hospitalized in a control PICU, where providers practiced abusive head trauma screening as usual. The yellow boxes track abusive head trauma screening and evaluation practices—and relevant clinical impacts of those practices—in patients the CDR categorized as higher risk. Green boxes track the equivalent practices and outcomes in the remaining lower risk patients. Red boxes highlight corroborated cases of abusive head trauma and estimates of missed abusive head trauma in higher vs. lower risk patients in each arm of the CRT.
Abbreviations: CDR=clinical decision rule, CNS=central nervous system, CRT=cluster randomized trial, PediBIRN=pediatric brain injury research network, PICU=pediatric intensive care unit, MVA=motor vehicle accident
Physicians.
Ninety (99%) of 91 PICU and CAP physicians practicing at intervention PICUs consented to participate and completed initial training. They included 57 pediatric intensivists, 18 CAP physicians, and 15 pediatric trauma- or neuro-surgeons. Their demographics are summarized in Table 4. Fourteen (16%) of 90 consented physicians relocated or retired prior to trial completion.
Interventions.
Over the course of the 32-month CRT, intervention coordinators facilitated 333 physician prompts to apply the PediBIRN-4 CDR as a directive abusive head trauma screening tool, 86 (96%) of 90 consented physicians acknowledged 1979 (76%) of 2594 booster training emails, 50 (56%) accessed the abusive head trauma probability calculator, 38 (42%) attended >2 of 4 information sharing sessions, and 66 (73%) had an opportunity to apply the CDR during patient care.
CDR application.
The PediBIRN-4 stratifies acutely head-injured children under 3 years of age into higher and lower risk cohorts, and directs physicians to evaluate every higher risk patient thoroughly for abuse (Table 1). Intervention physicians and coordinators correctly categorized 158 (86%) of 183 eligible patients as higher risk and 25 (14%) as lower risk. None were categorized incorrectly. At control PICUs, where the CRT was strictly observational, CDR application would have categorized 175 (74%) of 237 eligible patients as higher risk and 62 (26%) as lower risk (Figure 1).
Outcomes.
Figure 1 demonstrates the approach used to calculate outcome measures (Table 3) at intervention vs. control PICUs. Equivalent methods were applied to calculate these same outcome measures at baseline, in comparable patient cohorts (n=172 and n=165 at intervention and control PICUs respectively) and using equivalent data from prior observational studies.14,15 Complete results are detailed in Table 5 and Figure 2 (available at www.jpeds.com). Key results include the following:
Table 5.
The impacts of changing abusive head trauma evaluation practices on relevant clinical outcomes.
| Columns | A | B | C | D | |
| Intervention PICUs (n=4) | Matched Control PICUs (n=4) | ||||
| At Baseline | During the CRT | At Baseline | During the CRT | ||
| (n=172) | (n=183) | (n=165) | (n=237) | ||
| All patients evaluated at least partially for abuse, n (%) a | 126 (73) of 172 | 163 (89) of 183 | 135 (82) of 165 | 189 (80) of 237 | |
| Higher risk patients evaluated thoroughly for abuse, n (%) b | 89 (67) of 132 | 128(81)of 158 | 97 (78) of 124 | 128 (73) of 175 | |
| Lower risk patients evaluated even partially for abuse, n (%) a | 17 (43) of 40 | 17 (68) of 25 | 28 (68) of 41 | 37 (60) of 62 | |
| Patients with corroborating findings of abuse, n (%) c | 63 (50) of 126 | 75(46)of 163 | 54 (40) of 135 | 93 (49) of 189 | |
| Potential cases of missed abusive head trauma, n (%) d | 68 (40) of 172 | 38 (21) of 183 | 48 (29) of 165 | 75 (32) of 237 | |
| Mean estimate of abusive head trauma probability, (%) e | (17) | (16) | (14) | (19) | |
| Estimated cases of missed abusive head trauma, n f | 11 | 6 | 7 | 14 | |
