Abstract
Background:
High amounts of coronary artery calcium (CAC) pose challenges in interpretation of coronary CT angiography (CCTA). The accuracy of stenosis assessment by CCTA in patients with very extensive CAC is uncertain.
Methods:
Retrospective study was performed including patients who underwent clinically directed CCTA with CAC score >1000 and invasive coronary angiography within 90 days. Segmental stenosis on CCTA was graded by visual inspection with two-observer consensus using categories of 0%, 1–24%, 25–49%, 50–69%, 70–99%, 100% stenosis, or uninterpretable. Blinded quantitative coronary angiography (QCA) was performed on all segments with stenosis ≥25% by CCTA. The primary outcome was vessel-based agreement between CCTA and QCA, using significant stenosis defined by diameter stenosis ≥ 70%. Secondary analyses on a per-patient basis and inclusive of uninterpretable segments were performed.
Results:
726 segments with stenosis ≥25% in 346 vessels within 119 patients were analyzed. Median coronary calcium score was 1616 (1221–2118). CCTA identification of QCA-based stenosis resulted in a per-vessel sensitivity of 79%, specificity of 75%, positive predictive value (PPV) of 45%, negative predictive value (NPV) of 93%, and accuracy 76% (68 false positive and 15 false negative). Per-patient analysis had sensitivity 94%, specificity 55%, PPV 63%, NPV 92%, and accuracy 72% (30 false-positive and 3 false-negative). Inclusion of uninterpretable segments had variable effect on sensitivity and specificity, depending on whether they are considered as significant or non-significant stenosis.
Conclusions:
In patients with very extensive CAC (>1000 Agatston units), CCTA retained a negative predictive value > 90% to identify lack of significant stenosis on a per-vessel and per-patient level, but frequently overestimated stenosis.
Keywords: Coronary CT angiography, coronary calcium score, coronary artery calcium, accuracy, stenosis, quantitative coronary angiography
INTRODUCTION
Coronary Computed Tomography angiography (CCTA) is rapidly assuming a central role for assessing symptomatic patients for coronary artery disease (CAD). A high burden of coronary artery calcium (CAC) is known to affect the diagnostic information from CCTA due to partial volume effects and beam hardening.1, 2 Recent generation CT systems with faster temporal resolution, increased spatial resolution, improved x-ray filtering, and new reconstruction algorithms have provided opportunity to improve imaging in highly calcified coronary arteries.3 However, the diagnostic accuracy of CCTA, even with modern equipment and reconstructions in the presence of very extensive calcification, has not been assessed. It is important for clinicians ordering CCTA and cardiac imagers protocoling studies and reading images to understand the limitations of scan interpretation and the reliability of reporting of coronary stenosis in order to effectively use this technology in these patients.
The aim of our study was to assess the performance of clinical CCTA using second and third generation dual-source CT scanner platforms in patients with coronary artery calcium score (CACs) exceeding 1000. We compared stenoses (≥ 25% diameter) identified by CCTA to invasive quantitative coronary angiography (QCA) measurement to determine the accuracy of clinical CCTA interpretation in patients with high CACs. Recognizing the known challenges to CT imaging of highly calcified vessels,4–7 we hypothesized that CCTA with contemporary approaches would be effective at ruling out significant stenosis, but that overall accuracy would remain limited by low specificity.
METHODS
Study Sample