| Estimated rate of missed abusive head trauma, n (%), g | 11 (15) of 74 | 6 (7) of 81 | 7 (11) of 61 | 14 (13) of 107 | |
| Odds Ratio (95% CI), P values | |||||
| Comparing columns... | A vs. B | C vs. D | A vs. C | B vs. D | ΔA→B vs. ΔC→D |
| All patients evaluated at least partially for abuse a |
2.97
(1.67–5.29) P <.001 |
0.88 (0.53–1.45) P =.61 |
1.64 (0.95–2.83) P =.06 |
0.48
(0.27–0.85) P =.01 |
0.29
(0.14–0.64) P =.002 |
| Higher risk patients evaluated thoroughly for abuse b |
2.02 (1.16–3.52) P = .01 |
0.75 (0.43–1.30) P =.30 |
1.74 (0.96–3.13) P =.07 |
0.64 (0.37–1.11) P =.11 |
0.37
(0.17–0.80) P =.01 |
| Lower risk patients evaluated even partially for abuse a | 2.84 (0.98–8.19) P =.05 |
0.70 (0.30–1.63) P =.41 |
2.79
(1.01–7.70) P =.05 |
0.69 (0.24–1.98) P =.49 |
0.25
(0.60–0.96) P =.04 |
| Patients with corroborating findings of abuse c | 0.94 (0.58–1.53) P =.81 |
1.27 (0.79–2.06) P =.32 |
0.81 (0.32–2.02) P =.65 |
1.09 (0.46–2.60) P =.84 |
1.35 (0.68–2.67) P =.39 |
| Potential cases of missed abusive head trauma d |
0.42
(0.26–0.67) P <.001 |
1.13 (0.73–1.76) P =.58 |
0.68 (0.36–1.27) P =.22 |
1.84
(1.00–3.38) P =.05 |
2.71
(1.41–5.21) P =.003 |
| Estimated rate of missed abusive head trauma g | 0.46 (0.16–1.31) P =.14 |
1.16 (0.44–3.05) P =.76 |
0.74 (0.27–2.05) P =.20 |
1.88 (0.69–5.13) P =.22 |
2.53 (0.61–10.56) P =.22 |
Abbreviations: CI=confidence interval, P=probability, CRT=cluster randomized trial, PICU=pediatric intensive care unit
With skeletal survey AND/OR retinal examination by an ophthalmologist.
With skeletal survey AND retinal exam by an ophthalmologist.
Including rib fracture(s), classic metaphyseal lesion fracture(s), epiphyseal separation(s), fracture(s) of the scapula or sternum, fracture(s) of digit(s), vertebral body fracture(s) or dislocation(s), fracture(s) of spinous process(es), retinoschisis, and/or retinal hemorrhages described as dense, extensive, covering a large surface area, and/or extending to the ora serrata.
Including patients lacking skeletal survey AND retinal exam, and, patients whose partial abuse evaluation (with skeletal survey OR retinal exam) revealed no corroborating findings of abuse.
The patient-specific estimates of abuse probability used to calculate the means were accessed by applying the PediBIRN-4 as a prediction tool (see reference 16).
Calculated as [potential cases of missed abusive head trauma] x [their mean estimate of abuse probability].
Calculated as [estimated cases of missed abusive head trauma] / [estimated cases of missed abusive head trauma + patients with corroborating findings of abusive head trauma].
From baseline to CRT, intervention physicians significantly increased the proportions of their higher risk patients evaluated thoroughly for abuse (67%→81%, P =.01), and their lower risk patients evaluated at least partially (43%→68%, P =.05), thus significantly lowering their rate of potential missed abusive head trauma (40%→21%, P <.001). At control PICUs, there were no significant changes from baseline in these same outcome measures. The divergence (from baseline to CRT) in potential cases of missed abusive head trauma at intervention vs. control PICUs was significant (40%→21% vs. 29%→32%; P = .003), as was the difference in potential cases of missed abusive head trauma at intervention vs. control PICUs over the course of the CRT (21% vs. 32%, P =.05).
Post hoc analyses re-confirmed that, applied accurately and consistently, the PediBIRN 4-variable CDR would have screened effectively for abusive head trauma, with sensitivity 94%−99% and negative predictive value 90%−96% (Table 6; available at www.jpeds.com).
Harms.
Over the course of the CRT, no harms or adverse events were reported by physicians, coordinators, or patients’ families.