Subjects were selected post-hoc from a prospective cohort of patients who underwent CCTA for clinical purposes using second and third generation dual source scanners at a single center from 2013–2018 (Figure 1). One-hundred and nineteen consecutive patients who had a clinical CCTA, CACs > 1000 Agatston units, and underwent invasive coronary angiography (ICA) within 90 days after CCTA were included. Patients who had previous stenting (n=43) or coronary artery bypass grafts (n=12) were excluded. The study was approved by the local IRB and all patients gave written informed consent.
Figure 1.
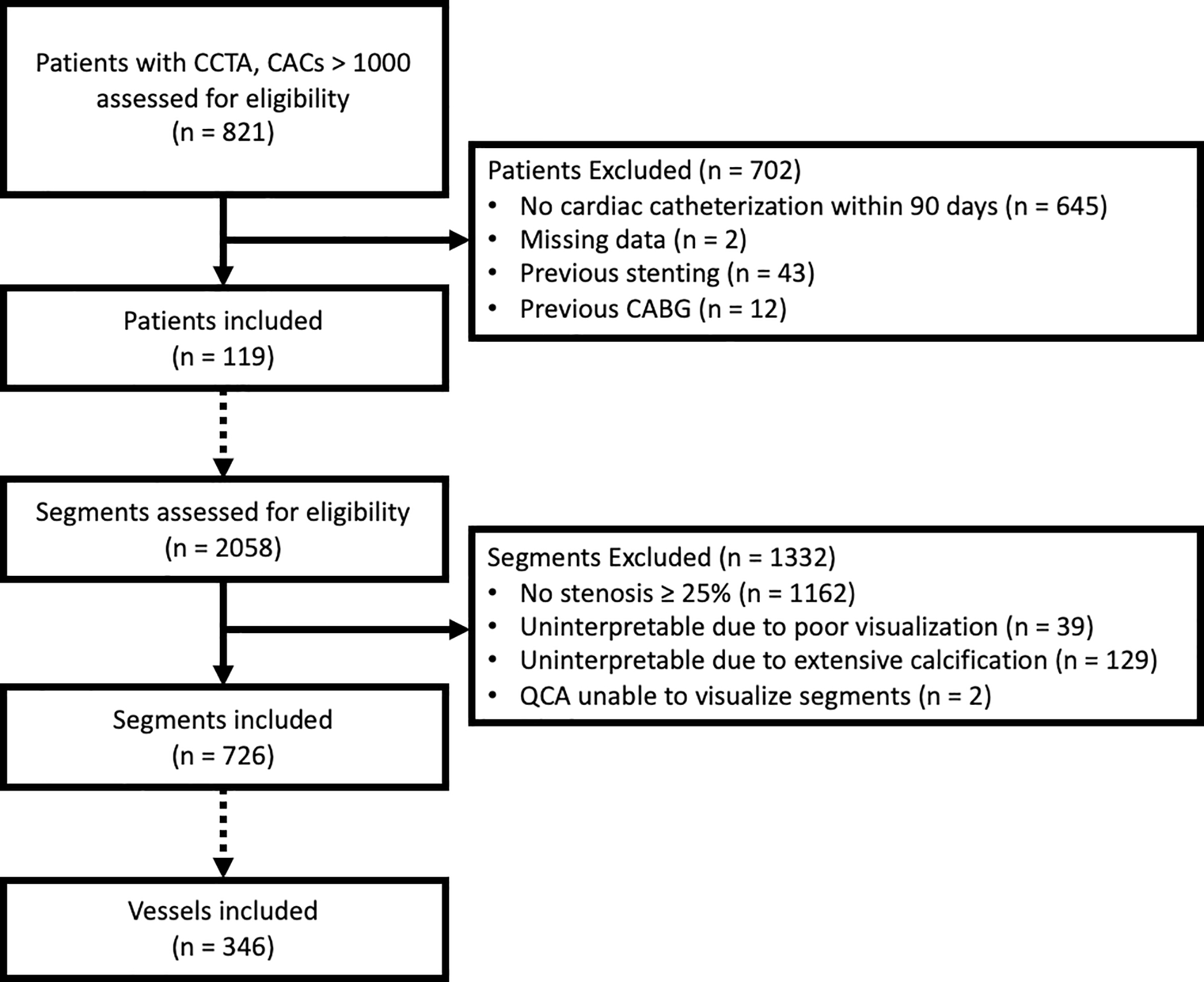
Consort diagram of screening and inclusion. Dashed arrows when changing screening criteria from patient to segment and segment to vessel.
Outcome Measures
The primary outcome was per-vessel agreement of CCTA with QCA for presence of ≥ 70% maximum diameter stenosis. All ≥ 2 mm diameter vessels with a qualitative CCTA-graded stenosis ≥ 25% were included in the primary analysis. Secondary analyses included per-patient agreement, as well as per-vessel and per-patient analyses with inclusion of segments deemed by CCTA to be “uninterpretable due to calcification.” The uninterpretable segment analyses were twofold, assessing testing characteristics if uninterpretable segments were arbitrarily assigned to either ≥ 70% or < 70% CCTA stenosis category and compared to measured QCA. In order to assess for selection bias due to restriction of the study population to those who received ICA with 90 days, the proportion of patients with uninterpretable calcification in significant locations was compared between the study population and all patients during the same timeframe with CACs > 1000 regardless of subsequent referral to ICA. In order to assess for causes of incorrect interpretation we compared factors between correctly and incorrectly graded vessels and patients. Tested variables included demographics: age and sex; risk factors: BMI, CACs, CACs plus total valvular and aortic calcium, vessel-based calcium, symptoms, hypertension, hyperlipidemia, diabetes, insulin use, family history of coronary artery disease, dialysis status, peripheral arterial disease, history of smoking and history of stroke; and acquisition factors: contrast volume, max heart rate, acquisition mode, use of beta blockade, nitroglycerin, and kVp.
Imaging Acquisition
CAC scanning and CCTA was performed on two dual-source CT platforms (Siemens Flash and Force scanners, Siemens Healthineers, Erlangen, Germany), using standard clinical coronary imaging protocols as previously described.8 Prior to the exam, beta blockers were administered in patients with heart rates >65 beats per minute. Sublingual nitroglycerin spray (0.4–0.8mg) was given for coronary vasodilation. A non-contrast ECG-gated coronary calcium scan was acquired prior to CCTA at 60–70% of the R-R interval using 120 kVp and mAs 80) and was reconstructed with 2.5–3.0mm slice thickness. Patients received prospectively gated protocols if feasible, and helical scans if body mass index (BMI) >35 kg/m2 or atrial fibrillation was present. Kilovoltage peak ranged between 80–120 kV at the discretion of the technologist. Automatic exposure control was used with further adjustment at the discretion of the technologists. The standard contrast agent was Iohexol (Omipaque 350mg/mL, GE Healthcare, Buckinghamshire, UK), administered via large-bore intravenous catheter. Contrast volume varied between 85 to 200 mL with injection rate 5 to 8 mL/s, adapted to BMI and followed by saline flush. Automated bolus tracking or timing bolus was used to trigger acquisition. Imaging reconstruction was performed with a 0.6 mm slice thickness and default medium-sharp cardiac reconstruction kernel (B36) with iterative reconstruction (SAFIRE or ADMIRE, Siemens Healthineers, Erlangen, Germany) with option for additional sharper kernels or non-iterative reconstruction at the request of the reading physician to use in an integrated assessment.