Discussion
Our results support the following impressions: (1) our interventions facilitated CDR application as an abusive head trauma screening tool, (2) CDR application effected changes in abusive head trauma evaluation practices that reflected heightened overall concern for missed abusive head trauma (rather than strict fidelity to the CDR’s recommendations), (3) the changes in abusive head trauma evaluation practices reduced potential cases of missed abusive head trauma, (4) the estimated rate of missed abusive head trauma at intervention PICUs (7%) was higher than that predicted with full adherence to the PediBIRN-4’s recommendations (4%), and (5) the CDR’s potential abusive head trauma screening accuracy was preserved.
The PediBIRN-4’s screening recommendations must be interpreted in the context of other relevant information, such as the presenting history, past and family medical history, psychosocial risk assessment, the results of tests to confirm or exclude medical mimics, and input from investigators. The CDR was designed to inform and enhance clinical judgement, not supplant it.
Toward that end, the CDR focuses attention on patients it stratifies as higher risk. In prior studies14,15, intervention physicians evaluated 67% (89 of 132) of higher risk patients thoroughly for abuse, and their estimated rate of missed abusive head trauma was 15% (11 of 74, Table 5). During the CRT, they evaluated 81% (128 of 158), and the estimated rate of missed abusive head trauma dropped to 7% (6 of 81, Table 5). Had they evaluated all 158 higher risk patients thoroughly for abuse during the CRT, the estimated rate of missed abusive head trauma would have been 1% (1 of 81, Table 6). Our estimates suggest that recognition of these 5 additional victims of abuse required thorough abuse evaluations of 30 additional higher risk patients, 18 of whom had already undergone skeletal survey or retinal examination, but not both (Figure 1).
Multiple investigators have developed CDRs for child physical abuse. Like the PediBIRN-4, some were designed to function as screening tools. Examples include Berger et al PIBIS (Pittsburgh brain injury score), which identifies infants at risk for brain injury or abusive head trauma who might benefit from neuroimaging23, and Pierce et al BCDR (bruising clinical decision rule), which identifies young children with bruising who need further evaluation for abuse24. Other CDRs apply the (positive or negative) results of completed abuse evaluations to estimate abuse probability. Examples include Maguire et al PredAHT (predicting abusive head trauma)25,26 and the PediBIRN-7 prediction tool27 for abusive head trauma. The PediBIRN-4 is the first of these CDRs to be introduced into clinical practice via a randomized trial.
We hypothesized incorrectly that the CDR’s focus on higher risk patients would prompt fewer abuse evaluations of lower risk patients. Those lower risk patients selected for evaluation may have presented with historical, psychosocial, and/or physical findings that elevated concerns for abuse. And yet, over time and across trial arms, only 3 (3%) of 99 lower risk patients evaluated at least partially for abuse revealed corroborating findings. It follows that many evaluations of lower risk patients were avoidable. Decreasing the number of avoidable abuse evaluations will lessen parental stress and distrust, reduce costs, and increase the accuracy of abusive head trauma screening.
Our study had multiple strengths. Its overall design embedded a pre-post study into the larger CRT. We calculated outcome measures reflective of both patient safety and practice efficiency. Decisions regarding PICU group assignment, patient risk stratification, and abusive head trauma evaluations were blinded to baseline differences between intervention groups. The biostatistician assessing outcomes was blinded to PICU randomization.
Our study also had limitations. By chance, baseline rates of abuse evaluations were lower in intervention PICUs than control PICUs, suggesting that regression toward the mean may have affected our results. CDR implementation supports may not have been delivered or received with fidelity across intervention PICUs and physicians. Because patient participants 2 to 3 years of age are not routinely evaluated for abuse with skeletal survey, calculations of potential and estimated cases of missed abusive head trauma may have been inflated. We failed to capture prospective data that might explain some absent or incomplete abuse evaluations (eg, early death). Absent a gold standard, we relied on patient-specific estimates of abusive head trauma probability16 to predict the results of abuse evaluations never completed. Finally, our results are not generalizable to non-PICU settings, to PICUs in non-academic centers, or to PICUs lacking access to CAP physicians.
Physician failures to recognize, diagnose, and report suspected abusive head trauma place young victims at risk for repetitive abuse and even death.12,13 Application of the PediBIRN 4-variable CDR facilitated changes in abuse evaluations that reduced potential cases of missed abusive head trauma in PICU settings. In anticipation of future CDR effectiveness studies (in non-PICU settings and/or under less controlled conditions), secondary analyses are planned to discern which interventions were most impactful; to identify site-, physician-, and patient-specific variables that impacted providers’ adherence to the CDR’s recommendations; and to estimate the cost-effectiveness and sustainability of our interventions.
Supplementary Material
Figure 2
From baseline in prior observational studies to the CRT, intervention physicians increased the proportions of all patients evaluated at least partially for abuse (73%→89%), higher risk patients evaluated thoroughly for abuse (67%→81%), and lower risk patients evaluated at least partially for abuse (43%→68%). These changes in abusive head trauma evaluation practices resulted in lower proportions of potential (40%→21%) and estimated cases of missed abusive head trauma (15%→7%), and a lower overall diagnostic yield of completed abuse evaluations (50%→46%). At control PICUs, the opposite trends were observed. The small brackets identify changes from baseline to CRT that were statistically significant (P ≤.05). The large brackets identify divergent changes (from baseline to CRT) at intervention vs. control PICUs that were statistically significant. The red boxes identify differences at intervention vs. control PICUs that were statistically significant during the CRT.
Acknowledgments
Funded by The Eunice Kennedy Shriver National Institute of Child Health and Human Development (P50HD089922. Additional support was provided by The Penn State Clinical & Translational Research Institute, Pennsylvania State University CTSA (NIH/CTSA UL1 TR002014). The National Institutes of Health and Pennsylvania State University had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Pennsylvania State University. The authors declare no conflicts of interest.
Abbreviations:
- CAP
child abuse pediatrics
- CDR
clinical decision rule
- CI
confidence interval
- CRT
cluster randomized trial
- IRB
institutional review board
- PediBIRN
pediatric brain injury research network
- PI
principle investigator
- PICU
pediatric intensive care unit
- OR
odds ratio
- P
probability
- REDCap
research electronic data capture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duhaime AC, Christian CW, Rorke LB, Zimmerman RA. Nonaccidental head injury in infants--the “shaken-baby syndrome”. N Engl J Med 1998; 338:1822–9. [DOI] [PubMed] [Google Scholar]
- 2.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA 2003;290:621–6. [DOI] [PubMed] [Google Scholar]
- 3.Eisele JA, Keglar SR, Trent RB, Coronado VG. Nonfatal traumatic brain injury-related hospitalization in very young children−−15 states. J Head Trauma Rehabil 2006;21:537–43. [DOI] [PubMed] [Google Scholar]
- 4.Minns RA, Jones PA, Mok JY. Annual incidence of shaken impact syndrome in young children. Am J Prev Med 2008;34:S126–33. [DOI] [PubMed] [Google Scholar]
- 5.Barlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet 2000;356:1571–2. [DOI] [PubMed] [Google Scholar]
- 6.Ellingson KD, Leventhal JM, Weiss HB. Using hospital data to track inflicted traumatic brain injury. Am J Prev Med 2008;34:S157–62. [DOI] [PubMed] [Google Scholar]
- 7.Jayawant S, Rawlinson A, Gibbon F, Price J, Schulte J, Sharples P, et al. Subdural haemorrhages in infants: population based study. BMJ 1998;317:1558–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acker SN, Roach JP, Partrick DA, Karrer FM, Bensard DD, Sirotnak AP. Beyond morbidity and mortality: the social and legal outcomes of non-accidental trauma. J Pediatr Surg 2015;50:604–7. [DOI] [PubMed] [Google Scholar]
- 9.Amagasa S, Matsui H, Tsuji S, Uematsu S, Moriya T, Kinoshita K. Characteristics distinguishing abusive head trauma from accidental head trauma in infants with traumatic intracranial hemorrhage in Japan. Acute Med Surg 2018;5:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nat Rev Neurol 2014;10:156–66. [DOI] [PubMed] [Google Scholar]
- 11.Selassie AW, Borg K, Busch C, Russell WS. Abusive head trauma in young children: a population-based study. J Trauma Nurs 2014;21:72–82. [DOI] [PubMed] [Google Scholar]
- 12.Jenny C, Hymel KP, Ritzen A, Reinert SE, Hay T. Analysis of missed cases of abusive head trauma. JAMA 1999;281:621–6. [DOI] [PubMed] [Google Scholar]