Coronary Calcium Score and CT Angiography Analysis
The Agatston scoring was performed by an experienced technologist using semiautomated software (NetraMD, ScImage, Los Altos, California) and was reviewed by the interpreting physicians.9 CCTA interpretation was performed as per standard laboratory protocol on a separate workstation (Syngo.Via, SiemensHealthineers, Erlangen, Germany) by two clinicians, with initial reading by an advanced cardiac imaging fellow and over-read by a senior imaging cardiologist with extensive experience with CCTA. The coronary artery tree was divided into standard segments10 which were graded into categories of stenosis by viewing of axial imaging, curved multiplanar images, and double-oblique views for all vessels ≥ 2 mm in diameter. Diameter stenosis categories for this study were: no stenosis, 1–24%, 25–49%, 50–69%, 70–99%, 100% stenosis or uninterpretable. Uninterpretable segments were coded as being due to calcification or not well seen. For the primary analyses, segments which were deemed uninterpretable were excluded. Segments that were uninterpretable due to calcification were included in a secondary analysis; segments that were not well seen for other reasons remained excluded. For per-vessel analyses, segments were assigned to four vessel categories: left main, left anterior descending artery plus ramus intermedius and diagonal branches, left circumflex and obtuse marginal branches, and right coronary artery with posterior descending artery and posterolateral branches. The maximum stenosis in each vessel was classified as greater or less than 70% stenosis. For patient-level analyses, the maximum stenosis in any segment was identified as greater or less than 70% stenosis.
Quantitative Coronary Angiography Analysis
All ≥ 2 mm vessel regions with a ≥ 25% stenosis or labeled as “uninterpretable due to calcification” on CCTA were identified. The location of the coronary segment involved, with blinding to the degree of stenosis, was provided to readers to guide invasive quantitative coronary angiography (QCA) analysis. The most recent ICA within 90 days after the CCTA was used for analysis if multiple ICA cases were performed on a patient. Fluoroscopy images were analyzed with dedicated software (CAAS Workstation, V8.1, Pie Medical Imaging, Maastricht, Netherlands). View and frame for each segment was selected to minimize foreshortening and overlying vessels and maximize contrast enhancement. If a lesion was clearly eccentric, the view with the greatest stenosis was chosen. Reference diameter was the nearest healthy segment proximal to the lesion. Stenosis percent was 1-(max diameter stenosis within segment / diameter of reference segment) and organized by ordinal categories (stenosis of 1–24%, 25–49%, 50–69%, 70–99%, or 100%). After initial analysis, vessels where QCA and CCTA differed by more than one ordinal category were individually assessed for contributing factors including calcification, motion, or other artifact. Examples of highly calcified lesions resulting in erroneous classification are shown in Figure 2.
Figure 2.
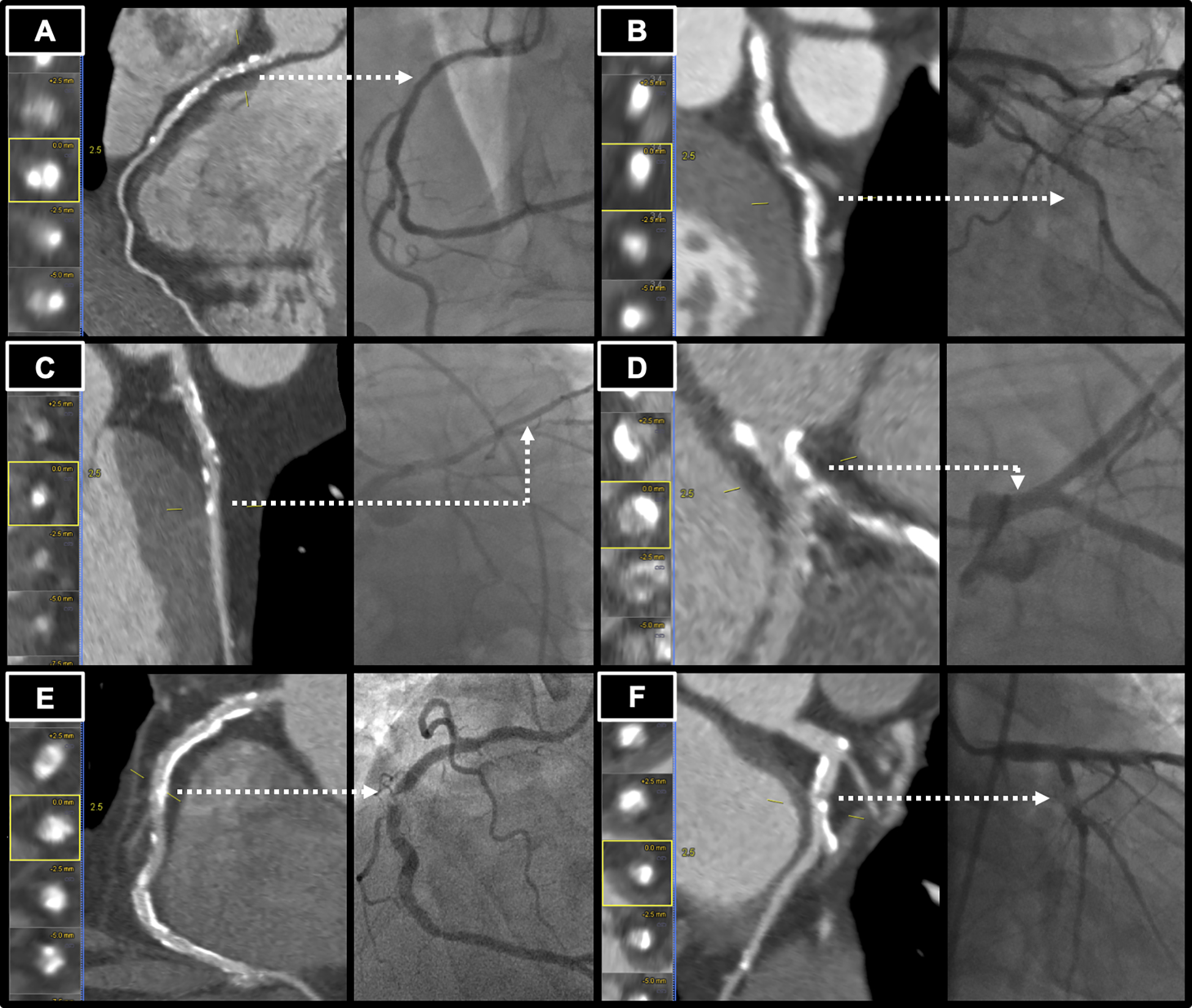
Examples of calcified lesions resulting in incorrect estimate by coronary CT angiography (CCTA). Dashed arrow indicates same lesion in CCTA and cardiac catheterization. (A) Left Panel: Calcified stenosis in the proximal right coronary artery (RCA) graded as 70–99% stenosis by CCTA. Right Panel: Cardiac catheterization view with 46% stenosis by quantitative coronary angiography (QCA). (B) Left Panel: Calcified stenosis in the left circumflex artery (LCx) graded as 70–99% stenosis by CCTA. Right Panel: Cardiac catherization view with 11% stenosis by QCA. (C) Left Panel: Calcified stenosis in the left anterior descending artery graded as 70–99% stenosis by CCTA. Right Panel: Cardiac catherization view with 32% stenosis by QCA. (D) Left Panel: Calcified stenosis in the left main coronary artery graded as 50–69% stenosis by CCTA. Right Panel: Cardiac catherization view with 6% stenosis by QCA. (E) Left Panel: Calcified stenosis in the mid RCA, graded as 25–49% stenosis by CCTA. Right Panel: Cardiac catheterization view with 97% stenosis by QCA. (F) Left Panel: Calcified stenosis in the LCx graded as 25–49% stenosis by CCTA. Right Panel: Cardiac catherization view with 93% stenosis by QCA.
Statistical Analysis
Patient and vessel demographic data were reported as mean and standard deviation for normally distributed variables, median and interquartile range for non-normal variables, and as a percent for categorical variables. Agreement for significant vessel-based stenosis was tested using unweighted Cohen’s kappa and Kendall’s tau. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for prediction of significant stenosis (greater than 70%). Comparison of correctly and incorrectly assessed vessels and patients was performed using two-sample t-test for continuous variables and chi-square test for categorical variables. Analysis was performed with R (v 3.6.1) / R Studio (v 1.2.5019). Statistical significance was pre-specified as p < 0.05.
RESULTS
The population consisted of 119 patients with mean age 69 years (Table 1) with 2058 coronary artery segments coded on CCTA. The patients were predominantly male (82%) and frequently overweight or obese. Acquisition energies included 1 (1%) patient with 80 kVp, 4 (3%) patients with 100 kVp, 1 (1%) patient with 110 kVp, and 113 (95%) with 120 kVp. Of the total coded coronary segments, 1162 (56%) had no disease or stenosis between 1–24%, 39 (2%) were coded as uninterpretable due to inadequate visualization, 129 (6%) were uninterpretable due to extensive calcification, and 728 had graded stenosis ≥ 25%. Of the 728 segments, QCA analysis could be performed on all but two segments. Our primary analysis included 346 vessels with 726 (35%) CCTA segments with stenosis ≥ 25%. Secondary analysis added 125 of the 129 segments coded as uninterpretable due to calcification, as four segments were inadequately visualized by QCA. The median CACs was 1616 (IQR 1221–2118) and 72% of the patients had CACs 1000–1999.
Table 1.
Baseline demographic information for included patients, scan parameters, and coronary segment distribution.
| Characteristic | Value |
|---|---|
| N (Patients) | 119 |
| N (Vessels) | 346 |
| Age (years) | 68.8±9.8 |
| Male | 97 (82%) |
| Median CAC (Agatston) | 1616 (1221–2118) |
| CAC 1000–1999 (N Patients) | 86 (72%) |
| CAC 2000–2999 (N Patients) | 21 (18%) |
| CAC > 3000 (N Patients) | 12 (10%) |
| BMI (kg/m2) | 26.6 (24.4–30.1) |
| Obese, BMI ≥ 30 | 31 (26%) |
| Presence of symptoms | 86 (72%) |
| Heart Rate (beats per min) | 65.6±14.1 |
| Contrast Volume (ml) | 100 (100–100) |
| Beta Blocker Premedication | 29 (24%) |
| Nitroglycerin Premedication | 104 (87%) |
| N (Segments) | 726 |
| Left Main | 38 (5%) |
| LAD | 280 (39%) |
| LCX | 171 (24%) |
| RCA | 237 (33%) |
CACs: Coronary Artery Calcium Score, BMI: Body Mass Index, LAD: Left anterior descending coronary artery, LCX: Left circumflex coronary artery, RCA: Right coronary artery.
By CCTA and QCA, the highest proportion of vessels were in the 25–49% stenosis group, with descending frequency as stenosis severity increased (Table 2). Comparison of CCTA categories to QCA showed that the proportion of patients with 70–99% was over 50% lower by QCA than by CCTA. On a per-vessel basis, by CCTA there were 223 (64%) vessels with stenosis < 70% and 123 (36%) with stenosis ≥ 70%. By invasive QCA, there were 276 (80%) vessels with <70% and 70 (20%) ≥ 70% stenosis.
Table 2.
Distribution of per-vessel stenosis segments by ordinal category and corresponding QCA measurements.
| CCTA Per-Vessel Maximum Stenosis Frequency (percent) | QCA Per-Vessel Maximum Stenosis Frequency (percent) | %QCA Per-Vessel Maximum Stenosis by CCTA group | |
|---|---|---|---|
| 1–24% | N/A | 12 (3%) | N/A |
| 25–49% | 118 (34%) | 163 (47%) | 42 ± 15% |
| 50–69% | 105 (30%) | 102 (29%) | 48 ± 14% |
| 70–99% | 99 (29%) | 44 (13%) | 63 ± 21% |
| 100% | 24 (7%) | 25 (7%) | 86 ± 21% |
CCTA: Coronary CT angiography, QCA: Quantitative coronary angiography.