- 13.Letson MM, Cooper JN, Deans KJ, Scribano PV, Makoroff Kl, Feldman KW, et al. Prior opportunities to identify abuse in children with abusive head trauma. Child Abuse Negl 2016;60:36–45. [DOI] [PubMed] [Google Scholar]
- 14.Hymel KP, Willson DF, Boos SC, Pullin DA, Homa K, Lorenz DJ, et al. Derivation of a clinical prediction rule for pediatric abusive head trauma. Pediatr Crit Care Med 2013;14:210–20. [DOI] [PubMed] [Google Scholar]
- 15.Hymel KP, Armijo-Garcia V, Foster R, Frazier TN, Stoiko M, Christie LM, et al. Validation of a clinical prediction rule for pediatric abusive head trauma. Pediatrics 2014;134:e1537–44. [DOI] [PubMed] [Google Scholar]
- 16.Hymel KP, Herman BE, Narang SK, Graf JM, Frazier TN, Stoiko M, et al. Potential impact of a validated screening tool for pediatric abusive head trauma. J Pediatr 2015;167:1375–81. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer H, Smith A, Kemp AM, Cowley LE, Cheek JA, Dalziel SR, et al. External validation of the PediBIRN clinical prediction rule for abusive head trauma. Pediatrics 2018;141:e20173674. [DOI] [PubMed] [Google Scholar]
- 18.Hymel KP, Fingarson AK, Pierce MC, Kaczor K, Makoroff KL, Wang M. External validation of the PediBIRN screening tool for abusive head trauma in pediatric emergency department settings. Pediatr Emer Care, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? a framework for improvement. JAMA 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 20.Reilly BM, Evans AT. Translating clinical research into clinical practice: impact of using prediction rules to make decisions. Ann Intern Med 2006;144:201–9. [DOI] [PubMed] [Google Scholar]
- 21.Wallace E, Smith SM, Perera-Salazar R, Vaucher P, McCowan C, Collins G, et al. Framework for the imapct analysis and implementation of clinical prediction rules. BMC Med Inform Decis Mak 2011;11:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toll DB, Janssen KJM, Vergouwe Y, Moons KGM. Validation, updating and impact of clinical prediction rules: a review. J Clin Epidemiol 2008;61:1085–94. [DOI] [PubMed] [Google Scholar]
- 23.Berger RP, Fromkin J, Herman B, Pierce MC, Saladino RA, Flom L, et al. Validation of the Pittsburgh infant brain injury score for abusive head trauma. Pediatrics 2016;138:e20153756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce MC, Kaczor K, Aldridge S, O’Flynn J, Lorenz DJ. Bruising characteristics discriminating physical child abuse from accidental trauma. Pediatrics 2010;125:67–74. [DOI] [PubMed] [Google Scholar]
- 25.Cowley LE, Morris CB, Maguire SA, Farewell DM, Kemp AM. Validation of a prediction tool for abusive head trauma. Pediatrics 2015;136:290–298. [DOI] [PubMed] [Google Scholar]
- 26.Maguire SA, Kemp AM, Lumb RC, Farewell DM. Estimating the probability of abusive head trauma: A pooled analysis. Pediatrics 2011;128:e550–e564. [DOI] [PubMed] [Google Scholar]
- 27.Hymel KP, Wang M, Chinchilli VM, Karst WA, Willson DF, Dias MS, et al. Estimating the probability of abusive head trauma after abuse evaluation. Child Abuse Negl 2019;88:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 2
From baseline in prior observational studies to the CRT, intervention physicians increased the proportions of all patients evaluated at least partially for abuse (73%→89%), higher risk patients evaluated thoroughly for abuse (67%→81%), and lower risk patients evaluated at least partially for abuse (43%→68%). These changes in abusive head trauma evaluation practices resulted in lower proportions of potential (40%→21%) and estimated cases of missed abusive head trauma (15%→7%), and a lower overall diagnostic yield of completed abuse evaluations (50%→46%). At control PICUs, the opposite trends were observed. The small brackets identify changes from baseline to CRT that were statistically significant (P ≤.05). The large brackets identify divergent changes (from baseline to CRT) at intervention vs. control PICUs that were statistically significant. The red boxes identify differences at intervention vs. control PICUs that were statistically significant during the CRT.