For detection of significant stenosis, agreement between CCTA and QCA was present in 263 vessels (76%) and disagreement in 83 vessels (24%) (Table 3A). In vessels with disagreement, CCTA showed stenosis ≥ 70% when QCA did not in 68 vessels, and QCA identified stenosis ≥ 70% when CCTA did not in 15 vessels. By category of stenosis, CCTA overestimated stenosis by >1 category in 29 vessels (8%) and underestimated stenosis by >1 category in 6 vessels (2%) (Table 3B). In these vessels, the discrepancy was in regions with excessive calcification alone in 11, calcification plus other artifact in 15, due to other artifact alone in 8, and due to overcall without artifact in 1 (Supplemental Table 1). Measures of agreement were both significantly correlated with Cohen’s Kappa 0.42, p<0.0001, and Kendall’s Tau 0.45, p<0.0001. Per-vessel comparison of ordinal stenosis categories (1–24%, 25–49%, 50–69%, 70–99%, and 100%) showed exact agreement 43% of the time and agreement within one category of the QCA measurement 90% of the time. CCTA overestimated QCA in 145 vessels (42%) and underestimated QCA in 51 vessels (15%). For identification of QCA stenosis ≥ 70%, CCTA had a sensitivity of 79%, specificity of 75%, PPV 45%, NPV 93%, and accuracy 76% (Table 4).
Table 3.
Comparison of stenosis categories by per-vessel maximum stenosis. Coronary CT angiography versus invasive quantitative coronary angiography.
| A. | QCA | ||
| CCTA | < 70% | ≥ 70% | |
| < 70% | 208 | 15 | |
| ≥ 70% | 68 | 55 | |
| B. | QCA | |||||
| 1–24% | 25–49% | 50–69% | 70–99% | 100% | ||
| 1–24% | N/A | N/A | N/A | N/A | N/A | |
| CCTA | 25–50% | 9 | 78 | 25 | 6 | 0 |
| 50–69% | 1 | 61 | 34 | 9 | 0 | |
|
| ||||||
| 70–99% | 2 | 21 | 41 | 24 | 11 | |
| 100% | 0 | 3 | 2 | 5 | 14 | |
A: Comparison by <70% and ≥ 70% stenosis.
B: Comparison by ordinal stenosis categories; black-shaded squares with exact match, grey within one category, and white outside of one category.
CCTA: Coronary CT angiography, QCA: Quantitative coronary angiography.
Table 4.
Testing characteristics of analyses using per-vessel and per-patient approaches.
| Per-Vessel | Per-Patient | |
|---|---|---|
| Sensitivity | 79% | 94% |
| Specificity | 75% | 55% |
| Positive Predictive Value | 45% | 63% |
| Negative Predictive Value | 93% | 92% |
| Accuracy | 76% | 72% |
Reference standard quantitative coronary angiography stenosis ≥ 70%.
Secondary Analyses
On a per-patient analysis, by CCTA, 39 (32%) of 119 patients had no stenosis ≥ 70% and 80 (68%) had at least one stenosis ≥ 70% (Table 5). QCA identified 66 (55%) patients without stenosis ≥ 70% and 53 (45%) with stenosis ≥ 70%. Agreement between CCTA and QCA was present in 88 patients (74%), and disagreement in 33 patients (26%) (Table 6A). In the patients with disagreement, CCTA showed stenosis ≥ 70% when QCA did not in 30 (25%) cases and showed no stenosis ≥ 70% when QCA identified significant stenosis in 3 (3%) cases. Examination by ordinal categories revealed no cases where CCTA underestimated the maximum stenosis by more than one category and 12 patients where the category was overestimated by more than one category (Table 6B). CCTA had a sensitivity 94%, specificity of 55%, PPV of 63%, NPV of 92%, and accuracy of 72% for predicting QCA ≥ 70% within patients (Table 4).
Table 5.
Distribution of per-patient stenosis segments by ordinal category and corresponding QCA measurements.
| CCTA Per-Patient Stenosis Frequency (percent) | QCA Per-Patient Stenosis Frequency (percent) | %QCA Per-Patient Stenosis by CCTA group | |
|---|---|---|---|
| 1–24% | N/A | 0 (0%) | N/A |
| 25–49% | 6 (5%) | 24 (20%) | 45.2 ± 11.3% |
| 50–69% | 33 (28%) | 42 (35%) | 53.9 ± 12.8% |
| 70–99% | 59 (50%) | 31 (26%) | 71.2 ± 18.8% |
| 100% | 21 (18%) | 22 (18%) | 90.2 ± 16.6% |
Table 6.
Comparison of stenosis categories by per-patient maximum stenosis. Coronary CT angiography versus invasive quantitative coronary angiography.
| A. | QCA | ||
| CCTA | < 70% | ≥ 70% | |
| < 70% | 36 | 3 | |
| ≥ 70% | 30 | 50 | |
| B. | QCA | ||||
| 25–49% | 50–69% | 70–99% | 100% | ||
| CCTA | 25–50% | 3 | 3 | 0 | 0 |
| 50–69% | 13 | 17 | 3 | 0 | |
|
| |||||
| 70–99% | 8 | 18 | 25 | 8 | |
| 100% | 0 | 4 | 3 | 14 | |
A: Comparison by <70% and ≥ 70% stenosis.
B: Comparison by ordinal stenosis categories; black-shaded squares with exact match, grey within one category, and white outside of one category.
CCTA: Coronary CT angiography, QCA: Quantitative coronary angiography.
In the 168 segments in 105 vessels and 61 patients (51%) deemed uninterpretable by CCTA, 129 (76%) segments were due to calcification and 39 (24%) were due to inadequate visualization. Of the segments deemed uninterpretable due to calcification, 4 (2%) could not be assessed by QCA and were excluded from analysis. Inclusion of uninterpretable calcification segments in the analyses in the analysis yielded decreases in accuracy in all groups except for per-patient with uninterpretable as CCTA < 70% (Tables 7a and 7b). On a per vessel basis, when vessels with uninterpretable segments were considered as having obstruction, per-vessel accuracy was significantly reduced (8%) as was the specificity (11%), with minor increase in sensitivity (2%). On a per-patient basis, accuracy was reduced by 10%, specificity by 17%, with a 1% increase in sensitivity. (Table 7a). In contrast, if these vessels were considered as non-significant, accuracy fell by only 1% per-vessel basis and increased by 2% on a per-patient basis, with the largest worsening being per-vessel sensitivity (6%) and per-patient negative predictive value (5%) and modest (1–2%) improvement in per-vessel PPV, and per-patient specificity and PPV (Table 7b). Overall, the effect of inclusion of the segments uninterpretable due to calcium was minimal when considering the segments as non-significant, which is concordant with the observation that by QCA, including these segments only increased the maximum in 8 (2%) vessels and 6 (5%) patients.
Table 7.
Testing characteristics of analyses using per-vessel and per-patient approaches with the addition of segments deemed uninterpretable by CCTA due to calcification.
| A. | ||
| Per-Vessel + Uninterpretable | Per-Patient + Uninterpretable | |
| Sensitivity | 81% | 95% |
| Specificity | 64% | 38% |
| Positive Predictive Value | 40% | 60% |
| Negative Predictive Value 1 | 92% | 88% |
| Accuracy | 68% | 66% |
| B. | ||
| Per-Vessel + Uninterpretable | Per-Patient + Uninterpretable | |
| Sensitivity | 73% | 92% |
| Specificity | 75% | 57% |
| Positive Predictive Value | 46% | 68% |
| Negative Predictive Value 1 | 91% | 87% |
| Accuracy | 75% | 74% |
A: Analysis with uninterpretable CCTA segments assigned to ≥ 70% stenosis.
B. Analysis with uninterpretable CCTA segments assigned to < 70% stenosis.
Reference standard quantitative coronary angiography stenosis ≥ 70%.
Regarding assessment for selection bias due to ICA, the proportion of patients with uninterpretable segments in significant locations (31 out of 119, 26%) was slightly higher in our population versus the population of patients with CACs > 1000 excluded from the study during the same time period (147 out of 702, 21%). Regarding differences between correctly and incorrectly scored vessels and patients, on a per-patient basis, the only significant variables were circumflex coronary calcium score and history of stroke; however, both of these were in an unexpected direction, that is the group with agreement had both higher circumflex CACs and more frequent stroke history, suggesting that this may not be meaningful. On a per-vessel basis, the vessel analyzed was significant, with left main having a much higher proportion of correctly assessed disease (36 correct, 2 incorrect). Notably, total and vessel-specific calcium measures, BMI, and acquisition factors all were not significantly different between correctly and incorrectly assessed groups (Supplemental table 2).
DISCUSSION
With increasing literature validation and changing guidelines, CCTA is undergoing a rapid increase in utilization.11 It is likely that there will be a broadening of the pool of patients that are referred, including patients with higher calcification burden as the study becomes more widely used in patients with a high likelihood of CAD or known CAD. Further, in the earlier use of CCTA, patients with very high CAC scores (e.g., over 1000) often had CCTA studies canceled, while at the present time, most laboratories are performing CCTA studies in these patients. The main findings of this study of CCTA accuracy in patients with very extensive CAC were three-fold. First, the negative predictive value on the per-vessel and per-patient basis for stenosis on QCA remained high as was the sensitivity in the per-patient analysis; however, positive predictive value and specificity were low. Secondly, CCTA stenosis grading was within 1 category of the QCA in 90% of vessels, but not in a balanced manner: CCTA was significantly more likely to overestimate stenosis on QCA than to underestimate stenosis, and particularly in highly calcified vessels. Third, a large proportion of patients had uninterpretable segments, including in significant locations. Inclusion of these segments as significant resulted in large reductions in accuracy with only minor improvements in sensitivity, but inclusion as non-significant had minimal effect on testing characteristics. Given the higher proportion of patients with uninterpretable segments in significant locations versus the larger population, we may have some degree of selection bias towards more difficult to interpret cases within the CACs > 1000 population.
Previous studies have investigated the ability of CCTA to rule out significant stenosis in patients with varying levels of CAC, have also shown high sensitivity and negative predictive value but limited specificity and accuracy.4, 12–23 Most of these studies, however, examined a range of calcification, but had small proportions of patients in high calcification groups. Only two studies were confined to high calcification (> 400 Agatston Units).17, 23 In comparison with prior studies, with the entry criterion of CACs >1000 and an average coronary calcium score of over 1600, the patients in the current study had a much higher degree of calcification and over double the average score of the majority of the prior studies. The overall sensitivities on a per-patient basis of CCTA compared to QCA for ≥ 70% stenosis were high (94% excluding and 95% including uninterpretable segments), and comparable to studies in which patients with extensive calcification have been excluded; however, specificity was lower.
Causes for CCTA inaccuracy have been previously assessed by Kruk et al., and included anatomical calcium factors including total calcium, length of calcium, thickness, volume, arc, and luminal diameter.24 We did not have a similar granular anatomical detail in our study, but examined the effects of risk factors, demographics, and acquisition factors, which included the vessel-based calcium burden. The significant factors were limited to left circumflex calcium score and stroke on a per-patient basis; however, the directionality of this association (higher disease burden with better accuracy) leads us to doubt the relevance of this finding. On a per-vessel basis, while it was reassuring that left main assessments had high reliability, we were surprised that vessel-based calcium score did not affect accuracy. This may be related to the quantity of calcium being above a threshold where a difference could be observed – the mean per-vessel calcium score was 569, which given the inclusion of the left main as a vessel, may be underestimated for other territories. The mean total CACs in Kruk et al. was 433. We also observe that there was a strong tendency of our interpreters, despite their experience, toward an overestimation of percent stenosis. This overestimation might be expected to be higher in centers with less experienced interpreters and could result in over-referral to invasive angiography compared to functional studies.25–27 The effect may be even more than our data suggest, as we have observed that identification of CCTA stenosis ≥ 50% (not only ≥ 70%) often prompts invasive angiography in patients with elevated risk at our center. Of further note, the scanners utilized in this study had higher spatial (0.24–0.33 mm) and temporal resolution (≤75 msec) than in prior studies. Both of these factors reduce calcium blooming by reduction of motion blur and partial volume effects.1 The findings regarding overestimation of stenosis by CCTA might be greater if scanners with lower spatial and temporal resolution were utilized. On the other hand, impending novel hardware advances in CCTA technology using higher resolution CT or photon-counting CT detectors will likely improve visualization in highly calcified coronary arteries in the future.28–31
Our study has limitations. The necessary inclusion criteria of patients who had both CCTA and ICA is likely to result in selection bias. In comparing our study population to the overall population of patients with CCTA and CACs > 1000, there were moderate differences in the frequency of uninterpretable segments in significant locations. The effect of this bias may falsely elevate the sensitivity of our results at the cost of specificity. In our analysis of factors related to incorrect assessment, our data did not have enough granularity to examine the effect of very high CAC scores in individual plaques, which may have revealed different results than our per-vessel calcium burden. The distribution of CACs was heavily weighted to the 1000–1999 population, and therefore the study results may be less applicable to patients with higher CACs. The use of CCTA stenoses < 25% as an exclusion has a significant theoretical limitation: if underestimation by CCTA is frequent, these segments would have been excluded from the analyzed dataset. This would be particularly concerning if segments with >70% stenosis were underestimated; however, due to CCTA’s high sensitivity, this is unlikely. This study measures anatomic relationships of CCTA and QCA. As known, degree of anatomical stenosis by either method does not necessarily correspond to functional significance measured by fractional flow reserve or perfusion methods. The clinical reading of CCTA employed a semiquantitative visual assessment which was compared to a quantitative invasive angiographic interpretation. Automated software assessment of the degree of stenosis on CCTA might optimize the comparison to QCA. We opted for a vessel-based analysis, instead of direct segmental comparisons to fully blind QCA readers to the CCTA results. Our primary analysis excluded uninterpretable segments. Including these segments in a secondary analysis resulted in significant differences in accuracy; though we recognize that arbitrary assignment to a positive or negative category does not reflect clinical uncertainty that may be conveyed in a report. Since optimal views for all segments were not uniformly performed in the clinical invasive angiography procedures, exact correspondence between segments on CCTA and QCA might have been compromised due to foreshortening or overlying vessels.
In conclusion, our study shows that CCTA in patients with CACs > 1000 remains effective to rule out significant stenosis on a per-vessel and per-patient basis but is associated with a systematic overestimation of stenosis, resulting in low positive predictive value. While performing CCTA in patients with very high CAC is reasonable and inevitable given expansion of guidelines regarding use of CCTA patients with a high likelihood of CAD, our results show that the limitations of accuracy and increased uncertainty in this patient population must be appreciated. Given concerns regarding over-referral to invasive angiography by CCTA, these results underscore the need for cardiac imagers to recognize and communicate reduced certainty when present in the categorization of percent stenosis in patients with severely elevated CACs. While there is a tendency of readers to avoid the category of “uninterpretable” regarding coronary segments, the results of this study suggest that this designation is frequently appropriate, and possibly underutilized. Strict interpretation of these segments as necessitating invasive assessment should not be advocated, as QCA measurements affecting maximal stenosis was relatively infrequent from these segments. High coronary calcium remains a significant barrier to accurate CCTA assessment.
Supplementary Material
Sources of Funding
Alan Kwan reports funding from NIH T32HL116273 and the Doris Duke Charitable Foundation Grant 2020059. Adele Pope was supported by a grant from the Heart Foundation of New Zealand Research. The work was supported by a grant from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Abbreviations:
- BMI
Body mass index
- CAC
Coronary artery calcium
- CACs
Coronary artery calcium score
- CAD
Coronary artery disease
- CCTA
Coronary CT angiography
- ICA
Invasive coronary angiography
- NPV
Negative predictive value
- PPV
Positive predictive value
- QCA
Quantitative coronary angiography
- SCCT
Society of Cardiovascular CT
Footnotes
Conflict of Interest
All authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kalisz K, Buethe J, Saboo SS, Abbara S, Halliburton S, Rajiah P. Artifacts at cardiac CT: physics and solutions. Radiographics. 2016;36:2064–2083. [DOI] [PubMed] [Google Scholar]
- 2.Cheng V, Gutstein A, Wolak A, et al. Moving beyond binary grading of coronary arterial stenoses on coronary computed tomographic angiography: insights for the imager and referring clinician. JACC: Cardiovascular Imaging. 2008;1:460–471. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, Ng CK, Xu L, Fan Z, Lei J. Coronary CT angiography in heavily calcified coronary arteries: Improvement of coronary lumen visualization and coronary stenosis assessment with image postprocessing methods. Medicine. 2015;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vavere AL, Arbab-Zadeh A, Rochitte CE, et al. Coronary artery stenoses: accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification—a subanalysis of the CORE-64 trial. Radiology. 2011;261:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong TK, Chin SP, Liew CK, et al. Accuracy of 64-row multidetector computed tomography in detecting coronary artery disease in 134 symptomatic patients: influence of calcification. American heart journal. 2006;151:1323. e1321–1323. e1326. [DOI] [PubMed] [Google Scholar]
- 6.Noll D, Kruk M, Demkow M, et al. Patterns of Coronary Calcification and Their Impact on the Diagnostic Accuracy of Computed Tomography Coronary Angiography. Journal of computer assisted tomography. 2018;42:263–268. [DOI] [PubMed] [Google Scholar]
- 7.Laggoune J, Nerlekar N, Munnur K, et al. The utility of coronary computed tomography angiography in elderly patients. Journal of geriatric cardiology: JGC. 2019;16:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RJ, Eisenberg E, Friedman J, et al. Impact of heart rate on coronary computed tomographic angiography interpretability with a third-generation dual-source scanner. International journal of cardiology. 2019;295:42–47. [DOI] [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 10.Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. Journal of cardiovascular computed tomography. 2014;8:342–358. [DOI] [PubMed] [Google Scholar]
- 11.Levin DC, Parker L, Halpern EJ, Rao VM. Coronary CT Angiography: Reversal of Earlier Utilization Trends. Journal of the American College of Radiology. 2019;16:147–155. [DOI] [PubMed] [Google Scholar]
- 12.Cademartiri F, Mollet NR, Lemos PA, et al. Impact of coronary calcium score on diagnostic accuracy for the detection of significant coronary stenosis with multislice computed tomography angiography. The American journal of cardiology. 2005;95:1225–1227. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro MA, Miller JM, Schmidt A, et al. Non-invasive half millimetre 32 detector row computed tomography angiography accurately excludes significant stenoses in patients with advanced coronary artery disease and high calcium scores. Heart. 2006;92:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pundziute G, Schuijf JD, Jukema JW, et al. Impact of coronary calcium score on diagnostic accuracy of multislice computed tomography coronary angiography for detection of coronary artery disease. Journal of nuclear cardiology. 2007;14:36–43. [DOI] [PubMed] [Google Scholar]
- 15.Brodoefel H, Burgstahler C, Tsiflikas I, et al. Dual-source CT: effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology. 2008;247:346–355. [DOI] [PubMed] [Google Scholar]
- 16.Chen C-C, Chen C-C, Hsieh I-C, et al. The effect of calcium score on the diagnostic accuracy of coronary computed tomography angiography. The international journal of cardiovascular imaging. 2011;27:37–42. [DOI] [PubMed] [Google Scholar]
- 17.Park MJ, Im Jung J, Choi Y-S, et al. Coronary CT angiography in patients with high calcium score: evaluation of plaque characteristics and diagnostic accuracy. The international journal of cardiovascular imaging. 2011;27:43–51. [DOI] [PubMed] [Google Scholar]
- 18.Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification: the CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. Journal of the American College of Cardiology. 2012;59:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westwood ME, Raatz HD, Misso K, et al. Systematic review of the accuracy of dual-source cardiac CT for detection of arterial stenosis in difficult to image patient groups. Radiology. 2013;267:387–395. [DOI] [PubMed] [Google Scholar]
- 20.Qi L, Tang L-J, Xu Y, et al. The Diagnostic Performance of Coronary CT Angiography for the Assessment of Coronary Stenosis in Calcified Plaque. PloS one. 2016;11:e0154852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghekiere O, Nchimi A, Djekic J, et al. Coronary Computed Tomography Angiography: Patient-related factors determining image quality using a second-generation 320-slice CT scanner. International journal of cardiology. 2016;221:970–976. [DOI] [PubMed] [Google Scholar]
- 22.Schuhbaeck A, Schmid J, Zimmer T, et al. Influence of the coronary calcium score on the ability to rule out coronary artery stenoses by coronary CT angiography in patients with suspected coronary artery disease. Journal of cardiovascular computed tomography. 2016;10:343–350. [DOI] [PubMed] [Google Scholar]
- 23.Ahn SJ, Kang DK, Sun JS, Yoon M-H. Accuracy and predictive value of coronary computed tomography angiography for the detection of obstructive coronary heart disease in patients with an Agatston calcium score above 400. Journal of Computer Assisted Tomography. 2013;37:387–394. [DOI] [PubMed] [Google Scholar]
- 24.Kruk M, Noll D, Achenbach S, et al. Impact of coronary artery calcium characteristics on accuracy of CT angiography. JACC: Cardiovascular Imaging. 2014;7:49–58. [DOI] [PubMed] [Google Scholar]
- 25.Foy AJ, Dhruva SS, Peterson B, Mandrola JM, Morgan DJ, Redberg RF. Coronary computed tomography angiography vs functional stress testing for patients with suspected coronary artery disease: a systematic review and meta-analysis. JAMA internal medicine. 2017;177:1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann U, Truong QA, Schoenfeld DA, et al. Coronary CT angiography versus standard evaluation in acute chest pain. New England Journal of Medicine. 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. New England Journal of Medicine. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motoyama S, Ito H, Sarai M, et al. Ultra-High-Resolution Computed Tomography Angiography for Assessment of Coronary Artery Stenosis. Circulation Journal. 2018:CJ-17–1281. [DOI] [PubMed] [Google Scholar]
- 29.Takagi H, Tanaka R, Nagata K, et al. Diagnostic performance of coronary CT angiography with ultra-high-resolution CT: Comparison with invasive coronary angiography. European journal of radiology. 2018;101:30–37. [DOI] [PubMed] [Google Scholar]
- 30.Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, Fleischmann D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology. 2018;289:293–312. [DOI] [PubMed] [Google Scholar]
- 31.Kwan AC, Pourmorteza A, Stutman D, Bluemke DA, Lima JA. Next-Generation Hardware Advances in CT: Cardiac Applications. Radiology. 2020:192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


